Abstract
In this study, we report our contribution to the application of the copper-catalyzed azide-alkyne cycloaddition (CuAAC) reaction for the synthesis of β-keto-1,2,3-triazole derivatives 3a-f from ethinylestradiol and their application in the inhibition of two human cancer cells lines: human breast adenocarcinoma (MCF-7) and human hepatocellular carcinoma (HepG2). The β-keto-1,2,3-triazole derivates 3a-f exhibited moderate cytotoxic activity for the HepG2 cells with IC50 values of 29.7 μM (3a), 16.4 μM (3b), 17.8 μM (3c), 20.4 μM (3d), 28.1 μM (3e) and 28.2 μM (3f). The semi-synthetic β-keto-1,2,3-triazoles derivatives 3a-f were all characterized by FT-IR, NMR, HRMS and [α]D.
Keywords: Organic chemistry; Pharmaceutical chemistry; Toxicology; β-keto-1,2,3-triazoles; Ethinylestradiol; Click reaction; Hepatocellular carcinoma; Breast adenocarcinoma
1. Introduction
Steroids are organic compounds that contain a tetracyclic ring system. They are present in a wide variety of plants, animals and fungi [1, 2]. Members of this class of compounds differ in their oxidation state, chains and functional groups that are attached to the tetracyclic core [3, 4].
Steroidal compounds are widely used as anti-inflammatory, immunosuppressive, anabolic and contraceptive agents [1, 2, 5, 6]. They have also been used against leishmaniasis and to treat breast and prostate cancer [2, 6]. Due to their wide variety of biological activities, many natural steroidal compounds, together with synthetic and semi-synthetic steroids, are routinely prepared and evaluated as drug candidates [1, 7].
Addition or replacement of one or more carbon atoms in a steroidal compound by nitrogen atoms changes its chemical and biological properties [8]. Thus, 1,2,3-triazole scaffolds are of interest for drug development because they do not readily undergo metabolic degradation [4, 7]. These compounds have a wide variety of biological activity including antimicrobial, antiviral, antiepileptic and anti-HIV activity, and they are also active against leishmaniasis [8, 9].
Among the methodologies used in the synthesis of 1,2,3-triazole compounds is the Huisgen 1,3-dipolar cycloaddition. This reaction occurs between azides and terminal alkynes through a concerted mechanism. However, the Huisgen reaction has some drawbacks, such as long reaction time and high temperatures, as well as the formation of 1,4-disubstituted and 1,5-disubstituted regioisomers [10].
In 2002, Sharpless's and Meldal's groups independently discovered the copper-catalyzed azide-alkyne cycloaddition (CuAAC) for the synthesis of 1,2,3-triazole compounds. The reaction can be performed under mild conditions with high regioselectivity and yield. This reaction became also known as the click reaction [11, 12, 13, 14]. Deobald's group reported the application of the copper-catalyzed azide-alkyne cycloaddition reaction in the synthesis of 1,2,3-triazole compounds containing steroids, saponins and digitalis analogues [7]. Conner and co-workers reported the application of the click reaction in the synthesis of 17α-(2H-2,3,4-triazolyl)-estradiol from ethinylestradiol and their interactions in Cytochrome P450 [15].
Recently, Liu's group synthesized a new estradiol derivate, 18F-17-(1-(2-(dimethyl((trifluoro-14-boranyl)methyl)-14-azanyl)ethyl)-1H-1,2,3-triazol-4-yl)-13-methyl-7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthrene-3,17-diol as a potential Positron Emission Tomography (PET) imaging agent for estrogen receptor-positive breast cancer [16]. Additionally, the synthesis of 1,2,3-triazole derivates possessing a carbonyl group at β-position is also known. For example, Mareddy and co-workers synthesized a new class of 1,2,3-triazole derivates from nimesulide as potential inhibitors of phosphodiesterases 4 (PDE 4B). Synthesis of these compounds was carried out via of the copper-catalyzed azide-alkyne cycloaddition reaction [17].
In this study, we report our contribution to the application of the copper-catalyzed azide-alkyne cycloaddition reaction for the semi-synthesis of β-keto-1,2,3-triazole derivatives 3a-f from ethinylestradiol 2 and their application in the inhibition of growth of two human cancer cells lines: human breast adenocarcinoma (MCF-7) and human hepatocellular carcinoma (HepG2).
2. Experimental
2.1. General
Deuterated chloroform (CDCl3, 99.8% with 0.5% tetramethylsilane-TMS), deuterated methanol (CD3OD, 99.9%), deuterated dimethylsulfoxide (DMSO-d6, 99.9%) and deuterated acetone (acetone-d6, 99.9%) were purchased from Sigma-Aldrich. Ethyl acetate and hexane were purchased from Synth. Sodium azide was purchased from Merck. The reagents 2-bromo-1-phenylethanone (98%), 2-bromo-1-(4-methoxyphenyl)ethanone (97%), 2-bromo-1-(4-chlorophenyl)ethanone (98%), 2-bromo-1-(4-bromophenyl)ethanone (98%), 2-bromo-1-(4-fluorinephenyl)ethanone (98%), 2-bromo-1-(3-fluorinephenyl)ethanone (98%), (+)-sodium L-ascorbate (98%) and ethinylestradiol (≥98%) were purchased from Sigma-Aldrich.
2.2. General procedure for the synthesis of 2-azido-1-phenylethanone derivatives (1a-f)
In a round-bottomed flask (100 mL) was added 2-bromo-1-phenylethanone 1a (5.02 mmol), sodium azide (15.38 mmol) and acetone (30 mL). The reaction mixture was kept under magnetic stirring (450 rpm) at room temperature for 5 h and monitored by TLC. After the reaction finished, the acetone was evaporated under reduced pressure and water was added to the crude reaction. Then, the mixture was extracted with EtOAc (2 × 30 mL), dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure. The same reaction conditions were applied in the synthesis of the 2-azido-1-phenylethanones 1b-f. These compounds were purified by column chromatography on silica gel eluted with solution of hexane and EtOAc (8:2). The 2-azido-1-phenylethanones 1a-f were characterized by 1H NMR and 13C NMR, FTIR and mp (see Supplementary material). The spectroscopy data of the compounds 1a-f are in accordance to the literature. [18, 19].
2-azido-1-phenylethanone (1a): Molecular formula: C8H7N3O; MM: 161.16 g mol−1; yield: 87%, yellow liquid; IR (silicon plate) ν (cm−1): 3062, 2900, 2096, 1692, 1597, 1449, 1214, 752, 686; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.56 (s, 2H), 7.50 (t, 2H, J = 7.7 Hz), 7.63 (tt, 1H, J = 7.6 and 1.2 Hz), 7.91 (dd, 2H, J = 8.6 and 1.2 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 55.0, 128.1, 129.1, 134.3, 134.5, 193.3.
2-azido-1-(4-methoxyphenyl)ethanone (1b): Molecular formula: C9H9N3O2; MM: 191.19 g mol−1; yield: 89%, cream solid; mp 61–64 °C; IR (KBr) ν (cm−1): 3032, 2905, 2124, 1686, 1599, 1516, 1466, 1271, 1238, 1179, 826; 1H NMR (400 MHz, CDCl3) δ (ppm): 3.88 (s, 3H), 4.50 (s, 2H), 6.95 (d, 2H, J = 8.9 Hz), 7.88 (d, 2H, J = 9.1 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 54.7, 55.7, 114.3, 127.5, 130.4, 164.4, 191.8.
2-azido-1-(4-chlorophenyl)ethanone (1c): Molecular formula: C8H6ClN3O; MM: 195.61 g mol−1; yield: 84%, yellow solid; mp 59–62 °C; IR (silicon plate) ν (cm−1): 3089, 2907, 2098, 1690, 1592, 1571, 1489, 1216, 814; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.53 (s, 2H), 7.48 (d, 2H, J = 8.6 Hz), 7.85 (d, 2H, J = 8.6 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 55.0, 129.5, 129.6, 132.8, 140.8, 192.2.
2-azido-1-(4-bromophenyl)ethanone (1d): Molecular formula: C8H6BrN3O; MM: 240.06 g mol−1; yield: 78%, orange solid; mp 65–68 °C; IR (KBr) ν (cm−1): 3086, 2903, 2114, 1694, 1585, 1485, 1219, 814; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.52 (s, 2H), 7.64 (d, 2H, J = 8.7 Hz), 7.77 (d, 2H, J = 8.7 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 54.9, 129.5, 129.6, 132.5, 133.2, 192.4.
2-azido-1-(4-fluorophenyl)ethanone (1e): Molecular formula: C8H6FN3O; MM: 179.15 g mol−1; yield: 80%, yellow solid; mp 57–60 °C; IR (KBr) ν (cm−1): 3062, 2900, 2096, 1692, 1597, 1449, 1214, 907, 752; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.53 (s, 2H), 7.17 (ddd, 2H, J = 2.3, 8.7 and 9.1 Hz), 7.94 (ddd, 2H, J = 4.6, 5.3 and 9.1 Hz); 13C NMR (100 MHz, CDCl3) δ (ppm): 54.9, 116.5 (d, 2JCF = 22.1 Hz), 130.8 (d, 3JCF = 9.5 Hz), 131 (4JCF = 3.1 Hz), 166.4 (1JCF = 256.8 Hz), 191.8.
2-azido-1-(3-fluorophenyl)ethanone (1f): Molecular formula: C8H6FN3O; MM: 179.15 g mol−1; yield: 70%, orange solid; mp 59–62 °C; IR (KBr) ν (cm−1): 2978, 2908, 2096, 1692, 1593, 1488, 1342, 1216, 1090, 730; 1H NMR (400 MHz, CDCl3) δ (ppm): 4.54 (s, 2H), 7.33 (tdd, 1H, J = 1.0, 2.6 and 8.2 Hz), 7.46–7.52 (m, 1H), 7.61 (ddd, 1H, J = 1.6, 2.6 and 9.1 Hz), 7.67 (ddd, 1H, J = 1.0, 1.6 and 7.7 Hz)); 13C NMR (100 MHz, CDCl3) δ (ppm): 55.1, 115.0 (d, 2JCF = 22.5 Hz), 121.4 (d, 3JCF = 21.5 Hz), 123.8 (d, 6JCF = 3.3 Hz), 130.9 (d, 4JCF = 7.8 Hz), 136.5 (d, 5JCF = 6.4 Hz), 164.0 (d, 1JCF = 249.4 Hz), 192.2.
2.3. General procedure for the semi-synthesis of β-keto-1,2,3-triazole derivatives 3a-f from ethinylestradiol 2
In a round-bottomed flask (10 mL) was added 2-azido-1-phenylethanone (0.3 mmol) 1a, ethinylestradiol 2 (0.33 mmol) and acetone (1 mL). Then, a solution of sodium ascorbate (20 mol%) and copper sulfate (10 mol%) in distilled water (1 mL) was added to the mixture. The reaction mixtures was kept under magnetic stirring (450 rpm) at room temperature for 24 h and monitored by TLC. After the reaction went to completion, water was added to the crude reaction. Then, the mixture was extracted with EtOAc (2 × 30 mL), dried over anhydrous Na2SO4, filtered and evaporated under reduced pressure. The same reaction conditions were applied in the synthesis of the β-keto-1,2,3-triazoles 3a-f. These compounds were purified by column chromatography on silica gel eluted with solution of hexane and EtOAc (3:7). The β-keto-1,2,3-triazole derivatives 3a-f were characterized by 1H NMR and 13C NMR, FTIR, HRMS, mp and [α]D (see Supplementary Material).
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8, 9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a] phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-phenylethan-1-one (3a): Molecular formula: C28H31N3O3; MM: 457.57 g mol−1; yield: 92%, white solid; mp 201–204 °C; [α]D [23] +34 (c 0.05, CH3OH); IR (silicon plate) ν (cm−1): 3346, 2924, 2855, 1692, 1596, 1498, 1449, 1225, 1180, 1059, 817; 1H NMR (500 MHz, CD3OD) δ (ppm): 0.77–0.85 (m, 1H), 1.06 (s, 3H), 1.31–1.48 (m, 4H), 1.53–1.60 (m, 1H), 1.64–1.67 (m, 1H), 1.82–1.96 (m, 3H), 2.10–2.22 (m, 2H), 2.48–2.55 (m, 1H), 2.74–2.81 (m, 2H), 6.08 (d, 2H, J = 2.9 Hz), 6.47 (d, 1H, J = 2.6 Hz), 6.51 (dd, 1H, J = 8.4 and 2.7 Hz), 7.01 (d, 1H, J = 8.5 Hz), 7.57 (t, 2H, J = 7.8 Hz), 7.69 (tt, 1H, J = 7.6 and 1.2 Hz), 7.84 (s, 1H), 8.08 (dd, 2H, J = 8.4 and 1.2 Hz); 13C NMR (125 MHz, CD3OD) δ (ppm): 14.9. 24.7, 27.6, 28.7, 30.7, 34.2, 38.4, 41.1, 44.9, 48.5, 49.6, 57.0, 83.2, 113.7, 116.1, 126.0, 127.2, 129.4, 130.1, 132.6, 135.3, 135.7, 138.9, 155.4, 155.9, 193.1. HRMS calcd for C28H31N3O3 [M + H]+ 458.2438, found 458.2439.
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-(4-methoxyphenyl)ethan-1-one (3b): Molecular formula: C29H33N3O4; MM: 486.60 g mol−1; yield: 83%, white solid; mp 250–254 °C; [α]D [23] +32 (c 0.05, CH3OH); IR (KBr) ν (cm−1): 3194, 2926, 2855, 1688, 1603, 1508, 1460, 1240, 1180, 1055, 826; 1H NMR (500 MHz, DMSO-d6) δ (ppm): 0.64–0.72 (m, 1H), 0.94 (s, 3H), 1.24–1.52 (m, 5H), 1.63–1.72 (m, 1H), 1.74–1.88 (m, 3H), 1.92–1.98 (m, 1H), 2.09–2.15 (m, 1H), 2.35–2.43 (m, 3H), 2.64–2.77 (m, 2H), 3.87 (s, 3H), 5.14 (s, 1H), 6.07 (d, 2H, J = 2.9 Hz), 6.42 (d, 1H, J = 2.5 Hz), 6.48 (dd, 2H, J = 8.4 and 2.6 Hz), 6.98 (d, 1H, J = 8.5 Hz), 7.11 (d, 2H, J = 9 Hz), 7.8 (s, 1H), 8.04 (d, 2H, J = 9 Hz), 8.96 (s, 1H); 13C NMR (125 MHz, DMSO-d6) δ (ppm): 14.4, 23.6, 26.1, 27.2, 29.3, 32.6, 37.2, 39.8, 43.2, 46.8, 47.5, 55.3, 55.7, 81.1, 112.7, 114.2, 114.9, 124.4, 126.0, 127.1, 130.4, 130.5, 137.2, 154.0, 154.9, 163.8, 190.6. HRMS calcd for C29H33N3O4 [M + H]+ 488.2549, found 488.2541.
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-(4-chlorophenyl)ethan-1-one (3c): Molecular formula: C28H30ClN3O3; MM: 492.02 g mol−1; yield: 89%, white solid; mp 225–228 °C; [α]D [23] +104 (c 0.05, CH3OH); IR (silicon plate) ν (cm−1): 3365, 2925, 2855, 1665, 1591, 1490, 1452, 1227, 1173, 1090, 1013, 847; 1H NMR (500 MHz, CD3OD) δ (ppm): 0.76–0.84 (m, 1H), 1.06 (s, 3H), 1.28–1.48 (m, 4H), 1.51–1.61 (m, 1H), 1.63–1.68 (m, 1H), 1.82–1.99 (m, 3H), 2.10–2.21 (m, 2H), 2.47–2.55 (m, 1H), 2.71–2.81 (m, 2H), 6.06 (d, 2H, J = 1.7 Hz), 6.46 (d, 1H, J = 2.6 Hz), 6.51 (dd, 1H, J = 8.4 and 2.7 Hz), 7.01 (d, 1H, J = 8.4 Hz), 7.60 (d, 2H, J = 8.8 Hz), 7.83 (s, 1H), 8.06 (d, 2H, J = 8.7 Hz); 13C NMR (125 MHz, CD3OD) δ (ppm): 14.9, 24.7, 27.6, 28.8, 30.7, 34.3, 38.4, 41.1, 44.9, 48.5, 49.6, 56.9, 83.2, 113.7, 116.0, 126.0, 127.2, 130.3, 131.0, 132.5, 134.3, 138.8, 141.6, 155.5, 155.9, 192.0. HRMS calcd for C28H30ClN3O3 [M + H]+ 492.2054, found 492.2044.
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-(4-bromophenyl)ethan-1-one (3d): Molecular formula: C28H30BrN3O3; MM: 536.47 g mol−1; yield: 90%, white solid; mp 230–234 °C; [α]D [23] +42 (c 0.05, CH3OH); IR (KBr) ν (cm−1): 3136, 2928, 2864, 1688, 1584, 1499, 1454, 1288, 1229, 1069, 993, 816; 1H NMR (500 MHz, acetone-d6) δ (ppm): 0.76–0.85 (m, 1H), 1.06 (s, 3H), 1.27–1.47 (m, 4H), 1.51–1.61 (m, 2H), 1.84–1.95 (m, 3H), 2.08–2.21 (m, 2H), 2.44–2.54 (m, 1H), 2.66–2.87 (m, 2H), 6.11 (s, 2H), 6.51 (d, 1H, J = 2.6 Hz), 6.56 (dd, 1H, J = 8.4 and 2.7 Hz), 7.03 (d, 1H, J = 8.1 Hz), 7.79 (d, 2H, J = 8.8 Hz), 7.85 (s, 1H), 8.04 (d, 2H, J = 8.6 Hz); 13C NMR (125 MHz, acetone-d6) δ (ppm): 14.9, 24.4, 27.3, 28.4, 30.1, 33.8, 38.5, 40.7, 44.5, 48.1, 49.0, 56.4, 82.7, 113.6, 115.9, 124.8, 127.0, 129.3, 130.9, 132.0, 133.0, 134.5, 138.4, 155.2, 156.0, 191.9. HRMS calcd for C28H30BrN3O3 [M + H]+ 536.1549, found 536.1543.
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-(4-fluorinephenyl)ethan-1-one (3e): Molecular formula: C28H30FN3O3; MM: 475.56 g mol−1; yield: 65%, white solid; mp 232–235 °C; [α]D [23] +52 (c 0.05, CH3OH); IR (KBr) ν (cm−1): 3279, 2926, 2857, 1695, 1599, 1506, 1456, 1288, 1234, 1055, 1001, 828; 1H NMR (500 MHz, CD3OD) δ (ppm): 0.77–0.85 (m, 1H), 1.06 (s, 3H), 1.28–1.49 (m, 4H), 1.51–1.60 (m, 1H), 1.63–1.68 (m, 1H), 1.85–2.00 (m, 3H), 2.09–2.20 (m, 2H), 2.47–2.56 (m, 1H), 2.70–2.81 (m, 2H), 6.06 (d, 2H, J = 1.7 Hz), 6.46 (d, 1H, J = 2.6 Hz), 6.51 (dd, 1H, J = 2.7 and 8.4 Hz), 7.01 (d, 1H, J = 8.4 Hz), 7.30 (ddd, 2H, J = 2.3, 8.7 and 9.1 Hz); 13C NMR (125 MHz, CD3OD) δ (ppm): 14.9, 24.7, 27.6, 28.7 30.8, 34.3, 38.4, 41.1, 44.9, 48.5, 49.6, 56.8, 83.2, 113.7, 116.0, 117.1 (d, 2JCF = 22.4 Hz), 126.0, 127.1, 132.2 (d, 3JCF = 9.5 Hz), 132.3, 132.5, 138.8, 155.4, 155.9, 167.7 (d, 1JCF = 254.6 Hz), 191.6. HRMS calcd for C28H30FN3O3 [M + H]+ 476.2344, found 476.2343.
2-(4-((8R,9S,13S,14S,17S)-3,17-dihydroxy-13-methyl-7,8,7,8,9,11,12,13,14,15,16,17-decahydro-6H-cyclopenta[a]phenanthren-17-yl)-1H-1,2,3-triazol-1-yl)-1-(3-fluorinephenyl)ethan-1-one (3f): Molecular formula: C28H30FN3O3; MM: 475.56 g mol−1; yield: 63%, white solid; mp 225–228 °C; [α]D [23] +26 (c 0.05, CH3OH); IR (KBr) ν (cm−1): 2926, 2856, 1704, 1589, 1498, 1447, 1286 1255, 1057, 1004, 872; 1H NMR (500 MHz, CD3OD) δ (ppm): 0.78–0.84 (m, 1H), 1.06 (s, 3H), 1.32–1.49 (m, 4H), 1.54–1.60 (m, 1H), 1.63–1.70 (m, 1H), 1.86–2.00 (m, 3H), 2.09–2.20 (m, 2H), 2.48–2.54 (m, 1H), 2.72–2.80 (m, 2H), 6.08 (d, 2H, J = 2.0 Hz), 6.46 (d, 1H, J = 2.6 Hz), 6.51 (dd, 1H, J = 2.7 and 8.4 Hz), 7.02 (d, 1H, J = 8.4 Hz), 7.45 (tdd, 1H, J = 0.8, 2.6 and 8.4 Hz), 7.59–7.63 (m, 1H), 7.79 (ddd, 1H, J = 1.6, 2.5 and 9.4 Hz), 7.84 (s, 1H), 7.92 (ddd, 1H, J = 1.0, 1.4 and 7.8 Hz); 13C NMR (125 MHz, CD3OD) δ (ppm): 13.5, 23.3, 26.2, 27.3, 29.3, 32.8, 37.0, 39.7, 43.5, 47.0, 48.1, 55.7, 81.8, 112.3, 114.4 (d, 2JCF = 23 Hz), 114.6, 120.8 (d, 3JCF = 21.7 Hz), 123.9 (d, 6JCF = 3.0 Hz), 124.6, 125.7, 130.8 (d, 4JCF = 7.7 Hz), 131.1, 136.5 (d, 5JCF = 6.6 Hz), 137.4, 154.1, 163.9 (d, 1JCF = 244.1 Hz), 190.6. HRMS calcd for C28H30FN3O3 [M + H] + 476.2344, found 476.2343.
2.4. Fourier transform infrared analysis (FTIR)
The FTIR spectra of the purified compounds were recorded on a Shimadzu IRAffinity-1 spectrometer model. Analyses were performed using KBr for solid samples and silicon plates for liquid samples. The transmittance was measured in cm−1 in the 4000-600 cm−1 region.
2.5. Nuclear magnetic resonance (NMR)
The 1H and 13C nuclear magnetic resonance (NMR) spectra of the purified compounds were recorded on an Agilent Technologies 400/54 Premium Shielded (1H NMR and 13C NMR at 400 and 100 MHz) or Agilent Technologies 500/54 Premium Shielded (1H NMR and 13C at 500 and 125 MHz) spectrometer. The samples were solubilized in acetone-d6, CDCl3, CD3OD or DMSO-d6, and the chemical shifts were reported in ppm relative to an internal standard, TMS. Coupling constants (J) were expressed in hertz (Hz).
2.6. High resolution mass spectrometry (HRMS)
The HRMS spectra were recorded on a micro Tof-QII hybrid quadrupole/time-of-flight (QqToF) mass spectrometer, from Daltonics (Bremen, Germany), equipped with an electrospray ionization (ESI) source. ESI source conditions used in the positive ionization mode included a capillary voltage of 4.0 kV, drying gas flow rate of 8.0 L/min, nebulizing gas pressure set at 4 bar and source temperature set at 200 °C. Data acquisition was performed using full MS mode (quadrupole m/z range was set from 50 to 3000 Da) at 1.0 Hz rate. Data processing was performed with software (version 4.2), also from Bruker Daltonics.
2.7. Absolute configuration
The optical rotations ([α]DT) of the β-keto-1,2,3-triazoles 3a-f were measured at 23 °C with a JASCO P2000 polarimeter equipped with a 589 nm-lamp Na in 1 dm cuvette. The samples were prepared with 1 mg of the purified compound diluted in 2.0 mL of CH3OH (0.05 g/100 mL).
2.8. Cytotoxic assay
Cancer cell lines, MCF-7 (human breast adenocarcinoma) and HepG2 (human hepatocellular carcinoma) and non-cancer cell line, MRC-5 (human lung fibroblast), were obtained from the American Type Culture Collection (ATCC) [20]. The cells were cultured in cell culture bottles (75 cm3, 250 mL volume) in RPMI 1640 medium and supplemented with 10% fetal bovine serum. The cells were maintained in incubators under a 5% CO2 atmosphere at 37 °C. Cellular growth was monitored daily using an inverted microscope. The medium was changed whenever the cell growth reached the necessary confluence for nutrient renewal. For the maintenance of adherent cells, trypsin (0.25%) was used detach the cells from the surface of the bottles. Cell cultures were mycoplasma negative, as assessed by incubation with Hoechst (Mycoplasma Stain Kit, Cat, MYC1, Sigma-Aldrich, St. Louis, MO, USA).
Cell viability was quantified using alamar blue assay, as previously described [21]. Initially, the cells were plated in 96-well plates (100 μL per well of a solution of 0.3 × 106 cells per mL for cells in suspension and 0.7 × 105 cells per mL for adhered cells). After 24 h of incubation, the compounds 3a-f solubilized in DMSO were added to the cells and incubated for 72 h. Doxorubicin (purity >95%, Laboratórios IMA S.A.I.C., Buenos Aires, Argentina) was used as a positive control and the negative control received the same amount of DMSO. Then, 4 h before the end of the incubation period, 20 μL of stock solution (0.312 mg mL−1) of alamar blue (resazurin) was added to each well. Absorbance was measured at wavelengths of 570 nm (reduced) and 595 nm (oxidized) using a plate reader. IC50 values were determined from the non-linear regression of the percentage of inhibition × log of the concentration, using the program Prisma version 5.0 (GraphPad Software).
3. Results and discussion
3.1. Semi-synthesis of β-keto-1,2,3-triazole derivatives 3a-f from ethinylestradiol 2
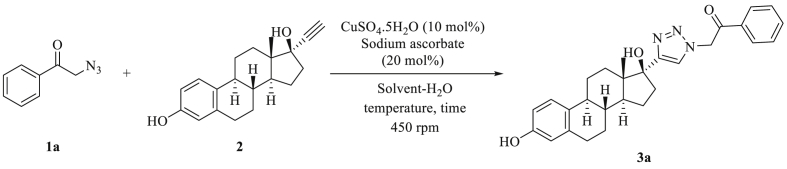
To obtain the optimal reaction conditions for 1,3-dipolar cycloaddition of 2-azido-1-phenylethanone 1a with ethinylestradiol 2, the reaction was performed in a mixture of organic solvent and H2O (1:1) in the presence of CuSO4.5H2O (10 mol%) and sodium ascorbate (20 mol%) (Scheme 1).
Scheme 1.
The 1,3-dipolar cycloaddition reaction using 2-azido-1-phenylethanone 1a and ethinylestradiol 2.
The first reaction condition tested was the organic solvent. The reaction was performed at room temperature for 14 h (Table 1, entries 1–4). Using the H2O and isopropanol (1:1) system, compound 3a was obtained with a 38% yield (Table 1, entry 1). A mixture of ethanol and H2O (1:1) produced the desired product 3a with a 56% yield (Table 1, entry 2). However, when the reaction was performed in THF and H2O (1:1) or acetone and H2O (1:1), product 3a was obtained with 72% and 83% yields, respectively (Table 1, entries 3 and 4). Therefore, the acetone and H2O solution was chosen as the solvent for the reaction because it afforded a higher reaction yield and good solubility of 2-azido-1-phenylethanone 1a and ethinylestradiol 2 among the reaction conditions studied.
Table 1.
Optimization of the 1,3-dipolar cycloaddition reaction conditions using 2-azido-1-phenylethanone 1a and ethinylestradiol 2 in the presence of CuSO4.5H2O and sodium ascorbate.
| Entry | Solvente-H2O (1:1) | Temperature (°C) | Yielda (%) | Reaction time (h) |
|---|---|---|---|---|
| 1b | Isopropanol | 26 | 38 | 14 |
| 2b | Ethanol | 26 | 56 | 14 |
| 3b | THF | 26 | 72 | 14 |
| 4b | Acetone | 26 | 83 | 14 |
| 5c | Acetone | 26 | 70 | 6 |
| 6c | Acetone | 40 | 66 | 6 |
| 7c | Acetone | 50 | 63 | 6 |
| 8d | Acetone | 26 | 92 | 24 |
Isolated yield.
CuSO4.5H2O (10 mol%), sodium ascorbate (10 mol%), reaction time of 14 h.
CuSO4.5H2O (10 mol%), sodium ascorbate (10 mol%), reaction time of 6 h.
CuSO4.5H2O (10 mol%), sodium ascorbate (10 mol%), reaction time of 24 h.
To evaluate the influence of temperature on this reaction, a study was performed using three temperature conditions (26 °C, 40 °C, and 50 °C) (Table 1, entries 5–7). When the reaction was performed in a mixture of acetone and H2O (1:1) at room temperature for 6 h, the β-keto-1,2,3-triazole derivative 3a was obtained with a 70% yield (Table 1, entry 5). When the reaction was performed at 40 °C or 50 °C for 6 h, compound 3a was obtained with 66% and 63% yields, respectively (Table 1, entries 6 and 7). Investigation of the temperature effects on the reaction revealed that the increase in temperature has no significant influence on the yield of product 3a.
Another condition tested was the reaction time (Table 1, entries 4, 5, and 8). When the reaction time was decreased from 14 to 6 h, the chemical yield decreased from 83% to 70% (Table 1, entries 4 and 5). The reaction was performed for 24 h and its chemical yield increased to 92% (Table 1, entries 4 and 8).
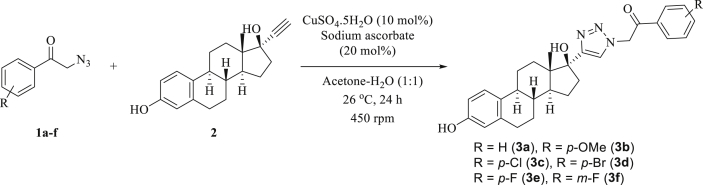
The optimized reaction conditions were used to synthesize the five different β-keto-1,2,3-triazole derivatives 3b-f (Scheme 2). High isolated yields were obtained for compounds 3a (92%), 3b (83%), 3c (89%), and 3d (90%). Good isolated yields were obtained for compounds 3e (65%) and 3f (63%), as reported in Table 2. Compounds 3a-3f were isolated using column chromatography containing silica gel as a stationary phase and characterized using Fourier transform infrared (FTIR), nuclear magnetic resonance (NMR), high resolution mass spectrometry (HRMS), optical rotation [α]D, and melting point (mp).
Scheme 2.
The 1,3-dipolar cycloaddition reaction using 2-azido-1-phenylethanones 1a-f and ethinylestradiol 2.
Table 2.
Yields for the β-keto-1,2,3-triazole derivatives 3b-f obtained by click reaction.
| Entry | R | Yielda (%) |
|---|---|---|
| 1 | H (3a) | 92 |
| 2 | p-OCH3 (3b) | 83 |
| 3 | p-Cl (3c) | 89 |
| 4 | p-Br (3d) | 90 |
| 5 | p-F (3e) | 65 |
| 6 | m-F (3f) | 63 |
Isolated yield.
Compound 3a was purified as a white solid (mp 201–204 °C). The molecular formula C28H31N3O3 was established using HRESIMS data (m/z for 458.2438 [M + H]+, establishing an index of hydrogen deficiency (IDH) of 15. The IR spectrum of compound 3a showed an absorption band at 3200 cm−1 for a hydroxyl group and an absorption band at 1691 cm−1 for a carbonyl group. It was also showed a C–H stretching band at 2926 cm−1 and a C–H asymmetrical band at 2840 cm−1.
The 1H NMR spectrum of compound 3a showed a singlet at δH 7.84 for the vinyl hydrogen. Additionally, three aromatic signals were integrated for four protons with chemical shifts at δH 7.57, 7.69, and 8.08, which confirms the aromatic moiety vicinal to carbonyl. The signals at δH 6.47, 6.51, 7.01, and 7.69 were integrated for three protons, which confirms the aromatic moiety from ethinylestradiol.
The 13C NMR spectrum of compound 3a showed a signal at δC 57.0 for the methylene carbon vicinal to triazole nucleus. The characteristic signal at δC 83.2 results from the carbinolic carbon vicinal to the triazole nucleus. The presence of ten signals from δC 126.0–155.9 for twelve carbons, with two of these signals having double intensity, confirm the two aromatic moieties. The signals at δC 126.0 and 155.4 are a result of the carbons from triazole nucleus and the typical signal at δC 193.1 is a result of the carbonyl carbon of a ketone. The spectra of all compounds 3a-f were similar, except for some signals in the aromatic regions that resulted from different 2-azido-1-phenylethanones substitutions on the aromatic ring.
3.2. Cytotoxic activity
The synthesized compounds 3a-f were evaluated against two human cancer cell lines, MCF-7 and HepG2, and one non-cancer cell line, MRC-5. Table 3 shows the IC50 data and the respective 95% confidence interval obtained by non-linear regression using the GraphPad Prism version 5.0 program. Doxorubicin was used as a positive control for the cytotoxicity assay, with an IC50 value of 1.9 μM for MCF-7 cells, 0.2 μM for HepG2 cells and 2.4 μM for MRC-5 cells.
Table 3.
The IC50 values obtained for cytotoxic activity in human cancer cell lines versus non-cancera for the β-keto-1,2,3-triazole compounds 3a-f.
| β-keto-1,2,3-triazoles | IC50 (μM) |
||
|---|---|---|---|
| MCF-7 | HepG2 | MRC-5 | |
| 3a | >54.6 | 29.7 24.1–36.5 |
>54.6 |
| 3b | 43.6 30.5–62.3 |
16.4 12.7–21.2 |
>52.5 |
| 3c | 44.4 31.4–62.8 |
17.8 13.1–24.3 |
>50.8 |
| 3d | 46.1 29.8–71.1 |
20.4 15.0–27.7 |
>46.6 |
| 3e | 39.3 32.2–47.9 |
28.1 20.3–38.7 |
>52.5 |
| 3f | >52.5 | 28.2 18.7–42.7 |
>52.5 |
| Doxorubicin | 1.9 1.4–2.5 |
0.20 0.1–0.3 |
2.4 1.9–3.0 |
Data are presented as IC50 values in μM and 95% confidence interval obtained by nonlinear regression from at the least three independent experiments performed in duplicate, measured by alamar blue assay after 72 h of incubation. Cancer cell lines: MCF-7 (human breast adenocarcinoma) and HepG2 (human hepatocellular carcinoma). Non-cancer cell line: MRC-5 (human lung fibroblast). Doxorubicin was used as a positive control.
As shown in Table 3, the β-keto-1,2,3-triazole compounds 3a-f exhibited moderate cytotoxic activity against the cancer cell line HepG2 with IC50 values of 29.7, 16.4, 17.8, 20.4, 28.1 and 28.2 μM, respectively. The IC50 values found for the cancer cell line MCF-7 were >54.6, 43.6, 44.4, 46.1, 39.3 and >52.5 μM, respectively, while no cytotoxic activity against the non-cancer cell line MRC-5 was found at the experimental concentrations tested.
This is an unpublished study describing the evaluation of cytotoxic activity in semi-synthetic β-keto-1,2,3-triazole derivates 3a-f from ethinylestradiol 2. In the literature there are few studies describing the synthesis and evaluation of cytotoxic activity of 1,2,3-triazole compounds from steroids. Recently, Ortiz and co-workers reported the synthesis of two pregnane derivates with a triazole (3β-hydroxy-21-(1H-1,2,4-triazol-1-yl)pregna-5,16-dien-20-one) or imidazole (3β-hydroxy-21-(1H-imidazol-1-yl)pregna-5,16-dien-20-one) ring and their application in inhibiting three human cancer cells lines: prostate cancer (PC-3), breast cancer (MCF7) and lung cancer (SK-LU-1). The results showed that the 3β-hydroxy-21-(1H-1,2,4-triazol-1-yl)pregna-5,16-dien-20-one compound exhibited cytotoxic activity for the PC-3, MCF7 and SK-LU-1 cancer cell lines with IC50 values of 17, 360 and 230 μM, respectively [4].
Also there are studies in the literature describing the synthesis and evaluation of biological activity of other 1,2,3-triazole derivates [22, 23, 24, 25]. The coumarin-1,2,3-triazole-dithiocarbamate hybrids were designed, synthesized and evaluated for their inhibitory activity towards lysine specific demethylase 1 (LSD1). Several of these compounds presented potent activity against LSD1 [26]. Kuntala's group synthesized novel benzoxepine-1,2,3-triazole hybrids and applied them as antibacterial and anticancer agents. Some of these compounds showed antibacterial activity against gram-positive and gram-negative species. These compounds also showed cytotoxicities against lung and colon cancer cell lines [27].
The 1,2,3-triazole-nimesulide hybrids were designed, synthesized and evaluated as anticancer agents. Several of these compounds showed growth inhibition of A549 (lung cancer), HepG2 (liver cancer), HeLa (cervical cancer) and DU145 (prostate cancer) cancer cell lines [28]. Neeraja and co-workers synthesized 1H-1,2,3-triazolyl-substituted 1,3,4-oxadiazole derivates containing structural features of ibuprofen/naproxen as antibacterial agents. Several of these compounds showed good to reasonable antibacterial activities when tested against three gram-positive (Staphylococcus aureus, Staphylococcus epidermidis and Bacillus subtilis) and three gram-negative (Pseudomonas aeruginosa, Klebsiella pneumoniae and Escherichia coli) species.
The 2-(4-((5-(1-(4-isobutylphenyl)ethyl)-1,3,4-oxadiazol-2-ylthio)methyl)-1H-1,2,3-triazol-1-yl)-N-(2-nitrophenyl)acetamide compound showed promising activities across both the species [29].
Our results demonstrated that compounds 3a-f were active against HepG2 cell proliferation. Therefore, the β-keto-1,2,3-triazole compounds 3a-f may be promising for the development of novel therapeutic alternatives to treat cancer. However, new derivatives can be synthesized that may provide better results.
4. Conclusions
Synthesis of the six β-keto-1,2,3-triazole derivatives 3a-f were obtained with good isolated yields (63–92%) were obtained through optimization of the 1,3-dipolar cycloaddition reaction in the presence of CuSO4.5H2O and sodium ascorbate using different 2-azido-1-phenylethanones 1a-f and ethinylestradiol 2. These compounds were investigated against two human cancer cells lines, MCF-7 and HepG2. Compounds 3a-f showed moderate cytotoxic activity against the HepG2 cancer cells.
Declarations
Author contribution statement
Thayane M. Queiroz: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Erika V.M. Orozco: Conceived and designed the experiments; Analyzed and interpreted the data. Valdenizia R. Silva, Luciano S. Santos: Performed the experiments; Analyzed and interpreted the data. Milena B.P. Soares, Daniel P. Bezerra: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data. André L.M. Porto: Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
Thayane M. Queiroz was supported by a fellowship provided by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES/Proc. 1732109). Erika V.M. Orozco was supported by a fellowship provided by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/140824/2015-4). André L.M. Porto was supported by a grant 2014/18257-0 and grant 2016/20155-7, São Paulo Research Foundation (FAPESP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq/Proc. 302528/2017-2). This work was supported by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. The author also acknowledge the Chromatography Group (Instituto de Química de São Carlos - USP), including Dr. Guilherme M. Titato for the QqToF analysis (grant 2004/09498-2, São Paulo Research Foundation).
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- 1.Dewick P.M. Wiley; New York: 2002. Medicinal Natural Product: A Biosynthetic Approach. [Google Scholar]
- 2.Bhatti H.N., Khera R.A. Biological transformations of steroidal compounds: A review. Steroids. 2012;77:1267–1290. doi: 10.1016/j.steroids.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 3.Payne A.H., Hales D.B. Overview of steroidogenic enzymes in the pathway from cholesterol to active steroid hormones. Endocr. Rev. 2004;25:947–970. doi: 10.1210/er.2003-0030. [DOI] [PubMed] [Google Scholar]
- 4.Ortiz A.V.S., Bratoeff E., Apan M.T.R., Becerra R.G., Rosado D.O., Martínez N.N., Bocanegra R.C., Barrera D. Synthesis and biological activity of two pregnane derivatives with a triazole or imidazole ring at C-21. J. Steroid Biochem. 2016;159:8–18. doi: 10.1016/j.jsbmb.2016.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Baydoun E., Wahab A.T., Shoaib N., Ahmad M.S., Massih R.A., Smith C., Naveed N., Choudhary M.I. Microbial transformation of contraceptive drug etonogestrel into new metabolites with Cunninghamella blakesleeana and Cunninghamella echinulata. Steroids. 2016;115:56–61. doi: 10.1016/j.steroids.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 6.Siddiqui M., Ahmad M.S., Wahab A.T., Yousuf S., Fatima N., Shaikh N.N., Rahman A.U., Choudhary M.I. Biotransformation of a potent anabolic steroid, mibolerone, with Cunninghamella blakesleeana, C. echinulata, and Macrophomina phaseolina, and biological activity evaluation of its metabolites. PLoS One. 2017;12 doi: 10.1371/journal.pone.0171476. e0171476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deobald A.M., Camargo L.R.S., Alves D., Schpector J.Z., Corrêa A.G. Click chemistry: An efficient synthesis of heterocycles substituted with steroids, saponins, and digitalis analogues. Synthesis. 2011;24:4003–4010. [Google Scholar]
- 8.Yadav J.S., Reddy P.T., Nanda S., Rao A.B. A facile synthesis of (R)-(−)-2-azido-1-arylethanols from 2-azido-1-arylketones using baker's yeast. Tetrahedron: Asymmetry. 2001;12:63–67. [Google Scholar]
- 9.Silva F.C., Cardoso M.F.C., Ferreira P.G., Ferreira V.F. In: Dehaen W., Bakulev V.A., editors. Springer International Publishing; Berlin: 2015. pp. 117–165. (Chemistry of 1,2,3-Triazoles). [Google Scholar]
- 10.Rostovtsev V.V., Green L.G., Fokin V.V., Sharpless K.B. A stepwise huisgen cycloaddition process: Copper(I)-catalyzed regioselective “Ligation” of Azides and terminal alkynes. Angew. Chem. 2002;114:2708–2711. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 11.Lutz J.F. 1,3-dipolar cycloadditions of azides and alkynes: a universal ligation tool in polymer and materials science. Angew. Chem. Int. Ed. 2007;46:1018–1025. doi: 10.1002/anie.200604050. [DOI] [PubMed] [Google Scholar]
- 12.Hein J.E., Fokin V.V. Copper catalyzed azide–alkyne cycloaddition (CuAAC) and beyond: new reactivity of copper(I) acetylides. Chem. Soc. Rev. 2010;39:1302–1315. doi: 10.1039/b904091a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agalave S.G., Maujan S.R., Pore V.S. Click chemistry: 1,2,3-Triazoles as pharmacophores. Chem. Asian J. 2011;6:2696–2718. doi: 10.1002/asia.201100432. [DOI] [PubMed] [Google Scholar]
- 14.Chang K.H., Lee L., Chen J., Li W.S. Lithocholic acid analogues, new and potent α-2,3-sialyltransferase inhibitors. Chem. Commun. 2006;6:629–631. doi: 10.1039/b514915k. [DOI] [PubMed] [Google Scholar]
- 15.Conner K.P., Vennam P., Krzyaniak M.D., Woods C.M., Bowman M.K., Atkins W.M. 1,2,3-Triazole-Heme interactions in cytochrome P450: functionally competent Triazole-Water- Heme complexes. Biochemistry. 2012;51:6441–6457. doi: 10.1021/bi300744z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu G., Wang W., Lin J., Li K., Lv G., Zhao X., Wang S., Luo S., Qiu L. Kit-like 18F-labeling of an estradiol derivative as a potential PET imaging agent for estrogen receptor-positive breast cancer. J. Radioanal. Nucl. Chem. 2017;312:599–607. [Google Scholar]
- 17.Mareddy J., Suresh N., Jayasree A., Devi Y.P., Mangamoori L.N., Kapavarapu R., Pal S. Synthesis and biological evaluation of nimesulide based new class of triazole derivatives as potential PDE4B inhibitors against cancer cells. Bioorg. Med. Chem. Lett. 2013;23:6721–6727. doi: 10.1016/j.bmcl.2013.10.035. [DOI] [PubMed] [Google Scholar]
- 18.Patonay T., Hoffman R.V. A General and efficient synthesis of α-Azido ketones. J. Org. Chem. 1994;59(10):2902–2905. [Google Scholar]
- 19.Rocha L.C., Rosset I.G., Melgar G.Z., Raminelli C., Porto A.L.M., Jeller A.H. Chemoenzymatic resolution of β-Azidophenylethanols by Candida antarctica and their application for the synthesis of chiral benzotriazoles. J. Braz. Chem. Soc. 2013;24(9):1427–1432. [Google Scholar]
- 20.Boulevard U., Manassas V.U. American Type Culture Collection (ATCC) https://www.atcc.org/
- 21.Ahmed S.A., Gogal R.M., Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H] thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- 22.Lauria A., Delisi R., Mingoia F., Terenzi A., Martorana A., Barone G., Almerico A.M. 1,2,3-Triazole in heterocyclic compounds, Endowed with biological activity, through 1,3-Dipolar Cycloadditions. Eur. J. Org. Chem. 2014;16:3289–3306. [Google Scholar]
- 23.D’hooghe M., Vandekerckhove S., Mollet K., Vervisch K., Dekeukeleire S., Lehoucq L., Lategan C., Smith P.J., Chibale K., De Kimpe N. Synthesis of 2-amino-3-arylpropan-1-ols and 1-(2,3-diaminopropyl)-1,2,3-triazoles and evaluation of their antimalarial activity. Beilstein J. Org. Chem. 2011;7:1745–1752. doi: 10.3762/bjoc.7.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Babu P.V., Mukherjee S., Gorja D.R., Yellanki S., Medisetti R., Kulkarni P., Mukkanti K., Pal M. Zebrafish based strategy for the identification of a potential pharmacophore for apoptosis: a greener CuAAC approach for novel 1,2,3-triazoles derived from mefenamic acid. RSC Adv. 2014;4:4878–4882. [Google Scholar]
- 25.Nallapati S.B., Sreenivas B.Y., Bankala R., Parsa K.V.L., Sripelly S., Mukkanti K., Pal M. 1,2,3-Triazoles derived from olanzapine: their synthesis via an ultrasound assisted CuAAC method and evaluation as inhibitors of PDE4B. RSC Adv. 2015;5:94623–94628. [Google Scholar]
- 26.Ye X.W., Zheng Y.C., Duan Y.C., Wang M.M., Yu B., Ren J.L., Ma J.L., Zhang E., Liu H.M. Synthesis and biological evaluation of coumarin–1,2,3-triazole–dithiocarbamate hybrids as potent LSD1 inhibitors. Med. Chem. Commun. 2014;5:650–654. [Google Scholar]
- 27.Kuntala N., Telu J.R., Banothu V., Babu N.S., Anireddy J.S., Pal S. Novel benzoxepine-1,2,3-triazole hybrids: synthesis and pharmacological evaluation as potential antibacterial and anticancer agents. Med. Chem. Commun. 2015;6:1612–1619. [Google Scholar]
- 28.Mareddy J., Suresh N., Kumar C.G., Kapavarapu R., Jayasree A., Pal S. 1,2,3-Triazole-nimesulide hybrid: Their design, synthesis and evaluation as potential anticancer agents. Bioorg. Med. Chem. Lett. 2017;27:518–523. doi: 10.1016/j.bmcl.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Neeraja P., Srinivas S., Mukkanti K., Dubey P.K., Pal S. 1H-1,2,3-Triazolyl-substituted 1,3,4-oxadiazole derivatives containing structural features of ibuprofen/naproxen: Their synthesis and antibacterial evaluation. Bioorg. Med. Chem. Lett. 2016;26:5212–5217. doi: 10.1016/j.bmcl.2016.09.059. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.