Abstract
Background
Life-threatening central venous catheter-related infections are primarily initiated by biofilm formation on the catheter surface. Antibiotic lock therapy is recommended for eradicating intraluminal biofilm. In the era of antibiotic resistance, antibiotics of natural origins provide an effective and cheap option for combating resistant strains. Garlic especially stole the spotlight because of its impressive antimicrobial effectiveness against such superbugs.
Aim
Is to estimate the potential use of fresh garlic extract (FGE) as a lock agent against multi-drug resistant (MDR) bacteria.
Methods
The agar well diffusion and broth microdilution techniques were employed to test the antimicrobial activities of FGE against five MDR strains; E. coli, Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae), Serratia marscens (S. marscens) and Methicillin-resistant Staphylococcus aureus (MRSA). Then the protective and therapeutic efficiencies of FGE against bacterial biofilms were in-vitro evaluated; at concentrations of 100, 75, 50 and 25%; in tissue culture plate (TCP) and on the polyurethane (PU) sheets using the crystal violet (CV) assay and colony-forming unit (CFU), respectively. Scanning electron microscopy (SEM) was also used to confirm eradication of biofilms on PU sheets. Finally, systemic and deep tissue infections by P. aeruginosa and MRSA were induced in mice that were then treated by FGE at either 100 or 200 mg/kg for seven days. Where the antibacterial activity was assessed by tissue and blood culturing at the end of the treatment period. Biochemical, hematological and histological parameters were also investigated.
Results
FGE exhibited potent in-vitro and in-vivo antibacterial and antibiofilm activities against MDR strains. It not only didn’t exhibit toxicological effects at the hematological and the histological levels but also provided protective effects as demonstrated by the significant drop in the biochemical parameters.
Conclusion
FGE has the potential to be used as a prophylactic and/or therapeutic lock agent against biofilm-associated infections caused by MDR bacteria.
Keywords: Biofilm, Antibacterial, Resistance, Garlic, Lock therapy, Central venous catheter-related infections, Antibiofilm, Histological
1. Introduction
Central venous catheter (CVC) is an indispensable tool for managing critically ill patients; such as hemodialysis and cancer patients (Gahlot et al., 2014). In spite of its crucial role, it poses patients to a high risk of catheter-related bloodstream infections (CRBSIs) resulting in high rates of morbidity, mortality and increased cost (Haddadin and Regunath, 2018). Catheters could be contaminated via four different routes including; migration of skin normal flora along the external surface at the insertion time, catheter hub contamination during catheter manipulation, hematogenous seeding of the catheter from another infected site, and the contamination of the catheter lumen with contaminated fluids (Trautner and Darouiche, 2004). The commonest culprits in CRBSI are P. aeruginosa, S. aureus, E. coli, K. pneumoniae, Acinetobacter baumannii, Enterobacter spp., Serratia spp., and Candida spp. (Hosny et al., 2014, Trautner and Darouiche, 2004). CRBSIs due to central venous catheterization are up to 64 times greater than peripheral venous catheters (Gahlot et al., 2014). Such infections are initiated by biofilm formation (Gominet et al., 2017), where the inert catheter surface acts as a substratum for microbial biofilms (Justo and Bookstaver, 2014).
A biofilm is a microbial community of sessile bacteria encased in a self-produced matrix of exopolysaccharides, protein, and eDNA (Chang, 2018). Biofilm formation process encompasses sequential steps; starting with attachment to a surface leading to micro-colony formation, giving rise to three-dimensional structures and ending up; after maturation; with detachment (Tewari et al., 2018). Biofilm bacteria are highly resistant to antibiotics and the human immune system (Fleming et al., 2017, Tewari et al., 2018). CVC is extraluminally colonized by cutaneous microorganisms in short-term catheterization (<10 days). Meanwhile, intraluminal colonization; from the hub; is common with long-term catheterization (>10 days) (Haddadin and Regunath, 2018). Preventive measures such as insertion under aseptic conditions and insertion site care mainly decrease the extra-luminal colonization, meanwhile antibiotic lock therapy (ALT) is recommended for eradicating intraluminal biofilm through filling the catheter lumen with a highly concentrated (100–1000 times MIC) antibiotic solution and leaving it indwelling (lock) for hours or days to eradicate biofilms (Justo and Bookstaver, 2014). In spite of proven efficiency in eradicating microbial biofilms, the emergence of resistant strains due to ALT was reported by many investigators (Dixon et al., 2012, Justo and Bookstaver, 2014). Moreover, ALT failed to treat infections caused by S. aureus, P. aeruginosa or Candida spp. (Blackwood et al., 2017, Funalleras et al., 2011).
In the era of antibiotic resistance, natural products could substitute for antibiotics to treat infections with low potential for resistance. Garlic (Allium sativum) was put to focus as a weapon against multi-drug resistant pathogens (Gupta et al., 2015). In addition to many other compounds, allicin is the main bioactive antibacterial compound in garlic (Gupta et al., 2015). The ability of garlic essential oils to penetrate the microbial cellular membranes and the sub-cellular organelles membranes was well documented (Li et al., 2016). Intracellularly, it interferes with RNA synthesis and blocks vital enzymes; within bacteria, fungi and viruses; such as cysteine proteinases, alcohol dehydrogenases, acetate kinase, phosphotransacetyl-CoA synthetase and thioredoxin reductases (Ratthawongjirakul and Thongkerd, 2016). This explains the broad-spectrum activity of garlic against different bacterial, viral and fungal infections with low potential for developing bacterial resistance because of the multiple sites of action (Reiter et al., 2017). Its potent antimicrobial and antibiofilm activities were reported against a big number of MDR Gram-positive and Gram-negative bacteria in addition to Candida spp. (Mendoza-Juache et al., 2017, Nidadavolu et al., 2012, Rammo, 2017), including resistant strains of Helicobacter pylori, E Coli-0124, E Coli-0111, Salmonella spp., Shigella spp. and S. aureus (Abiy and Berhe, 2016, Bayati, 2018). Even fatal diseases such as anthrax and tuberculosis were found to be sensitive to garlic components (Abiy and Berhe, 2016). The aim of this study was directed to assess the antibacterial and antibiofilm activities of fresh garlic extract (FGE) against MDR bacterial species in both in-vitro and in-vivo models to assess its potential use in antibiotic lock therapy.
2. Materials & methods
2.1. Test strains, culture conditions, and inoculum preparation
Five strong biofilm-forming MDR clinical isolates namely, Pseudomonas aeruginosa (P. aeruginosa), Klebsiella pneumoniae (K. pneumoniae), Serratia marscens (S. marscens) and Methicillin-resistant Staphylococcus aureus (MRSA), were used. Test strains were isolated from the catheter tips of central venous catheters collected from oncology patients within an Egyptian University Hospital. Strains were identified using the VITEK 2® system and stored in tryptic soy broth (Oxoid, England) with 20% (vol/vol) glycerol at −80 °C. Their ability for in-vitro biofilm formation was assessed by the crystal violet (CV) staining assay in tissue culture plates (TCP) as previously described (Stepanović et al., 2000). For propagation, bacteria were spread over sheep blood agar, incubated aerobically at 37 °C/24 h. Colonies were suspended in tryptic soy broth (TSB), incubated till the logarithmic growth phase, then standardized according to EUCAST (Hasselmann and Diseases, 2003).
2.2. Preparation of fresh garlic extract (FGE)
Fresh garlic bulbs were purchased from a public food store, peeled, and homogenized using sterile mortar and pestle. Then filtered through a cheesecloth, centrifuged at 12,000 rpm for 10 min and filtered twice through a 0.22 μm filter (Millipore TM; MA, USA) to obtain raw garlic extract. This represented the 100% concentration that was then diluted with sterile distilled water to get concentrations of 75%, 50%, and 25%, then stored in the refrigerator for subsequent uses (Khan et al., 2014).
2.3. Testing the antibacterial activity of FGE
FGE at concentrations of 100%, 75%, 50%, and 25% were tested for antibacterial activity by the agar well diffusion assay as described elsewhere (Andualem, 2013) using chloramphenicol (32 µg/mL) and sterile distilled water as positive and negative controls, respectively. Inhibition zones with diameter less than 12 mm were considered as negative for antibacterial activity.
2.4. Determination of the minimal inhibitory concentration (MIC) of FGE against test strains
The MIC of FGE against test strains was tested by the microdilution method according to the guidelines (Hasselmann and Diseases, 2003) using two-fold serial dilution (1–1024 µg/mL), where untreated bacterial broth and uninoculated broth were used as positive and negative controls, respectively.
2.5. Estimation of the antibiofilm activity of sub-inhibitory concentrations of FGE
To assess the antibiofilm efficiency of FGE, the crystal violet assay was used as previously described (Yang et al., 2017) with minor modifications. Briefly, bacterial isolates were exposed to FGE at 1/2, 1/4 or 1/8 MIC prepared in TSB supplemented with 1% glucose (TSB/1% glucose) in a sterile tissue culture plate (TCP), 200 μl each using untreated inoculated broth (cells + broth) as growth control. Un-inoculated FGE-free and un-inoculated FGE-supplemented media were used to define the background OD570 values. Plates were aerobically incubated on a shaker (100 rpm) at 37 °C for 24 h. Culture media were then removed, wells were washed twice with sterile phosphate buffer saline (PBS, pH 7.2)., fixed with methanol, stained for 20 min with 1% w/v crystal violet, washed and then solubilized by 95% ethanol. The OD570 was determined using ELISA microplate reader ELX800 (Biotek/USA). The antibiofilm activity was calculated as the percentage of reduction using the previously described formula (Mathur et al., 2013) as follow:
2.6. Testing for the antibiofilm efficiency of test concentrations against test strains
To assess the efficiency of test concentrations of FGE in inhibiting biofilm formation, the above-mentioned protocol was used with the exception of replacing the sub-MICs of FGE with test concentrations (100%, 75%, 50% & 25%). To assess the biofilm eradication efficiency, mature biofilms were first formed by inoculating wells with a heavy bacterial suspension (1 × 108 CFU/ml) prepared in TSB/1% glucose, then aerobically incubated in an incubator shaker at 37°/48 h. Media were discarded and wells were washed with sterile PBS (pH 7.2). Biofilms were then treated with test concentrations and aerobically incubated at 37°/24 h. Biofilms were detected by crystal violet staining and quantified as mentioned above. All tests were performed in independent triplicates.
2.7. In-vitro lock models
Test concentrations were evaluated as lock solutions for either inhibition or eradication of bacterial colonization of CVC. For assessing the inhibitory efficiency, the previously described method (Bradford, 2011) was used with minor modifications. Briefly; 3 mm segments of a 4-French Polyurethane (PU) CVC (Amecath, Egypt) were inserted into a 24-well Costar plate containing 0.5 ml test concentrations of FGE in TSB/1% glucose along with bacterial cultures and incubated aerobically at 37° for 24 h in a shaker (100 rpm). Segments were then picked up, washed and prepared for viable counting. Where adhered cells were released via sonication using a VCX-400 sonicator (Sonics & Materials Inc., Danbury, CT, USA) (120 s, 30% cycle, pulse 3.5 s), vortexed, serially diluted, and spread over Mueller-Hinton agar. The eradication efficiency was assessed according to Ko et al. (Ko et al., 2010) with modifications. CVC sheets were incubated in human plasma at 37 °C for 24 h (Kuhn et al., 2002). Plasma was then replaced by microbial suspensions (1x108 CFU/ml) of test strain in TSB/1% glucose in an incubator shaker (100 rpm) at 37 °C/5 days. Sheets were then picked up, washed with PBS, placed in 10 ml of FGE test concentration. Garlic-free broth was used as a control. After 1, 3, 5, 7 & 10 days, segments were processed for viable counting as mentioned above. Experiments were performed in triplicates. Scanning electron microscopy (SEM) was used for confirming the effectiveness of garlic lock in eradicating biofilms of P. aeruginosa and MRSA according to the published protocol (Lazaro-Diez et al., 2016). Briefly; segments were fixed for 2 h at 4 °C with 3% glutaraldehyde, then dehydrated in a graded ethanol series (30%, 50%, 70%, 90% and 100%, 10 min each), dried, sputter coated with gold and observed with a high-resolution scanning microscope (JEOL-Japan Electron Optics Laboratory) at 30 KV.
2.8. In-vivo antimicrobial activity of FGE
2.8.1. Animals
Female albino mice (weight 25–30 g) were acclimatized for two weeks in a temperature and light-controlled room (25 °C, 12-h light/dark cycle) in the animal house at the NCRRT with free access to water and food.
2.8.2. Ethical considerations
All procedures in this study were approved by the Ethics Committee, Faculty of Pharmacy, Cairo University (MI-1082).
2.8.3. Selection of garlic doses
The corresponding MIC values against P. aeruginosa and MRSA were prepared in human serum, then 20 µl were inoculated on a blank filter paper disk, placed on inoculated agar, incubated at 35–37 °C for 24 h and the diameter of the inhibition zone was determined. At the same time, mice were intraperitoneally injected with different doses (50, 100, 200 & 400 mg/kg) of FGE, 2-hours post-injection; and at 6-hours interval over 24 h; the antibacterial activity of serum garlic was estimated by the Kirby-Bauer method as previously described (Driscoll et al., 2012). Inhibitions zones were compared to that obtained by the disc holding the MIC.
2.8.4. Bacterial inoculum and induction of infections
P. aeruginosa and MRSA were grown in TSB/1% glucose till the log phase. Bacterial concentration was determined by measuring the OD600 and comparing to the growth curve constructed by plotting OD600 of bacterial suspension and colony-forming units (CFU) plated on Mueller-Hinton agar. Different bacterial doses (106-1012 CFU) were injected into the animals. Over a 24 h period and at 6-hours intervals, animal’s body temperatures were measured and blood samples were collected from the tail and processed for total white blood cells (WBCs) count.
Mice were divided into 12 groups of 7 each, six groups were allocated for each test strain. Out of which, group A was used as a negative control (saline-treated). Groups B through F were injected intraperitoneally with the corresponding infective dose and categorized as follow, group B was used as a positive control (infected and treated with normal saline), groups C and D were infected and simultaneously treated with either 100 mg/kg or 200 mg/kg of FGE, respectively. To ascertain metastatic infections and assess the efficiency of FGE in eradicating tissue biofilms, the other two groups were treated after 48 h with 100 mg/kg (group E) or 200 mg/kg of FGE (group F). Extra two groups were only injected with FGE, 100 mg/kg (group G) or 200 mg/kg of FGE (group H) without being infected for estimating the toxicity profiles. Animals received treatments once a day for seven days and observed for any physical change during the treatment period.
2.8.5. Terminal harvest and processing of blood and tissues
2.8.5.1. In-vivo antimicrobial efficiency of garlic treatment
One day after the end of the experiment, mice were euthanized under aseptic conditions, blood samples and organs (heart, liver, kidney, and spleen) were collected from infected groups. Blood and minced body organs (1 g each) were cultured for detecting the total viable count by the spread plate technique (Sanders, 2012), any growth was recorded.
2.8.5.2. Evaluation of toxicity profile of garlic
Blood and tissue samples from groups A, G &H were assessed for biochemical, hematological and histopathological changes. Biochemical profiles of heart, kidney, and liver functions were assessed by measuring the serum aspartate aminotransferase (AST), alanine aminotransferase (ALT), albumin, bilirubin, total protein, creatine kinase (CK-MB), lactate dehydrogenase (LDH), creatinine and blood urea. All tests were performed according to the standard methods (Scott et al., 2012). Hematological parameters; hemoglobin, hematocrit (HCT), red blood cells (RBC), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), mean corpuscular hemoglobin concentration (MCHC), white blood cell (WBC), granulocyte count and lymphocytes were determined using Animal Blood Counter-ABC vet (Horiba ABX, France).
For histopathological studies, at autopsy, one gram from liver, heart, lung, kidney, and spleen was fixed in 10% formaldehyde; dehydrated through ascending grades of alcohol, cleared in xylene, wax-impregnated and embedded in paraffin wax. They were sectioned using microtome (Leica RM 2125, Leica Biosystems Nussloch GmbH, Germany) and stained with haematoxylin and Eosin and examined for histopathological changes using light microscope (Olympus BH-2, Olympus, Tokyo, Japan) (Fowotade et al., 2017).
2.9. Statistical analysis
Experiments were performed in triplicates. All data are expressed as means ± standard deviation (mean ± SD). ANOVA test (SPSS, version 18) was used for comparing data. P < 0.05 was considered significant.
3. Results
3.1. Antibacterial activity of FGE
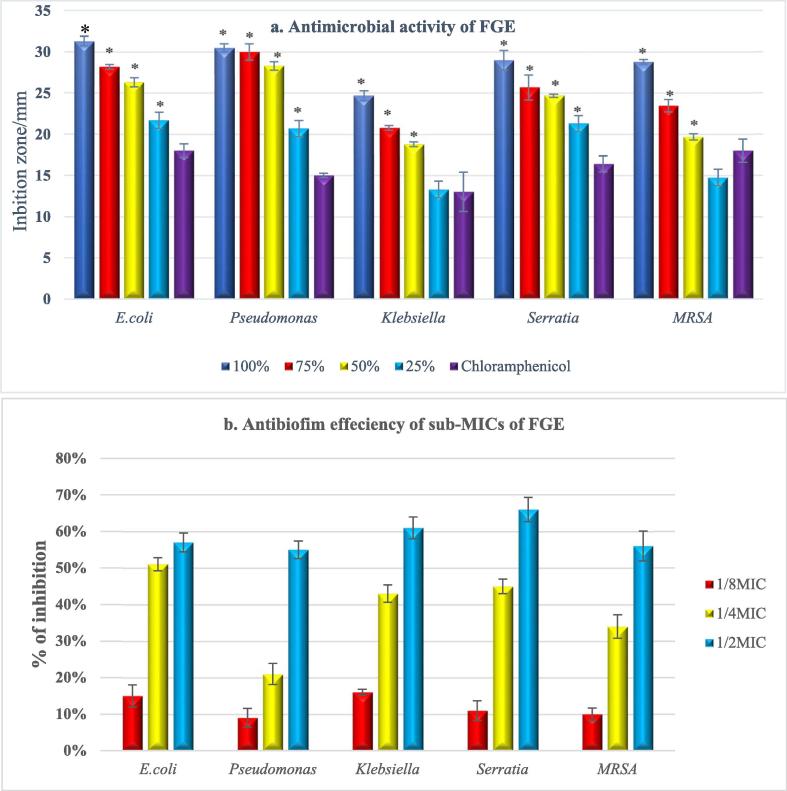
With the exception of MRSA and K. pneumoniae, inhibition zone measurement showed a significant (P value <0.001) higher antibacterial activity with all test concentrations; compared to chloramphenicol; that was concentration and strain-dependent (Fig. 1a). The MIC values were found to be 16 mg/ml for E. coli and P. aeruginosa, 32 mg/ml for S. marscens and MRSA, while K. pneumoniae recorded 64 mg/ml.
Fig. 1.
(a) Antibacterial activity of FGE, (b) Antibiofilm efficiency of FGE at sub-MICs against test strains, Data are presented as means of three trials (n = 3), error bars represent SD.
3.2. Estimation of the antibiofilm activity of FGE
Crystal violet staining in tissue culture plate (TCP) was used for evaluating the antibiofilm efficiency of FGE. FGE at sub-inhibitory concentrations exhibited a concentration (P < 0.001) and strain-dependent (P < 0.05) antibiofilm activity with the highest efficiency observed with 1/2 MIC against all test strains with predominance against S. marscens (66%) followed by K. pneumoniae (61%), E. coli (57%) and MRSA (56%). However, P. aeruginosa recorded the lowest inhibition rate (55%) (Fig. 1b).
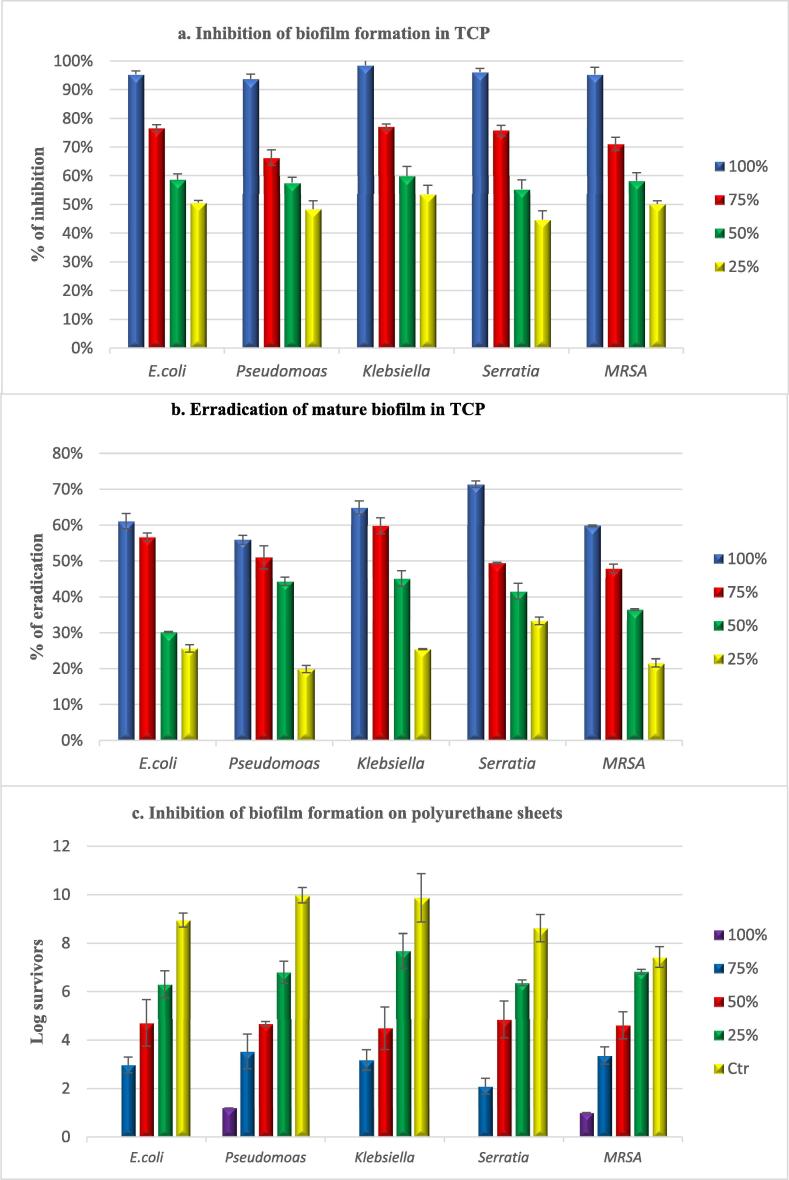
Additionally, test concentrations (100%, 75%, 50% & 25%) showed concentration and strain-dependent effectiveness in inhibition and eradication of biofilms (P-value < 0.001). Where the inhibitory effect of pure FGE (100%) recorded inhibition rates exceeded 90% with test strains as follow; 98.50, 96.10, 95.30, 95.30 &93.70% with K. penumoniae, S. marscens, E. coli, MRSA and P. aeruginosa, respectively. While at a concentration of 25%, FGE exhibited inhibition rates of 53.60, 44.70, 50.60, 50.50 and 48.40%, respectively (Fig. 2a). On the other hand, when evaluating Fig. 2(b), it was observed with the concentration of 100% that garlic was able to eradicate over 70% of the mature biofilm of S. marcescens, whereas for P. aeruginosa, its activity was not very accentuated.
Fig. 2.
Efficiency of test concentrations of FGE in: Inhibition of biofilm in tissue culture plates (a) Eradication of mature biofilms in tissue culture plates (b), and Inhibition of biofilms on Polyurethane sheets (c). Data are presented as means of three trials (n = 3), error bars represent SD.
3.3. In-vitro lock models
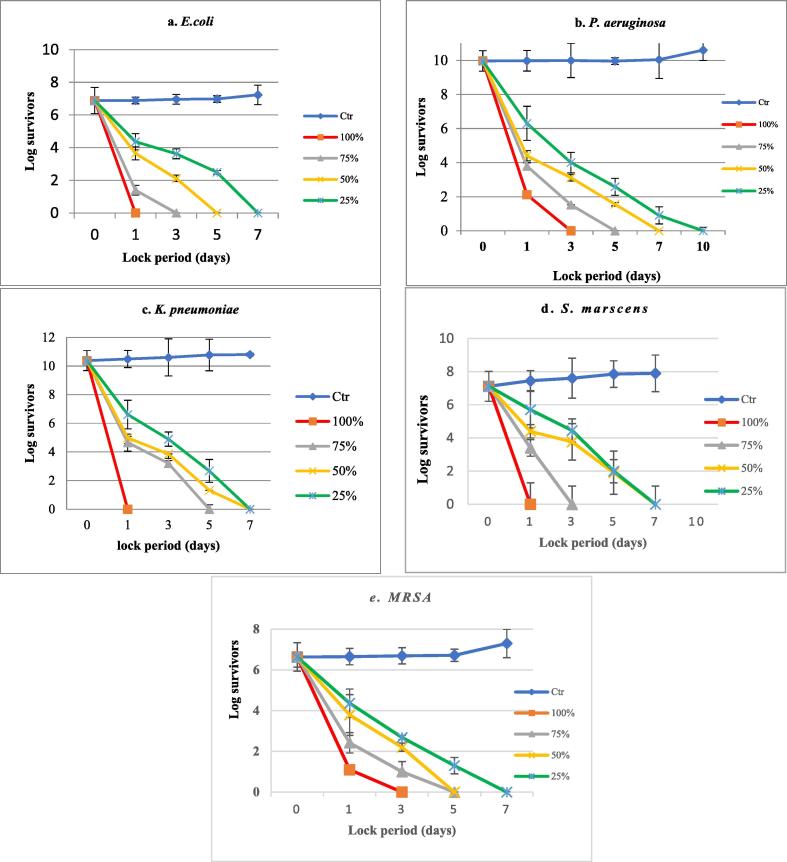
Upon evaluating the chemoprotective efficacy of garlic lock against catheter colonization, pure garlic almost inhibited biofilm formation by test strains on the catheter sheets after 24 h. however, low growth was detected with P. aeruginosa and MRSA. While concentration-dependent differences (P value < 0.001) in inhibition rates were observed with other concentrations (Fig. 2c). Similarly, the chemotherapeutic efficacy of FGE showed significant concentration and time-dependent variations among test strains (P-value < 0.001). Where the average initial biofilm count was 7.6 × 106, 9.2 × 109, 2.4 × 1010, 1.3 × 107 and 4.3 × 106 CFU/segment for E. coli, P. aeruginosa, K. penumoniae, S. marscens and MRSA, respectively. Pure garlic extract (100%) completely eradicated biofilms formed by S. marscens, K. penumoniae and E. coli after one day lock. Meanwhile, P. aeruginosa and MRSA required three days for complete eradication. The lowest concentration (25%) achieved zero survivors after 7 days lock period with all test strains, except P. aeruginosa required 10 days (Fig. 3).
Fig. 3.
Efficiency of garlic lock in the eradication of biofilms by test strains at different lock periods. Data are presented as means of three trials (n = 3), error bars show the SD.
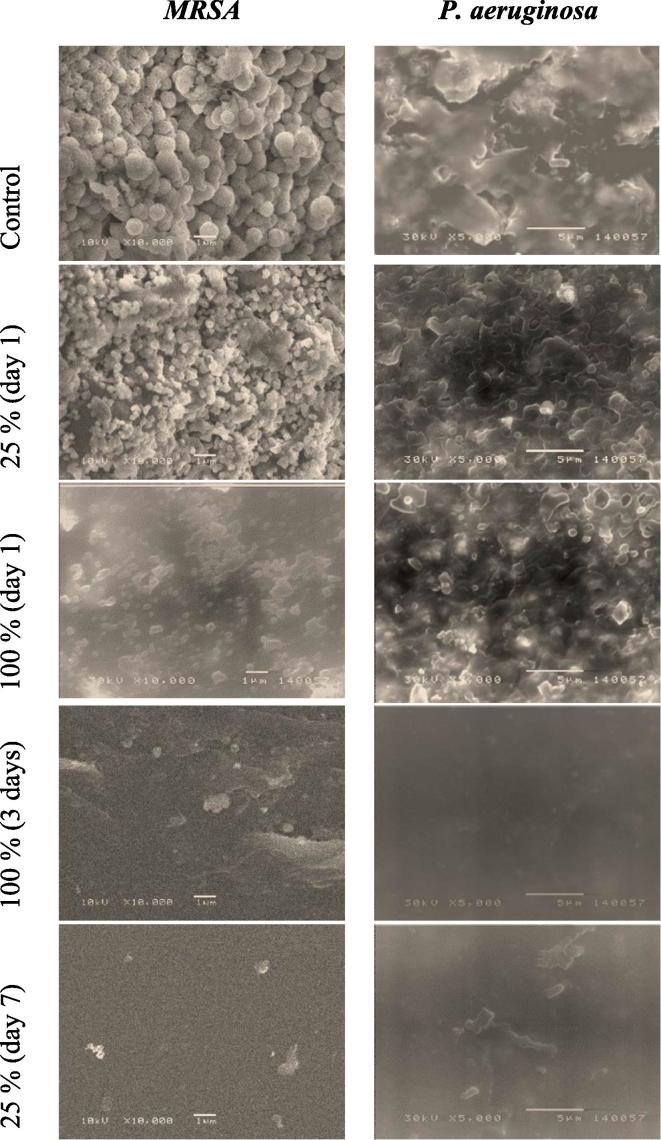
Garlic locks induced morphological changes in bacterial biofilms of MRSA and P. aeruginosa; as revealed by the SEM; comprising cell-shape deformation and cellular breakdown. Untreated samples showed clumps of heavy biofilms composed of aggregates of bacterial cells embedded in a matrix. However, sheets treated with 100% FGE showed clean surface almost free from any adhered cells after a three-days lock period, representative images for SEM are shown in Fig. 4.
Fig. 4.
SEM micrographs showing the effects of the highest (100%) and the lowest (25%) garlic lock concentrations on biofilms of MRSA and Pseudomonas at different lock periods.
3.4. Bacterial inoculum and induction of infections
Doses of 1.0 × 106 and 1.0 × 108 CFU were found enough to induce infections by P. aeruginosa and MRSA, respectively after 12 h as confirmed by leukocytosis.
3.5. In-vivo antimicrobial activity of FGE
By comparing the inhibition zones achieved by the MIC and test doses, doses of 100 and 200 mg/kg produced comparable results sustained for 24 h. Systemic infections with P. aeruginosa and MRSA were induced in mice via intraperitoneal injection. In case of P. aeruginosa, groups C and D recorded survival rate of 85.7%; where one mouse died after 4 and 1.5 h, respectively. For groups E and F; in spite of the high fever (40.2 ± 0.9 C0) developed; their body temperature dropped to (36.5 ± 0.44 C0) after being treated and survived till the end of the experiment. However, the untreated group (B) showed an increase in their body temperatures, and recorded a 100% mortality by the 5th day. In case of MRSA, all animals in groups C and D survived till the end of the experiment. Groups E and F developed fever (39.2 ± 0.54 C0), but dropped to (35.6 ± 0.24 C0) after treatment and survived till the end of the experiment. However, only one mouse (14.3%) survived in group B.
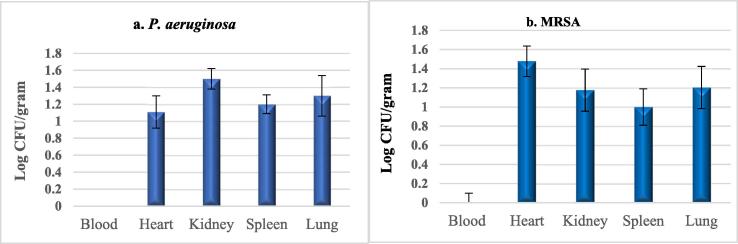
One day after the end of the treatment period, blood samples and tissues were harvested for viable counting from survived animals. No growth was detected in the blood or tissues of groups C, D or F in both infections. However, for E groups, low growth levels were detected within the tissues, but their blood was growth-free (Fig. 5 a &b).
Fig. 5.
Log surviving cells within blood and organs of animals in group E of: (a) P. aeruginosa, and (b) MRSA. Data are presented as means (n = 3), error bars represent SD.
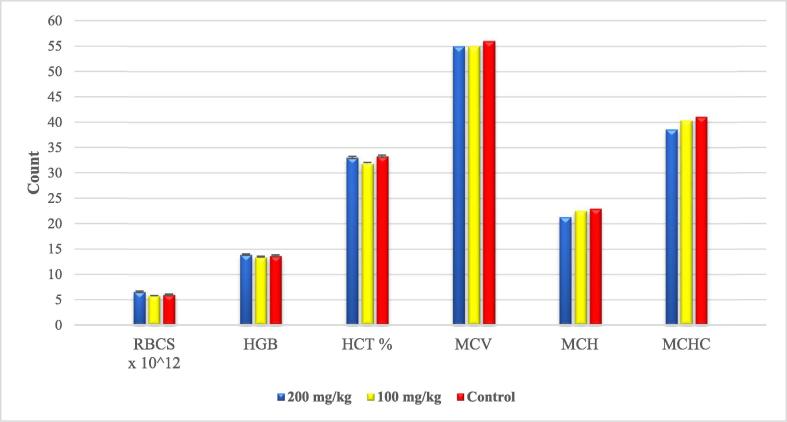
By comparing the biochemical parameters of groups G&H with that of the control (group A), both doses exhibited cardio and hepatoprotective effects as noticed by the significant (P-value < 0.001 & <0.05) drop in the cardiac and hepatic biomarkers. No alteration in the kidney biomarkers was recorded, except for the blood urea which significantly decreased upon garlic treatment (P-value < 0.005) (Table 1). Upon evaluating the hematological parameters, no significant alterations (P value > 0.05) were found in the tested parameters (Fig. 6).
Table 1.
Serum biochemical parameters in mice treated with FGE.
| Parameter | Control | 100 mg/kg | 200 mg/kg |
|---|---|---|---|
| CK-MB | 624 ± 1 | 244 ± 3* | 615 ± 1* |
| LDH | 171.9 ± 1 | 174 ± 1.5* | 159 ± 0.9* |
| Creatinine | 1 ± 0.0 | 0.6 ± 0.03* | 0.95 ± 0.0 |
| Blood urea | 29 ± 1 | 22 ± 0.8* | 24 ± 1* |
| Serum Albumin | 3.2 ± 0.0 | 3.6 ± 0.36 | 2.8 ± 0 |
| Bilirubin | 0.27 ± 0.01 | 0.25 ± 0.0 | 0.29 ± 0.01 |
| AST (SGOT) | 36 ± 1 | 31 ± 1.6* | 27 ± 1* |
| ALT (SGPT) | 47 ± 1 | 42 ± 0.5* | 39 ± 0* |
Results are presented as means ± SD (n = 7).
Indicates a significant reduction in the treated group compared to control.
Fig. 6.
Effect of garlic on the hematological parameters. Data are presented as means of three trials (n = 7).
3.6. Histological investigation
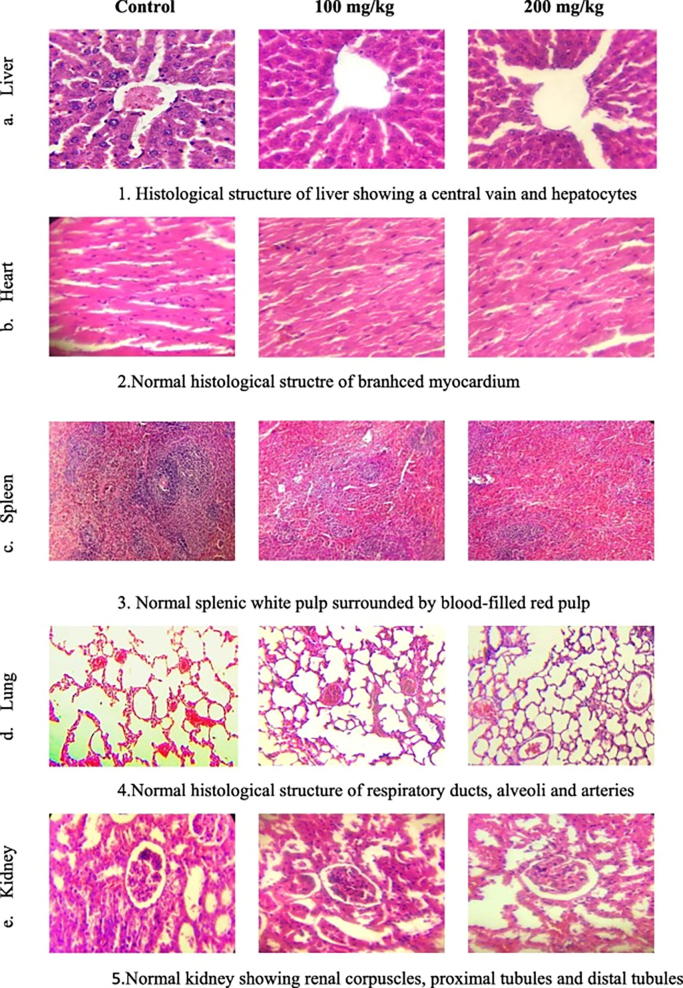
The H & E staining showed no histological changes in the architecture of the heart, spleen, lung, kidneys or liver of animals treated with tested doses; when compared to the control ones (Fig. 7).
Fig. 7.
Light micrographs of the mice organs showing the control and treated ones (X400).
4. Discussion
Natural organosulfur compounds within garlic provide the basis for innovative sources of novel antibiotics against resistant pathogens (Haina Wang, 2014). This study was directed to assess the potential use of garlic in the prevention and treatment of CVCRIs. Where FGE was in-vitro evaluated; in relation to its antibacterial activity; against five MDR clinical bacterial strains. Additionally, the in-vivo antibacterial activity was further evaluated against P. aeruginosa and MRSA infections. We also highlighted the potential toxic effects of garlic on mice. We prepared fresh raw garlic extract to be used, as its antimicrobial activity was reported to be superior to its isolated compounds (Venâncio et al., 2017). In agreement with others (Bayati, 2018), FGE showed potent antibacterial activity that was concentration and strain-dependent. Among the five test strains, the highest MIC value was recorded with K. pneumoniae. Its capsule might block the access of plant essential oils to the fragile inner membrane (Fournomiti et al., 2015). Garlic allicin interferes with crucial bacterial enzymes through the inactivation of thiol group (Ratthawongjirakul and Thongkerd, 2016, Reiter et al., 2017). Other investigators (Mohsenipour, 2015, Pakdel et al., 2017) didn’t record prominent antibacterial activity for either the aqueous or alcoholic extract of garlic by the disk diffusion methods. However, many factors could affect the results; such as the plant origin, period of the year, extraction methods, microbial test strains (Bayati, 2018, Pakdel et al., 2017), testing methodology (agar well, filter paper, macro-broth dilution or micro-broth dilution) and storage conditions. The antibacterial activity of fresh garlic was found to be superior to garlic powder, garlic oil and butylated hydroxyanisole (Sallam et al., 2004). Drying temperature and time might also affect the results (Rahman et al., 2006). Finally, testing strains might have variable results; clinical strains are more resistant than standard ones (Abiy and Berhe, 2016).
Biofilm plays a vital role in pathogenicity, virulence and antimicrobial resistance of microbial pathogens (Bayati, 2018, Tewari et al., 2018). We found that sub-MICs of FGE showed an evident antibiofilm activity in a concentration-dependent fashion. Allicin at sub-MIC levels was found to inhibit adherence and biofilm (Pérez-Giraldo et al., 2003). Our findings revealed that the inhibitory effect was more prominent than the eradication effect on mature biofilm. However, both effects on biofilm were concentration and strain-dependent. These findings agree with that of Mohsenipour (Mohsenipour, 2015). In the same way, the antibiofilm activity of garlic on Gram-positive and Gram-negative bacteria under chemopreventive and chemotherapeutic conditions was reported (Nidadavolu et al., 2012, Ratthawongjirakul and Thongkerd, 2016). The higher preventive effect could be due to the bactericidal effect of garlic on the planktonic phase and subsequently, interference with the initial attachment step which is essential for biofilm formation (Nidadavolu et al., 2012). Additionally, allicin down-regulates biofilm genes expression (Wu et al., 2015), reflecting the chemotherapeutic efficiency of garlic on mature biofilm.
For the in-vitro evaluation of garlic lock, PU catheter sheets were used because PU; compared to other biomaterials; is more susceptible for microbial colonization (Wildgruber et al., 2016). Generally, the results of the lock model matched with that of the CV assay in TCP regarding the preventive and therapeutic efficiencies of garlic, where pure garlic extract showed the highest efficiency against biofilms in both cases. Similar observations were documented by others (Mathur et al., 2013, Mohsenipour, 2015). Morphological deformation noticed by the SEM could be due to the interaction of allicin with the cellular thiol groups (Fujisawa et al., 2009). The increased exposed surface area of bacterial rods might explain the fragmentation noticed in Pseudomonas. Other investigators documented similar observations with other bacilli (Lu et al., 2011).
Because bacterial behaviors, as well as the effectiveness of antimicrobial agents, might differ in-vivo from that of in-vitro (Venâncio et al., 2017), we designed an in-vivo model to assess the effectiveness of FGE in managing infections. Systemic infections were induced in mice, then treated daily with 100 or 200 mg/kg of FGE for seven days. A 7-day catheter-lock period was reported to be an effective period for managing CVC-related infections (Oncu et al., 2004). The in-vivo results demonstrated promising antibacterial effectiveness for FGE. The early death occurred in Pseudomonas-infected mice; simultaneously treated with garlic; demonstrates the potent antibacterial activity of garlic on planktonic cells that provoked an inflammatory response and endotoxic shock due to massive cell destruction and the release of endotoxins (Fink, 2014). Being less sensitive to lipopolysaccharides (LPS), mice require a high dose to elicit an inflammatory response (Popov and Pavlov, 2013), this indicates the high bioburden within infected mice. The in-vivo antimicrobial activity of garlic against MRSA (Karunanidhi et al., 2017) and P. aeruginosa (Bjarnsholt et al., 2005) was previously documented. Moreover, the prophylactic garlic treatment was found to be associated with reduced mortality (Bjarnsholt et al., 2005). Biofilm-related infections could affect internal organs (Lebeaux et al., 2013). We established such infections by delayed garlic treatment 2-days post-infections. In spite of the complete clearance of infecting pathogens from the bloodstream, few cell numbers were detected in the organs in both infections treated with 100 mg/kg. This might recommend a longer treatment duration to completely eradicate organs biofilm. However, this low number explains the antibiofilm activity of garlic treatment; especially organs biofilms were completely eradicated when treated with 200 mg/kg. Tsao and colleagues (Tsao et al., 2003) found that oral garlic treatment of MRSA-infected mice reduced bacterial bioburden within internal organs. Additionally, the in-vivo antibiofilm activities against Pseudomonas spp. as well as Staphylococcus spp. were reported (Bjarnsholt et al., 2005, Zhai et al., 2014). Moreover, garlic exhibited antibiofilm activity in a burn model against Gram-negative and Gram-positive bacteria (Nidadavolu et al., 2012). On the contrary, A. sativum failed to eradicate MRSA infections in induced granulation tissues in rats upon oral administration of 100 or 400 mg/kg (Venâncio et al., 2017). The discrepancies may be due to the short treatment durations used by the investigators (6–24 h), especially they recorded an in-vitro antimicrobial activity against the test strains. This supports our explanation for the low detected CFU in organs.
The toxic effects of garlic could be a barrier against its potential usefulness, so we assessed the potential toxic effects of the two selected doses at the biochemical, hematological and histological levels. Regarding the biochemical profile, a cardioprotective effect was found with both doses, where a significant lowering in the cardiac enzymes was reported; compared to the control. This could be due to the cardiac glycosides within garlic (Fowotade et al., 2017). In agreement with others, garlic treatment exhibited dose-dependent hepatoprotective effect as exhibited by the drop in liver biomarkers (Fowotade et al., 2017) which could be due to the antioxidant nature of garlic (Haina Wang, 2014). Garlic doses up to 350 mg/kg were reported to have protective effects on liver, heart, and kidneys (Fowotade et al., 2017). No changes in the hematological parameters were recorded in our study. Garlic dosing at 300 mg/kg was previously reported to be safe for use based on the biochemical and hematological investigations (Lawal et al., 2016). Finally; and in agreement with others (Fowotade et al., 2017); no prominent changes in the histological architecture of the heart, lung, kidneys, spleen, and livers were observed in our study, such findings support the biochemical measurements.
5. Conclusions
Considering the results, FGE demonstrated a unique antibacterial activity against clinical pathogenic bacteria; either in the planktonic or biofilm forms; in both in-vitro and in-vivo models without observed toxic effects. This supports its potential use as a prophylactic and/or therapeutic lock agent against biofilm-associated infections caused by MDR bacteria.
5.1. Recommendations for future researches
Despite the breadth of studies on the antimicrobial properties of garlic, there remains a paucity of the in-vivo researches and clinical trials. Furthermore, the majority of studies were in-vitro performed on mono-species biofilms, so it’s difficult to extrapolate the in-vitro results to the complex, multispecies biofilms in patients. So, we recommend further in-vivo studies on multispecies biofilms on catheter models for verifying the effectiveness of garlic in lock therapy.
Declaration of Competing Interest
The authors declare no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abiy E., Berhe A. Antibacterial effect of garlic (Allium sativum) against clinical isolates of Staphylococcus aureus and Escherichia coli from patients attending Hawassa Referral Hospital. Ethiopia. J. Infect. Dis. Treat. 2016;02:1–5. doi: 10.21767/2472-1093.100023. [DOI] [Google Scholar]
- Andualem B. Combined antibacterial activity of stingless bee (Apis mellipodae) honey and garlic (Allium sativum) extracts against standard and clinical pathogenic bacteria. Asian Pac. J. Trop. Biomed. 2013;3:725–731. doi: 10.1016/S2221-1691(13)60146-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayati S. Al. Antibacterial effect of ethanolic extract of Allium sativum on biofilm forming Staphylococcus aureus which causes folliculitis. Int. J. Curr. Microbiol. App. Sci. 2018;7(1):1904–1913. [Google Scholar]
- Bjarnsholt T., Jensen P.Ø., Rasmussen T.B., Christophersen L., Calum H., Hentzer M., Hougen H.P., Rygaard J., Moser C., Eberl L., Høiby N., Givskov M. Garlic blocks quorum sensing and promotes rapid clearing of pulmonary Pseudomonas aeruginosa infections. Microbiology. 2005;151:3873–3880. doi: 10.1099/mic.0.27955-0. [DOI] [PubMed] [Google Scholar]
- Blackwood R.A., Issa M., Klein K., Mody R., Willers M., Teitelbaum D. Ethanol lock therapy for the treatment of intravenous catheter infections that have failed standard treatment. J. Pediatric Infect. Dis. Soc. 2017;6:94–97. doi: 10.1093/jpids/piv060. [DOI] [PubMed] [Google Scholar]
- Bradford C. The use of commercially available alpha-amylase compounds to inhibit and remove Staphylococcus aureus biofilms. Open Microbiol. J. 2011;5:21–31. doi: 10.2174/1874285801105010021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C.-Y. Surface sensing for biofilm formation in Pseudomonas aeruginosa. Front. Microbiol. 2018;8:2671. doi: 10.3389/fmicb.2017.02671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon J.J., Steele M., Makanjuola A.D. Antimicrobial locks increase the prevalence of Staphylococcus aureus and antibiotic-resistant Enterobacter: Observational retrospective cohort study. Nephrol. Dial. Transplant. 2012;27:3575–3581. doi: 10.1093/ndt/gfs081. [DOI] [PubMed] [Google Scholar]
- Driscoll A.J., Bhat N., Karron R.A., O’Brien K.L., Murdoch D.R. Disk diffusion bioassays for the detection of antibiotic activity in body fluids: Applications for the pneumonia etiology research for child health project. Clin. Infect. Dis. 2012;54:159–164. doi: 10.1093/cid/cir1061. [DOI] [PubMed] [Google Scholar]
- Fink M.P. Animal models of sepsis. Virulence. 2014;5:143–153. doi: 10.4161/viru.26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming D., Chahin L., Rumbaugh K. Glycoside hydrolases degrade polymicrobial bacterial biofilms in wounds. Antimicrob. Agents Chemother. 2017;61:1–9. doi: 10.1128/AAC.01998-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournomiti M., Kimbaris A., Mantzourani I., Plessas S., Theodoridou I., Papaemmanouil V., Kapsiotis I., Panopoulou M., Stavropoulou E., Bezirtzoglou E.E., Alexopoulos A. Antimicrobial activity of essential oils of cultivated oregano (Origanum vulgare), sage (Salvia officinalis), and thyme (Thymus vulgaris) against clinical isolates of Escherichia coli, Klebsiella oxytoca, and Klebsiella pneumoniae. Microb. Ecol. Health Dis. 2015;1:1–7. doi: 10.3402/mehd.v26.23289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowotade, A.A., Fowotade, A., Enaibe, B.U., Avwioro, G.O., 2017. Evaluating toxicity profile of garlic (Allium sativum) on the liver, kidney and heart using Wistar rat model 26, 1–12. 10.9734/IJTDH/2017/36282. [DOI]
- Fujisawa H., Watanabe K., Suma K., Origuchi K., Matsufuji H., Seki T., Ariga T. Antibacterial potential of garlic-derived allicin and its cancellation by sulfhydryl compounds. Biosci. Biotechnol. Biochem. 2009 doi: 10.1271/bbb.90096. [DOI] [PubMed] [Google Scholar]
- Funalleras G., Fernández-Hidalgo N., Borrego A., Almirante B., Planes A.M., Rodríguez D., Ruiz I., Pahissa A. Effectiveness of antibiotic-lock therapy for long-term catheter-related bacteremia due to gram-negative bacilli: A prospective observational study. Clin. Infect. Dis. 2011;53:129–132. doi: 10.1093/cid/cir551. [DOI] [PubMed] [Google Scholar]
- Gahlot R., Nigam C., Kumar V., Yadav G., Anupurba S., Gahlot R., Nigam C., Kumar V., Yadav G., Anupurba S. Catheter-related bloodstream infections. Int. J. Crit. Illn. Inj. Sci. 2014;4:162–167. doi: 10.4103/2229-5151.134184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gominet M., Compain F., Beloin C., Lebeaux D. Central venous catheters and biofilms: where do we stand in 2017? Apmis. 2017;125:365–375. doi: 10.1111/apm.12665. [DOI] [PubMed] [Google Scholar]
- Gupta S., Kapur S., Padmavathi D.V., Verma A. Garlic: An effective functional food to combat the growing antimicrobial resistance. Pertanika J. Trop. Agric. Sci. 2015;38:271–278. [Google Scholar]
- Haddadin Y., Regunath H. StatPearls; 2018. Central line-associated bloodstream infections (CLABSI) [Google Scholar]
- Haina Wang X.J. Drug metabolism and pharmacokinetics of organosulfur compounds from garlic. J. Drug Metab. Toxicol. 2014;04:1–10. [Google Scholar]
- Hasselmann, C., Diseases, I., 2003. EUCAST discussion document E. Dis 5. 1 March 2003 Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution 1–7.
- Hosny, A.E.D.M.S., Farrag, H.A., Soheir A. Issa, S.A Hagras, S.A.A. 2014. Prevalence of central venous catheter-related infections in catheterized ICUs’ patients. ijphc. 10.13140/RG.2.2.18720.07687. [DOI]
- Justo J.A., Bookstaver P.B. Antibiotic lock therapy: Review of technique and logistical challenges. Infect. Drug Resist. 2014;7:343–363. doi: 10.2147/IDR.S51388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karunanidhi A., Ghaznavi-Rad E., Nathan J.J., Abba Y., Van Belkum A., Neela V. Allium stipitatum extract exhibits in vivo antibacterial activity against Methicillin-Resistant Staphylococcus aureus and accelerates burn wound healing in a full-thickness murine burn model. Evidence-based Complement. Altern. Med. 2017 doi: 10.1155/2017/1914732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan L., Paulino E.M., Lim D., Nadela F., Yadav R., Birring O.S. Antimicrobial efficacy of Allium sativum against Streptococcus mutans biofilm formation on orthodontic mini-implants. J. Orthod. Res. 2014;2:129. [Google Scholar]
- Ko K.S., Lee J.Y., Song J.H., Peck K.R. In-vitro evaluation of antibiotic lock technique for the treatment of Candida albicans, C. glabrata, and C. tropicalis biofilms. J. Korean Med. Sci. 2010 doi: 10.3346/jkms.2010.25.12.1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn D.M., George T., Chandra J., Mukherjee P.K., Ghannoum M.A. Antimicrob; Agents Chemother: 2002. Antifungal susceptibility of Candida biofilms: Unique efficacy of amphotericin B lipid formulations and echinocandins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawal B., Shittu O.K., Oibiokpa F.I., Mohammed H., Umar S.I., Haruna G.M. Antimicrobial evaluation, acute and sub-acute toxicity studies of Allium sativum. J. Acute Dis. 2016;5:296–301. [Google Scholar]
- Lazaro-Diez M., Remuzgo-Martinez S., Rodriguez-Mirones C., Acosta F., Icardo J.M., Martinez-Martinez L., Ramos-Vivas J. Effects of subinhibitory concentrations of ceftaroline on Methicillin-Resistant Staphylococcus aureus (MRSA) biofilms. PLoS One. 2016;11:1–15. doi: 10.1371/journal.pone.0147569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeaux D., Chauhan A., Rendueles O., Beloin C. From in-vitro to in-vivo models of bacterial biofilm-related infections. Pathogens. 2013;2:288–356. doi: 10.3390/pathogens2020288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.-R., Shi Q.-S., Dai H.-Q., Liang Q., Xie X.-B., Huang X.-M., Zhao G.-Z., Zhang L.-X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Rasco B.A., Kang D.H., Jabal J.M.F., Aston D.E., Konkel M.E. Infrared and Raman spectroscopic studies of the antimicrobial effects of garlic concentrates and diallyl constituents on foodborne pathogens. Anal. Chem. 2011 doi: 10.1021/ac2001498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur S., Gutte M., Paul D., Udgire M. Study the effect of essential oils on microbial biofilm formation by Klebsiella pneumoniae. Sch. Acad. J. Biosci. 2013;1:76–79. [Google Scholar]
- Mendoza-Juache A., Aranda-Romo S., Bermeo-Escalona J.R., Gómez-Hernández A., Pozos-Guillén A., Sánchez-Vargas L.O. The essential oil of Allium sativum as an alternative agent against Candida isolated from dental prostheses. Rev. Iberoam. Micol. 2017 doi: 10.1016/j.riam.2016.11.008. [DOI] [PubMed] [Google Scholar]
- Mohsenipour Z. The effects of Allium sativum extracts on biofilm formation and activities of six pathogenic bacteria. Jundishapur. J. Microbiol. 2015 doi: 10.5812/jjm.18971v2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nidadavolu P., Amor W., Tran P.L., Dertien J., Colmer-Hamood J.A., Hamood A.N. Garlic ointment inhibits biofilm formation by bacterial pathogens from burn wounds. J. Med. Microbiol. 2012;61:662–671. doi: 10.1099/jmm.0.038638-0. [DOI] [PubMed] [Google Scholar]
- Oncu S., Kurt I., Sakarya S., Ozturk B., Oncu S. Elimination of intraluminal colonization by antibiotic lock in catheters. Tohoku J. Exp. Med. 2004 doi: 10.1620/tjem.203.1. [DOI] [PubMed] [Google Scholar]
- Pakdel F., Ghasemi S., Babaloo A., Javadzadeh Y., Momeni R., Ghanizadeh M., Moaddab S.R., Fathi F.Y. Antibacterial effects of garlic extracts and ziziphora essential oil on bacteria associated with peri-implantitis. J. Clin. Diagnostic Res. 2017;11:ZC16-ZC19. doi: 10.7860/JCDR/2017/24786.9620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Giraldo C., Cruz-Villalón G., Sánchez-Silos R., Martínez-Rubio R., Blanco M.T., Gómez-García A.C. In-vitro activity of allicin against Staphylococcus epidermidis and influence of subinhibitory concentrations on biofilm formation. J. Appl. Microbiol. 2003;95:709–711. doi: 10.1046/j.1365-2672.2003.02030.x. [DOI] [PubMed] [Google Scholar]
- Popov D., Pavlov G. Sepsis models in experimental animals. Trakia J. Sci. 2013;11:13–23. [Google Scholar]
- Rahman M.S., Al-Sheibani H.I., Al-Riziqi M.H., Mothershaw A., Guizani N., Bengtsson G. Assessment of the antimicrobial activity of dried garlic powders produced by different methods of drying. Int. J. Food Prop. 2006 [Google Scholar]
- Rammo R.N.N. Bactericidal and antibiofilm formation of aqueous plant extracts against pathogenic bacteria. Asian J. Pharm. Res. 2017 [Google Scholar]
- Ratthawongjirakul P., Thongkerd V. Fresh garlic extract inhibits Staphylococcus aureus biofilm formation under chemopreventive and chemotherapeutic conditions. Songklanakarin J. Sci. Technol. 2016;38:381–389. [Google Scholar]
- Reiter J., Levina N., Van Der Linden M., Gruhlke M., Martin C., Slusarenko A.J. Diallylthiosulfinate (Allicin), a volatile antimicrobial from garlic (Allium sativum), kills human lung pathogenic bacteria, including MDR strains, as a vapor. Molecules. 2017;22:1–14. doi: 10.3390/molecules22101711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallam K.I., Ishioroshi M., Samejima K. Antioxidant and antimicrobial effects of garlic in chicken sausage. LWT - Food Sci. Technol. 2004 doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders, E.R., 2012. Aseptic Laboratory Techniques: Plating Methods 2. Streak Plate Procedure: Isolation of bacterial colonies using the quadrant method 1–18. 10.3791/3064. [DOI]
- Scott, M.G., LeGrys, V.A., Hood, J.L., 2012. Electrolytes and blood gases, tietz textbook of clinical chemistry and molecular diagnostics. 10.1016/B978-1-4160-6164-9.00028-7. [DOI]
- Stepanović S., Vuković D., Dakić I., Savić B., Švabić-Vlahović M. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J. Microbiol. Methods. 2000;40:175–179. doi: 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- Tewari A., Jain B., Dhamannapatil P.S., Saxena M.K. Biofilm resistance to antimicrobial agents and novel approaches to combat biofilm mediated resistance in bacteria. EC Microbiol. 2018;3:71–77. [Google Scholar]
- Trautner B.W., Darouiche R.O. Catheter-associated infections. Arch. Intern. Med. 2004;164:842. doi: 10.1001/archinte.164.8.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao S.M., Hsu C.C., Yin M.C. Garlic extract and two diallyl sulphides inhibit Methicillin-Resistant Staphylococcus aureus infection in BALB/cA mice. J. Antimicrob. Chemother. 2003;52:974–980. doi: 10.1093/jac/dkg476. [DOI] [PubMed] [Google Scholar]
- Venâncio P.C., Del Fiol F.de S., Sartoratto A., Nani B.D., Ferreira L.E., Muniz B.V., Ribeiro Rosa E.A., Figueroba S.R., Groppo F.C. Antimicrobial activity of two garlic species (Allium sativum and A. tuberosum) against Staphylococci infection. in vivo study in rats. Adv. Pharm. Bull. 2017;7:115–121. doi: 10.15171/apb.2017.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildgruber M., Lueg C., Borgmeyer S., Karimov I., Braun U., Kiechle M., Meier R., Koehler M., Ettl J., Berger H. Polyurethane versus silicone catheters for central venous port devices implanted at the forearm. Eur. J. Cancer. 2016 doi: 10.1016/j.ejca.2016.02.011. [DOI] [PubMed] [Google Scholar]
- Wu X., Santos R.R., Fink-Gremmels J. Analyzing the antibacterial effects of food ingredients: model experiments with allicin and garlic extracts on biofilm formation and viability of Staphylococcus epidermidis. Food Sci. Nutr. 2015;3:158–168. doi: 10.1002/fsn3.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang B., Lei Z., Zhao Y., Ahmed S., Wang C., Zhang S., Fu S., Cao J., Qiu Y. Combination susceptibility testing of common antimicrobials in vitro and the effects of Sub-MIC of antimicrobials on Staphylococcus aureus biofilm formation. Front. Microbiol. 2017;8:1–14. doi: 10.3389/fmicb.2017.02125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai H., Pan J., Pang E., Bai B. Lavage with allicin in combination with vancomycin inhibits biofilm formation by Staphylococcus epidermidis in a rabbit model of prosthetic joint infection. PLoS One. 2014 doi: 10.1371/journal.pone.0102760. [DOI] [PMC free article] [PubMed] [Google Scholar]