Abstract
Symptoms of functional gastrointestinal disorders (FGIDs), including fullness, bloating, abdominal pain, and altered gastrointestinal (GI) motility, present a significant clinical problem, with a reported prevalence of 25%–40% within the general population. More than 60% of those affected seek and require healthcare, and affected individuals report a significantly decreased quality of life. FGIDs are highly correlated with episodes of acute and chronic stress and are increased in prevalence and reported severity in women compared with men. Although there is evidence that sex and stress interact to exacerbate FGID symptoms, the physiological mechanisms that mediate these sex-dependent disparities are incompletely understood, although hormonal-related differences in GI motility and visceral sensitivity have been purported to play a significant role in the etiology. In this mini review, we will discuss brain-gut axis control of GI motility and sensitivity, the influence of estrogen on GI motility and sensitivity, and stress modulation of the brain-gut axis.
INTRODUCTION
The gastrointestinal (GI) tract performs the processes of digestion under semiautonomous control of the enteric nervous system (ENS). Parasympathetic (vagal and pelvic) and sympathetic (thoracolumbar) pathways convey sensory information to the central nervous system (CNS) and modulate motility via descending brain-gut pathways impinging onto ENS neurons. Disorders of the brain-gut axis contribute to the development of functional GI disorders (FGIDs), such as functional dyspepsia (FD) and irritable bowel syndrome (IBS), that involve altered motility and/or altered sensitivity (21).
Multiple epidemiological studies have established that the prevalence of FGIDs is higher among women (25). Although the exact pathophysiology is largely unknown, clinical evidence suggests that GI dysmotility, including impaired gastric accommodation, delayed gastric emptying, and gastric hypersensitivity, contributes to FD symptoms (25). Women are more likely to report symptoms of FD, such as nausea, early satiety, bloating, and both upper and lower abdominal pain, and meet diagnostic criteria for FGIDs, suggesting the involvement of circulating gonadal hormones, estrogen, and progesterone (25). GI motility is decreased in women, including a shorter migrating motor complex, prolonged proximal gastric relaxation, altered distal gastric motor function, and attenuated postprandial antral contractions, during the follicular phase when estrogen levels are high (4). These observations suggest that circulating female hormones play a major role in the delayed gastric emptying observed in women, although the effects of the menstrual cycle on gastric emptying rate seem inconclusive, likely because of a disparity in measurement methodology as well as the size, age, and intrinsic variation of the selected sample (44, 46, 92). Notably, pre- as well as postmenopausal women receiving hormone therapy replacement have gastric emptying rates slower than that of postmenopausal women without hormone therapy, which is similar to that of age-matched men (46). Studies in animals have also shown that gastric emptying rates are slower in intact compared with ovariectomized females and that estradiol administration delays gastric emptying and inhibits GI motility (9, 19, 36). Conversely, testosterone, or androgens in general, does not appear to have any effect on GI motility or gastric hypersensitivity (3, 19, 36).
Abdominal pain is one of the major symptoms in patients with IBS (24). Clinical evidence suggests that patients with IBS exhibit abnormal bowel habits in part because of altered smooth muscle function, abnormal mucosal transport, and/or increased epithelial permeability (24). There is a strong sex-related bias in the prevalence of IBS, with a female-to-male ratio ranging from 2:1 to 4:1 in developed countries (16). The observed sex difference in the prevalence and severity of GI symptoms in IBS could be, at least in part, explained by circulating ovarian hormones (65), since many symptoms, such as bloating, change in bowel habits, and abdominal pain, vary during the menstrual cycle and pain severity scores are reduced in patients with IBS following menopause (22, 39, 71). Furthermore, in rodent models, colonic sensitivity is increased during proestrus and estrus and diminished during diestrus or metestrus (37, 48), whereas estrogen replacement in ovariectomized rats increases visceromotor response to nociceptive stimuli (18, 47).
BRAIN-GUT AXIS REGULATION OF MOTILITY
The coordinated autonomic processes of the GI tract, from the lower esophagus to the transverse colon, are under a prominent extrinsic, parasympathetic modulatory control (Fig. 1). Upper GI functions are regulated by the efferent vagus nerve, the output of which is controlled by neurons of the dorsal vagal complex (DVC), consisting of the dorsal motor nucleus of the vagus (DMV), the nucleus tractus solitarius (NTS), and the area postrema. Sensory signals from the upper GI tract are relayed by afferent vagal fibers to the NTS, where they are integrated with information from other CNS centers involved in the regulation of autonomic and homeostatic functions. The integrated signal is then transmitted from the NTS to the efferent preganglionic neurons of the DMV, which project to either cholinergic (excitatory) or nonadrenergic noncholinergic [inhibitory, mainly vasoactive intestinal peptide and nitric oxide (NO) but also ATP] postganglionic myenteric neurons (12, 32, 85).
Fig. 1.
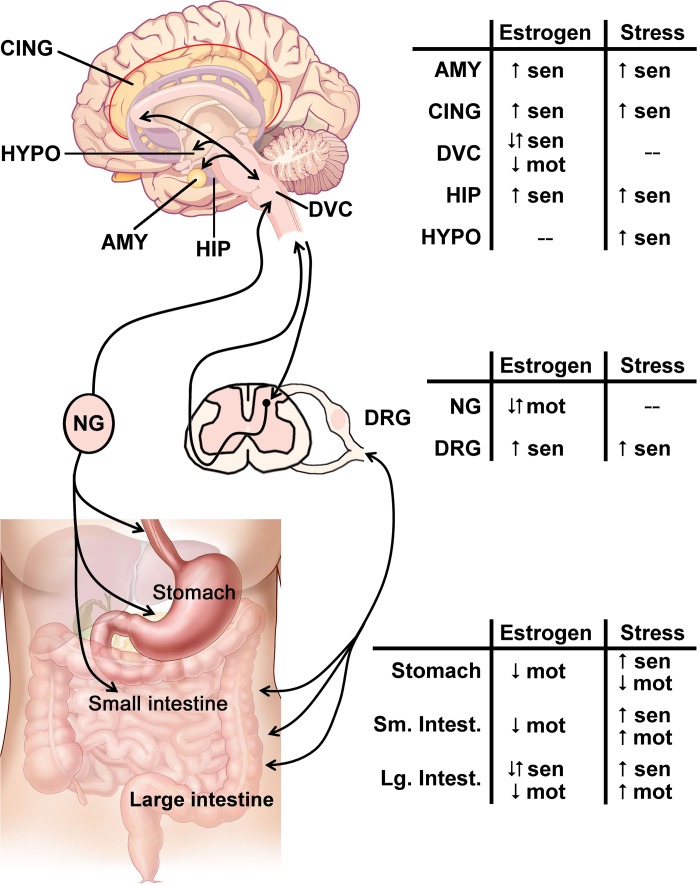
The effect of estrogen or stress in the brain-gut axis. The brain-gut axis, illustrated on the left, comprises bidirectional communication from the visceral organs to the brain via spinal and parasympathetic connections. Within the stress and pain responsive areas in the brain, such as the amygdala (AMY), cingulate cortex (CING), hippocampus (HIP), and hypothalamus (HYPO), integrate signals from the gastrointestinal (GI) tract are transmitted through brainstem areas such as the dorsal vagal complex (DVC). The bidirectional communication is relayed and modified within parasympathetic ganglia, such as the nodose ganglia (NG), and/or sympathetic dorsal root ganglia (DRG), with further regulation of noxious signals within the dorsal horn of the spinal cord. Within the GI tract, the stomach and small intestine (Sm. Intest.) are primarily innervated by vagal afferents, whereas the majority of the large intestine (Lg. Intest.) is innervated by spinal afferents. For each region of the brain-gut axis, the summarized effect of estrogen signaling or stress on sensation (sen) or motility (mot) is indicated with up arrows (↑) for increased responses, down arrows (↓) for decreased responses, or both arrows (↑↓) when the response can both increase and decrease depending on the receptor subtype. Changes are measured compared with ovariectomized females for estrogen or nonstressed baselines for stress. A “−” indicates that there is no literature consensus on the effect at the listed region. Brain and GI images modified from CNX OpenStax/Wikimedia Commons/CC-BY-4.0. Available at https://commons.wikimedia.org/wiki/File:Figure_35_03_06.jpg and https://commons.wikimedia.org/wiki/File:GI_normal.jpg.
NTS neurons project to the DMV primarily via GABAergic, glutamatergic, and catecholaminergic synapses, with GABAergic inputs exerting the strongest influence on the activity of gastric-projecting DMV neurons. Microinjection of the GABAA receptor antagonist bicuculline into the DVC increases gastric motility, for example, whereas microinjections of glutamatergic or catecholaminergic antagonists have limited effects on gastric motility and tone under basal conditions. Using a brainstem slice preparation, we and others have shown that bicuculline increases the firing rate in the majority of DMV neurons, suggesting a robust GABAergic synaptic input onto these neurons, which tonically regulates their excitability. Notably, these GABAergic NTS-DMV synapses are not static but undergo a great deal of plastic changes that enable an appropriate response of vagally regulated gastric motility to variable physiological and pathophysiological conditions. The vagal output that modulates gastric motility, or smooth muscle contractility, is thus largely dependent on the activity of DMV neurons. Both the intrinsic spontaneous pacemaking properties as well as the synaptic inputs to the DMV neurons shape their excitability and, by consequence, determine the vagal motor output to the stomach (85). Lower GI motility is modulated by the parasympathetic fibers originating in the pelvic ganglia that innervate the distal colon.
Both the upper and lower GI tract are also innervated by sympathetic fibers from the prevertebral ganglia, which project to the esophagus, stomach, and proximal small intestine (celiac ganglia), duodenum (superior mesenteric ganglia), and distal small intestine and colon (inferior mesenteric ganglia). These ganglia play an essential role in the inhibition of motility via activation of presynaptic α2 receptors (29).
BRAIN-GUT AXIS REGULATION OF SENSITIVITY
Sensory information, including noxious somatic stimuli, visceral pain, and responses to neuromodulators released from the enteric neurons, is detected by nociceptors located throughout the layers of the GI tract (35). Although vagal afferent fibers play a significant role in upper GI pain signaling, the majority of nociceptive signaling occurs via thoracolumbar sympathetic afferents (30). The nociceptive neurons have cell bodies located in the dorsal root ganglia and transmit the noxious signal to the dorsal horn of the spinal cord (2, 8). Ascending fibers transmit pain signals to higher centers via various tracts and are relayed by the thalamus to cortical areas for localization of pain and to limbic areas, such as the amygdala, insula, and nucleus accumbens, for the processing of the emotional component of pain (14). Descending inhibitory brainstem pathways are activated by outputs from both the cortical and the limbic systems in response to the pain signals, decreasing noxious signaling by inhibiting dorsal horn neurons (38).
Chronic visceral pain is associated with sensitization that occurs in both peripheral sensory receptors and in the neuronal network, mediating pain responses in the brain. Peripheral sensitization in response to injury or infection is associated with receptor activation by inflammatory mediators such as cytokines, chemokines, or prostaglandins and/or algesic chemicals such as bradykinin or histamine (75). The downstream signaling further sensitizes visceral afferents via modification of existing cell-membrane receptors that increase excitability of the afferent fibers as well as via changes in gene expression that lead to insertion of more or different classes of receptors into the cell membrane. These changes in sensory neurons modify the amount and pattern of neurotransmitters released within the dorsal horn of the spinal cord and amplify pain signals via both increased centripetal synaptic transmission and decreased descending inhibitory modulation (26).
In the brain, a similar mechanism to promote and maintain chronic pain can be evoked in the thalamus and brainstem. Increased afferent nociceptive neurotransmission because of peripheral or spinal sensitization leads to hypersensitivity and central remodeling in the thalamus and enhances signaling to the other cortical and limbic regions (74). The integration nuclei, including prefrontal cortex, cingulate cortex, amygdala, and insula, are subsequently sensitized in response to increased afferent stimulation, which can produce an enhanced negative emotional response and/or disrupt the descending inhibitory pathways (89).
ESTROGEN RECEPTOR SIGNALING AND EXPRESSION
The biological effects of estrogen are mediated through two subtypes of genomic/nuclear receptors, estrogen receptor (ER)α and ERβ, as well as membrane-bound/nongenomic receptors, G protein-coupled receptor 30/G protein-coupled ER 1. The mechanisms of estrogen action involve a long-term, slow genomic effect via actions at nuclear receptors and a rapid, nongenome action via activation of membrane-bound G protein-coupled ER 1 receptors (40).
ERs are expressed throughout the brain, including the hypothalamus, amygdala, and midbrain, all of which have been shown to send extensive projections to preganglionic vagal neurons of the DMV, and hence modulate GI functions (12, 57, 63, 80). ERs are also expressed on the myenteric plexus of both rodents and humans (1, 58, 59, 90, 93).
Estrogen or its nonselective agonist, 17β-estradiol, inhibits voltage-gated potassium channels in CNS regions (23) as well as in the GI tract, resulting in inhibition of smooth muscle contractility in both stomach and colon (1, 59, 90, 93) and modulates synaptic transmission and neuronal firing rate via actions on both glutamate and GABAergic transmission (45, 64, 70, 88).
ESTROGEN EFFECT ON GI MOTILITY
Recent evidence indicates that ERs are abundant in the brainstem neuronal population, including NTS and DMV neurons, thus providing the neuroanatomical support for the direct effect of estrogen on either the DMV membrane or the critical GABAergic synapses between NTS and DMV, hence vagal efferent output to the stomach (76, 87). Furthermore, estrogen promotes increased density of vagal afferent projections to the NTS (20), suggesting that estrogen may also facilitate GABAergic neurotransmission to the gastric-projecting neurons of the DMV, thereby decreasing their excitability and vagal efferent output to the stomach. Additionally, direct administration of estrogen onto isolated gastric smooth muscle decreases gastric contractions, likely via a cGMP-dependent NO production (1, 77). Importantly, such effects of estrogen are also sex-dependent, since the relaxation in response to estrogen is greater in females compared with males (1).
In general, estrogen has been shown to delay colonic motility in in vivo and in vitro rodent models via the release of NO (7, 58, 93). However, short-term sex hormone supplementation and withdrawal in healthy postmenopausal women was not found to affect colonic transit, suggesting the effects of estrogen on GI motility may be influenced by the dosage and timing of hormonal exposure (31).
ESTROGEN EFFECT ON GI SENSITIVITY
ERs are distributed at all levels of the visceral pain sensation pathways, including the ENS, spinal cord, and the brain centers mediating pain responses (81). In peripheral visceral afferent terminals, estrogen can modulate nociception by altering ion channel opening and regulation of receptor expression. Furthermore, estrogen also activates colonic tachykinin neurokinin 1 receptor and probably induces substance P release, in addition to modulating inflammatory pathways, secretion, and barrier function (10, 61, 78). Intrathecal administration of an ERα agonist increases the visceromotor behavioral response to colonic distension in ovariectomized rats, suggesting an important role of spinal ER in mediating visceral sensation (17, 47).
An emerging body of evidence suggests that estrogen modulates not only pain perception but also the processing of visceral information in the CNS. Brain imaging studies have shown that, compared with men with IBS, women with IBS have increased activation in emotional circuits, including the amygdala and locus coeruleus, in response to aversive visceral stimuli (54, 55). Elevation of estrogen levels by implantation of estradiol in the amygdala has been shown to increase visceromotor pain response to colorectal distension in ovariectomized rats (67). Although the underlying mechanisms of the central estrogen actions have not been fully investigated, several studies have suggested that estrogen may alter the expression of specific receptors related to pain signaling, such as the glucocorticoid receptor (GR) (73). The estrogen-mediated mechanism may also involve opioid systems, as evidence suggests that estrogen can promote µ-opioid receptor activation in several brain areas, such as the amygdala and bed nucleus of the stria terminalis, related to pain processing (15).
STRESS MODULATION OF GI MOTILITY
Stress can be defined as a stimulus or event that challenges the physiological and psychological homeostasis of an individual (27, 86). A rapid, appropriate response to stress is a reflexive mechanism that allows for necessary adaptive processes of relatively brief duration to maintain physiological homeostasis. Conversely, prolonged stress represents a more serious challenge and requires more sustained modifications. Stressful situations promote a complex and integrated rearrangement of neuroendocrine and autonomic stress systems, including the vagal neurocircuits that control GI motility (41, 80, 85). Stress activates the hypothalamic-pituitary-adrenocortical axis, resulting in release of corticotrophin-releasing hormone (CRH) from the paraventricular nucleus of the hypothalamus (PVN) and elevations in circulating glucocorticoids. CRH release delays gastric emptying and inhibits gastric motility profoundly through actions that involve vagal motoneurons in the DVC (57). Indeed, functional GI disorders, including FD and IBS, are correlated highly with stress, and stressful situations trigger and exacerbate GI symptoms in susceptible individuals (21, 28). A lack of resilience, habituation, or adaptation to stress results in dysfunction of both stomach (delayed gastric emptying) and colon (accelerated colonic motility) (6). The response of individuals to stress, however, differs such that some individuals exhibit high level of resistance, whereas other individuals show vulnerability. It is crucial and urgent to recognize and elucidate the underlying mechanisms that determine the degree of stress resilience or susceptibility to enable a better understanding of stress-associated GI-related dysfunctions.
Cumulative evidence strongly supports the anxiolytic and stress-attenuating effects of oxytocin, including the restoration of impaired gastric and colonic motility by oxytocin. A series of studies pioneered by Takahashi’s group (5, 6, 91) has highlighted the essential role of central oxytocin in adaptive GI response following chronic repetitive stress. Furthermore, oxytocin is involved in restoring the delayed gastric emptying and impaired gastric motility following acute stress or chronic stress maladaptation (91). Although several beneficial effects of oxytocin on GI motility are attributed to its action to reduce the expression and release of CRH in the PVN and, by consequence, the prominent systemic effect on the hypothalamic-pituitary-adrenocortical axis (13, 53, 68), one cannot downplay the direct influence of hypothalamic oxytocin on vagal neurocircuits innervating the GI tract. In fact, oxytocin projections from the PVN are present in the DVC at birth and increase markedly with age. In adult rats, oxytocin axons occur throughout the rostrocaudal extent of the DVC and appose closely to GI-projecting DMV neurons (60). This anatomical evidence suggests that oxytocin may regulate the activity of vagal neurocircuits directly, thus influencing the vagal output to the peripheral organs including the GI tract (60). Indeed, upon its release onto the brainstem vagal neurons, oxytocin excites DMV neurons and inhibits glutamate but not GABAergic neurotransmission, resulting in gastric relaxation through the activation of a postganglionic NO-mediated pathway (11, 42, 72).
It is important to note that the oxytocinergic connection from the PVN to the DVC undergoes a high level of neuroplasticity in both morphology and physiology, especially in conditions related to stressful stimuli. In naïve, nonstressed rats, oxytocin-mediated modulation of previously unresponsive NTS-DMV GABAergic neurotransmission is uncovered by pretreatment with CRH. Furthermore, the gastric relaxation induced by microinjection of oxytocin into the DVC is attenuated, abolished, or even reversed in CRH-exposed rats, possible via a cAMP-dependent translocation of oxytocin receptors to the terminals of GABAergic NTS-DMV synapses. Interestingly, following stress load, the mechanism of action of oxytocin engages another distinct pathway; in fact, in naïve conditions, the oxytocin-mediated effects occur via activation of a nonadrenergic noncholinergic-NO pathway, whereas after stress, they involve the activation of postganglionic VIP- and cholinergic-vagal pathways (11, 42).
Furthermore, we demonstrated recently that rats that undergo chronic repetitive stress display a higher number of oxytocin-immunoreactive neurons that project from the PVN to the DVC, as well as an increased density of oxytocin-immunoreactive fibers in the DVC (50). Such an upregulation of oxytocin in the hypothalamic-vagal neurocircuits may contribute to stress adaptation and restoration of GI motility, although its precise physiological effect and the modulation by sex hormones need further investigation.
Although the mechanisms of neuroplasticity in vagal neurocircuits induced by chronic stress are still largely unknown, the receptor translocation seems to be one important candidate that can explain the rearrangement of brainstem wiring that determines the level of adaptive response following chronic stress exposure. Indeed, we have shown recently that, following chronic stress exposure, the response of vagal neurocircuits to α2-adreneceptor activation varies according to the type of chronic stress. Rats that underwent chronic variable stress showed a larger inhibition of antrum tone in response to α2-adreneceptor activation compared with control or rats that underwent chronic repetitive stress. The translocation of α2-adreneceptor on GABAergic terminal of NTS-DMV synapses, combined with changes in intrinsic DMV neuronal excitability, may be responsible for the maladaptive response to α2-adreneceptor activation on gastric tone and motility (49). More detailed investigations on the mechanisms of neuroplasticity of vagal neurocircuits that occurred following chronic stress, as well as how these changes contribute to the adaptive or maladaptive response to stress, are certainly needed.
STRESS MODULATION OF GI SENSITIVITY
Stress maladaptation and negative emotions also play a significant role in the modulation of colorectal hypersensitivity, which contributes to IBS. Clinically, evidence implicates that periods of stress exhibit a high comorbidity with anxiety, depression, and other psychiatric disorders in the exacerbation of IBS symptoms.
An emerging body of evidence has shown that stress enhances visceral hypersensitivity through multifactorial mechanisms, e.g., psychological stress increases colonic permeability, epithelial secretion, and the structure and composition of the ENS, likely via CRH1-mediated actions (56, 69, 80).
In addition to peripheral mechanisms that mediated stress-induced visceral hypersensitivity, activation of central neuroendocrine and pain facilitatory mechanisms by stress appears to play a prominent role in colonic hypersensitivity (34). Neuroimaging studies in patients with IBS have shown a greater response to nociceptive stimuli in limbic regions (62) that regulate sensory processing and emotion. In particular, several studies have suggested that neuronal remodeling in the central nucleus of the amygdala (CeA) following chronic stress exposure exacerbates nociception and promotes visceral hypersensitivity (34). This neuronal remodeling involves regulation of CRH expression as well as the corticosterone (CORT) receptors, mineralocorticoid receptors (MRs), and GRs. Chronic stress or stereotaxic delivery of CORT in the CeA induces visceral hypersensitivity, which can be attenuated by central application of GR or MR antagonist to the CeA (66) or systemic administration of a GR antagonist (43). Furthermore, a persistent decrease in GR expression in the CeA and an upregulation of CRH has also been observed following visceral hypersensitivity induced by either stress or CeA administration of CORT (33, 82, 83). Selective knockdown of GR or MR in the absence of CORT exposure in the CeA is sufficient to promote visceral hypersensitivity in stress-naïve rats, indicating a significant role of GR and MR signaling in the CeA for modulation of colonic sensitivity (51). In addition, CRH expression in the CeA is a further regulator in mediating stress-induced visceral hypersensitivity. Indeed, intra-CeA CRH administration increases colonic sensitivity via CRH1 receptor activation, and similar findings were demonstrated in female rats that had undergone an early life stressor (73) (79). Knockdown of CRH in the CeA attenuates visceral hypersensitivity induced by adult or early life stress, as does exposure of CeA to elevated CORT (52, 73). Furthermore, recent evidence also suggests that stress-induced visceral hypersensitivity involves central epigenetic mechanisms within the CeA (82, 84).
SUMMARY AND CONCLUSION
The incidence of FGIDs is disproportionately higher in women, possibly because of a complex interaction between sex hormone signaling and stress reactivity on the function of the brain-gut axis. Specifically, both preclinical and clinical evidence has demonstrated that estrogen can affect GI motility and sensitivity via direct activation of its receptors, which are located throughout the brain-gut axis, and indirectly via modulation of other receptor systems. Many women with FGIDs have also experienced multiple stressors across their lifespan, the additive effects of which can lead to peripheral and central sensitization along the brain-gut axis to affect motility and sensitivity throughout the GI tract. By further investigating sex- or stress-specific mechanisms underlying FGID pathophysiology, targeted therapies can be developed to provide relief for these patient populations.
GRANTS
This work was supported by National Institute of Health Grants DK-99350 and DK-120170 to R. A. Travagli. G.-Van Meerveld is a Senior Research Career Scientist with the Department of Veterans Affairs (IK6-BX003610 and I01 BX001195). A. C. Johnson is a Career Development Award Fellow with the Department of Veterans Affairs (IK2-BX003630). The material in this review does not represent the views of the US Department of Veterans Affairs or the United States Government.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Y.J., B.G.-V.M., A.C.J., and R.A.T. drafted manuscript; Y.J., B.G.-V.M., A.C.J., and R.A.T. edited and revised manuscript; Y.J., B.G.-V.M., A.C.J., and R.A.T. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Cesare M. Travagli and Zoraide Travagli for support and encouragement and Dr. Kirsteen N. Browning for comments on previous versions of the manuscript.
REFERENCES
- 1.Al-Shboul OA, Nazzal MS, Mustafa AG, Al-Dwairi AN, Alqudah MA, Abu Omar A, Alfaqih MA, Alsalem MI. Estrogen relaxes gastric muscle cells via a nitric oxide- and cyclic guanosine monophosphate-dependent mechanism: a sex-associated differential effect. Exp Ther Med 16: 1685–1692, 2018. doi: 10.3892/etm.2018.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res 1000: 40–56, 2004. doi: 10.1016/j.brainres.2003.10.073. [DOI] [PubMed] [Google Scholar]
- 3.Aloisi AM, Affaitati G, Ceccarelli I, Fiorenzani P, Lerza R, Rossi C, Pace MC, Chiefari M, Aurilio C, Giamberardino MA. Estradiol and testosterone differently affect visceral pain-related behavioural responses in male and female rats. Eur J Pain 14: 602–607, 2010. doi: 10.1016/j.ejpain.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 4.Aytuğ N, Giral A, Imeryüz N, Enç FY, Bekiroğlu N, Aktaş G, Ulusoy NB. Gender influence on jejunal migrating motor complex. Am J Physiol Gastrointest Liver Physiol 280: G255–G263, 2001. doi: 10.1152/ajpgi.2001.280.2.G255. [DOI] [PubMed] [Google Scholar]
- 5.Babygirija R, Bülbül M, Yoshimoto S, Ludwig K, Takahashi T. Central and peripheral release of oxytocin following chronic homotypic stress in rats. Auton Neurosci 167: 56–60, 2012. doi: 10.1016/j.autneu.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 6.Babygirija R, Zheng J, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Sustained delayed gastric emptying during repeated restraint stress in oxytocin knockout mice. J Neuroendocrinol 22: 1181–1186, 2010. doi: 10.1111/j.1365-2826.2010.02069.x. [DOI] [PubMed] [Google Scholar]
- 7.Baumgartner C, Hubacher T, Krayer M, Gschossmann J. In vitro spontaneous contractile activity of colonic smooth muscle in naive Lewis rats: acute effect of gonadal hormones. J Dig Dis 18: 13–22, 2017. doi: 10.1111/1751-2980.12438. [DOI] [PubMed] [Google Scholar]
- 8.Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev 67: 67–186, 1987. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Bond EF, Heitkemper MM, Perigo R. Gastric emptying and gastric-intestinal transit in rats with varying ovarian hormone status. Nurs Res 45: 218–224, 1996. doi: 10.1097/00006199-199607000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Bradesi S, Eutamene H, Garcia-Villar R, Fioramonti J, Bueno L. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain 102: 227–234, 2003. doi: 10.1016/S0304-3959(02)00056-8. [DOI] [PubMed] [Google Scholar]
- 11.Browning KN, Babic T, Toti L, Holmes GM, Coleman FH, Travagli RA. Plasticity in the brainstem vagal circuits controlling gastric motor function triggered by corticotropin releasing factor. J Physiol 592: 4591–4605, 2014. doi: 10.1113/jphysiol.2014.278192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browning KN, Travagli RA. Central nervous system control of gastrointestinal motility and secretion and modulation of gastrointestinal functions. Compr Physiol 4: 1339–1368, 2014. doi: 10.1002/cphy.c130055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bülbül M, Babygirija R, Cerjak D, Yoshimoto S, Ludwig K, Takahashi T. Hypothalamic oxytocin attenuates CRF expression via GABA(A) receptors in rats. Brain Res 1387: 39–45, 2011. doi: 10.1016/j.brainres.2011.02.091. [DOI] [PubMed] [Google Scholar]
- 14.Bushnell MC, Ceko M, Low LA. Cognitive and emotional control of pain and its disruption in chronic pain. Nat Rev Neurosci 14: 502–511, 2013. doi: 10.1038/nrn3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cairns BE, Gazerani P. Sex-related differences in pain. Maturitas 63: 292–296, 2009. doi: 10.1016/j.maturitas.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Canavan C, West J, Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol 6: 71–80, 2014. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cao DY, Bai G, Ji Y, Traub RJ. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen-induced visceral hypersensitivity. Gut 64: 1913–1920, 2015. doi: 10.1136/gutjnl-2014-307748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chaloner A, Greenwood-Van Meerveld B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J Pain 14: 270–280, 2013. doi: 10.1016/j.jpain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 19.Chen TS, Doong ML, Chang FY, Lee SD, Wang PS. Effects of sex steroid hormones on gastric emptying and gastrointestinal transit in rats. Am J Physiol Gastrointest Liver Physiol 268: G171–G176, 1995. doi: 10.1152/ajpgi.1995.268.1.G171. [DOI] [PubMed] [Google Scholar]
- 20.Ciriello J, Caverson MM. Effect of estrogen on vagal afferent projections to the brainstem in the female. Brain Res 1636: 21–42, 2016. doi: 10.1016/j.brainres.2016.01.041. [DOI] [PubMed] [Google Scholar]
- 21.Drossman DA. Functional gastrointestinal disorders: history, pathophysiology, clinical features and Rome IV. Gastroenterology 150: 1262–1279.e2, 2016. doi: 10.1053/j.gastro.2016.02.032. [DOI] [PubMed] [Google Scholar]
- 22.Drossman DA, Chang L, Bellamy N, Gallo-Torres HE, Lembo A, Mearin F, Norton NJ, Whorwell P. Severity in irritable bowel syndrome: a Rome Foundation Working Team report. Am J Gastroenterol 106: 1749–1759, 2011. doi: 10.1038/ajg.2011.201. [DOI] [PubMed] [Google Scholar]
- 23.Druzin M, Malinina E, Grimsholm O, Johansson S. Mechanism of estradiol-induced block of voltage-gated K+ currents in rat medial preoptic neurons. PLoS One 6: e20213, 2011. doi: 10.1371/journal.pone.0020213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Enck P, Aziz Q, Barbara G, Farmer AD, Fukudo S, Mayer EA, Niesler B, Quigley EM, Rajilić-Stojanović M, Schemann M, Schwille-Kiuntke J, Simren M, Zipfel S, Spiller RC. Irritable bowel syndrome. Nat Rev Dis Primers 2: 16014, 2016. doi: 10.1038/nrdp.2016.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enck P, Azpiroz F, Boeckxstaens G, Elsenbruch S, Feinle-Bisset C, Holtmann G, Lackner JM, Ronkainen J, Schemann M, Stengel A, Tack J, Zipfel S, Talley NJ. Functional dyspepsia. Nat Rev Dis Primers 3: 17081, 2017. doi: 10.1038/nrdp.2017.81. [DOI] [PubMed] [Google Scholar]
- 26.Fornasari D. Pain mechanisms in patients with chronic pain. Clin Drug Investig 32, Suppl 1: 45–52, 2012. doi: 10.2165/11630070-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 27.Franklin TB, Saab BJ, Mansuy IM. Neural mechanisms of stress resilience and vulnerability. Neuron 75: 747–761, 2012. doi: 10.1016/j.neuron.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 28.Fukudo S. Stress and visceral pain: focusing on irritable bowel syndrome. Pain 154, Suppl 1: S63–S70, 2013. doi: 10.1016/j.pain.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 29.Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 9: 286–294, 2012. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- 30.Gebhart GF, Bielefeldt K. Physiology of visceral pain. Compr Physiol 6: 1609–1633, 2016. doi: 10.1002/cphy.c150049. [DOI] [PubMed] [Google Scholar]
- 31.Gonenne J, Esfandyari T, Camilleri M, Burton DD, Stephens DA, Baxter KL, Zinsmeister AR, Bharucha AE. Effect of female sex hormone supplementation and withdrawal on gastrointestinal and colonic transit in postmenopausal women. Neurogastroenterol Motil 18: 911–918, 2006. doi: 10.1111/j.1365-2982.2006.00808.x. [DOI] [PubMed] [Google Scholar]
- 32.Goyal RK, Guo Y, Mashimo H. Advances in the physiology of gastric emptying. Neurogastroenterol Motil 31: e13546, 2019. doi: 10.1111/nmo.13546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greenwood-Van Meerveld B, Gibson M, Gunter W, Shepard J, Foreman R, Myers D. Stereotaxic delivery of corticosterone to the amygdala modulates colonic sensitivity in rats. Brain Res 893: 135–142, 2001. doi: 10.1016/S0006-8993(00)03305-9. [DOI] [PubMed] [Google Scholar]
- 34.Greenwood Van Meerveld B, Johnson AC. Mechanisms of stress-induced visceral pain. J Neurogastroenterol Motil 24: 7–18, 2018. doi: 10.5056/jnm17137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Grundy D. Neuroanatomy of visceral nociception: vagal and splanchnic afferent. Gut 51, Suppl 1: i2–i5, 2002. doi: 10.1136/gut.51.suppl_1.i2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Günal O, Bozkurt A, Deniz M, Sungur M, Yeğen BC. Effect of sex steroids on colonic distension-induced delay of gastric emptying in rats. J Gastroenterol Hepatol 19: 975–981, 2004. doi: 10.1111/j.1440-1746.2004.03409.x. [DOI] [PubMed] [Google Scholar]
- 37.Gustafsson JK, Greenwood-Van Meerveld B. Amygdala activation by corticosterone alters visceral and somatic pain in cycling female rats. Am J Physiol Gastrointest Liver Physiol 300: G1080–G1085, 2011. doi: 10.1152/ajpgi.00349.2010. [DOI] [PubMed] [Google Scholar]
- 38.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: Specificity, recruitment and plasticity. Brain Res Brain Res Rev 60: 214–225, 2009. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heitkemper MM, Cain KC, Jarrett ME, Burr RL, Hertig V, Bond EF. Symptoms across the menstrual cycle in women with irritable bowel syndrome. Am J Gastroenterol 98: 420–430, 2003. doi: 10.1111/j.1572-0241.2003.07233.x. [DOI] [PubMed] [Google Scholar]
- 40.Heldring N, Pike A, Andersson S, Matthews J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M, Gustafsson JA. Estrogen receptors: how do they signal and what are their targets. Physiol Rev 87: 905–931, 2007. doi: 10.1152/physrev.00026.2006. [DOI] [PubMed] [Google Scholar]
- 41.Herman JP, Tasker JG. Paraventricular Hypothalamic Mechanisms of Chronic Stress Adaptation. Front Endocrinol (Lausanne) 7: 137, 2016. doi: 10.3389/fendo.2016.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Holmes GM, Browning KN, Babic T, Fortna SR, Coleman FH, Travagli RA. Vagal afferent fibres determine the oxytocin-induced modulation of gastric tone. J Physiol 591: 3081–3100, 2013. doi: 10.1113/jphysiol.2013.253732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hong S, Zheng G, Wu X, Snider NT, Owyang C, Wiley JW. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology 140: 627–637.e624, 2011. doi: 10.1053/j.gastro.2010.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Horowitz M, Maddern GJ, Chatterton BE, Collins PJ, Petrucco OM, Seamark R, Shearman DJ. The normal menstrual cycle has no effect on gastric emptying. Br J Obstet Gynaecol 92: 743–746, 1985. doi: 10.1111/j.1471-0528.1985.tb01458.x. [DOI] [PubMed] [Google Scholar]
- 45.Hu P, Liu J, Yasrebi A, Gotthardt JD, Bello NT, Pang ZP, Roepke TA. Gq protein-coupled membrane-initiated estrogen signaling rapidly excites corticotropin-releasing hormone neurons in the hypothalamic paraventricular nucleus in female mice. Endocrinology 157: 3604–3620, 2016. doi: 10.1210/en.2016-1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hutson WR, Roehrkasse RL, Wald A. Influence of gender and menopause on gastric emptying and motility. Gastroenterology 96: 11–17, 1989. doi: 10.1016/0016-5085(89)90758-0. [DOI] [PubMed] [Google Scholar]
- 47.Ji Y, Bai G, Cao DY, Traub RJ. Estradiol modulates visceral hyperalgesia by increasing thoracolumbar spinal GluN2B subunit activity in female rats. Neurogastroenterol Motil 27: 775–786, 2015. doi: 10.1111/nmo.12549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ji Y, Tang B, Traub RJ. The visceromotor response to colorectal distention fluctuates with the estrous cycle in rats. Neuroscience 154: 1562–1567, 2008. doi: 10.1016/j.neuroscience.2008.04.070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang Y, Browning KN, Toti L, Travagli RA. Vagally mediated gastric effects of brain stem α2-adrenoceptor activation in stressed rats. Am J Physiol Gastrointest Liver Physiol 314: G504–G516, 2018. doi: 10.1152/ajpgi.00382.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang Y, Coleman FH, Kopenhaver Doheny K, Travagli RA. Stress adaptation upregulates oxytocin within hypothalamo-vagal neurocircuits. Neuroscience 390: 198–205, 2018. doi: 10.1016/j.neuroscience.2018.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Johnson AC, Greenwood-Van Meerveld B. Knockdown of steroid receptors in the central nucleus of the amygdala induces heightened pain behaviors in the rat. Neuropharmacology 93: 116–123, 2015. doi: 10.1016/j.neuropharm.2015.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Johnson AC, Tran L, Greenwood-Van Meerveld B. Knockdown of corticotropin-releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl Psychiatry 5: e517, 2015. doi: 10.1038/tp.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jurek B, Neumann ID. The oxytocin receptor: from intracellular signaling to behavior. Physiol Rev 98: 1805–1908, 2018. doi: 10.1152/physrev.00031.2017. [DOI] [PubMed] [Google Scholar]
- 54.Labus JS, Dinov ID, Jiang Z, Ashe-McNalley C, Zamanyan A, Shi Y, Hong JY, Gupta A, Tillisch K, Ebrat B, Hobel S, Gutman BA, Joshi S, Thompson PM, Toga AW, Mayer EA. Irritable bowel syndrome in female patients is associated with alterations in structural brain networks. Pain 155: 137–149, 2014. doi: 10.1016/j.pain.2013.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Labus JS, Gupta A, Coveleskie K, Tillisch K, Kilpatrick L, Jarcho J, Feier N, Bueller J, Stains J, Smith S, Suyenobu B, Naliboff B, Mayer EA. Sex differences in emotion-related cognitive processes in irritable bowel syndrome and healthy control subjects. Pain 154: 2088–2099, 2013. doi: 10.1016/j.pain.2013.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lauffer A, Vanuytsel T, Vanormelingen C, Vanheel H, Salim Rasoel S, Tóth J, Tack J, Fornari F, Farré R. Subacute stress and chronic stress interact to decrease intestinal barrier function in rats. Stress 19: 225–234, 2016. doi: 10.3109/10253890.2016.1154527. [DOI] [PubMed] [Google Scholar]
- 57.Lewis MW, Hermann GE, Rogers RC, Travagli RA. In vitro and in vivo analysis of the effects of corticotropin releasing factor on rat dorsal vagal complex. J Physiol 543: 135–146, 2002. doi: 10.1113/jphysiol.2002.019281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li Y, Xu J, Jiang F, Jiang Z, Liu C, Li L, Luo Y, Lu R, Mu Y, Liu Y, Xue B. G protein-coupled estrogen receptor is involved in modulating colonic motor function via nitric oxide release in C57BL/6 female mice. Neurogastroenterol Motil 28: 432–442, 2016. doi: 10.1111/nmo.12743. [DOI] [PubMed] [Google Scholar]
- 59.Liu JYH, Lin G, Fang M, Rudd JA. Localization of estrogen receptor ERα, ERβ and GPR30 on myenteric neurons of the gastrointestinal tract and their role in motility. Gen Comp Endocrinol 272: 63–75, 2019. doi: 10.1016/j.ygcen.2018.11.016. [DOI] [PubMed] [Google Scholar]
- 60.Llewellyn-Smith IJ, Kellett DO, Jordan D, Browning KN, Travagli RA. Oxytocin-immunoreactive innervation of identified neurons in the rat dorsal vagal complex. Neurogastroenterol Motil 24: e136–e146, 2012. doi: 10.1111/j.1365-2982.2011.01851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Looijer-van Langen M, Hotte N, Dieleman LA, Albert E, Mulder C, Madsen KL. Estrogen receptor-β signaling modulates epithelial barrier function. Am J Physiol Gastrointest Liver Physiol 300: G621–G626, 2011. doi: 10.1152/ajpgi.00274.2010. [DOI] [PubMed] [Google Scholar]
- 62.Mayer EA, Labus JS, Tillisch K, Cole SW, Baldi P. Towards a systems view of IBS. Nat Rev Gastroenterol Hepatol 12: 592–605, 2015. doi: 10.1038/nrgastro.2015.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McEwen BS, Akama KT, Spencer-Segal JL, Milner TA, Waters EM. Estrogen effects on the brain: actions beyond the hypothalamus via novel mechanisms. Behav Neurosci 126: 4–16, 2012. doi: 10.1037/a0026708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mukherjee J, Cardarelli RA, Cantaut-Belarif Y, Deeb TZ, Srivastava DP, Tyagarajan SK, Pangalos MN, Triller A, Maguire J, Brandon NJ, Moss SJ. Estradiol modulates the efficacy of synaptic inhibition by decreasing the dwell time of GABAA receptors at inhibitory synapses. Proc Natl Acad Sci USA 114: 11763–11768, 2017. doi: 10.1073/pnas.1705075114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mulak A, Taché Y, Larauche M. Sex hormones in the modulation of irritable bowel syndrome. World J Gastroenterol 20: 2433–2448, 2014. doi: 10.3748/wjg.v20.i10.2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Myers B, Greenwood-Van Meerveld B. Differential involvement of amygdala corticosteroid receptors in visceral hyperalgesia following acute or repeated stress. Am J Physiol Gastrointest Liver Physiol 302: G260–G266, 2012. doi: 10.1152/ajpgi.00353.2011. [DOI] [PubMed] [Google Scholar]
- 67.Myers B, Schulkin J, Greenwood-Van Meerveld B. Sex steroids localized to the amygdala increase pain responses to visceral stimulation in rats. J Pain 12: 486–494, 2011. doi: 10.1016/j.jpain.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 68.Neumann ID, Wigger A, Torner L, Holsboer F, Landgraf R. Brain oxytocin inhibits basal and stress-induced activity of the hypothalamo-pituitary-adrenal axis in male and female rats: partial action within the paraventricular nucleus. J Neuroendocrinol 12: 235–243, 2000. doi: 10.1046/j.1365-2826.2000.00442.x. [DOI] [PubMed] [Google Scholar]
- 69.Nozu T, Miyagishi S, Kumei S, Nozu R, Takakusaki K, Okumura T. Lovastatin inhibits visceral allodynia and increased colonic permeability induced by lipopolysaccharide or repeated water avoidance stress in rats. Eur J Pharmacol 818: 228–234, 2018. doi: 10.1016/j.ejphar.2017.10.056. [DOI] [PubMed] [Google Scholar]
- 70.Oberlander JG, Woolley CS. 17β-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci 36: 2677–2690, 2016. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Palsson OS, Whitehead WE, Barghout V, Levy R, Field A, Von Korff M, Garner M, Drossman DA, Turner MJ. IBS severity and health-related quality of life improve with age in women but not in men. Am J Gastroenterol 98: S272, 2003. [Google Scholar]
- 72.Peters JH, McDougall SJ, Kellett DO, Jordan D, Llewellyn-Smith IJ, Andresen MC. Oxytocin enhances cranial visceral afferent synaptic transmission to the solitary tract nucleus. J Neurosci 28: 11731–11740, 2008. doi: 10.1523/JNEUROSCI.3419-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Prusator DK, Greenwood-Van Meerveld B. Amygdala-mediated mechanisms regulate visceral hypersensitivity in adult females following early life stress: importance of the glucocorticoid receptor and corticotropin-releasing factor. Pain 158: 296–305, 2017. doi: 10.1097/j.pain.0000000000000759. [DOI] [PubMed] [Google Scholar]
- 74.Saab CY. Pain-related changes in the brain: diagnostic and therapeutic potentials. Trends Neurosci 35: 629–637, 2012. doi: 10.1016/j.tins.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 75.Schaible HG, Ebersberger A, Natura G. Update on peripheral mechanisms of pain: beyond prostaglandins and cytokines. Arthritis Res Ther 13: 210, 2011. doi: 10.1186/ar3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res 1123: 89–100, 2006. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 77.Shah S, Nathan L, Singh R, Fu YS, Chaudhuri G. E2 and not P4 increases NO release from NANC nerves of the gastrointestinal tract: implications in pregnancy. Am J Physiol Regul Integr Comp Physiol 280: R1546–R1554, 2001. doi: 10.1152/ajpregu.2001.280.5.R1546. [DOI] [PubMed] [Google Scholar]
- 78.Straub RH. The complex role of estrogens in inflammation. Endocr Rev 28: 521–574, 2007. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 79.Su J, Tanaka Y, Muratsubaki T, Kano M, Kanazawa M, Fukudo S. Injection of corticotropin-releasing hormone into the amygdala aggravates visceral nociception and induces noradrenaline release in rats. Neurogastroenterol Motil 27: 30–39, 2015. doi: 10.1111/nmo.12462. [DOI] [PubMed] [Google Scholar]
- 80.Tache Y, Larauche M, Yuan PQ, Million M. Brain and gut CRF signaling: biological actions and role in the gastrointestinal tract. Curr Mol Pharmacol 11: 51–71, 2018. doi: 10.2174/1874467210666170224095741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ter Horst GJ, Wichmann R, Gerrits M, Westenbroek C, Lin Y. Sex differences in stress responses: focus on ovarian hormones. Physiol Behav 97: 239–249, 2009. doi: 10.1016/j.physbeh.2009.02.036. [DOI] [PubMed] [Google Scholar]
- 82.Tran L, Chaloner A, Sawalha AH, Greenwood Van-Meerveld B. Importance of epigenetic mechanisms in visceral pain induced by chronic water avoidance stress. Psychoneuroendocrinology 38: 898–906, 2013. doi: 10.1016/j.psyneuen.2012.09.016. [DOI] [PubMed] [Google Scholar]
- 83.Tran L, Greenwood-Van Meerveld B. Altered expression of glucocorticoid receptor and corticotropin-releasing factor in the central amygdala in response to elevated corticosterone. Behav Brain Res 234: 380–385, 2012. doi: 10.1016/j.bbr.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 84.Tran L, Schulkin J, Ligon CO, Greenwood-Van Meerveld B. Epigenetic modulation of chronic anxiety and pain by histone deacetylation. Mol Psychiatry 20: 1219–1231, 2015. doi: 10.1038/mp.2014.122. [DOI] [PubMed] [Google Scholar]
- 85.Travagli RA, Anselmi L. Vagal neurocircuitry and its influence on gastric motility. Nat Rev Gastroenterol Hepatol 13: 389–401, 2016. doi: 10.1038/nrgastro.2016.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10: 397–409, 2009. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.VanderHorst VG, Gustafsson JA, Ulfhake B. Estrogen receptor-alpha and -beta immunoreactive neurons in the brainstem and spinal cord of male and female mice: relationships to monoaminergic, cholinergic, and spinal projection systems. J Comp Neurol 488: 152–179, 2005. doi: 10.1002/cne.20569. [DOI] [PubMed] [Google Scholar]
- 88.Wang L, Burger LL, Greenwald-Yarnell ML, Myers MG Jr, Moenter SM. Glutamatergic transmission to hypothalamic kisspeptin neurons is differentially regulated by estradiol through estrogen receptor α in adult female mice. J Neurosci 38: 1061–1072, 2018. doi: 10.1523/JNEUROSCI.2428-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wilder-Smith CH. The balancing act: endogenous modulation of pain in functional gastrointestinal disorders. Gut 60: 1589–1599, 2011. doi: 10.1136/gutjnl-2011-300253. [DOI] [PubMed] [Google Scholar]
- 90.Winborn WB, Sheridan PJ, McGill HC Jr. Sex steroid receptors in the stomach, liver, pancreas, and gastrointestinal tract of the baboon. Gastroenterology 92: 23–32, 1987. doi: 10.1016/0016-5085(87)90835-3. [DOI] [PubMed] [Google Scholar]
- 91.Zheng J, Babygirija R, Bülbül M, Cerjak D, Ludwig K, Takahashi T. Hypothalamic oxytocin mediates adaptation mechanism against chronic stress in rats. Am J Physiol Gastrointest Liver Physiol 299: G946–G953, 2010. doi: 10.1152/ajpgi.00483.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zia JK, Heitkemper MM. Upper gastrointestinal tract motility disorders in women, gastroparesis, and gastroesophageal reflux disease. Gastroenterol Clin North Am 45: 239–251, 2016. doi: 10.1016/j.gtc.2016.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Zielińska M, Fichna J, Bashashati M, Habibi S, Sibaev A, Timmermans JP, Storr M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol Motil 29: e13025, 2017. doi: 10.1111/nmo.13025. [DOI] [PubMed] [Google Scholar]