Abstract
Diabetes compromises bone cell metabolism and function, resulting in increased risk of fragility fracture. Advanced glycation end products (AGEs) interact with the receptor for AGEs (RAGE) and can make a meaningful contribution to bone cell metabolism and/or alter function. Searches in PubMed using the key words “advanced glycation end-product,” “RAGE,” “sRAGE,” “bone,” and “diabetes” were made to explain some of the clinical outcomes of diabetes in bone metabolism through the AGE–RAGE signaling pathway. All published clinical studies were included in tables. The AGE–RAGE signaling pathway participates in diabetic complications, including diabetic osteopathy. Some clinical results in diabetic patients, such as reduced bone density, suppressed bone turnover markers, and bone quality impairment, could be potentially due to AGE–RAGE signaling consequences. However, the AGE–RAGE signaling pathway has some helpful roles in the bone, including an increase in osteogenic function. Soluble RAGE (sRAGE), as a ligand decoy, may increase in either conditions of RAGE production or destruction, and then it cannot always reflect the AGE–RAGE signaling. Recombinant sRAGE can block the AGE–RAGE signaling pathway but is associated with some limitations, such as accessibility to AGEs, an increase in other RAGE ligands, and a long half-life (24 hours), which is associated with losing the beneficial effect of AGE/RAGE. As a result, sRAGE is not a helpful marker to assess activity of the RAGE signaling pathway. The recombinant sRAGE cannot be translated into clinical practice due to its limitations.
Keywords: advanced glycation end product, diabetes, osteoporosis, RAGE
Diabetes is a metabolic disease that is associated with increased risk of fracture. Diabetic bone is characterized by changes in bone metabolism, decreased bone mineral content and density, increased fracture rate, and delayed fracture healing. Measuring bone mineral density (BMD) by dual-energy X-ray absorptiometry is the usual method of predicting bone fragility [1]. However, older age, reduced muscle mass or poor balance, duration of type 2 diabetes mellitus (T2DM), complications of DM, poor glycemic control, hypoglycemia, and medications (thiazolidinedione, SGLT2 inhibitors, and insulin) are reported as further compounding the fracture risk of patients with T2DM [1, 2]. The pathophysiology of bone impairment includes compromised bone metabolism (due to oxidant injury, lower IGF-1, higher sclerostin, and lower circulating osteoprogenitor cells), impairment of cell function, and extracellular matrix complications (due to the factors such as increased collagen glycation and accumulation of AGEs). Subsequently, diabetes leads to increased rate of bone loss, alteration of bone structure (increased cortical porosity, inhomogeneity, and thinning of cortex), reduced bone turnover, and then a predisposition to bone fragility [1, 2].
Obesity, especially central obesity, as a component of metabolic syndrome has important consequences for morbidity and increases the risk of developing T2DM. Association of fat mass and bone health is bidirectional. The body mass index has a positive correlation with BMD of all sites, as well as anegative correlation with bone turnover markers (BTMs) [1, 3]. However, after assessing femoral neck strength by using the femoral neck composite index, obesity shows negative correlation with femoral neck strength [4]. Additionally, obesity and T2DM have negative effects on bone turnover, trabecular bone volume, and bone microarchitecture, irrespective of diet in rats [5]. Conclusively, the mechanical load resulting from excessive weight along with hyperinsulinemia have positive effects on bone quality. However, hyperglycemia, inflammation, and a change of adipokines in the setting of obesity and insulin resistance have detrimental effects on bone [1].
Generally, the route of obesity, insulin resistance, and T2DM progress in the setting of the integrated metabolic and inflammatory events. Advanced glycation end products (AGEs) and their receptor (RAGE) are considered as important parts of inflammatory events that can cause diabetes and its complications. They are mainly produced due to a high-fat diet or hyperglycemia [6, 7]. Then, it is important to discuss the role of collagen glycation, accumulation of AGEs, and the AGE–RAGE signaling pathway in the changes of bone matrix properties.
In this review, we discuss the alteration of bone health in diabetes, the correlation of the current clinical and paraclinical information with AGE–RAGE interaction, and summarize our current understanding regarding the connection between AGE/RAGE and different signaling pathways that may play a role in bone metabolism. Finally, on the basis of the found reported evidence, we discuss the benefits and limitations of soluble RAGE (sRAGE) as a potential therapeutic intervention.
1. AGE/RAGE Production
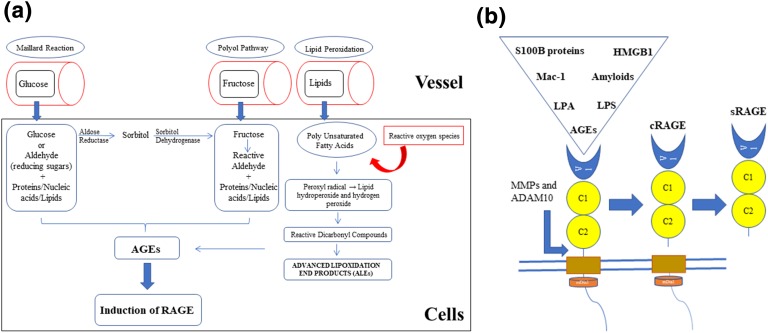
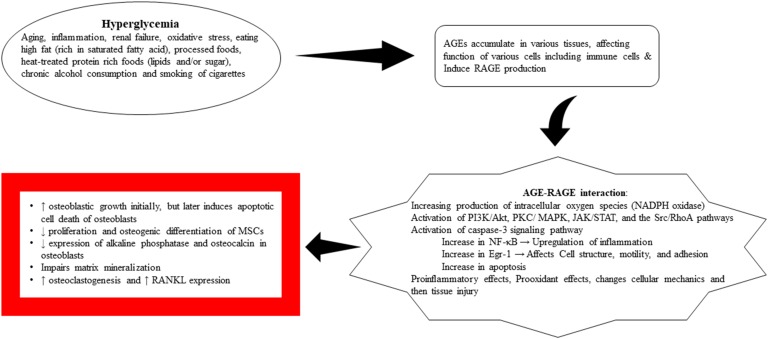
Hyperglycemia leads to increased production of AGEs, which are the products of nonenzymatic glycation of macromolecules (proteins, lipids, and nucleic acids) with a reducing sugar, known as the Maillard reaction. Excess oxidative stress and a marked rise in reactive oxygen species (ROS) can injure proteins and stimulate their modification. Increased production of oxidized lipids and glucose, in addition to the damaged proteins, can lead to AGE formation. AGEs are generally considered as structurally heterogeneous molecules. The common route of the generation of AGEs in humans with diabetes initially starts with the combination of the carbonyl group of a reducing sugar or aldehyde with lysine and arginine amino acid residues. AGEs can induce an intrinsic signaling pathway inside the cells and promote the development of RAGE on cell membranes. In humans with diabetes, the carboxymethllysine ligands of AGEs are the usual ligands of RAGE. Other than this nonenzymatic reaction of glucose with macromolecules, AGEs can be formed endogenously through the polyol pathway and lipid peroxidation. Furthermore, AGEs can be produced in situations other than hyperglycemia and diabetes [6, 7]. Aging, inflammation, renal failure, increase in intracellular and extracellular stress, oxidative stress, eating high-fat processed foods (rich in saturated fatty acid), heat-treated protein-rich foods (lipids and/or sugar), cigarette smoking, and chronic alcohol consumption can cause production of AGEs [6–10] (Fig. 1a).
Figure 1.
Formation of AGEs, induction of RAGE (a), and sRAGE production (b). (a) The three different pathways leading to the formation of endogenous AGEs consist of the Maillard reaction, the polyol pathway, and lipid peroxidation. Reducing sugar (glucose, fructose, glyceraldehyde) or reactive dicarbonyl compounds (products of lipid peroxidation) reacts with the macromolecules (such as the amino group of proteins), and then, after the modification process, results in the production of AGEs. Advanced lipoxidation end products (ALEs) are produced by reactive dicarbonyl compounds, which are generated by lipid peroxidation. Polyunsaturated fatty acids (membrane lipids) will produce reactive carbonyl species after being damaged by ROS and further oxidation. Additional modification of ALEs can advance AGE production unless the detoxification becomes dominant. A daily diet including high fat, high sugar, alcohol, and processed foods are important sources of the AGEs. (b) sRAGE production through enzymatic cleavage of external of the RAGE [6, 7, 11]. LPA, lysophosphatidic acid; MMP, matrix metalloproteinase.
Anecdotally, it seems that the same conditions noted to increase AGE/RAGE production have been reported as risk factors for osteoporosis and fracture, including aging, diabetes, inflammation, renal failure, high-fat diet, smoking, and chronic alcohol consumption.
2. RAGE and sRAGE Production
AGEs induce different intrinsic signaling pathways mediated mainly through RAGE. RAGE is a multiligand transmembrane receptor that is structurally similar to immunoglobulin. It is composed of an extracellular portion, a transmembrane part, and an intracellular domain. The extracellular domain is constructed by a variable domain (V) and two constant domains (C1 and C2). The variable domain and V-C1 are important for interaction of ligands with RAGE [6, 11] (Fig. 1b).
AGE–RAGE interaction leads to multiple biological effects, including microvascular and macrovascular complications of diabetes (neuropathy, nephropathy, retinopathy, cardiomyopathy, and atherosclerosis). RAGE can be expressed on various cells, including inflammatory cells (monocytes, macrophages, and lymphocytes), endothelial cells, smooth muscle cells, neurons, osteoblasts, and osteoclasts [11, 12]. AGE–RAGE interaction can affect cellular function, motility, and metabolism. AGEs can directly injure cells and tissues through inflammatory and oxidant damages. Additionally, it interacts with variable receptors, especially RAGE, and results in activation of multiple signals, including PKC, phosphatidylinositol 3-kinase (PI3K)/Akt, MAPK/ERK, Src/RhoA, JAK/STAT, and the reduced form of NAD phosphate oxidase. This complex activation of the signals increases the level of nuclear factor κB (NF-κB), Egr-1, or other transcription factors, along with the production of ROS. The results of these events include upregulation of inflammation, induction of oxidative injury, interference with cell motility, and changes in cell metabolism. Additionally, increased cell oxidant stress can further augment AGE production and activate RAGE signaling pathways, leading to altered cell function [11, 13].
Other receptors can interact with AGEs containing AGE–receptor complexes (AGE-R1/OST-48, AGE-R2/80K-H, AGE-R3/galectin-3) and the scavenger receptor family (SR-A, SR-B/CD36, SR-BI, SR-E/LOX-1, FEEL-1, FEEL-2). Scavenger receptors, especially CD36, are thought to participate in endocytosis of AGE proteins. Endocytosed AGEs can be modified by binding to the lysosome and cleared by the kidney [7]. RAGE also has different endogenous and exogenous ligands. It binds endogenous damage-associated proteins, such as AGEs, high-mobility group box 1 (HMGB1), S100/calgranulins, amyloid-β peptide, and other forms of amyloid and macrophage adhesion ligand-1 (MAC-1). Other RAGE ligands include complement proteins (C3a and C1q), lysophosphatidic acid, phosphatidylserine, lipopolysaccharide, transthyretin, heparin sulfate, and heat shock proteins [11, 14–16].
sRAGE is a form of the RAGE that can circulate and be measured by ELISA. In humans, two types of sRAGE have been reported. The first form is originated from splicing the external domain of RAGE that contains N-terminal extracellular portion (V-C1-C2 domains). AGEs can induce secretion of matrix metalloproteinases or a disintegrin and metalloproteinase domain-containing protein 10 (ADAM10) that splices RAGE and produces sRAGE (Fig. 1b). Endogenous secretory RAGE (esRAGE) is another form of sRAGE, which is expressed by alternatively spliced precursor mRNA and contains the C-terminal part of RAGE. The main source of sRAGE production is not completely known; however, vascular and immune cells are thought to contribute to the production of sRAGE [17, 18].
sRAGE can bind AGEs without inducing intrinsic signals, due to lacking internal and/or transmembrane parts of the RAGE, leads to potential blocking of AGE–RAGE interaction, and so plays a protective role in the setting of RAGE accumulation [17, 18]. sRAGE has been considered an inhibitor of receptor activator for NF-κB ligand (RANKL)–induced osteoclastogenesis [19]. Furthermore, an increase in the amount of esRAGE release into bone by applying acidic oligopeptide–tagged esRAGE enhanced esRAGE affinity to hydroxyapatite and improved the therapeutic effects of esRAGE on synovial hyperplasia, cartilage, and bone destruction, in addition to its negative effects on inflammation in rheumatoid arthritis [20].
There is an abundance of published articles that report a correlation between sRAGE and/or esRAGE with different diseases or conditions. However, clinical data around sRAGE are bidirectional. In humans, circulating sRAGE is reduced in the disease conditions such as atherosclerosis, coronary artery disease, hypertension, hypercholesterolemia, chronic obstructive lung disease, heart failure, and Alzheimer’s disease [11, 21]. However, heart failure patients, diabetic and nondiabetic, have higher serum HMGB1 and cleaved RAGE, but lower esRAGE [22]. In terms of correlation of sRAGE with bone parameters, it has been reported that sRAGE had no significant correlation with BMD or fractures in postmenopausal women with T2DM [23], but it had positive link with bone formation [24]. It was also positively correlated with osteopenia and osteoporosis [25], but low esRAGE was reported as a risk factor for vertebral fracture in patients with T2DM [26]. Furthermore, elevated serum levels of sRAGE and reduced esRAGE levels were reported in patients with type 1 DM (T1DM) and T2DM [21]. The bidirectional correlation of sRAGE with different clinical situations can be explained by the fact that excess production of AGEs induces ADAM10 and then breaks down part of the overproduced RAGE, resulting in more sRAGE secretion. Under other circumstances, alternative splicing of pre-mRNA and/or enzymatic cleavage of RAGE can limit RAGE availability while increasing sRAGE production. Additionally, contribution of the immune system in the production of sRAGE is another factor that may lead to an increase in sRAGE production. Finally, in either situation of AGE/RAGE overproduction or RAGE destruction we might have higher sRAGE levels (Tables 1 and 2) [23–53].
Table 1.
Studies Reporting Blood/Urine Ages, sRAGE and Bone Changes in Humans in Reverse Order of the Year of Publication Between 2003 and 2018
| Author/Journal and Year | Participants | Results | Comments |
|---|---|---|---|
| Choi et al., 2018 [27] | 40 (7 men, 33 women) | Serum PEN levels are higher in the vertebral fracture group and positively correlated with FRAX | Serum PEN is a possible biochemical marker for vertebral fractures |
| 68–76 y of age (mean age, 70.6 y) | |||
| 11 vertebral fracture (2 men, 9 women) | |||
| 29 no fracture (5 men, 24 women) | |||
| Lamb et al., 2018 [24] | 3384 men | Plasma CML, methylglyoxal, glyoxal, and esRAGE were similar in men with and without DM | Higher blood glucose is positively associated with CML and is reciprocally associated with esRAGE |
| 70–89 y of age | CML had a positive correlation and esRAGE was inversely associated with FBS | esRAGE can modify or control bone turnover in older men, and CML can predict hip fracture incidence | |
| esRAGE had a positive correlation with bone formation | |||
| Tamaki et al., 2018 [28] | 1285 men | 25 fragility fractures | Decreased risk of fragility fractures is noted with higher esRAGE/PEN ratios and this is independent of BMD |
| ≥65 y of age | The crude fragility fracture HRs (95% CI) for the following are | ||
| PEN 1.56 (1.05–2.31) | |||
| esRAGE 0.79 (0.54–1.15) | |||
| esRAGE/PEN 0.65 (0.44–0.95) | |||
| Miyazawa et al., 2017 [29] | 46 prostate cancer patients receiving antiandrogen treatment | Decrease in serum PEN and increase in BMD in the denosumab receiver | Denosumab inhibited the rise in PEN levels in patients with prostate cancer antiandrogen therapy |
| 20 received denosumab | |||
| 26 no denosumab | |||
| Galliera et al., 2017 [25] | 84 postmenopausal women | There were 12 subjects with osteoporosis, 32 with osteopenia, and 40 with normal BMD | Serum level of sRAGE could potentially be used to monitor osteoporosis progression and fracture risk |
| Mean age, 53 ± 6 y | Higher sRAGE was noted in osteopenic and osteoporotic patients | ||
| Raška et al., 2017 [23] | Postmenopausal women | No association between sRAGE and BMD | No association between RAGE polymorphisms and BMD/fractures in postmenopausal women with T2DM |
| 112 with T2DM | No association between sRAGE and fracture | ||
| 171 control nondiabetics | |||
| Barzilay et al., 2015 [30] | 3373 patients | Unadjusted HR of hip fracture increased with each 1 SD increase of serum levels of the AGE CML level | Increased levels of CML are associated with risk of hip fracture in and older population independent of hip BMD |
| Age 78 y (range, 68–102 y) | BMD of the total hip was not correlated with CML levels | ||
| 39.8% men | |||
| Median follow-up of 9.22 y | |||
| Neumann et al., 2014 [31] | 128 men and premenopausal women with T1D with and without prevalent fractures | Higher PEN levels in patients with fractures | Increase in AGEs impairs bone quality in T1D |
| No difference in CML and esRAGE | |||
| Kuroda et al., 2013 [32] | 1475 postmenopausal women (66.6 ± 9.0 y) | Urinary PEN and serum homocysteine were associated with vertebral fractures | Severity of vertebral fractures had minor correlation with PEN levels |
| Tanaka et al., 2011 [33] | 765 postmenopausal women | Increase in urinary PEN has a positive correlation and linear relationship with long bone and vertebral fractures | Applying urinary PEN may improve fracture risk classification |
| Yamamoto et al., 2009 [26] | Japanese T2DM patients: | T2DM patients with VFs had a lower esRAGE/PEN ratio | In T2DM, serum esRAGE and the esRAGE/PEN ratio are better than BMD in assessing VF risk |
| 137 men >50 y of age | Serum esRAGE and the esRAGE/PEN ratio are correlated with VFs independent of BMD | ||
| 140 postmenopausal women with and without VFs | |||
| Schwartz et al., 2009 [34] | 928 men and women 70–79 y of age | In the patients with diabetes, PEN was associated with increased clinical fracture incidence and vertebral fracture prevalence | Higher PEN levels may represent decreased bone strength in T2DM, leading to an increase in fracture risk |
| 501 with DM | |||
| 427 without DM | |||
| Subjects matched on sex, race, and study site | |||
| Pullerits et al., 2009 [35] | 88 postmenopausal RA patients received vitamin D3/calcium with or without HRT (estradiol plus norethisterone acetate) | HRT decreased levels of serum sRAGE | sRAGE may play a direct or indirect role in bone metabolism |
| Serum sRAGE was correlated with BMD and markers of bone/cartilage metabolism | |||
| Yamamoto et al. 2008 [36] | Japanese T2DM patients: | T2DM women with VFs had higher serum PEN levels independent of their BMD; PEN levels are thus associated with VFs in postmenopausal women with T2DM | Serum PEN levels instead of BMD could potentially be a helpful biomarker for assessing the VF risk in postmenopausal women with T2DM; PEN levels may help determine a patient’s bone quality |
| 77 men >50 y of age | |||
| 76 postmenopausal women | |||
| Shiraki et al., 2008 [37] | 432 Japanese women | Increased urine PEN levels were associated with vertebral fractures | AGEs are a potential risk factor for vertebral fracture |
| Followed for 5.2 ± 3.3 SD | |||
| Hein et al., 2003 [38] | 116 osteoporotic patients (34 men and 82 women; mean age, 55 ± 10 y) | Higher PEN and CML serum levels were noted in the group with osteoporosis | Bone remodeling may be affected by AGE-modified proteins |
| 44 age-matched healthy controls (18 men and 26 women; mean age, 55 ± 8 y) | Serum PEN was correlated with osteoclast activity/bone resorption |
Abbreviations: CML, Nε-carboxymethyllysine; FRAX, fracture risk assessment; HR, hazard ratio; HRT, hormone replacement therapy; PEN, pentosidine; P1NP, N-terminal propeptide of type I collagen; RA, rheumatoid arthritis; VF, vertebral fracture.
Table 2.
Studies Reporting Tissue/Serum AGEs and Bone Changes in Humans in Reverse Order of the Year of Publication Between 2005 and 2018
| Author/Journal and Year | Participants | Results | Comments |
|---|---|---|---|
| Rabelo et al., 2018 [39] | 35 postmenopausal women | Increase in PEN in femoral neck of osteoporotic fractures independent of age | Increase in PEN contributes to a decrease bone in quality and an increased risk of hip fracture in postmenopausal women |
| Femoral neck sample | |||
| 17 fracture (79 ± 2 y) | |||
| 18 osteoarthritis (66 ± 2 y) | |||
| Vaculik et al., 2016 [40] | 111 patients hip surgery | Both serum and bone PEN levels were increased in patients with hip fractures | PEN can be a potential biomarker to assess bone quality and strength |
| 70 femoral neck fracture | |||
| 41 advanced hip osteoarthritis | |||
| Furst et al., 2016 [41] | 35 postmenopausal women | Increase in AGEs (determined by SAF) was associated with reduced BMSi and lower bone formation marker (P1NP) in T2DM | T2DM: |
| 16 with T2DM | Impaired bone material properties | ||
| 19 matched controls | The accumulation of AGEs may lead to lower BMSi | ||
| Farlay et al., 2016 [42] | Iliac crest bone biopsies from: | Fracturing T1DM had higher levels of PEN in trabecular bone | High PEN and bone mineralization could lead to a less flexible and more rigid bone matrix in fracturing T1DM |
| 5 fracturing T1DM | Positive correlations noted between: | ||
| 5 T1DM with no fracture | HbA1c and PEN | ||
| 5 healthy subjects | HbA1c and bone mineralization | ||
| All age and sex matched | |||
| Karim et al., 2013 [43] | 170 human bone samples | More PEN and total AGEs were noted in cancellous bone compared with cortical bone | PEN and total AGEs accumulate differently in cancellous and cortical bone. Quantifying total AGEs and PEN is important for a complete understanding of the AGEs in bone |
| PEN was related to total AGEs in cancellous bone but was weakly correlated in cortical bone | |||
| Karim et al., 2012 [44] | 42 cancellous bone obtain from | More trabecular rods than plates and more microdamage were noted in highly glycated samples | AGEs can heterogeneously modify cancellous bone trabecular microarchitecture, which can affect bone fragility |
| 24 men | High levels of AGEs decrease bone mechanical measures against fracture (yield strain, ultimate strain, and toughness) | ||
| 18 women | |||
| Age 18 to 97 y (mean, 59.3 ± 22.1 y) | |||
| Momma et al., 2012 [45] | 193 Japanese men | Negative correlation between SAF and osteo sono assessment index | AGE accumulation can potentially affect bone strength |
| Median age, 43 y (range, 37.0–55.0 y) | |||
| Dong et al., 2011 [46] | 18 cortical bone from cadaveric femur of men | The concentration of AGEs depends on age of donor and biological tissue ages | AGEs accumulation in human cortical bone can potentially affect bone remodeling |
| 6 young (31 ± 6 y old) | AGEs concentration has a positive correlation with osteoclast activities | ||
| 6 middle-aged (51 ± 3 y old) | |||
| 6 elderly (76 ± 4 y old) | |||
| Oren et al., 2011 [47] | 20 total knee arthroplasty | Higher PEN levels in tissue of patients with diabetes | The inverse relationship between synovial fluid osteocalcin levels and the levels of AGEs in the joint may indicate that AGEs can affect bone healing in individuals with diabetes |
| 10 with diabetes, 10 controls | Negative correlation between osteocalcin and HP in cartilage | ||
| Synovial fluid markers and collagen crosslinks in bone and cartilage were assessed | Negative correlation between osteocalcin and PEN in cartilage of patients with diabetes | ||
| Tang et al., 2007 [48] | 8 fresh human cadaver femoral heads were paired for ribosylation and control treatments | AGEs content increased with age in control group | AGEs can increase the tendency of cancellous bone to fracture |
| AGEs in cancellous bone cores have correlation with damage in treated group | |||
| Viguet-Carrin et al., 2006 [49] | 19 L3 vertebrae after necropsy; age 26–93 y; 10 men, 9 women | BMD and trabecular PEN were correlated with failure load and work to fracture | PEN has a negative impact on vertebral mechanical properties |
| Posttranslational modification of type 1 collagen can affect skeletal fragility | |||
| Hein et al., 2006 [50] | 8 patients with osteoporosis | AGEs imidazolone and CML were found in osteoporotic bone specimens (iliac crest) | There is an inverse correlation between the AGEs and the number of osteoblasts on the surface of a trabecular bone |
| Iliac crest bone biopsy | Advanced age was associated with the higher intensity of AGEs | AGEs modify bone proteins and may impair bone remodeling | |
| Saito et al., 2006 [51] | 16 women (78 ± 6 y of age) with intracapsular hip fracture | High-mineralized bone had a higher PEN content than did low-mineralized | Poor bone quality in osteoporosis could be due to reduction in bone mineralization, lower enzymatic cross-links, and excessive PEN formation |
| 16 age- and sex-matched postmortem controls (76 ± 6 y of age) | Low-mineralized bone with fracture had higher PEN content than did control | ||
| Hernandez et al., 2005 [52] | 32 thoracic vertebral bodies from cadavers (16 men and 16 women; 54–94 y of age) | PEN was correlated with structural ductility | Ductility of trabeculae is weakly affected by nonenzymatic glycation |
| Odetti et al., 2005 [53] | 104 nondiabetic subjects (74 women and 30 men), 72 ± 1 y of age | Samples of human leg bone (femur or knee) Advanced age was associated with increase in PEN concentration in cortical bone | PEN could potentially be used as a biomarker for bone density loss |
Abbreviations: BMSi, bone material strength index; CML, Nε-carboxymethyllysine; HP, hydroxylysylpyridinoline; PEN, pentosidine; P1NP, N-terminal propeptide of type I collagen; SAF, skin autofluorescence.
As a result, sRAGE is not a useful marker for assessment of activity of the AGE–RAGE signaling pathway, because it may increase in either excess RAGE production or destruction. However, based on the effects of AGEs on ADAM10, the immune system, and sRAGE production, we may suggest that the sRAGE/AGE ratio is a helpful marker to correlate the activity of the AGE–RAGE signaling pathway with clinical outcomes [28, 54].
3. AGE/RAGE and the Bone Remodeling Process
The bone mass is determined by the balance between osteoblast and osteoclast activity, which is orchestrated by osteocytes in reaction to endocrine and mechanical stimuli. Hematopoietic stem cells differentiate into mononuclear cells and then osteoclasts under the influence of macrophage colony-stimulating factor and RANKL. Bone marrow mesenchymal stem cells (BMSCs) differentiate into osteoblasts, osteocytes, adipocytes, and chondrocytes. Osteoblasts produce bone matrix proteins, including type I collagen [55, 56]. Osteoblasts are involved in cross talk with osteoclasts through cytokines, the extracellular matrix, and direct connections; they interact through OPG/RANKL/RANK, RANKL/LGR4/RANK, Ephrin2/ephB4, and Fas/FasL pathways. This interaction between osteoblasts and osteoclasts is important and eventually leads to osteoclast formation, differentiation, or apoptosis. Osteoclasts also participate in bone formation by communicating with osteoblasts via the d2 isoform of the vacuolar (H+) ATPase V0 domain (Atp6v0d2), complement component 3a, semaphorin 4D, or miRNAs [56, 57]. Furthermore, participation of the AGE–RAGE signaling pathway and RAGE ligands (such as HMGB1, S100/calgranulin proteins, and amyloid precursor protein) in bone remodeling [55] and the effects of cytokines such as TGF-β and IGF-1 on the osteoblast’s function [57] can explain the outcomes of diabetes and diabetic complications on the determinants of bone strength (including bone mass, composition, microstructure, and material properties).
AGEs (pentosidine, a biomarker for AGEs) can accumulate in human diabetic bone [47]. Evaluation of postmenopausal women with T2DM showed that a lower bone material strength index correlated with the accumulation of AGEs, measured by skin autofluorescence [41]. Generally, AGEs not only induce osteoclastogenesis by upregulation of RANKL mRNA, but they also affect osteoblasts by suppressing cell growth, promoting apoptosis, and downregulating differentiation, which impair mineralization (data from primary human osteoblast culture, human MSCs, and mouse stromal ST2 cells) [58–60]. They can increase [58] or decrease [61] mRNA expression of RAGE in human osteoblasts. However, they increase RAGE mRNA expression in the mouse stromal cell line ST2 (differentiated into osteoblast-like cells) [59]. It was reported that AGEs increase the mRNA expression of RANKL and osterix (transcription factors for osteoblast differentiation) but downregulate alkaline phosphatase and osteocalcin in human osteoblasts [58]. However, they are also reported to increase sclerostin protein but decrease the RANKL expression in osteocyte-like MLO-Y4-A2 cells [62]. They are also shown to reduce Runx2 and osterix protein expression in the mouse stromal cell line ST2 (differentiated into osteoblast-like cells) [59] and decrease not only alkaline phosphatase, but also collagen I mRNA expression, in MSCs [63]. Alternatively, pentosidine was shown to have no effect on human osteoblast expression of osteocalcin, but it does affect human osteoblast function by decreasing alkaline phosphatase and collagen Iα1 [61]. Generally, AGE has biphasic effects on the human fetal osteoblastic cell (hFOB1) survival. Low concentration of AGE in a short period seems to have a protective role by increasing osteogenic function and decreasing osteoclastic function, but the results became reversed with increasing the duration of treatment of hFOB1 by AGE [64]. Accordingly, it has been demonstrated that diabetes increases MSC number initially but later causes reductions in MSCs, leading to trabecular bone loss [65]. In other words, AGEs can increase osterix expression in human osteoblasts [58] and promote osteoblastic growth and cellular alkaline phosphatase activity initially [66], but later induce apoptotic cell death of osteoblasts mainly by interacting with RAGE, activating caspase-3 signaling pathways, increasing production of intracellular ROS, and reducing alkaline phosphatase activity and activation of MAPKs [65, 67–71]. The different attained results of AGEs on osterix, alkaline phosphatase, and RANKL could potentially be due to the timing of assessment of AGEs on cell function and/or the dose of AGEs.
Additionally, by testing ST2 cells and human MSCs, it was shown that AGEs interact with RAGE and increase expression and secretion of TGF-β, which can suppress stromal cell mineralization [60]. The interaction of AGE–RAGE seems to be the dominant way, as their inhibitory effects on Wnt signaling pathway is reversible with the RAGE receptor antagonist FPS-ZM1 [72]. RAGE suppresses cell proliferation through suppression of Wnt, PI3K, and ERK signaling pathways [73]. Concerning bone remodeling, it seems that RAGE signaling pathway participates in the regulation of osteoclast development and activity, but its role in osteoblasts/osteocytes is less studied. It is seems that RANKL stimulates RAGE expression, which is associated with osteoclast differentiation [55, 74], and knocking out RAGE attenuates RANKL-mediated osteoclast differentiation [75]. Consequently, consistent with the effect of RAGE on osteoclast differentiation and activity, RAGE knockout mice showed decreased osteoclast number, reduced bone resorption, increased bone mass, and improved biomechanical strength [76]. Alternatively, with regard to the effects of RAGE on osteoblasts/osteocytes, it is reported that the loss of RAGE decreased femoral cancellous bone accrual, altered architecture, and was associated with reduced expression of alkaline phosphatase, cola1, Runx2, and osterix (osteoblast genes) [12]. Additionally, AGEs have harmful effects on human MSCs [77], and RAGE signaling seems to impair BMSC maintenance under the chronic pathologic conditions, such as diabetes, but not under physiologic conditions [78]. As a result, inhibiting RAGE signaling is a potential approach to improve capacity of BMSCs for differentiation into adipocytes, osteoblasts, and osteocytes in the diabetic condition.
Importantly, note that high glucose levels have synergistic effects with AGEs in impairing the mineralization process [79], but RAGE knockout did not affect bone metabolism in the diabetic condition. The reduction of osteoclast formation due to RAGE deletion was reported only under physiological, but not the diabetic, condition [80], which may indicate a partial protective role of RAGE in diabetes or highlight the roles of other pathophysiologic mechanisms in bone metabolism of the diabetic condition.
Furthermore, we should not underestimate the roles of other RAGE ligands, other than AGEs, in the pathophysiology of diabetic osteopathy. Myeloid cells, osteoblasts, and osteoclasts can secrete HMGB1. HMGB1, which is a ligand for RAGE and Toll-like receptor (TLR)2 and TLR4, works similar to a chemotactic agent for osteoblasts and osteoclasts during the bone remodeling process. Apoptotic bone cells release HMGB1 into the bone marrow and increase levels of RANKL, TNFα, and IL-6 in osteoblasts and stromal cells. Additionally, HMGB1 plays an important role in inflammatory reactions and the bone remodeling process, especially bone resorption [81].
HMGB1 activates PI3K, Akt, and AP-1 pathways and increases integrin (α5β1 integrin) expression through the RAGE/PI3K/Akt/c-Jun/AP-1–dependent pathway [82]. HMGB1, S100, and amyloid precursor protein (APP) are the RAGE ligands that increase osteoclast differentiation through RANKL [19, 74, 75]. S100A8 and S100A9 are initiators and promoters of the inflammatory response. N-glycans, RAGE, and TLR4 are known receptors of S100A8. However, S100A8, but not S100A9, is able to stimulate osteoclast differentiation and increase osteoclast number. Additionally, it was reported that S100A8-mediated bone resorption happens through TLR4, given evidence that S100A8-mediated osteoclast stimulation cannot be blocked by either using RAGE-blocking antibody or sRAGE [83], but we should consider that sRAGE can bind AGEs and block AGE–RAGE interaction, which technically has no effects of S100A8/RAGE interaction. Furthermore, S100A7 can interact with RAGE and increase activity of matrix metalloproteinases of osteosarcoma cells, promoting migration and invasion of these cells [84].
Generally, RAGE has an important role in diabetic complications, including diabetic osteopathy. As mentioned earlier, it interacts with different ligands such as AGEs, HMGB1, S100 proteins, β-amyloids, β2-integrin Mac-1, and pyridinoline (a collagen crosslink) and then activates NF-κB and Erg1, which are involved in inflammation, activation of innate immune system, cell survival signaling, tissue regeneration, and immune modulation [11, 55, 85–87] (Fig. 1b).
However, AGE–RAGE signaling pathway seems to have some protective roles in the skeletal system. It is necessary for the skeleton’s response to anabolic effects of PTH. Absence of RAGE weakened PTH-mediated increases in femoral cancellous bone formation and trabecular number but had no effects on the response of vertebral cancellous bone to PTH [12]. Additionally, during development of the skeleton and endochondral ossification, PTH/PTH-related peptide receptor and Indian hedgehog (Ihh) participate in proliferation and maturation of chondrocytes. AGEs have negative effects on tissue repair capacity and reduce cartilage matrix production and chondrocyte differentiation through a Rho family GTPase mechanism. AGEs downregulate Ihh and Col10a1, but upregulate PTH-related peptide receptor [88] (Fig. 2).
Figure 2.
The process of development and function of AGE–RAGE signaling [6, 7, 11, 12, 56, 59–64, 79].
4. AGE/RAGE, Bone Matrix, and BTMs
Bone turnover includes resorption of damaged bone by osteoclasts and replacing new bone by osteoblasts. BTMs generally consist of bone proteins (the products of collagen degradation/production) or enzymes that are presumed to reflect the rate of bone formation and resorption. Increased osteoclast activity leads to the resorption and the release of bone soft tissue constituents into serum and urine. C-telopeptide of type I collagen (CTX-I) is the product of collagen degradation that shows osteoclast activity. Osteoblasts secrete collagen and other molecules that participate in osteoid formation. N-terminal propeptide of type I collagen (P1NP) is a bone protein that reflects osteoblast activity and function [89]. Measuring the concentration of BTMs in blood and urine helps to assess the process of resorption and formation. P1NP and CTX-I are recommended by the International Osteoporosis Foundation and the International Federation of Clinical Chemistry to assess fracture risk and response to treatment in patients with osteoporosis [89, 90]. Diabetes, obesity (visceral obesity), and insulin resistance are associated with lower BTM levels (P1NP and CTX-I) [1, 91], which are the result of AGE–RAGE interactions and reduced bone formation in the diabetic condition, but is opposite to the fact that diabetes increases osteoclast activity.
Posttranslational modification of collagen is crucial for collagen stability and plays an important role in bone biology and strength. The collagen crosslinking is not only important for bone quality, but it also participates in remodeling process by affecting the differentiation of bone cells and regulation of their behavior [92]. The collagen crosslinks include lysyl oxidase–mediated enzymatic and glycation-induced nonenzymatic crosslinks. The lysyl oxidase–mediated enzymatic crosslink stabilizes the collagen fibers and makes them stronger. However, the nonenzymatic crosslinks, produced by the reaction of reducing sugar with protein (AGEs), is associated with decreased bone strength. Collagen of diabetic bone contains fewer enzymatic crosslinks, which can affect bone strength without a reduced BMD [93]. Importantly, note that osteoblast interaction with collagen leads to an increase in lysyl oxidase expression, but glycated collagen cannot increase lysyl oxidase production. Integrins and discoidin domain receptors are two main binding sites for collagen on osteoblasts. Glycation of collagen can diminish binding capacity of collagen with osteoblasts through discoidin domain receptor 2 (DDR2) and integrin receptors [94, 95], which probably leads to less lysyl oxidase production. However, AGEs can potentially increase collagen production, but with enhancing degradation they lead to less collagen [96]. Alternatively, AGE-modified proteins, such as AGE-modified β2-microglobulin, decreased collagen synthesis in fibroblasts through interaction with RAGE. Additionally, they have proinflammatory effects that enhance collagenase [97]. Lastly, modification of collagen through the glycation processes changes the charge profile of collagen (eliminates the positive charge of lysine) and affects the mass and architecture of fibers, which end up having less solubility and flexibility, but more toughness to degradation by proteases [18]. As a result, resistance to degradation of glycated collagen could be the reason for lower CTX-I levels in patients with DM, despite that diabetes increases osteoclast activity. Additionally, reduction of osteoblast activity and lowered collagen production lead to a lower P1NP concentration in patients with diabetes (Fig. 2).
5. Current Treatments and Limitations of sRAGE
Growing efforts have been made to find an effective solution that can inhibit or reduce the detrimental effects of AGE–RAGE interaction. Current reported therapeutic interventions against the AGE–RAGE signaling pathway include treatments targeting AGEs, RAGE, postreceptor signaling pathways, or the complications of AGE–RAGE interactions. The list of reported therapeutic interventions for AGE- and RAGE-associated pathology includes: AGE inhibitor (aminoguanidine), AGE crosslink breaker (alagebrium and related compounds), antioxidants, medications, and natural substances with anti-AGE/RAGE properties, such as bisphosphonates, statin, metformin, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonist, pyridoxamine, ascorbic acid, N-acetylcysteine, and vitamins D and K [14, 98].
Additionally, the effects of antidiabetic medications on RAGE expression and bone health could be different. There are antidiabetic medications, such as metformin [99, 100] and glucagon-like peptide-1 (GLP-1) agonist [101, 102], that have negative effects on the AGE–RAGE signaling pathway and some beneficial effects on BMD and/or fracture [1]. However, thiazolidinediones [peroxisome proliferator-activated receptor-γ (PPAR-γ) agonist] induce adipogenesis and osteoclastogenesis that lead to increases in fracture rate [1], but the nuclear receptor PPAR-γ activation can inhibit RAGE expression [103].
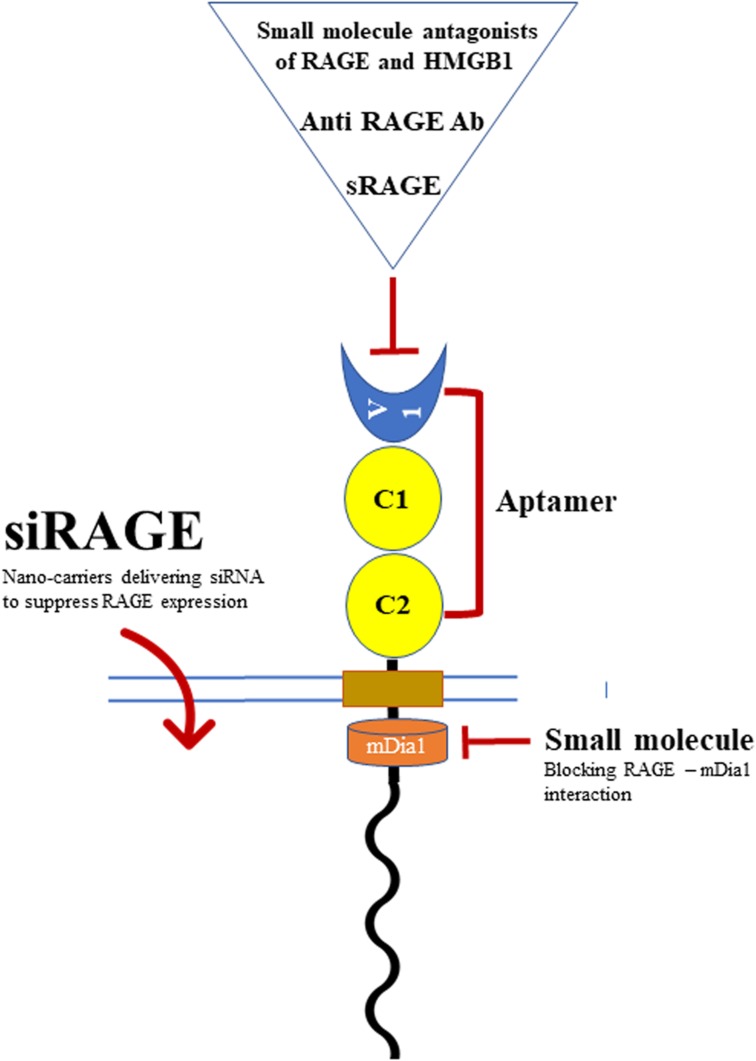
Targeting RAGE is a potential approach to prevent diabetic complications. Mainly animal experiments have shown some benefits of different products to target RAGE, including (Fig. 3):
sRAGE as a ligand decoy [11]
Anti-RAGE antibody [11]
Small-molecule RAGE antagonists [11]
Longistatin, which blocks RAGE stimulation by binding to the RAGE V domain [104]
Aptamers (RAGE aptamers) [11]
Inhibitors of the cytoplasmic domain of RAGE (ctRAGE) include 13 small molecules [105]
Genetic suppression of RAGE by using RAGE siRNA (siRAGE) [106]
Figure 3.
Diverse strategies to target RAGE function and expression.
Despite the impressive improvement in the landscape of our understanding regarding the AGE–RAGE signaling pathway and the presence of variable therapeutic interventions, no clinically successful human study was found to be able to block the pathway efficiently and probably alleviate diabetes-related complications. However, putting different pieces of this amazing puzzle together in a goal-oriented fashion may give us insight into the limitations of the therapeutic approaches fighting against the AGE–RAGE signaling pathway.
Among all of the reported therapeutic options to alleviate activity of the AGE–RAGE signaling pathway, recombinant sRAGE, as a ligand decoy, was thought to be the most effective method. sRAGE can inhibit RANKL-induced osteoclastogenesis [19], reduce inflammatory stresses [107], and protect against weight gain and insulin resistance in high-fat diet–fed mice, but it can increase the levels of other RAGE ligands, such as Hmgb1 mRNA [108]. Furthermore, the important part of RAGE for interaction with RAGE ligands is the variable domain [6, 11], which is AGE specific, and AGEs are generally complex and heterogenic compounds [7]. As a result, recombinant sRAGE, with a fixed variable domain, can partially block the produced AGEs.
RAGE and RAGE ligands, such as amyloid-β, AGEs, and HMGB1 lipopolysaccharide, play a crucial role in engaging macrophages [6, 14, 109], but AGEs increase lipid accumulation in macrophages, which can potentially disable macrophages [110]. Alternatively, activated macrophages have an important role in the accumulation of AGE-albumin in tissues as a defense mechanism [14]. Then, we can suspect that sRAGE can block the AGEs and improve macrophage defensive role, but it is also reported that sRAGE reduces macrophage phagocytosis, perhaps by opposing RAGE-mediated and other phosphatidylserine receptor–mediated phagocytosis [111].
Moreover, the accessibility of sRAGE to the AGEs seems to be important, as transfecting BMSCs [107] or umbilical cord–derived MSCs [112] by sRAGE leads to better suppressive effects on inflammation [107] and improved protection against RAGE-induced neuronal cell death [112].
The last thing about RAGE is its possible protective role alongside AGEs. Obesity has positive correlations with both fat and lean mass in humans, which means that an increase in body weight is naturally associated with an increase in lean and fat mass. However, knocking out RAGE leads not only to significantly lower insulin resistance and fat mass, but also to reduced lean mass in high-fat diet–fed mice [108]. Additionally, in terms of bone pathophysiology, AGEs initially increase osteoblastic osterix expression [58] and promote osteoblastic growth [68], and their receptor (RAGE) improves the skeleton’s response to anabolic effects of PTH [12]. As a result, blocking the AGE–RAGE interaction by recombinant sRAGE that has a >24-hour elimination half-life [113] not only has limitations, such as heterogeneity of AGEs, accessibility of sRAGE, and an increase in the levels of other RAGE ligands, but it also can lead to loss of the beneficial effects of AGE–RAGE signaling.
6. Conclusion
AGEs are heterogeneous molecules that mainly result from the nonenzymatic reaction of a sugar with macromolecules. AGEs induce intrinsic cellular signaling that leads to the development of RAGE. The AGE/RAGE production and osteoporosis share common risk factors. AGE–RAGE interaction could be a potential reason for suppression of BTMs (P1NP and CTX) in the setting of diabetes. sRAGE may be elevated in either excess RAGE production or destruction, and it does not always reflect AGE–RAGE signaling activities. sRAGE is a beneficial option for alleviating the effects of the AGE–RAGE signaling pathway in bone, but because of the limitations it cannot be translated into clinical practice.
Acknowledgments
The authors express their gratitude to Svetlana Bagdasarov who kindly edited the article and references.
Author Contributions: K.A. reviewed the literature and wrote the manuscript. E.M.U. helped prepare the tables and edit the manuscript.
Glossary
Abbreviations:
- ADAM10
a disintegrin and metalloproteinase domain-containing protein 10
- AGE
advanced glycation end product
- BMD
bone mineral density
- BMSC
bone marrow mesenchymal stromal cell
- BTM
bone turnover marker
- CTX-I
C-telopeptide of type I collagen
- DM
diabetes mellitus
- esRAGE
endogenous secretory RAGE
- HMGB1
high-mobility group box 1
- MSC
mesenchymal stem cell
- NF-κB
nuclear factor κB
- PI3K
phosphatidylinositol 3-kinase
- P1NP
N-terminal propeptide of type I collagen
- RAGE
receptor for AGEs
- RANKL
receptor activator for NF-κB ligand
- ROS
reactive oxygen species
- sRAGE
soluble RAGE
- T1DM
type 1 diabetes mellitus
- T2DM
type 2 diabetes mellitus
- TLR
Toll-like receptor
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability:
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References and Notes.
References and Notes
- 1. Shanbhogue VV, Mitchell DM, Rosen CJ, Bouxsein ML. Type 2 diabetes and the skeleton: new insights into sweet bones. Lancet Diabetes Endocrinol. 2016;4(2):159–173. [DOI] [PubMed] [Google Scholar]
- 2. Farr JN, Khosla S. Determinants of bone strength and quality in diabetes mellitus in humans. Bone. 2016;82:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wang J, Yan D, Hou X, Chen P, Sun Q, Bao Y, Hu C, Zhang Z, Jia W. Association of adiposity indices with bone density and bone turnover in the Chinese population. Osteoporos Int. 2017;28(9):2645–2652. [DOI] [PubMed] [Google Scholar]
- 4. Kim H, Lee SH, Kim BJ, Koh JM. Association between obesity and femoral neck strength according to age, sex, and fat distribution. Osteoporos Int. 2017;28(7):2137–2146. [DOI] [PubMed] [Google Scholar]
- 5. Ortinau LC, Linden MA, Dirkes R, Rector RS, Hinton PS. Obesity and type 2 diabetes, not a diet high in fat, sucrose, and cholesterol, negatively impacts bone outcomes in the hyperphagic Otsuka Long Evans Tokushima Fatty rat. Bone. 2017;105:200–211. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt AM. 22016 ATVB Plenary Lecture: receptor for advanced glycation endproducts and implications for the pathogenesis and treatment of cardiometabolic disorders: spotlight on the macrophage [published correction appears in Arterioscler Thromb Vasc Biol. 2017;37(6):e66]. Arterioscler Thromb Vasc Biol. 2017;37(4):613–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;2:411–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Leung C, Herath CB, Jia Z, Andrikopoulos S, Brown BE, Davies MJ, Rivera LR, Furness JB, Forbes JM, Angus PW. Dietary advanced glycation end-products aggravate non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22(35):8026–8040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lopez-Moreno J, Quintana-Navarro GM, Camargo A, Jimenez-Lucena R, Delgado-Lista J, Marin C, Tinahones FJ, Striker GE, Roche HM, Perez-Martinez P, Lopez-Miranda J, Yubero-Serrano EM. Dietary fat quantity and quality modifies advanced glycation end products metabolism in patients with metabolic syndrome. Mol Nutr Food Res. 2017;61(8):1601029. [DOI] [PubMed] [Google Scholar]
- 10. Hayashi N, George J, Takeuchi M, Fukumura A, Toshikuni N, Arisawa T, Tsutsumi M. Acetaldehyde-derived advanced glycation end-products promote alcoholic liver disease. PLoS One. 2013;8(7):e70034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Litwinoff E, Hurtado Del Pozo C, Ramasamy R, Schmidt AM. Emerging targets for therapeutic development in diabetes and its complications: the RAGE signaling pathway. Clin Pharmacol Ther. 2015;98(2):135–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Philip BK, Childress PJ, Robling AG, Heller A, Nawroth PP, Bierhaus A, Bidwell JP. RAGE supports parathyroid hormone-induced gains in femoral trabecular bone. Am J Physiol Endocrinol Metab. 2010;298(3):E714–E725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wautier MP, Guillausseau PJ, Wautier JL. Activation of the receptor for advanced glycation end products and consequences on health. Diabetes Metab Syndr. 2017;11(4):305–309. [DOI] [PubMed] [Google Scholar]
- 14. Byun K, Yoo Y, Son M, Lee J, Jeong GB, Park YM, Salekdeh GH, Lee B. Advanced glycation end-products produced systemically and by macrophages: a common contributor to inflammation and degenerative diseases. Pharmacol Ther. 2017;177:44–55. [DOI] [PubMed] [Google Scholar]
- 15. Merhi Z, Kandaraki EA, Diamanti-Kandarakis E. Implications and future perspectives of AGEs in PCOS pathophysiology. Trends Endocrinol Metab. 2019;30(3):150–162. [DOI] [PubMed] [Google Scholar]
- 16. Plotkin LI, Essex AL, Davis HM. RAGE signaling in skeletal biology. Curr Osteoporos Rep. 2019;17(1):16–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schmidt AM. Soluble RAGEs—prospects for treating & tracking metabolic and inflammatory disease. Vascul Pharmacol. 2015;72:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar Pasupulati A, Chitra PS, Reddy GB. Advanced glycation end products mediated cellular and molecular events in the pathology of diabetic nephropathy. Biomol Concepts. 2016;7(5–6):293–309. [DOI] [PubMed] [Google Scholar]
- 19. Cui S, Xiong F, Hong Y, Jung JU, Li XS, Liu JZ, Yan R, Mei L, Feng X, Xiong WC. APPswe/Aβ regulation of osteoclast activation and RAGE expression in an age-dependent manner. J Bone Miner Res. 2011;26(5):1084–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Takahashi T, Katsuta S, Tamura Y, Nagase N, Suzuki K, Nomura M, Tomatsu S, Miyamoto K, Kobayashi S. Bone-targeting endogenous secretory receptor for advanced glycation end products rescues rheumatoid arthritis. Mol Med. 2013;19(1):183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prasad K. Low levels of serum soluble receptors for advanced glycation end products, biomarkers for disease state: myth or reality. Int J Angiol. 2014;23(1):11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang LJ, Lu L, Zhang FR, Chen QJ, De Caterina R, Shen WF. Increased serum high-mobility group box-1 and cleaved receptor for advanced glycation endproducts levels and decreased endogenous secretory receptor for advanced glycation endproducts levels in diabetic and non-diabetic patients with heart failure. Eur J Heart Fail. 2011;13(4):440–449. [DOI] [PubMed] [Google Scholar]
- 23. Raška I Jr, Rašková M, Zikán V, Škrha J. Prevalence and risk factors of osteoporosis in postmenopausal women with type 2 diabetes mellitus. Cent Eur J Public Health. 2017;25(1):3–10. [DOI] [PubMed] [Google Scholar]
- 24. Lamb LS, Alfonso H, Norman PE, Davis TME, Forbes J, Müench G, Irrgang F, Almeida OP, Golledge J, Hankey GJ, Flicker L, Yeap BB. Advanced glycation end products and esRAGE are associated with bone turnover and incidence of hip fracture in older men. J Clin Endocrinol Metab. 2018;103(11):4224–4231. [DOI] [PubMed] [Google Scholar]
- 25. Galliera E, Marazzi MG, Gazzaruso C, Gallotti P, Coppola A, Montalcini T, Pujia A, Corsi Romanelli MM. Evaluation of circulating sRAGE in osteoporosis according to BMI, adipokines and fracture risk: a pilot observational study. Immun Ageing. 2017;14:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yamamoto M, Yamaguchi T, Yamauchi M, Sugimoto T. Low serum level of the endogenous secretory receptor for advanced glycation end products (esRAGE) is a risk factor for prevalent vertebral fractures independent of bone mineral density in patients with type 2 diabetes. Diabetes Care. 2009;32(12):2263–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Choi DH, Lee SM, Lim SA, Choi YS. Feasibility of serum pentosidine level as a potential risk factor for osteoporotic vertebral compression fracture. Asian Spine J. 2018;12(6):992–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tamaki J, Kouda K, Fujita Y, Iki M, Yura A, Miura M, Sato Y, Okamoto N, Kurumatani N. Ratio of endogenous secretory receptor for advanced glycation end products to pentosidine predicts fractures in men. J Clin Endocrinol Metab. 2018;103(1):85–94. [DOI] [PubMed] [Google Scholar]
- 29. Miyazawa Y, Sekine Y, Syuto T, Nomura M, Koike H, Matsui H, Shibata Y, Ito K, Suzuki K. Evaluation of bone turnover/quality markers and bone mineral density in prostate cancer patients receiving androgen deprivation therapy with or without denosumab. Anticancer Res. 2017;37(7):3667–3671. [DOI] [PubMed] [Google Scholar]
- 30. Barzilay JI, Buzkova P, Zieman SJ, Kizer JR, Djousse L, Ix JH, Tracy RP, Siscovick DS, Cauley JA, Mukamal KJ. Circulating levels of carboxy-methyl-lysine (CML) are associated with hip fracture risk: the Cardiovascular Health Study. J Bone Miner Res. 2014;29(5):1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Neumann T, Lodes S, Kastner B, Franke S, Kiehntopf M, Lehmann T, Muller UA, Wolf G, Samann A. High serum pentosidine but not esRAGE is associated with prevalent fractures in type 1 diabetes independent of bone mineral density and glycaemic control. Osteoporos Int. 2014;25(5):1527–1533. [DOI] [PubMed] [Google Scholar]
- 32. Kuroda T, Tanaka S, Saito M, Shiraki Y, Shiraki M. Plasma level of homocysteine associated with severe vertebral fracture in postmenopausal women. Calcif Tissue Int. 2013;93(3):269–275. [DOI] [PubMed] [Google Scholar]
- 33. Tanaka S, Kuroda T, Saito M, Shiraki M. Urinary pentosidine improves risk classification using fracture risk assessment tools for postmenopausal women. J Bone Miner Res. 2011;26(11):2778–2784. [DOI] [PubMed] [Google Scholar]
- 34. Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, Resnick HE, Tylavsky FA, Black DM, Cummings SR, Harris TB, Bauer DC; Health, Aging, and Body Composition Study. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J Clin Endocrinol Metab. 2009;94(7):2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pullerits R, d’Elia HF, Tarkowski A, Carlsten H. The decrease of soluble RAGE levels in rheumatoid arthritis patients following hormone replacement therapy is associated with increased bone mineral density and diminished bone/cartilage turnover: a randomized controlled trial. Rheumatology (Oxford). 2009;48(7):785–790. [DOI] [PubMed] [Google Scholar]
- 36. Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J Clin Endocrinol Metab. 2008;93(3):1013–1019. [DOI] [PubMed] [Google Scholar]
- 37. Shiraki M, Kuroda T, Tanaka S, Saito M, Fukunaga M, Nakamura T. Nonenzymatic collagen cross-links induced by glycoxidation (pentosidine) predicts vertebral fractures. J Bone Miner Metab. 2008;26(1):93–100. [DOI] [PubMed] [Google Scholar]
- 38. Hein G, Wiegand R, Lehmann G, Stein G, Franke S. Advanced glycation end-products pentosidine and Nε-carboxymethyllysine are elevated in serum of patients with osteoporosis. Rheumatology (Oxford). 2003;42(10):1242–1246. [DOI] [PubMed] [Google Scholar]
- 39. Rabelo GD, Roux JP, Portero-Muzy N, Gineyts E, Chapurlat R, Chavassieux P. Cortical fractal analysis and collagen crosslinks content in femoral neck after osteoporotic fracture in postmenopausal women: comparison with osteoarthritis. Calcif Tissue Int. 2018;102(6):644–650. [DOI] [PubMed] [Google Scholar]
- 40. Vaculík J, Braun M, Dungl P, Pavelka K, Stepan JJ. Serum and bone pentosidine in patients with low impact hip fractures and in patients with advanced osteoarthritis. BMC Musculoskelet Disord. 2016;17(1):308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Furst JR, Bandeira LC, Fan WW, Agarwal S, Nishiyama KK, McMahon DJ, Dworakowski E, Jiang H, Silverberg SJ, Rubin MR. Advanced glycation endproducts and bone material strength in type 2 diabetes. J Clin Endocrinol Metab. 2016;101(6):2502–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Farlay D, Armas LA, Gineyts E, Akhter MP, Recker RR, Boivin G. Nonenzymatic glycation and degree of mineralization are higher in bone from fractured patients with type 1 diabetes mellitus. J Bone Miner Res. 2016;31(1):190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Karim L, Tang SY, Sroga GE, Vashishth D. Differences in non-enzymatic glycation and collagen cross-links between human cortical and cancellous bone. Osteoporos Int. 2013;24(9):2441–2447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Karim L, Vashishth D. Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS One. 2012;7(4):e35047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Momma H, Niu K, Kobayashi Y, Guan L, Sato M, Guo H, Chujo M, Otomo A, Yufei C, Tadaura H, Saito T, Mori T, Miyata T, Nagatomi R. Skin advanced glycation end-product accumulation is negatively associated with calcaneal osteo-sono assessment index among non-diabetic adult Japanese men. Osteoporos Int. 2012;23(6):1673–1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dong XN, Qin A, Xu J, Wang X. In situ accumulation of advanced glycation endproducts (AGEs) in bone matrix and its correlation with osteoclastic bone resorption. Bone. 2011;49(2):174–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Oren TW, Botolin S, Williams A, Bucknell A, King KB. Arthroplasty in veterans: analysis of cartilage, bone, serum, and synovial fluid reveals differences and similarities in osteoarthritis with and without comorbid diabetes. J Rehabil Res Dev. 2011;48(10):1195–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Tang SY, Zeenath U, Vashishth D. Effects of non-enzymatic glycation on cancellous bone fragility. Bone. 2007;40(4):1144–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Viguet-Carrin S, Roux JP, Arlot ME, Merabet Z, Leeming DJ, Byrjalsen I, Delmas PD, Bouxsein ML. Contribution of the advanced glycation end product pentosidine and of maturation of type I collagen to compressive biomechanical properties of human lumbar vertebrae. Bone. 2006;39(5):1073–1079. [DOI] [PubMed] [Google Scholar]
- 50. Hein G, Weiss C, Lehmann G, Niwa T, Stein G, Franke S. Advanced glycation end product modification of bone proteins and bone remodelling: hypothesis and preliminary immunohistochemical findings. Ann Rheum Dis. 2006;65(1):101–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saito M, Fujii K, Soshi S, Tanaka T. Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int. 2006;17(7):986–995. [DOI] [PubMed] [Google Scholar]
- 52. Hernandez CJ, Tang SY, Baumbach BM, Hwu PB, Sakkee AN, van der Ham F, DeGroot J, Bank RA, Keaveny TM. Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone. 2005;37(6):825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Odetti P, Rossi S, Monacelli F, Poggi A, Cirnigliaro M, Federici M, Federici A. Advanced glycation end products and bone loss during aging. Ann N Y Acad Sci. 2005;1043(1):710–717. [DOI] [PubMed] [Google Scholar]
- 54. Asadipooya K. Letter to the Editor: “Advanced glycation end products and esRAGE are associated with bone turnover and incidence of hip fracture in older men.” J Clin Endocrinol Metab. 2019;104(3):682–683. [DOI] [PubMed] [Google Scholar]
- 55. Zhou Z, Xiong WC. RAGE and its ligands in bone metabolism. Front Biosci (Schol Ed). 2011;3:768–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Clarke B. Normal bone anatomy and physiology. Clin J Am Soc Nephrol. 2008;3(Suppl 3):S131–S139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen X, Wang Z, Duan N, Zhu G, Schwarz EM, Xie C. Osteoblast–osteoclast interactions. Connect Tissue Res. 2018;59(2):99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Franke S, Siggelkow H, Wolf G, Hein G. Advanced glycation endproducts influence the mRNA expression of RAGE, RANKL and various osteoblastic genes in human osteoblasts. Arch Physiol Biochem. 2007;113(3):154–161. [DOI] [PubMed] [Google Scholar]
- 59. Okazaki K, Yamaguchi T, Tanaka K, Notsu M, Ogawa N, Yano S, Sugimoto T. Advanced glycation end products (AGEs), but not high glucose, inhibit the osteoblastic differentiation of mouse stromal ST2 cells through the suppression of osterix expression, and inhibit cell growth and increasing cell apoptosis. Calcif Tissue Int. 2012;91(4):286–296. [DOI] [PubMed] [Google Scholar]
- 60. Notsu M, Yamaguchi T, Okazaki K, Tanaka K, Ogawa N, Kanazawa I, Sugimoto T. Advanced glycation end product 3 (AGE3) suppresses the mineralization of mouse stromal ST2 cells and human mesenchymal stem cells by increasing TGF-β expression and secretion. Endocrinology. 2014;155(7):2402–2410. [DOI] [PubMed] [Google Scholar]
- 61. Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann N Y Acad Sci. 2008;1126(1):166–172. [DOI] [PubMed] [Google Scholar]
- 62. Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T. Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun. 2015;461(2):193–199. [DOI] [PubMed] [Google Scholar]
- 63. Sun N, Yang L, Li Y, Zhang H, Chen H, Liu D, Li Q, Cai D. Effect of advanced oxidation protein products on the proliferation and osteogenic differentiation of rat mesenchymal stem cells. Int J Mol Med. 2013;32(2):485–491. [DOI] [PubMed] [Google Scholar]
- 64. Meng HZ, Zhang WL, Liu F, Yang MW. Advanced glycation end products affect osteoblast proliferation and function by modulating autophagy via the receptor of advanced glycation end products/Raf protein/mitogen-activated protein kinase/extracellular signal-regulated kinase kinase/extracellular signal-regulated kinase (RAGE/Raf/MEK/ERK) pathway. J Biol Chem. 2015;290(47):28189–28199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Stolzing A, Sellers D, Llewelyn O, Scutt A. Diabetes induced changes in rat mesenchymal stem cells. Cells Tissues Organs. 2010;191(6):453–465. [DOI] [PubMed] [Google Scholar]
- 66. McCarthy AD, Etcheverry SB, Bruzzone L, Cortizo AM. Effects of advanced glycation end-products on the proliferation and differentiation of osteoblast-like cells. Mol Cell Biochem. 1997;170(1-2):43–51. [DOI] [PubMed] [Google Scholar]
- 67. Liu J, Mao J, Jiang Y, Xia L, Mao L, Wu Y, Ma P, Fang B. AGEs induce apoptosis in rat osteoblast cells by activating the caspase-3 signaling pathway under a high-glucose environment in vitro. Appl Biochem Biotechnol. 2016;178(5):1015–1027. [DOI] [PubMed] [Google Scholar]
- 68. Mercer N, Ahmed H, Etcheverry SB, Vasta GR, Cortizo AM. Regulation of advanced glycation end product (AGE) receptors and apoptosis by AGEs in osteoblast-like cells. Mol Cell Biochem. 2007;306(1-2):87–94. [DOI] [PubMed] [Google Scholar]
- 69. Schurman L, McCarthy AD, Sedlinsky C, Gangoiti MV, Arnol V, Bruzzone L, Cortizo AM. Metformin reverts deleterious effects of advanced glycation end-products (AGEs) on osteoblastic cells. Exp Clin Endocrinol Diabetes. 2008;116(6):333–340. [DOI] [PubMed] [Google Scholar]
- 70. Zhu SY, Zhuang JS, Wu Q, Liu ZY, Liao CR, Luo SG, Chen JT, Zhong ZM. Advanced oxidation protein products induce pre-osteoblast apoptosis through a nicotinamide adenine dinucleotide phosphate oxidase-dependent, mitogen-activated protein kinases-mediated intrinsic apoptosis pathway. Aging Cell. 2018;17(4):e12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Zhang M, Li Y, Rao P, Huang K, Luo D, Cai X, Xiao J. Blockade of receptors of advanced glycation end products ameliorates diabetic osteogenesis of adipose-derived stem cells through DNA methylation and Wnt signalling pathway. Cell Prolif. 2018;51(5):e12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Li G, Xu J, Li Z. Receptor for advanced glycation end products inhibits proliferation in osteoblast through suppression of Wnt, PI3K and ERK signaling. Biochem Biophys Res Commun. 2012;423(4):684–689. [DOI] [PubMed] [Google Scholar]
- 74. Zhou Z, Immel D, Xi CX, Bierhaus A, Feng X, Mei L, Nawroth P, Stern DM, Xiong WC. Regulation of osteoclast function and bone mass by RAGE. J Exp Med. 2006;203(4):1067–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Zhou Z, Han JY, Xi CX, Xie JX, Feng X, Wang CY, Mei L, Xiong WC. HMGB1 regulates RANKL-induced osteoclastogenesis in a manner dependent on RAGE. J Bone Miner Res. 2008;23(7):1084–1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Ding KH, Wang ZZ, Hamrick MW, Deng ZB, Zhou L, Kang B, Yan SL, She JX, Stern DM, Isales CM, Mi QS. Disordered osteoclast formation in RAGE-deficient mouse establishes an essential role for RAGE in diabetes related bone loss. Biochem Biophys Res Commun. 2006;340(4):1091–1097. [DOI] [PubMed] [Google Scholar]
- 77. Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, Okawa T, Kojiro M, Nagata K. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Miner Res. 2005;20(9):1647–1658. [DOI] [PubMed] [Google Scholar]
- 78. Aikawa E, Fujita R, Asai M, Kaneda Y, Tamai K. Receptor for advanced glycation end products-mediated signaling impairs the maintenance of bone marrow mesenchymal stromal cells in diabetic model mice. Stem Cells Dev. 2016;25(22):1721–1732. [DOI] [PubMed] [Google Scholar]
- 79. Ogawa N, Yamaguchi T, Yano S, Yamauchi M, Yamamoto M, Sugimoto T. The combination of high glucose and advanced glycation end-products (AGEs) inhibits the mineralization of osteoblastic MC3T3-E1 cells through glucose-induced increase in the receptor for AGEs. Horm Metab Res. 2007;39(12):871–875. [DOI] [PubMed] [Google Scholar]
- 80. Hamada Y, Kitazawa S, Kitazawa R, Kono K, Goto S, Komaba H, Fujii H, Yamamoto Y, Yamamoto H, Usami M, Fukagawa M. The effects of the receptor for advanced glycation end products (RAGE) on bone metabolism under physiological and diabetic conditions. Endocrine. 2010;38(3):369–376. [DOI] [PubMed] [Google Scholar]
- 81. Yang J, Shah R, Robling AG, Templeton E, Yang H, Tracey KJ, Bidwell JP. HMGB1 is a bone-active cytokine. J Cell Physiol. 2008;214(3):730–739. [DOI] [PubMed] [Google Scholar]
- 82. Tang CH, Keng YT, Liu JF. HMGB-1 induces cell motility and α5β1 integrin expression in human chondrosarcoma cells. Cancer Lett. 2012;322(1):98–106. [DOI] [PubMed] [Google Scholar]
- 83. Grevers LC, de Vries TJ, Vogl T, Abdollahi-Roodsaz S, Sloetjes AW, Leenen PJ, Roth J, Everts V, van den Berg WB, van Lent PL. S100A8 enhances osteoclastic bone resorption in vitro through activation of Toll-like receptor 4: implications for bone destruction in murine antigen-induced arthritis. Arthritis Rheum. 2011;63(5):1365–1375. [DOI] [PubMed] [Google Scholar]
- 84. Kataoka K, Ono T, Murata H, Morishita M, Yamamoto KI, Sakaguchi M, Huh NH. S100A7 promotes the migration and invasion of osteosarcoma cells via the receptor for advanced glycation end products. Oncol Lett. 2012;3(5):1149–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bidwell JP, Yang J, Robling AG. Is HMGB1 an osteocyte alarmin? J Cell Biochem. 2008;103(6):1671–1680. [DOI] [PubMed] [Google Scholar]
- 86. Ramasamy R, Vannucci SJ, Yan SS, Herold K, Yan SF, Schmidt AM. Advanced glycation end products and RAGE: a common thread in aging, diabetes, neurodegeneration, and inflammation. Glycobiology. 2005;15(7):16R–28R. [DOI] [PubMed] [Google Scholar]
- 87. Murakami Y, Fujino T, Kurachi R, Hasegawa T, Usui T, Hayase F, Watanabe H. Identification of pyridinoline, a collagen crosslink, as a novel intrinsic ligand for the receptor for advanced glycation end-products (RAGE). Biosci Biotechnol Biochem. 2018;82(9):1508–1514. [DOI] [PubMed] [Google Scholar]
- 88. Kosaka T, Fukui R, Matsui M, Kurosaka Y, Nishimura H, Tanabe M, Takakura Y, Iwai K, Waki T, Fujita T. RAGE, receptor of advanced glycation endoproducts, negatively regulates chondrocytes differentiation. PLoS One. 2014;9(9):e108819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Eastell R, Szulc P. Use of bone turnover markers in postmenopausal osteoporosis. Lancet Diabetes Endocrinol. 2017;5(11):908–923. [DOI] [PubMed] [Google Scholar]
- 90. Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET. Use of CTX-I and PINP as bone turnover markers: National Bone Health Alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int. 2017;28(9):2541–2556. [DOI] [PubMed] [Google Scholar]
- 91. Tonks KT, White CP, Center JR, Samocha-Bonet D, Greenfield JR. Bone turnover is suppressed in insulin resistance, independent of adiposity. J Clin Endocrinol Metab. 2017;102(4):1112–1121. [DOI] [PubMed] [Google Scholar]
- 92. Ida T, Kaku M, Kitami M, Terajima M, Rosales Rocabado JM, Akiba Y, Nagasawa M, Yamauchi M, Uoshima K. Extracellular matrix with defective collagen cross-linking affects the differentiation of bone cells. PLoS One. 2018;13(9):e0204306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Saito M, Fujii K, Mori Y, Marumo K. Role of collagen enzymatic and glycation induced cross-links as a determinant of bone quality in spontaneously diabetic WBN/Kob rats. Osteoporos Int. 2006;17(10):1514–1523. [DOI] [PubMed] [Google Scholar]
- 94. McCarthy AD, Uemura T, Etcheverry SB, Cortizo AM. Advanced glycation endproducts interefere with integrin-mediated osteoblastic attachment to a type-I collagen matrix. Int J Biochem Cell Biol. 2004;36(5):840–848. [DOI] [PubMed] [Google Scholar]
- 95. Khosravi R, Sodek KL, Faibish M, Trackman PC. Collagen advanced glycation inhibits its discoidin domain receptor 2 (DDR2)-mediated induction of lysyl oxidase in osteoblasts. Bone. 2014;58:33–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Li W, Ling W, Teng X, Quan C, Cai S, Hu S. Effect of advanced glycation end products, extracellular matrix metalloproteinase inducer and matrix metalloproteinases on type-I collagen metabolism. Biomed Rep. 2016;4(6):691–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Owen WF Jr, Hou FF, Stuart RO, Kay J, Boyce J, Chertow GM, Schmidt AM β2-Microglobulin modified with advanced glycation end products modulates collagen synthesis by human fibroblasts. Kidney Int. 1998;53(5):1365–1373. [DOI] [PubMed] [Google Scholar]
- 98. Sanguineti R, Monacelli F, Parodi A, Furfaro AL, Borghi R, Pacini D, Pronzato MA, Odetti P, Molfetta L, Traverso N. Vitamins D3 and K2 may partially counterbalance the detrimental effects of pentosidine in ex vivo human osteoblasts. J Biol Regul Homeost Agents. 2016;30(3):713–726. [PubMed] [Google Scholar]
- 99. Tolosa MJ, Chuguransky SR, Sedlinsky C, Schurman L, McCarthy AD, Molinuevo MS, Cortizo AM. Insulin-deficient diabetes-induced bone microarchitecture alterations are associated with a decrease in the osteogenic potential of bone marrow progenitor cells: preventive effects of metformin. Diabetes Res Clin Pract. 2013;101(2):177–186. [DOI] [PubMed] [Google Scholar]
- 100. Zhou Z, Tang Y, Jin X, Chen C, Lu Y, Liu L, Shen C. Metformin inhibits advanced glycation end products-induced inflammatory response in murine macrophages partly through AMPK activation and RAGE/NFκB pathway suppression. J Diabetes Res. 2016;2016:4847812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Nguyen DV, Linderholm A, Haczku A, Kenyon N. Glucagon-like peptide 1: a potential anti-inflammatory pathway in obesity-related asthma. Pharmacol Ther. 2017;180:139–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Li P, Tang Z, Wang L, Feng B. Glucagon-like peptide-1 analogue liraglutide ameliorates atherogenesis via inhibiting advanced glycation end product-induced receptor for advanced glycosylation end product expression in apolipoprotein-E deficient mice. Mol Med Rep. 2017;16(3):3421–3426. [DOI] [PubMed] [Google Scholar]
- 103. Wang K, Zhou Z, Zhang M, Fan L, Forudi F, Zhou X, Qu W, Lincoff AM, Schmidt AM, Topol EJ, Penn MS. Peroxisome proliferator-activated receptor γ down-regulates receptor for advanced glycation end products and inhibits smooth muscle cell proliferation in a diabetic and nondiabetic rat carotid artery injury model. J Pharmacol Exp Ther. 2006;317(1):37–43. [DOI] [PubMed] [Google Scholar]
- 104. Anisuzzaman HT, Hatta T, Miyoshi T, Matsubayashi M, Islam MK, Alim MA, Anas MA, Hasan MM, Matsumoto Y, Yamamoto Y, Yamamoto H, Fujisaki K, Tsuji N. Longistatin in tick saliva blocks advanced glycation end-product receptor activation. J Clin Invest. 2014;124(10):4429–4444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Manigrasso MB, Pan J, Rai V, Zhang J, Reverdatto S, Quadri N, DeVita RJ, Ramasamy R, Shekhtman A, Schmidt AM. Small molecule inhibition of ligand-stimulated RAGE-DIAPH1 signal transduction. Sci Rep. 2016;6(1):22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ojima A, Matsui T, Nakamura N, Higashimoto Y, Ueda S, Fukami K, Okuda S, Yamagishi S.. DNA aptamer raised against advanced glycation end products (AGEs) improves glycemic control and decreases adipocyte size in fructose-fed rats by suppressing AGE-RAGE axis. Horm Metab Res. 2015;47(4):253–258. [DOI] [PubMed] [Google Scholar]
- 107. Wang J, Wang H, Shi J, Ding Y. Effects of bone marrow MSCs transfected with sRAGE on the intervention of HMGB1 induced immuno-inflammatory reaction. Int J Clin Exp Pathol. 2015;8(10):12028–12040. [PMC free article] [PubMed] [Google Scholar]
- 108. Song F, Hurtado del Pozo C, Rosario R, Zou YS, Ananthakrishnan R, Xu X, Patel PR, Benoit VM, Yan SF, Li H, Friedman RA, Kim JK, Ramasamy R, Ferrante AW Jr, Schmidt AM. RAGE regulates the metabolic and inflammatory response to high-fat feeding in mice. Diabetes. 2014;63(6):1948–1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Lee RH, Bergmeier W. Sugar makes neutrophils RAGE: linking diabetes-associated hyperglycemia to thrombocytosis and platelet reactivity. J Clin Invest. 2017;127(6):2040–2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Xu L, Wang YR, Li PC, Feng B. Advanced glycation end products increase lipids accumulation in macrophages through upregulation of receptor of advanced glycation end products: increasing uptake, esterification and decreasing efflux of cholesterol. Lipids Health Dis. 2016;15(1):161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. He M, Kubo H, Morimoto K, Fujino N, Suzuki T, Takahasi T, Yamada M, Yamaya M, Maekawa T, Yamamoto Y, Yamamoto H. Receptor for advanced glycation end products binds to phosphatidylserine and assists in the clearance of apoptotic cells. EMBO Rep. 2011;12(4):358–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Son M, Oh S, Park H, Ahn H, Choi J, Kim H, Lee HS, Lee S, Park HJ, Kim SU, Lee B, Byun K. Protection against RAGE-mediated neuronal cell death by sRAGE-secreting human mesenchymal stem cells in 5xFAD transgenic mouse model. Brain Behav Immun. 2017;66:347–358. [DOI] [PubMed] [Google Scholar]
- 113. Renard C, Chappey O, Wautier MP, Nagashima M, Lundh E, Morser J, Zhao L, Schmidt AM, Scherrmann JM, Wautier JL. Recombinant advanced glycation end product receptor pharmacokinetics in normal and diabetic rats. Mol Pharmacol. 1997;52(1):54–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article or in the data repositories listed in References and Notes.