Abstract
Idiopathic pulmonary fibrosis (IPF) is a pernicious lung disease characterized by alveolar epithelial apoptosis, dysregulated repair of epithelial injury, scar formation, and respiratory failure. In this study, we identified phospholipase D (PLD)-generated phosphatidic acid (PA) signaling in the development of pulmonary fibrosis (PF). Of the PLD isoenzymes, the protein expression of PLD2, but not PLD1, was upregulated in lung tissues from IPF patients and bleomycin challenged mice. Both PLD1 (Pld1−/−)- and PLD2 (Pld2−/−)-deficient mice were protected against bleomycin-induced lung inflammation and fibrosis, thereby establishing the role of PLD in fibrogenesis. The role of PLD1 and PLD2 in bleomycin-induced lung epithelial injury was investigated by infecting bronchial airway epithelial cells (Beas2B) with catalytically inactive mutants of PLD (hPLD1-K898R or mPld2-K758R) or downregulation of expression of PLD1 or PLD2 with siRNA. Bleomycin stimulated mitochondrial (mt) superoxide production, mtDNA damage, and apoptosis in Beas2B cells, which was attenuated by the catalytically inactive mutants of PLD or PLD2 siRNA. These results show a role for PLD1 and PLD2 in bleomycin-induced generation of mt reactive oxygen species, mt DNA damage, and apoptosis of lung epithelial cells in mice. Thus, PLD may be a novel therapeutic target in ameliorating experimental PF in mice.
Keywords: epithelial cell apoptosis, mitochondrial DNA damage, mitochondrial ROS, phosphatidic acid, phospholipase D, pulmonary fibrosis
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a progressive, chronic, and fatal interstitial lung disease of unknown etiology (1) that has a prevalence of over 50 per 100,000 persons with a post-diagnosis median survival rate of 2–5 yr (53). The disease is characterized by abnormal lung architecture and scarring of the lung tissue due to excessive extracellular matrix deposition resulting in dysregulated wound repair and ultimately to respiratory failure (24). Several factors, such as cigarette smoke, bacterial and viral infection of the lung, and radio- and chemotherapy may be involved in the development and progression of lung fibrosis. Currently only two FDA-approved drugs, pirfenidone and nintedanib, are available for the treatment of IPF (54), which do not cure the disease but may slow the progression of the disease. Hence, understanding molecular mechanisms and identifying new target(s) might advance additional therapeutic interventions to treat IPF.
The alveolar epithelium plays an important role in the pathogenesis of pulmonary fibrosis (PF), as injury and impaired repair of the alveolar epithelial cells leads to epithelial-mesenchymal transition and activation of fibroblast to myofibroblast differentiation (49). An important molecular mechanism underlying lung fibrosis in IPF patients and the bleomycin mouse model is excessive generation of reactive oxygen species (ROS) in lung cells and specifically excess generation of mitochondrial superoxide (O2·−) from alveolar epithelial cells and macrophages (41) resulting in defective mitochondrial electron transport (70), loss of mitochondrial membrane potential (50), and mitochondrial DNA damage (42). Furthermore, the damaged mitochondrial DNA fragments, as mitochondrial damage-associated molecular patterns, could play a role in alveolar epithelial cell apoptosis and development of PF (35, 56, 57).
During our investigations on the role of sphingosine kinase-1 and sphingosine 1-phosphate on PF (26), we observed enhanced expression of a key lipid metabolizing enzyme, namely phospholipase D (PLD)2 in lung specimens from IPF patients and bleomycin-treated mice; however, the role of PLD in lung fibrosis is unknown. PLD hydrolyzes the phosphodiester bond of phospholipids and specifically phosphatidylcholine to phosphatidic acid (PA) and choline. PA is an important second messenger generated inside the cell that directly regulates several cellular processes including apoptosis, cytoskeletal organization, cell morphogenesis, membrane biogenesis, and vesicular trafficking (33). Also, PA is catabolized to diacylglycerol (DAG) by lipid phosphate phosphatases or lysophosphatidic acid (LPA) by PA-specific phospholipase A1 or A2 (20) in cells. Both DAG and LPA are signaling lipid molecules; DAG activates PKC (25) and LPA signals via G protein-coupled LPA1–6 receptors in mammalian cells (73). Thus, activation of PLD and generation of PA is central to cellular signal transduction under normal and pathological conditions. There are six isoforms of PLD, PLD1–6, of which PLD1 and PLD2 isoforms have been widely recognized in several human pathophysiologies, including cancer, hypertension, neurodisorders, diabetes, and acute lung injury (2, 46). A recent study suggests a novel role for PLD4 in the development of kidney fibrosis. Genetic deletion of PLD4, a transmembrane glycoprotein and an isoform of PLD that lacks any enzyme activity, in kidney tubular epithelial cells attenuated the development of kidney fibrosis (63), but the mechanism of protection in the absence of PA generation was not addressed. In this study, our data show that global genetic deletion of Pld1 or Pld2 in mice protected against bleomycin-induced lung inflammation and PF in vivo and in vitro inhibition of PLD2, and to a lesser extent PLD1 activity attenuated bleomycin-mediated mitochondrial (mt) O2·− generation, mtDNA damage, and apoptosis of lung epithelial cells.
MATERIALS AND METHODS
Cells and reagents.
Human bronchial epithelial (Beas2B) cells were purchased from American Type Culture Collection (Manassas, VA) and cultured in DMEM (Corning, Corning, NY) supplemented with 10% FBS (Millipore Sigma, St. Louis, MO) and 1% penicillin-streptomycin. All experiments were performed between passage 10 and 20, with passage 1 defined as the thawed cells from the supplier. Primary human bronchial epithelial cells (HBEpCs) (passage 2) were purchased from Lonza (Rockville, MD) and cultured in serum-free basal essential growth medium and supplemented with growth factors provided by the supplier. Cells were incubated at 37°C in 5% CO2 and 95% air to ~80% confluence and subsequently propagated in 100-mm or 35-mm fibronectin-coated dishes. All experiments with primary HBEpCs were carried out between passages 3 and 6. The identities of the Beas2B cell line and primary HBEpCs were confirmed using forensic DNA fingerprinting based on polymorphic STR analysis. Beas2B cells were analyzed for mycoplasma contamination by using the MycoAlert system (Lonza, Rockville, MD). PLD1 inhibitor VU0155069 and PLD2 inhibitor VU0364739 were obtained from Cayman Chemical (Ann Arbor, MI).
Human lung tissue specimens.
Lung tissue lysates from normal and IPF patients undergoing biopsy were obtained from C. Feghali-Bostwick at the Medical University of South Carolina, with a collaborative agreement. Specimens of IPF lung tissues were obtained from patients who had undergone lung transplantation, and normal lung tissue specimens were obtained from donors whose lungs were not used for transplant surgery at the University of Pittsburgh Medical Center, under a protocol approved by the University of Pittsburgh Institutional Review Board, and from normal lung tissue obtained from organ donors. Protein concentration was determined from the lung tissue homogenates, and equal amounts of proteins were probed with anti-PLD1 antibody (rabbit polyclonal, no. 3832), PLD2 (rabbit monoclonal, E1Y9L, no. 13891, 1:1,000 dilution; Cell Signaling, Danvers, MA) and anti-β-actin antibody (Clone RM112, MAB T523, 1:10,000 dilution; Millipore Sigma).
Immunostaining.
Tissue sections were stained on BondRX autostainer (Leica Biosystems) following a preset protocol. In brief, sections were deparaffinized, subjected to citric acid-based (Bond ER1 solution, pH6) antigen retrieval for 20 min at 99°C, and blocked with protein block (Background Sniper, Biocare Medical) for 30 min. After washing with Bond Wash Solution, sections were sequentially stained with anti-PLD2-antibody (rabbit monoclonal, E1Y9L, no. 13891, 1:50, Cell Signaling) followed by the detection with goat-anti-rabbit Alexa 594 antibody (Molecular Probes, no. A-11037, 1:400) and ready-to-use Multi-cytokeratin AE1/AE3 antibody (NCL-L-AE1/AE3, Leica Biosystems) followed by detection with goat-anti-mouse Alexa 488 antibody (1:400, Molecular Probes, no. R37120). All slides were incubated with DAPI (Invitrogen, no. D3571) for 10 min and mounted with Prolong Diamond Antifade mounting medium (Life Technologies, no. P36961). Images were taken at ×20 magnification on the PerkinElmer Vectra 3 multispectral imaging system. A spectral library was created using slides with single stains (Alexa 488, Alexa 594, DAPI, and background autofluorescence) to unmix channels and create clean images of the targets of interest.
Animals.
PLD1 (Pld1+/−) and PLD2 (Pld2+/−) heterozygous mice were obtained from G. Di Paolo (Columbia University). The heterozygous mice were intercrossed to generate homozygous mice. Wild-type, Pld1−/− and Pld2−/− male and female mice of ~8–12 wk were used for all the experiments. All animal procedures were approved by the University of Illinois Chicago Institutional Animal Care and Use Committee.
Murine model of bleomycin-induced pulmonary fibrosis.
The mice were anesthetized with 100 mg/kg ketamine plus 5 mg/kg xylazine by intraperitoneal injection, and bleomycin (1.5 U/kg body wt; Hospira, Lake Forest, IL) was instilled intratracheally to the mice with a maximum volume of 50 µl. The mice were euthanized after 0, 7, 14, 21, or 28 days of post-bleomycin injection for analysis.
Bronchoalveolar lavage fluid collection and analysis.
Bronchoalveolar lavage (BAL) fluid was collected by intratracheal injection of 1 ml of phosphate-buffered saline (PBS) and spun at 500 g for 20 min at 4°C, supernatant was collected, the pellet was resuspended in 200 μl of PBS, and total cell counts were done using a TC20 automated cell counter. For differential cell counts, 20-μl aliquots were spun onto microscope slides and stained using Diff-Quik stain set (Dade-Behring, Newark, DE). The supernatant was further centrifuged at 10,000 g for 10 min at 4°C and stored as BAL fluid at −80°C for analysis of cytokines, protein concentration, and other secreted proteins. Protein in BAL fluid, a measure of alveolar permeability, was measured using a BCA protein assay kit (Pierce Thermo Scientific, Rockford, IL). Cytokine levels in the cell-free BAL fluid samples were measured using ELISA kits (Peprotech, Rocky Hill, NJ), according to the manufacturer’s instructions.
Mouse lung tissue harvesting and analysis.
After BAL fluid collection, the right lung of the mouse was excised, snap-frozen in liquid nitrogen, and stored at −80°C. The left lung was fixed with formalin for histological analysis. The frozen lung tissue was diced, and lung homogenates were prepared in 350 μl of radioimmunoprecipitation assay (RIPA) buffer containing protease and phosphatase inhibitors. The homogenized samples were sonicated and spun at 10,000 g at 4°C for 10 min to remove cell debris, and the clear supernatant of the lung homogenate was used for total protein measurement.
Histopathological analysis.
Formalin-fixed lung tissues were embedded in paraffin and later sectioned onto glass slides (3–5 μm). The lung tissue sections were stained with hematoxylin and eosin (H&E) to assess for lung injury and with Masson’s trichrome staining to assess for collagen deposition in the lung, which is a marker of fibrosis. Lung injury was scored from the H&E staining on the basis of the official workshop report by the American Thoracic society for distinguishing features during acute lung injury (44). The extent of lung fibrosis was determined by evaluating the Ashcroft score as described previously (28). The mean score from each lung section was computed after observing and assessing multiple fields from each lung section separately and averaging them in a blinded manner.
Sircol assay.
Acid-soluble collagen levels in the lung tissue were measured using the Sircol Soluble Collagen Assay (Biocolor, Newtownabbey, Northern Ireland). The right lungs from the mice were minced and homogenized in 5 ml of 0.5 M acetic acid in PBS containing 0.6% pepsin. The lung homogenate was mixed with Sircol dye and rotated overnight at 4°C. They were cleared by centrifugation at 12,000 g for 15 min. The pellet was washed with acid salt wash reagent and finally suspended in alkali reagent supplied by the manufacturer. Absorbance measurement was taken at 555 nm, and the values are presented as micrograms of acid-soluble collagen levels per right lung.
Phospholipase D activity.
Total PLD activity was measured using the Amplex Red Phospholipase D Assay Kit (ThermoFisher Scientific, Waltham, MA). The cell lysates were incubated with the reaction mixture at 37°C in the dark. PLD activity present in the cell lysates cleaves the lecithin (phosphatidylcholine) substrate in the reaction mixture to produce choline and phosphatidic acid. The choline oxidase that is also present in the reaction mixture oxidizes choline to betaine and H2O2. The kit indirectly measures PLD activity by measuring the fluorescence intensity at 540 nm of the resorufin generated from reaction with H2O2.
Western blotting.
The lung tissue or cell lysates were subjected to SDS-PAGE on a 10% or 4–20% precast gel (Invitrogen, Carlsbad, CA) run at constant voltage (225 V) and transferred to nitrocellulose membranes (Bio-Rad, Hercules, CA). The membrane was blocked for 1 h at room temperature in blocking buffer [Tris-buffered saline + Tween 20 (TBST) with 1% BSA] to reduce nonspecific binding. The membranes were then incubated with the respective primary antibodies overnight. All primary antibodies used were either validated by the supplier or in our laboratory. The primary antibodies used were PLD1 (no. 3832), PLD2 (no. 13891), polyADP ribose polymerase (PARP; no. 9542) (72), and caspase-3 (61) (no. 9662, 1:1,000 dilution; Cell Signaling, Danvers, MA); β-actin (1:10,000 dilution, no. A5441, Millipore Sigma), acetylated MnSOD K68 (58) (1:1,000 dilution, no. 137037, Abcam, Cambridge, UK), fibronectin (26) (no. 9068), collagen type I α2 chain [COL1A2 (5) no. 166865], and SOD (66) (no. 18504, 1:1,000 dilution; Santa Cruz Biotechnology, Dallas, TX). After four 10-min washes with TBST, membranes were incubated for 1 h with the respective species specific horseradish peroxidase-conjugated secondary antibodies (Bio-Rad, Hercules, CA) in TBST containing 1% BSA. The membranes were rinsed again four times with TBST for 10 min each time, and bands were detected using Supersignal luminol enhancer (Perbio Science UK, Cheshire, UK) followed by exposure to blue light-sensitive X-ray film (Hyperfilm; Amersham Biosciences, Little Chalfont, UK). Equal protein loading was verified by reprobing membranes with anti-β-actin antibody. The relative intensities of protein bands (relative density units) were quantified by scanning densitometry using ImageJ software (Molecular Dynamics, Sunnyvale, CA).
Transfection and viral infection.
Beas2B cells were transfected with scrambled RNA or PLD2 siRNA (50 nM) for 5 h in serum-free DMEM; the medium was replaced with complete DMEM containing 10% fetal bovine serum, and cells were cultured for an additional 48 h. Beas2B cells (~60% confluence) were infected with adenoviral constructs of vector control [25 MOI (multiplicity of infection)], and catalytically inactive mutants of hPLD1 (K898R, 50 MOI) and mPld2 (K758R, 50 MOI) in 1 ml of complete DMEM. After 24 h, the virus-containing medium was replaced with DMEM containing 1% FBS, and cells were challenged with vehicle or bleomycin. The expressions of PLD1 and PLD2 in cell lysates were determined by Western blotting.
Mitochondrial ROS generation.
Bleomycin-induced mitochondrial O2·− generation in Beas2B cells was determined using MitoSOX red, a mitochondrial O2·− indicator (ThermoFisher Scientific). The effect of inhibiting PLD activity on bleomycin-induced O2·− generation was evaluated by infecting cells with adenoviral constructs of vector control, PLD1, and PLD2 mutants (50 MOI) for 24 h before bleomycin challenge for 1 h. After bleomycin challenge, cells were loaded with 5 µM MitoSOX reagent for 15 min at 37°C and then washed two times in phenol red-free medium. The cells were examined under a Nikon Eclipse TE 2000-S fluorescence microscope, and pictures were captured on a Hamamatsu digital charge-coupled device camera (Japan) using a ×60 objective lens.
Mitochondrial DNA damage.
Mitochondrial and nuclear DNA damage were assessed by quantitative PCR (Q-PCR) as previously described (36). Genomic DNA, including both nuclear and mitochondrial DNA (mtDNA), was extracted using Qiagen Genomic-Tip 20G and Qiagen DNA Buffer Set (Qiagen, Gaithersburg, MD) and was assessed by Q-PCR (Thermo Fisher Scientific). DNA was quantified by Pico-green (Thermo Fisher Scientific) using the FL600 Microplate Fluorescence Reader (Tecan, Mannendorf, Switzerland) at excitation and emission wavelengths 485 and 530 nm, respectively. Data obtained from the small fragments were subsequently used to normalize the results of the mitochondrial long fragment. To compare the levels of DNA lesion in each tested region of the mitochondrial genome, two mtDNA fragments of different lengths (long fragments, ranging from 972 to 1,037 bp and small fragments from 54 to 87 bp, respectively), located in the same mitochondrial genomic region were used. The number of mitochondrial lesions was calculated using the following equation: D = [1 – 2−(Δlong−Δshort)] × 10,000 (bp)/size of the long fragment (bp). The effect of PLD1 or PLD2 inhibition on mtDNA damage was be evaluated by preincubating the cells with 250 nm PLD1 inhibitor (VU0155069) and 500 nM PLD2 inhibitor (VU0364739) and a combination of both for 3 h. In some experiments, PLD2 expression was downregulated by PLD2 siRNA and mitochondrial DNA damage was assessed (35). In separate experiments, the cells were also preincubated with MitoTEMPO, a scavenger of mitochondrial ROS 3 h before bleomycin challenge (24 h), to study the impact of blocking mt O2·− on mtDNA damage.
Bronchial epithelial cell apoptosis.
The effect of inhibiting PLD activity on bleomycin-induced apoptosis was evaluated by preincubating the cells with 250 nM PLD1 inhibitor (VU0155069), 500 nM PLD2 inhibitor (VU0364739), and a combination of both the inhibitors for 3 h before bleomycin challenge (24 h). The cell lysates were subjected to Western blotting for caspase-3, PARP, cleaved caspase-3, and PARP fragments, which are markers of apoptosis. Another alternative approach to determine apoptosis was by flow cytometry analysis using an FITC Annexin V Apoptosis Detection Kit (BD Biosciences, San Jose, CA). After bleomycin challenge for 24 h, the Beas2B cells were collected using dissociation buffer and washed with ice-cold PBS. The cells were suspended in 300 μl of binding buffer before loading with 10 μl of annexin V-FITC and then gently vortexed and incubated for 10 min at 4 C in the dark. Propidium iodide (PI; 3 μl) was added to each tube and incubated for 5 min at 4°C in the dark. The cells were evaluated for annexin V-FITC and PI binding by Beckman Coulter's Gallios flow cytometer.
Statistical analysis.
Experimental results are expressed as means ± SD of triplicate values from three independent experiments. All results were subjected to statistical analysis using one-way ANOVA and, whenever appropriate, analyzed by Student-Newman-Keuls test. P < 0.05 was considered statistically significant.
RESULTS
PLD2 is upregulated in lung tissues from IPF patients and bleomycin-challenged mice.
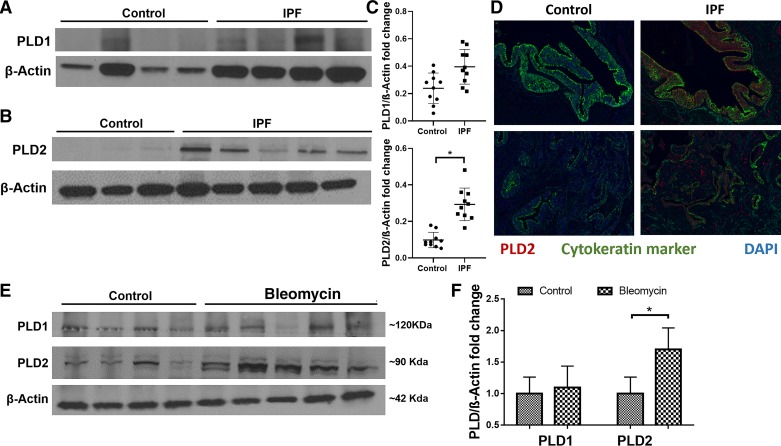
Recent studies suggest a role for PLD activation in dimethylnitrosamine-induced liver fibrosis (74) and bleomycin-mediated cytotoxicity in bovine lung microvascular endothelial cells (51); however, the expression and role of PLD1 and PLD2 in lung tissues from IPF or murine models of PF has not been investigated. Expression of PLD2, but not PLD1, was significantly elevated in lung tissues of IPF patients compared with control subjects (Fig. 1, A–C). The increase in the expression of PLD2 in lung tissues of IPF patients was further confirmed by immunohistochemistry of tissue sections from control and IPF patients (Fig. 1D). Further, to determine whether the increased PLD2 expression was predominantly in epithelial cells of the lung, paraffin-embedded lung sections from normal and IPF patients were subjected to immunohistochemical staining for PLD2 and cytokeratin marker to stain for epithelial cells. Enhanced PLD2 expression in IPF lung tissue sections was observed and compared with normal lung sections (Fig. 1D). Interestingly, PLD2 expression was increased not only in epithelial cells of the fibrotic lung but also in other cell types present in the fibrotic foci. Similarly, in a murine model of bleomycin-induced pulmonary fibrosis, the protein expression of PLD2 was elevated in the lung tissues compared with PLD1 (Fig. 1, E and F). These results suggest enhanced expression of PLD2, but not PLD1, in lung tissues from IPF patients and a murine model of experimental lung fibrosis.
Fig. 1.
Phospholipase D2 (PLD2), but not PLD1, is upregulated in lung tissues obtained from patients with idiopathic pulmonary fibrosis (IPF) and bleomycin-challenged mice. A and B: protein expression of PLD1 (A) and PLD2 (B) in lung homogenates from patients (n = 10) with IPF and donor control subjects (n = 10) as determined by Western blotting. C: band intensities of PLD1 and PLD2 were quantified and normalized to β-actin using ImageJ software, as indicated in the dot plots. D: PLD2 staining in IPF paraffin-embedded lung tissue sections: Immunohistochemical staining was performed to determine expression and localization of PLD2 in lung tissues from control subject and patients with IPF. Red regions indicate PLD2 expression; green is a cytokeratin marker, and blue stain is from DAPI. Images were acquired at ×20 magnification. E: C57BL/6 mice (6–8 wk, male and female; n = 4, control; n = 5, bleomycin) were challenged with sterile PBS or bleomycin (1.5 U/kg in mouse) intratracheally, and lungs were harvested on day 14 post-bleomycin challenge. Protein expression of PLD1 and PLD2 in whole lung homogenates (30 µg of protein) was determined by Western blotting. F: band intensities of PLD1 and PLD2 were quantified and normalized to β-actin with ImageJ software. *P < 0.05.
Genetic deletion of Pld1 and Pld2 in mice attenuates bleomycin-induced lung inflammation.
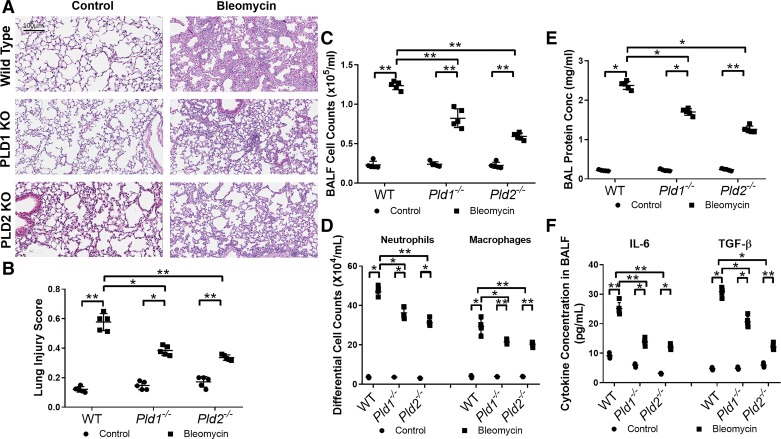
Although PLD1 expression was not altered in lungs of IPF patients and bleomycin-challenged mice (Fig. 1), bleomycin has been shown to enhance total PLD activity in endothelial cells (51). Therefore, the role of PLD1 and PLD2 in bleomycin-mediated inflammation in mouse lung was studied in Pld1−/− and PLD2 Pld2−/− knockout (KO) mice, which had similar phenotype to wild-type (WT) mice. WT, Pld1−/−, and Pld2−/− mice were challenged intratracheally with bleomycin (1.5 U/kg body wt) or sterile physiological saline and were evaluated for lung inflammation on day 7 post-bleomycin challenge. Bleomycin challenge of WT mice significantly increased lung damage as evaluated by H&E staining (Fig. 2, A and B), pulmonary leak as determined by infiltration of proinflammatory cells such as neutrophils and macrophages (Fig. 2, C and D), elevated protein levels (Fig. 2E), and enhanced secretion of interleukin-6 (IL-6) and transforming growth factor-β (TGF-β) (Fig. 2F) in BAL fluid, which were attenuated in both Pld1 and Pld2 KO mice (Fig. 2, B–G).
Fig. 2.
Genetic deletion (KO) of phospholipase D1/2 genes Pld1 and Pld2 (Pld1−/− and Pld2−/−) protects mice against bleomycin-induced inflammation and lung injury. Male and female C57BL/6 wild-type (WT), Pld1−/−, and Pld2−/− mice (n = 6 per group) were challenged intratracheally with bleomycin (1.5 U/kg in 50 μl PBS) or sterile PBS, were euthanized on day 7 post-bleomycin administration. Lungs were lavaged with sterile PBS solution, and bronchoalveolar lavage (BAL) fluids were collected and analyzed as described in materials and methods. Lung tissue sections were fixed in formalin and stained with hematoxylin and eosin (H&E). A: representative H&E photomicrographs of lung sections obtained from WT, Pld1−/−, and Pld2−/− mice; scale bar, 100 μm. B: acute lung injury score for each group. C–F: BAL fluids (BALF) were analyzed for total cell counts (C), differential cell counts (D), total protein concentration (E), and IL-6 and transforming growth factor-β (TGF-β) levels (F). *P < 0.05, **P < 0.005.
Genetic deletion of Pld1 and Pld2 in mice confers protection against bleomycin-induced pulmonary fibrosis.
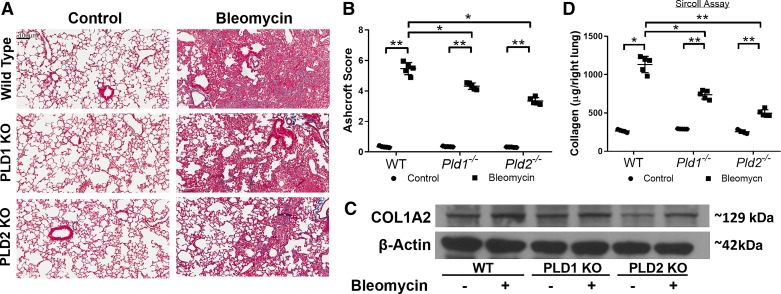
In bleomycin-induced PF, after the initial inflammatory cascade there is onset of fibrosis from days 10 to 21 followed by reversal after day 28 post-bleomycin challenge (69). To assess the role of PLD1 and PLD2 in bleomycin-induced fibrosis, wild type, Pld1−/−, and Pld2−/− mice were treated with bleomycin, and lung tissues were harvested on days 14, 21, and 28 post-bleomycin challenge. On day 21, the bleomycin challenge induced significant lung tissue scarring in WT mice, whereas the lung tissues from Pld1−/− and Pld2−/− mice were trending toward normal lung architecture, as seen from the Trichrome staining (Fig. 3A), and quantified Ashcroft score (Fig. 3B). Both Pld1−/− and Pld2−/− mice showed decreased collagen levels in the lung tissue compared with wild-type mice (Fig. 3C). Furthermore, soluble collagen levels, as measured by Sircol assay, were reduced in the Pld1−/− and Pld2−/− mice compared with WT mice challenged with bleomycin (Fig. 3D). These results showed that genetic deletion of Pld1 or Pld2 conferred protection against bleomycin-induced PF in mice.
Fig. 3.
Genetic deletion (KO) of phospholipase D1/2 genes Pld1 and Pld2 (Pld1−/− and Pld2−/−) protects against bleomycin-induced lung fibrosis in mice. Wild-type (WT), Pld1−/−, and Pld2−/− mice were challenged intratracheally with bleomycin (1.5 U/kg in 50 μl PBS) or sterile PBS and euthanized on day 21 post-bleomycin administration. A: representative photomicrographs of Masson’s trichrome staining of lung tissue sections from WT, Pld1−/−, and Pld2−/− mice with or without bleomycin challenge, blue indicating collagen deposition; scale bar, 100 μm. B: Ashcroft score of lung sections. C: representative Western blot showing protein expression of collagen type I α2 (COL1A2) in lung tissues in mice with and without bleomycin challenge on day 21. D: acid-soluble collagen level in lung tissue determined by Sircol assay. *P < 0.05, **P < 0.005.
Genetic deletion of Pld1 and Pld2 reduces bleomycin-induced apoptosis in mouse lung.
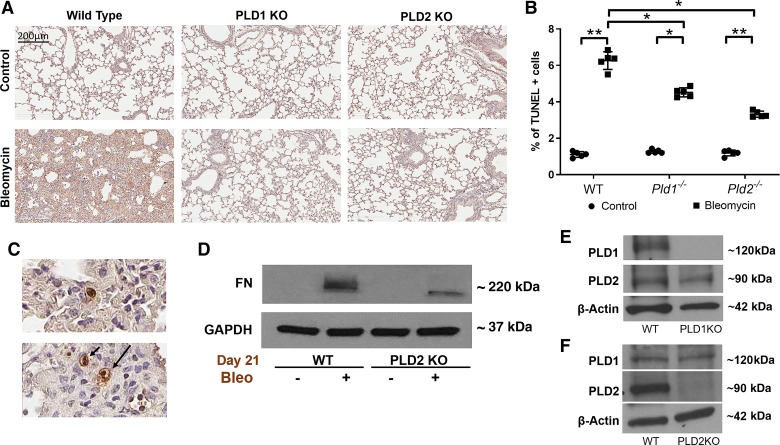
Apoptosis of lung epithelial and endothelial cells plays an important role in the initiation and development of PF in murine models of lung fibrosis (21). Here, we investigated the role of PLD in bleomycin-induced apoptosis in mouse lung. Bleomycin-induced apoptosis in lung cells of WT mice, as determined by TUNEL-positive cells in lung tissues at day 7 post-bleomycin challenge, which was significantly reduced in Pld1−/− and Pld2−/− mouse lungs (Fig. 4, A and B). Further, at higher magnification of the sections (×40), the majority of TUNEL-positive cells were epithelial cells (Fig. 4C), with some staining of endothelial cells. The expression of fibrogenic proteins like fibronectin (FN) in the whole lung tissue lysates after bleomycin administration for 21 days was significantly reduced in Pld2−/− mice compared with WT mice (Fig. 4D). Western blot analysis of the lung tissues showed knockdown of PLD1 or PLD2 protein expression in Pld1−/− and Pld2−/− mice had no effect on the expression of the other isoform (Fig. 4, E and F). These results suggested that both PLD1 and PLD2 are involved in bleomycin-induced apoptosis of mouse lung epithelial cells.
Fig. 4.
Genetic deletion (KO) of phospholipase D1/2 genes Pld1 and Pld2 (Pld1−/− and Pld2−/−) reduces bleomycin-induced apoptosis in mouse lung. Wild-type (WT), Pld1−/−, and Pld2−/− mice were challenged intratracheally with bleomycin (1.5 U/kg in 50 μl PBS) or sterile PBS and euthanized on day 7 post-bleomycin administration as described in Fig. 2. A: representative photomicrographs of TUNEL-positive staining of lung tissue sections. Apoptotic nuclei are stained brown and marked by arrows. B: number of TUNEL-positive cells per field in lung sections. *P < 0.05, **P < 0.005. C: higher magnification, at ×40, indicates that TUNEL-positive cells are mostly epithelial. D: representative Western blot showing protein expression of fibrotic marker fibronectin (FN) in lung tissue as described in Fig. 3 with and without bleomycin (Bleo;) challenge on day 21. E and F: representative Western blot showing PLD1 and PLD2 expression, respectively, in lung tissue lysates from WT, Pld1−/−, and Pld2−/− mice.
Bleomycin enhances total PLD activity and expression of PLD2 in bronchial epithelial cells.
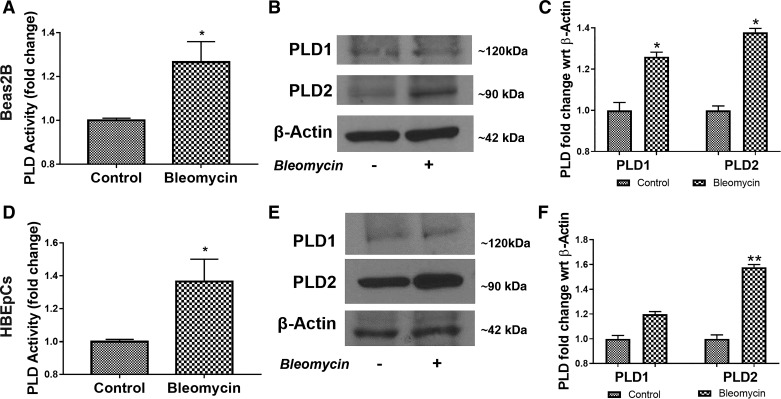
Having established a role for PLD1 and PLD2 in bleomycin-induced PF and apoptosis of lung epithelial cells in vivo, we next investigated the mechanism(s) by which PLD regulated bleomycin-induced apoptosis of lung epithelial cells. Exposure of primary human bronchial epithelial cells (HBEpCs) and a human bronchial epithelial cell line, Beas2B, to bleomycin for 24 h increased total PLD activity and expression of PLD2, but not PLD1 (Fig. 5, A–F). These results showed enhanced PLD activity and PLD2 expression in primary epithelial cells and an epithelial cell line challenged with bleomycin.
Fig. 5.
Bleomycin enhances phospholipase D (PLD) activity, and PLD2 protein expression in bronchial airway epithelial (Beas2B) cells. Beas2B cells grown in 35-mm dishes (~90% confluence) were challenged with 10 mU bleomycin for 24 h. A: total PLD activity was measured by Amplex Red assay, as indicated in materials and methods. B: representative Western blot of PLD1, PLD2, and β-actin expression in total cell lysates from Beas2B cells of 3 independent experiments in triplicate. C: quantification of Western blots by densitometry/ImageJ analysis and fold changes were normalized to β-actin. D: primary human bronchial epithelial cells (HBEpCs) in 35-mm dishes were treated with bleomycin as in A, and total PLD activity was determined with and without bleomycin challenge, as indicated above. E: representative Western blot of PLD1, PLD2, and β-actin in total cell lysates from HBEpCs of 3 independent experiments in triplicate. F: quantification of Western blots by densitometry/ImageJ analysis and fold changes were normalized to β-actin. *P < 0.05, **P < 0.005.
PLD regulates bleomycin-induced mtSOD generation in bronchial epithelial cells.
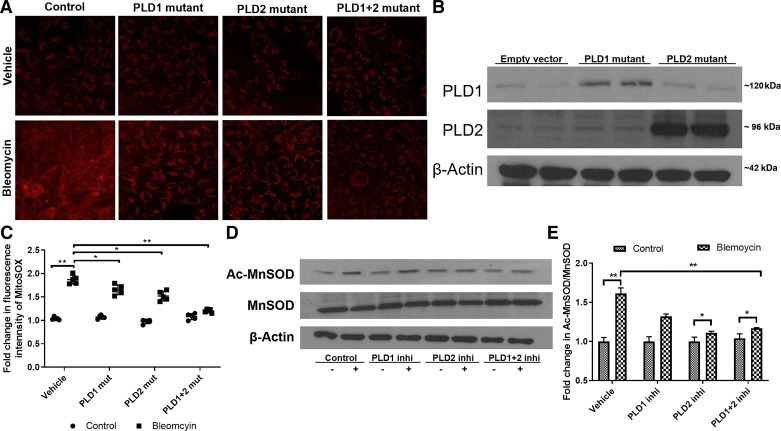
Recent studies suggest a role for NADPH oxidase (NOX)4 and mitochondrial ROS in promoting lung fibrosis in IPF and the bleomycin murine model of lung fibrosis (4, 23, 34, 67). Also, we and others (8, 64) have demonstrated a role for PLD-mediated generation of phosphatidic acid signaling in NOX-dependent ROS production in mammalian cells. Since bleomycin challenge resulted in alveolar epithelial injury, leading to activation and proliferation of myofibroblasts (38), we investigated the role of PLD in bleomycin-mediated mitochondrial O2·− production and regulation of apoptosis in lung epithelial cells. Infection of Beas2B cells with adenoviral mutants of PLD1 or PLD2 increased the expression of PLD1 or PLD2 as determined by Western blotting (Fig. 6C). Challenge of Beas2B cells with bleomycin enhanced mitochondrial O2·− production as quantified by MitoSOX, which was blocked by catalytically inactive PLD1 or PLD2 mutant (Fig. 6, A and B). Since blocking PLD1 or PLD2 reduced bleomycin-induced mtROS generation by ~30% compared with cells with empty vector, we next infected cells with both adenoviral PLD1 and PLD2 mutants, which reduced the mtROS by bleomycin ~60% compared with control cells (Fig. 6, A and B). Increased acetylation of MnSOD, a mitochondrial superoxide dismutase, has been shown to regulate mtROS production (47). Therefore, we determined whether bleomycin challenge modulated acetylation of MnSOD and the effect of PLD1 or PLD2 inhibitor on MnSOD acetylation. Inhibition of PLD2 activity with VU0364739 (500 nM), but not PLD1 activity with VU155069 (250 nM), blocked the bleomycin-induced increase in acetylation of MnSOD in Beas2B cells (Fig. 6, D and E). These results suggested a role for PLD2 in bleomycin-induced mitochondrial O2·− generation and modulation of the acetylation of the antioxidant enzyme MnSOD in Beas2B cells.
Fig. 6.
Inhibition (Inhi) of phospholipase D1 (PLD1) and PLD2 activity attenuates bleomycin-induced mitochondrial reactive oxygen species (ROS) generation and acetylation (Ac-) of manganese-superoxide dismutase (MnSOD). Bronchial airway epithelial (Beas2B) cells grown in 35-mm dishes (~60% confluence) were infected with adenoviral control or catalytically inactive PLD1 [50 MOI (multiplicity of infection)], PLD2 mutant (mut; 50 MOI), or a combination of PLD1 + PLD2 (50 MOI each) for 24 h before bleomycin challenge (10 mU/ml) for 1 h. Cells were then incubated with MitoSOX red reagent for 15 min and later subjected to 2 washes with phenol red-free medium. A: representative images of MitoSOX staining, a measure of mitochondrial superoxide (O2·−) generated. B: Western blot showing overexpression of PLD1 and PLD2 in Beas2B cells after infection with catalytically inactive mutants. C: quantification of fluorescence intensity of MitoSOX staining by ImageJ. D: Beas2B cells grown in 35-mm dishes (~90% confluence) were pretreated with 250 nM PLD1 inhibitor (VU0155609), 500 nM PLD2 inhibitor (VU0364739), or 250 nM VU0155609 + 500 nM VU0364739 for 3 h before bleomycin challenge (10 mU/ml) for 1 h. Cell lysates (30 µg protein) were subjected to Western blotting and stained for Ac-MnSOD and total MnSOD with antibodies. Shown is a representative blot from 3 independent experiments. E: quantification of Western blots (D) by densitometry/ImageJ analysis, and fold changes were normalized to total MnSOD. Values are means ± SD of 3 independent experiments. *P < 0.05, **P < 0.005.
Inhibition of PLD attenuates bleomycin-induced mitochondrial DNA damage in bronchial epithelial cells.
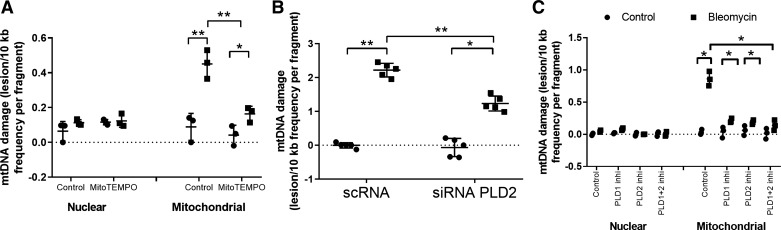
Recent evidence implicates excess generation of mitochondrial O2·− by agents such as bleomycin and asbestos induce mtDNA damage (35, 70). Having demonstrated a role for PLD in bleomycin-induced mitochondrial O2·− generation, we next assessed whether PLD regulates mtDNA damage by using a Q-PCR-based assay, which determines both nuclear and mtDNA (36). Treatment of Beas2B cells with MitoTEMPO (100 μM), a scavenger of mtROS, decreased mt O2·− and mtDNA damage (Fig. 7A). Knockdown of Pld2 using Pld2 siRNA also showed a reduction in bleomycin-induced mtDNA damage in Beas2B cells (Fig. 7B). Similarly, pretreatment of Beas2B cells with VU0155069 (PLD1-selective) or VU0364739 (PLD2-selective) inhibitor reduced bleomycin-induced mtDNA damage almost fourfold (Fig. 7C). Simultaneous addition of both PLD1 and PLD2 inhibitors to cells did not further reduce bleomycin-induced mtDNA damage (Fig. 7C). Collectively, these data showed that activation of PLD by bleomycin enhanced mtROS-mediated mtDNA damage in lung bronchial epithelial cells.
Fig. 7.
Downregulation or inhibition (Inhi) of phospholipase D1 (PLD1) or PLD2 attenuates bleomycin-induced mitochondrial (mt)DNA damage in bronchial epithelial cells. A: bronchial airway epithelial (Beas2B) cells grown in 35-mm dishes (~90% confluence) were treated with MitoTempo (100 μM) for 3 h before exposure to bleomycin (10 mU/ml) for 24 h. B: Beas2B cells grown in 35-mm dishes (~60% confluence) were transfected with scrambled (sc)RNA or PLD2 small interfering (si)RNA (50 nM, 24 h) before bleomycin challenge as in A. C: Beas2B cells in 35-mm dishes (~90% confluence) were pretreated with 250 nM PLD1 inhibitor VU0155609, 500 nM PLD2 inhibitor VU0364739, or a mixture of 250 nM PLD1 + 500 nM PLD2 inhibitors for 3 h prior to bleomycin challenge (10 mU/ml) for 24 h. mtDNA damage was determined by Q-PCR-based measurement using isolated whole genomic DNA, both mitochondrial and nuclear DNA, as indicated in materials and methods. DNA damage was expressed as the ratio of lesion frequency to fragment. Values are means ± SD from 3 independent experiments in triplicate. *P < 0.05, **P < 0.005.
Inhibition of PLD attenuates bleomycin-induced apoptosis of bronchial epithelial cells.
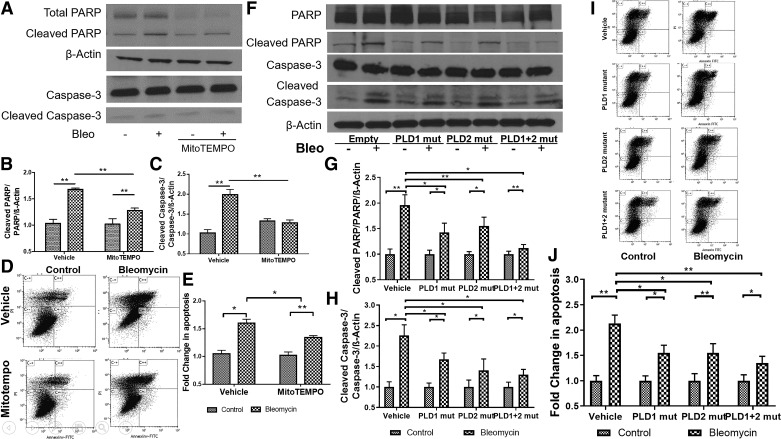
There is compelling evidence implicating that oxidative stress-mediated mtDNA damage promotes epithelial cell apoptosis and PF (35). Having demonstrated a role for PLD in bleomycin-induced mtDNA damage via mitochondrial O2·−, we next studied the effect of inhibiting PLD1 and PLD2 activities on bleomycin-induced epithelial apoptosis. Pretreatment of Beas2B cells with MitoTEMPO (100 μM) attenuated bleomycin-induced apoptosis (Fig. 8, A–D), as determined by cleaved PARP and caspase 3 and flow cytometry of annexin V- and PI-positive cells. Further, adenoviral-mediated overexpression of catalytically inactive PLD1 or PLD2 mutant attenuated bleomycin-induced apoptosis in Beas2B cells (Fig. 8, E–J). Inhibition of both PLD1 and PLD2 activities at the same time with the mutants was more effective in attenuating bleomycin-induced apoptosis (Fig. 8, E–J). These results supported the role for PLD1 and PLD2 in oxidative stress-dependent bleomycin-induced lung epithelial cell apoptosis.
Fig. 8.
Inhibition of mitochondrial superoxide generation and phospholipase D (PLD) activity attenuates bleomycin (Bleo)-induced apoptosis in bronchial airway epithelial (Beas2B) cells. Beas2B cells grown in 35-mm dishes (~60% confluence) were infected with vector, PLD1 [50 MOI (multiplicity of infection)], PLD2 (50 MOI), or a combination of PLD1 + PLD2 mutants (mut; 50 MOI each) for 24 h. Cells were treated with MitoTempo (100 μM) for 1 h and challenged with bleomycin (10 mU/ml) for 24 h. Programmed cell death following bleomycin challenge was determined by cleavage of caspase-3 and polyADP ribose polymerase (PARP), indicators for apoptosis, and flow cytometry of propidium iodide (PI)/annexin V-positive cells. A: protein expression of caspase-3 and PARP in cell lysates (20 µg of protein) from cells with or without MitoTempo pretreatment. Shown is a representative blot from 3 independent experiments in triplicate. B and C: quantification of cleaved caspase-3 and PARP from A by densitometry and ImageJ analysis; data were normalized to β-actin levels. D and E: Beas2B cells grown in 35-mm dishes (~90% confluence) were exposed to bleomycin in the absence or presence of MitoTempo as in A, and apoptosis was quantified by flow cytometry analysis. Early apoptotic and late apoptotic cells were sorted out by labeling the cells with annexin V and PI and quantified. F–H: cell lysates from Beas2B cells infected with empty vector, PLD1, PLD2, and PLD1 + PLD2 mutant with and without bleomycin challenge as in A were analyzed by Western blotting for cleaved PARP and caspase-3 and total β-actin. Band intensities were quantified by densitometry and ImageJ and normalized to total actin. Shown is a representative blot from 3 independent experiments. I: Beas2B cells transfected with PLD1 or PLD2 mutants or PLD1 +PLD2 mutants as in F in the presence or absence of bleomycin (10 mU/ml) for 24 h were analyzed by flow cytometry for apoptosis. Shown is a representative dot plot of annexin V and PI staining. Late-apoptotic cells stained as annexin V+/PI+ cells seen on C++, and early-apoptotic cells were stained as annexin V+/PI− cells seen on C+/−. J: percentage of late-apoptotic cells were quantified from I. *P < 0.05, **P < 0.005.
DISCUSSION
Idiopathic pulmonary fibrosis is a progressive disease of unknown etiology, leading to mortality due to lack of effective therapeutic strategies. One of the most important mechanisms in the development of IPF is repeated injury to the lung epithelium that leads to its dysfunction through dysregulated wound repair. Abnormal bronchoalveolar and hyperplastic type II epithelial cells that line the honeycomb regions are distinctly present in IPF tissues in addition to fibroblastic foci containing fibroblasts and myofibroblasts (75). The lung epithelium being the first line of injury during any insult to the lung, elucidating the dysregulated signaling pathways regulating wound repair is essential to identify new and novel therapeutic strategies for the disease. Here, we provide in vivo evidence demonstrating that genetic deletion of Pld1 and Pld2 in mice rendered protection against bleomycin-induced lung injury and inflammation, as seen by the histological changes in the lung after bleomycin instillation, cytokine analysis, and infiltration of cells into the bronchoalveolar lavage fluid. Further, bleomycin-induced fibrosis in the lung was attenuated in Pld1−/− and Pld2−/− mice, as seen in the reduction in collagen deposition and FN expression on day 21 post-bleomycin challenge of the lung. Also, the recovery from bleomycin-induced fibrosis as seen on day 28 after bleomycin instillation was enhanced in Pld2−/− mice more than in wild-type mice (data not shown). Both Pld1 and Pld2−/− mice were only partially protected against bleomycin-induced PF, which might have been due to the compensatory role of PLD1 or PLD2. Further, we demonstrated, for the first time, that downregulation of PLD1/PLD2 or inhibition of PLD1/PLD2 activity attenuated bleomycin-induced mtROS and mtDNA damage and apoptosis of lung epithelial cells, suggesting that PLD may be a potential therapeutic target against lung fibrosis.
Of the six PLD isoforms, PLD1–6, only PLD1 and PLD2 exhibit catalytic activity (19) and hydrolyze phosphatidylcholine (PC) to phosphatidic acid and choline (10). In addition to PC, PLD1 and PLD2 can also use other phospholipids such as phosphatidylethanolamine, and phosphatidylserine as phospholipid substrates, and mitochondrial PLD6 utilizes cardiolipin to generate PA. In the present study, lung tissues from IPF patients and the bleomycin-induced murine model of fibrosis showed enhanced PLD2 expression compared with PLD1; however, PLD1, but not PLD2, expression was increased in dimethylnitrosamine (DMN)-induced rat liver fibrosis (74). Furthermore, blocking PLD activity with N-methylethanolamine reduced rat liver fibrosis by DMN by modulating tissue inhibitor of metalloproteinases-1 (TIMP1) and COL1A1 expression (74), with no change in TGF-β and monocyte chemoattractant protein-1expression. Costaining for PLD2 and alveolar type 2 cell marker such as surfactant protein C would clarify the localization of the increased PLD2 expression in alveolar epithelial cells of lung tissues from IPF patients compared with control patients; however, this was not feasible due to the limited availability of IPF paraffin-embedded tissues. Genetic deletion of Pld1 or Pld2 in mice reduced bleomycin-induced COL1A2 and FN expression, suggesting a role for PLD1 and PLD2 in regulating bleomycin mediated expression of fibrogenic and extracellular matrix proteins. In contrast to requirement of PLD1 or PLD2 activity in the development of liver or lung fibrosis, mice subjected to either folic acid (FA) or surgical unilateral ureteral obstruction (UUO) developed kidney fibrosis, which was dependent on expression of PLD4 (63). PLD4 is a transmembrane glycoprotein, has no enzyme activity, and, unlike PLD1 and PLD2, lacks Phox and pleckstrin homology (PH) domains in the NH2-terminal region and two conserved His-x-Lys-x-x-x-x-Asp sequence (x is HKD) motifs in the COOH-terminal region. Global or proximal tubule epithelial cell-specific PLD4 knockdown conferred protection against FA- or UUO-induced kidney fibrosis in mice (63). While the mechanism(s) of PLD4 in the development of FA- or UUO-induced kidney fibrosis is unclear, PLD4 was found to interact with neutral elastase and neurotropic receptor tyrosine kinase-1 to modulate mitogen-activated protein kinase in proximal tubule epithelial cells (63). PA generated through the PLD pathway is an important second messenger and has been implicated in cell migration (29), proliferation (18), cytoskeletal reorganization (3), NOX activation (52), and vesicular trafficking (11). In addition, PLD-derived PA serves as a precursor for DAG and LPA, which are bioactive lipid mediators involved in cell signaling and function. Lipid phosphate phosphatases hydrolyze PA to DAG (9), which activates classical and atypical PKC isoforms including PKC-α, -β, and -δ in mammalian cells. TGF-β-stimulated collagen synthesis was dependent on PKC-δ activation in human lung fibroblast (71), while PKC-βII- and PKC-δ-dependent inactivation of p38 MAPK signaling regulated cardiac fibroblast proliferation and collagen deposition (14). However, in human lung fibroblasts, C-C ligand 18 (CCL18)-stimulated collagen production was PKC-α dependent, but not PKC-δ or PKC-ε dependent (43). The contradictory role of the various PKC isoforms in collagen production in fibroblasts is unclear but may be related to different stimuli and types of fibroblasts used. LPA is another lipid second messenger that has been implicated in IPF and bleomycin-induced pulmonary fibrosis (27). LPA is generated either from PA by the action of PA-specific phospholipase A1 or A2 (17, 59) or from lyso-PC by autotaxin (ATX) (40). Genetic deletion of LPA1 and LPA2 in mice or inhibition of ATX protected mice from bleomycin-induced pulmonary fibrosis (27, 47, 62). However, a recent study has demonstrated that ATX activity, although increased following bleomycin-induced lung injury, may not be required for pulmonary LPA production or fibrosis (6). In lung epithelial cells, exogenous addition of dioleoyl PA (C18:1 PA) (50 µM) stimulated mtROS generation (data not shown). The physiological relevance of exogenous PA-induced mtROS is unclear, and further studies are necessary to determine the role of PLD1 and/or PLD2 in spatiotemporal generation of PA and its role pulmonary fibrosis.
A novel finding of this study is the role of PLD in regulating bleomycin-induced mitochondrial O2·− generation, which can lead to mtDNA damage and apoptosis in lung epithelial cells. Superoxide is an important oxidant generated in the mitochondria via electron transport chain (ETC) that regulates oxidative stress, apart from hydrogen peroxide, and hydroxyl radical (65). Physiologically, low levels of ROS are generated in cells, which are essential for various cellular processes like gene expression, migration, proliferation, and differentiation (55). However, in case of excess mitochondrial O2·− generation, as in the case of IPF, the redox equilibrium is disrupted, and there is oxidative stress-induced damage in the cells and tissues (68). Enhanced generation of ROS resulting in oxidative stress has been implicated in bleomycin-induced pulmonary fibrosis (13) and IPF (7). Oxidative stress causes apoptosis of lung epithelial cells, differentiation and proliferation of lung fibroblasts, and modulation of TGF-β signaling through the Smad pathway (31), which is central for fibrogenesis. Hydrogen peroxide derived from NOX4 and O2·− from mitochondrial ETC are two major source of ROS generated in the bleomycin model of lung fibrosis and IPF (4, 13, 42). Recent evidence shows that inhibition of TGF-β-induced mtROS generation attenuates profibrotic gene expression, and targeting mtROS might be a therapeutic approach in treating excessive fibrosis-associated pathologies (31). In lung epithelial cells, bleomycin stimulated mtROS production, which was attenuated by inhibition of PLD1 or PLD2 activity (Fig. 6, A and C). Interestingly, inhibition of both PLD1 and PLD2 by catalytically inactive mutants was more effective in reducing bleomycin-induced mt O2·− generation, suggesting a compensatory role for PLD1 or PLD2 in generation of PA and signal transduction. The mechanism(s) of PA-mediated regulation of mitochondrial ETC and O2·− generation is unclear. Cell surface receptor(s) for PA has not been identified, and PA generated intracellularly modulates signaling cascades via non-receptor-mediated pathways. PA generated by the activation of PLD1 or PLD2 has been shown to activate PKC-ζ (60), phosphatidylinositol 4-kinase (32, 33, 45), PLC-γ (15), and sphingosine kinase-1 (16) in mammalian cells. More recently, a role for the PLD/PA signaling axis in activation of IQ motif-containing GTPase-activating protein-1 through Rac1 in hyperoxia-mediated ROS generation in lung endothelial cells was demonstrated, suggesting PA generated by the PLD pathway to be a potent activator of NOX proteins (64).
Superoxide dismutase (SOD) is a key antioxidant defense enzyme in the lung (39), and specifically mitochondrial manganese SOD (mtMnSOD) plays an important role in maintaining mitochondrial redox status. This enzyme detoxifies O2·− by catalyzing its dismutation to H2O2. Bleomycin increased acetylation of MnSODK68 in Beas2B cells (Fig. 6, D and E). Acetylation of MnSOD has been shown to inactive the enzyme, and enhance oxidative stress-induced damage to the lung (48). Inhibition of PLD2 activity with PLD2-specific inhibitor VU0364739 blocked bleomycin-induced acetylation of MnSODK68, whereas blocking PLD1 activity had no effect on acetylation of MnSOD (Fig. 6, D and E). Alveolar type II cells isolated from IPF lungs showed increased MnSODK68 acetylation compared with controls due to decreased sirtuin 3 (SIRT3) protein expression (30). SIRT3 is a mitochondrial member of the sirtuin family of NAD-dependent deacetylases (12, 37), and blocking SIRT3 reduced oxidant-induced mtDNA damage and apoptosis (30). The results presented here show a role for PLD/PA signaling in MnSOD acetylation; however, they do not address the role of PLD1 or PLD2 in modulating SIRT3 expression or activity and fibrosis.
Another important finding in this study is that inhibition of PLD1 or PLD2 ameliorated bleomycin-induced mtDNA damage (Fig. 7) and apoptosis (Fig. 8) in lung epithelial cells. The role of mtROS in mtDNA damage and apoptosis was verified by using a mtROS-specific scavenger, MitoTempo. MitoTempo pretreatment of lung epithelial cells attenuated bleomycin-mediated mtROS, mtDNA damage, and apoptosis (Figs. 7 and 8). These findings confirm the earlier reports on increased mitochondrial O2·− production, leading to mtDNA damage and apoptosis of alveolar epithelial cells (35). The near proximity of mtDNA to ETC, inadequate DNA repair mechanism, and lack of histone protective shield to cover mtDNA makes it more susceptible to damage induced by oxidative stress (~50% higher) than nuclear DNA (57). mtDNA damage leads to reduction in mitochondrial membrane potential and thereby alters the efficiency of ETC, thus contributing to the enhanced outer mitochondrial membrane permeability (22). PLD6 associated with mitochondria generates PA by using CL, which is localized predominantly in the inner mitochondrial membrane; however, our study does not address the role of PLD6 in bleomycin-induced mtROS, mtDNA damage, and apoptosis of lung epithelial cells.
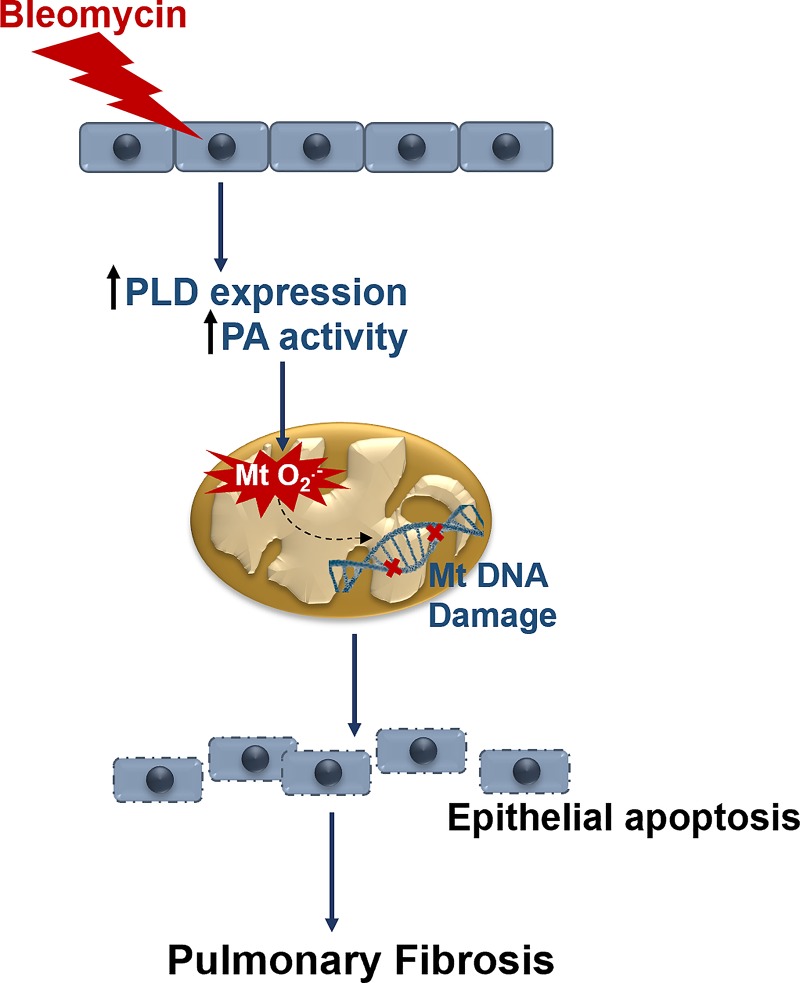
In summary, we have demonstrated a key role of PLD1 and PLD2 in bleomycin-induced PF in mice. We showed that genetic deletion of Pld1 and Pld2 in mice was protective against bleomycin-induced fibrogenesis in mice. Both PLD1 and PLD2 were found to regulate lung fibrosis by enhancing mitochondrial oxidative stress, and mtDNA damage that resulted in epithelial cell apoptosis (Fig. 9). Thus, targeting PLD in the lung may be a viable therapeutic strategy against PF.
Fig. 9.
Proposed role of phospholipase D (PLD) in bleomycin-induced activation of mitochondrial reactive oxygen species (mtROS), mtDNA damage, and apoptosis of bronchial epithelial cells. Bleomycin challenge stimulates expression and activity of PLD in bronchial epithelial cells. Activated PLD generates phosphatidic acid (PA), which enhances mitochondrial superoxide (mt O2·−) production, which leads to mtDNA damage and apoptosis of bronchial epithelial cells. These events result in increased expression of fibrogenic markers and development of lung fibrosis in a bleomycin murine model of pulmonary fibrosis.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant P01 HL-098050 (to V. Natarajan) and a VA Merit Award 2I01BX000786-05A2 (to D. W. Kamp).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
V.S., C.F.-B., D.W.K., and V.N. conceived and designed research; V.S., L.H., S.-J.K., M.S., and M.B. performed experiments; V.S. analyzed data; V.S. and P.F. interpreted results of experiments; V.S. prepared figures; V.S. drafted manuscript; V.S., P.C., C.F.-B., G.D.P., and V.N. edited and revised manuscript; V.S. and V.N. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. Yasunori Kanaho (Univ. of Tsukuba, Japan), for the generous gift of rabbit polyclonal PLD1 and PLD2 antibodies.
Present address of G. Di Paolo: Denali Therapeutics, 161 Oyster Point Blvd., South San Francisco, CA 94080 (e-mail: dipaolo@dnli.com).
REFERENCES
- 1.American Thoracic Society American Thoracic Society. Idiopathic pulmonary fibrosis: diagnosis and treatment. International consensus statement. American Thoracic Society (ATS), and the European Respiratory Society (ERS). Am J Respir Crit Care Med 161: 646–664, 2000. doi: 10.1164/ajrccm.161.2.ats3-00. [DOI] [PubMed] [Google Scholar]
- 2.Abdulnour RE, Howrylak JA, Tavares AH, Douda DN, Henkels KM, Miller TE, Fredenburgh LE, Baron RM, Gomez-Cambronero J, Levy BD. Phospholipase D isoforms differentially regulate leukocyte responses to acute lung injury. J Leukoc Biol 103: 919–932, 2018. doi: 10.1002/JLB.3A0617-252RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ali WH, Chen Q, Delgiorno KE, Su W, Hall JC, Hongu T, Tian H, Kanaho Y, Di Paolo G, Crawford HC, Frohman MA. Deficiencies of the lipid-signaling enzymes phospholipase D1 and D2 alter cytoskeletal organization, macrophage phagocytosis, and cytokine-stimulated neutrophil recruitment. PLoS One 8: e55325, 2013. doi: 10.1371/journal.pone.0055325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amara N, Goven D, Prost F, Muloway R, Crestani B, Boczkowski J. NOX4/NADPH oxidase expression is increased in pulmonary fibroblasts from patients with idiopathic pulmonary fibrosis and mediates TGFbeta1-induced fibroblast differentiation into myofibroblasts. Thorax 65: 733–738, 2010. doi: 10.1136/thx.2009.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arellanes-Robledo J, Reyes-Gordillo K, Shah R, Domínguez-Rosales JA, Hernández-Nazara ZH, Ramirez F, Rojkind M, Lakshman MR. Fibrogenic actions of acetaldehyde are β-catenin dependent but Wingless independent: a critical role of nucleoredoxin and reactive oxygen species in human hepatic stellate cells. Free Radic Biol Med 65: 1487–1496, 2013. doi: 10.1016/j.freeradbiomed.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 6.Black KE, Berdyshev E, Bain G, Castelino FV, Shea BS, Probst CK, Fontaine BA, Bronova I, Goulet L, Lagares D, Ahluwalia N, Knipe RS, Natarajan V, Tager AM. Autotaxin activity increases locally following lung injury, but is not required for pulmonary lysophosphatidic acid production or fibrosis. FASEB J 30: 2435–2450, 2016. doi: 10.1096/fj.201500197R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bocchino M, Agnese S, Fagone E, Svegliati S, Grieco D, Vancheri C, Gabrielli A, Sanduzzi A, Avvedimento EV. Reactive oxygen species are required for maintenance and differentiation of primary lung fibroblasts in idiopathic pulmonary fibrosis. PLoS One 5: e14003, 2010. doi: 10.1371/journal.pone.0014003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandes RP, Kreuzer J. Vascular NADPH oxidases: molecular mechanisms of activation. Cardiovasc Res 65: 16–27, 2005. doi: 10.1016/j.cardiores.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 9.Brindley DN, Pilquil C. Lipid phosphate phosphatases and signaling. J Lipid Res 50, Suppl: S225–S230, 2009. doi: 10.1194/jlr.R800055-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown HA, Thomas PG, Lindsley CW. Targeting phospholipase D in cancer, infection and neurodegenerative disorders. Nat Rev Drug Discov 16: 351–367, 2017. doi: 10.1038/nrd.2016.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cazzolli R, Shemon AN, Fang MQ, Hughes WE. Phospholipid signalling through phospholipase D and phosphatidic acid. IUBMB Life 58: 457–461, 2006. doi: 10.1080/15216540600871142. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Fu LL, Wen X, Wang XY, Liu J, Cheng Y, Huang J. Sirtuin-3 (SIRT3), a therapeutic target with oncogenic and tumor-suppressive function in cancer. Cell Death Dis 5: e1047, 2014. doi: 10.1038/cddis.2014.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheresh P, Kim SJ, Tulasiram S, Kamp DW. Oxidative stress and pulmonary fibrosis. Biochim Biophys Acta 1832: 1028–1040, 2013. doi: 10.1016/j.bbadis.2012.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chichger H, Vang A, O’Connell KA, Zhang P, Mende U, Harrington EO, Choudhary G. PKC δ and βII regulate angiotensin II-mediated fibrosis through p38: a mechanism of RV fibrosis in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 308: L827–L836, 2015. doi: 10.1152/ajplung.00184.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cockcroft S. Signalling roles of mammalian phospholipase D1 and D2. Cell Mol Life Sci 58: 1674–1687, 2001. doi: 10.1007/PL00000805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Delon C, Manifava M, Wood E, Thompson D, Krugmann S, Pyne S, Ktistakis NT. Sphingosine kinase 1 is an intracellular effector of phosphatidic acid. J Biol Chem 279: 44763–44774, 2004. doi: 10.1074/jbc.M405771200. [DOI] [PubMed] [Google Scholar]
- 17.Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA)-induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res 6: 2482–2491, 2000. [PubMed] [Google Scholar]
- 18.Foster DA, Xu L. Phospholipase D in cell proliferation and cancer. Mol Cancer Res 1: 789–800, 2003. [PubMed] [Google Scholar]
- 19.Frohman MA. The phospholipase D superfamily as therapeutic targets. Trends Pharmacol Sci 36: 137–144, 2015. doi: 10.1016/j.tips.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaits F, Fourcade O, Le Balle F, Gueguen G, Gaigé B, Gassama-Diagne A, Fauvel J, Salles JP, Mauco G, Simon MF, Chap H. Lysophosphatidic acid as a phospholipid mediator: pathways of synthesis. FEBS Lett 410: 54–58, 1997. doi: 10.1016/S0014-5793(97)00411-0. [DOI] [PubMed] [Google Scholar]
- 21.Hagimoto N, Kuwano K, Miyazaki H, Kunitake R, Fujita M, Kawasaki M, Kaneko Y, Hara N. Induction of apoptosis and pulmonary fibrosis in mice in response to ligation of Fas antigen. Am J Respir Cell Mol Biol 17: 272–278, 1997. doi: 10.1165/ajrcmb.17.3.2893. [DOI] [PubMed] [Google Scholar]
- 22.Hebert SL, Lanza IR, Nair KS. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech Ageing Dev 131: 451–462, 2010. doi: 10.1016/j.mad.2010.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, Pennathur S, Martinez FJ, Thannickal VJ. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med 15: 1077–1081, 2009. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hewlett JC, Kropski JA, Blackwell TS. Idiopathic pulmonary fibrosis: Epithelial-mesenchymal interactions and emerging therapeutic targets. Matrix Biol 71-72: 112–127, 2018. doi: 10.1016/j.matbio.2018.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang KP. The mechanism of protein kinase C activation. Trends Neurosci 12: 425–432, 1989. doi: 10.1016/0166-2236(89)90091-X. [DOI] [PubMed] [Google Scholar]
- 26.Huang LS, Berdyshev E, Mathew B, Fu P, Gorshkova IA, He D, Ma W, Noth I, Ma SF, Pendyala S, Reddy SP, Zhou T, Zhang W, Garzon SA, Garcia JG, Natarajan V. Targeting sphingosine kinase 1 attenuates bleomycin-induced pulmonary fibrosis. FASEB J 27: 1749–1760, 2013. doi: 10.1096/fj.12-219634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang LS, Fu P, Patel P, Harijith A, Sun T, Zhao Y, Garcia JG, Chun J, Natarajan V. Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice. Am J Respir Cell Mol Biol 49: 912–922, 2013. doi: 10.1165/rcmb.2013-0070OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hübner RH, Gitter W, El Mokhtari NE, Mathiak M, Both M, Bolte H, Freitag-Wolf S, Bewig B. Standardized quantification of pulmonary fibrosis in histological samples. Biotechniques 44: 507–517, 2008. doi: 10.2144/000112729. [DOI] [PubMed] [Google Scholar]
- 29.Itoh T, Hasegawa J, Tsujita K, Kanaho Y, Takenawa T. The tyrosine kinase Fer is a downstream target of the PLD-PA pathway that regulates cell migration. Sci Signal 2: ra52, 2009. doi: 10.1126/scisignal.2000393. [DOI] [PubMed] [Google Scholar]
- 30.Jablonski RP, Kim SJ, Cheresh P, Williams DB, Morales-Nebreda L, Cheng Y, Yeldandi A, Bhorade S, Pardo A, Selman M, Ridge K, Gius D, Budinger GRS, Kamp DW. SIRT3 deficiency promotes lung fibrosis by augmenting alveolar epithelial cell mitochondrial DNA damage and apoptosis. FASEB J 31: 2520–2532, 2017. doi: 10.1096/fj.201601077R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jain M, Rivera S, Monclus EA, Synenki L, Zirk A, Eisenbart J, Feghali-Bostwick C, Mutlu GM, Budinger GR, Chandel NS. Mitochondrial reactive oxygen species regulate transforming growth factor-β signaling. J Biol Chem 288: 770–777, 2013. doi: 10.1074/jbc.M112.431973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jenkins CM, Waterman MR. Flavodoxin and NADPH-flavodoxin reductase from Escherichia coli support bovine cytochrome P450c17 hydroxylase activities. J Biol Chem 269: 27401–27408, 1994. [PubMed] [Google Scholar]
- 33.Jenkins GM, Frohman MA. Phospholipase D: a lipid centric review. Cell Mol Life Sci 62: 2305–2316, 2005. doi: 10.1007/s00018-005-5195-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim SJ, Cheresh P, Jablonski RP, Morales-Nebreda L, Cheng Y, Hogan E, Yeldandi A, Chi M, Piseaux R, Ridge K, Michael Hart C, Chandel N, Scott Budinger GR, Kamp DW. Mitochondrial catalase overexpressed transgenic mice are protected against lung fibrosis in part via preventing alveolar epithelial cell mitochondrial DNA damage. Free Radic Biol Med 101: 482–490, 2016. doi: 10.1016/j.freeradbiomed.2016.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim SJ, Cheresh P, Jablonski RP, Williams DB, Kamp DW. The role of mitochondrial DNA in mediating alveolar epithelial cell apoptosis and pulmonary fibrosis. Int J Mol Sci 16: 21486–21519, 2015. doi: 10.3390/ijms160921486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim SJ, Cheresh P, Williams D, Cheng Y, Ridge K, Schumacker PT, Weitzman S, Bohr VA, Kamp DW. Mitochondria-targeted Ogg1 and aconitase-2 prevent oxidant-induced mitochondrial DNA damage in alveolar epithelial cells. J Biol Chem 289: 6165–6176, 2014. doi: 10.1074/jbc.M113.515130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kincaid B, Bossy-Wetzel E. Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front Aging Neurosci 5: 48, 2013. doi: 10.3389/fnagi.2013.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.King TE Jr, Pardo A, Selman M. Idiopathic pulmonary fibrosis. Lancet 378: 1949–1961, 2011. doi: 10.1016/S0140-6736(11)60052-4. [DOI] [PubMed] [Google Scholar]
- 39.Kinnula VL, Crapo JD. Superoxide dismutases in the lung and human lung diseases. Am J Respir Crit Care Med 167: 1600–1619, 2003. doi: 10.1164/rccm.200212-1479SO. [DOI] [PubMed] [Google Scholar]
- 40.Knowlden S, Georas SN. The autotaxin-LPA axis emerges as a novel regulator of lymphocyte homing and inflammation. J Immunol 192: 851–857, 2014. doi: 10.4049/jimmunol.1302831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson-Casey JL, Deshane JS, Ryan AJ, Thannickal VJ, Carter AB. Macrophage Akt1 kinase-mediated mitophagy modulates apoptosis resistance and pulmonary fibrosis. Immunity 44: 582–596, 2016. doi: 10.1016/j.immuni.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu X, Chen Z. The pathophysiological role of mitochondrial oxidative stress in lung diseases. J Transl Med 15: 207, 2017. doi: 10.1186/s12967-017-1306-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luzina IG, Highsmith K, Pochetuhen K, Nacu N, Rao JN, Atamas SP. PKCα mediates CCL18-stimulated collagen production in pulmonary fibroblasts. Am J Respir Cell Mol Biol 35: 298–305, 2006. doi: 10.1165/rcmb.2006-0033OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM; Acute Lung Injury in Animals Study Group . An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol 44: 725–738, 2011. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moritz A, De Graan PN, Gispen WH, Wirtz KW. Phosphatidic acid is a specific activator of phosphatidylinositol-4-phosphate kinase. J Biol Chem 267: 7207–7210, 1992. [PubMed] [Google Scholar]
- 46.Nelson RK, Frohman MA. Physiological and pathophysiological roles for phospholipase D. J Lipid Res 56: 2229–2237, 2015. doi: 10.1194/jlr.R059220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oikonomou N, Mouratis MA, Tzouvelekis A, Kaffe E, Valavanis C, Vilaras G, Karameris A, Prestwich GD, Bouros D, Aidinis V. Pulmonary autotaxin expression contributes to the pathogenesis of pulmonary fibrosis. Am J Respir Cell Mol Biol 47: 566–574, 2012. doi: 10.1165/rcmb.2012-0004OC. [DOI] [PubMed] [Google Scholar]
- 48.Ozden O, Park SH, Kim HS, Jiang H, Coleman MC, Spitz DR, Gius D. Acetylation of MnSOD directs enzymatic activity responding to cellular nutrient status or oxidative stress. Aging (Albany NY) 3: 102–107, 2011. doi: 10.18632/aging.100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pardo A, Selman M. Lung fibroblasts, aging, and idiopathic pulmonary fibrosis. Ann Am Thorac Soc 13, Suppl 5: S417–S421, 2016. doi: 10.1513/AnnalsATS.201605-341AW. [DOI] [PubMed] [Google Scholar]
- 50.Patel AS, Song JW, Chu SG, Mizumura K, Osorio JC, Shi Y, El-Chemaly S, Lee CG, Rosas IO, Elias JA, Choi AM, Morse D. Epithelial cell mitochondrial dysfunction and PINK1 are induced by transforming growth factor-beta1 in pulmonary fibrosis. PLoS One 10: e0121246, 2015. doi: 10.1371/journal.pone.0121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Patel RB, Kotha SR, Sherwani SI, Sliman SM, Gurney TO, Loar B, Butler SO, Morris AJ, Marsh CB, Parinandi NL. Pulmonary fibrosis inducer, bleomycin, causes redox-sensitive activation of phospholipase D and cytotoxicity through formation of bioactive lipid signal mediator, phosphatidic acid, in lung microvascular endothelial cells. Int J Toxicol 30: 69–90, 2011. doi: 10.1177/1091581810388850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pendyala S, Usatyuk PV, Gorshkova IA, Garcia JG, Natarajan V. Regulation of NADPH oxidase in vascular endothelium: the role of phospholipases, protein kinases, and cytoskeletal proteins. Antioxid Redox Signal 11: 841–860, 2009. doi: 10.1089/ars.2008.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ; ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis . An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 183: 788–824, 2011. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Raghu G, Selman M. Nintedanib and pirfenidone. New antifibrotic treatments indicated for idiopathic pulmonary fibrosis offer hopes and raises questions. Am J Respir Crit Care Med 191: 252–254, 2015. doi: 10.1164/rccm.201411-2044ED. [DOI] [PubMed] [Google Scholar]
- 55.Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24: 981–990, 2012. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryu C, Sun H, Gulati M, Herazo-Maya JD, Chen Y, Osafo-Addo A, Brandsdorfer C, Winkler J, Blaul C, Faunce J, Pan H, Woolard T, Tzouvelekis A, Antin-Ozerkis DE, Puchalski JT, Slade M, Gonzalez AL, Bogenhagen DF, Kirillov V, Feghali-Bostwick C, Gibson K, Lindell K, Herzog RI, Dela Cruz CS, Mehal W, Kaminski N, Herzog EL, Trujillo G. Extracellular mitochondrial DNA is generated by fibroblasts and predicts death in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 196: 1571–1581, 2017. doi: 10.1164/rccm.201612-2480OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schumacker PT, Gillespie MN, Nakahira K, Choi AM, Crouser ED, Piantadosi CA, Bhattacharya J. Mitochondria in lung biology and pathology: more than just a powerhouse. Am J Physiol Lung Cell Mol Physiol 306: L962–L974, 2014. doi: 10.1152/ajplung.00073.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi H, Deng HX, Gius D, Schumacker PT, Surmeier DJ, Ma YC. Sirt3 protects dopaminergic neurons from mitochondrial oxidative stress. Hum Mol Genet 26: 1915–1926, 2017. doi: 10.1093/hmg/ddx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sonoda H, Aoki J, Hiramatsu T, Ishida M, Bandoh K, Nagai Y, Taguchi R, Inoue K, Arai H. A novel phosphatidic acid-selective phospholipase A1 that produces lysophosphatidic acid. J Biol Chem 277: 34254–34263, 2002. doi: 10.1074/jbc.M201659200. [DOI] [PubMed] [Google Scholar]
- 60.Stasek JE Jr, Natarajan V, Garcia JG. Phosphatidic acid directly activates endothelial cell protein kinase C. Biochem Biophys Res Commun 191: 134–141, 1993. doi: 10.1006/bbrc.1993.1194. [DOI] [PubMed] [Google Scholar]
- 61.Suryadevara V, Fu P, Ebenezer DL, Berdyshev E, Bronova IA, Huang LS, Harijith A, Natarajan V. Sphingolipids in ventilator induced lung injury: role of sphingosine-1-phosphate lyase. Int J Mol Sci 19: 114, 2018. doi: 10.3390/ijms19010114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tager AM, LaCamera P, Shea BS, Campanella GS, Selman M, Zhao Z, Polosukhin V, Wain J, Karimi-Shah BA, Kim ND, Hart WK, Pardo A, Blackwell TS, Xu Y, Chun J, Luster AD. The lysophosphatidic acid receptor LPA1 links pulmonary fibrosis to lung injury by mediating fibroblast recruitment and vascular leak. Nat Med 14: 45–54, 2008. doi: 10.1038/nm1685. [DOI] [PubMed] [Google Scholar]
- 63.Trivedi P, Kumar RK, Iyer A, Boswell S, Gerarduzzi C, Dadhania VP, Herbert Z, Joshi N, Luyendyk JP, Humphreys BD, Vaidya VS. Targeting phospholipase D4 attenuates kidney fibrosis. J Am Soc Nephrol 28: 3579–3589, 2017. doi: 10.1681/ASN.2016111222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Usatyuk PV, Gorshkova IA, He D, Zhao Y, Kalari SK, Garcia JG, Natarajan V. Phospholipase D-mediated activation of IQGAP1 through Rac1 regulates hyperoxia-induced p47phox translocation and reactive oxygen species generation in lung endothelial cells. J Biol Chem 284: 15339–15352, 2009. doi: 10.1074/jbc.M109.005439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 7: 65–74, 2009. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vega-Naredo I, Caballero B, Sierra V, García-Macia M, de Gonzalo-Calvo D, Oliveira PJ, Rodríguez-Colunga MJ, Coto-Montes A. Melatonin modulates autophagy through a redox-mediated action in female Syrian hamster Harderian gland controlling cell types and gland activity. J Pineal Res 52: 80–92, 2012. doi: 10.1111/j.1600-079X.2011.00922.x. [DOI] [PubMed] [Google Scholar]
- 67.Veith C, Boots AW, Idris M, van Schooten FJ, van der Vliet A. Redox imbalance in idiopathic pulmonary fibrosis: a role for oxidant cross-talk between NADPH oxidase enzymes and mitochondria. Antioxid Redox Signal. 5 Apr 2019. doi: 10.1089/ars.2019.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Walters DM, Cho HY, Kleeberger SR. Oxidative stress and antioxidants in the pathogenesis of pulmonary fibrosis: a potential role for Nrf2. Antioxid Redox Signal 10: 321–332, 2008. doi: 10.1089/ars.2007.1901. [DOI] [PubMed] [Google Scholar]
- 69.Xiao J, Meng XM, Huang XR, Chung AC, Feng YL, Hui DS, Yu CM, Sung JJ, Lan HY. miR-29 inhibits bleomycin-induced pulmonary fibrosis in mice. Mol Ther 20: 1251–1260, 2012. doi: 10.1038/mt.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zank DC, Bueno M, Mora AL, Rojas M. Idiopathic pulmonary fibrosis: aging, mitochondrial dysfunction, and cellular bioenergetics. Front Med (Lausanne) 5: 10, 2018. doi: 10.3389/fmed.2018.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang L, Keane MP, Zhu LX, Sharma S, Rozengurt E, Strieter RM, Dubinett SM, Huang M. Interleukin-7 and transforming growth factor-β play counter-regulatory roles in protein kinase C-δ-dependent control of fibroblast collagen synthesis in pulmonary fibrosis. J Biol Chem 279: 28315–28319, 2004. doi: 10.1074/jbc.C400115200. [DOI] [PubMed] [Google Scholar]
- 72.Zhang N, Chen Y, Jiang R, Li E, Chen X, Xi Z, Guo Y, Liu X, Zhou Y, Che Y, Jiang X. PARP and RIP 1 are required for autophagy induced by 11′-deoxyverticillin A, which precedes caspase-dependent apoptosis. Autophagy 7: 598–612, 2011. doi: 10.4161/auto.7.6.15103. [DOI] [PubMed] [Google Scholar]
- 73.Zhao Y, Natarajan V. Lysophosphatidic acid (LPA) and its receptors: role in airway inflammation and remodeling. Biochim Biophys Acta 1831: 86–92, 2013. doi: 10.1016/j.bbalip.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zhu X, Liu R, Kuang D, Liu J, Shi X, Zhang T, Zeng Y, Sun X, Zhang Y, Yang W. The role of phospholipase D1 in liver fibrosis induced by dimethylnitrosamine in vivo. Dig Dis Sci 59: 1779–1788, 2014. doi: 10.1007/s10620-014-3130-6. [DOI] [PubMed] [Google Scholar]
- 75.Zoz DF, Lawson WE, Blackwell TS. Idiopathic pulmonary fibrosis: a disorder of epithelial cell dysfunction. Am J Med Sci 341: 435–438, 2011. doi: 10.1097/MAJ.0b013e31821a9d8e. [DOI] [PMC free article] [PubMed] [Google Scholar]