Keywords: CMMCs, colon, GECI, motor neurons, optogenetics
Abstract
The enteric nervous system in the large intestine generates two important patterns relating to motility: 1) propagating rhythmic peristaltic smooth muscle contractions referred to as colonic migrating motor complexes (CMMCs) and 2) tonic inhibition, during which colonic smooth muscle contractions are suppressed. The precise neurobiological substrates underlying each of these patterns are unclear. Using transgenic animals expressing the genetically encoded calcium indicator GCaMP3 to monitor activity or the optogenetic actuator channelrhodopsin (ChR2) to drive activity in defined enteric neuronal subpopulations, we provide evidence that cholinergic and nitrergic neurons play significant roles in mediating CMMCs and tonic inhibition, respectively. Nitrergic neurons [neuronal nitric oxide synthase (nNOS)-positive neurons] expressing GCaMP3 exhibited higher levels of activity during periods of tonic inhibition than during CMMCs. Consistent with these findings, optogenetic activation of ChR2 in nitrergic neurons depressed ongoing CMMCs. Conversely, cholinergic neurons [choline acetyltransferase (ChAT)-positive neurons] expressing GCaMP3 markedly increased their activity during the CMMC. Treatment with the NO synthesis inhibitor Nω-nitro-l-arginine also augmented the activity of ChAT-GCaMP3 neurons, suggesting that the reciprocal patterns of activity exhibited by nitrergic and cholinergic enteric neurons during distinct phases of colonic motility may be related.
NEW & NOTEWORTHY Correlating the activity of neuronal populations in the myenteric plexus to distinct periods of gastrointestinal motility is complicated by the difficulty of measuring the activity of specific neuronal subtypes. Here, using mice expressing genetically encoded calcium indicators or the optical actuator channelrhodopsin-2, we provide compelling evidence that cholinergic and nitrergic neurons play important roles in mediating coordinated propagating peristaltic contractions or tonic inhibition, respectively, in the murine colon.
INTRODUCTION
The motility of the murine large intestine consists of two major states of electromechanical activity: rhythmic propagating contractions of smooth muscle that propel fecal matter, referred to in the mouse as colonic migrating motor/myoelectric complexes (CMMCs) (4, 5, 16, 48, 59), and periods of quiescence in between CMMCs during which smooth muscle is inactive, called tonic inhibition (58, 59). CMMCs occur once every 3–4 min and are mediated by neurons within the myenteric plexus, as they are blocked by the sodium channel antagonist tetrodotoxin (TTX) (5). Electrically, CMMCs are preceded by a brief hyperpolarization of smooth muscle, followed by a series of fast electrical oscillations superimposed on a slow depolarization (5). The hyperpolarization is thought to reflect an enhancement of the mechanisms underlying tonic inhibition, discussed below. The fast oscillations are mediated by the stimulation of postjunctional muscarinic ACh receptors by cholinergic excitatory motor neurons (EMNs) within the plexus (5). The slow depolarization is mediated by the stimulation of postjunctional tachykinin receptors by these same EMNs (4, 13) and/or by the termination of tonic inhibition (52, 53). Increasing evidence points to a critical role for myenteric pacemaker interstitial cells of Cajal (ICC-MY) in the translation of neural activity into physiological smooth muscle cell responses (47). Clinically, CMMCs appear similar to high-amplitude propagating contractions observed in the human colon, which have a similar duration to CMMCs (50).
The propagation of CMMCs characteristically begins in the proximal colon and propagates in the anal direction during the propulsion of fecal pellets (26). This coordinated pattern of CMMCs is initiated, in part, by serotonin (5-HT) released from enterochromaffin cells (ECs) in response to chemical or mechanical stimulation of the mucosa, since stroking the mucosa with a brush generates CMMCs (26), since removing the mucosa or genetically depleting EC-derived 5-HT abolishes or reduces the oral to anal coordination of CMMCs, respectively (26, 28, 33). EC-derived 5-HT activates the mucosal endings of intrinsic primary afferent neurons (IPANs) of the Dogiel type II category, which, in turn, drive the firing of serotonergic and/or cholinergic descending interneurons (2, 3, 6, 35). The inhibitory activity of these neurons ensures the sequential oral to anal coordination of CMMCs (27, 56).
Seminal studies by Wood (59) demonstrated that activity within enteric inhibitory motor neurons (IMNs) is required to maintain the state of tonic inhibition of the inherently excitable musculature (37). Electrically, tonic inhibition is characterized by a biphasic postjunctional smooth muscle cell response. The initial phase is a fast hyperpolarization referred to as a fast inhibitory junctional potential (IJP), mediated by the release of the purines ATP and β-NAD from IMNs (31, 38), followed by a smaller and longer duration IJP, mediated by the release of nitric oxide by IMNs (34). Studies of mice lacking the neuronal isoform of the biosynthetic enzyme of NO, neuronal nitric oxide synthase (nNOS), suggest that the nitrergic component of tonic inhibition is also important for gastric emptying (30, 42), as well as for normal pellet formation, maintenance of the hyperpolarized resting membrane potential in colonic smooth muscle, and regulation of CMMC amplitude and frequency (13). However, CMMCs in mice caused by pharmacological or genetic inhibition of NO synthesis are not coordinated and do not propagate (14). In contrast, the increased number of contractions observed in the human colon in response to NO inhibition do propagate (51).
Soluble guanylate cyclase (sGC; or NO-sensitive guanylyl cyclase; NO-GC) represents the main molecular target for NO, producing cGMP, which modulates the activity of a variety of effectors, including cGMP-dependent protein kinase I (PKGI) (18). Deletion of the genes encoding NO-GC or PKGI phenocopies nNOS mutants and produces severe defects in gastrointestinal motility (19, 30, 42, 44). Interestingly, restricted deletion of NO-GC in smooth muscle cells (SMCs) produces limited changes to NO-induced smooth muscle relaxation, whereas combined elimination in SMCs and ICCs recapitulates the effect of global NO-GC deletion (21, 22).
These studies suggest that activity within cholinergic and nitrergic enteric neurons is responsible for CMMCs and tonic inhibition, respectively. Direct evidence supporting this idea comes from the imaging of Ca2+ transients within each of these enteric subpopulations identified by post hoc immunohistochemical staining (2, 10). However, several questions remain. First, identifying the cellular origin of Ca2+ transients after bath application of chemical Ca2+ binding dyes by the post hoc techniques described above is challenging. It would be advantageous to evaluate nitrergic and cholinergic activity during specific colonic motility patterns using new mouse genetic tools that target the expression of fluorescent reporters, genetically encoded calcium indicators (GECI) or genetically encoded optical actuators to these enteric subpopulations (25, 32, 39). These tools permit the unequivocal assignment of neurochemical identity to large subpopulations of enteric neurons active during distinct periods of colonic motility. Furthermore, because cholinergic and nitrergic neurons themselves each represent several different functional subclasses of enteric neurons within the myenteric plexus of the colon, many of which exhibit distinct patterns of activity and/or express distinct combinations of other proteins (e.g., ascending and descending interneurons, IPANs, EMNs, and IMNs) (7, 45, 46, 57), evaluating the spatio-temporal activity signatures or post hoc expression profile of each of these two enteric neuronal subpopulations is likely to further elucidate the identity of distinct enteric neuronal subclasses. Finally, the ability to selectively manipulate the activity of cholinergic and nitrergic neuronal subpopulations using these mouse genetic tools provides the opportunity to study the role of this activity during CMMCs or periods of tonic inhibition.
Using mice expressing the GECI GCaMP3 in cholinergic and nitrergic enteric neurons or the optogenetic actuator channel rhodopsin-2 (ChR2) in nitrergic enteric neurons (9), we studied the activity of these subpopulations during spontaneous and evoked CMMCs, as well as during tonic inhibition. We found that most nitrergic enteric neurons were active during the period of tonic inhibition and inactive during CMMCs, whereas most cholinergic enteric neurons exhibited a marked increase of activity during CMMCs, relative to tonic inhibition. To determine whether these two patterns were related, we blocked the production of NO and observed a robust enhancement in the activity of cholinergic neurons. These findings raise the possibility that nitrergic enteric neurons regulate tonic inhibition through the indirect inhibition of cholinergic EMNs in addition to or instead of the direct inhibition of ICC cells/smooth muscle cells (15, 21, 22, 43). Finally, we optogenetically stimulated nitrergic neurons and observed an immediate inhibition of ongoing CMMCs for the duration of optical stimulation. Together, these studies provide insight into the spatiotemporal pattern and physiological function of activity in defined enteric neuronal subpopulations.
METHODS
Mice.
Conditional GCaMP3 (Jax no. 029043) or channelrhodpsin-2 (Jax no. 024109) mice, which allow for cell-specific expression of the optical sensor GCaMP3 or the optical actuator ChR2, were crossed to ChAT-Cre mice (MMRRC no. 37336), which drive expression in cholinergic enteric neurons; and nNOS-CreERT2 mice (Jax no.014541), which drive expression, upon tamoxifen injection, in nitrergic enteric neurons. For convenience, these are referred to as ChAT-GCaMP3 or nNOS-GCaMP3/ChR2 mice. For tamoxifen injections into nNOS-GCaMP3 or nNOS-ChR2 mice, 200 mg of tamoxifen was first dissolved in 2 mL of 100% ethanol and incubated at 37°C for 30 min, vortexed for 1 min, incubated again at 37°C for 5–10 min and vortexed again for 1 min. This was repeated 3 or 4 times until the drug was completely dissolved. Then, 8 mL of safflower oil was added, and the same cycles were repeated 2 or 3 times. This solution was kept at 4° in the dark for up to 2 wk. Just before administration, this solution was sonicated for 30 min and vortexed for 1 min; then 100 μl (2 mg) was drawn into a 1-mL syringe with a 25-gauge needle and injected intraperitoneally. Three consecutive daily injections were administered to mice at least 8 wk old. Mice were euthanized 2 wk later to allow Cre-mediated gene expression.
Adult mice of each sex (at least 7–9 wk of age) were killed by isoflurane inhalation, and cervical dislocation, in accordance with the requirements of the Animal Ethics Committee at the University of Nevada, Reno. A ventral midline incision was made, and the entire colon was removed and placed in a Sylgard (Dow-Corning, Midland, MI)-lined dish that was perfused with Krebs-Ringer buffer solution [KRB; composition containing (in mM) 120.35 NaCl, 5.9 KCl, 15.5 NaHCO3, 1.2 MgCl2, 1.2 NaH2PO4, 11.5 glucose, 2.5 CaCl2, with pH at 7.4; 21°C], bubbled with 97% O2-3% CO2, and the bath was heated to 37°C.
Imaging the spatial distribution of GCaMP3-expressing neurons in the colon.
The entire colon was cut into proximal, transverse, and distal segments and was pinned and laid in flat sheets. The mucosa was removed by sharp dissection (26), and the tissue was fixed in 4% paraformaldehyde overnight, rinsed, and mounted onto a slide. GCaMP3-expressing myenteric neurons were identified by fluorescence on an inverted microscope (Keyence, Itaska, IL) and counted in 500-μm2 areas across the entire colonic circumference of each colonic segment.
Imaging the activity of GCaMP3-labeled neurons in the colon.
Ca2+-imaging recordings were performed on an Olympus BX51WI upright fluorescence microscope using 10 and 20 Fluor water immersion lenses (Olympus, Center Valley, PA). The tissue was excited at 488 nm, using an X-Cite series 120Q (Lumen Dynamics, ON, Canada) and a modified GFP dichroic cube (excitation 488 nm; emission 543 nm; Chroma Technology, Bellows Falls, VT). Movies were captured with an Andor iXon +897 EMCCD camera (normal recording ~2,000 frames at 32.4 Hz) using Andor Solis 4.14 software (Andor Technology, Belfast, UK). Solis (16-bit, tif) files were analyzed on a MacPro desktop computer (Apple, Cupertino, CA) using in-house analysis software (Volumetry G8c; developed by G. W. Hennig, University of Vermont).
For Ca2+ imaging of ChAT-GCaMP3 and nNOS-GCaMP3 neurons, the proximal and middle colon (∼25 mm length) were cut along the mesenteric border and pinned as a flat sheet, with the serosal side up. The distal portion (∼10 mm) of the colon was also opened along the mesenteric border but was pinned mucosal side up (with the mucosa intact) to evoke CMMCs via mucosal stimulation (2, 12, 26).
To correlate Ca2+ transients to periods of tonic inhibition or a CMMC, movement was tracked using Volumetry G8c. CMMCs can be readily distinguished from slow waves by the magnitude of their movement. A region of interest was drawn around a nonfiring, fluorescently labeled cell that remained in frame for the duration of the recording. A tracking routine followed the object while maintaining a tolerance of at least three pixels in plotting x, y movement.
For quantifying cell response percentages, we simply counted the total number of fluorescent cells in several myenteric ganglia within a field at ×20 magnification, and then quantified the percentage of them that exhibited activity during tonic inhibition, during a spontaneous or an evoked CMMC, or in response to pharmacological treatments. For quantifying frequency, the number of Ca2+ transients captured by Volumetry was counted within a minute. We were often unable to perform adequate spatio-temporal mapping of Ca2+ transient responses to different treatments, because of interference by motion induced by the CMMC, which we needed to track in order to assess the state of motility. Future experiments in the presence of the smooth muscle paralytic nicardipine will be instrumental in evaluating responses with this parameter.
The following supplemental videos are available on http://doi.org/10.5281/zenodo.2696195: 1) spontaneous CMMC in a ChAT-GCaMP3 mouse colon; 2) evoked CMMC in same ChAT-GCaMP3 mouse colon as Video 1; 3) evoked CMMC in a ChAT-GCaMP3 mouse colon; 4) treatment with L-NNA in same ChAT-GCaMP3 mouse colon as Video 3; 5) tonic inhibition in a nNOS-GCaMP3 mouse colon; and 6) tonic inhibition in same nNOS-GCaMP3 mouse colon as Video 5 after treatment with odantseron and hexamethonium.
Optogenetic stimulation of nitrergic enteric neurons during a CMMC.
To measure the effect of nitreregic neuron activation on the magnitude of CMMCs in nNOS-ChR2 mice, the intact isolated colon was dissected in the dark under infrared illumination, and then drawn over a 1.5-mm diameter fire-polished capillary tube, whose length exceeded that of the colon. An artificial pellet was mounted to the capillary glass, and the colon was positioned with the pellet in the middle. The capillary glass was then fixed to the bottom of the organ bath by the ends protruding from each colonic opening. Suture silk was used to connect three force transducers (model TST125C; Biopac Systems, Santa Barbara, CA) to the proximal, transverse and distal segments of the colon. Resting tension was initially set at 8 mN and monitored using an MP100 interface and recorded on a PC running Acqknowledge software 3.2.6 (Biopac Systems). An epoxy-coated pellet was inserted into the proximal end of the colon, where it was allowed to propagate to the midcolon, and then it was held in place using pins. When a CMMC was detected, blue light was shined continuously for 15 s or 25 s by a hand-held laser (450 nm Sapphire Galaxy 3; ZBolt; Happy Valley, OR) mounted on a clamp stand, positioned 30 cm above the preparation.
Drugs.
Hexamethonium bromide, ondansetron, and Nω-nitro-l-arginine (l-NNA) were purchased from Sigma-Aldrich (St. Louis, MO).
Statistical methods.
Statistical comparisons of data were performed using Student’s (paired or unpaired) t-tests, ANOVA, or Wilcoxon rank sum test, and a minimum level of significance was reached at P < 0.05. In the results, N refers to the number of animals, and n refers to the number of cells used. We examined male and female animals in equal numbers and failed to observe any sex-dependent differences, so we pooled these data within our N. All data are presented as means ± SE.
RESULTS
Distribution and activity of colonic nNOS-GCaMP3 neurons.
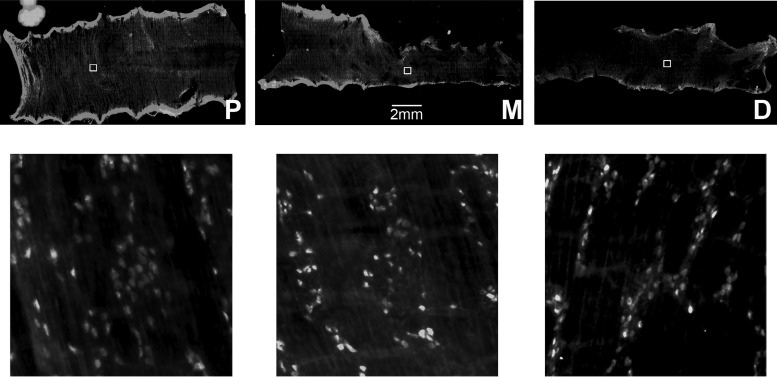
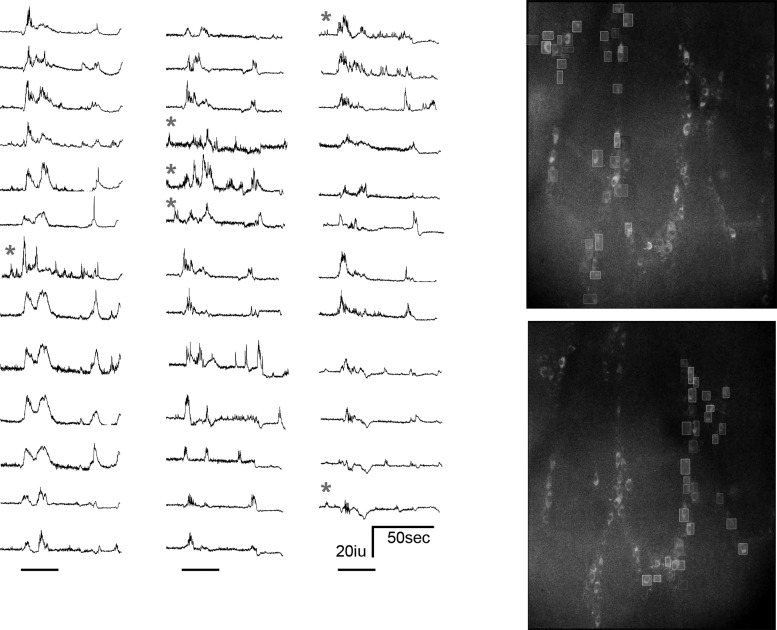
First, we examined the spatial distribution of nitrergic neurons expressing GCaMP3 in the myenteric ganglia of the colon of nNOS-GCaMP3 mice (nNOS-GCaMP3 neurons). nNOS-GCaMP3 neurons were observed within ganglia across the entire circumference of proximal, middle, and distal colon (Fig. 1). The average number of nNOS-GCaMP3 neurons was quantified by counting fluorescent cells within 500-μm2 squares selected from different regions of each colonic segment. Nitrergic neurons were more numerous in proximal and middle than distal colon (80.3 ± 4.1 vs. 81.5 ± 3.7 vs. 54.6 ± 4.8; proximal vs. middle vs. distal; neurons counted in five squares per segment; n = 4). We then imaged spontaneous Ca2+ transients of nNOS-GCaMP3 neurons within individual myenteric ganglia in the middle colon during a period of tonic inhibition, in which there was limited movement (Fig. 2). The patterns of activity between neighboring cells were rarely well synchronized [28.3 ± 7% of nNOS-GCaMP3 neurons with peaks within 1 s of each other during tonic inhibition vs. 86.1 ± 13 of ChAT-GCaMP3 neurons with peaks within 1 s of each other at the onset of a CMMC (see below); average frequency of nNOS-GCaMP3 neurons during tonic inhibition = 17.3 ± 8.8/min; n = 74 cells, N = 4 animals]. Next, we imaged this activity in nNOS-GCaMP3 neurons (size 41.4 ± 0.98 μm long and 17.6 ± 0.43 μm wide) in response to a CMMC evoked by stimulation of the anal mucosa with five paintbrush strokes (Fig. 3). Many nNOS-GCAMP3 neurons exhibited an initial increase in the amplitude and frequency of their Ca2+ transient during the earliest portion of the CMMC (37.1 ± 6.3%; n = 53 cells, N = 4 animals; e.g., cell 8), followed by a reduction in the firing of these cells during the remainder of the CMMC, followed by a resumption of activity after cessation of the CMMC.
Fig. 1.
Representative image of colon of nNOS-GCaMP3 mice that was opened along the mesenteric border, divided into proximal (P), middle (M) and distal (D) segments, and pinned as a circumferential sheet (top). The number of fluorescently labeled neurons was quantified in higher-magnification images (bottom) of 500 μm2 squares (inset boxes in upper row).
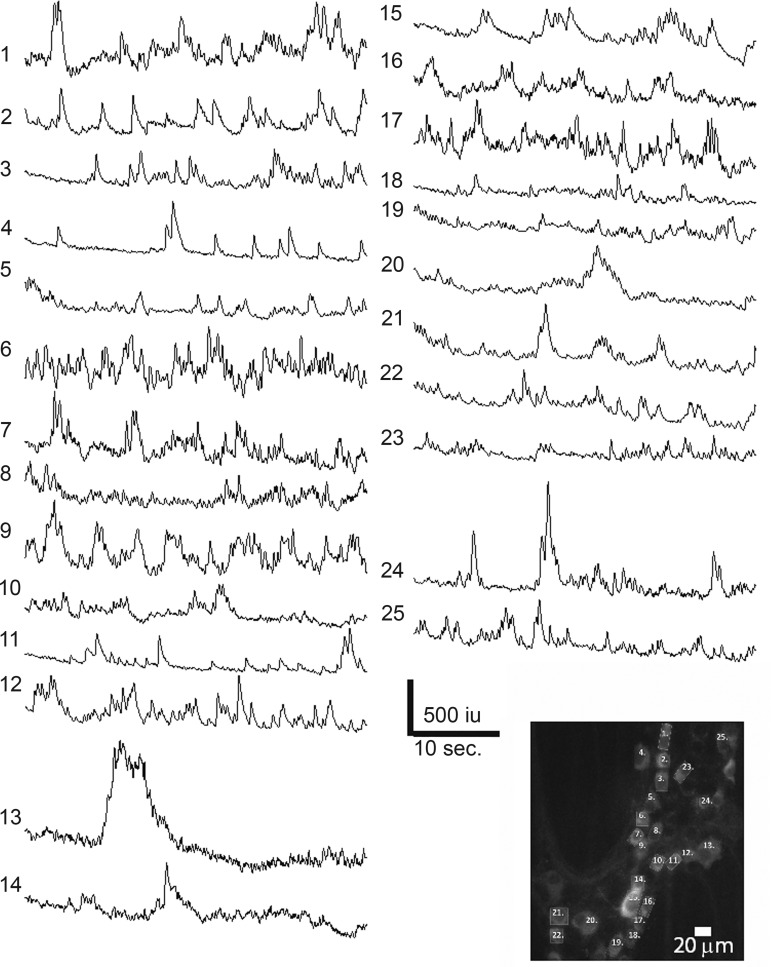
Fig. 2.
Representative spontaneous Ca2+ transients from nNOS-GCaMP3 neurons within an individual myenteric ganglia (bottom right) from the proximal colon during a period of limited movement characteristic of tonic inhibition. Although transients were observed in the majority of nNOS-GCaMP3 neurons, they were rarely correlated between different neurons.
Fig. 3.
Cessation of Ca2+ transients within most nNOS-GCaMP3 neurons during an evoked colonic migrating motor complex (CMMC), the movement of which is illustrated in the bottom trace (C). Note the relative increase in activity in cells 2, 3, 4, 6, 7, and 8 at the beginning of the CMMC, a time when smooth muscle cells exhibit hyperpolarization.
Distribution and activity of colonic ChAT-GCaMP3 neurons.
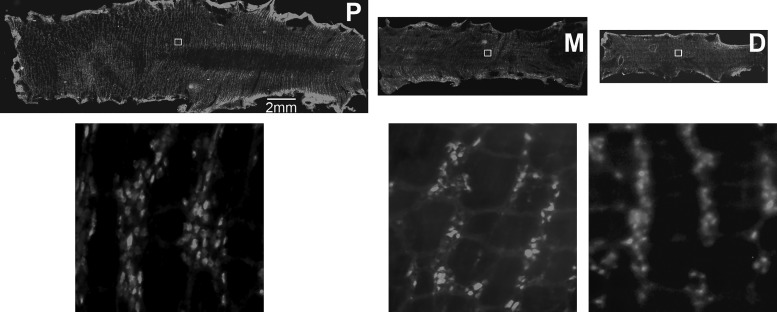
We then examined the spatial distribution of cholinergic neurons expressing GCaMP3 throughout the colon (ChAT-GCaMP3 neurons; Fig. 4), including EMNs, IPANs, and ascending and descending interneurons (INs). Cholinergic neurons were more numerous in proximal than middle and distal colonic segments (130.9 ± 12.8 vs. 90.1 ± 5.1 vs. 74.3 ± 5.3; proximal vs. middle vs. distal colon, respectively; neurons counted in five squares per segment; N = 4 animals). Based the observation that 95% of all enteric neurons in mouse colon are either nitrergic or cholinergic (32), we quantified the percentage of neurons that were nitrergic or cholinergic by expressing them as a percentage of their summed numbers. The percentages of nitrergic and cholinergic neurons in proximal, middle, and distal colon were 37.9, 47.4, and 42.2 versus 62.1, 52.6, and 57.8, respectively, demonstrating a higher percentage of cholinergic than nitrergic neurons in each region of the colon, similar to results generated from the entire colon (32).
Fig. 4.
Representative image of colon of ChAT-GCaMP3 mice that was opened along the mesenteric border, divided into proximal (P), middle (M), and distal (D) segments, and pinned as a circumferential sheet (upper row of images). The number of fluorescently labeled neurons was quantified in higher-magnification images (lower row) of 500 μm2 squares (inset boxes in upper row).
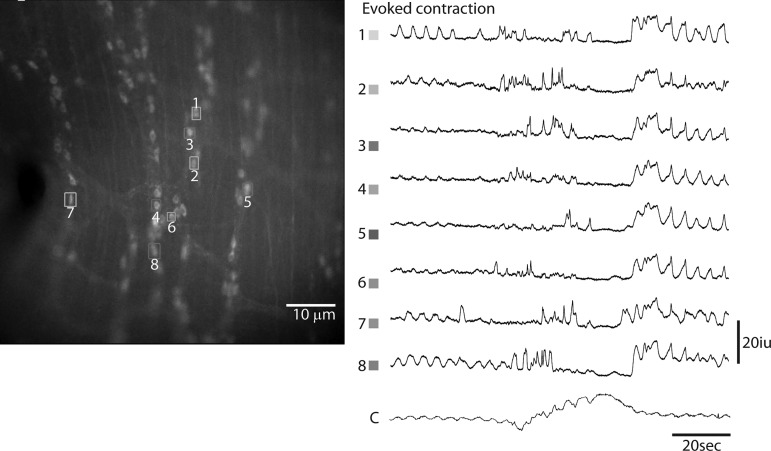
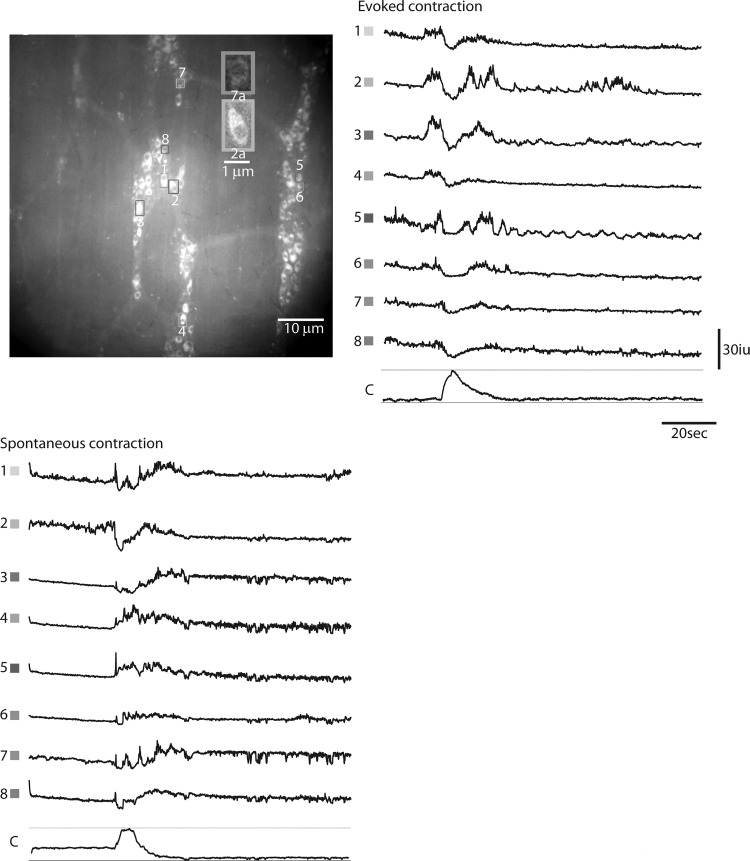
Next, we recorded spontaneous activity of the same cohorts of ChAT-GCaMP3 neurons in the middle colon during tonic inhibition (i.e., periods in between CMMCs) and in response to a spontaneous or evoked CMMC (Figs. 5 and 6; Supplemental Videos S1 and S2). Every fluorescent cell was tracked (Fig. 5), and greater than 95% of fluorescent cells exhibited activity (denoted as an increase of fluorescence greater than 10% of initial fluorescence values, in 16-bit intensity units (iu) (16). Active neurons were divided into groups based on morphology and size. During tonic inhibition, 19 ± 6.2% of ChAT-GCaMP3 neurons observed within a field at ×20, mostly smaller in size (long axis 24.2 ± 1.43 μm; width 18.6 ± 1.01 μm), exhibited Ca2+ transients at a variety of frequencies (average = 0.93 ± 0.35 Hz; n = 135 cells, N = 4 animals; e.g., traces with gray asterisks, Fig. 5). The relative smallness, together with the activity during tonic inhibition, suggests that these are cholinergic INs. In response to a spontaneous CMMC, 54.3 ± 11% of ChAT-GCaMP3 neurons exhibited Ca2+ transients. The average frequency was 1.2 ± 0.62 Hz (n = 220 cells, N = 4 animals). The increased number of responding cells is compatible with the activation of cholinergic EMNs during the CMMC. The duration of this enhanced activity was 22.3 ± 4.8 s (n = 220 cells, N = 4 animals; e.g., cells 5–8, Fig. 6). In response to a CMMC evoked by anal mucosal stimulation, the number of cells exhibiting Ca2+ transients increased to 72.4 ± 13%. A population of cells that were active only after the onset of a spontaneous CMMC became intensely active before the onset of an evoked CMMC (e.g., cells 1–4 in Fig. 6). Based on this response pattern and size (long axis 41.4 ± 0.98 μm; width 17.6 ± 0.43 μm; n = 20 cells; N = 4 animals), these cells are likely IPANs/Dogiel Type II sensory neurons. The intensity of the Ca2+ transients within these cells during evoked CMMCs precluded an easy analysis of frequency. The duration of enhanced activity in these cells was 38.5 ± 5.3 s (n = 154 cells, N = 4 animals).
Fig. 5.
Ca2+ transients in ChAT-GCaMP3 neurons recorded from several myenteric ganglia of the proximal colon during an evoked colonic migrating motor complex (CMMC). Every fluorescent cell located in the upper image to the right was outlined as a box and selected for analysis. Transients were plotted on the left. Only transients from the upper image are shown. The bars below each set of transients represents the onset of movement and is equal to 30 s. *Cells that exhibit transients before the onset of movement associated with the CMMC and are, therefore, active during the period of tonic inhibition.
Fig. 6.
Ca2+ transients in ChAT-GCaMP3 neurons recorded from several myenteric ganglia of the proximal colon during an evoked or spontaneous colonic migrating motor complex (CMMC). These transients were observed in most cholinergic neurons over several ganglia. Top left inset shows the morphology of putative excitatory motor neurons (EMNs) or interneurons (7a) versus putative intrinsic primary afferent neurons (2a). Note that this latter population of cells (1–4) exhibit a transient whose onset precedes the CMMC in response to the evoked, but not spontaneous, CMMC. The downward deflections in Ca2+ transients triggered by an evoked CMMC reflect movement artifact.
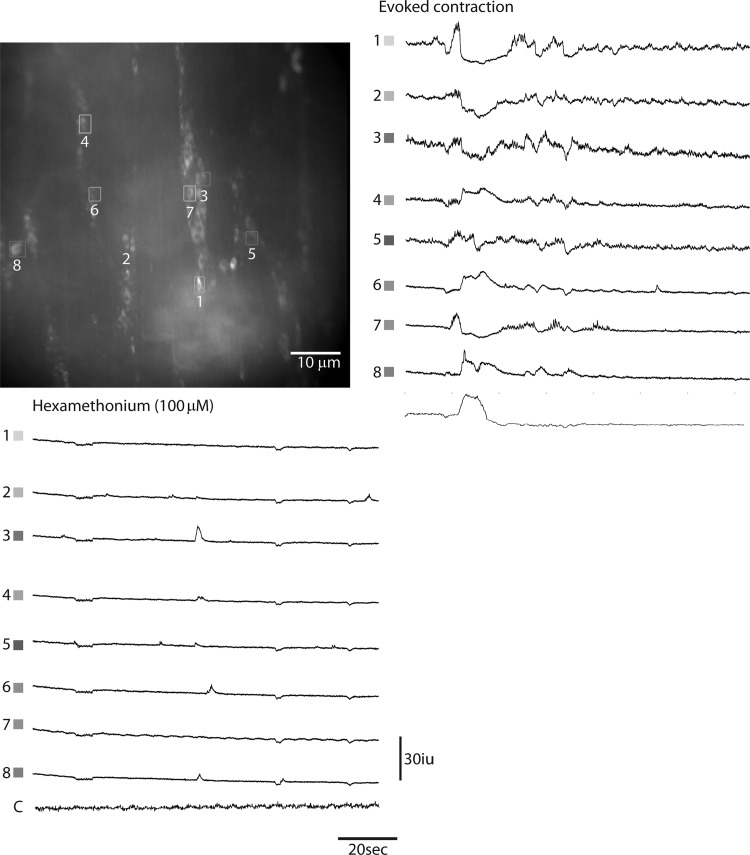
Cholinergic EMNs express nicotinic ACh receptor (nAChRs), and treatment with the nAChR antagonist hexamethonium inhibits the generation of CMMCs (2, 5, 26, 52). IPANs also reportedly express nAChRs (36), and a recent result showed that hexamethonium blocks the activation of these cells by fast synaptic inputs (29). Similarly, Ca2+ transients in ChAT-GCaMP3 neurons induced by anal mucosal stimulation were nearly completely abolished by hexamethonium (62.7 + 13% vs. 1.5 + % responsive ChAT-GCaMP3 neurons; 100 μM, n = 45 cells, N = 4 animals; P < 0.0001), suggesting that the activity within cholinergic IPANs, EMNs, and INs is almost entirely dependent on nAChR-mediated synaptic transmission (Fig. 7).
Fig. 7.
Ca2+ transients in ChAT-GCaMP3 neurons recorded from several myenteric ganglia of the proximal colon during an evoked CMMC in the absence or presence of the nicotinic acetylcholine receptor (nAChR) antagonist hexamethonium. Note the almost complete block of responses, suggesting the presence of these receptors on multiple cholinergic enteric neuronal subtypes.
Effect of blocking nitric oxide synthesis on activity of colonic ChAT-GCaMP3 neurons.
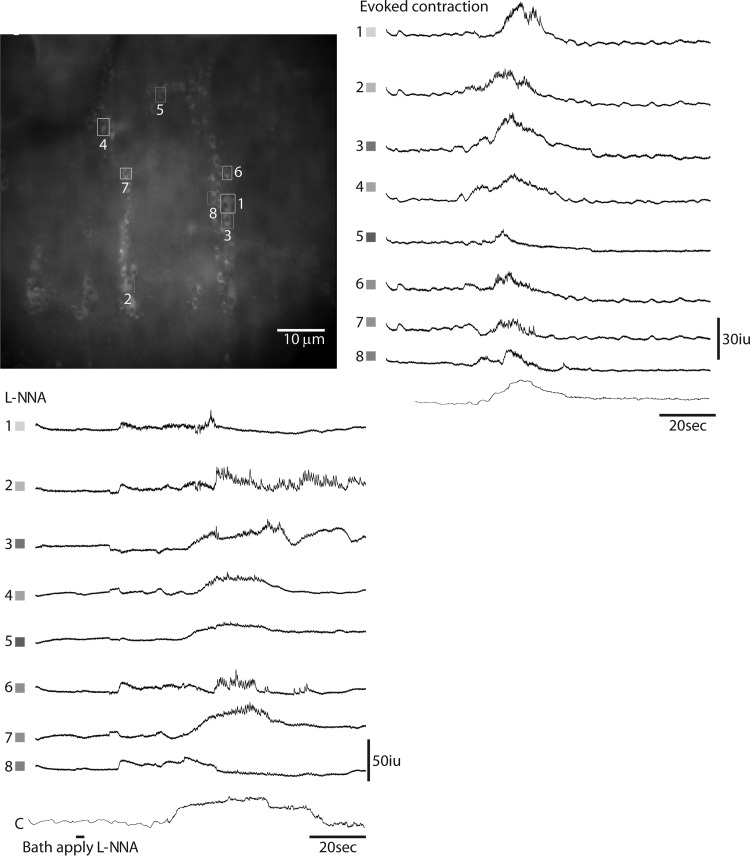
Blocking the production of NO leads to a dramatic increase of contractile activity, suggesting that the nNOS-positive IMNs projecting to colonic smooth muscle trigger tonic inhibition by releasing NO. nNOS is also expressed by a population of descending interneurons (41). These neurons are activated by elongation (longitudinal stretch) of the colon, a stimulus which shuts down the coordinated activation of proximal and inhibition of distal colon induced by circumferential stretch (54, 55) and underlies colonic slow transit (10, 11). The targets of NO produced by these INs likely include both ascending and descending cholinergic INs that drive EMNs in the proximal and IMNs in the anal colon, respectively (10). Accordingly, we imaged Ca2+ transients in a cohort of ChAT-GCaMP3 neurons in response to an evoked CMMC and then after bath application of the NO synthesis inhibitor l-NNA (10 μM) during a period of tonic inhibition. We found that even in the absence of longitudinal stretch, blockade of NO production produced a CMMC-like contraction together with a CMMC-like response of cholinergic activity (onset of each = 38.9 + 7.2 s after treatment, N = 5 animals; number of active ChAT-GCaMP3 neurons before versus after l-NNA = 27 + 5.6% vs. 74 + 11.2%; n = 73 cells, N = 5 animals; P < 0.01) (Fig. 8; Supplemental Videos S3 and S4). These results suggest that nitrergic neurons regulate the activity of cholinergic neurons, although whether this regulation is direct is unclear.
Fig. 8.
Ca2+ transients in ChAT-GCaMP3 neurons recorded from several myenteric ganglia of the proximal colon during an evoked colonic migrating motor complex (CMMC) or in response to acute treatment with the nitric oxide (NO) inhibitor Nω-nitro-l-arginine (l-NNA). Note the increase in firing frequency of selected cholinergic neurons in response to NO blockade.
Effect of blocking 5-HT3R and nAChR on the activity of colonic nNOS-GCaMP3 neurons.
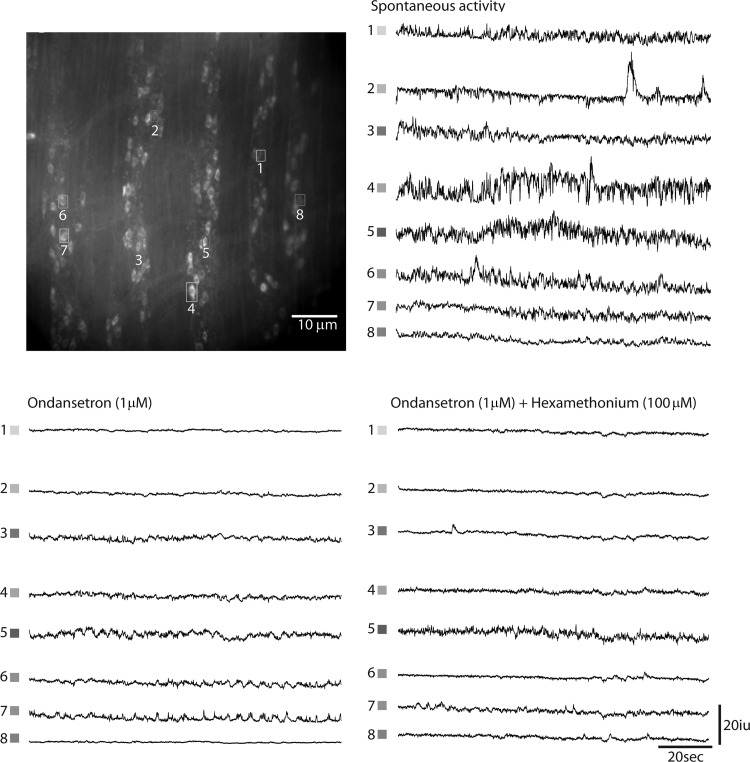
Tonic inhibition is characterized by spontaneous IJPs and membrane hyperpolarization of smooth muscle. In addition to their effects on cholinergic activity, EJPs, and CMMCs, the 5-HT3R antagonist ondansetron and the nAChR antagonist hexamethonium each reduce and together block spontaneous IJPs in circular muscle (11), suggesting that the activity of nitrergic IMNs is not intrinsic but rather driven by 5-HT- and/or ACh-expressing descending interneurons (51). To determine whether the effects of ondansetron and hexamethonium on IJPs occur by inhibiting nitrergic neuronal activity, we examined Ca2+ transients within nNOS-GCaMP3 neurons in the presence of each of these agents (Fig. 9; Supplemental Videos S5 and S6). In response to ondansetron (1 μM), 32 ± 11.2% of nNOS-GCaMP3 neurons active during a period of tonic inhibition exhibited a complete loss of activity (e.g., cells 1, 2, and 8), 58 ± 12% displayed a reduction of activity (e.g., cells 3–6), and 9 ± 4% showed no difference in activity (e.g., cell 7; n = 53 cells, N = 4 animals). After subsequent treatment with hexamethonium (100 μM), the frequency of Ca2+ transients within these neurons during a period of tonic inhibition was further, but not completely, suppressed (e.g., cells 6 and 7).
Fig. 9.
Ca2+ transients in nNOS-GCaMP3 neurons recorded from several myenteric ganglia of the proximal colon during a period of tonic inhibition before or after treatment with the 5-HT receptor antagonist ondansetron and cotreatment with the nicotinic acetylcholine receptor (nAChR) antagonist hexamethonium. Some nitrergic neurons ceased (cells 1, 2, and 8), while others reduced their firing in response to odansetron. Combined treatment with hexamethonium further reduced the amplitudes and frequency of activity within most nNOS-GCaMP3 neurons.
Effects of optogenetic stimulation of nNOS-ChR2 neurons during a CMMC.
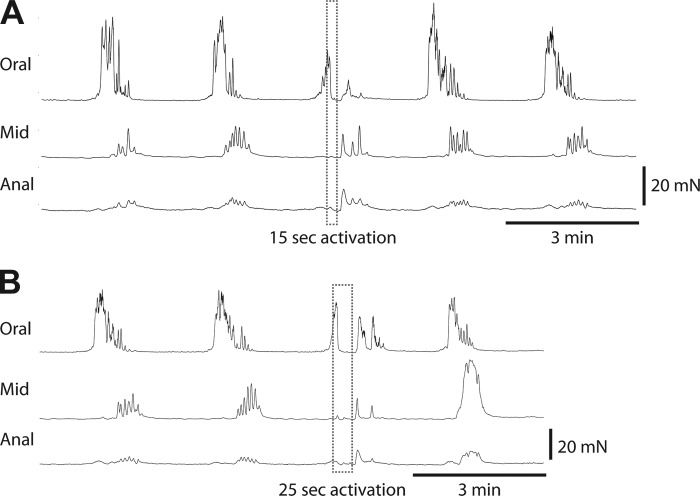
Most nNOS-GCaMP3 neurons within myenteric ganglia are IMNs, as only 1–2 per ganglion respond to longitudinal stretch in the absence of synaptic transmission and, thus, function as INs (10). These results are similar to the finding that the vast majority of nNOS-GCaMP3 neurons cease their activity during a CMMC (Fig. 3) and are, thus, IMNs. Together, these data are consistent with the idea that the slow depolarizing portion of the CMMC is mediated by the termination of postjunctional NO release by nitrergic IMNs. If true, then the reestablishment of IMN activity and concomitant postjunctional NO release during an ongoing CMMC should abbreviate the CMMC. To perform this experiment, we used mice expressing the optogenetic actuator ChR2, which opens a nonspecific cation channel and depolarizes the cell when activated with blue light (9), in nitrergic neurons. We recorded spontaneous CMMCs in proximal, middle, and distal colon with force transducers. When one such CMMC was initiated in the proximal colon, we shined blue light for either 15 or 25 s and found that the CMMC was abolished immediately for the duration of blue light stimulation (98.7 ± 2% loss of tension; CMMCs: n = 23, N = 6 animals). When the light was shut off, a CMMC-like contraction immediately resumed for a period of time similar to that of unperturbed CMMCs (CMMCs: n = 23, N = 6 animals; Fig. 10). These results suggest that the experimental induction of NO secretion by nitrergic IMNs is sufficient to relax the muscle, even during a CMMC, although the maintenance of this effect depends on continued stimulation of these neurons throughout the CMMC.
Fig. 10.
Colonic migrating motor complexes (CMMCs) (peaks of contraction) and tonic inhibition (periods in between CMMCs) recorded with force transducers on the proximal (oral), middle (mid), and distal (anal) segments of the colon from nNOS-ChR2 mice, before, during, and after optical stimulation. Tonic optical stimulation of nitrergic neurons during a spontaneous CMMC for 15 s (A) or 25 s (B) immediately and reversibly shut the CMMC down for the duration of the stimulation. CMMCs restored after the cessation of optical stimulation lasted only as long as they would have without such optogenetic stimulation.
DISCUSSION
The overall objective of this study was to examine the rostrocaudal distribution and activity of cholinergic and nitrergic neurons in the myenteric plexus of the murine colon during defined motility patterns. The advent of molecular genetic techniques in the mouse permits the targeting of various transgenes such as the GECI GCaMP3 into these defined enteric neuronal subpopulations, greatly facilitating the identification of individual cell activation patterns, compared with incubation of gastrointestinal tissue with chemical calcium-binding dyes (1). GECIs offer superior signal to noise and are more resistant to the bleaching effects of excessive imaging, thereby allowing repeated imaging of the same cells in response to multiple stimuli, despite retaining sensitivity to the phototoxic effects of such repeat imaging. This approach accordingly allows for the simultaneous recording of activity from large populations of a specific neurochemical or other specific cell subtypes in response to multiple stimuli. This fact is particularly useful when imaging cholinergic or nitrergic enteric neurons, which themselves include several different subclasses (i.e., sensory and motor neurons, interneurons) that have previously identified morphological, molecular, or functional signatures (20). We used this feature to begin to identify these subtypes (e.g., cholinergic sensory versus motor neurons).
Our most significant finding is that the majority of nitregic enteric neurons within the myenteric plexus of the murine colon are active during periods of tonic inhibition. Although not surprising, these results are an important confirmation and expansion of previous studies (2, 25, 51). For example, although most nitrergic neurons exhibit this pattern, several also continue to display Ca2+ transients during the middle of CMMCs. Whether these are IMNs “inappropriately” continuing to fire during movement, or instead are descending INs involved in colonic coordination, is unclear, but could potentially be addressed through post-hoc immunostaining. For example, in the guinea pig colon, nitrergic IMNs coexpress vasoactive intestinal peptide (VIP) and fail to express ChAT, but the two populations of descending nitrergic INs either express VIP and ChAT or fail to express VIP (41). An additional finding is that a small number of nNOS-GCaMP3 neurons appeared to enhance their firing just before and overlapping with the onset of the CMMC. This may represent a quantitatively different level of NO release that helps drive the hyperpolarization of SMCs, which represents the first portion of the CMMC (5).
We also demonstrate that a larger number of ChAT-GCaMP3 enteric neurons become active at the onset and throughout the duration of CMMCs than during tonic inhibition. Moreover, using a combination of morphological, functional, and pharmacological approaches, we identify several subtypes of cholinergic neurons, such as presumptive IPANs with Dogiel Type II morphology, robust response to evoked CMMCs, and sensitivity to hexamethonium.
In addition to their robust activation by an evoked CMMC, ChAT-GCaMP3 neurons are activated by the pharmacological inhibition of NO production. Although previous studies have provided data consistent with this idea (10), this is the first direct demonstration that NO normally inhibits the activity of cholinergic neurons within the plexus, even independently of longitudinal stretch. Several questions follow, such as, which cholinergic subtype is most affected? On the one hand, Dickson et al. (10) postulated that ascending cholinergic INs represented a likely direct target of nitrergic INs. Consistent with this idea, robust staining of the NO target enzyme NO-GC or its product cGMP is observed in a small subset of colonic myenteric neurons (40, 49). However, the CMMC-like activation of a large number of cholinergic neurons seems to indicate that a greater population than just these INs is affected. Therefore, either cGMP or NO-GC staining underestimates the number of NO-responsive neurons, or this approach is valid, and the response by cholinergic neurons that are NO-GC/cGMP-negative is indirectly caused by 1) the response of NO-SC/cGMP-positive cholinergic INs, which may provide a chain of excitation to cholinergic EMNs (8), or 2) other effects of l-NNA, such as those on postjunctional ICC/SMCs. We favor the former possibility, because the onset of l-NNA -induced CMMC-like contractions is contemporaneous with that of the increased l-NNA -induced response of cholinergic Ca2+ transients.
A second question is, if NO-GC/cGMP staining underestimates the number of direct NO-responsive cells, does the large cholinergic response, in fact, reflect the activity of nitrergic INs, which purportedly constitute only ~10% of all nNOS-expressing enteric neurons (46)? If so, this would mean that each nitrergic IN is connected to a large number of postsynaptic cholinergic neurons, since only 1 or 2 nitrergic INs exist per myenteric ganglion (10). Alternatively, the ability of NO to rapidly diffuse in a synapse-independent fashion may underlie these effects (17). Subcellular staining with antibodies that distinguish the various nNOS splice variants could be useful to address this idea. Or, more heretically, could the enhanced activation of cholinergic neurons observed by treatment with l-NNA reflect innervation by the collaterals of nitrergic IMNs? While these Dogiel Type I cells are typically defined by a single axon projecting to smooth muscle and dendrites within the plexus (23), it remains formally possible that collateral axons could project to different neurons within the plexus, such as cholinergic INs/EMNs, similar to the recurrent collaterals of somatic motoneurons that innervate Renshaw INs in the spinal cord. If so, one could test this idea by examining whether the effects of NO inhibition on smooth muscle contractility (i.e., enhanced number of CMMC-like, non-propagating contractions) are abolished by blocking cholinergic postjunctional responses (i.e., treatment with postjunctional specific muscarinic receptor subtype antagonists) (24). If so, this could suggest that nitrergic IMNs mediate tonic inhibition indirectly through effects on cholinergic EMNs, as well as or instead of, on postjunctional ICCs/SMCs.
The present study also supports the idea that CMMCs depend on the termination of tonic inhibition mediated by nitrergic IMNs, because the restoration of NO release via the optogenetic activation of these cells immediately arrests an ongoing CMMC. This relaxation of the colonic smooth muscle lasts only as long as the nNOS-ChR2 neurons are optically stimulated, suggesting that once a CMMC is inhibited, continuous NO is required to maintain its suppression. Interestingly, once a CMMC is restored in response to the cessation of optogenetic stimulation and concomitant NO release, it appears to persist only through its originally specified duration. These results may support the idea that the duration of CMMCs is determined by enteric neuronal pattern generators (60), independently of manipulations to the postjunctional smooth muscle.
Conclusions.
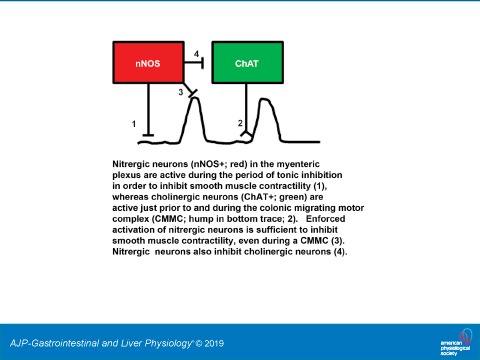
We have shown for the first time that populations of specific enteric neuronal subtypes with unique neurochemical identities within the myenteric plexus of the colon exhibit distinct patterns of activity during defined periods of colonic motility. Specifically, nitrergic neuronal activity is associated with the period of tonic inhibition, while cholinergic activity is more correlated with the CMMC. Blocking the production of NO by nitrergic neurons leads to an increase in the activity of cholinergic neurons, suggesting that the reciprocal patterns of activity within these two populations during tonic inhibition and CMMCs are related. Finally, using optogenetic techniques, we show that activation of nitrergic neurons during a CMMC shuts it down, demonstrating that the experimental induction of their firing, during a time when they are normally quiescent, is sufficient to restore tonic inhibition.
GRANTS
This study was funded by National Institute of Health Diabetes and Digestive and Kidney Diseases Grant R56DK109277 (to T. K. Smith) and Stimulating Peripheral Activity to Relieve Conditions Grant 10T20D0204899-01 (to T. K. Smith and T. W. Gould).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
T.W.G. and T.K.S. conceived and designed research; T.W.G., W.A.S., and T.K.S. interpreted results of experiments; T.W.G. and W.A.S. drafted manuscript; T.W.G., W.A.S., and T.K.S. edited and revised manuscript; T.W.G., W.A.S., D.J.H., R.D.C., and T.K.S. approved final version of manuscript; W.A.S., D.J.H., R.D.C., and T.K.S. performed experiments; W.A.S., D.J.H., and R.D.C. analyzed data; W.A.S., D.J.H., and R.D.C. prepared figures.
REFERENCES
- 1.Badura A, Sun XR, Giovannucci A, Lynch LA, Wang SS. Fast calcium sensor proteins for monitoring neural activity. Neurophotonics 1: 025008, 2014. doi: 10.1117/1.NPh.1.2.025008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bayguinov PO, Hennig GW, Smith TK. Calcium activity in different classes of myenteric neurons underlying the migrating motor complex in the murine colon. J Physiol 588: 399–421, 2010. doi: 10.1113/jphysiol.2009.181172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bertrand PP, Kunze WA, Furness JB, Bornstein JC. The terminals of myenteric intrinsic primary afferent neurons of the guinea-pig ileum are excited by 5-hydroxytryptamine acting at 5-hydroxytryptamine-3 receptors. Neuroscience 101: 459–469, 2000. doi: 10.1016/S0306-4522(00)00363-8. [DOI] [PubMed] [Google Scholar]
- 4.Brierley SM, Nichols K, Grasby DJ, Waterman SA. Neural mechanisms underlying migrating motor complex formation in mouse isolated colon. Br J Pharmacol 132: 507–517, 2001. doi: 10.1038/sj.bjp.0703814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bywater RA, Small RC, Taylor GS. Neurogenic slow depolarizations and rapid oscillations in the membrane potential of circular muscle of mouse colon. J Physiol 413: 505–519, 1989. doi: 10.1113/jphysiol.1989.sp017666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cooke HJ, Sidhu M, Wang YZ. Activation of 5-HT1P receptors on submucosal afferents subsequently triggers VIP neurons and chloride secretion in the guinea-pig colon. J Auton Nerv Syst 66: 105–110, 1997. doi: 10.1016/S0165-1838(97)00075-1. [DOI] [PubMed] [Google Scholar]
- 7.Costa M, Brookes SJ, Steeled PA, Gibbins I, Burcher E, Kandiah CJ. Neurochemical classification of myenteric neurons in the guinea-pig ileum. Neuroscience 75: 949–967, 1996. doi: 10.1016/0306-4522(96)00275-8. [DOI] [PubMed] [Google Scholar]
- 8.Costa M, Brookes SH. Architecture of enteric neural circuits involved in intestinal motility. Eur Rev Med Pharmacol Sci 12, Suppl 1: 3–19, 2008. [PubMed] [Google Scholar]
- 9.Deisseroth K, Hegemann P. The form and function of channelrhodopsin. Science 357: 357, 2017. doi: 10.1126/science.aan5544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dickson EJ, Spencer NJ, Hennig GW, Bayguinov PO, Ren J, Heredia DJ, Smith TK. An enteric occult reflex underlies accommodation and slow transit in the distal large bowel. Gastroenterology 132: 1912–1924, 2007. doi: 10.1053/j.gastro.2007.02.047. [DOI] [PubMed] [Google Scholar]
- 11.Dickson EJ, Hennig GW, Heredia DJ, Lee HT, Bayguinov PO, Spencer NJ, Smith TK. Polarized intrinsic neural reflexes in response to colonic elongation. J Physiol 586: 4225–4240, 2008. doi: 10.1113/jphysiol.2008.155630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dickson EJ, Heredia DJ, Smith TK. Critical role of 5-HT1A, 5-HT3, and 5-HT7 receptor subtypes in the initiation, generation, and propagation of the murine colonic migrating motor complex. Am J Physiol Gastrointest Liver Physiol 299: G144–G157, 2010. doi: 10.1152/ajpgi.00496.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickson EJ, Heredia DJ, McCann CJ, Hennig GW, Smith TK. The mechanisms underlying the generation of the colonic migrating motor complex in both wild-type and nNOS knockout mice. Am J Physiol Gastrointest Liver Physiol 298: G222–G232, 2010. doi: 10.1152/ajpgi.00399.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinning PG, Szczesniak M, Cook IJ. Removal of tonic nitrergic inhibition is a potent stimulus for human proximal colonic propagating sequences. Neurogastroenterol Motil 18: 37–44, 2006. doi: 10.1111/j.1365-2982.2005.00724.x. [DOI] [PubMed] [Google Scholar]
- 15.Drumm BT, Rembetski BE, Baker SA, Sanders KM. Tonic inhibition of murine proximal colon is due to nitrergic suppression of Ca2+ signaling in interstitial cells of Cajal. Sci Rep 9: 4402, 2019. doi: 10.1038/s41598-019-39729-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fida R, Lyster DJ, Bywater RA, Taylor GS. Colonic migrating motor complexes (CMMCs) in the isolated mouse colon. Neurogastroenterol Motil 9: 99–107, 1997. doi: 10.1046/j.1365-2982.1997.d01-25.x. [DOI] [PubMed] [Google Scholar]
- 17.Folasire O, Mills KA, Sellers DJ, Chess-Williams R. Three gaseous neurotransmitters, nitric oxide, carbon monoxide, and hydrogen sulfide, are involved in the neurogenic relaxation responses of the porcine internal anal sphincter. J Neurogastroenterol Motil 22: 141–148, 2016. doi: 10.5056/jnm15036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res 93: 96–105, 2003. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- 19.Friebe A, Mergia E, Dangel O, Lange A, Koesling D. Fatal gastrointestinal obstruction and hypertension in mice lacking nitric oxide-sensitive guanylyl cyclase. Proc Natl Acad Sci USA 104: 7699–7704, 2007. doi: 10.1073/pnas.0609778104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst 81: 87–96, 2000. doi: 10.1016/S0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- 21.Groneberg D, König P, Koesling D, Friebe A. Nitric oxide-sensitive guanylyl cyclase is dispensable for nitrergic signaling and gut motility in mouse intestinal smooth muscle. Gastroenterology 140: 1608–1617, 2011. doi: 10.1053/j.gastro.2011.01.038. [DOI] [PubMed] [Google Scholar]
- 22.Groneberg D, Lies B, König P, Jäger R, Seidler B, Klein S, Saur D, Friebe A. Cell-specific deletion of nitric oxide-sensitive guanylyl cyclase reveals a dual pathway for nitrergic neuromuscular transmission in the murine fundus. Gastroenterology 145: 188–196, 2013. doi: 10.1053/j.gastro.2013.03.042. [DOI] [PubMed] [Google Scholar]
- 23.Hao MM, Young HM. Development of enteric neuron diversity. J Cell Mol Med 13: 1193–1210, 2009. doi: 10.1111/j.1582-4934.2009.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harrington AM, Hutson JM, Southwell BR. Cholinergic neurotransmission and muscarinic receptors in the enteric nervous system. Prog Histochem Cytochem 44: 173–202, 2010. doi: 10.1016/j.proghi.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 25.Hennig GW, Gould TW, Koh SD, Corrigan RD, Heredia DJ, Shonnard MC, Smith TK. Use of genetically encoded calcium indicators (GECIs) combined with advanced motion tracking to examine the behavior of neurons and glia in the enteric nervous system of the intact murine colon. Front Cell Neurosci 9: 436, 2015. doi: 10.3389/fncel.2015.00436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heredia DJ, Dickson EJ, Bayguinov PO, Hennig GW, Smith TK. Localized release of serotonin (5-hydroxytryptamine) by a fecal pellet regulates migrating motor complexes in murine colon. Gastroenterology 136: 1328–1338, 2009. doi: 10.1053/j.gastro.2008.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Heredia DJ, Grainger N, McCann CJ, Smith TK. Insights from a novel model of slow-transit constipation generated by partial outlet obstruction in the murine large intestine. Am J Physiol Gastrointest Liver Physiol 303: G1004–G1016, 2012. doi: 10.1152/ajpgi.00238.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Heredia DJ, Gershon MD, Koh SD, Corrigan RD, Okamoto T, Smith TK. Important role of mucosal serotonin in colonic propulsion and peristaltic reflexes: in vitro analyses in mice lacking tryptophan hydroxylase 1. J Physiol 591: 5939–5957, 2013. doi: 10.1113/jphysiol.2013.256230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hibberd TJ, Travis L, Wiklendt L, Costa M, Brookes SJH, Hu H, Keating DJ, Spencer NJ. Synaptic activation of putative sensory neurons by hexamethonium-sensitive nerve pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol 314: G53–G64, 2018. doi: 10.1152/ajpgi.00234.2017. [DOI] [PubMed] [Google Scholar]
- 30.Huang PL, Dawson TM, Bredt DS, Snyder SH, Fishman MC. Targeted disruption of the neuronal nitric oxide synthase gene. Cell 75: 1273–1286, 1993. doi: 10.1016/0092-8674(93)90615-W. [DOI] [PubMed] [Google Scholar]
- 31.Hwang SJ, Durnin L, Dwyer L, Rhee PL, Ward SM, Koh SD, Sanders KM, Mutafova-Yambolieva VN. β-nicotinamide adenine dinucleotide is an enteric inhibitory neurotransmitter in human and nonhuman primate colons. Gastroenterology 140: 608–617.e6, 2011. doi: 10.1053/j.gastro.2010.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang Y, Dong H, Eckmann L, Hanson EM, Ihn KC, Mittal RK. Visualizing the enteric nervous system using genetically engineered double reporter mice: Comparison with immunofluorescence. PLoS One 12: e0171239, 2017. doi: 10.1371/journal.pone.0171239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keating DJ, Spencer NJ. Release of 5-hydroxytryptamine from the mucosa is not required for the generation or propagation of colonic migrating motor complexes. Gastroenterology 138: 659–670, 2010. doi: 10.1053/j.gastro.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 34.Keef KD, Du C, Ward SM, McGregor B, Sanders KM. Enteric inhibitory neural regulation of human colonic circular muscle: role of nitric oxide. Gastroenterology 105: 1009–1016, 1993. doi: 10.1016/0016-5085(93)90943-7. [DOI] [PubMed] [Google Scholar]
- 35.Kirchgessner AL, Tamir H, Gershon MD. Identification and stimulation by serotonin of intrinsic sensory neurons of the submucosal plexus of the guinea pig gut: activity-induced expression of Fos immunoreactivity. J Neurosci 12: 235–248, 1992. doi: 10.1523/JNEUROSCI.12-01-00235.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kirchgessner AL, Liu MT. Immunohistochemical localization of nicotinic acetylcholine receptors in the guinea pig bowel and pancreas. J Comp Neurol 390: 497–514, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- 37.Koh SD, Ward SM, Sanders KM. Ionic conductances regulating the excitability of colonic smooth muscles. Neurogastroenterol Motil 24: 705–718, 2012. doi: 10.1111/j.1365-2982.2012.01956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh SD, Dick GM, Sanders KM. Small-conductance Ca2+-dependent K+ channels activated by ATP in murine colonic smooth muscle. Am J Physiol 273: C2010–C2021, 1997. doi: 10.1152/ajpcell.1997.273.6.C2010. [DOI] [PubMed] [Google Scholar]
- 39.Li Z, Hao MM, Van den Haute C, Baekelandt V, Boesmans W, Vanden Berghe P. Regional complexity in enteric neuron wiring reflects diversity of motility patterns in the mouse large intestine (Abstract). eLife 8: e42914, 2019. doi: 10.7554/eLife.42914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iino S, Horiguchi K, Nojyo Y. Interstitial cells of Cajal are innervated by nitrergic nerves and express nitric oxide-sensitive guanylate cyclase in the guinea-pig gastrointestinal tract. Neuroscience 152: 437–448, 2008. doi: 10.1016/j.neuroscience.2007.12.044. [DOI] [PubMed] [Google Scholar]
- 41.Lomax AE, Furness JB. Neurochemical classification of enteric neurons in the guinea-pig distal colon. Cell Tissue Res 302: 59–72, 2000. doi: 10.1007/s004410000260. [DOI] [PubMed] [Google Scholar]
- 42.Mashimo H, Kjellin A, Goyal RK. Gastric stasis in neuronal nitric oxide synthase-deficient knockout mice. Gastroenterology 119: 766–773, 2000. doi: 10.1053/gast.2000.16509. [DOI] [PubMed] [Google Scholar]
- 43.Middleton SJ, Cuthbert AW, Shorthouse M, Hunter JO. Nitric oxide affects mammalian distal colonic smooth muscle by tonic neural inhibition. Br J Pharmacol 108: 974–979, 1993. doi: 10.1111/j.1476-5381.1993.tb13494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pfeifer A, Klatt P, Massberg S, Ny L, Sausbier M, Hirneiss C, Wang GX, Korth M, Aszódi A, Andersson KE, Krombach F, Mayerhofer A, Ruth P, Fässler R, Hofmann F. Defective smooth muscle regulation in cGMP kinase I-deficient mice. EMBO J 17: 3045–3051, 1998. doi: 10.1093/emboj/17.11.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter AJ, Wattchow DA, Brookes SJH, Costa M. Cholinergic and nitrergic interneurones in the myenteric plexus of the human colon. Gut 51: 70–75, 2002. doi: 10.1136/gut.51.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qu ZD, Thacker M, Castelucci P, Bagyánszki M, Epstein ML, Furness JB. Immunohistochemical analysis of neuron types in the mouse small intestine. Cell Tissue Res 334: 147–161, 2008. doi: 10.1007/s00441-008-0684-7. [DOI] [PubMed] [Google Scholar]
- 47.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 94: 859–907, 2014. doi: 10.1152/physrev.00037.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sarna SK. Cyclic motor activity; migrating motor complex: 1985. Gastroenterology 89: 894–913, 1985. doi: 10.1016/0016-5085(85)90589-X. [DOI] [PubMed] [Google Scholar]
- 49.Shuttleworth CW, Xue C, Ward SM, de Vente J, Sanders KM. Immunohistochemical localization of 3′,5′-cyclic guanosine monophosphate in the canine proximal colon: responses to nitric oxide and electrical stimulation of enteric inhibitory neurons. Neuroscience 56: 513–522, 1993. doi: 10.1016/0306-4522(93)90350-O. [DOI] [PubMed] [Google Scholar]
- 50.Smith TK, Park KJ, Hennig GW. Colonic migrating motor complexes, high amplitude propagating contractions, neural reflexes and the importance of neuronal and mucosal serotonin. J Neurogastroenterol Motil 20: 423–446, 2014. doi: 10.5056/jnm14092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith TK, Koh SD. A model of the enteric neural circuitry underlying the generation of rhythmic motor patterns in the colon: the role of serotonin. Am J Physiol Gastrointest Liver Physiol 312: G1–G14, 2017. doi: 10.1152/ajpgi.00337.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Spencer NJ, Bywater RAR, Taylor GS. Disinhibition during myoelectric complexes in the mouse colon. J Auton Nerv Syst 71: 37–47, 1998. doi: 10.1016/S0165-1838(98)00063-0. [DOI] [PubMed] [Google Scholar]
- 53.Spencer NJ. Control of migrating motor activity in the colon. Curr Opin Pharmacol 1: 604–610, 2001. doi: 10.1016/S1471-4892(01)00103-5. [DOI] [PubMed] [Google Scholar]
- 54.Spencer NJ, Hennig GW, Smith TK. A rhythmic motor pattern activated by circumferential stretch in guinea-pig distal colon. J Physiol 545: 629–648, 2002. doi: 10.1113/jphysiol.2002.028647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spencer NJ, Hennig GW, Smith TK. Stretch-activated neuronal pathways to longitudinal and circular muscle in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol 284: G231–G241, 2003. doi: 10.1152/ajpgi.00291.2002. [DOI] [PubMed] [Google Scholar]
- 56.Spencer NJ, Hennig GW, Dickson E, Smith TK. Synchronization of enteric neuronal firing during the murine colonic MMC. J Physiol 564: 829–847, 2005. doi: 10.1113/jphysiol.2005.083600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Steele PA, Brookes SJ, Costa M. Immunohistochemical identification of cholinergic neurons in the myenteric plexus of guinea-pig small intestine. Neuroscience 45: 227–239, 1991. doi: 10.1016/0306-4522(91)90119-9. [DOI] [PubMed] [Google Scholar]
- 58.Wood JD. Excitation of intestinal muscle by atropine, tetrodotoxin, and xylocaine. Am J Physiol 222: 118–125, 1972. doi: 10.1152/ajplegacy.1972.222.1.118. [DOI] [PubMed] [Google Scholar]
- 59.Wood JD. Electrical activity of the intestine of mice with hereditary megacolon and absence of enteric ganglion cells. Am J Dig Dis 18: 477–488, 1973. doi: 10.1007/BF01076598. [DOI] [PubMed] [Google Scholar]
- 60.Wood JD. Enteric nervous system: reflexes, pattern generators and motility. Curr Opin Gastroenterol 24: 149–158, 2008. doi: 10.1097/MOG.0b013e3282f56125. [DOI] [PubMed] [Google Scholar]