Abstract
Acidosis is common among critically ill patients, but current approaches to correct pH do not improve disease outcomes. During systemic acidosis, cells are either passively exposed to extracellular acidosis that other cells have generated (extrinsic acidosis) or they are exposed to acid that they generate and export into the extracellular space (intrinsic acidosis). Although endothelial repair following intrinsic acidosis has been studied, the impact of extrinsic acidosis on migration and angiogenesis is unclear. We hypothesized that extrinsic acidosis inhibits metabolism and migration but promotes capillary-like network formation in pulmonary microvascular endothelial cells (PMVECs). Extrinsic acidosis was modeled by titrating media pH. Two types of intrinsic acidosis were compared, including increasing cellular metabolism by chemically inhibiting carbonic anhydrases (CAs) IX and XII (SLC-0111) and with hypoxia. PMVECs maintained baseline intracellular pH for 24 h with both extrinsic and intrinsic acidosis. Whole cell CA IX protein expression was decreased by extrinsic acidosis but not affected by hypoxia. When extracellular pH was equally acidic, extrinsic acidosis suppressed glycolysis, whereas intrinsic acidosis did not. Extrinsic acidosis suppressed migration, but increased Matrigel network master junction and total segment length. CRISPR-Cas9 CA IX knockout PMVECs revealed an independent role of CA IX in promoting glycolysis, as loss of CA IX alone was accompanied by decreased hexokinase I and pyruvate dehydrogenase E1α expression and decreasing migration. 2-deoxy-d-glucose had no effect on migration but profoundly inhibited network formation and increased N-cadherin expression. Thus, we report that while extrinsic acidosis suppresses endothelial glycolysis and migration, it promotes network formation.
Keywords: carbonic anhydrase IX (CA IX), Matrigel, N-cadherin, SLC-0111, 2-deoxy-d-glucose (2DG)
INTRODUCTION
Acidosis is common among critically ill patients, but the effects of acidosis on acute respiratory distress syndrome (ARDS) pathophysiology are poorly understood. In a study of 851 critically ill patients, metabolic acidosis was present in 64% of patients, and it was associated with a twofold higher mortality rate (18). Recently, Calfee et al. (6) classified ARDS into two categories based on clinical features, and acidosis was a major characteristic of the hyperinflammatory subphenotype, which portends higher mortality. On the other hand, a secondary analysis of the ARDS Network trial involving 861 acute lung injury patients showed that respiratory acidosis was associated with reduced 28-day mortality in the high tidal volume group after controlling for comorbidities and severity of lung injury (26). Because of these conflicting data, current guidelines lack high-level (i.e., randomized placebo-controlled double-blind) studies, recommendations for targeting pH, and acidosis management strategies in ARDS patients (13).
ARDS severity is heterogeneously distributed in the lung. Two mortality-reducing ventilator strategies, low tidal volume high positive end-expiratory pressure ventilation and prone positioning, mainly aim to homogenize alveolar stress and strain and minimize ventilator-induced lung injury (13, 16, 17). As a potential consequence of lung protective ventilation, respiratory acidosis may ensue but be accepted as the cost of low tidal volume ventilation (permissive hypercapnia). Whether such permissive hypercapnia in this patient population is beneficial or harmful remains controversial (10), particularly if it is associated with other highly prevalent acid-base disorders (1). Regionally heterogeneous involvement in ARDS causes variable degrees of hypoxia-induced cellular metabolic changes and proton production, with variability in tissue pH. Such microenvironmental pH variation has been described in tumors, infection, and inflammation in other organs (12), but it has not been studied in the lung and in the context of ARDS pathophysiology. Furthermore, at an earlier stage of disease when local pH perturbations do not overwhelm systemic acid regulatory mechanisms, arterial blood pH, which is the only clinically available method to assess acid-base status, cannot sensitively or accurately reflect tissue level pH status (2, 23).
Cells within a tissue are either passively exposed to extracellular acidosis from acid generated by other tissues or they actively produce and secrete protons into their surrounding extracellular space. Here, we define two types of extracellular acidosis based on the primary proton source: 1) extrinsic acidosis is determined by passive extracellular proton exposure, and 2) intrinsic acidosis is determined by local cellular metabolism leading to increased proton production with subsequent extracellular proton exposure (Fig. 1A). Extrinsic acidosis impairs pulmonary neutrophil phagocytosis (15) and epithelial cell migration (30) and increases vascular endothelial growth factor transcription in tumor cell lines (14). If these observations are germane to endothelial cell physiology, then we would predict that extrinsic acidosis would impair migration but stimulate angiogenesis, which may seem paradoxical given that cell migration is a prerequisite for angiogenesis. To date, no study has compared how extrinsic and intrinsic extracellular acidosis affects pulmonary endothelial cell metabolism and repair mechanisms, including migration and network formation. Here, we hypothesized that extrinsic acidosis inhibits metabolism and migration but promotes network formation in pulmonary microvascular endothelial cells (PMVECs).
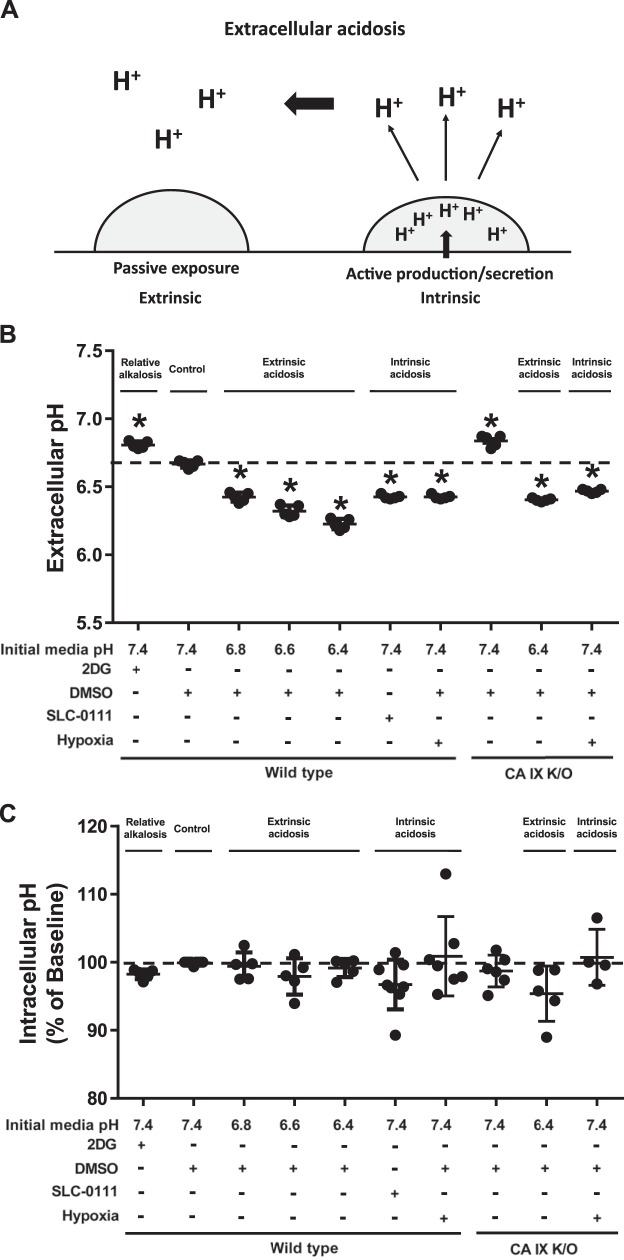
Fig. 1.
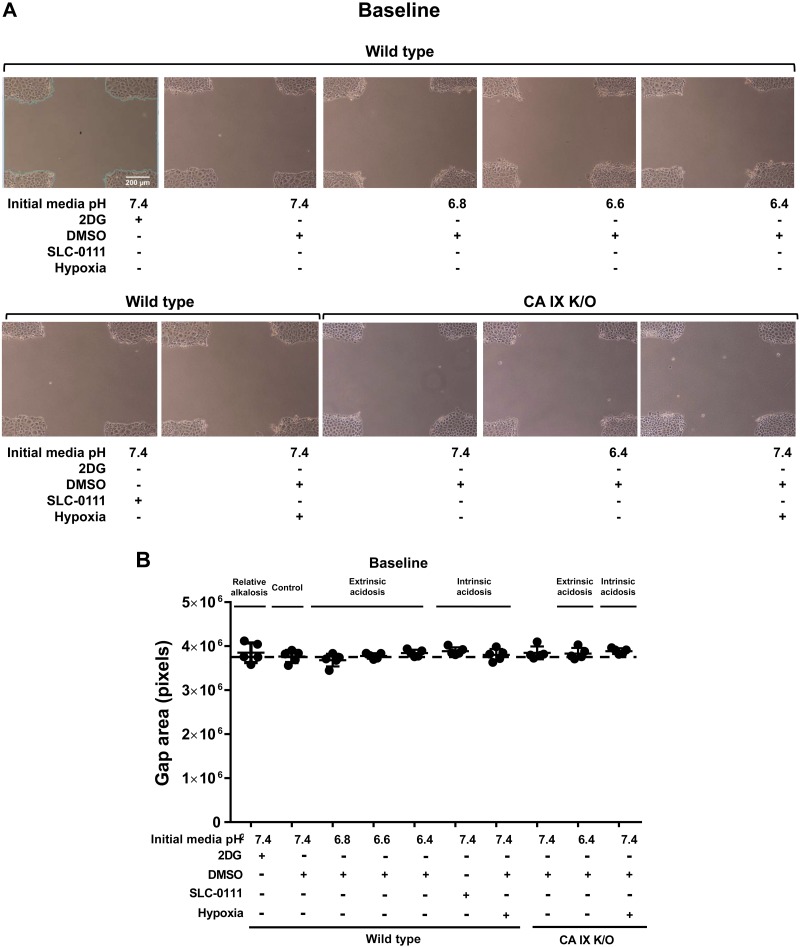
Twenty-four hours after extrinsic and intrinsic extracellular acidosis exposure, pulmonary microvascular endothelial cells (PMVECs) maintain intracellular pH. A: schematic representation of extrinsic and intrinsic extracellular acidosis: extrinsic acidosis is determined by passive extracellular proton exposure, and intrinsic acidosis is determined by increased cellular proton production and/or secretion. B and C: wild-type and carbonic anhydrase (CA) IX knockout (K/O) PMVECs were seeded at 5.0 × 105 cells/well on 6-well plates (B) or 4.0 × 104 cells/well on 96-well plates (C) on bicarbonate buffered media. Two days after cell seeding, media were changed to HEPES-buffered media with pH 7.4, 6.8, 6.6, and 6.4 and treated with 2-deoxy-d-glucose (2DG; 5 mM), DMSO (0.5%), and SLC-0111 (150 µM) in normoxia and hypoxia (1% oxygen). Extracellular acidosis is attenuated with 2DG treatment but enhanced with SLC-0111 or hypoxia (B). No significant change occurs in intracellular pH levels 24 h after extrinsic or intrinsic acidosis in PMVECs (C). Data represent means ± SD. Two-way ANOVA and Bonferroni post hoc tests were used to compare between different groups. At least five separate experiments were performed. *Significant difference (P < 0.05) from wild-type pH 7.4.
MATERIALS AND METHODS
Isolation of rat lung endothelial cells.
Procedures for isolation of rat endothelial cells were approved by the University of South Alabama Institutional Animal Care and Use Committee. Pulmonary microvascular endothelial cells were isolated from male Sprague-Dawley rats (CD strain, 350–400 g; Charles River) as previously described (39). PMVECs were verified with a panel of lectin-binding criteria, and all cells stained positive for DiI-LDL and factor VIII. Since carbonic anhydrase (CA) IX knockout (K/O) cells were extensively used in this study, we used cells isolated from one animal that matches with the K/O cells for adequate comparison, to add mechanistic insight into the role of CA IX on endothelial acid sensing. Generalizability of this principle across animals and species has not been tested here and will be the focus of our future work.
Generation of CA IX-depleted cells.
CA IX knockout PMVECs were generated using CRISPR-Cas9 gene editing technology as previously described (5, 27).
In vitro extrinsic and intrinsic acidosis models.
Cells were seeded at a density of 5.0 × 105 cells per well in 6-well plates in DMEM (Thermo Fisher, Grand Island, NY), 10% FCS, and 1% penicillin-streptomycin at 37°C in ambient air, 5% CO2. Two days later, media was replaced with bicarbonate-free media (Thermo Fisher) buffered with 30 mM HEPES at pH 7.4 and incubated at 37°C in ambient air, 0% CO2. Cells treated with 5 mM 2-deoxy-d-glucose (2DG), which partially inhibits glycolysis, were used as a control group that only causes minimal media acidification. For extrinsic acidosis, media pH was decreased by titrating with 1 N HCl to achieve a pH of 6.8, 6.6, or 6.4. For intrinsic acidosis, cells were either treated with 150 µM SLC-0111 (specific CA IX and CA XII inhibitor, MedKoo Biosciences, Morrisville, NC and gift from Dr. Supuran, Italy), which decreases both intra- and extracellular pH (27), or 1% hypoxia. One day after treatments, media pH was measured using a pH meter (Denver Instrument Company, Bohemia, NY). For intrinsic acidosis reversal, 1 N NaOH was delivered at a rate of 1 µl/h per well (2 ml media volume) for 24 h using osmotic pumps (Alzet Osmotic Pumps, Cupertino, CA).
Intracellular pH measurement.
Cells were seeded at a density of 4.0 × 104 cells per well in 96-well plates. Two days later, media was changed to bicarbonate-free HEPES-buffered media with different treatments as specified above. The next day, cells were rinsed with HBSS, incubated in 1 µM BCECF-AM (Thermo Fisher) for 15 min. After two rinses with HBSS, intracellular pH was assessed by ratiometric measurements using a SpectraMax iD5 Multi-Mode Microplate Reader (Molecular Devices, San Jose, CA) with dual excitation (440 and 490 nm) and single emission (535 nm) wavelengths. For calibration, 10 µM nigericin sodium (Adipogen, San Diego, CA) with pH 6.0 to 8.0 solutions containing 120 mM KCl, 2 mM CaCl2·2H2O, 1 mM MgCl2, and 10 mM glucose in 20 mM HEPES (or MES) was used as previously described (27).
Western blotting.
Cells were seeded at 5.0 × 105 cells/well on six-well plates on bicarbonate-buffered media. Two days after cell seeding, media was changed to bicarbonate-free HEPES-buffered media. One day after treatments with different conditions, cells were collected and subjected to immunoblot analysis as previously described (31). Primary antibodies for CA IX (M75, 1:700 dilution) (27) and GAPDH (1:1,000 dilution) were obtained from BioScience Slovakia (Bratislava, Slovakia) and Cell Signaling (Danvers, MA), respectively, and antibodies for Na+/H+ exchanger-1 (NHE-1, 1:200 dilution) (3), hexokinase-1 (HXK I, 1:200 dilution) (42), pyruvate kinase M-type (PKM, 1:500 dilution) (44), pyruvate dehydrogenase E1α subunit (PDH-E1α, 1:300 dilution) (45), and N-cadherin (1:200 dilution) (34) were obtained from Santa Cruz Biotechnology (Dallas, TX).
Lactate and glucose measurement.
Lactate and glucose levels were measured in the media using the YSI 2300 STAT Plus Glucose & Lactate Analyzer (Yellow Springs, OH) (31).
Scratch wound migration assay.
Cells were seeded at a density of 5.0 × 105 cells per well in 6-well plates. Two days later, monolayers were scratched with a sterile 200-μl pipette tip, and baseline wounds were imaged. Then, the media was replaced with bicarbonate-free media and treated with the above-mentioned conditions. One day after treatments, wounds were reimaged. Images were analyzed using the “MRI_Wound_Healing_Tool” programmed in ImageJ’s macro language.
Apoptosis assay.
Cells were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, media was changed to bicarbonate-free HEPES-buffered media. Then, media pH was decreased by titrating with 1 N HCl to achieve a pH of 6.4 or cells were treated with 150 µM SLC-0111. As a positive control for apoptosis induction, cells were treated with 500 nM of staurosporine obtained from LC Laboratories (Woburn, MA). One day after treatments, cell lysates were collected, and apoptosis activity was assessed using a Caspase-3/CPP32 colorimetric assay kit obtained from Biovision (Milpitas, CA) following manufacturer’s instructions, and 405-nm wavelength absorbance was measured using a SpectraMax iD5 Multi-Mode Microplate Reader.
Matrigel network formation assay.
Matrigel (356231, Corning) was thawed overnight at 4°C. On the day of the experiment, Matrigel was loaded (30 µl per well) in 96-well plates while the plates were maintained on ice. The plates were incubated at 37°C with 0% CO2 ambient air for 1 h. Cells were trypsinized from 10-cm dishes at ~70% confluence and seeded at a density of 4.0 × 104 cells per well in bicarbonate-free HEPES-buffered media with a range of pH and inhibitors and a total cell solution volume of 100 µl per well. Cells were incubated at 37°C with 0% CO2 ambient air for 24 h. Pictures were taken at 24 h. Images were analyzed using the “Angiogenesis Analyzer” tool programmed in ImageJ’s macro language.
Statistics.
One-way ANOVA and Bonferroni post hoc tests were used. Significance was denoted as P < 0.05.
RESULTS
Neither extrinsic nor intrinsic extracellular acidosis change PMVEC intracellular pH at 24 h.
We have previously shown that PMVECs are highly glycolytic and rapidly acidify culture media at baseline (27, 28, 31, 32). We compared 2DG against the DMSO control group, which develops a mild degree of extracellular acidosis without any treatment due to baseline glycolytic activity [Fig. 1B; 2DG vs. wild-type (WT) pH 7.4, P < 0.05]; 2DG reduces extracellular acidification and therefore serves as a relative control group. To model extrinsic acidosis, we titrated the media to achieve a relatively physiologic pH range of 7.4, 6.8, 6.6, and 6.4. To model intrinsic acidosis, cells were treated with SLC-0111 [inhibits CA IX and XII and shown to induce acidosis in our previous study (27)] or hypoxia (enhances anaerobic glycolysis). After 24 h, media pH of extrinsic acidosis groups further decreased, but the pH gradient among the groups was maintained (Fig. 1B; WT pH 7.4 vs. 6.8, 6.6, and WT 6.4, P < 0.05). SLC-0111 and hypoxia lowered media pH (Fig. 1B; WT pH 7.4 vs. SLC-0111 and WT hypoxia, P < 0.05) to a similar degree as the pH 6.8 group (Fig. 1B; WT pH 6.8 vs. SLC-0111 and WT hypoxia, P = ns). Unlike chemical CA IX inhibition or possibly that of other membrane-bound CAs (SLC-0111) that accelerated media acidification, CA IX K/O attenuated media acidification (Fig. 1B; WT pH 7.4 vs. CA IX K/O pH 7.4, P < 0.05) but responded similarly to extrinsic and hypoxia-induced intrinsic acidosis (Fig. 1B; CA IX K/O pH 7.4 vs. CA IX K/O pH 6.4 and CA IX K/O hypoxia, P < 0.05). Despite the variable extracellular pHs among groups, there was no difference in intracellular pH at 24 h [Fig. 1C; WT pH 7.4 vs. all other groups, P = not significant (ns)]. Using these models of extrinsic and intrinsic acidosis with constant intracellular pH, we further investigated relevant acid regulatory protein expression, metabolism, migration, and network formation.
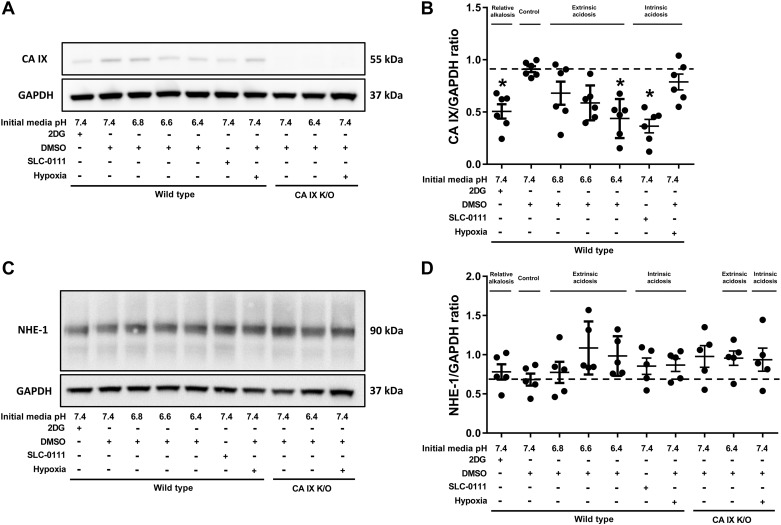
2DG, extrinsic acidosis (pH 6.4), and SLC-0111 decrease CA IX protein expression but do not alter NHE-1.
CA IX and NHE-1 are major regulators of intracellular pH, and their expression is increased in highly glycolytic cells such as cancer cells (41). It is not known whether CA IX and NHE-1 expression are affected by extrinsic versus intrinsic acidosis in nontransformed (nonmalignant) PMVECs. Western blot analysis of whole cell lysates of WT and CA IX K/O PMVECs exposed to extrinsic and intrinsic acidosis revealed a decrease in CA IX expression by 2DG, pH 6.4, and SLC-0111 (Fig. 2, A and B; WT pH 7.4 vs. 2DG, WT pH 6.4, and SLC-0111, P < 0.05) but no change in NHE-1 expression across groups (Fig. 2, C and D; WT pH 7.4 vs. all other groups, P = ns). Therefore, PMVECs dynamically regulate CA IX expression in response to pH and metabolic status.
Fig. 2.
Carbonic anhydrase (CA) IX protein expression is decreased by 2-deoxy-d-glucose (2DG), pH 6.4, and SLC-0111 but not by hypoxia, while Na+/H+ exchanger-1 (NHE-1) expression remains constant. Wild-type and CA IX knockout (K/O) pulmonary microvascular endothelial cells (PMVECs) were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, media were changed to HEPES-buffered media with pH 7.4, 6.8, 6.6, and 6.4 and treated with 2DG (5 mM), DMSO (0.5%), and SLC-0111 (150 µM) in normoxia and hypoxia (1% oxygen). Twenty-four hours later, whole cell lysates were collected, and Western blotting was performed to assess CA IX (A and B) and NHE-1 (C and D) protein abundance. CA IX was decreased by 2DG, pH 6.4, and SLC-0111 but was not changed by hypoxia. NHE-1 expression remained unchanged across groups. Data represent means ± SD. Two-way ANOVA and Bonferroni post hoc tests were used to compare between different groups. At least five separate experiments were performed. *Significant difference (P < 0.05) from wild-type pH 7.4.
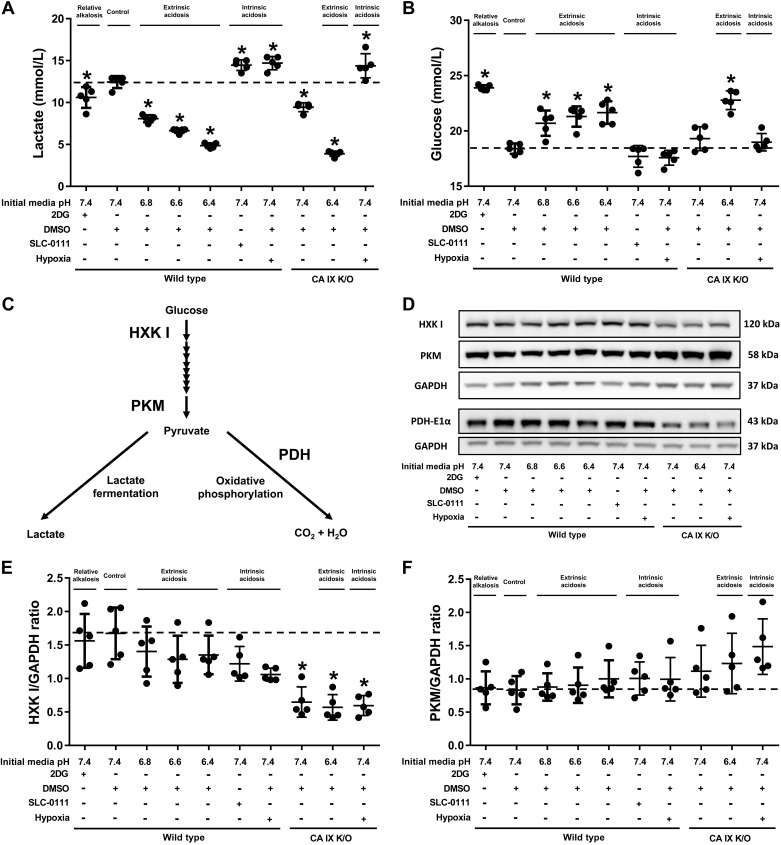
Extrinsic acidosis suppresses glycolysis.
Metabolism is a major proton source for extracellular acidosis, but little is known about the feedback effect of extrinsic and intrinsic extracellular acidosis on metabolism. To address this question, we measured lactate and glucose in culture media using the extrinsic and intrinsic acidosis models. 2DG decreased glycolysis, as evidenced by decreased lactate production and glucose consumption, which is consistent with its attenuating effect on extracellular acidification. Extrinsic acidosis suppressed glycolysis in a dose-dependent manner (Fig. 3, A and B; WT pH 7.4 vs. 6.8, 6.6, and 6.4, P < 0.05). On the other hand, intrinsic acidosis, induced by either SLC-0111 or with hypoxia, increased lactate production (Fig. 3A; WT vs. SLC-0111 and WT hypoxia, P < 0.05) without significantly changing glucose consumption. Extracellular pH was equivalent among pH 6.8, SLC-0111, and hypoxia groups in WT PMVECs (Fig. 1B; pH 6.8 vs. SLC-0111 and WT hypoxia; mean extracellular pH 6.38 vs. 6.42 and 6.41, respectively, P = ns). Therefore, the primary source of extracellular proton, i.e., whether it is extrinsic or intrinsic, determines how PMVECs adjust their metabolic activity.
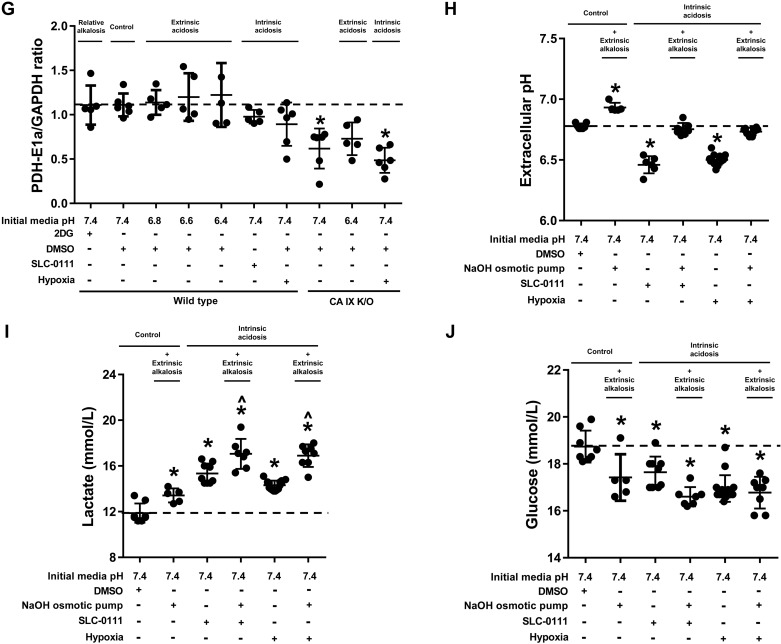
Fig. 3.
Extrinsic acidosis suppresses glycolysis. Wild-type and carbonic anhydrase (CA) IX knockout (K/O) pulmonary microvascular endothelial cells (PMVECs) were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, media were changed to HEPES-buffered media with pH 7.4, 6.8, 6.6, and 6.4 and treated with 2-deoxy-d-glucose (2DG; 5 mM), DMSO (0.5%), and SLC-0111 (150 µM) in normoxia and hypoxia (1% oxygen). Twenty-four hours later, media were collected for lactate and glucose measurements (A and B), and whole cell lysates were collected for Western blot analysis of main metabolic enzymes (C) pyruvate kinase M-type (PKM), hexokinase I (HXK I), and pyruvate dehydrogenase E1α subunit (PDH-E1α) (D–G). Extrinsic acidosis decreased lactate production and glucose consumption, while intrinsic acidosis increased lactate production (A and B). CA IX K/O PMVECs have decreased baseline lactate production and HXK I and PDH-E1α expression (A, B, E, F, and G). The same experimental setting was used to test the effect of extrinsic alkalosis on wild-type PMVECs in the presence of intrinsic acidosis, utilizing an osmotic pump to deliver NaOH (1 N NaOH 1 µl/h for 24 h). Media pH (H), lactate (I), and glucose (J) were measured, revealing glycolysis-enhancing effect of extrinsic alkalosis. Data represent means ± SD. Two-way ANOVA and Bonferroni post hoc tests were used to compare between different groups. At least five separate experiments were performed. *Significant difference (P < 0.05) from wild type pH 7.4.
In our previous study, we suggested that the SLC-0111-induced extracellular acidosis was due to impaired cellular buffering capacity (27). However, our current study reveals that SLC-0111 independently increases glycolysis, which leads to development of extracellular acidosis in PMVECs. This finding indicates the presence of a direct regulatory role of either CA IX or CA XII on glycolysis. While chemical inhibition of CA IX and CA XII by SLC-0111 increased lactate production, genetic CA IX inhibition decreased lactate levels (Fig. 3A; WT DMSO vs. CA IX K/O DMSO, P < 0.05) as low as the 2DG group (Fig. 3A; 2DG vs. CA IX K/O DMSO, P = ns), suggesting that CA IX promotes, while CA XII inhibits, glycolysis in PMVECs.
CA IX independently promotes PMVEC glycolysis mediated by HXK I and PDH-E1α.
Having identified that CA IX promotes glycolysis, we further investigated whether it affects the protein expression level of key enzymes involved in glycolysis and oxidative phosphorylation. HXK I and PKM regulate the first and last step, respectively, of glycolysis before entering lactate fermentation, and PDH-E1α mediates the first reaction of oxidative phosphorylation after pyruvate enters the mitochondria. HXK I, PKM, and PDH-E1α were measured by Western blotting 24 h after extrinsic and intrinsic acidosis exposure. PKM expression remained constant across groups (Fig. 3, D and E; WT pH 7.4 vs. all other groups, P = ns), but HXK I and PDH-E1α were significantly lower in CA IX K/O PMVECs (Fig. 3, D, F, and G; WT pH 7.4 vs. CA IX K/O pH 7.4, P < 0.05). The findings are consistent with the functional assay results showing decreased glycolysis in CA IX K/O PMVECs and support the idea that CA IX contributes to the cellular signals that control PMVEC glycolytic flux.
Glycolysis drives intrinsic acidosis, which has a negative feedback on glycolysis.
Although we demonstrated that SLC-0111 and hypoxia induce intrinsic acidosis via enhancing glycolysis, we have not excluded potential confounding effects originating from CA- or hypoxia-specific pathways. To confirm the independent effect of extracellular pH on metabolism, we introduced extrinsic alkalosis to intrinsic acidosis groups to achieve extracellular pH equivalent to that of the control group, using osmotic pumps that continuously release small amounts of NaOH (Fig. 3H; WT pH 7.4 vs. SLC-0111 + NaOH and hypoxia + NaOH, P = ns). In contrast to extrinsic acidosis, which decreased glycolysis, extrinsic alkalosis increased glycolysis, evidenced by increased lactate production (Fig. 3I; WT pH 7.4 vs. NaOH, P < 0.05) and glucose consumption (Fig. 3J; WT pH 7.4 vs. NaOH, P < 0.05). This glycolysis-enhancing effect of extrinsic alkalosis was maintained in the setting of intrinsic acidosis by SLC-0111 treatment (Fig. 3I; SLC-0111 vs. SLC-0111 + NaOH, P < 0.05) and hypoxia exposure (Fig. 3I; hypoxia vs. hypoxia + NaOH, P < 0.05). Considering our PMVECs have a significant degree of baseline glycolysis, which is consistent with intrinsic acidosis, current findings suggest that intrinsic acidosis generates some degree of feedback inhibition.
Extrinsic acidosis inhibits PMVEC migration.
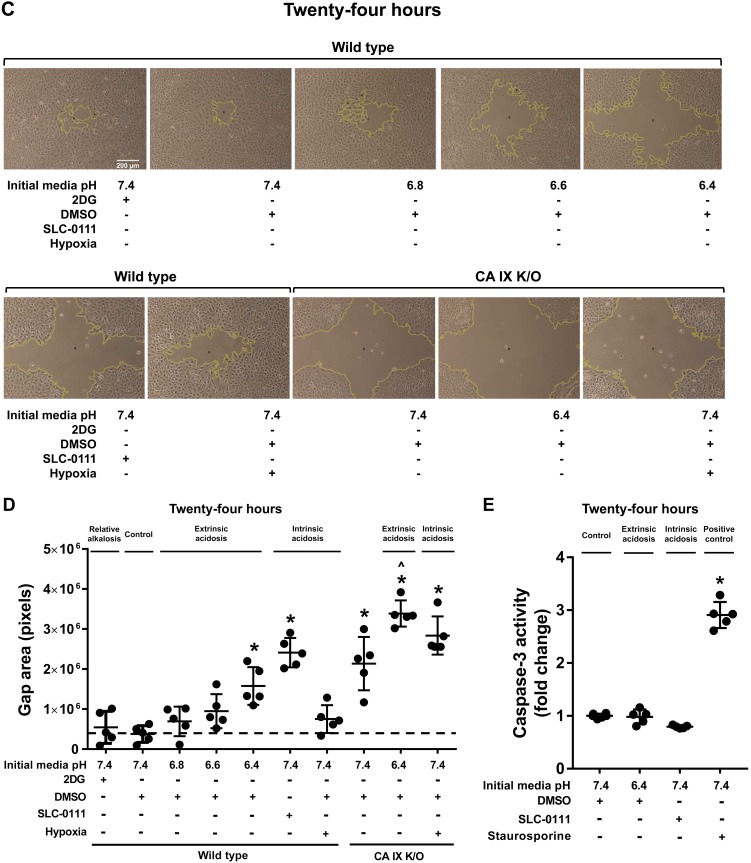
A primary goal in managing critically ill patients, in addition to correcting the underlying causes of their illness, is offering supportive care to facilitate wound healing. Since acidosis is common among this patient population, we investigated how either extrinsic acidosis, which is relevant to current therapeutic measures, or intrinsic acidosis affect cellular migration. Using the above described extrinsic and intrinsic acidosis models, we performed a scratch wound migration assay on WT and CA IX K/O PMVECs. We generated a cross wound rather than a single vertical wound to increase accuracy and minimize potential selection bias during imaging. At baseline, wound areas were similar across all groups (Fig. 4, A and B; WT pH 7.4 vs. all other groups, P = ns). Twenty-four hours later, wound areas were significantly larger in the extrinsic acidosis pH 6.4 group (Fig. 4, C and D; WT pH 7.4 vs. WT pH 6.4, P < 0.05) but not in the 2DG, mild extrinsic acidosis (pH 6.8 and 6.6) or hypoxia groups (Fig. 4, C and D; WT pH 7.4 vs. 2DG vs. pH 6.8 vs. pH 6.6 vs. WT hypoxia, P = ns). The finding indicates an inhibitory effect of extrinsic acidosis on PMVEC migration. Hypoxia neither promoted nor impaired PMVEC migration in this experimental setup.
Fig. 4.
Extrinsic acidosis and carbonic anhydrase (CA) IX inhibition suppresses migration. Wild-type and CA IX knockout (K/O) pulmonary microvascular endothelial cells (PMVECs) were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, monolayers were scratched with a sterile 200-μl pipette tip. Media were changed to HEPES-buffered media with pH 7.4, 6.8, 6.6, and 6.4 and treated with 2-deoxy-d-glucose (2DG; 5 mM), DMSO (0.5%), and SLC-0111 (150 µM) in normoxia and hypoxia (1% oxygen). Baseline and 24-h time point wounds were imaged and analyzed using ImageJ. At baseline, there was no difference in wound areas across groups (A and B). At 24 h, wound areas were bigger in extrinsic acidosis and CA IX inhibition (C and D). On separate experiments, cells were treated with DMSO (0.5%), pH 6.4, SLC-0111 (150 µM), and staurosporine (500 nM), lysates were collected after 24 h, and caspase-3 activity assays were performed. pH 6.4 or SLC-0111 had no effect on caspase-3 activity. Data represent means ± SD. Two-way ANOVA and Bonferroni post hoc tests were used to compare between different groups. At least five separate experiments were performed. *Significant difference (P < 0.05) from wild type pH 7.4; ^significant difference (P < 0.05) from CA IX K/O pH 7.4.
CA IX is critical to PMVEC migration.
CA IX plays an important role in cancer cell invasion and metastasis (40), but it is not known whether it is involved in PMVEC migration and wound healing. Both chemical and genetic CA IX inhibition similarly suppressed PMVEC migration (Fig. 4, C and D; WT pH 7.4 vs. SLC-0111 vs. CA IX K/O pH 7.4, P < 0.05), and the inhibitory effect of extrinsic acidosis at pH 6.4 was additive to the already decreased CA IX K/O PMVEC migration (Fig. 4, C and D; CA IX K/O pH 7.4 vs. CA IX K/O pH 6.4, P < 0.05). The data support an important role of CA IX in PMVEC migration, in addition to its control of pH.
Extrinsic acidosis- and CA IX depletion-induced PMVEC migratory inhibition is not due to caspase-3-dependent apoptosis.
To assess whether severe cell injury/apoptosis confounds PMVEC migratory characteristics, we conducted a caspase-3 activity assay 24 h after representative extrinsic and intrinsic acidosis exposure. Extrinsic acidosis of pH 6.4 or SLC-0111 treatment had no effect (Fig. 4E; WT DMSO vs. pH 6.4 and SLC-0111, P = ns) on caspase-3 activity. There was an increase in caspase-3 activity in response to staurosporine, an apoptosis-inducing agent (Fig. 4E; WT DMSO vs. staurosporine, P < 0.05), suggesting that the migratory impairment in extrinsic acidosis and CA IX depletion is not due to caspase-3-mediated apoptosis.
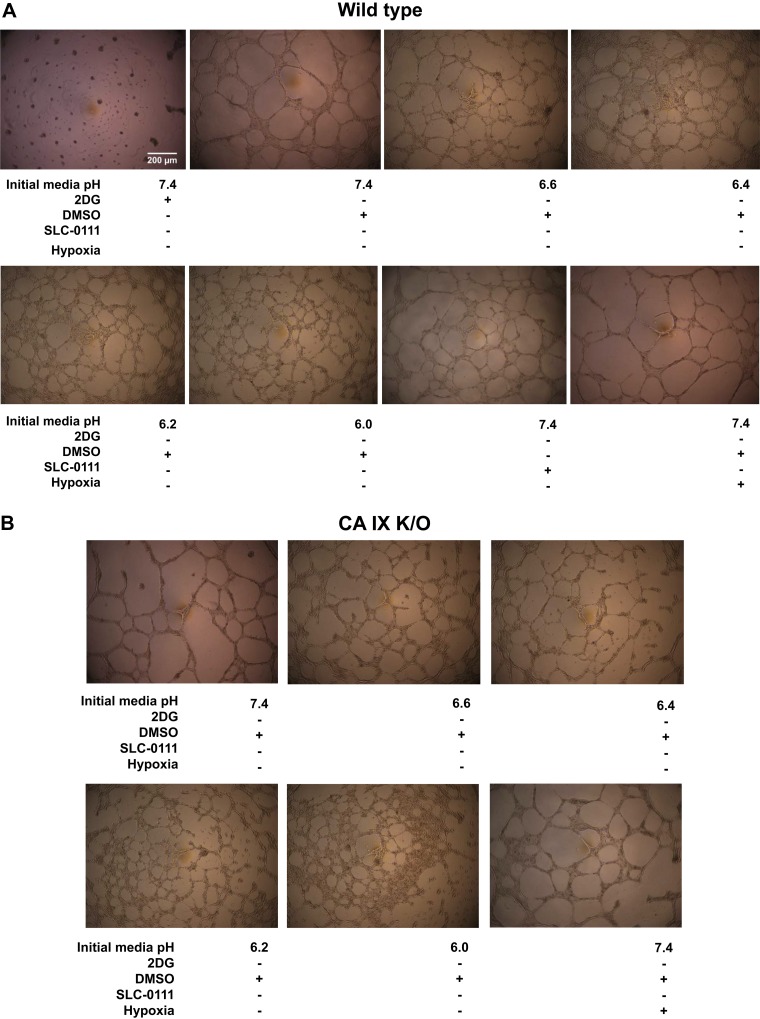
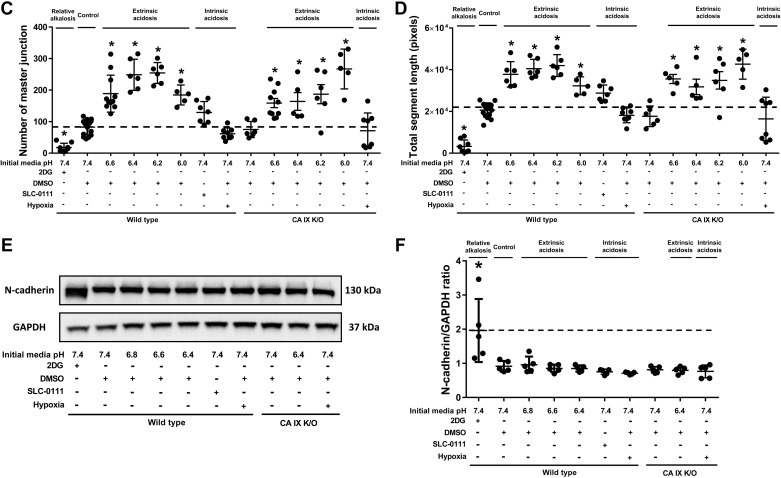
Extrinsic acidosis increases the number of master junctions and total segment length of Matrigel networks.
Previously we have reported that pH 6.2 decreases the number of junctions and branches in WT, and CA IX stabilizes PMVEC capillary-like network formation in acidosis (27). However, the study was limited by historically comparing pH 7.4 to pH 6.2. In this study, we tested the effect of extrinsic acidosis on PMVEC angiogenesis more rigorously with an extended range of acidic pH and in a side by side comparison with neutral pH groups. Interestingly, extrinsic acidosis, which inhibited migration at pH 6.4, did not decrease but rather increased both the master junction number and total segment length in WT (Fig. 5, A–D; WT pH 7.4 vs. pH 6.6 vs. pH 6.4 vs. pH 6.2 vs. pH 6.0, P < 0.05) and CA IX K/O PMVECs (Fig. 5, A–D; CA IX K/O pH 7.4 vs. CA IX K/O pH 6.6 vs. CA IX K/O pH 6.4 vs. CA IX K/O pH 6.2 vs. CA IX K/O pH 6.0, P < 0.05). Furthermore, network formation continued even at lower pH, as low as 6.0. However, on gross microscopic exam, the networks that formed in extreme acidosis tended to collapse after a few days, more so in CA IX K/O cells, unlike networks in neutral pH which were stable beyond a week (data not shown). Therefore, it appears that acidosis promotes network formation, but extreme acidity adversely affects network stability.
Fig. 5.
2-Deoxy-d-glucose (2DG) decreases and extrinsic acidosis increases Matrigel network master junction number and total segment length, and 2DG modifies N-cadherin expression. A–D: 96-well plates were loaded with Matrigel, 30 µl per well, and incubated at 37°C with 0% CO2 room air for 1 h. Wild-type and carbonic anhydrase (CA) IX knockout (K/O) cells were seeded at a density of 4.0 × 104 cells per well in bicarbonate-free HEPES-buffered media with a range of pH and inhibitors and a total cell solution volume of 100 µl per well. Cells were incubated at 37°C with 0% CO2 room air for 24 h. Pictures were taken at 24 h and analyzed using ImageJ. Total master junction number and segment length were decreased by 2DG and increased by extrinsic acidosis. E and F: wild type and CA IX K/O pulmonary microvascular endothelial cells (PMVECs) were seeded at 5.0 × 105 cells/well on 6-well plates on bicarbonate-buffered media. Two days after cell seeding, media was changed to HEPES-buffered media with pH 7.4, 6.8, 6.6, and 6.4 and treated with 2DG (5 mM), DMSO (0.5%), and SLC-0111 (150 µM) in normoxia and hypoxia (1% oxygen). Twenty-four hours later, whole cell lysates were collected, and Western blotting was performed to assess N-cadherin protein abundance. N-cadherin expression was increased, and its molecular weight was decreased by 2DG. Data represent means ± SD. Two-way ANOVA and Bonferroni post hoc tests were used to compare between different groups. At least five separate experiments were performed. *Significant difference (P < 0.05) from wild-type pH 7.4.
2DG does not affect PMVEC migration but profoundly inhibits network formation.
Throughout our experiments, 2DG served as a negative control for extracellular acidosis and rapid glycolysis. 2DG showed no significant inhibitory effect on PMVEC migration. However, 2DG had a profound effect on Matrigel network formation as noted by the presence of clumped cells that failed to branch and the decreased number of master junctions and total segment lengths (Fig. 5, A–D; WT pH 7.4 vs. 2DG, P < 0.05). We identified N-cadherin as a potentially relevant cell adhesion molecule that may contribute to the network forming pattern of 2DG-treated PMVECs. Western blotting revealed increased thickness and shifted molecular weight N-cadherin bands (Fig. 5, E and F; WT pH 7.4 vs. 2DG, P < 0.05). However, further studies will be needed to confirm any functional role of N-cadherin in 2DG-related network forming pattern changes. Together with extrinsic acidosis data, the findings suggest that extrinsic acidosis promotes, while 2DG inhibits network formation in PMVECs.
DISCUSSION
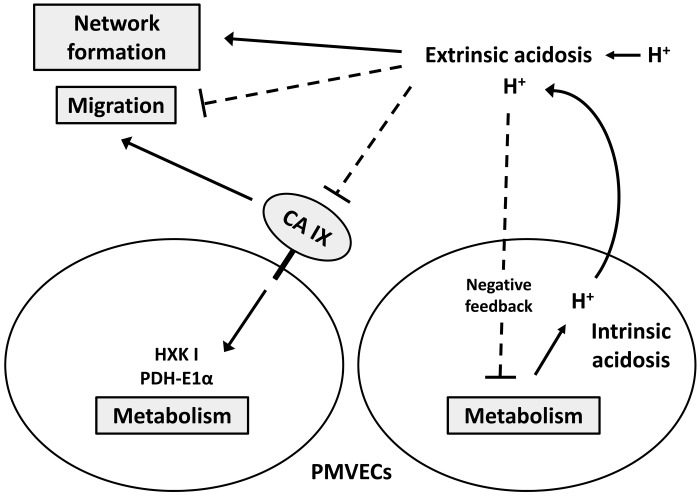
In this study, we classify extracellular acidosis into two distinct categories, including extrinsic and intrinsic acidosis, as determined by the primary proton source. We examined intracellular pH, metabolism, migration, and network formation of PMVECs in extrinsic and intrinsic acidosis at 24 h. We report three important findings, that 1) extrinsic acidosis inhibits glycolysis, 2) CA IX, independent of extracellular pH, promotes metabolic enzyme expression (HXK I and PDH-E1α) and cell migration, and 3) extrinsic acidosis inhibits migration, while it does not impair network formation. The key results are summarized in Table 1 and Fig. 6.
Table 1.
Summary of key findings
| Relative Alkalosis (2DG) | Extrinsic Acidosis | Intrinsic Acidosis (SLC-0111) | Intrinsic Acidosis (Hypoxia) | CA IX K/O | |
|---|---|---|---|---|---|
| Extracelluar pH | ↑ | ↓ | ↓ | ↓ | ↑ |
| Intracelluar pH | |||||
| CA IX | ↓ | ↓ | ↓ | ↓ | |
| NHE1 | |||||
| Metabolism | ↓ | ↓ | ↑ | ↑ | ↓ |
| Migration | ↓ | ↓ | ↓ | ||
| Network formation | ↓ | ↑ | |||
| N-cadherin | ↑ |
Up arrow indicates an increase, down arrow indicates a decrease in each variable. 2DG, 2-deoxy-d-glucose; CA, carbonic anhydrase; K/O, knockout; NHE-1, Na+/H+ exchanger 1.
Fig. 6.
Extrinsic acidosis suppresses intrinsic metabolism via negative feedback. Metabolism drives intrinsic acidosis which subsequently results in extrinsic acidosis for neighboring cells. Extrinsic acidosis inhibits migration and enhances network formation. Carbonic anhydrase (CA) IX independently promotes metabolism via upregulating hexokinase I (HXK I) and pyruvate dehydrogenase E1α subunit (PDH-E1α) and plays a critical role in pulmonary microvascular endothelial cell (PMVEC) migration.
Current management strategies for acid-base disorders are focused on measuring and correcting systemic blood pH. Based on our findings, extracellular pH poorly reflects intracellular pH, and such an extracellular pH-guided approach may variably affect cellular metabolism, migration, and angiogenesis depending on the underlying metabolic status and diseases involved. Furthermore, our evidence for an important independent role of CA IX in regulating metabolism and migration, and evidence that its expression is decreased by extrinsic acidosis and not changed by hypoxia-induced intrinsic acidosis, indicates the need to develop more focused strategies to regulate local pH before overwhelming systemic acidosis ensues. Together, our study reveals important links between regulatory mechanisms involved in pH regulation, metabolism, migration, and network formation in PMVECs.
Intracellular pH is a critical determinant of the structure and function of macromolecules and is tightly regulated against extracellular pH changes by multiple mechanisms (7). The bicarbonate buffer system with CAs serves as a first responder to minimize rapid pH swings, but it has finite capacity. Therefore, ion transporters and exchangers such as NHE-1 closely work with carbonic anhydrases (CAs) to achieve sustained stabilization of intracellular pH. Cell type-dependent variability in resistance to extrinsic acidosis (36) may be related to how cells utilize their acid-regulating mechanisms. In cancer cells, CA IX is upregulated by extrinsic and hypoxia-induced intrinsic acidosis, which is thought to play a protective and facilitating role by increasing intracellular pH and promoting cancer invasion and metastases (4, 9, 22, 24). NHE-1 is downregulated by extrinsic acidosis in mouse epidermis and in vitro keratinocytes (19) and upregulated by hypoxia-induced intrinsic acidosis in mouse pulmonary arterial myocytes, which mediate vascular remodeling in pulmonary hypertension (37, 46). Unlike the findings from previous studies with other cell types, CA IX was decreased by extrinsic acidosis and was not affected by hypoxia-induced intrinsic acidosis in our study. There also was no significant change in NHE-1 expression across groups. PMVECs maintained intracellular pH over the entire 24-h time period, the time point when most studies report a decrease in intracellular pH. However, this acid resistance cannot be sustained indefinitely; we observed a decrease in intracellular pH in PMVECs if acidosis exposure is extended beyond 48 h (data not shown). The contrasting CA IX and NHE-1 expression patterns in PMVECs may suggest a unique acid handling strategy that potentially contributes to high acid resistance. We speculate that this acid handling capacity of PMVECs may be advantageous for their survival in the unique environment of the alveolar gas exchange interface, where pH regulation is critical.
We report that extrinsic acidosis suppresses glycolytic metabolism, consistent with previous reports. Guinea pig cerebral cortex tissue decreases oxygen and glucose consumption in response to extrinsic acidosis (pH 6.5) (33). Lowering of the extracellular pH from 7.9 to 7.0 decreases lactate accumulation in isolated frog gastrocnemius muscle (43). Extrinsic acidosis (pH 6.8) modulates fatty acid metabolism to confer growth benefits to cancer cells (25). Extrinsic acidosis (pH 6.3) decreases ATP turnover and protein synthesis, and hypoxia (5%, 1%, 0.1%, 0.01%, or 0% oxygen) further augments this suppressive effect (38). Although these studies and our results suggest inhibitory effects of extrinsic acidosis on cellular metabolism, it is questionable whether our study results are due to abrupt introduction of extrinsic acidosis rather than a slowly developing acid environment. However, when we gradually induced extrinsic acidosis using an osmotic pump, time-matched with intrinsic acidosis, the same glycolysis suppressing effect occurred (data not shown), suggesting that the inhibitory effect of extrinsic acidosis is not due to rapid development of acidosis. Using these osmotic pumps, we demonstrated that extrinsically alkalinizing pH during intrinsic acidosis enhances metabolism, which would otherwise have been attenuated by negative feedback. Metabolism induces variable degrees of intrinsic acidosis that ultimately cause negative feedback, which maintains metabolic and pH homeostasis. If this phenomenon holds true in vivo, then extrinsically correcting acidic pH in clinical settings may complicate the disease course by unnecessarily enhancing metabolism; this is an important question to address in future studies.
The regulatory role of CAs on cellular metabolism has been studied in the context of their involvement in generation of bicarbonate substrate. For example, Lynch et al. (29) suggested that the carbonic anhydrase inhibitor, trifluoromethyl sulfonamide, via its role in offering bicarbonate substrate for pyruvate carboxylase activity, mediates de novo lipogenesis. Our study shows that knocking out CA IX in PMVECs downregulates important regulatory enzymes of glycolysis and oxidative phosphorylation, HXK I and PDH-E1α. To our knowledge, this is the first study to report regulatory effects of CA IX on metabolic enzyme expression, and it again supports an important regulatory link between metabolism and pH regulation. Interestingly, however, there was an opposite effect on metabolism by chemically versus genetically inhibiting CA IX; SLC-0111 increased, whereas CA IX K/O decreased, glycolysis. We speculate that this may be due to an inhibitory role of SLC-0111 on CA IX and XII and perhaps other membrane-bound isoforms such as CA IV and XIV. This idea is further supported by our observation that SLC-0111 enhances glycolysis in CA IX K/O PMVECs (data not shown). In addition to SLC-0111, we tested other CA inhibitors including acetazolamide (AZ; nonspecific CA inhibitor), benzolamide (nonspecific extracellular CA inhibitor) (20), and N-methyl-AZ (AZ analogue with no CA-inhibiting activity) (35). Unlike SLC-0111, none of the other tested CA inhibitors showed a significant regulatory effect on metabolism, suggesting a complex interplay of vascular endothelial cell CA isoforms (data not shown).
Migration and angiogenesis are generally believed to be complementary mechanisms in wound healing following tissue injury. Studies have shown that extrinsic acidosis inhibits pulmonary epithelial cell (30), human umbilical vein endothelial cell (11), and tumor cell (8) migration and human bone marrow-derived endothelial progenitor cell migration and Matrigel network formation (21). Our finding suggests that migration and network formation do not necessarily occur together, with distinct cellular mechanisms controlling the latter. A striking effect of 2DG on network formation inhibition and N-cadherin expression/modification, while preserving migratory capacity, is evidence to support these distinctive processes. More interestingly, both extrinsic acidosis and CA IX inhibition resulted in migratory inhibition, but only extrinsic acidosis increased master junctions and segment lengths in Matrigel assays. We speculate that such a small-lumen network formation pattern induced by extrinsic acidosis may indicate a potential role of acidosis during the early stages of vessel formation in vivo.
As a constituent of the alveolar-capillary membrane, PMVECs are on the frontline of acid-base regulation, yet little is known about their acid regulatory mechanisms. Here, we aimed to distinguish two types of extracellular acidosis, including extrinsic and intrinsic acidosis, based on the primary source of protons. We demonstrated that extrinsic acidosis inhibits glycolysis, while intrinsic acidosis does not. CA IX, in addition to pH regulation, independently promoted glycolysis and migration. Lastly, we identified that extrinsic acidosis inhibits migration, but it does not impair network formation. These findings suggest an important functional link between pH regulation, migration, and angiogenesis, which may help guide studies answering the impact of acidosis during lung injury and repair and potentially identify new therapeutic strategies.
GRANTS
This work was supported in part by American Heart Association Grant 18CDA34080151 (J. Y. Lee), NIH Office of the Director Grant OD010944 (M. Alexeyev), and National Heart, Lung, and Blood Institute Grants HL-66299 and HL-60024 (T. Stevens).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.Y.L. and T.S. conceived and designed research; J.Y.L., M.O., I.G., R.W., M.C., M.F.A., N.K., and V.P. performed experiments; J.Y.L., M.F.A., E.R.S., C.T.S., and T.S. analyzed data; J.Y.L., M.F.A., E.R.S., C.T.S., and T.S. interpreted results of experiments; J.Y.L. and T.S. prepared figures; J.Y.L. and T.S. drafted manuscript; J.Y.L., M.F.A., E.R.S., C.T.S., and T.S. edited and revised manuscript; J.Y.L., M.F.A., E.R.S., C.T.S., and T.S. approved final version of manuscript.
REFERENCES
- 1.Al-Jaghbeer M, Kellum JA. Acid-base disturbances in intensive care patients: etiology, pathophysiology and treatment. Nephrol Dial Transplant 30: 1104–1111, 2015. doi: 10.1093/ndt/gfu289. [DOI] [PubMed] [Google Scholar]
- 2.Albert TJ, Swenson ER. Circumstances when arterial blood gas analysis can lead us astray. Respir Care 61: 119–121, 2016. doi: 10.4187/respcare.04556. [DOI] [PubMed] [Google Scholar]
- 3.Andersen AP, Samsøe-Petersen J, Oernbo EK, Boedtkjer E, Moreira JMA, Kveiborg M, Pedersen SF. The net acid extruders NHE1, NBCn1 and MCT4 promote mammary tumor growth through distinct but overlapping mechanisms. Int J Cancer 142: 2529–2542, 2018. doi: 10.1002/ijc.31276. [DOI] [PubMed] [Google Scholar]
- 4.Andreucci E, Peppicelli S, Carta F, Brisotto G, Biscontin E, Ruzzolini J, Bianchini F, Biagioni A, Supuran CT, Calorini L. Carbonic anhydrase IX inhibition affects viability of cancer cells adapted to extracellular acidosis. J Mol Med (Berl) 95: 1341–1353, 2017. doi: 10.1007/s00109-017-1590-9. [DOI] [PubMed] [Google Scholar]
- 5.Balczon R, Morrow KA, Zhou C, Edmonds B, Alexeyev M, Pittet JF, Wagener BM, Moser SA, Leavesley S, Zha X, Frank DW, Stevens T. Pseudomonas aeruginosa infection liberates transmissible, cytotoxic prion amyloids. FASEB J 31: 2785–2796, 2017. doi: 10.1096/fj.201601042RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA; NHLBI ARDS Network . Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med 2: 611–620, 2014. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casey JR, Grinstein S, Orlowski J. Sensors and regulators of intracellular pH. Nat Rev Mol Cell Biol 11: 50–61, 2010. doi: 10.1038/nrm2820. [DOI] [PubMed] [Google Scholar]
- 8.Castellone RD, Leffler NR, Dong L, Yang LV. Inhibition of tumor cell migration and metastasis by the proton-sensing GPR4 receptor. Cancer Lett 312: 197–208, 2011. doi: 10.1016/j.canlet.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 9.Chiche J, Ilc K, Laferrière J, Trottier E, Dayan F, Mazure NM, Brahimi-Horn MC, Pouysségur J. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res 69: 358–368, 2009. doi: 10.1158/0008-5472.CAN-08-2470. [DOI] [PubMed] [Google Scholar]
- 10.Curley G, Hayes M, Laffey JG. Can ‘permissive’ hypercapnia modulate the severity of sepsis-induced ALI/ARDS? Crit Care 15: 212, 2011. doi: 10.1186/cc9994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faes S, Uldry E, Planche A, Santoro T, Pythoud C, Demartines N, Dormond O. Acidic pH reduces VEGF-mediated endothelial cell responses by downregulation of VEGFR-2; relevance for anti-angiogenic therapies. Oncotarget 7: 86026–86038, 2016. doi: 10.18632/oncotarget.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fais S, Venturi G, Gatenby B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev 33: 1095–1108, 2014. [Erratum in Cancer Metastasis Rev 34: 165, 2015]. doi: 10.1007/s10555-014-9531-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, Adhikari NKJ, Amato MBP, Branson R, Brower RG, Ferguson ND, Gajic O, Gattinoni L, Hess D, Mancebo J, Meade MO, McAuley DF, Pesenti A, Ranieri VM, Rubenfeld GD, Rubin E, Seckel M, Slutsky AS, Talmor D, Thompson BT, Wunsch H, Uleryk E, Brozek J, Brochard LJ; American Thoracic Society, European Society of Intensive Care Medicine, and Society of Critical Care Medicine . An official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine clinical practice guideline: mechanical ventilation in adult patients with acute respiratory distress syndrome. Am J Respir Crit Care Med 195: 1253–1263, 2017. doi: 10.1164/rccm.201703-0548ST. [DOI] [PubMed] [Google Scholar]
- 14.Fukumura D, Xu L, Chen Y, Gohongi T, Seed B, Jain RK. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res 61: 6020–6024, 2001. [PubMed] [Google Scholar]
- 15.Gates KL, Howell HA, Nair A, Vohwinkel CU, Welch LC, Beitel GJ, Hauser AR, Sznajder JI, Sporn PHS. Hypercapnia impairs lung neutrophil function and increases mortality in murine pseudomonas pneumonia. Am J Respir Cell Mol Biol 49: 821–828, 2013. doi: 10.1165/rcmb.2012-0487OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gattinoni L, Pelosi P, Crotti S, Valenza F. Effects of positive end-expiratory pressure on regional distribution of tidal volume and recruitment in adult respiratory distress syndrome. Am J Respir Crit Care Med 151: 1807–1814, 1995. doi: 10.1164/ajrccm.151.6.7767524. [DOI] [PubMed] [Google Scholar]
- 17.Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L; PROSEVA Study Group . Prone positioning in severe acute respiratory distress syndrome. N Engl J Med 368: 2159–2168, 2013. doi: 10.1056/NEJMoa1214103. [DOI] [PubMed] [Google Scholar]
- 18.Gunnerson KJ, Saul M, He S, Kellum JA. Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit Care 10: R22, 2006. doi: 10.1186/cc3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hachem JP, Behne M, Aronchik I, Demerjian M, Feingold KR, Elias PM, Mauro TM. Extracellular pH Controls NHE1 expression in epidermis and keratinocytes: implications for barrier repair. J Invest Dermatol 125: 790–797, 2005. doi: 10.1111/j.0022-202X.2005.23836.x. [DOI] [PubMed] [Google Scholar]
- 20.Höhne C, Pickerodt PA, Francis RC, Boemke W, Swenson ER. Pulmonary vasodilation by acetazolamide during hypoxia is unrelated to carbonic anhydrase inhibition. Am J Physiol Lung Cell Mol Physiol 292: L178–L184, 2007. doi: 10.1152/ajplung.00205.2006. [DOI] [PubMed] [Google Scholar]
- 21.Huang S, He P, Xu D, Li J, Peng X, Tang Y. Acidic stress induces apoptosis and inhibits angiogenesis in human bone marrow-derived endothelial progenitor cells. Oncol Lett 14: 5695–5702, 2017. doi: 10.3892/ol.2017.6947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ihnatko R, Kubes M, Takacova M, Sedlakova O, Sedlak J, Pastorek J, Kopacek J, Pastorekova S. Extracellular acidosis elevates carbonic anhydrase IX in human glioblastoma cells via transcriptional modulation that does not depend on hypoxia. Int J Oncol 29: 1025–1033, 2006. doi: 10.3892/ijo.29.4.1025. [DOI] [PubMed] [Google Scholar]
- 23.Kellum JA, Kramer DJ, Lee K, Mankad S, Bellomo R, Pinsky MR. Release of lactate by the lung in acute lung injury. Chest 111: 1301–1305, 1997. doi: 10.1378/chest.111.5.1301. [DOI] [PubMed] [Google Scholar]
- 24.Koltai T. Cancer: fundamentals behind pH targeting and the double-edged approach. OncoTargets Ther 9: 6343–6360, 2016. doi: 10.2147/OTT.S115438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kondo A, Yamamoto S, Nakaki R, Shimamura T, Hamakubo T, Sakai J, Kodama T, Yoshida T, Aburatani H, Osawa T. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep 18: 2228–2242, 2017. doi: 10.1016/j.celrep.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 26.Kregenow DA, Rubenfeld GD, Hudson LD, Swenson ER. Hypercapnic acidosis and mortality in acute lung injury. Crit Care Med 34: 1–7, 2006. doi: 10.1097/01.CCM.0000194533.75481.03. [DOI] [PubMed] [Google Scholar]
- 27.Lee JY, Alexeyev M, Kozhukhar N, Pastukh V, White R, Stevens T. Carbonic anhydrase IX is a critical determinant of pulmonary microvascular endothelial cell pH regulation and angiogenesis during acidosis. Am J Physiol Lung Cell Mol Physiol 315: L41–L51, 2018. doi: 10.1152/ajplung.00446.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee JY, McMurtry SA, Stevens T. Single cell cloning generates lung endothelial colonies with conserved growth, angiogenic, and bioenergetic characteristics. Pulm Circ 7: 777–792, 2017. doi: 10.1177/2045893217731295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lynch CJ, Fox H, Hazen SA, Stanley BA, Dodgson S, Lanoue KF. Role of hepatic carbonic anhydrase in de novo lipogenesis. Biochem J 310: 197–202, 1995. doi: 10.1042/bj3100197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Toole D, Hassett P, Contreras M, Higgins BD, McKeown ST, McAuley DF, O’Brien T, Laffey JG. Hypercapnic acidosis attenuates pulmonary epithelial wound repair by an NF-kappaB dependent mechanism. Thorax 64: 976–982, 2009. doi: 10.1136/thx.2008.110304. [DOI] [PubMed] [Google Scholar]
- 31.Parra-Bonilla G, Alvarez DF, Al-Mehdi AB, Alexeyev M, Stevens T. Critical role for lactate dehydrogenase A in aerobic glycolysis that sustains pulmonary microvascular endothelial cell proliferation. Am J Physiol Lung Cell Mol Physiol 299: L513–L522, 2010. doi: 10.1152/ajplung.00274.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parra-Bonilla G, Alvarez DF, Alexeyev M, Vasauskas A, Stevens T. Lactate dehydrogenase a expression is necessary to sustain rapid angiogenesis of pulmonary microvascular endothelium. PLoS One 8: e75984, 2013. doi: 10.1371/journal.pone.0075984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patel KK, Hartmann JF, Cohen MM. Effect of pH on metabolism and ultrastructure of guinea pig cerebral cortex slices. Stroke 4: 221–231, 1973. doi: 10.1161/01.STR.4.2.221. [DOI] [PubMed] [Google Scholar]
- 34.Pei G, Li B, Ma A. Suppression of Hiwi inhibits the growth and epithelial-mesenchymal transition of cervical cancer cells. Oncol Lett 16: 3874–3880, 2018. doi: 10.3892/ol.2018.9056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pickerodt PA, Francis RC, Höhne C, Neubert F, Telalbasic S, Boemke W, Swenson ER. Pulmonary vasodilation by acetazolamide during hypoxia: impact of methyl-group substitutions and administration route in conscious, spontaneously breathing dogs. J Appl Physiol (1985) 116: 715–723, 2014. doi: 10.1152/japplphysiol.01235.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Salameh AI, Ruffin VA, Boron WF. Effects of metabolic acidosis on intracellular pH responses in multiple cell types. Am J Physiol Regul Integr Comp Physiol 307: R1413–R1427, 2014. doi: 10.1152/ajpregu.00154.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimoda LA, Fallon M, Pisarcik S, Wang J, Semenza GL. HIF-1 regulates hypoxic induction of NHE1 expression and alkalinization of intracellular pH in pulmonary arterial myocytes. Am J Physiol Lung Cell Mol Physiol 291: L941–L949, 2006. doi: 10.1152/ajplung.00528.2005. [DOI] [PubMed] [Google Scholar]
- 38.Sørensen BS, Busk M, Overgaard J, Horsman MR, Alsner J. Simultaneous hypoxia and low extracellular pH suppress overall metabolic rate and protein synthesis in vitro. PLoS One 10: e0134955, 2015. doi: 10.1371/journal.pone.0134955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stevens T, Creighton J, Thompson WJ. Control of cAMP in lung endothelial cell phenotypes. Implications for control of barrier function. Am J Physiol Lung Cell Mol Physiol 277: L119–L126, 1999. doi: 10.1152/ajplung.1999.277.1.L119. [DOI] [PubMed] [Google Scholar]
- 40.Supuran CT. Carbonic anhydrase inhibition and the management of hypoxic tumors. Metabolites 7: 48, 2017. doi: 10.3390/metabo7030048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Swayampakula M, McDonald PC, Vallejo M, Coyaud E, Chafe SC, Westerback A, Venkateswaran G, Shankar J, Gao G, Laurent EMN, Lou Y, Bennewith KL, Supuran CT, Nabi IR, Raught B, Dedhar S. The interactome of metabolic enzyme carbonic anhydrase IX reveals novel roles in tumor cell migration and invadopodia/MMP14-mediated invasion. Oncogene 36: 6244–6261, 2017. doi: 10.1038/onc.2017.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tseng PL, Wu WH, Hu TH, Chen CW, Cheng HC, Li CF, Tsai WH, Tsai HJ, Hsieh MC, Chuang JH, Chang WT. Decreased succinate dehydrogenase B in human hepatocellular carcinoma accelerates tumor malignancy by inducing the Warburg effect. Sci Rep 8: 3081, 2018. doi: 10.1038/s41598-018-21361-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vezzoli A, Gussoni M, Greco F, Zetta L. Effects of temperature and extracellular pH on metabolites: kinetics of anaerobic metabolism in resting muscle by 31P- and 1H-NMR spectroscopy. J Exp Biol 206: 3043–3052, 2003. doi: 10.1242/jeb.00521. [DOI] [PubMed] [Google Scholar]
- 44.Wei Y, Wang D, Jin F, Bian Z, Li L, Liang H, Li M, Shi L, Pan C, Zhu D, Chen X, Hu G, Liu Y, Zhang CY, Zen K. Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat Commun 8: 14041, 2017. doi: 10.1038/ncomms14041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yin C, He D, Chen S, Tan X, Sang N. Exogenous pyruvate facilitates cancer cell adaptation to hypoxia by serving as an oxygen surrogate. Oncotarget 7: 47494–47510, 2016. doi: 10.18632/oncotarget.10202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu L, Quinn DA, Garg HG, Hales CA. Deficiency of the NHE1 gene prevents hypoxia-induced pulmonary hypertension and vascular remodeling. Am J Respir Crit Care Med 177: 1276–1284, 2008. doi: 10.1164/rccm.200710-1522OC. [DOI] [PMC free article] [PubMed] [Google Scholar]