Abstract
Antibody-drug conjugates (ADC) represent a distinct family of chemoimmunotherapy agents. ADCs are composed of monoclonal antibodies conjugated to cytotoxic payloads via specialized chemical linkers. ADCs therefore combine the immune therapy with targeted chemotherapy. Due to the distinct biomarkers associated with lymphocytes and plasma cells, ADCs have emerged as a promising treatment option for lymphoid malignancies and multiple myeloma. Several ADCs have been approved for clinical applications: brentuximab vedotin, inotuzumab ozogamicin, moxetumomab pasudotox, and polatuzumab vedotin. More novel ADCs are under clinical development. In this article, we summarized the general principles for ADC design, and updated novel ADCs under various stages of clinical trials for lymphoid malignancies and multiple myeloma.
Keywords: Antibody-drug conjugate, B cell maturation antigen, Brentuximab vedotin, Inotuzumab ozogamicin, Polatuzumab vedotin
Introduction
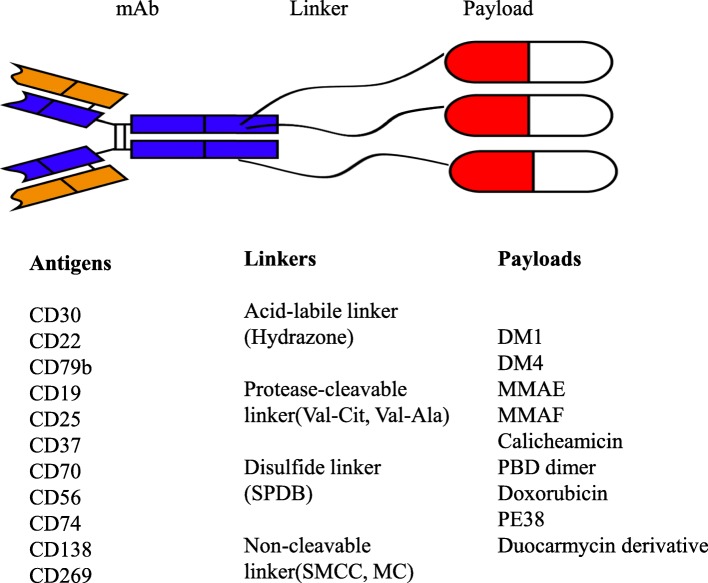
Monoclonal antibodies such as rituximab and obinutuzumab are a major component in the combination regimens for the therapy of lymphoid malignancies [1–6]. Antibody-drug conjugates (ADC) are a new class of agents in the treatment of various malignancies. ADC consists of three fundamental elements: a tumor-specific monoclonal antibody (mAb), a cytotoxic small molecule referred to as payload, and a specialized chemical linker that connects the mAb and payload (Fig. 1). Upon binding to the corresponding antigen on the surface of tumor cells, the ADC/antigen complex is internalized and then the payloads are released, leading to cytotoxicity and cell death. ADC represents a novel class of anticancer agents that theoretically enhance targeted killing of tumors while sparing normal tissues, thereby maximizing efficacy and minimizing systemic toxicity [7].
Fig. 1.
The schematic diagram of the structure of an antibody-drug conjugate. Antigens for the monoclonal antibody (mAb), linker types, and payloads that are in clinical development were listed
Brentuximab vedotin, inotuzumab ozogamicin, moxetumomab pasudotox, and polatuzumab vedotin are FDA-approved ADCs for lymphoid malignancies [8–11]. More ADCs are under clinical development over the past decade. In this review, we discussed the general principles of ADC design and updated on novel ADCs in clinical trials for the treatment of lymphoid malignancies and multiple myeloma.
Engineering ADCs
Selection of antigens and antibodies
An ideal antigen for targeted therapy should have a high copy number on tumor cells with limited or no expression in normal tissues to minimize off-target ADC uptake [12]. The antigen should be able to trigger intracellular internalization upon ADC binding. When an antigen target has heterogenous expression, optimal antitumor activity relies more on the bystander effect, which is referred to as the ADC’s ability to diffuse across cell membranes and exert cytotoxicity on the neighboring cells. This bystander effect is frequently influenced by the chemical nature of the payloads and linkers of the ADCs [13, 14].
Immunoglobulin G (IgG) is the most frequently used subtype in ADCs due to the longer half-life, and the most chosen isotype is IgG1 [15]. IgG1 can induce stronger antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC), which further enhance the antitumor effect of ADCs [16]. However, the ADCC and CDC activities intrinsic to the antibodies may add additional toxicities to the cytotoxic payloads. One solution is to engineer the Fc portion of IgG1 heavy chain by introducing mutations to silent the intrinsic effector immune function [17]. IgG2 is believed to conjugate more payloads because it contains four reducible interchain disulfide bonds, whereas IgG1 and IgG4 have only two such bonds [18]. IgG4 has the tendency to exchange with other antibodies, therefore IgG4-based ADCs, such as inotuzumab ozogamicin, often contain a stabilizing mutation in the hinge region to prevent half antibody exchange [19].
Characteristics of payloads
The payloads used in ADCs are selected small molecules with high potency and proper hydropholicity [20, 21]. Another important parameter is drug-antibody ratio (DAR), which is defined as the average number of payload molecules attached to a single mAb. The ideal level of DAR is between 3 and 4. The DAR affects the drug stability in the circulation, tumor penetration capability, antitumor efficacy, and toxicity of an ADC [22].
The payloads commonly used in ADCs can be divided into two main categories: microtubule inhibitors and DNA-damaging agents. Two currently employed microtubule inhibitors are maytansinoids and auristatins. Maytansinoids were initially derived from maytansine, a natural benzoansamacrolide discovered in the plant maytenus ovatus [23]. There are two maytansinoids derivatives: DM1 and DM4. DM1 includes emtansine and mertansine. DM4 includes soravtansine and ravtansine. Auristatins are extracted from the sea hare Dolabella auricularia. Two auristatin derivatives are commonly used for ADC constructions: monomethyl auristatin E (MMAE, vedotin) and monomethyl auristatin F (MMAF, mafodotin) [24–31]. MMAE is toxic to the neighboring cells through the bystander effect due to its neutral charge that allows diffusion across cell membranes. MMAE has been used in brentuximab vedotin and polatuzumab vedotin. MMAF lacks the ability of bystander killing [32]. DNA-damaging agents include calicheamicin, pyrrolobenzodiazepines (PBD) dimer, indolinobenzodiazepines, duocarmycins, doxorubicin, etc. [33]. Calicheamicin has been used in inotuzumab ozogamicin and gemtuzumab ozogamycin [11, 34–36].
Linker selections and conjugation strategies
An ideal linker should not allow premature deconjugation in the circulation which triggers off-target toxicity. Linkers currently used in ADCs fall into two broad categories: cleavable and non-cleavable linkers. Cleavable linkers are sensitive to several intracellular conditions. Here are some examples. Hydrazone, an acid-labile linker used in inotuzumab ozogamicin, can be selectively hydrolyzed in the acidic pH environment inside the lysosomes and endosomes. However, slow hydrolysis under physiologic condition in circulation has been reported [37]. Protease-cleavable linkers contain dipeptide sequences like valine-citrulline (Val-Cit) and valine-alanine (Val-Ala) that can be recognized by cathepsin B. These linkers are often coupled with p-aminobenzyloxycarbonyl (PABC) which serves as a spacer between the dipeptide and payload. Protease cleavable linkers show relatively higher stability in plasma. Val-Cit linker has been used to construct brentuximab vedotin [38]. Typical disulfide linkers include N-succinimidyl-4-(2-pyridylthio) butanoate (SPDB) and N-succinimidyl-4-(2-pyridyldithio) pentanoate (SPP) [39].
Non-cleavable linkers are more stable but rely on complete proteolytic degradation of the whole mAb backbone by the lysosomes to release active payloads. Most common examples of non-cleavable linkers are thioether linkers, N-succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate SMCC) and maleimidocaproyl (MC) [40].
Multiple conjugation strategies have been developed to attach linkers to a specific amino acid residue on the mAb. Lysine is one of the most common amino acid residues used to connect linkers with activated carboxylic acid esters [41]. Cysteine-based conjugation has been used in brentuximab vedotin and a variety of ADCs under development. It has been further improved by a new engineered cysteine technology, THIOMAB, that generates highly homogeneous ADCs with a controlled DAR of 2 [42].
Homogeneous ADCs can also be achieved through site-specific conjugation by incorporating genetically engineered non-natural amino acid (nnAA) [43, 44]. Other conjugation methods include enzymatic conjugations such as transpeptidation mediated by sortase or bacterial transglutaminases, N-glycan engineering by β-1,4-galactosyltransferase and α-2,6-sialyltransferase, etc. [45–47].
ADCs approved for lymphoid malignancies
Brentuximab vedotin (Adcetris®, SGN-35)
Brentuximab (BV) is composed of an anti-CD30 chimeric IgG1 mAb conjugated to MMAE via a protease-cleavable linker [48]. CD30 is a tumor necrosis factor (TNF) receptor superfamily member, characteristically expressed on the surface of Reed–Sternberg cells in Hodgkin lymphoma (HL) [49], anaplastic large cell lymphoma (ALCL) cells, and a subset of cutaneous T cell lymphoma (CTCL) cells, with limited expression on normal cells [50].
Single-agent BV has been approved by the US FDA in 2011 for the treatment of HL after failure of autologous stem cell transplantation (ASCT) or after failure of at least two prior multiagent chemotherapy regimens [8, 49, 51, 52] (Table 1). In a pivotal phase II trial (NCT00848926), 102 patients with relapsed/refractory (R/R) HL who failed ASCT were treated with intravenous BV 1.8 mg/kg every 3 weeks at a maximum of 16 cycles in the absence of disease progression or unacceptable toxicity. The overall response rate (ORR) was 75% with 34% complete remission (CR) and the median duration of response (DOR) was 6.7 months. The most common treatment-emerging adverse events (TEAE) were peripheral sensory neuropathy (42%), nausea (35%), fatigue (34%), and neutropenia (19%). Fifty-five percent of patients experienced grade ≥ 3 AEs (SAE), majority of which included neutropenia (20%) and peripheral sensory neuropathy (8%). Most of these AEs are manageable with dose reductions and/or delays [8, 53]. At the 5-year follow-up, the overall survival (OS) and progression-free survival (PFS) rate for all patients were 41% and 22% respectively. The estimated median OS and PFS were 40.5 and 9.3 months respectively, suggesting long-term disease control provided by single-agent BV [54]. However, many patients still eventually developed resistance partially because of the upregulation of multidrug resistance pump (MDR1), a drug export pump. Currently, a phase I trial (NCT03013933) is investigating the combination of BV and cyclosporine (CsA), an MDR1 inhibitor, for R/R HL. The interim result was encouraging, showing a 67% ORR (33 % CR) and a manageable toxicity profile at the maximum tolerated dose (MTD) [55].
Table 1.
FDA approved antibody-drug conjugates for B cell malignancies and multiple myeloma
| ADC names | Target | Indications | Dosage and schedule | Year of approval |
|---|---|---|---|---|
| Brentuximab vedotin (Adcetrix®) | CD30 | R/R HL |
- 1.8 mg/kg (maximum 180 mg) - Every 3 weeks until disease progression or unacceptable toxicity |
2011 |
| Frontline stage III & IV HL (+ AVD) |
- 1.2 mg/kg (maximum 120 mg) combined with chemotherapy - Every 2 weeks until a maximum of 12 doses, disease progression, or unacceptable toxicity |
2018 | ||
| Post-ASCT consolidation for HL |
- 1.8 mg/kg (maximum 180 mg) - Initiate within 4–6 weeks post-ASCT or upon recovery from ASCT - Every 3 weeks until a maximum of 16 cycles, disease progression, or unacceptable toxicity |
2015 | ||
| R/R systemic ALCL |
- 1.8 mg/kg (maximum 180 mg) - Every 3 weeks until disease progression or unacceptable toxicity |
2011 | ||
| R/R PTCL (+ CHP) |
- 1.8 mg/kg (maximum 180 mg) combined with chemotherapy - Every 3 weeks with each cycle of chemotherapy for 6 to 8 doses |
2018 | ||
| R/R CTCL |
- 1.8 mg/kg (maximum 180 mg) - Every 3 weeks until a maximum of 16 cycles, disease progression, or unacceptable toxicity |
2017 | ||
| Inotuzumab ozogamicin (Besponsa®) | CD22 | R/R B-cell ALL |
- Cycle 1 (21 day-cycle): 1.8 mg/m2 [day 1 (0.8 mg/m2), day 8 (0.5 mg/m2), and day 15 (0.5 mg/m2)] - Subsequent cycles (28 day-cycle): 1) Patients who have achieved CR or CRi: 1.5 mg/m2 [day 1 (0.5 mg/m2), day 8 (0.5 mg/m2), and day 15 (0.5 mg/m2)] per cycle 2) Patients who have not achieved CR or CRi: repeat cycle 1 - Duration: 1) Patients proceeding to ASCT: 2 cycles 2) Patients not proceeding to ASCT: maximum 6 cycles |
2017 |
| Moxetumomab pasudotox (Lumoxiti®) | CD22 | R/R HCL |
- 0.04 mg/kg on days 1, 3, and 5 of each 28-day cycle. - Maximum of 6 cycles, disease progression, or unacceptable toxicity |
2018 |
| Polatuzumab vedotin (Polivy®) | CD79b | R/R DLBCL (+ BR) |
- 1.8 mg/kg per cycle, combined with BR - every 21 days for 6 cycles |
2019 |
ALCL anaplastic large cell lymphoma, ALL acute lymphoblastic leukemia, ASCT autologous stem cell transplant, BR bendamustine and rituximab, CR complete remission, CTCL cutaneous T cell lymphoma, DLBCL diffuse large cell lymphoma, HCL hairy cell leukemia, HL Hodgkin lymphoma, NHL non-Hodgkin lymphoma, PTCL peripheral T cell lymphoma, R/R relapsed/refractory
BV plus bendamustine (BVB) has been studied as a salvage regimen for R/R HL with favorable toxicity profile in phase I/II trials. One study (NCT01657331) revealed that BVB achieved a 78% ORR in heavily pretreated HL patients. Grade 3 to 4 neutropenia were found in 25% of patients across the trial [56]. Another study (NCT01874054) used BVB as the first salvage regimen for 55 HL patients who failed the frontline therapy. A better outcome was reported with a 92.5% ORR (73.6% CR) and a 62.6% estimated 2-year PFS. Further, 75.4% patients proceeded to ASCT. Most frequently reported SAEs included rash (16.3%), lymphopenia (10.9%), and hypotension (7.3%) [57]. There is another ongoing trial (NCT01657331) that identified 23 out of 65 patients treated with BVB who experienced prolonged median PFS of more than 1 year [58]. BV in combination with nivolumab (Nivo) represents another ongoing clinical trial for R/R HL. In the phase I/II trial (NCT02572167), BV + Nivo achieved 82% ORR and 61% CR, almost doubled the CR rate of BV monotherapy in the pivotal phase II trial. There were mostly grade 1 and 2 AEs: nausea (49%), fatigue (41%), infusion-related reactions (44%) [59]. Currently, BVB and BV + Nivo are being compared in an ongoing phase II trial (NCT02927769).
BV is an effective option of consolidation therapy both before and after ASCT for HL at a high risk of relapse or progression. A randomized, double-blind, multinational, phase III trial (AETHERA, NCT01100502) enrolled 329 eligible patients to receive either 16 cycles of 1.8 mg/kg intravenous BV or placebo every 3 weeks, starting 30–45 days after transplantation. Median PFS was 42.9 months for patients in the BV group (n = 165), significantly better than the 24.1 months in the placebo group (n = 164) [60]. There was continued benefit at 5-year follow-up, with PFS rate for patients receiving BV and placebo at 59% and 41% respectively [61]. The toxicity profile mainly consisted of peripheral neuropathy (56%) and neutropenia (35%), with neutropenia (29%) being the most common SAE [60]. Recently, a retrospective multicenter study revealed that chemo-refractory HL patients receiving BV prior to allogeneic SCT (AlloSCT) presented with a better outcome and a lower incidence of chronic graft versus host disease (GVHD) at 3-year follow-up compared to those without BV (PFS 53% vs. 33%; OS 62% vs. 44%; GVHD incidence 43% vs. 47%) [62].
BV in combination with chemotherapy has been reported to optimize the frontline treatment of newly diagnosed advanced stage HL. This report was from the international randomized phase III trial (ECHELON-1, NCT01712490) which assigned patients with previously untreated stage III or IV classic HL to receive either BV (adcetris) plus doxorubicin, vinblastine, and dacarbazine (AAVD) (n = 664) or doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD) (n = 670) [51]. The outcome of the AAVD cohort appeared to be superior to the ABVD cohort in terms of the 2-year modified PFS (81.0% vs. 74.4%), which was further confirmed by sensitivity analysis, even though the response rate was not significantly different between the two cohorts: ORR (86% vs. 83%), CR (73% vs. 70%). Of note, patients receiving AAVD presented with a higher incidence of peripheral neuropathy (29% vs. 17%) but a lower incidence of pulmonary toxicity (< 1% vs. 3%) than patients receiving ABVD. Neutropenia (54% vs. 39%) was the most frequently encountered SAE in both cohorts. Prophylaxis with granulocyte colony-stimulating factor (G-CSF) effectively decreased the rate of neutropenia and febrile neutropenia [51, 63].
BV plus etoposide, cyclophosphamide, doxorubicin, dacarbazine, and dexamethasone (BrECADD), a modified first-line treatment for advanced classical HL by incorporating BV was reported with an 88% CR rate and a more favorable toxicity profile (NCT01569204) [64]. This BrECADD regimen is currently being compared to the standard BEACOPP chemotherapy in a phase III randomized trial (HD21, NCT02661503).
BV has been studied in a few subtypes of non-Hodgkin lymphoma (NHL), including systemic ALCL, an aggressive CD30+ subtype of peripheral T cell lymphoma (PTCL). In a phase II multicenter trial (NCT00866047), 58 patients with systemic ALCL after failure of at least one prior multiagent chemotherapy regimen received intravenous BV 1.8 mg/kg every 3 weeks. Fifty (86%) patients achieved ORR, and 33 (57%) achieved CR [65]. For all enrolled patients, the estimated 5-year OS and PFS were 60% and 39% respectively. Among those who achieved CR, the 5-year OS and PFS were 79% and 57% respectively. Of the 50 patients with an objective response, the median duration of response (DOR) was 25.6 months [66]. In 2018, FDA approved BV in combination with cyclophosphamide, doxorubicin, and prednisone (CHAP) for the treatment of CD30-expressing PTCL including systemic ALCL, angioimmunoblastic T cell lymphoma, and PTCL not otherwise specified, based on the positive result of a randomized, double-blind phase III trial (ECHELON-2, NCT01777152). In this study, 452 PTCL patients were randomized to receive either CHAP or cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP). The patients in the CHAP group had longer PFS (48.2 months vs. 20.8 months), a higher ORR (83% vs. 72%), and CR rate (68% vs. 56%). Most common AEs of CHAP recipients were nausea (46%), peripheral neuropathy (45%), neutropenia (38%), and diarrhea (38%), comparable to those of CHOP recipients. Neutropenia (35%) was the most common SAE [67]. BV has also been investigated in cutaneous T cell lymphoma (CTCL). In a multicenter randomized phase III trial (ALCANZA, NCT01578499) that enrolled adult patients with CD30-positive mycosis fungoides or primary cutaneous ALCL who had received prior systemic therapy, patients receiving BV demonstrated more favorable outcome than those receiving conventional therapy (methotrexate or bexarotene): ORR lasting at least 4 months (56.3% vs. 12.5%), CR (16% vs 2%), and median PFS (17.2 vs. 3.5 months). A higher level of CD30 expression seemed to be associated with a better response to treatment with BV. There were mostly grade 1 to 2 AEs in BV-treated patients. Of note, the incidence of reported peripheral neuropathy (45%; grade ≥ 3: 5%) was much higher than that in patients receiving conventional therapy. Other common AEs included nausea (36%), diarrhea (29%), and fatigue (29%) [68].
In addition, several studies suggested that BV could provide additional treatment options for patients suffering from R/R B-cell NHL [69, 70]. In one phase II trial (NCT01421667) that enrolled 49 patients with heavily pretreated diffuse large B cell lymphoma (DLBCL), there was a 44% ORR with a 17% CR, and the DOR was 16.6 months [69].
Inotuzumab ozogamicin (Besponsa®, CMC-544)
Inotuzumab ozogamicin (InO) is composed of a humanized anti-CD22 IgG4 mAb conjugated to calicheamicin via an acid-labile linker [71]. CD22 is a B cell-restricted transmembrane sialoglycoprotein, present on the surface of mature B cells, and thought to be involved in signal transduction, B cell activation and regulation [11, 71]. CD22 is also expressed by most B cell malignancies, including leukemic blasts in > 90% patients with B cell ALL, as well as chronic lymphocytic leukemia (CLL), NHL, and hairy cell leukemia (HCL) [72, 73]. Therefore, CD22 represents an important therapeutic target for ALL and other B cell malignancies.
InO has been approved by the US FDA in 2017 for the treatment of R/R CD22-positive B cell ALL [9, 10]. The approval was mainly based on a phase III, international, randomized trial (INO-VATE, NCT01564784) designed to compare single-agent InO with chemotherapy regimens as first or second salvage therapy for R/R ALL patients. Three hundred thwenty-six adult patients were randomized to receive either InO (n = 164) or standard of care (SoC, intensive chemotherapy; n = 162) [74]. The CR/CR with incomplete hematologic recovery (CRi) rate was significantly higher in patients in the InO arm (73.8%) compared to those in the SoC arm (30.9%). Both PFS and OS were longer with InO than SoC: the median PFS was 5.0 vs. 1.7 months, the median OS was 7.7 vs. 6.2 months, and the 2-year OS rate was 22.8% vs. 10.0%. Subset analyses revealed that remission rates remained consistent for patients with Philadelphia chromosome-positive (Ph+) or -negative (Ph−) ALL. Interestingly, more patients in the InO arm proceeded directly to AlloSCT after achieving CR/CRi (39.6% vs. 10.5%), suggesting InO as an effective bridging therapy to transplantation [75]. Besides, InO-treated patients reported a better quality of life than those receiving SoC [76]. Frequent AEs reported by InO recipients were neutropenia (36%), thrombocytopenia (29%), anemia (18%), febrile neutropenia (16%), nausea (15%), and pyrexia (11%), with lower incidences than SoC recipients [74]. However, there is a higher incidence of hepatotoxicity especially veno-occlusive disease (VOD) (14% vs. 2.1%), which is also the most common SAE found in InO recipients. Of note, among patients who proceeded to AlloSCT, 22% of InO recipients developed VOD after transplantation compared to only 3% of SoC recipients [77]. In view of this risk, expert opinions recommended that for patients planning to receive AlloSCT, InO administration should be limited to two cycles. Transplantation conditioning regimens containing two alkylating agents or any concomitant hepatotoxic drugs should be avoided [78]. Besides, patients receiving InO should also be monitored for prolonged QTc and tumor lysis syndrome. Interestingly, weekly low doses of InO has been revealed to result in less AEs than single-dose InO [79]. Currently, there is an ongoing trial evaluating the efficacy of weekly low-dose InO for R/R CD22-positive ALL (NCT03094611).
In addition to monotherapy, InO has been studied in combination with chemotherapy for R/R B cell ALL. InO was added to the treatment cycles of mini-hyper-CVD (miniHCVD), which was a modified hyper-CVAD regimen (hyper-fractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone) with no anthracycline, whereas cyclophosphamide and dexamethasone were given at 50% dose reduction, methotrexate at 75% dose reduction, and cytarabine at 0.5 mg/m2 for four doses [80]. InO was added on day 3 of each cycle with 1.3 mg/m2 in cycle 1 and 1 mg/m2 in subsequent cycles. A phase II trial (NCT01371630) enrolled 59 patients with R/R B cell ALL receiving InO-miniHCVD. The regimen showed a 78% ORR (59% CR) and a minimal residual disease (MRD) negativity of 82% among responders. The median OS and PFS were 11 and 8 months respectively, and the 1-year overall OS and PFS rates were 46% and 40% respectively. Subset analysis showed that the OS rate was much higher in patients who were treated in first salvage than those in subsequent salvages. Notably, 44% patients proceeded to subsequent ASCT suggesting InO + miniHCVD as an option for bridging therapy. The most common SAEs reported were thrombocytopenia (81%) and infections (73%). VOD still occurred in 15% of patients [81]. Even though InO + miniHCVD seemed to present with more favorable outcomes compared to the INO-VATE trial, further phase III studies are still warranted to ascertain the value of this combination therapy.
InO-miniHCVD has also been investigated as a frontline therapy in older patients (≥ 60 years) with newly diagnosed Ph− B cell ALL [82]. A single-arm phase II trial (NCT01371630) conducted in MD Anderson Cancer Center first reported that InO-miniHCVD demonstrated robust activity (ORR 98%, CR 85%, 3-year OS 56%, and 3-year PFS 49%). Frequently reported SAEs were thrombocytopenia (81%), infections occurred during induction (52%) or consolidation (69%), hyperglycemia (54%), and hepatic events including VOD (33%) [82]. In an attempt to further reduce toxicity and length of maintenance, blinatumomab (blina), a bispecific T cell-engaging antibody targeting both CD19 and CD3 [83–86], was added to the regimen. This new regimen, InO-miniHCVD with or without blina, achieved a 98% ORR (87% CR) and a 54% 3-year OS according to the interim result of an ongoing phase II trial (NCT03249870), comparable to the previous one [87]. A recently published retrospective propensity score analysis reported that older patients with newly diagnosed Ph− ALL who received InO-miniHCVD ± blina demonstrated better outcomes and lower risks than those who received hyper-CVAD (ORR 98% vs. 88%; 3-year OS 64% vs. 34%; early death 0% vs. 8%) [88]. Currently, there is another ongoing trial examining combination of InO with hyper-CVAD as a frontline therapy for ALL patients (NCT03488225).
The development of InO in B cell lymphoma has made relatively less progress. Single-agent InO was administered to 81 patients with R/R B cell NHL, predominantly follicular lymphoma (FL), who failed rituximab, rituximab plus chemotherapy, or radioimmunotherapy in a phase II trial (NCT00868608). The study reported a 67% ORR, a 31% CR, and a median PFS of 12.7 months. Bone marrow suppression was reported with most frequently thrombocytopenia (74%) and neutropenia (56%). Of note, 58% of patients with AEs discontinued the treatment [89]. InO combined with rituximab (R-InO) is thought to be a treatment option for patients with R/R B cell NHL who are not candidates for high-dose chemotherapy. One phase I/II trial (NCT00299494) reported that the R-InO regimen at MTD (1.8 mg/m2 InO plus 375 mg/m2 rituximab) yielded an 87% ORR for R/R FL and 74% ORR for R/R DLBCL. R-InO demonstrated a similar toxicity profile to the single-agent InO [90]. A separate phase II trial (NCT00867087) reported only 29% ORR after 3 cycles of R-InO for patients with R/R DLBCL [91]. However, a recent randomized phase III trial (NCT01232556) revealed that the outcome of the R-InO recipients appeared not to be superior to those receiving rituximab plus chemotherapy of bendamustine or gemcitabine in terms of ORR (41% vs. 44%), OS (35% vs. 37%), and PFS (19% vs. 17%) [92]. Several clinical trials are exploring different new regimens. InO at 0.8 mg/m2 combined with full dose of rituximab, cyclophosphamide, vincristine, and prednisolone (R-CVP) was reported safe and effective for CD22+ R/R B cell NHL in a phase I trial (NCT01055496). The ORR was 84% with 24% CR. Subset analysis showed that ORR was 100% for patients with indolent lymphoma and 57% for those with aggressive histology. The toxicity profile was also similar to InO monotherapy [93]. Currently, InO + R-CVP is being evaluated in patients with DLBCL not suitable for anthracycline-based chemotherapy in a phase II trial (NCT01679119). InO at 0.8 mg/m2 plus full dose rituximab, gemcitabine, dexamethasone, and cisplatin (R-GDP) is a regimen proposed by another phase I trial (NCT01055496) for patients with R/R CD22+ B cell NHL. The preliminary result was less encouraging (53% ORR; 20% CR) [94].
Moxetumomab pasudotox (Lumoxiti®, CAT-8015)
Moxetumomab pasudotox (MP) is the second anti-CD22 ADC that comes into clinical practice. It has the Fv fragment of a recombinant murine mAb with higher affinity for CD22 than the parent compound [95]. The fragment is genetically fused to a truncated form of Pseudomonas aeruginosa exotoxin (PE38) [96]. MP has been approved by the US FDA in September 2018 for the treatment of adult patients with R/R hairy cell leukemia (HCL) who have received at least two prior systemic therapies, including two courses of a purine analog or one course of rituximab or a BRAF inhibitor following a single course of a purine analog. The approval was mainly based on a pivotal, multicenter, open-label, single-arm trial (NCT01829711) that enrolled 80 patients who received 40 μg/kg MP intravenously on days 1, 3, and 5 every 28 days for a maximum of 6 cycles. The treatment led to a 75% ORR with 41% CR. Eighty-five percent of CR patients achieved MRD negativity. PFS was not yet reached at a median follow-up of 16.7 months. The median DOR for the MRD-positive patients was 5.9 months and has not been reached for MRD-negative patients [97]. Elimination of MRD has been shown to be associated with prolonged CR duration [98]. The most common treatment-emerging AEs (TEAE) were peripheral edema (39%), nausea (35%), fatigue (34%), and headache (33%). Serious AEs included hemolytic uremic syndrome (HUS) (8%) and capillary leak syndrome (CLS) (5%). Although four of HUS and two of CLS patients ended up with discontinuation of therapy, most of the above AEs were manageable with dose reduction and supportive care [97, 99]. It was noted that antidrug titers increased after repeated administration of the ADC [99]. Currently, a phase I trial (NCT03805932) is looking at whether the combination of MP and rituximab is safe and effective for R/R HCL. The development of MP for precursor cell lymphoblastic leukemia/lymphoma, NHL, and CLL has been discontinued [99].
Polatuzumab vedotin (Polivy®, DCDTS4501A)
CD79b is a component of the B cell receptor (BCR) complex [100]. It is a promising therapeutic target because of its restricted expression on mature B cells and B cell malignancies [101]. Polatuzumab vedotin (Pola) is an anti-CD79b mAb conjugated to MMAE via a protease-cleavable linker similar to the structure of anti-CD22 ADC, pinatuzumab vedotin (Pina). Phase I trials demonstrated that single-agent Pola at a recommended dose of 2.4 mg/kg was effective against NHL but not for CLL [102]. Both pola and pina were often studied in combination with rituximab. The ROMULUS trial showed that irrespective of CD79b expression, R-Pola was associated with a 54% ORR (21% CR), and a median PFS of 5.6 months for DLBCL patients, which was comparable to those from R-Pina; but for FL patients, R-Pola was associated with a 70% ORR (45% CR), and a median PFS of 15.3 months, much more effective than R-Pina. R-Pola shared a similar profile of AEs with R-Pina, but less incidence of SAEs [103]. Therefore, the overall risk-benefit ratio favored Pola for further investigation in B cell NHL.
In an ongoing randomized phase II trial (NCT02257567), Pola was added to the regimen of bendamustine plus rituximab (BR) or obinutuzumab (BO) for patients with R/R DLBCL or R/R FL ineligible for autologous stem cell transplantation (ASCT). The interim result showed that the addition of Pola significantly improve ORR (45% vs. 18%), CR (40% vs. 18%), median OS (12.4 vs. 4.7 months), and PFS (7.6 vs. 2.0 months) [104]. For the R/R DLBCL patients in this randomized phase II trial, 40 patients received Pola-BR, and 40 patients were randomized to BR only arm. In the Pola-BR arm, 25 (63%) patients achieved a PR/CR, versus 25% in the BR arm. The DOR was also longer in the pola-BR arm. Pola has been approved by FDA in June 2019 for R/R DLBCL who have failed at least two prior therapy and are not eligible for ASCT (Table 1). Pola is infused at 1.8 mg/kg over 90 min in combination with BR for the first cycle. Subsequent infusions may be administered over 30 min if the previous infusion is tolerated. The Pola-BR regimen is given every 21 days for a total of 6 cycles. One phase I/II trial (NCT01992653) reported that Pola plus obinutuzumab, cyclophosphamide, doxorubicin, and prednisone (Pola-G-CHP) yielded a 91% ORR (81% CR) with manageable toxicity profile. Common SAEs were neutropenia (38%) and febrile neutropenia (33%) [105]. Another phase I/II trial (NCT01992653) investigated the combination of Pola with rituximab-cyclophosphamide, doxorubicin, and prednisone (Pola-R-CHP). An encouraging response with 91% ORR (78% CR) was also achieved. Most common SAEs were neutropenia (27%) and febrile neutropenia (11%) [106]. A multicenter, randomized, double-blind, placebo-controlled phase III trial (POLARIX, NCT03274492) is currently ongoing to compare Pola-R-CHP to rituximab-cyclophosphamide, doxorubicin, vincristine, and prednisone (R-CHOP) as the first-line treatment for patients with newly diagnosed DLBCL.
ADCs under clinical development for lymphoid malignancies
Anti-CD19 ADCs
CD19 is a transmembrane glycoprotein that is essential in modulating both B cell receptor-dependent and independent signaling. It is ubiquitously expressed in the B lymphocyte lineage, from the earliest B lineage committed cells, and continuing through pre-B and mature B cell stage. CD19 is present in the majority of B cell malignancies. CD19 is therefore a genuine biomarker for normal and malignant B cells, making it an ideal therapeutic target [107].
Coltuximab ravtansine (SAR3419) is a humanized anti-CD19 IgG1 mAb conjugated to DM4 via a cleavable disulfide linker [108] (Table 2). In a phase II trial (NCT01472887), SAR3419 was administered to patients with R/R DLBCL previously treated with rituximab-containing regimen. The dose used in this study was 55 mg/m2 weekly for 4 weeks, then every 2 weeks for the next four doses. The study achieved a 43.9% ORR (14.6% CR), and a median OS and PFS of 9.2 and 4.4 months respectively. In contrast to limited efficacy, the safety profile was favorable. Common AEs were mainly gastrointestinal disorders such as diarrhea and nausea (43%), asthenia (30%), and ocular toxicity (25%). Most common SAEs were hepatotoxicity (3%) and abdominal pain (3%) [109]. Another phase II trial combined SAR3419 with rituximab for R/R DLBCL, but the trial resulted in a less favorable clinical response compared to the previous single-agent trial [119]. SAR3419 has also been evaluated in patients with R/R ALL in a phase II trial (NCT01440179), but ORR was only 25.5%. This trial was terminated [120].
Table 2.
Antibody-drug conjugates in clinical trials for lymphoid malignancies
| ADC names | Target | Linker | Payload | Indications | Major responses | Status [reference] |
|---|---|---|---|---|---|---|
| Brentuximab vedotin (Adcetrix®) | CD30 | Protease-cleavable (Val-Cit) | MMAE | R/R HL | ORR 75%, CR 34%; OS 40.5 months, PFS 9.3 months; 5-year OS 41%, PFS 22%; | Approved [54] |
| Frontline stage III & IV HL (+ AVD) | ORR 86%, CR 73%, 2-year PFS 81% | Approved [51] | ||||
| Post-ASCT consolidation for HL | PFS 42.9 months, 5-year PFS 59% | Approved [60, 61] | ||||
| R/R systemic ALCL | ORR 86%, CR 57%, 5-year OS 79%, PFS 57%, DOR 25.6 months | Approved [65, 66] | ||||
| R/R PTCL (+ CHP) | ORR 83%, CR 68%, PFS 48.2 months | Approved [67] | ||||
| R/R CTCL | ORR 56.3%, CR 16%, PFS 17.2 months | Approved [68] | ||||
| Inotuzumab ozogamicin (Besponsa®) | CD22 | Acid-labile (hydrazone) | Calicheamicin | R/R B-cell ALL, | CR/CRi 73.8%, OS 7.7 mo, PFS 5 months | Approved [74, 75] |
| Frontline Ph- B-cell ALL (+ miniHCVD) | ORR 98%, CR 85%, 3-year OS 56%, PFS 49% | Phase II [82] | ||||
| R/R B-cell NHL | ORR 67%, CR 31%, PFS 12.7 months | Phase II [89] | ||||
| Moxetumomab pasudotox (Lumoxiti®) | CD22 | Disulfide bond | PE38 | R/R HCL | ORR 75%, CR 41%, PFS not reached at 16.7-month follow up | Approved [97, 98] |
| Polatuzumab vedotin (DCDTS4501A) | CD79b | Protease-cleavable (Val-Cit) | MMAE | R/R DLBCL (+ BR) | ORR 63%, PFS 7.6 months, OS 12.4 months | Approved [103] |
| Newly diagnosed DLBCL (+ G-CHP or + R-CHP) |
G-CHP: ORR 91%, CR 81%; R-CHP: ORR 91%, CR 78%; |
Phase III [105, 106] | ||||
| Coltuximab ravtansine (SAR3419) | CD19 | Disulfide bond (SPDB) | DM4 | R/R DLBCL | ORR 43.9%, CR 14.6%, OS 9.2 months, PFS 4.4 months | Phase II [109] |
| Denintuzumab mafodotin (SGN-CD19A) | CD19 | Non-cleavable (MC) | MMAF | R/R B-cell ALL | ORR 35%, CR 19%, DOR 27 weeks | Phase I [110] |
| R/R DLBCL | ORR 33%, CR 22%, DOR 40 weeks | Phase I [111] | ||||
| Loncastuximab Tesirine (ADCT-402) | CD19 | Protease-cleavable (Val-Ala) | PBD dimer | R/R B-cell ALL | CR 8.7% | Phase I [112] |
| R/R NHL |
DLBCL: ORR 40.2%, CR 22% MCL: ORR 46.7%, CR 26.7%; FL: ORR 80%, CR 53.3% |
Phase I [113, 114] | ||||
| Pinatuzumb vedotin (DCDT2980S) | CD22 | Protease-cleavable (Val-Cit) | MMAE | R/R B-cell NHL (+ Rituximab) | DLBCL: ORR 60%, CR 26%, PFS 5.4 months; FL: ORR 62%, CR 5%, PFS 12.7 months | Phase II [103] |
| Camidanlumab tesirine (ADCT-301) | CD25 | Protease-cleavable (Val-Ala) | PBD dimer | R/R HL | ORR 80.8%, CR 50%, DOR 7.7 months, PFS 6.7 months | Phase I [115] |
| R/R B and T cell NHL |
B: ORR 31.3%, CR 18.8%; T: ORR 50%, CR 0 |
Phase I [116] | ||||
| Naratuximab emtansine (IMGN529) | CD37 | Non-cleavable (SMCC) | DM1 | R/R B-cell NHL | ORR 13%, CR 2.6% | Phase I [117] |
| AGS67E | CD37 | Protease-cleavable (Val-Cit) | MMAE | R/R B and T cell NHL | ORR 22%, CR 14% | Phase I [118] |
ADC antibody-drug conjugate, ALCL anaplastic large cell lymphoma, ALL acute lymphoblastic leukemia, CTCL cutaneous T cell lymphoma, HL Hodgkin lymphoma, NHL non-Hodgkin lymphoma, R/R relapsed/refractory, MM multiple myeloma, CR complete remission, DLBCL diffuse large cell lymphoma, DOR duration of response, FL follicular lymphoma, MCL mantle cell lymphoma, ORR overall response rate, OS overall survival, PFS progression-free survival, PTCL peripheral T cell lymphoma
Denintuzumab mafodotin (SGN-CD19A) is a humanized anti-CD19 IgG1 mAb conjugated to MMAF via a non-cleavable MC linker. A phase I trial (NCT01786096) of SGN-CD19A showed a 35% ORR (19% CR) and a median DOR of 27 weeks in patients with R/R B cell ALL. Most frequently reported AEs were pyrexia (54%), nausea (52%), fatigue (51%), headache (44%), chills (38%), vomiting (37%), and blurry vision (35%) [110]. A separate phase I trial (NCT01786135) in patients with R/R DLBCL revealed a 33% ORR (22% CR) and a median DOR of 40 weeks. Of note, ocular toxicity, such as blurry vision (65%), dry eye (52%), and keratopathy (35%), was prominent [111]. Two phase II trials were evaluating the combination of SGN-CD19A and other regimens (NCT02855359: R-CHP or R-CHOP; NCT02592876: RICE [rituximab, ifosfamide, carboplatin, and etoposide]) in DLBCL, but were currently on hold.
Loncastuximab tesirine (ADCT-402) is a humanized anti-CD19 IgG1 mAb conjugated through a protease-cleavable Val-Ala linker to a PDB dimer, a DNA crosslinking agent. ADCT-402 was investigated in a phase I trial (NCT02669264) of adult patients with R/R B-cell ALL. The interim result showed that only two out of 23 patients (8.7%) achieved CR with MRD negativity. The most frequently reported TEAEs were nausea (30%), fatigue (26%), and febrile neutropenia (22%), with febrile neutropenia being the most common SAE [112]. Another ongoing phase I trial (NCT02669017) of ADCT-402 in R/R B cell NHL recently reported its interim result. ADCT-402 yielded a 40.2% ORR (22% CR), with a median DOR and PFS of 4.17 and 2.79 months respectively at doses > 120 μg/kg in a subpopulation of 132 evaluable patients with R/R DLBCL. Most common SAEs were neutropenia (42%), thrombocytopenia (27.3%), anemia (11.7%), and increased gamma-glutamyltransferase (GGT) (8.8%) [113]. Among the 132 patients, 15 evaluable patients with mantle cell lymphoma (MCL) treated with ADCT-402 achieved a 46.7% ORR (26.7% CR) and a median DOR and PFS of 5.3 and 4.8 months respectively; while for the 15 evaluable patients with FL, ADCT-402 therapy achieved an 80% ORR (53.3% CR), the median DOR and PFS have not been reached at the 7.56-months follow-up. The most common SAEs were elevated GGT (26.7%), neutropenia (20%), and anemia (13.3%) [114]. Another phase II trial (NCT03589469) in patients with R/R DLBCL is ongoing to evaluate the efficacy and safety of single agent ADCT-402. In addition, there are phase II trials looking at the combination therapy of ADCT-402 and ibrutinib (NCT03684694), ADCT-402 and durvalumab, a PD-L1 blocker (NCT03685344) for R/R DLBCL, FL, or MCL.
Anti-CD22 ADCs
Pinatuzumab vedotin (Pina, DCDT2980S) is another anti-CD22 ADC currently under clinical development. Pina is a humanized anti-CD22 IgG1 mAb conjugated to MMAE via a protease-cleavable linker [121]. Pina has been revealed effective with or without rituximab for R/R DLBCL and R/R indolent NHL by a phase I trial (NCT01209130), but little effect was seen in CLL [122]. A multicenter phase II trial (ROMULUS, NCT01691898) randomized 81 patients with R/R DLBCL and 42 patients with R/R FL to receive rituximab plus either Pina (R-Pina) or polatuzumab vedotin (anti-CD79b ADC, discussed above). The recommended dosage was Pina or Pola 2.4 mg/kg with rituximab 375 mg/m2 every 21 days up to 1 year or until disease progression or severe toxicity. The regimen of R-Pina generated a 60% ORR (26% CR) and a median PFS of 5.4 months for the DLBCL cohort, whereas a 62% ORR (5% CR) and a median PFS of 12.7 months were seen for the FL cohort. Neutropenia (29%) was the most common SAE. Peripheral neuropathy was a major AE that led to discontinuation of treatment in 21% of patients with DLBCL and 48% of patients with FL [103].
Anti-CD25 ADCs
CD25 is the α subunit of IL-2 receptor (IL-2Rα) involved in the signal transduction for the growth and survival of immune cells. CD25 overexpression has been found in multiple hematologic malignancies including both HL and NHL, as well as ALL, B cell CLL, HCL, and adult T cell leukemia (ATL) [123]. Camidanlumab tesirine (ADCT-301) is the first ADC developed targeting CD25. ADCT-301 consists of a humanized IgG1 mAb (HuMax®-TAC) conjugated to a PBD dimer warhead via a protease-cleavable linker. ADCT-301 is being investigated in R/R HL and NHL in a phase I trial (NCT02432235). Among 26 patients with R/R HL who failed prior BV treatment, therapy with ADCT-301 at a dose of 45 μg/kg achieved an 80.8% ORR (50% CR). Median DOR and PFS were 7.7 and 6.7 months, respectively. Major SAEs included GGT elevation (16.7%) and maculopapular rash (13.3%) [115]. The same trial also enrolled 16 patients with B cell NHL and ten patients with T cell NHL who failed a median of three prior therapies. These patients were treated with ADCT-301 at a dose range of 60–150 μg/kg. The B cell NHL cohort demonstrated a 31.3% ORR (18.8% CR), while the T cell NHL cohort demonstrated a 50% ORR (all PR) [116]. ADCT-301 has also been evaluated in patients with ALL and acute myeloid leukemia (AML) in another phase I trial (NCT02588092). Even though the safety profile was acceptable, no response has been reported to date [124].
Anti-CD37 ADCs
CD37 is a member of transmembrane tetraspanin protein family. Similar to CD19, CD37 is almost exclusively expressed on B cells, but absent from hematopoietic stem cells and plasma cells. It plays a role in signal transduction and immune cell interactions that are important for B cell activation and survival [125]. CD37 is also highly expressed on malignant B cells including most histological subtypes of NHL [126]. Two ADCs targeting CD37, IMGN529 and AGS67E, are currently under clinical development.
Naratuximab emtansine (IMGN529) is a humanized anti-CD37 IgG1 antibody conjugated to the maytansinoid DM1 via a nonreducible thioether linker, succinimidyl-4-(N-maleimidomethyl)-cyclohexane-1-carboxylate (SMCC) [127]. Of note, IMGN529 exhibited significant intrinsic ADCC activity against targeted cells [128]. A phase I trial (NCT01534715) evaluated the safety and efficacy of IMGN529 monotherapy with escalating doses in R/R B cell NHL. The MTD was 1.4 mg/kg every 3 weeks. Only five out of 39 (13%) evaluable patients achieved ORR (one CR), four of whom were patients with DLBCL. The most common AEs were neutropenia (37%), thrombocytopenia (37%), and pyrexia (37%) [117]. Rituximab has been reported to potentiate the antitumor effect of IMGN529 in multiple xenograft models [128]. The combination of IMGN529 and rituximab for patients with R/R NHL is being explored in a phase II trial (NCT02564744).
AGS67E is a fully human anti-CD37 IgG2 mAb conjugated to MMAE via a protease-cleavable linker. Preclinical study discovered that AGS67E was able to alter cell cycle and induce apoptosis in vitro and in xenograft models of NHL [129]. AGS67E demonstrated some effects in a phase I trial (NCT02175433) in 50 patients with R/R B and T cell NHL. Eleven (22%) patients experienced ORR, and seven (14%) patients achieved CR, four of whom had DLBCL. Peripheral neuropathy (16%) and neutropenia (8%) were the major AEs [118].
Anti-CD70 ADCs
CD70, also known as CD27L, is a member of TNF receptor superfamily. The interaction between CD70 and CD27 is critical for B cell activation, T helper 1 (Th1)/Th2 switching, and cell differentiation. CD70 is highly expressed on several malignancies including NHL and renal cell carcinoma (RCC) [130]. Three anti-CD70 ADCs have been investigated in phase I trials in patients with CD70-positive R/R B-cell NHL and metastatic RCC.
SGN-CD70A is a humanized anti-CD70 IgG1 mAb conjugated to a PBD dimer via a protease-cleavable linker. Severe (grade ≥ 3) early-onset thrombocytopenia was reported in 75% of patients with DLBCL and MCL treated by SGN-CD70A and led to treatment termination (NCT02216890) [131]. MDX-1203 (BMS-936561) consists of a fully human anti-CD70 IgG1 mAb conjugated to a duocarmycin derivative through a protease-cleavable linker. In a dose-escalation phase I trial (NCT00944905), dose-limiting toxicity of grade 3 hypersensitivity (13%) and delayed toxicities featured by facial edema and pleural/pericardial effusions (38%) were recorded. Eighteen of 26 patients (69%) maintained stable diseases. There was no dose-response correlation [132]. Vorsetuzumab mafodotin (SGN-75) is a humanized anti-CD70 IgG1 mAb conjugated to MMAF via a non-cleavable linker. However, the development of SGN-75 for NHL was discontinued due to idiopathic thrombocytopenia purpura in two out of 19 NHL patients in a phase I trial (NCT01015911) [133]. It appears that these three anti-CD70 ADCs have major concerns over the toxicity profiles which led to termination of further clinical development.
ADCs under clinical development for multiple myeloma
Anti-CD56 ADC
CD56, also called neural cell adhesion molecule 1 (NCAM1), is a marker for cells of neuroendocrine origin, as well as natural killer cells and a subset of T cells. It is also expressed on 75% of malignant plasma cells but less than 15% of normal plasma cells. These features make it an attractive therapeutic target for multiple myeloma (MM) [134]. Lorvotuzumab mertansine (IMGN901) is a humanized anti-CD56 IgG1 mAb conjugated to DM1 via a stable disulfide linker. IMGN901 retains the ADCC activity [135] (Table 3). Single-agent IMGN901 has been evaluated in patients with CD56-positive MM and small-cell lung cancer. In a dose-escalating phase I trial (NCT00346255) that enrolled 37 patients with R/R MM, there was only a 5.7% ORR with no CR, but 42.9% of patients remained in stable disease for an average duration of 15.5 months. The AE profile was acceptable, with the MTD at 112 mg/m2 [136]. IMGN901 is being studied in combination with lenalidomide and dexamethasone in a phase I trial (NCT00991562) in patients with R/R MM. Among 32 patients, the ORR was 59% with 1 CR [140].
Table 3.
Antibody-drug conjugates in clinical trials for multiple myeloma
| ADC names | Target | Linker | Payload | Disease | Major responses | Status [reference] |
|---|---|---|---|---|---|---|
| Lorvotuzumab mertansine (IMGN901) | CD56 | Disulfide bond (SPP) | DM1 | R/R MM | ORR 5.7%, CR 0; 42.9% stable disease for 15.5 mo | Phase I [136] |
| Milatuzumab doxorubicin (hLL1-DOX) | CD74 | Acid-labile (hydrazine) | Doxorubicin | R/R MM | Data not reported | Phase I [137] |
| Indatuximab ravtansine (BT062) | CD138 | Disulfide bond (SPDB) | DM4 | R/R MM | ORR 5.9%, CR 0, stable disease 61.8%, OS 26.7 mo, PFS 3 mo | Phase I/II [138] |
| GSK2857916 | CD269 (BCMA) | Non-cleavable (MC) | MMAF | R/R MM | ORR 60%, CR 14%, PFS 12 mo, DOR 14.3 mo | Phase I [139] |
ADC antibody-drug conjugate, BCMA B cell maturation antigen, R/R relapsed /refractory, MM multiple myeloma, CR complete remission, DOR duration of response, ORR overall response rate, OS overall survival, PFS progression free survival, Mo month
Anti-CD74 ADC
CD74 is a type II transmembrane glycoprotein involved in major histocompatibility complex class II antigen presentation, B cell maturation, and T cell response. CD74 is expressed in more than 90% of B cell malignancies. It becomes an attractive therapeutic target because of its rapid internalization and recycling [141]. Milatuzumab doxorubicin (hLL1-DOX) is a humanized CD74 mAb conjugated to doxorubicin via an acid-labile hydrazone linker [137]. Unconjugated milatuzumab effectively maintained five out of 19 patients with MM (26%) in stable disease for more than 3 months in a phase I trial (NCT00421525) [142]. hLL1-DOX has been administered to 17 patients with R/R MM in a phase I trial (NCT01101594) which has not reported any updates so far.
Anti-CD138 ADC
CD138, also known as syndecan-1, is a transmembrane protein receptor for extracellular matrix involved in cell-cell adhesion. It is upregulated on malignant plasma cells, as well as various epithelial neoplasms [143]. Indatuximab ravtansine (BT062) is a chimeric B-B4, an afucosylated IgG4 mAb specific for CD138, conjugated to maytansinoid DM4 via a SPDB disulfide cleavable linker. Preclinical studies have demonstrated the efficacy of BT062 against the growth of MM cells in vivo [144]. In a phase I trial (NCT01001442) conducted in 35 patients with R/R MM who have failed previous immunomodulatory drugs and proteasome inhibitor therapies, BT062 was administered at the MTD of 140 mg/m2 on days 1, 8, and 15 of each 28-day cycle. The therapy resulted in a 5.9% ORR with no CR. Then, 61.8% of patients achieved stable diseases. The median OS and PFS were 26.7 and 3 months respectively. Eight-eight percent reported AEs were grade 1–2, with the most common ones being fatigue (47.7%) and diarrhea (43.2%) [138]. The same regimen was investigated in combination with dexamethasone (20–40 mg on days 1, 8, 15, and 22) and lenalidomide (25 mg, daily on days 1–21) in another phase I/IIa trial (NCT01638936). Among 43 evaluable patients who completed at least 2 cycles of treatment, 33 (77%) achieved ORR (no CR) with a median DOR of 21.0 months. Frequently reported AEs were fatigue, diarrhea, and nausea [145].
Anti-CD269 (BCMA) ADC
CD269, also known as B cell maturation antigen (BCMA), is a transmembrane receptor required for B cell maturation. It is universally expressed on malignant plasma cells. Increased plasma level of BCMA is associated with a worse prognosis of MM [146, 147]. GSK2857916 is a humanized anti-BCMA IgG1 mAb conjugated to MMAF via a MC non-cleavable linker. Additionally, GSK2857916 is able to induce enhanced activity of ADCC against MM cells [148]. A multicenter phase I trial (NCT02064387) enrolled 35 adult patients with R/R MM after ASCT, alkylators, proteasome inhibitors, and immunomodulators. At a recommended dose of 3.4 mg/kg, 21 (60%) patients showed ORR with 5 (14%) achieving CR in a recent update. The median PFS and DOR were 12 and 14.3 months respectively. There were mostly grade 1-2 AEs, with thrombocytopenia (35%) being the most common SAE. Corneal events such as blurry vision (52%), dry eyes (37%), and photophobia (29%) were reported [139]. Several phase II trials are currently recruiting to investigate combination regimens incorporating GSK2857916 for patients with R/R MM. One study is exploring GSK2857916 plus pembrolizumab (NCT03848845). Another ongoing study is looking at GSK2857916 administered in combination with dexamethasone plus either lenalidomide or bortezomib (NCT03544281).
Future perspectives
Careful clinical trials are needed for ADCs since several clinical trials of new ADCs have been terminated due to minimal efficacy and unacceptable toxicity. Efforts are being made on identifying better target antigens exclusively expressed on tumor cells to increase the specificity for targeted killing; exploring more potent payloads with high penetration ability and better bystander effect to enhance the antitumor activity; and optimizing the linker and conjugation technology to achieve a homogeneous DAR to enhance drug stability and minimize off-target toxicity. In addition, it is important to discover payloads that can avoid organ-specific toxicity, such as ozogamicin-related hepatic VOD.
A variety of antigen targets used for ADCs, such as CD19, CD22, and BCMA, are also being explored for engineering bispecific antibodies (BiTE) and chimeric antigen receptors (CAR) [149–153]. A CD 19 BiTE, blinatumomab, and two CAR T cell products have been approved for acute lymphoblastic leukemia [154, 155]. It remains to be determined whether any particular target (CD19, CD22, or BCMA) or form of targeted agents (ADC, BiTE, or CAR T) offers better therapeutic index. It is equally intriguing whether these immunotargeted agents can be combined concurrently or consequentially.
Conclusion
Proper selections of antigen targets, linkers, and payloads are critical in ADC designs. Robust antitumor activity and favorable toxicity profiles of ADCs have made them an important modality of cancer therapy. ADCs in combination with chemotherapy regimens and immune checkpoint inhibitors are in clinical trials to further improve the treatment of lymphoid malignancies as well as multiple myeloma.
Acknowledgement
We thank Dr. Juanjuan Zhao for her assistance in preparation of Fig. 1.
Abbreviations
- ADCC
Antibody-dependent cytotoxicity
- Ala
Alanine
- Cit
Citrulline
- CDC
Complement-dependent cytotoxicity
- DLT
Dose-limiting toxicity
- DM1
N20-deacetyl-N20-(3-mercapto-1-oxopropyl)maytansine
- DM4
N20-deacetyl-N20-(4-mercapto-4-methyl-1-oxopentyl)maytansine
- MC
Maleimidocaproyl
- MMAE
Monomethyl auristatin E
- MMAF
Monomethyl auristatin F
- PBD
Pyrrolobenzodiazepines
- PE38
Pseudomonas aeruginosa exotoxin
- SMCC
Succinimidyl-4-(N-maleimidomethyl)cyclohexane-1-carboxylate
- SPDB
N-Succinimidyl-4-(2-pyridylthio)butanoate
- SPP
N-Succinimidyl-4-(2-pyridyldithio)pentanoate
- Val
Valine
Authors’ contributions
DL designed the study. DL and BY drafted the manuscript. All authors participated in the revision of the manuscript. All authors read and approved the final manuscript.
Funding
The study is partly supported by the Affiliated First Hospital of Zhengzhou University, Zhengzhou, China.
Availability of data and materials
The material supporting the conclusion of this review has been included within the article.
Ethics approval and consent to participate
This is not applicable for this review.
Consent for publication
This is not applicable for this review.
Competing interests
The authors have no relevant conflicts.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bo Yu, Email: boyu051988@gmail.com.
Delong Liu, Email: delong_liu@nymc.edu.
References
- 1.Ruan J, Martin P, Shah B, Schuster SJ, Smith SM, Furman RR, Christos P, Rodriguez A, Svoboda J, Lewis J, Katz O, Coleman M, Leonard JP. Lenalidomide plus Rituximab as initial treatment for mantle-cell lymphoma. New England Journal of Medicine. 2015;373(19):1835–1844. doi: 10.1056/NEJMoa1505237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marcus R, Davies A, Ando K, Klapper W, Opat S, Owen C, Phillips E, Sangha R, Schlag R, Seymour JF, Townsend W, Trněný M, Wenger M, Fingerle-Rowson G, Rufibach K, Moore T, Herold M, Hiddemann W. Obinutuzumab for the first-line treatment of follicular lymphoma. New England Journal of Medicine. 2017;377(14):1331–1344. doi: 10.1056/NEJMoa1614598. [DOI] [PubMed] [Google Scholar]
- 3.Chen RW, Palmer JM, Tomassetti S, Popplewell LL, Alluin J, Chomchan P, Nademanee AP, Siddiqi T, Tsai N-C, Chen L, Zuo F, Abary R. Cai J-l, Herrera AF, Rossi JJ, Rosen ST, Forman SJ, Kwak LW, Holmberg LA: Multi-center phase II trial of bortezomib and rituximab maintenance combination therapy in patients with mantle cell lymphoma after consolidative autologous stem cell transplantation. Journal of Hematology & Oncology. 2018;11(1):87. doi: 10.1186/s13045-018-0631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Steiner RE, Romaguera J, Wang M. Current trials for frontline therapy of mantle cell lymphoma. Journal of Hematology & Oncology. 2018;11(1):13. doi: 10.1186/s13045-018-0556-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perini GF, Ribeiro GN, Pinto Neto JV, Campos LT, Hamerschlak N. BCL-2 as therapeutic target for hematological malignancies. Journal of Hematology & Oncology. 2018;11(1):65. doi: 10.1186/s13045-018-0608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Epperla N, Ahn KW, Ahmed S, Jagasia M, DiGilio A, Devine SM, Jaglowski S, Kennedy V, Rezvani AR, Smith SM, Sureda A, Fenske TS, Kharfan-Dabaja MA, Armand P, Hamadani M. Rituximab-containing reduced-intensity conditioning improves progression-free survival following allogeneic transplantation in B cell non-Hodgkin lymphoma. Journal of Hematology & Oncology. 2017;10(1):117. doi: 10.1186/s13045-017-0487-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;11(1):3–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Younes A, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Ramchandren R, Bartlett NL, Cheson BD, de Vos S, Forero-Torres A, Moskowitz CH, Connors JM, Engert A, Larsen EK, Kennedy DA, Sievers EL, Chen R. Results of a pivotal phase II study of brentuximab vedotin for patients with relapsed or refractory Hodgkin's lymphoma. J Clin Oncol. 2012;30(18):2183–2189. doi: 10.1200/JCO.2011.38.0410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yurkiewicz IR, Muffly L, Liedtke M. Inotuzumab ozogamicin: a CD22 mAb-drug conjugate for adult relapsed or refractory B-cell precursor acute lymphoblastic leukemia. Drug Des Devel Ther. 2018;12:2293–2300. doi: 10.2147/DDDT.S150317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeAngelo DJ, Stock W, Stein AS, Shustov A, Liedtke M, Schiffer CA, Vandendries E, Liau K, Ananthakrishnan R, Boni J, Laird AD, Fostvedt L, Kantarjian HM, Advani AS. Inotuzumab ozogamicin in adults with relapsed or refractory CD22-positive acute lymphoblastic leukemia: a phase 1/2 study. Blood Adv. 2017;1(15):1167–1180. doi: 10.1182/bloodadvances.2016001925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aujla A, Aujla R, Liu D. Inotuzumab ozogamicin in clinical development for acute lymphoblastic leukemia and non-Hodgkin lymphoma. Biomarker Research. 2019;7(1):9. doi: 10.1186/s40364-019-0160-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chari RV, Miller ML, Widdison WC. Antibody-drug conjugates: an emerging concept in cancer therapy. Angew Chem Int Ed Engl. 2014;53(15):3796–3827. doi: 10.1002/anie.201307628. [DOI] [PubMed] [Google Scholar]
- 13.Denning C, Pitts JD. Bystander effects of different enzyme-prodrug systems for cancer gene therapy depend on different pathways for intercellular transfer of toxic metabolites, a factor that will govern clinical choice of appropriate regimes. Hum Gene Ther. 1997;8(15):1825–1835. doi: 10.1089/hum.1997.8.15-1825. [DOI] [PubMed] [Google Scholar]
- 14.Kovtun YV, Audette CA, Ye Y, Xie H, Ruberti MF, Phinney SJ, Leece BA, Chittenden T, Blattler WA, Goldmacher VS. Antibody-drug conjugates designed to eradicate tumors with homogeneous and heterogeneous expression of the target antigen. Cancer Res. 2006;66(6):3214–3221. doi: 10.1158/0008-5472.CAN-05-3973. [DOI] [PubMed] [Google Scholar]
- 15.Zhang A, Fang J, Chou RY, Bondarenko PV, Zhang Z. Conformational difference in human IgG2 disulfide isoforms revealed by hydrogen/deuterium exchange mass spectrometry. Biochemistry. 2015;54(10):1956–1962. doi: 10.1021/bi5015216. [DOI] [PubMed] [Google Scholar]
- 16.Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Bioscience Reports. 2015;35(4):e00225. doi: 10.1042/BSR20150089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlothauer T, Herter S, Koller CF, Grau-Richards S, Steinhart V, Spick C, Kubbies M, Klein C, Umana P, Mossner E. Novel human IgG1 and IgG4 Fc-engineered antibodies with completely abolished immune effector functions. Protein Eng Des Sel. 2016;29(10):457–466. doi: 10.1093/protein/gzw040. [DOI] [PubMed] [Google Scholar]
- 18.Wiggins B, Liu-Shin L, Yamaguchi H, Ratnaswamy G. Characterization of cysteine-linked conjugation profiles of immunoglobulin G1 and immunoglobulin G2 antibody-drug conjugates. Journal of Pharmaceutical Sciences. 2015;104(4):1362–1372. doi: 10.1002/jps.24338. [DOI] [PubMed] [Google Scholar]
- 19.Prabhu S, Boswell CA, Leipold D, Khawli LA, Li D, Lu D, Theil FP, Joshi A, Lum BL. Antibody delivery of drugs and radionuclides: factors influencing clinical pharmacology. Ther Deliv. 2011;2(6):769–791. doi: 10.4155/tde.11.41. [DOI] [PubMed] [Google Scholar]
- 20.Abdollahpour-Alitappeh M, Lotfinia M, Gharibi T, Mardaneh J, Farhadihosseinabadi B, Larki P, Faghfourian B, Sepehr KS, Abbaszadeh-Goudarzi K, Abbaszadeh-Goudarzi G, Johari B, Zali MR, Bagheri N. Antibody-drug conjugates (ADCs) for cancer therapy: Strategies, challenges, and successes. J Cell Physiol. 2019;234(5):5628–5642. doi: 10.1002/jcp.27419. [DOI] [PubMed] [Google Scholar]
- 21.Xie H, Adjei AA. Antibody-drug conjugates for the therapy of thoracic malignancies. J Thorac Oncol. 2019;14(3):358–376. doi: 10.1016/j.jtho.2018.11.034. [DOI] [PubMed] [Google Scholar]
- 22.Hamblett KJ, Senter PD, Chace DF, Sun MM, Lenox J, Cerveny CG, Kissler KM, Bernhardt SX, Kopcha AK, Zabinski RF, Meyer DL, Francisco JA. Effects of drug loading on the antitumor activity of a monoclonal antibody drug conjugate. Clin Cancer Res. 2004;10(20):7063–7070. doi: 10.1158/1078-0432.CCR-04-0789. [DOI] [PubMed] [Google Scholar]
- 23.Nittoli T, Kelly MP, Delfino F, Rudge J, Kunz A, Markotan T, Spink J, Chen Z, Shan J, Navarro E, Tait M, Provoncha K, Giurleo J, Zhao F, Jiang X, Hylton D, Makonnen S, Hickey C, Kirshner JR, Thurston G, Papadopoulos N. Antibody drug conjugates of cleavable amino-alkyl and aryl maytansinoids. Bioorg Med Chem. 2018;26(9):2271–2279. doi: 10.1016/j.bmc.2018.02.025. [DOI] [PubMed] [Google Scholar]
- 24.Cunningham D, Parajuli KR, Zhang C, Wang G, Mei J, Zhang Q, Liu S, You Z. Monomethyl Auristatin E Phosphate inhibits human prostate cancer growth. Prostate. 2016;76(15):1420–1430. doi: 10.1002/pros.23226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gebleux R, Stringhini M, Casanova R, Soltermann A, Neri D. Non-internalizing antibody-drug conjugates display potent anti-cancer activity upon proteolytic release of monomethyl auristatin E in the subendothelial extracellular matrix. Int J Cancer. 2017;140(7):1670–1679. doi: 10.1002/ijc.30569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Yu C, Jiang J, Huang C, Yao X, Xu Q, Yu F, Lou L, Fang J. An anti-HER2 antibody conjugated with monomethyl auristatin E is highly effective in HER2-positive human gastric cancer. Cancer Biol Ther. 2016;17(4):346–354. doi: 10.1080/15384047.2016.1139248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sochaj-Gregorczyk AM, Serwotka-Suszczak AM, Otlewski J. A novel affibody-auristatin E conjugate with a potent and selective activity against HER2+ cell lines. J Immunother. 2016;39(6):223–232. doi: 10.1097/CJI.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 28.Sommer A, Kopitz C, Schatz CA, Nising CF, Mahlert C, Lerchen HG, Stelte-Ludwig B, Hammer S, Greven S, Schuhmacher J, Braun M, Zierz R, Wittemer-Rump S, Harrenga A, Dittmer F, Reetz F, Apeler H, Jautelat R, Huynh H, Ziegelbauer K, Kreft B. Preclinical efficacy of the auristatin-based antibody-drug conjugate BAY 1187982 for the Treatment of FGFR2-Positive Solid Tumors. Cancer Res. 2016;76(21):6331–6339. doi: 10.1158/0008-5472.CAN-16-0180. [DOI] [PubMed] [Google Scholar]
- 29.Waight AB, Bargsten K, Doronina S, Steinmetz MO, Sussman D. Prota AE: structural basis of microtubule destabilization by potent auristatin anti-mitotics. PLoS One. 2016;11(8):e0160890. doi: 10.1371/journal.pone.0160890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Woitok M, Klose D, Niesen J, Richter W, Abbas M, Stein C, Fendel R, Bialon M, Puttmann C, Fischer R, Barth S. Kolberg K: the efficient elimination of solid tumor cells by EGFR-specific and HER2-specific scFv-SNAP fusion proteins conjugated to benzylguanine-modified auristatin F. Cancer Lett. 2016;381(2):323–330. doi: 10.1016/j.canlet.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Yoshida S, Tuscano E, Duong C, Chung J, Li Y, Beckett L, Tuscano JM, Satake N. Efficacy of an anti-CD22 antibody-monomethyl auristatin E conjugate in a preclinical xenograft model of precursor B-cell acute lymphoblastic leukemia. Leuk Lymphoma. 2017;58(5):1254–1257. doi: 10.1080/10428194.2016.1235273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vezina HE, Cotreau M, Han TH, Gupta M. Antibody-drug conjugates as cancer therapeutics: past, present, and future. J Clin Pharmacol. 2017;57(Suppl 10):S11–S25. doi: 10.1002/jcph.981. [DOI] [PubMed] [Google Scholar]
- 33.Jackson PJM, Kay S, Pysz I, Thurston DE. Use of pyrrolobenzodiazepines and related covalent-binding DNA-interactive molecules as ADC payloads: is mechanism related to systemic toxicity? Drug Discov Today Technol. 2018;30:71–83. doi: 10.1016/j.ddtec.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Wang J. Precision therapy for acute myeloid leukemia. Journal of Hematology & Oncology. 2018;11(1):3. doi: 10.1186/s13045-017-0543-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saygin C, Carraway HE. Emerging therapies for acute myeloid leukemia. Journal of Hematology & Oncology. 2017;10(1):93. doi: 10.1186/s13045-017-0463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wynne J, Wright D, Stock W. Inotuzumab: from preclinical development to success in B-cell acute lymphoblastic leukemia. Blood Adv. 2019;3(1):96–104. doi: 10.1182/bloodadvances.2018026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsuchikama K, An Z. Antibody-drug conjugates: recent advances in conjugation and linker chemistries. Protein Cell. 2018;9(1):33–46. doi: 10.1007/s13238-016-0323-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dubowchik GM, Firestone RA, Padilla L, Willner D, Hofstead SJ, Mosure K, Knipe JO, Lasch SJ, Trail PA. Cathepsin B-labile dipeptide linkers for lysosomal release of doxorubicin from internalizing immunoconjugates: model studies of enzymatic drug release and antigen-specific in vitro anticancer activity. Bioconjug Chem. 2002;13(4):855–869. doi: 10.1021/bc025536j. [DOI] [PubMed] [Google Scholar]
- 39.Saito G, Swanson JA, Lee KD. Drug delivery strategy utilizing conjugation via reversible disulfide linkages: role and site of cellular reducing activities. Adv Drug Deliv Rev. 2003;55(2):199–215. doi: 10.1016/S0169-409X(02)00179-5. [DOI] [PubMed] [Google Scholar]
- 40.Lu Jun, Jiang Feng, Lu Aiping, Zhang Ge. Linkers Having a Crucial Role in Antibody–Drug Conjugates. International Journal of Molecular Sciences. 2016;17(4):561. doi: 10.3390/ijms17040561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lazar AC, Wang LT, Blattler WA, Amphlett G, Lambert JM, Zhang W. Analysis of the composition of immunoconjugates using size-exclusion chromatography coupled to mass spectrometry. Rapid Communications in Mass Spectrometry. 2005;19(13):1806–1814. doi: 10.1002/rcm.1987. [DOI] [PubMed] [Google Scholar]
- 42.Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, Chen Y, Simpson M, Tsai SP, Dennis MS, Lu Y, Meng YG, Ng C, Yang J, Lee CC, Duenas E, Gorrell J, Katta V, Kim A, McDorman K, Flagella K, Venook R, Ross S, Spencer SD, Lee Wong W, Lowman HB, Vandlen R, Sliwkowski MX, Scheller RH, Polakis P, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26(8):925–932. doi: 10.1038/nbt.1480. [DOI] [PubMed] [Google Scholar]
- 43.Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, Halder R, Forsyth JS, Santidrian AF, Stafin K, Lu YC, Tran H, Seller AJ, Biroce SL, Szydlik A, Pinkstaff JK, Tian F, Sinha SC, Felding-Habermann B, Smider VV, Schultz PG. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(40):16101–16106. doi: 10.1073/pnas.1211023109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zimmerman ES, Heibeck TH, Gill A, Li X, Murray CJ, Madlansacay MR, Tran C, Uter NT, Yin G, Rivers PJ, Yam AY, Wang WD, Steiner AR, Bajad SU, Penta K, Yang W, Hallam TJ, Thanos CD, Sato AK. Production of site-specific antibody-drug conjugates using optimized non-natural amino acids in a cell-free expression system. Bioconjug Chem. 2014;25(2):351–361. doi: 10.1021/bc400490z. [DOI] [PubMed] [Google Scholar]
- 45.Antos JM, Ingram J, Fang T, Pishesha N, Truttmann MC, Ploegh HL. Site-Specific Protein Labeling via Sortase-Mediated Transpeptidation. Curr Protoc Protein Sci. 2017;89:1531–15319. doi: 10.1002/cpps.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jeger S, Zimmermann K, Blanc A, Grunberg J, Honer M, Hunziker P, Struthers H, Schibli R. Site-specific and stoichiometric modification of antibodies by bacterial transglutaminase. Angew Chem Int Ed Engl. 2010;49(51):9995–9997. doi: 10.1002/anie.201004243. [DOI] [PubMed] [Google Scholar]
- 47.Zhou Q, Stefano JE, Manning C, Kyazike J, Chen B, Gianolio DA, Park A, Busch M, Bird J, Zheng XY, Simonds-Mannes H, Kim J, Gregory RC, Miller RJ, Brondyk WH. Dhal PK. Pan CQ: Site-specific Antibody-drug conjugation through glycoengineering. bioconjugate chemistry. 2014;25(3):510–520. doi: 10.1021/bc400505q. [DOI] [PubMed] [Google Scholar]
- 48.Sutherland MS, Sanderson RJ, Gordon KA, Andreyka J, Cerveny CG, Yu C, Lewis TS, Meyer DL, Zabinski RF, Doronina SO, Senter PD, Law CL, Wahl AF. Lysosomal trafficking and cysteine protease metabolism confer target-specific cytotoxicity by peptide-linked anti-CD30-auristatin conjugates. J Biol Chem. 2006;281(15):10540–10547. doi: 10.1074/jbc.M510026200. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Nowakowski GS, Wang ML, Ansell SM. Advances in CD30- and PD-1-targeted therapies for classical Hodgkin lymphoma. Journal of Hematology & Oncology. 2018;11(1):57. doi: 10.1186/s13045-018-0601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schirrmann T, Steinwand M, Wezler X, Ten Haaf A, Tur MK, Barth S. CD30 as a therapeutic target for lymphoma. BioDrugs. 2014;28(2):181–209. doi: 10.1007/s40259-013-0068-8. [DOI] [PubMed] [Google Scholar]
- 51.Connors JM, Jurczak W, Straus DJ, Ansell SM, Kim WS, Gallamini A, Younes A, Alekseev S, Illes A, Picardi M, Lech-Maranda E, Oki Y, Feldman T, Smolewski P, Savage KJ, Bartlett NL, Walewski J, Chen R, Ramchandren R, Zinzani PL, Cunningham D, Rosta A, Josephson NC, Song E, Sachs J, Liu R, Jolin HA, Huebner D, Radford J, Group E-S Brentuximab vedotin with chemotherapy for stage III or IV Hodgkin's lymphoma. N Engl J Med. 2018;378(4):331–344. doi: 10.1056/NEJMoa1708984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, Forero-Torres A. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. New England Journal of Medicine. 2010;363(19):1812–1821. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 53.Gopal AK, Chen R, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Chi X, Sievers EL, Younes A. Durable remissions in a pivotal phase 2 study of brentuximab vedotin in relapsed or refractory Hodgkin lymphoma. Blood. 2015;125(8):1236–1243. doi: 10.1182/blood-2014-08-595801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen R, Gopal AK, Smith SE, Ansell SM, Rosenblatt JD, Savage KJ, Connors JM, Engert A, Larsen EK, Huebner D, Fong A, Younes A. Five-year survival and durability results of brentuximab vedotin in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2016;128(12):1562–1566. doi: 10.1182/blood-2016-02-699850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chen RW, Chen L, Herrera AF, Mei M, McBride K, Abary R, Siddiqi T, Popplewell L, Forman SJ, Rosen ST, Kwak LW. Phase 1 Study of MDR1 inhibitor plus brentuximab vedotin in relapsed/refractory Hodgkin lymphoma. Blood. 2018;132(Suppl 1):1636. [Google Scholar]
- 56.O'Connor OA, Lue JK, Sawas A, Amengual JE, Deng C, Kalac M, Falchi L, Marchi E, Turenne I, Lichtenstein R, Rojas C, Francescone M, Schwartz L, Cheng B, Savage KJ, Villa D, Crump M, Prica A, Kukreti V, Cremers S, Connors JM, Kuruvilla J. Brentuximab vedotin plus bendamustine in relapsed or refractory Hodgkin's lymphoma: an international, multicentre, single-arm, phase 1-2 trial. Lancet Oncol. 2018;19(2):257–266. doi: 10.1016/S1470-2045(17)30912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.LaCasce AS, Bociek RG, Sawas A, Caimi P, Agura E, Matous J, Ansell SM, Crosswell HE, Islas-Ohlmayer M, Behler C, Cheung E, Forero-Torres A, Vose J, O'Connor OA, Josephson N, Wang Y, Advani R. Brentuximab vedotin plus bendamustine: a highly active first salvage regimen for relapsed or refractory Hodgkin lymphoma. Blood. 2018;132(1):40–48. doi: 10.1182/blood-2017-11-815183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sawas A, Kuruvilla J, Lue JK, Deng CC, Amengual JE, Montanari F, Savage KJ, Elgedawe H, Villa D, Crump M, Connors JM, O'Connor OA: Prolonged overall survival (OS) in a subset of responders to the combination of brentuximab vedotin (Bv) and bendamustine (B) in heavily treated patients with relapsed or refractory Hodgkin lymphoma (HL): results of an international multi- center phase I/II experience. Blood 2018, 132:2908.
- 59.Herrera AF, Moskowitz AJ, Bartlett NL, Vose JM, Ramchandren R, Feldman TA, LaCasce AS, Ansell SM, Moskowitz CH, Fenton K, Ogden CA, Taft D, Zhang Q, Kato K, Campbell M, Advani RH. Interim results of brentuximab vedotin in combination with nivolumab in patients with relapsed or refractory Hodgkin lymphoma. Blood. 2018;131(11):1183–1194. doi: 10.1182/blood-2017-10-811224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Gianni AM, Carella A, Osmanov D, Bachanova V, Sweetenham J, Sureda A, Huebner D, Sievers EL, Chi A, Larsen EK, Hunder NN, Walewski J, Group AS Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–1862. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 61.Moskowitz CH, Walewski J, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, Chen AI, Stiff P, Viviani S, Bachanova V, Sureda A, McClendon T, Lee C, Lisano J, Sweetenham J. Five-year PFS from the AETHERA trial of brentuximab vedotin for Hodgkin lymphoma at high risk of progression or relapse. Blood. 2018;132(25):2639–2642. doi: 10.1182/blood-2018-07-861641. [DOI] [PubMed] [Google Scholar]
- 62.Gaudio F, Mazza P, Mele A, Palazzo G, Carella AM, Delia M, Pisapia G, Pastore D, Cascavilla N, Pavone V, Specchia G. Brentuximab vedotin prior to allogeneic stem cell transplantation increases survival in chemorefractory Hodgkin's lymphoma patients. Ann Hematol. 2019;98(6):1449–1455. doi: 10.1007/s00277-019-03662-6. [DOI] [PubMed] [Google Scholar]
- 63.Connors JM, Younes A, Gallamini A, et al. Brentuximab vedotin plus chemotherapy in patients with advanced-stage classical hodgkin lymphoma: evaluation of modified progression-free survival and traditional PFS in the phase 3 ECHELON-1 study. Clinical Advances in Hematology & Oncology. 2019;17(2):19–20. [Google Scholar]
- 64.Eichenauer DA, Plutschow A, Kreissl S, Sokler M, Hellmuth JC, Meissner J, Mathas S, Topp MS, Behringer K, Klapper W, Kuhnert G, Dietlein M, Kobe C, Fuchs M, Diehl V, Engert A, Borchmann P. Incorporation of brentuximab vedotin into first-line treatment of advanced classical Hodgkin's lymphoma: final analysis of a phase 2 randomised trial by the German Hodgkin Study Group. Lancet Oncology. 2017;18(12):1680–1687. doi: 10.1016/S1470-2045(17)30696-4. [DOI] [PubMed] [Google Scholar]
- 65.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Yang Y, Sievers EL, Kennedy DA, Shustov A. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012;30(18):2190–2196. doi: 10.1200/JCO.2011.38.0402. [DOI] [PubMed] [Google Scholar]
- 66.Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, Matous J, Ramchandren R, Fanale M, Connors JM, Fenton K, Huebner D, Pinelli JM, Kennedy DA, Shustov A. Five-year results of brentuximab vedotin in patients with relapsed or refractory systemic anaplastic large cell lymphoma. Blood. 2017;130(25):2709–2717. doi: 10.1182/blood-2017-05-780049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Horwitz S, O'Connor OA, Pro B, Illidge T, Fanale M, Advani R, Bartlett NL, Christensen JH, Morschhauser F, Domingo-Domenech E, Rossi G, Kim WS, Feldman T, Lennard A, Belada D, Illes A, Tobinai K, Tsukasaki K, Yeh SP, Shustov A, Huttmann A, Savage KJ, Yuen S, Iyer S, Zinzani PL, Hua Z, Little M, Rao S, Woolery J, Manley T et al: Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. Lancet 2019, 393(10168):229-240. [DOI] [PMC free article] [PubMed]
- 68.Prince H Miles, Kim Youn H, Horwitz Steven M, Dummer Reinhard, Scarisbrick Julia, Quaglino Pietro, Zinzani Pier Luigi, Wolter Pascal, Sanches Jose A, Ortiz-Romero Pablo L, Akilov Oleg E, Geskin Larisa, Trotman Judith, Taylor Kerry, Dalle Stephane, Weichenthal Michael, Walewski Jan, Fisher David, Dréno Brigitte, Stadler Rudolf, Feldman Tatyana, Kuzel Timothy M, Wang Yinghui, Palanca-Wessels Maria Corinna, Zagadailov Erin, Trepicchio William L, Zhang Wenwen, Lin Hui-Min, Liu Yi, Huebner Dirk, Little Meredith, Whittaker Sean, Duvic Madeleine, Trotman Judith, Joske David, Prince H. Miles, Taylor Kerry, Lewis Ian D., Jonak Constanze, Trautinger Franz, Bechter Oliver, Wolter Pascal, Bron Dominique, de Lima Vladmir Claudio C., Sanches Jose Antonio, Klasa Richard, Bagot Martine, Beylot-Barry Marie, Dalle Stephane, D'Incan Michel, Dreno Brigitte, Grange Florent, Nicolay Jan, Stadler Rudolf, Weichenthal Michael, Wobser Marion, Assaf Chalid, Loquai Carmen, Quaglino Pietro, Spina Michele, Zinzani Pier Luigi, Bosi Alberto, Fattori Pier Paolo, Grzanka Aleksandra, Walewski Jan, Lopez-Hernandez Andres, Ortiz-Romero Pablo L., Roca Jose Juan Rifon, Canales Silvana Novelli, Dummer Reinhard, Illidge Timothy, Johnson Rod, Whittaker Sean, Morris Stephen, McKay Pam, Scarisbrick Julia, Duvic Madeleine, Feldman Tatyana, Akilov Oleg, Geskin Larisa, Horwitz Steve, Kim Youn H., Pro Barbara, Kuzel Timothy, Lerner Adam, Eradat Herbert, Sokol Lubomir, Fisher David C., Hughey Sarah. Brentuximab vedotin or physician's choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. The Lancet. 2017;390(10094):555–566. doi: 10.1016/S0140-6736(17)31266-7. [DOI] [PubMed] [Google Scholar]
- 69.Jacobsen ED, Sharman JP, Oki Y, Advani RH, Winter JN, Bello CM, Spitzer G, Palanca-Wessels MC, Kennedy DA, Levine P, Yang J, Bartlett NL. Brentuximab vedotin demonstrates objective responses in a phase 2 study of relapsed/refractory DLBCL with variable CD30 expression. Blood. 2015;125(9):1394–1402. doi: 10.1182/blood-2014-09-598763. [DOI] [PubMed] [Google Scholar]
- 70.Zinzani PL, Pellegrini C, Chiappella A, Di Rocco A, Salvi F, Cabras MG, Argnani L, Stefoni V. Brentuximab vedotin in relapsed primary mediastinal large B-cell lymphoma: results from a phase 2 clinical trial. Blood. 2017;129(16):2328–2330. doi: 10.1182/blood-2017-01-764258. [DOI] [PubMed] [Google Scholar]
- 71.Thota S, Advani A. Inotuzumab ozogamicin in relapsed B-cell acute lymphoblastic leukemia. Eur J Haematol. 2017;98(5):425–434. doi: 10.1111/ejh.12862. [DOI] [PubMed] [Google Scholar]
- 72.Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A, Sabattini E, Visani G, Pileri SA. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52(2):325–327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 73.Shor Boris, Gerber Hans-Peter, Sapra Puja. Preclinical and clinical development of inotuzumab-ozogamicin in hematological malignancies. Molecular Immunology. 2015;67(2):107–116. doi: 10.1016/j.molimm.2014.09.014. [DOI] [PubMed] [Google Scholar]
- 74.Kantarjian HM, DeAngelo DJ, Stelljes M, Martinelli G, Liedtke M, Stock W, Gokbuget N, O'Brien S, Wang K, Wang T, Paccagnella ML, Sleight B, Vandendries E, Advani AS. Inotuzumab Ozogamicin versus Standard Therapy for Acute Lymphoblastic Leukemia. N Engl J Med. 2016;375(8):740–753. doi: 10.1056/NEJMoa1509277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kantarjian HM, DeAngelo DJ, Stelljes M, Liedtke M, Stock W, Gokbuget N, O'Brien SM, Jabbour E, Wang T, Liang White J, Sleight B, Vandendries E, Advani AS. Inotuzumab ozogamicin versus standard of care in relapsed or refractory acute lymphoblastic leukemia: final report and long-term survival follow-up from the randomized, phase 3 INO-VATE study. Cancer. 2019. [DOI] [PMC free article] [PubMed]
- 76.Kantarjian HM, Su Y, Jabbour EJ, Bhattacharyya H, Yan E, Cappelleri JC, Marks DI. Patient-reported outcomes from a phase 3 randomized controlled trial of inotuzumab ozogamicin versus standard therapy for relapsed/refractory acute lymphoblastic leukemia. Cancer. 2018;124(10):2151–2160. doi: 10.1002/cncr.31317. [DOI] [PubMed] [Google Scholar]
- 77.Kantarjian HM, DeAngelo DJ, Advani AS, Stelljes M, Kebriaei P, Cassaday RD, Merchant AA, Fujishima N, Uchida T, Calbacho M, Ejduk AA, O'Brien SM, Jabbour EJ, Zhang H, Sleight BJ, Vandendries ER, Marks DI. Hepatic adverse event profile of inotuzumab ozogamicin in adult patients with relapsed or refractory acute lymphoblastic leukaemia: results from the open-label, randomised, phase 3 INO-VATE study. Lancet Haematol. 2017;4(8):e387–e398. doi: 10.1016/S2352-3026(17)30103-5. [DOI] [PubMed] [Google Scholar]
- 78.Kebriaei P, Cutler C, de Lima M, Giralt S, Lee SJ, Marks D, Merchant A, Stock W, van Besien K, Stelljes M. Management of important adverse events associated with inotuzumab ozogamicin: expert panel review. Bone Marrow Transplant. 2018;53(4):449–456. doi: 10.1038/s41409-017-0019-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M, York S, Ravandi F, Garris R, Kwari M, Faderl S, Cortes J, Champlin R, O'Brien S. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu D, Zhao J, Song Y, Luo X, Yang T. Clinical trial update on bispecific antibodies, antibody-drug conjugates, and antibody-containing regimens for acute lymphoblastic leukemia. Journal of Hematology & Oncology. 2019;12(1):15. doi: 10.1186/s13045-019-0703-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Jabbour E, Ravandi F, Kebriaei P, Huang X, Short NJ, Thomas D, Sasaki K, Rytting M, Jain N, Konopleva M, Garcia-Manero G, Champlin R, Marin D, Kadia T, Cortes J, Estrov Z, Takahashi K, Patel Y, Khouri MR, Jacob J, Garris R, O'Brien S, Kantarjian H. Salvage chemoimmunotherapy with inotuzumab ozogamicin combined with mini-hyper-CVD for patients with relapsed or refractory philadelphia chromosome-negative acute lymphoblastic leukemia: a phase 2 clinical trial. JAMA Oncol. 2018;4(2):230–234. doi: 10.1001/jamaoncol.2017.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kantarjian H, Ravandi F, Short NJ, Huang X, Jain N, Sasaki K, Daver N, Pemmaraju N, Khoury JD, Jorgensen J, Alvarado Y, Konopleva M, Garcia-Manero G, Kadia T, Yilmaz M, Bortakhur G, Burger J, Kornblau S, Wierda W, DiNardo C, Ferrajoli A, Jacob J, Garris R, O'Brien S, Jabbour E. Inotuzumab ozogamicin in combination with low-intensity chemotherapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukaemia: a single-arm, phase 2 study. Lancet Oncol. 2018;19(2):240–248. doi: 10.1016/S1470-2045(18)30011-1. [DOI] [PubMed] [Google Scholar]
- 83.Kantarjian H, Stein A, Gökbuget N, Fielding AK, Schuh AC, Ribera J-M, Wei A, Dombret H, Foà R, Bassan R, Arslan Ö, Sanz MA, Bergeron J, Demirkan F, Lech-Maranda E, Rambaldi A, Thomas X, Horst H-A, Brüggemann M, Klapper W, Wood BL, Fleishman A, Nagorsen D, Holland C, Zimmerman Z, Topp MS. Blinatumomab versus chemotherapy for advanced acute lymphoblastic leukemia. New England Journal of Medicine. 2017;376(9):836–847. doi: 10.1056/NEJMoa1609783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nägele V, Kratzer A, Zugmaier G, Holland C, Hijazi Y, Topp MS, Gökbuget N, Baeuerle PA, Kufer P, Wolf A, Klinger M. Changes in clinical laboratory parameters and pharmacodynamic markers in response to blinatumomab treatment of patients with relapsed/refractory ALL. Experimental Hematology & Oncology. 2017;6(1):14. doi: 10.1186/s40164-017-0074-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Topp MS, Gokbuget N, Stein AS, Zugmaier G, O'Brien S, Bargou RC, Dombret H, Fielding AK, Heffner L, Larson RA, Neumann S, Foa R, Litzow M, Ribera JM, Rambaldi A, Schiller G, Bruggemann M, Horst HA, Holland C, Jia C, Maniar T, Huber B, Nagorsen D, Forman SJ, Kantarjian HM. Safety and activity of blinatumomab for adult patients with relapsed or refractory B-precursor acute lymphoblastic leukaemia: a multicentre, single-arm, phase 2 study. Lancet Oncol. 2014;16(1):57–66. doi: 10.1016/S1470-2045(14)71170-2. [DOI] [PubMed] [Google Scholar]
- 86.Zhang X, Yang Y, Fan D, Xiong D. The development of bispecific antibodies and their applications in tumor immune escape. Experimental Hematology & Oncology. 2017;6(1):12. doi: 10.1186/s40164-017-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Short NJ, Jabbour EJ, Ravandi F, Huang XL, Jain N, Sasaki K, Pemmaraju N, Daver NG, Khoury JD, Jorgensen JL, Alvarado Y, Konopleva MY, Garcia-Manero G, Kadia TM, Yilmaz M, Borthakur G, Burger JA, Kornblau SM, Wierda WG, DiNardo CD, Ferrajoli A, Nasnas P, Jacob J, Garris RE, O'Brien SM, Kantarjian HM: Chemoimmunotherapy with Inotuzumab Ozogamicin Combined with Mini-Hyper-CVD, with or without Blinatumomab, for newly diagnosed older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: results from a phase II study. Blood 2018, 132(Suppl 1):36.
- 88.Jabbour EJ, Sasaki K, Ravandi F, Short NJ, Garcia-Manero G, Daver N, Kadia T, Konopleva M, Jain N, Cortes J, Issa GC, Jacob J, Kwari M, Thompson P, Garris R, Pemmaraju N, Yilmaz M, O'Brien SM, Kantarjian HM: Inotuzumab ozogamicin in combination with low-intensity chemotherapy (mini-HCVD) with or without blinatumomab versus standard intensive chemotherapy (HCVAD) as frontline therapy for older patients with Philadelphia chromosome-negative acute lymphoblastic leukemia: a propensity score analysis. Cancer 2019, 125:10.1002/cncr.32139. [DOI] [PMC free article] [PubMed]
- 89.Goy A, Forero A, Wagner-Johnston N, Christopher Ehmann W, Tsai M, Hatake K, Ananthakrishnan R, Volkert A, Vandendries E, Ogura M. A phase 2 study of inotuzumab ozogamicin in patients with indolent B-cell non-Hodgkin lymphoma refractory to rituximab alone, rituximab and chemotherapy, or radioimmunotherapy. Br J Haematol. 2016;174(4):571–581. doi: 10.1111/bjh.14094. [DOI] [PubMed] [Google Scholar]
- 90.Fayad L, Offner F, Smith MR, Verhoef G, Johnson P, Kaufman JL, Rohatiner A, Advani A, Foran J, Hess G, Coiffier B, Czuczman M, Gine E, Durrant S, Kneissl M, Luu KT, Hua SY, Boni J, Vandendries E, Dang NH. Safety and clinical activity of a combination therapy comprising two antibody-based targeting agents for the treatment of non-Hodgkin lymphoma: results of a phase I/II study evaluating the immunoconjugate inotuzumab ozogamicin with rituximab. J Clin Oncol. 2013;31(5):573–583. doi: 10.1200/JCO.2012.42.7211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wagner-Johnston ND, Goy A, Rodriguez MA, Ehmann WC, Hamlin PA, Radford J, Thieblemont C, Suh C, Sweetenham J, Huang Y, Sullivan ST, Vandendries ER, Gisselbrecht C. A phase 2 study of inotuzumab ozogamicin and rituximab, followed by autologous stem cell transplant in patients with relapsed/refractory diffuse large B-cell lymphoma. Leuk Lymphoma. 2015;56(10):2863–2869. doi: 10.3109/10428194.2015.1017821. [DOI] [PubMed] [Google Scholar]
- 92.Dang NH, Ogura M, Castaigne S, Fayad LE, Jerkeman M, Radford J, Pezzutto A, Bondarenko I, Stewart DA, Shnaidman M, Sullivan S, Vandendries E, Tobinai K, Ramchandren R, Hamlin PA, Gine E, Ando K. Randomized, phase 3 trial of inotuzumab ozogamicin plus rituximab versus chemotherapy plus rituximab for relapsed/refractory aggressive B-cell non-Hodgkin lymphoma. Br J Haematol. 2018;182(4):583–586. doi: 10.1111/bjh.14820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ogura M, Tobinai K, Hatake K, Davies A, Crump M, Ananthakrishnan R, Ishibashi T, Paccagnella ML, Boni J, Vandendries E, MacDonald D. Phase I study of inotuzumab ozogamicin combined with R-CVP for relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2016;22(19):4807–4816. doi: 10.1158/1078-0432.CCR-15-2488. [DOI] [PubMed] [Google Scholar]
- 94.Sangha R, Davies A, Dang NH, Ogura M, MacDonald DA, Ananthakrishnan R, Paccagnella ML, Vandendries E, Boni J, Goh YT. Phase 1 study of inotuzumab ozogamicin combined with R-GDP for the treatment of patients with relapsed/refractory CD22+ B-cell non-Hodgkin lymphoma. J Drug Assess. 2017;6(1):10–17. doi: 10.1080/21556660.2017.1315336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alderson RF, Kreitman RJ, Chen T, Yeung P, Herbst R, Fox JA, Pastan I. CAT-8015: a second-generation pseudomonas exotoxin A-based immunotherapy targeting CD22-expressing hematologic malignancies. Clin Cancer Res. 2009;15(3):832–839. doi: 10.1158/1078-0432.CCR-08-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kreitman RJ, Pastan I. Antibody fusion proteins: anti-CD22 recombinant immunotoxin moxetumomab pasudotox. Clin Cancer Res. 2011;17(20):6398–6405. doi: 10.1158/1078-0432.CCR-11-0487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kreitman RJ, Dearden C, Zinzani PL, Delgado J, Karlin L, Robak T, Gladstone DE, le Coutre P, Dietrich S, Gotic M, Larratt L, Offner F, Schiller G, Swords R, Bacon L, Bocchia M, Bouabdallah K, Breems DA, Cortelezzi A, Dinner S, Doubek M, Gjertsen BT, Gobbi M, Hellmann A, Lepretre S, Maloisel F, Ravandi F, Rousselot P, Rummel M, Siddiqi T, et al. Moxetumomab pasudotox in relapsed/refractory hairy cell leukemia. Leukemia. 2018;32(8):1768–1777. doi: 10.1038/s41375-018-0210-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, FitzGerald DJ, Santiago L, Gao G, Lanasa MC, Pastan I. Minimal residual hairy cell leukemia eradication with moxetumomab pasudotox: phase 1 results and long-term follow-up. Blood. 2018;131(21):2331–2334. doi: 10.1182/blood-2017-09-803072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dhillon S. Moxetumomab Pasudotox: First Global Approval. Drugs. 2018;78(16):1763–1767. doi: 10.1007/s40265-018-1000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Fichtner M, Dreyling M, Binder M, Trepel M: The role of B cell antigen receptors in mantle cell lymphoma. Journal of Hematology & Oncology 2017, 10(1):164. [DOI] [PMC free article] [PubMed]
- 101.Pfeifer M, Zheng B, Erdmann T, Koeppen H, McCord R, Grau M, Staiger A, Chai A, Sandmann T, Madle H, Dorken B, Chu YW, Chen AI, Lebovic D, Salles GA, Czuczman MS, Palanca-Wessels MC, Press OW, Advani R, Morschhauser F, Cheson BD, Lenz P, Ott G, Polson AG, Mundt KE, Lenz G. Anti-CD22 and anti-CD79B antibody drug conjugates are active in different molecular diffuse large B-cell lymphoma subtypes. Leukemia. 2015;29(7):1578–1586. doi: 10.1038/leu.2015.48. [DOI] [PubMed] [Google Scholar]
- 102.Palanca-Wessels MC, Czuczman M, Salles G, Assouline S, Sehn LH, Flinn I, Patel MR, Sangha R, Hagenbeek A, Advani R, Tilly H, Casasnovas O, Press OW, Yalamanchili S, Kahn R, Dere RC, Lu D, Jones S, Jones C, Chu YW, Morschhauser F. Safety and activity of the anti-CD79B antibody-drug conjugate polatuzumab vedotin in relapsed or refractory B-cell non-Hodgkin lymphoma and chronic lymphocytic leukaemia: a phase 1 study. Lancet Oncol. 2015;16(6):704–715. doi: 10.1016/S1470-2045(15)70128-2. [DOI] [PubMed] [Google Scholar]
- 103.Morschhauser F, Flinn IW, Advani R, Sehn LH, Diefenbach C, Kolibaba K, Press OW, Salles G, Tilly H, Chen AI, Assouline S, Cheson BD, Dreyling M, Hagenbeek A, Zinzani PL, Jones S, Cheng J, Lu D, Penuel E, Hirata J, Wenger M, Chu YW, Sharman J. Polatuzumab vedotin or pinatuzumab vedotin plus rituximab in patients with relapsed or refractory non-Hodgkin lymphoma: final results from a phase 2 randomised study (ROMULUS) Lancet Haematol. 2019;6(5):e254–e265. doi: 10.1016/S2352-3026(19)30026-2. [DOI] [PubMed] [Google Scholar]
- 104.Sehn LH, Herrera AF, Matasar MJ, Kamdar M, Assouline S, Hertzberg M, Kim TM, Kim WS, McMillan A, Ozcan M, Hirata JM, Penuel E, Cheng J, Ku G, Flowers CR. Polatuzumab vedotin (Pola) plus bendamustine (B) with rituximab (R) or obinutuzumab (G) in Relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL): updated results of a phase (Ph) Ib/II study. Blood. 2018;132(Suppl 1):1683. [Google Scholar]
- 105.Forero-Torres A, Kolibaba KS, Tilly H, Salles G, Wang LJ, Lee C, Sharman JP. Polatuzumab vedotin combined with obinutuzumab, cyclophosphamide, doxorubicin, and prednisone (G-CHP) for patients with previously untreated diffuse large B-cell lymphoma (DLBCL): updated results of a phase Ib/II study. Blood. 2017;130:4120. [Google Scholar]
- 106.Tilly H, Sharman J, Bartlett N, Morschhauser F, Haioun C, Munoz J, Chen A, Lamy T, Wang L, Penuel E, Hirata J, Lee C, Salles G. Pola-R-Chp: Polatuzumab Vedotin Combined with Rituximab, Cyclophosphamide, doxorubicin, prednisone for patients with previously untreated diffuse large B-cell lymphoma. Haematologica. 2017;102:4–4. doi: 10.3324/haematol.2016.155267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Scheuermann RH, Racila E. CD19 antigen in leukemia and lymphoma diagnosis and immunotherapy. Leuk Lymphoma. 1995;18(5-6):385–397. doi: 10.3109/10428199509059636. [DOI] [PubMed] [Google Scholar]
- 108.Hong EE, Erickson H, Lutz RJ, Whiteman KR, Jones G, Kovtun Y, Blanc V, Lambert JM. Design of coltuximab ravtansine, a CD19-targeting antibody-drug conjugate (ADC) for the treatment of B-cell malignancies: structure-activity relationships and preclinical evaluation. Mol Pharm. 2015;12(6):1703–1716. doi: 10.1021/acs.molpharmaceut.5b00175. [DOI] [PubMed] [Google Scholar]
- 109.Trnĕný Marek, Verhoef Gregor, Dyer Martin JS, Ben Yehuda Dina, Patti Caterina, Canales Miguel, Lopez Andrés, Awan Farrukh T, Montgomery Paul G, Janikova Andrea, Barbui Anna M, Sulek Kazimierz, Terol Maria J, Radford John, Guidetti Anna, Di Nicola Massimo, Siraudin Laure, Hatteville Laurence, Schwab Sandrine, Oprea Corina, Gianni Alessandro M. A phase II multicenter study of the anti-CD19 antibody drug conjugate coltuximab ravtansine (SAR3419) in patients with relapsed or refractory diffuse large B-cell lymphoma previously treated with rituximab-based immunotherapy. Haematologica. 2018;103(8):1351–1358. doi: 10.3324/haematol.2017.168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fathi AT, Borate U, DeAngelo DJ, O'Brien MM, Trippett T, Shah BD, Hale GA, Foran JM, Silverman LB, Tibes R, Cramer S, Pauly M, Kim S, Kostic A, Huang XH, Pan Y, Chen R. A phase 1 study of denintuzumab mafodotin (SGN-CD19A) in adults with relapsed or refractory B-lineage acute leukemia (B-ALL) and highly aggressive lymphoma. Blood. 2015;126(23):1328. [Google Scholar]
- 111.Moskowitz CH, Fanale MA, Shah BD, Advani RH, Chen R, Kim S, Kostic A, Liu TN, Peng J, Forero-Torres A. A phase 1 study of denintuzumab mafodotin (SGN-CD19A) in relapsed/refactory B-lineage non-Hodgkin lymphoma. Blood. 2015;126(23):182. [Google Scholar]
- 112.Jain N, Klisovic RB, Stock W, Ungar D, Zeidan AM, Atallah E, McCloskey J, Heffner L, Tomlinson B, Kantarjian HM, He S, Boni J, Wieduwilt MJ. Interim data from a phase 1 study evaluating pyrrolobenzodiazepine-based antibody drug conjugate ADCT-402 (Loncastuximab Tesirine) targeting CD19 for relapsed or refractory B-cell acute lymphoblastic leukemia. Blood. 2017;130:1321. doi: 10.1182/blood-2017-03-771576. [DOI] [Google Scholar]
- 113.Radford J, Kahl BS, Hamadani M, Carlo-Stella C, Caimi P, Ardeshna KM, Feingold J, He S, Reid E, Solh M, Chung KY, Heffner L, Ungar D, O'Connor OA. Interim results from the first-in-human clinical trial of Adct-402 (Loncastuximab Tesirine), a novel pyrrolobenzodiazepine-based antibody drug conjugate, in relapsed/refractory diffuse large B-cell lymphoma. Blood. 2018;132:398. [Google Scholar]
- 114.Caimi P, Kahl BS, Hamadani M, Carlo-Stella C, He S, Ungar D, Feingold J, Ardeshna KM, Radford J, Solh M, Heffner L, O'Connor OA. Safety and efficacy of Adct-402 (Loncastuximab Tesirine), a novel antibody drug conjugate, in relapsed/refractory follicular lymphoma and mantle cell lymphoma: interim results from the phase 1 first-in-human study. Blood. 2018;132:2874. [Google Scholar]
- 115.Hamadani M, Collins GP, Samaniego F, Spira AI, Davies A, Radford J, Caimi P, Menne T, Boni J, Cruz H, Feingold J, He S, Wuerthner J, Horwitz SM. Phase 1 study of Adct-301 (Camidanlumab Tesirine), a novel pyrrolobenzodiazepine-based antibody drug conjugate, in relapsed/refractory classical Hodgkin lymphoma. Blood. 2018;132:928. [Google Scholar]
- 116.Collins GP, Horwitz SM, Davies A, Karnad A, Samaniego F, Spira AI, Fields PA, Menne T, Boni J, Cruz H, Feingold J, He S, Wuerthner J, Hamadani M. Adct-301 (Camidanlumab Tesirine), a novel pyrrolobenzodiaze-based CD25-targeting antibody drug conjugate, in a phase 1 study of replased/refractory non-Hodgkin lymphoma shows activity in T-cell lymphoma. Blood. 2018;132:1658. [Google Scholar]
- 117.Stathis A, Flinn IW, Madan S, Maddocks K, Freedman A, Weitman S, Zucca E, Munteanu MC, Lia Palomba M. Safety, tolerability, and preliminary activity of IMGN529, a CD37-targeted antibody-drug conjugate, in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: a dose-escalation, phase I study. Invest New Drugs. 2018;36(5):869–876. doi: 10.1007/s10637-018-0570-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Sawas A, Savage KJ, Perez R, Advani RH, Butturini A, Lackey J, Trave F, Anand B, Huang Y, Reyno L, O'Connor OA. A phase 1 study of the anti-CD37 Antibody-drug conjugate AGS67E in advanced lymphoid malignancies. Interim results. Blood. 2015;126(23):3976. [Google Scholar]
- 119.Coiffier B, Thieblemont C, de Guibert S, Dupuis J, Ribrag V, Bouabdallah R, Morschhauser F, Navarro R, Le Gouill S, Haioun C, Houot R, Casasnovas O, Holte H, Lamy T, Broussais F, Payrard S, Hatteville L, Tilly H. A phase II, single-arm, multicentre study of coltuximab ravtansine (SAR3419) and rituximab in patients with relapsed or refractory diffuse large B-cell lymphoma. Br J Haematol. 2016;173(5):722–730. doi: 10.1111/bjh.13992. [DOI] [PubMed] [Google Scholar]
- 120.Kantarjian HM, Lioure B, Kim SK, Atallah E, Leguay T, Kelly K, Marolleau JP, Escoffre-Barbe M, Thomas XG, Cortes J, Jabbour E, O'Brien S, Bories P, Oprea C, Hatteville L, Dombret H. A phase II study of coltuximab ravtansine (SAR3419) monotherapy in patients with relapsed or refractory acute lymphoblastic leukemia. Clin Lymphoma Myeloma Leuk. 2016;16(3):139–145. doi: 10.1016/j.clml.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Li D., Poon K. A., Yu S.-F., Dere R., Go M., Lau J., Zheng B., Elkins K., Danilenko D., Kozak K. R., Chan P., Chuh J., Shi X., Nazzal D., Fuh F., McBride J., Ramakrishnan V., de Tute R., Rawstron A., Jack A. S., Deng R., Chu Y.-W., Dornan D., Williams M., Ho W., Ebens A., Prabhu S., Polson A. G. DCDT2980S, an Anti-CD22-Monomethyl Auristatin E Antibody-Drug Conjugate, Is a Potential Treatment for Non-Hodgkin Lymphoma. Molecular Cancer Therapeutics. 2013;12(7):1255–1265. doi: 10.1158/1535-7163.MCT-12-1173. [DOI] [PubMed] [Google Scholar]
- 122.Advani RH, Lebovic D, Chen A, Brunvand M, Goy A, Chang JE, Hochberg E, Yalamanchili S, Kahn R, Lu D, Agarwal P, Dere RC, Hsieh HJ, Jones S, Chu YW, Cheson BD. Phase I study of the anti-CD22 antibody-drug conjugate pinatuzumab vedotin with/without rituximab in patients with relapsed/refractory B-cell non-Hodgkin lymphoma. Clin Cancer Res. 2017;23(5):1167–1176. doi: 10.1158/1078-0432.CCR-16-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kuhn DJ, Dou QP. The role of interleukin-2 receptor alpha in cancer. Front Biosci. 2005;10:1462–1474. doi: 10.2741/1631. [DOI] [PubMed] [Google Scholar]
- 124.Goldberg AD, Tallman MS, Solh MM, Ungar D, Rizzieri DA, Walter RB, Spira AI, Chung KY, Stock W, He S, Boni J, Atallah E. Results from an ongoing phase 1 study indicate ACDT-301 (Camidanlumab Tesirine) is well-tolerated in patients with relapsed or refractory CD25-positive acute leukemia. Blood. 2017;130:2662. [Google Scholar]
- 125.Lapalombella R, Yeh YY, Wang L, Ramanunni A, Rafiq S, Jha S, Staubli J, Lucas DM, Mani R, Herman SE, Johnson AJ, Lozanski A, Andritsos L, Jones J, Flynn JM, Lannutti B, Thompson P, Algate P, Stromatt S, Jarjoura D, Mo X, Wang D, Chen CS, Lozanski G, Heerema NA, Tridandapani S, Freitas MA, Muthusamy N, Byrd JC. Tetraspanin CD37 directly mediates transduction of survival and apoptotic signals. Cancer Cell. 2012;21(5):694–708. doi: 10.1016/j.ccr.2012.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Zhao X, Lapalombella R, Joshi T, Cheney C, Gowda A, Hayden-Ledbetter MS, Baum PR, Lin TS, Jarjoura D, Lehman A, Kussewitt D, Lee RJ, Caligiuri MA, Tridandapani S, Muthusamy N, Byrd JC. Targeting CD37-positive lymphoid malignancies with a novel engineered small modular immunopharmaceutical. Blood. 2007;110(7):2569–2577. doi: 10.1182/blood-2006-12-062927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Deckert J, Park PU, Chicklas S, Yi Y, Li M, Lai KC, Mayo MF, Carrigan CN, Erickson HK, Pinkas J, Lutz RJ, Chittenden T, Lambert JM. A novel anti-CD37 antibody-drug conjugate with multiple anti-tumor mechanisms for the treatment of B-cell malignancies. Blood. 2013;122(20):3500–3510. doi: 10.1182/blood-2013-05-505685. [DOI] [PubMed] [Google Scholar]
- 128.Hicks SW, Lai KC, Gavrilescu LC, Yi Y, Sikka S, Shah P, Kelly ME, Lee J, Lanieri L, Ponte JF, Sloss CM, Romanelli A. The antitumor activity of IMGN529, a CD37-targeting antibody-drug conjugate, is potentiated by rituximab in non-Hodgkin lymphoma models. Neoplasia. 2017;19(9):661–671. doi: 10.1016/j.neo.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pereira DS, Guevara CI, Jin L, Mbong N, Verlinsky A, Hsu SJ, Avina H, Karki S, Abad JD, Yang P, Moon SJ, Malik F, Choi MY, An Z, Morrison K, Challita-Eid PM, Donate F, Joseph IB, Kipps TJ, Dick JE, Stover DR. AGS67E, an Anti-CD37 Monomethyl Auristatin E Antibody-drug conjugate as a potential therapeutic for B/T-cell malignancies and AML: a new role for CD37 in AML. Mol Cancer Ther. 2015;14(7):1650–1660. doi: 10.1158/1535-7163.MCT-15-0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Borst J, Hendriks J, Xiao Y. CD27 and CD70 in T cell and B cell activation. Curr Opin Immunol. 2005;17(3):275–281. doi: 10.1016/j.coi.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 131.Phillips T, Barr PM, Park SI, Kolibaba K, Caimi PF, Chhabra S, Kingsley EC, Boyd T, Chen R, Carret AS, Gartner EM, Li H, Yu C, Smith DC. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest New Drugs. 2019;37(2):297–306. doi: 10.1007/s10637-018-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Owonikoko TK, Hussain A, Stadler WM, Smith DC, Kluger H, Molina AM, Gulati P, Shah A, Ahlers CM, Cardarelli PM, Cohen LJ. First-in-human multicenter phase I study of BMS-936561 (MDX-1203), an antibody-drug conjugate targeting CD70. Cancer Chemother Pharmacol. 2016;77(1):155–162. doi: 10.1007/s00280-015-2909-2. [DOI] [PubMed] [Google Scholar]
- 133.Tannir NM, Forero-Torres A, Ramchandren R, Pal SK, Ansell SM, Infante JR, de Vos S, Hamlin PA, Kim SK, Whiting NC, Gartner EM, Zhao B, Thompson JA. Phase I dose-escalation study of SGN-75 in patients with CD70-positive relapsed/refractory non-Hodgkin lymphoma or metastatic renal cell carcinoma. Invest New Drugs. 2014;32(6):1246–1257. doi: 10.1007/s10637-014-0151-0. [DOI] [PubMed] [Google Scholar]
- 134.Ishitsuka K, Jimi S, Goldmacher VS, Ab O, Tamura K. Targeting CD56 by the maytansinoid immunoconjugate IMGN901 (huN901-DM1): a potential therapeutic modality implication against natural killer/T cell malignancy. Br J Haematol. 2008;141(1):129–131. doi: 10.1111/j.1365-2141.2008.07000.x. [DOI] [PubMed] [Google Scholar]
- 135.Skaletskaya A, Setiady YY, Park PU, Lutz RJ. Lorvotuzumab mertansine (IMGN901) immune effector activity and its effect on human NK cells. Cancer Research. 2011;71(Suppl 8):770. [Google Scholar]
- 136.Ailawadhi S, Kelly KR, Vescio RA, Jagannath S, Wolf J, Gharibo M, Sher T, Bojanini L, Kirby M, Chanan-Khan A. A phase I study to assess the safety and pharmacokinetics of single-agent lorvotuzumab mertansine (IMGN901) in patients with relapsed and/or refractory CD-56-positive multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19(1):29–34. doi: 10.1016/j.clml.2018.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Govindan SV, Cardillo TM, Sharkey RM, Tat F, Gold DV, Goldenberg DM. Milatuzumab-SN-38 Conjugates for the Treatment of CD74 Cancers. Molecular Cancer Therapeutics. 2013;12(6):968–978. doi: 10.1158/1535-7163.MCT-12-1170. [DOI] [PubMed] [Google Scholar]
- 138.Jagannath S, Heffner LT, Jr, Ailawadhi S, Munshi NC, Zimmerman TM, Rosenblatt J, Lonial S, Chanan-Khan A, Ruehle M, Rharbaoui F, Haeder T, Wartenberg-Demand A, Anderson KC. Indatuximab ravtansine (BT062) monotherapy in patients with relapsed and/or refractory multiple myeloma. Clin Lymphoma Myeloma Leuk. 2019;19(6):372–380. doi: 10.1016/j.clml.2019.02.006. [DOI] [PubMed] [Google Scholar]
- 139.Trudel S, Lendvai N, Popat R, Voorhees PM, Reeves B, Libby EN, Richardson PG, Hoos A, Gupta I, Bragulat V, He Z, Opalinska JB, Cohen AD. Antibody-drug conjugate, GSK2857916, in relapsed/refractory multiple myeloma: an update on safety and efficacy from dose expansion phase I study. Blood Cancer Journal. 2019;9:37. doi: 10.1038/s41408-019-0196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Berdeja JG, Hernandez-Ilizaliturri F, Chanan-Khan A, Patel M, Kelly KR, Running KL, Murphy M, Guild R, Carrigan C, Ladd S, Wolf BB, O'Leary JJ, Ailawadhi S. Phase I study of lorvotuzumab mertansine (LM, IMGN901) in combination with lenalidomide (Len) and dexamethasone (Dex) in patients with CD56-positive relapsed or relapsed/refractory multiple myeloma (MM) Blood. 2012;120(21):728. [Google Scholar]
- 141.Stein R., Mattes M. J., Cardillo T. M., Hansen H. J., Chang C.-H., Burton J., Govindan S., Goldenberg D. M. CD74: A New Candidate Target for the Immunotherapy of B-Cell Neoplasms. Clinical Cancer Research. 2007;13(18):5556s–5563s. doi: 10.1158/1078-0432.CCR-07-1167. [DOI] [PubMed] [Google Scholar]
- 142.Kaufman JL, Niesvizky R, Stadtmauer EA, Chanan-Khan A, Siegel D, Horne H, Wegener WA, Goldenberg DM. Phase I, multicentre, dose-escalation trial of monotherapy with milatuzumab (humanized anti-CD74 monoclonal antibody) in relapsed or refractory multiple myeloma. Br J Haematol. 2013;163(4):478–486. doi: 10.1111/bjh.12565. [DOI] [PubMed] [Google Scholar]
- 143.Sanderson RD, Lalor P, Bernfield M. Lymphocytes-B Express and Lose Syndecan at Specific Stages of Differentiation. Cell Regulation. 1989;1(1):27–35. doi: 10.1091/mbc.1.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schonfeld K, Zuber C, Pinkas J, Hader T, Bernoster K, Uherek C. Indatuximab ravtansine (BT062) combination treatment in multiple myeloma: pre-clinical studies. Journal of Hematology & Oncology. 2017;10:13. doi: 10.1186/s13045-016-0380-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kelly KR, Siegel DS, Chanan-Khan AA, Somlo G, Heffner LT, Jagannath S, Zimmerman T, Munshi NC, Madan S, Mohrbacher A, Lonial S, Barmaki-Rad F, Ruhle M, Herrmann E, Wartenberg-Demand A, Haeder T, Anderson KC. Indatuximab ravtansine (BT062) in combination with low-dose dexamethasone and lenalidomide or pomalidomide: clinical activity in patients with relapsed / refractory multiple myeloma. Blood. 2016;128(22):4486. [Google Scholar]
- 146.Sanchez E, Li MJ, Kitto A, Li J, Wang CS, Kirk DT, Yellin O, Nichols CM, Dreyer MP, Ahles CP, Robinson A, Madden E, Waterman GN, Swift RA, Bonavida B, Boccia R, Vescio RA, Crowley J, Chen HM, Berenson JR. Serum B-cell maturation antigen is elevated in multiple myeloma and correlates with disease status and survival. British Journal of Haematology. 2012;158(6):727–738. doi: 10.1111/j.1365-2141.2012.09241.x. [DOI] [PubMed] [Google Scholar]
- 147.Lee L, Bounds D, Paterson J, Herledan G, Sully K, Seestaller-Wehr LM, Fieles WE, Tunstead J, McCahon L, Germaschewski FM, Mayes PA, Craigen JL, Rodriguez-Justo M, Yong KL. Evaluation of B cell maturation antigen as a target for antibody drug conjugate mediated cytotoxicity in multiple myeloma. British Journal of Haematology. 2016;174(6):911–922. doi: 10.1111/bjh.14145. [DOI] [PubMed] [Google Scholar]
- 148.Tai YT, Mayes PA, Acharya C, Zhong MY, Cea M, Cagnetta A, Craigen J, Yates J, Gliddon L, Fieles W, Hoang B, Tunstead J, Christie AL, Kung AL, Richardson P, Munshi NC, Anderson KC. Novel anti-B-cell maturation antigen antibody-drug conjugate (GSK2857916) selectively induces killing of multiple myeloma. Blood. 2014;123(20):3128–3138. doi: 10.1182/blood-2013-10-535088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Zhang C, Liu J, Zhong JF, Zhang X. Engineering CAR-T cells. Biomarker Research. 2017;5(1):22. doi: 10.1186/s40364-017-0102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wei G, Ding L, Wang J, Hu Y, Huang H. Advances of CD19-directed chimeric antigen receptor-modified T cells in refractory/relapsed acute lymphoblastic leukemia. Experimental Hematology & Oncology. 2017;6(1):10. doi: 10.1186/s40164-017-0070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Yu S, Liu Q, Han X, Qin S, Zhao W, Li A, Wu K. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Experimental Hematology & Oncology. 2017;6(1):31. doi: 10.1186/s40164-017-0091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365(8):725–733. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wang J, Hu Y, Huang H. Current development of chimeric antigen receptor T-cell therapy. Stem Cell Investigation. 2018;5:44. doi: 10.21037/sci.2018.11.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Maude SL, Laetsch TW, Buechner J, Rives S, Boyer M, Bittencourt H, Bader P, Verneris MR, Stefanski HE, Myers GD, Qayed M, De Moerloose B, Hiramatsu H, Schlis K, Davis KL, Martin PL, Nemecek ER, Yanik GA, Peters C, Baruchel A, Boissel N, Mechinaud F, Balduzzi A, Krueger J, June CH, Levine BL, Wood P, Taran T, Leung M, Mueller KT, et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N Engl J Med. 2018;378(5):439–448. doi: 10.1056/NEJMoa1709866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Neelapu SS, Locke FL, Bartlett NL, Lekakis LJ, Miklos DB, Jacobson CA, Braunschweig I, Oluwole OO, Siddiqi T, Lin Y, Timmerman JM, Stiff PJ, Friedberg JW, Flinn IW, Goy A, Hill BT, Smith MR, Deol A, Farooq U, McSweeney P, Munoz J, Avivi I, Castro JE, Westin JR, Chavez JC, Ghobadi A, Komanduri KV, Levy R, Jacobsen ED, Witzig TE, et al. Axicabtagene ciloleucel CAR T-cell therapy in refractory large B-cell lymphoma. N Engl J Med. 2017;377(26):2531–2544. doi: 10.1056/NEJMoa1707447. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The material supporting the conclusion of this review has been included within the article.