Abstract
Background
Polymyxins (colistin, polymyxin B) have been first-line antibiotics against carbapenem-resistant Enterobacteriaceae (CRE) infections. New anti-CRE antibiotics (ceftazidime-avibactam, meropenem-vaborbactam, plazomicin) improve outcomes in CRE-infected patients and reduce toxicity compared with polymyxins. It is unclear how widely polymyxins and newer agents are used to treat CRE infections.
Methods
We conducted an online survey of US hospital-based pharmacists to determine antibiotic positioning against CRE infections. Numbers of all infections and CRE infections treated with different antibiotics in the United States were determined using IQVIA prescription data and Driving Re-investment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) estimates of CRE infections.
Results
Ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin were positioned as first-line agents against CRE pneumonia, bacteremia, intra-abdominal infections, and urinary tract infections at 87%, 90%, 83%, and 56% of surveyed US hospitals, respectively. From February 2018 to January 2019, an estimated 9437 and 7941 CRE infections were treated with an intravenous polymyxin or new agent, respectively; these figures represented ~28% (range, 19%–50%) and ~23% (range, 16%–42%) of CRE infections in the United States. Use of ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin exceeded that of intravenous polymyxins against CRE infections as of December 2018. Currently, the new drugs are estimated to treat 35% (23% to 62%) of CRE infections in which they were expected to be first-line agents.
Conclusions
New anti-CRE agents recently surpassed intravenous polymyxins as treatment for CRE infections, but use is less than expected from their positioning at US hospitals. Research on behavioral and economic factors that impact use of new antibiotics is needed, as are financial “pull” incentives that promote an economically viable marketplace.
Keywords: carbapenem-resistant Enterobacteriaceae, ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, polymyxins
Carbapenem-resistant Enterobacteriaceae (CRE) are classified as “urgent threat” pathogens by the US Centers for Disease Control and Prevention (CDC) [1]. A recent study by the Driving Re-investment in Research and Development and Responsible Antibiotic Use (DRIVE-AB) consortium estimated that carbapenem-resistant (CR)-Klebsiella pneumoniae and CR-Escherichia coli accounted for 3 million (interval range, 1.5 to 4.5 million) serious infections (ie, requiring hospitalization) worldwide in 2014 [2]. In the same study, CR-K pneumoniae and CR-E coli were estimated to cause 34 000 (interval range, 19 000 to 49 000) serious infections in the United States. The CDC estimated that 9300 hospital-acquired CRE infections occurred in the United States in 2013 [1]. Because approximately half of CRE infections in the United States from 2009 to 2013 were acquired outside of healthcare facilities [3, 4], the CDC data suggested a total number of CRE infections that was similar to lower range DRIVE-AB estimates.
The World Health Organization and Infectious Diseases Society of America have designated CRE as highest priority pathogens for development of new antibiotics [5, 6]. Polymyxins (colistin, polymyxin B) have been first-line agents against CRE infections despite treatment failure and nephrotoxicity rates of ~40% to 60% and ~20% to 50%, respectively [7–10]. Four antibiotics with anti-CRE activity have received US Food and Drug Administration (FDA) approval since 2015. Ceftazidime-avibactam was approved for treatment of (1) complicated urinary tract infection (cUTI) and complicated intra-abdominal infection (cIAI) in February 2015 and (2) hospital-acquired and ventilator-associated pneumonias in February 2018. Meropenem-vaborbactam and plazomicin were approved for treatment of cUTI in August 2017 and June 2018, respectively. Eravacycline was approved for treatment of cIAI in August 2018. Observational studies and randomized trials have shown that ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin are significantly more effective and less toxic than colistin and other older agents in treating CRE-infected patients [7–11].
It is unclear how widely new anti-CRE agents have been incorporated into patient care. Indeed, Allergan and The Medicines Company (developers of ceftazidime-avibactam and meropenem-vaborbactam, respectively) have sought to exit the antimicrobial field since introducing their drugs to market due to insufficient returns on investment [12]. Melinta slashed staff and reduced research investment in antibiotic discovery 1 year after buying The Medicines Company’s infectious diseases group [13]. Achaogen announced widespread layoffs and a shake-up in corporate leadership 1 month after securing FDA approval for plazomicin [12], and it subsequently filed for bankruptcy protection and auctioned its assets [14, 15]. These events suggest that use of the new agents has been less extensive than expected. It is plausible that clinical uptake of the drugs has been restrained by high acquisition costs compared with polymyxins, or by the fact that their superior efficacy and safety in treating CRE infections was demonstrated incrementally in relatively small studies that were published after FDA approval.
The objectives of this study were (1) to describe intravenous polymyxin and newly approved anti-CRE agent use in the United States and (2) to estimate numbers of CRE infections treated with these agents. We anticipated that results would provide important insights into the current positioning of anti-CRE drugs in US hospitals, antimicrobial treatment and stewardship practices against CRE infections, and the state of the marketplace for new anti-CRE agents.
METHODS
Survey of Hospital-Based Pharmacists
We conducted an online survey, through Qualtrics (Provo, UT), of hospital-based pharmacists in the United States who are members of the Society of Infectious Diseases Pharmacists (SIDP). Participants selected the antibiotic that was considered first-line against various CRE infections at their hospital from among a multiple-choice menu that included ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, or “other”; if other was chosen, specific agents could be entered as free text. Participants also provided information on percentages of intravenous polymyxin use at their hospital that were directed against CRE and other multidrug-resistant (MDR) bacterial infections. Survey questions are posted in the Supplementary Material. Responses were collected from November 27 through December 18, 2018.
Prescription Data for Anti-Carbapenem-Resistant Enterobacteriaceae Agents
We designed this project to compare intravenous use of polymyxins (colsitin, polymyxin B), the longstanding first-line antibiotics against CRE infections, with that of newer agents that have been shown to be more effective and less toxic than polymyxins in clinical studies (ceftazidime-avibactam, meropenem-vaborbactam, plazomicin) [7–11]. We recognize that tigecycline and aminoglycosides also are used to treat systemic CRE infections, but we have not included them because much of their use is against non-CRE infections, and their current roles in treating CRE infections are often as adjunctive agents in combination regimens. Eravacycline (available commercially in October 2018) is not included because 3-month moving average usage data were available only for December 2018 and January 2019, and clinical data for treatment of CRE infections thus far are limited. We obtained antibiotic prescription data for June 2011 through January 2019 from IQVIA (formerly IMS Health, Durham, NC), which were provided as numbers of vials of each agent prescribed by month for systemic injectable use in the United States. We converted prescription data into estimated numbers of infections treated with a drug, based on standard 14-day dosing regimens for patients with normal renal function who weigh 75 kilograms (details provided in Figure 1 legend and Supplementary Materials). To smooth out monthly fluctuations in antibiotic prescriptions and highlight longer-term trends, data were analyzed as 3-month moving averages.
Figure 1.
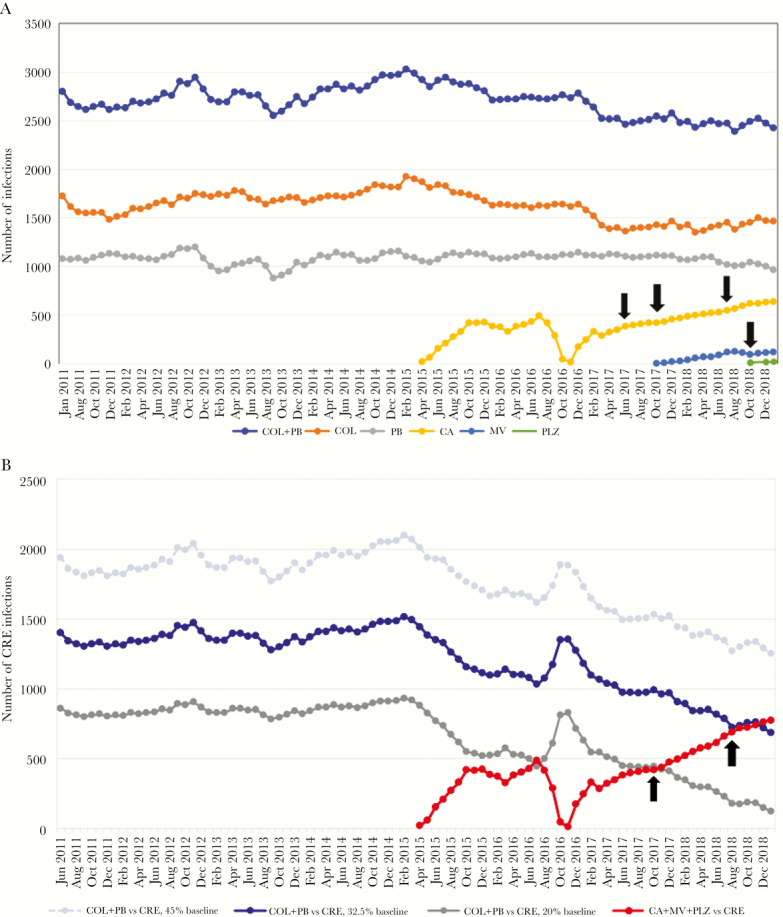
Estimated numbers of all infections (A) and carbapenem-resistant Enterobacteriacae (CRE) infections (B) treated by various agents in the United States. Data are presented as 3-month moving averages of numbers of infections treated intravenously by the indicated antibiotics. Black arrows in A indicate months immediately after the online publication of papers reporting that ceftazidime-avibactam (June 2017, October 2017, July 2018) or meropenem-vaborbactam (October 2018) were significantly more effective and less toxic than colistin or other best available regimens as treatment for CRE infections [7–9, 11]. There is no correlation between publication of these data and changes in the rate of uptake of the respective drug. B shows 3 estimates of numbers of CRE infections treated by intravenous polymyxins. The primary estimate was based on the assumption that 32.5% of all colistin or polymyxin B use before availability of ceftazidime-avibactam was as intravenous treatment of CRE infections (dark blue line in B, labeled as “32.5% baseline”). These data suggested that use of the new agents (ceftazidime-avibactam, meropenem-vaborbactam, plazomicin) against CRE infections exceeded that of intravenous polymyxins in December 2018 (red line). Other estimates were based on assumptions that 20% (gray line) or 45% (light blue, dashed line) of baseline polymyxin use was as intravenous treatment of CRE infections. In the former scenario, use of the new agents against CRE infections has exceeded that of intravenous polymyxins since November 2017. Black arrows in B indicate months that first sales of meropenem-vaborbactam (October 2017) and plazomicin (August 2018) were reported. There was a slight uptick in the overall use of new agents against CRE infections after introduction of meropenem-vaborbactam. Introduction of plazomicin did not impact the trajectory of the usage curve. Estimates of numbers of treated infections were based on the following 14-day regimens: colistin 150 mg twice a day (BID), polymyxin B 1 million units BID (12 500 to 15 000 units/kg BID), ceftazidime-avibactam 2.5 grams 3 times a day (TID), meropenem-vaborbactam 4 grams TID, plazomicin 1125 mg once daily (15 mg/kg per day). Per-vial concentrations of antibiotics are provided in Supplementary Material. (A) Estimated numbers of all infections treated by intravenous polymyxins and new anti-CRE agents. (B) Estimated numbers of CRE infections treated by intravenous polymyxins and new anti-CRE agents. CA, ceftazidime-avibactam; COL, colisitin; MV, meropenem-vaborbactam; PB, polymyxin B; PLZ, plazomicin.
IQVIA data do not account for the route of antibiotic administration to patients. Therefore, it was not possible to distinguish precisely between use of polymyxins as intravenous or inhaled agents. To our knowledge, there are no data that quantitate percentages of polymyxins given by these routes in the United States. Using IQVIA data on types of facilities that dispense polymyxins, we estimated that 65% and 35% of polymyxin use nationally was intravenous and inhalational, respectively. The rationale for these estimations and calculations for usage of various antibiotics are presented in the Supplementary Material.
Use of Anti-Carbapenem-Resistant Enterobacteriaceae Agents
Before FDA approval of ceftazidime-avibactam, 50% of intravenous polymyxin use was assumed to be against CRE infections. This assumption was based on data from our hospital (University of Pittsburgh Medical Center) that showed a 50% reduction in intravenous polymyxin consumption in the year after adoption of ceftazidime-avibactam as the first-line option against CRE infections, in lieu of colistin. We estimated that 32.5% of all polymyxin use nationally at the time was intravenous against CRE infections (0.325 = 0.65 (polymyxin use that was intravenous) × 0.50 (percentage of intravenous polymyxin use that was against CRE infections)). Therefore, through March 2015, the number of CRE infections treated intravenously by an agent of interest was calculated as follows: (all infections treated by colistin or polymyxin B) × 0.325.
Thereafter, we estimated the number of CRE infections treated by an agent of interest as follows: (all infections treated by intravenous colistin, polymyxin B, ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin) × 0.325. We assumed that all ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin use was against CRE infections. Our goal is using this approach was to identify the highest possible number of CRE infections that were treated with a new agent. The approach is reasonable because the vast majority of ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin use reported to date in routine clinical practice (as opposed to clinical trials that led to FDA approval) has been against CRE infections [16, 17], and most hospitals tightly restrict prescriptions for these agents. Because all ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin use was assumed to be against CRE infections, the number of such infections treated by intravenous polymyxins beginning in April 2015 was estimated as follows: ((all infections treated by colistin, polymyxin B, ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin) × 0.325) – (infections treated by ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin)). The percentage of intravenous polymyxin use that was directed against CRE infections was expected to decrease as use of newer agents increased. Representative calculations of antibiotic use against CRE infections are presented in the Supplementary Material. To determine a range of possible polymyxin use, we performed recalculations based on assumptions that 20% and 45% (rather than 32.5%) of all polymyxin use through March 2015 was intravenous against CRE infections.
Numbers of Carbapenem-Resistant Enterobacteriaceae (CRE) Infections for Which New Anti-CRE Agents Were Expected to Be First-Line Treatment
We used data from DRIVE-AB models and our survey to estimate the number of CRE infections that were expected to be treated with a new anti-CRE agent in the United States. Our primary estimate used DRIVE-AB’s middle-range calculation of 34 000 CRE infections per year; other estimates used DRIVE-AB’s high-range and low-range calculations of 49 000 and 19 000 CRE infections per year, respectively [2]. For each estimate, the maximum number of CRE infections that would be expected to be treated with a new anti-CRE agent was calculated as follows: (number of CRE infections) × 0.675. This formula was derived as follows: (1) data from our survey indicating that a new anti-CRE agent was considered first-line against ~50% of CRE UTIs and ~85% of other types of CRE infection (Table 1); (2) data indicating that UTIs accounted for ~50% of CRE infections in the United States [3]; (3) the assumption that if 50% and 85% of CRE UTIs and other infections, respectively, were treated with a new anti-CRE agent, then as many as 67.5% of all CRE infections would be treated with one of these agents (0.675 = (0.5 × 0.5) + (0.85 × 0.5)).
Table 1.
Results From a Survey of US Hospital-Based Pharmacists on First-Line Agents for Treatment of CRE Infections
| Type of CRE Infection, Percentage of Respondents (Number) | ||||
|---|---|---|---|---|
| Agent Positioned as First-Linea | Pneumonia | Bacteremia | Intra-abdominal | Urinary Tract |
| Ceftazidime-avibactam | 54% (51/95) | 58% (52/89) | 51% (47/93) | 36% (33/91) |
| Meropenem-vaborbactam | 32% (30/95) | 31% (28/89) | 31% (29/93) | 14% (13/91) |
| Plazomicin | 2% (2/95) | 1% (1/89) | 1% (1/93) | 5% (5/91) |
| Other | 13% (12/95) | 10% (9/89) | 17% (16/93) | 44% (40/91)b |
| Polymyxinc | 4% (4/95) | 4% (4/89) | 4% (4/93) | 3% (3/91) |
| Aminoglycosided | 2% (2/95) | 1% (1/89) | 2% (2/93) | 11% (10/91) |
| Tigecyclined | 2% (2/95) | 0 | 5% (5/93) | 3% (3/91) |
| Fosfomycin | 0 | 0 | 0 | 15% (14/95) |
| Nitrofurantoin | 0 | 0 | 0 | 5% (5/91) |
| Trimethoprim/sulfamethoxazole | 0 | 0 | 0 | 3% (3/91) |
| Miscellaneous | 3% (3/95)e | 2% (2/89)f | 3% (3/93)g | 7% (6/91)h |
| Not specifiedi | 1% (1/95) | 2% (2/89) | 2% (2/93) | 2% (2/91) |
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae.
aFor each type of CRE infection, survey participants selected the agent positioned as first-line against CRE infections at their hospital from a multiple-choice menu that included ceftazidime-avibactam, meropenem-vaborbactam, plazomicin, or “other”; a single choice was permitted. If other was chosen, participants could enter specific agents as free text.
bThe sum of “other” responses is 49 since survey participants could list more than 1 agent.
cIncludes polymyxins ± carbapenem.
dNot specified if administered as monotherapy or in combination.
eIncludes the following agents that were listed once each: aztreonam, minocycline, ceftolozane-tazobactam.
fIncludes the following agents that were listed once each: aztreonam, ceftolozane-tazobactam.
gIncludes the following agents that were listed once each: aztreonam, minocycline, ceftolozane-tazobactam.
hIncludes the following agents that were listed once each: minocycline, ceftolozane-tazobactam, cefepime, double carbapenems, levofloxacin, chlorpactin irrigation.
i“Not specified” indicates that no agent was listed, or the respondent stated that agents were selected based on susceptibility data without naming an agent.
RESULTS
Survey on the Positioning of Anti-Carbapenem-Resistant Enterobacteriacae Agents in United States Hospitals
We began our study by conducting a survey of pharmacists practicing in US hospitals, the goals of which were to understand where antibiotics with anti-CRE activity were positioned as treatment for various types of CRE infection, and the current role of intravenous polymyxins in treating infections by CRE and other MDR bacteria. Respondents were from 41 states and Puerto Rico; 89–110 pharmacists responded to individual questions.
Ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin were positioned as the first-line antibiotic against CRE pneumonia, bacteremia, and IAI by 87%, 90%, and 83% of respondents, respectively (Table 1). In contrast, one of these new agents was positioned as the first-line antibiotic against CRE UTI by 56% of respondents. Respondents (n = 110) indicated that 26.5% of intravenous polymyxin use at their hospitals was directed against CRE infections (mean value; range at individual hospitals, 0% to 100%). Corresponding figures for infections by MDR-Acinetobacter, MDR-Pseudomonas, or other bacteria were 36%, 26.5%, and 11%, respectively (ranges, 0% to 100% for each).
Use of Anticarbapenem-Resistant Enterobacteriaceae Agents
Numbers of all infections and CRE infections treated by various intravenous agents, as estimated from US prescription data, are presented in Figure 1A and B, respectively. In the 12 months before introduction of ceftazidime-avibactam to the market (April 2014 through March 2015), an estimated 34 901 infections and 17 450 CRE infections were treated with an intravenous polymyxin. The corresponding figures for the 12 months ending in January 2019 were 29 594 infections and 9437 CRE infections.
From February 2018 through January 2019, an estimated 7941 CRE infections were treated with ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin. A striking decrease and subsequent rebound in use of ceftazidime-avibactam in late 2016 and early 2017 corresponded to a nationwide supply shortage of the agent and its resolution. In January 2019, 689 and 777 CRE infections were estimated to be treated by an intravenous polymyxin and ceftazidime-avibactam/meropenem-vaborbactam/plazomicin, respectively (Figure 1B). In this month, 28.4% (689 CRE infections/2426 total infections) of intravenous polymyxin use nationally was against CRE infections. New agents were estimated to be used more commonly than intravenous polymyxins to treat CRE infections as of December 2018.
The foregoing estimates, combined with data from DRIVE-AB models [2], suggest that 28% (range, 19% to 50%) and 23% (range, 16% to 42%) of CRE infections in the United States were treated with an intravenous polymyxin and new anti-CRE agent, respectively, over the 12 months ending in January 2019 (Table 2). After adjusting for cases in which another antibiotic may be preferred over a new anti-CRE agent, we estimated that ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin were used against 35% (range, 23% to 62%) of CRE infections in which they were expected to be first-line agents (calculations in Supplementary Material).
Table 2.
Percentages of CRE Infections Treated With an intravenous Polymyxin or a New Anti-CRE Agent
| CRE Infections | CRE Infections Expected to Be Treated With New Agentb | ||||
|---|---|---|---|---|---|
| Estimates of Numbers of CRE Infectionsa | Number | Treated With Polymyxin, %c | Treated With New Agent, %d | Number | Treated With New Agent, %d |
| Middle estimate | 34 000 | 28% | 23% | 22 950 | 35% |
| High estimate | 49 000 | 19%e | 16%e | 34 055 | 23% |
| Low estimate | 19 000 | 50%f | 42%f | 12 825 | 62% |
Abbreviations: CRE, carbapenem-resistant Enterobacteriaceae.
aIn our primary calculation of percentages of CRE infections treated with a polymyxin or a new anti-CRE agent, we used the middle-range DIRECT-AB estimate of 34 000 CRE infections per year in the United States [2]. To define ranges of CRE infections treated by various agents, we used high- and low-range DIRECT-AB estimates of 49 000 and 19 000 CRE infections per year, respectively.
bNew anti-CRE agents of interest were ceftazidme-avibactam, meropenem-vaborbactam, and plazomcin. The maximum number of CRE that would be anticipated to be treated with a new anti-CRE agent was defined as follows: (total number of CRE infections) × 0.675.
cPercentages shown in this column were calculated using a numerator of 9437 CRE infections that were estimated to be treated with an intravenous polymyxin from February 2018 to January 2019 (9437/34 000 = 28%, 9437/49 000 = 19%, 9437/19 000 = 50%).
dPercentages shown in this column were calculated using a numerator of 7941 CRE infections that were estimated to be treated with ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin from February 2018 to January 2019.
eThese data signify that as few as 19% and 16% of CRE infections in the United States from February 2018 to January 2019 were treated with an intravenous polymyxin or new agent, respectively. Percentages define the lower limit of the range, and they are not designed to add up to 100%.
fThese data signify that as many as 50% and 42% of CRE infections in the United States from February 2018 to January 2019 were treated with an intravenous polymyxin or new agent, respectively. Percentages define the upper limit of the range, and they are not designed to add up to 100%.
Alternative assessments of polymyxin use against CRE infections, based on differing baseline assumptions about how colistin and polymyxin B were used before availability of the newer agents, are presented in Figure 1B. In low-range and high-range analyses, 5.2% (125 CRE infections/2426 total infections) and 51.6% (1253 CRE infections/2426 total infections) of intravenous polymyxin use in January 2019 was against CRE infections, respectively. In the former scenario, monthly new agent use exceeded that of intravenous polymyxins against CRE infections as of November 2017.
DISCUSSION
Antibiotic prescription data demonstrate that polymyxins remain widely used in the United States. Ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin have been incorporated into practice at a steady incremental rate since a supply shortage of ceftazidime-avibactam was resolved in late 2016–early 2017, and their use against CRE infections was estimated to exceed that of intravenous polymyxins in December 2018. Nevertheless, after adjusting for cases in which new agents may not constitute first-line treatment, we estimated that ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin were prescribed in only 35% (range, 23% to 62%) of CRE infections in which their use might have been expected. Therefore, treatment of CRE infections in the United States is still evolving, 4 years after FDA approval of ceftazidime-avibactam as the first new agent with anti-CRE activity.
Definitive interpretation of these findings is open to debate. On the one hand, it is unprecedented that newly released antibiotics captured such a sizeable slice of the market for a given indication in this short a timeperiod. As a comparison, linezolid and daptomycin, novel first-in-class agents with activity against Gram-positive bacteria, were estimated to command a combined 21% market share for treatment of methicillin-resistant Staphylococcus aureus and skin/soft tissue infections in 2012, approximately 12 and 9 years after FDA approval, respectively [18]. On the other hand, there was a particularly compelling case for rapid uptake of ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin against CRE infections. The need for new antibiotics with anti-CRE activity has been recognized for over a decade as a critical worldwide medical priority [1, 5, 6]. Given the inadequacies of existing treatment options and the extremely poor outcomes of CRE infections, the potential that new drugs would benefit patients was substantial. Ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin demonstrated excellent anti-CRE activity in vitro and in animal studies, and they have been shown to be superior to polymyxins or other best available regimens in treating CRE-infected patients [7–11]. The β-lactam/β-lactamase class of antibiotics, in particular, offers considerable advantages over polymyxins in pharmacokinetics-pharmacodynamics, ease of dosing, safety and need for monitoring of toxicity, and clinician familiarity.
Use of new agents likely has been constrained by concerns over cost. Current wholesale acquisition costs for 14-day courses of ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin in 75-kilogram adults are $15 070, $13 860, and $13 230, respectively, compared with $784 for colistin and $305 to $630 for polymyxin B [19]. Other issues that may have limited new drug use are reports of emerging resistance and unavailability of automated susceptibility testing [20–22]. Of note, publication of studies demonstrating ceftazidime-avibactam and meropenem-vaborbactam superiority over polymyxins in CRE-infected patients has not correlated with increased uptake of the newer drugs (Figure 1A). The data on treatment of CRE infections stand in contrast to our survey results. The SIDP member pharmacists indicated that new agents were positioned as first-line against CRE infections at a sizeable majority of hospitals, whereas polymyxins were first-line at only 3% to 4% of hospitals (Table 1). Our findings suggest that pharmacy cost savings or other concerns, such as limiting resistance, are prioritized over the existing effectiveness and toxicity data in treatment of CRE infections at many hospitals. The results raise questions about drug pricing, stewardship priorities, and types of clinical data that justify use of effective, but expensive antibiotics. It is plausible that many providers are unwilling to commit to new agents based on the relatively small studies of CRE-infected patients published to date. In this event, the field must consider novel clinical trial designs that (1) better reflect future real-world usage of antibiotics and (2) address challenges in enrolling and demonstrating outcome differences for MDR infections [23, 24]. It is clear that there is pressing need to understand issues that influence stewardship and treatment decisions for new antibiotics. We are currently addressing policies and rationales for endorsing or withholding anti-CRE agents at US hospitals.
Our estimates were predicated upon assumptions about how often various antibiotics were used against CRE infections. The fact that findings were in line with data from previously published studies and our survey of hospital-based pharmacists suggests that assumptions were reasonable and conservative. For example, our estimate that 17 450 CRE infections were treated with intravenous polymyxins in 2014 was in keeping with the overall number of CRE infections in the United States determined by DIRECT-AB. Likewise, our estimate that 28.4% of intravenous polymyxin use was against CRE infections in January 2019 aligned closely with survey results. We may have overestimated the number of CRE infections treated by ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin because at least some use of these agents is empiric or directed against Pseudomonas and other MDR bacteria [16]. In contrast, we may have underestimated the number of CRE infections treated by different drugs if typical durations of therapy were shorter than 14 days or dose reductions for impaired renal function were common. It is also possible that survey results were biased by the make-up of SIDP membership, which is drawn disproportionately from larger, academic hospitals with existing stewardship programs. Approximately half of MDR Enterobacteriaceae infections in the United States are treated at small community hospitals (<300 beds) [25], where positioning of ceftazidime-avibactam, meropenem-vaborbactam, or plazomicin may differ from that of most hospitals captured in our survey. This study and the DRIVE-AB study highlight the need for more comprehensive surveillance data on CRE infections and their treatment in the United States and globally.
We reanalyzed our data by varying baseline assumptions about polymyxin use (Figure 1B). Using our high-range assumptions, an estimated 51.6% rather than 28.4% of intravenous polymyxin use in January 2019 was against CRE infections. If these data are correct, the anti-CRE drug market has more room for new agents than suggested by our primary estimates. In contrast, our low-range assumptions indicated that only 5.2% of intravenous polymyxin use in January 2019 was against CRE infections. Although this analysis demonstrated that new agent use in CRE-infected patients has exceeded that of intravenous polymyxins since late 2017, it also implies that the new anti-CRE drug market has more limited room for further growth. Such a scenario is ominous from a drug development perspective because companies marketing ceftazidime-avibactam, meropenem-vaborbactam, and plazomicin have faced major financial challenges, even as other companies are poised to attain FDA approval for CRE-active agents [26]. We recognize that polymyxins or other older antibiotics may be preferred over new agents in certain CRE-infected patients, such as those infected with metallo-β-lactamase-producing isolates or in treatment of uncomplicated UTIs. Furthermore, new anti-CRE drugs are not necessarily interchangeable, because one agent may offer advantages over another in pharmacokinetics at sites of disease or in treating infections by isolates manifesting specific carbapenem resistance mechanisms [27]. We were unable to assess the impact of carrying more than 1 new anti-CRE agent at a hospital on treatment patterns. There was a slight uptick in overall use of new agents nationally after meropenem-vaborbactam entered the market (Figure 1B).
IQVIA data are derived from anonymized pharmaceutical sales information and serve as an industry standard for estimating US antibiotic prescriptions. Sales figures of ceftazidime-avibactam calculated by IQVIA in second quarter 2018 correlated closely with values reported by Allergan ($21.2 vs $23.5 million), suggesting that prescription estimates are accurate. IQVIA prescription data do not account for the use of antibiotics as part of combination regimens or provide patient-level information on dosages, dose adjustments, outcomes, or toxicities.
CONCLUSIONS
Moving forward, it will be important to validate our findings. A confirmation that new anti-CRE agents are used much less widely than expected would support our call for (1) an examination of clinical and stewardship practices at US hospitals and (2) research into behavioral and economic factors that have led to sluggish drug uptake. In contrast, if the percentage of CRE infections that are treated by new agents is significantly higher than estimated here, then the market for current anti-CRE agents may not be economically viable under present reimbursement schemes. Governmental and public-private “push” incentives that reduce companies’ research and development costs by providing financial and infrastructure support have been successful in getting new anti-CRE agents into the pipeline [28]. Now, the need is for “pull” incentives that fairly recognize the societal value of effective antibiotics, reward manufacturers after agents with activity against priority pathogens enter the marketplace, and delink revenues from volume of sales [29]. Infectious diseases specialists and professional societies have vital roles to play in shaping public policy, educating healthcare providers, and developing timely guidelines for the most appropriate use of new and older antibiotics [14]. At this point, guidelines should explicitly state that polymyxins are not standard first-line agents against CRE infections.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We thank Alan Carr for assistance with obtaining and interpreting antibiotic prescription and sales data. We also thank Lloyd Clarke for obtaining UPMC pharmacy data.
Potential conflicts of interest. C. J. C. has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for projects unrelated to this study, served on advisory boards or consulted for Astellas, Merck, the Medicines Company, Cidara, Scynexis, Shionogi, Qpex, and Needham & Company, and spoken at symposia sponsored by Merck and T2Biosystems. M. H. N. has been awarded investigator-initiated research grants from Astellas, Merck, Melinta, and Cidara for projects unrelated to this study and served on advisory boards for Astellas, Merck, the Medicines Company, Scynexis, and Shionogi. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. Available at: https://www.cdc.gov/drugresistance/pdf/ar-threats-2013-508.pdf. Accessed 14 June 2019.
- 2. Temkin E, Fallach N, Almagor J, et al. Estimating the number of infections caused by antibiotic-resistant Escherichia coli and Klebsiella pneumoniae in 2014: a modelling study. Lancet Glob Health 2018; 6:e969–79. [DOI] [PubMed] [Google Scholar]
- 3. Zilberberg MD, Nathanson BH, Sulham K, et al. Carbapenem resistance, inappropriate empiric treatment and outcomes among patients hospitalized with Enterobacteriaceae urinary tract infection, pneumonia and sepsis. BMC Infect Dis 2017; 17:279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. New York State Department of Health. Hospital-aquired infections, New York State 2014 Available at: https://www.health.ny.gov/statistics/facilities/hospital/hospital_acquired_infections/2014/docs/hospital_acquired_infection.pdf. Accessed 9 April 2019. [Google Scholar]
- 5. Tacconelli E, Carrara E, Savoldi A, et al. Discovery, research, and development of new antibiotics: the WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect Dis 2018; 18:318–27. [DOI] [PubMed] [Google Scholar]
- 6. Boucher HW, Talbot GH, Bradley JS, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 2009; 48:1–12. [DOI] [PubMed] [Google Scholar]
- 7. Shields RK, Nguyen MH, Chen L, et al. Ceftazidime-avibactam is superior to other treatment regimens against carbapenem-resistant Klebsiella pneumoniae bacteremia. Antimicrob Agents Chemother 2017; 61:e00883–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Duin D, Lok JJ, Earley M, et al. Colistin versus ceftazidime-avibactam in the treatment of infections due to carbapenem-resistant Enterobacteriaceae. Clin Infect Dis 2018; 66:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wunderink RG, Giamarellos-Bourboulis EJ, Rahav G, et al. Effect and safety of meropenem-vaborbactam versus best-available therapy in patients with carbapenem-resistant Enterobacteriaceae infections: the TANGO II randomized clinical trial. Infect Dis Ther 2018; 7:439–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKinnell JA, Dwyer JP, Talbot GH, et al. Plazomicin for infections caused by carbapenem-resistant Enterobacteriaceae. N Engl J Med 2019; 380:791–3. [DOI] [PubMed] [Google Scholar]
- 11. Tumbarello M, Trecarichi EM, Corona A, et al. Efficacy of ceftazidime-avibactam salvage therapy in patients with infections caused by Klebsiella pneumoniae carbapenemase-producing K. pneumoniae. Clin Infect Dis 2019; 68:355–64. [DOI] [PubMed] [Google Scholar]
- 12. Shlaes DM. You too, Achaogen? Available at: http://antibiotics-theperfectstorm.blogspot.com/2018/07/you-too-achaogen.html. Accessed 9 January 2019.
- 13. Tong A. Low sales, high cost: Melinta slashes HQ research staff as it struggles to grow antibiotics revenue. Endpoints News, 2018. Available at: https://endpts.com/low-sales-high-cost-melinta-slashes-hq-research-staff-as-it-struggles-to-grow-antibiotics-revenue/. Accessed 14 June 2019.
- 14. Rex JH. Scary, scarier, scariest (part 2): What can you do about it? 2019. Blog: AMR Solutions. Available at http://amr.solutions/blog/scary-scarier-scariest-part-2-what-can-you-do-about-it. Accessed 14 June 2019. [Google Scholar]
- 15. Carroll J. Once picked as a $500M winner, bankrupt Achaogen auctions off its antibiotic for a fraction of that. Endpoints News 2019. https://endpts.com/once-picked-as-a-500m-winner-bankrupt-achaogen-auctions-off-its-antibiotic-for-a-fraction-of-that/. Accessed 14 June 2019. [Google Scholar]
- 16. Santevecchi BA, Smith TT, MacVane SH. Clinical experience with ceftazidime/avibactam for treatment of antibiotic-resistant organisms other than Klebsiella pneumoniae. Int J Antimicrob Agents 2018; 51:629–35. [DOI] [PubMed] [Google Scholar]
- 17. Temkin E, Torre-Cisneros J, Beovic B, et al. Ceftazidime-avibactam as salvage therapy for infections caused by carbapenem-resistant organisms. Antimicrob Agents Chemother 2017; 61:e01964–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cubist. Corporate Presentation September 2012 Available at: http://www.snl.com/interactive/lookandfeel/4093793/cubistsept.pdf. Accessed 14 June 2019.
- 19. Medi-Span drug database. Wholesale acquisition costs pricing. 2019. WoltersKluwer. Available at: https://www.wolterskluwercdi.com/drug-data/average-wac-pricing/. Accessed 14 June 2019. [Google Scholar]
- 20. Shields RK, Clancy CJ, Pasculle AW, et al. Verification of ceftazidime-avibactam and ceftolozane-tazobactam susceptibility testing methods against carbapenem-resistant Enterobacteriaceae and Pseudomonas aeruginosa. J Clin Microbiol 2018; 56:e01093–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shields RK, Potoski BA, Haidar G, et al. Clinical outcomes, drug toxicity, and emergence of ceftazidime-avibactam resistance among patients treated for carbapenem-resistant Enterobacteriaceae infections. Clin Infect Dis 2016; 63:1615–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Haidar G, Clancy CJ, Shields RK, et al. Mutations in blaKPC-3 that confer ceftazidime-avibactam resistance encode novel KPC-3 variants that function as extended-spectrum beta-lactamases. Antimicrob Agents Chemother 2017; 61e02534–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rex JH, Talbot GH, Goldberger MJ, et al. Progress in the fight against multidrug-resistant bacteria 2005–2016: modern noninferiority trial designs enable antibiotic development in advance of epidemic bacterial resistance. Clin Infect Dis 2017; 65:141–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Infectious Diseases Society of America. White paper: recommendations on the conduct of superiority and organism-specific clinical trials of antibacterial agents for the treatment of infections caused by drug-resistant bacterial pathogens. Clin Infect Dis 2012; 55:1031–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gandra S, Trett A, Klein EY, Laxminarayan R. Is antimicrobial resistance a bigger problem in tertiary care hospitals than in small community hospitals in the United States? Clin Infect Dis 2017; 65:860–3. [DOI] [PubMed] [Google Scholar]
- 26. Petty LA, Henig O, Patel TS, et al. Overview of meropenem-vaborbactam and newer antimicrobial agents for the treatment of carbapenem-resistant Enterobacteriaceae. Infect Drug Resist 2018; 11:1461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Pogue JM, Bonomo RA, Kaye KS. Ceftazidime/avibactam, meropenem/vaborbactam, or both? Clinical and formulary considerations. Clin Infect Dis 2019; 68:519–24. [DOI] [PubMed] [Google Scholar]
- 28. Bhatti T, Lum K, Holland S, et al. A perspective on incentives for novel inpatient antibiotics: no one-size-fits-all. J Law Med Ethics 2018; 46:59–65. [DOI] [PubMed] [Google Scholar]
- 29. Sciarretta K, Røttingen JA, Opalska A, et al. Economic incentives for antibacterial drug development: literature review and considerations from the transatlantic task force on antimicrobial resistance. Clin Infect Dis 2016; 63:1470–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


