Abstract
The effects of maternal obesity on lung development have been recognized, and speculation is that these diseases are not simply because of accelerated pulmonary decline with aging but with a failure to achieve optimal lung development during early life. These studies tested the hypothesis that maternal obesity alters signaling pathways during the course of lung development that may affect life-long pulmonary health. Adult female mice were fed 60% fat [high-fat diet (HFD)] or 10% fat [control diet (CD)] for 8 wk before mating and through weaning. Pup lung tissues were collected at postnatal days (PN) 7, 21, and 90 (after receiving HFD or CD as adults). At PN7, body weights from HFD were greater than CD but lung weight-to-body weight ratios were lower. In lung tissues, NFκB-mediated inflammation was greater in HFD pups at PN21 and phospho-/total STAT3, phospho-/total VEGF receptor 2, and total AKT protein levels were lower with maternal HFD and protein tyrosine phosphatase B1 levels were increased. Decreased platelet endothelial cell adhesion molecule levels were observed at PN21 and at PN90 in the pups exposed to maternal HFD. Morphometry indicated that the pups exposed to maternal or adult HFD had fewer alveoli, and the effect was additive. Decreases in pulmonary resistance, elastance, and compliance were observed because of adult HFD diet and decreases in airway resistance and increases in inspiratory capacity because of maternal HFD. In conclusion, maternal HFD disrupts signaling pathways in the early developing lung and may contribute to deficiencies in lung function and increased susceptibility in adults.
Keywords: alveolarization, angiogenesis, high-fat diet, lung development, maternal obesity
INTRODUCTION
Obesity is an overwhelming global health concern, and the impact has not yet been fully appreciated. The influence of maternal obesity on the health of the offspring has become an area of interest in light of “fetal origins of adult disease” hypotheses. Recent statistics indicate that 60% of women are either overweight or obese at the time of conception and as many as 25% remain obese throughout pregnancy (24, 31). The fetal consequences of maternal exposure to obesity may result from direct intrauterine influence on organ development and metabolism or from subtle perturbations, including epigenetic alterations. Infants born to mothers with obesity are more likely to develop pulmonary pathologies such as chronic lung disease, reactive airways, and respiratory infections, and have altered glucose and fatty acid delivery to the lungs for surfactant production (9, 21, 23, 24, 27). In addition, infants born to mothers with obesity have altered metabolic function, which includes disruption of the hypothalamic pituitary adrenal (HPA) axis, increased leptin levels, insulin resistance, and increased blood pressure (11, 31).
Obstructive lung diseases such as chronic obstructive pulmonary disease (COPD) and asthma are on the rise globally (4, 15). Although environmental exposures such as smoking and air pollution are associated risk factors, they do not fully explain the etiology or prevalence of these diseases. Furthermore, the lack of curative options may be because of a disease origin that occurs long before the symptoms or actual disease manifests. There is speculation that development of COPD is not simply due to accelerated pulmonary decline with aging but with a failure to achieve optimal or peak lung development during early life (29). Several epidemiological studies have supported this speculation and linked maternal obesity to childhood wheezing, increases in the number of respiratory infections, and development of asthma (24). Children with asthma or respiratory symptoms have increased risk of adult COPD (6).
Adipose tissue is not metabolically inert but actively secretes hormone-like substances known as adipokines (1). One adipokine shown to influence endothelial function and angiogenesis is leptin (1, 12). Leptin is synthesized and secreted by white adipose tissue as a signal to the hypothalamus for the repletion of body energy stores, is secreted in proportion to fat mass, and as such, is elevated in the context of obesity (12). Leptin effects on angiogenesis are mediated via signaling through the cognate receptor Ob-R. Although acute increases in leptin levels can be proangiogenic, chronic elevation of leptin levels, such as that found in instances of obesity, are associated with attenuated angiogenic responses through upregulation of protein tyrosine phosphatase B1 (PTP1B) (16).
Although the effects of leptin on vascular function have been investigated in the context of adult obesity, little is known about its effects on offspring in the context of maternal obesity. Lung development and alveolarization are driven by angiogenesis and formation of a capillary network to provide the platform for gas exchange (32). Interruption of this temporally sensitive process could result in permanent deficits in lung development and/or function. Our data indicate that in newborn pups exposed to maternal high-fat diet (HFD), circulating leptin levels are increased, lung expression of p65 and PTP1B are markedly increased, and lung expression of proteins associated with angiogenic pathways are decreased. Overall, our data support the hypothesis that maternal HFD-induced obesity alters lung development in offspring and that this is due, in part, to disruption of normal pulmonary angiogenesis in the developing lung. Interestingly, these changes in lung development may go unnoticed early in life but may dramatically affect the timing of age-dependent lung function decline or increase susceptibility to lung diseases such as asthma or COPD.
METHODS
Animal model.
All animal studies were carried out in accordance with a protocol approved by the Institutional Animal Care and Use Committee at The Research Institute at Nationwide Children’s Hospital (Columbus, OH; Institutional Review Board no. AR13–00068). The animal model and the diets were described in detail previously and are depicted in Fig. 1 (14). Briefly, at 4 wk of age, female mice were randomly assigned to either the HFD (Harlan Teklad TD.06414, 60% calories from fat, Madison, WI) or control diet (CD; Harlan Teklad TD.08806, 10% calories from fat) for 8 wk and then bred. Male mice were maintained on standard chow except for the 2-wk breeding periods while they were housed with the females, when they were exposed to the respective experimental diet. After birth, pups were designated as CD and HFD based on the maternal diet during pregnancy and nursing. At postnatal day (PN) 7 or PN21 pups were weighed and euthanized or weaned and placed on adult diet (CD or HFD) until PN90. For the pups euthanized before weaning, there were two groups based on maternal diets (CD and HFD). For pups that were weaned, there were four distinct groups at PN90 depicting maternal diet/adult diet; CD/CD, CD/HFD, HFD/CD, and HFD/HFD. At the designated times, the mice were euthanized, and lungs were either collected and frozen in liquid nitrogen immediately or inflation-fixed as previously described (7). Both sexes were independently assessed, but analyses using sex as a variable did not identify any sex-specific differences. Consequently, both sexes were included in the data presented to simplify the presentation.
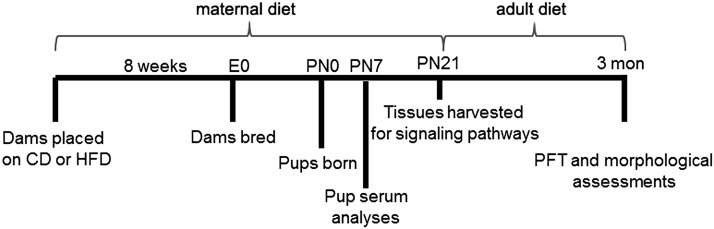
Fig. 1.
Experimental design. Dams were placed on diet for 8 wk before breeding and remained on diet through pregnancy and lactation (maternal diet). Pups were euthanized at PN0, PN7, and PN21. Weaned pups were placed on postnatal CD or HFD and euthanized at PN90 (adult diet). CD, control diet; HFD, high-fat diet; PFT, pulmonary function test; PN, postnatal day.
PCR.
RNA was isolated from lung tissues using TRIzol (Invitrogen) and RNeasy kit (Qiagen) following the manufacturer’s protocols. RNA (2 μg) was reverse-transcribed per instructions using Maxima First Strand cDNA Synthesis Kit (Thermo Scientific). PCR with Maxima SYBR green master mix (Thermo Scientific) was performed on an Eppendorf Realplex Master Cycler using custom DNA primers (Integrated DNA Technologies). Expression levels of TNFα, IL-6, and IL-5 were normalized to β-actin.
Immunohistochemistry.
Tissue sections were cut at 6 mm from inflation-fixed and paraffin-embedded lung tissues. Slides were deparaffinized at 60°C for 40 min and then submerged in heated citrate buffer for antigen retrieval before immunostaining. Immunostaining was performed on PN90 tissue sections using an anti-platelet endothelial cell adhesion molecule (PECAM) primary antibody (1:100; Abcam cat. no. ab-124432) and a donkey anti-rabbit secondary (1:600; Jackson Immunoresearch cat. no. 711-065-152) or MAC3 primary antibody (1:100; Biolegend cat. no. 108502) and a donkey anti-rabbit secondary (1:600; Jackson Immunoresearch cat. no. 711-065-152). Quantification of the total area stained darkest brown was performed on five fields each slide from four individual mice for all diet combinations by blinded individuals using ImageJ software. Data were then averaged and statistically analyzed.
Western blotting.
Lung homogenates obtained from mice at PN21 and PN90 were separated on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride membranes. Membranes were probed with antibodies to PECAM (Abcam, cat. no. ab124432), VCAM-1 (Cell Signaling Technologies, cat. no. 14694), pSTAT3 (Cell Signaling Technologies, cat. no. 9145), STAT3 (Cell Signaling Technologies, cat. no. 12640S), pAKT (New England Biolabs, cat. no. 9275), AKT (New England Biolabs, cat. no. 9272), phosphorylated vascular endothelial growth factor receptor (pVEGFR) 2 (Cell Signaling Technologies, cat. no. 9698), vascular endothelial growth factor receptor (VEGFR) 2 (Cell Signaling Technologies, cat. no. 2478), PTP1B (R&D Systems, cat. no. AF13661), IκBα (Cell Signaling Technologies, cat. no. 44D4), IκKβ (Cell Signaling Technologies, cat. no. 2370), and p65 (Cell Signaling Technologies cat. no. 3034). Blots were developed using enhanced chemiluminescence (ECL Western Blotting Detection, GE Healthcare, UK), and protein levels were quantified by densitometry using Bio-Rad Image Laboratory (Bio-Rad, Hercules, CA). For each target, the density of the band for the protein of interest was normalized to the density of the entire lane, stained with Ponceau’s (13, 25, 30).
Morphometry.
Following paraffin embedding, lung tissue sections (5 µm) were cut and slides stained with hematoxylin and eosin (H&E) for morphometric measurements as previously described (26). Five images per animal were analyzed and averaged using digital image analysis software (Image Pro Plus 6.3; Media Cybernetics, Silver Spring, MD).
Pulmonary function tests.
A SCIREQ FlexiVent (SCIREQ, Montreal, Canada) ventilator was used to perform pulmonary function analyses. Mice were anesthetized with ketamine (200 mg/kg, ip) and xylazine (20 mg/kg), tracheotomized with a 20-gage cannula (BD INTRAMEDIC, cat. no. 427564, Franklin Lakes, NJ), and connected to the FlexiVent ventilator. The plane of anesthesia was sufficient to prevent spontaneous breathing. The mice were ventilated with a tidal volume of 10 ml/kg at a frequency of 350 breaths/min and positive end-expiratory pressure of 2 cm H2O to achieve lung volume similar to spontaneous breathing. Forced oscillation (0.5–19.6 Hz) was applied for 8 s. Subsequently, dynamic pressure–volume maneuvers were performed stepwise, increasing airway pressure to 30 cm H2O and then reversing the process. For each parameter, three measurements were assessed and averaged. Measurements were excluded from analyses if disrupted by a spontaneous breath, and a coefficient of determination of 0.95 was used as the lower limit for each measurement.
Statistics.
Statistical analyses were performed using a GraphPad PRISM 5 (La Jolla, CA). Data were analyzed by unpaired t-test for PN21 assessments and by two-way ANOVA for PN90 assessments. Tukey’s was performed as a post hoc analysis for all two-way ANOVA analyses.
RESULTS
Body weights.
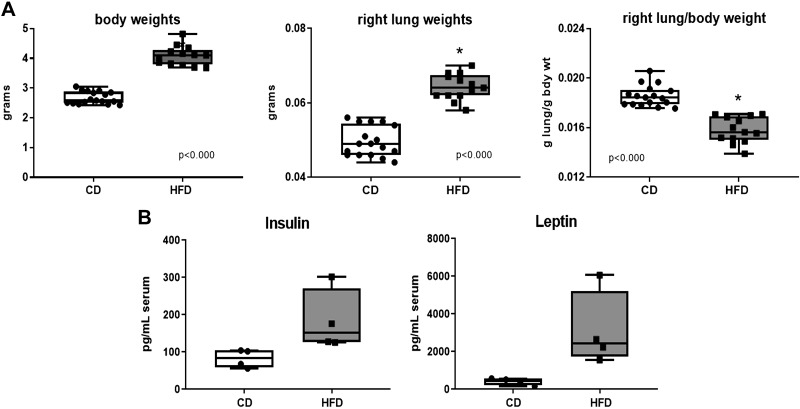
At PN7, body weights and right lung weights were higher and consequently, the right lung/body weight ratios were lower in the pups from the dams fed HFD. This would indicate an overnutrition status associated with maternal HFD early in lung development but a lighter lung relative to the increase in body mass (Fig. 2A). Although the same relative differences in body weight were evident at PN21 and PN90, the differences in right lung-to-body weight ratios were no longer different at P21 (0.0078 versus 0.0072, CD versus HFD, respectively) or at P90 (0.0041 versus 0.0050, CD versus HFD, respectively). Interestingly, serum obtained at PN7 indicated elevated insulin levels and substantially higher leptin levels in the pups from dams fed HFD (Fig. 2B).
Fig. 2.
Growth and metabolism at PN7. Pups were euthanized at PN7, and body weights and right lung weights were obtained. Blood was drawn by intracardiac puncture, and the blood from 3 to 4 pups from the same dam was pooled for analysis. A: weights were analyzed by unpaired t-test, n = 17 in CD group and n = 13 in HFD group. B: insulin and leptin levels were analyzed by Mann-Whitney test, n = 4 pooled samples in each group, each pooled sample originated from an independent litter. Statistical significance was set at P < 0.05 (*). CD, control diet; HFD, high-fat diet; PN, postnatal day.
Inflammatory pathways.
Cytokine levels were measured in pup lung tissues at PN21 by PCR and expressed as fold change above controls (mean ± SE). TNFα, IL-6, and IL-5 were chosen based on previously reported increased levels of these cytokines in pup serum (14). Levels of TNFα (1.21 ± 0.27 versus 1.92 ± 0.50; n = 10 and 8, respectively), IL-6 (1.18 ± 0.23 versus 1.03 ± 0.28; n = 10 and 9, respectively), and IL-5 (1.08 ± 0.15 versus 0.87 ± 0.01; n = 10 and 9, respectively) were not different between pups from dams fed CD and dams fed HFD.
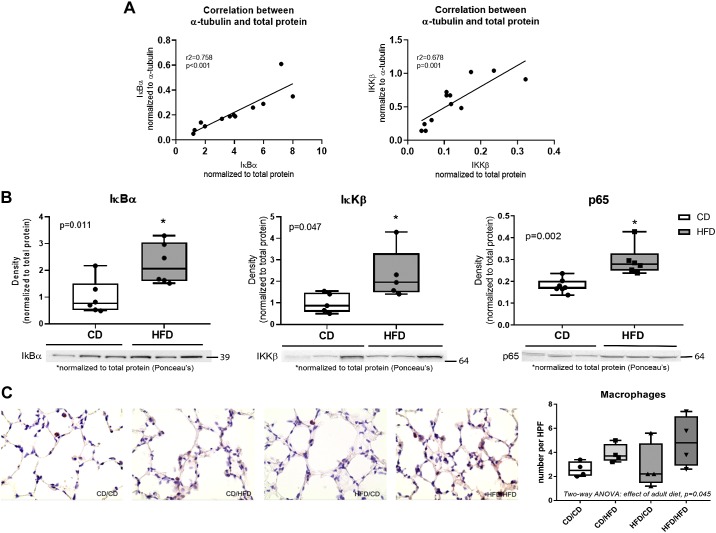
To validate the use of total protein as a normalizer for Western blots we compared the results of two sets of data, ΙκBα and IκKβ, using total protein or α-tubulin as denominators (Fig. 3A). We observed a linear correlation between the data, indicating that the two denominators yielded essentially the same relative results. NFκB-pathway markers were further assessed by Western blot. IκBα, IκKβ, and p65 were elevated in the pup tissues from HFD-fed dams compared with pups from CD-fed dams (Fig. 3B). Macrophage numbers were counted in lung tissue sections at PN90 using immunohistochemistry. An effect of adult diet was observed by two-way ANOVA, but no effect of maternal diet or individual differences was seen using post hoc analysis (Fig. 3C).
Fig. 3.
Markers of inflammation. A: normalization of Western blot density to total protein was compared with a-tubulin for IκBα and IκKβ protein. The data were analyzed by linear regression, and r2 and P values are indicated. B: markers of NFκB-mediated inflammation were measured by Western blot in tissues obtained from pups euthanized on PN21. Data were analyzed by unpaired t-test, n = 6–7 per group. C: tissues sections obtained from pups euthanized at PN90 were stained with MAC3 antibody, and macrophage numbers were counted using ImageJ software in five independent fields per slide (averaged as an individual point) with n = 4 individual mice from different litters. Data were analyzed by two-way ANOVA with Tukey’s post hoc. Statistical significance was set at P < 0.05 (*). CD, control diet; HFD, high-fat diet; PN, postnatal day.
Angiogenic pathways.
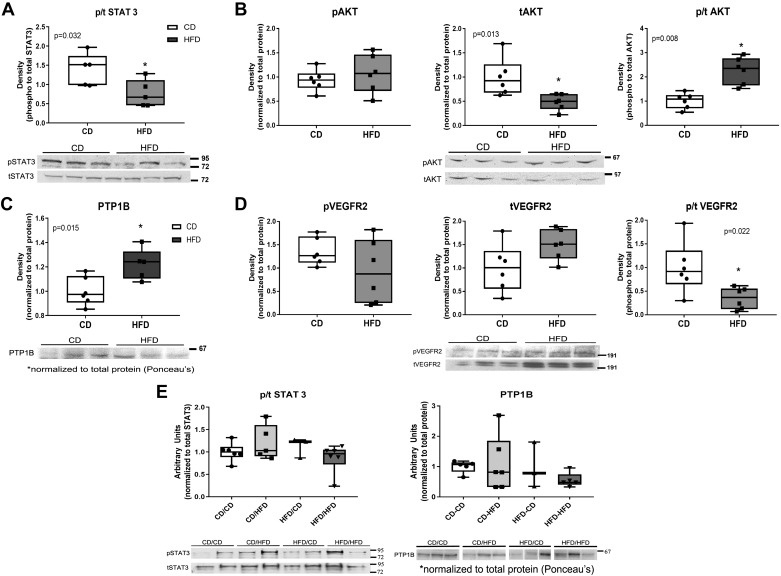
Components of the leptin-mediated angiogenic pathway were investigated. Using tissue homogenates from PN21, lower levels of phospho-/total STAT3 were observed in the pups from dams fed HFD than those from dams fed CD (Fig. 4A). Although levels of phospho-AKT were not different between groups, total AKT was substantially lower in the pups from the HFD group, which resulted in a higher ratio of phospho-/total AKT in the HFD group (Fig. 4B). Protein levels of PTP1B were elevated in pups from dams fed HFD compared with pups from dams fed CD (Fig. 4C). No differences were observed in phospho-VEGFR2 or total VEGFR2 independently, but the ratio of phospho-/total VEGFR2 was lower in the pups from dams fed HFD than those from dams fed CD (Fig. 4D). To determine whether these alterations in signaling persisted into adulthood, we assessed phospho-/total STAT3 and PTP1B by Western blot at PN90 (Fig. 4E). No differences in expression were observed in adulthood.
Fig. 4.
Expression of signaling pathway proteins. Pups were euthanized at PN21 (before weaning) (A–D) or PN90 (after adult diets) (E). Tissue homogenates were separated by SDS-PAGE and protein-quantified using Western blot followed by densitometry. Blots were normalized to total protein using Ponceau’s staining or the nonphosphorylated form of the protein as indicated and bands were quantified by densitometry. Samples obtained at PN21 were probed for phospho (p)/total STAT3, n = 5–6 (A); phospho (p), total, and phospho (p)/total AKT, n = 5–6 (B); PTP1B, n = 5–6 (C); and phospho (p), total, and phospho (p)/total VEGFR2, n = 5–6 (D). Data in A–D were analyzed by unpaired t-test; statistical significance was set at P = 0.05 (*). Samples obtained at PN90 were probed for phospho (p)/total STAT3 and PTP1B, n = 6 (E). Data in E were analyzed by two-way ANOVA with Tukey’s post hoc; no significant differences were observed. CD, control diet; HFD, high-fat diet; p, phospho; PN, postnatal day; PTPB1, protein tyrosine phosphatase B1; t, total; VEGFR, vascular endothelial growth factor receptor.
Lung vascular markers.
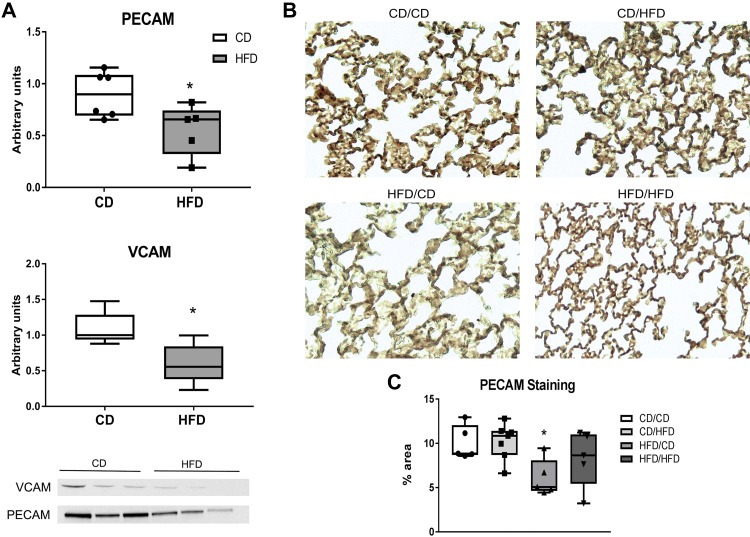
Protein levels of PECAM and VCAM were measured in lung tissue homogenates at PN21 by Western blot. Densitometry indicated that both PECAM and VCAM protein expression was decreased in the pups from dams receiving HFD (Fig. 5A). PECAM was further assessed in lung tissues at PN90 by immunohistochemistry. At PN90 the pups were divided into four groups with both maternal diet and pup adult diet indicated. There was an effect of maternal diet by two-way ANOVA, and pup tissues obtained from dams fed HFD and later fed CD as adults had less PECAM staining than the other three groups (post hoc analysis) (Fig. 5B).
Fig. 5.
Markers of vascularization. A: pups were euthanized at PN21 (before weaning), tissue homogenates were separated by SDS-PAGE, and proteins quantified using Western blot followed by densitometry. Blots were normalized to total protein using Ponceau’s staining and densitometry measurements where indicated. B: lung sections obtained from pups at PN90 were stained with platelet endothelial cell adhesion molecule (PECAM) antibody (see methods) and imaged at ×100 and subsequently enlarged ×4. C: total area of dark brown staining was quantified using ImageJ software measuring four fields per slide (averaged as an individual point). Data were analyzed by unpaired t-test (A), n = 6; or two-way ANOVA with Tukey’s post hoc (C), n = 5–7. Statistical significance was set at P < 0.05 (*). CD, control diet; HFD, high-fat diet; PECAM, platelet endothelial cell adhesion molecule; PN, postnatal day.
Morphometric analysis.
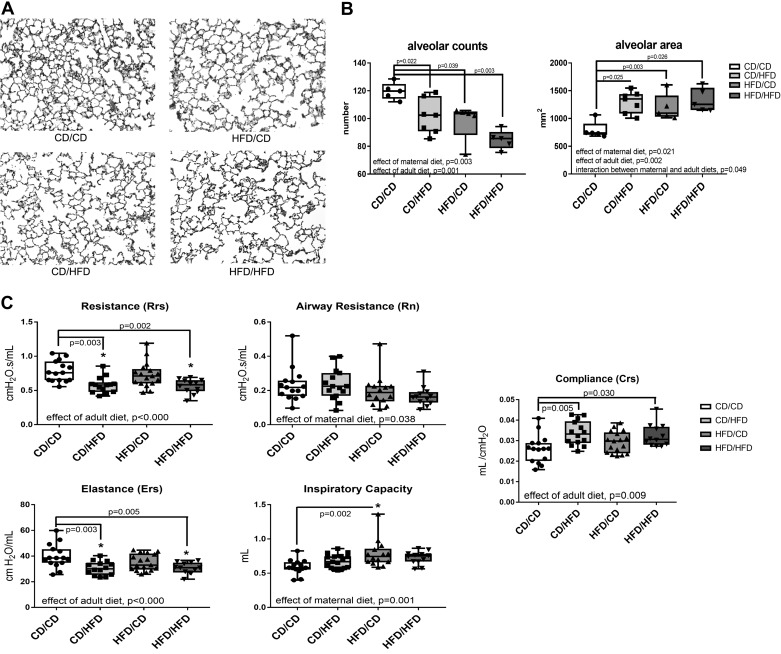
Alveolar number and area were quantified using H&E-stained lung tissue sections at PN90 (Fig. 6A). There were fewer alveoli and larger alveoli in mice from CD/HFD, HFD/CD, and HFD/HFD than in the CD/CD group (Fig. 6B).
Fig. 6.
Morphometric analysis and pulmonary function tests at PN90. A: lung tissues were inflation-fixed, and paraffin-embedded sections were stained with H&E. B: morphometric analysis were performed on H&E-stained tissue sections as described in methods and quantified using Image Pro software, n = 5–7 per group. C: pulmonary function tests, n = 12–17 per group, were performed using a SCIREQ FlexiVent. Data were analyzed by two-way ANOVA with Tukey’s post hoc, statistical significance was set at P < 0.05 (*). CD, control diet; H&E, hematoxylin-eosin; HFD, high-fat diet; PN, postnatal day.
Pulmonary function tests.
Pulmonary function was measured in mice at PN90. There was an effect of adult diet with the adult HFD-fed mice having lower resistance and elastance than the adult CD (Fig. 6C). There was an effect of maternal diet with the maternal HFD mice having higher inspiratory capacity and compliance (Fig. 6C).
DISCUSSION
The effects of maternal obesity on lung development have been identified in both humans (8, 9, 31) and in animal studies (3, 28). These include increased susceptibility to lung-related pathologies or overt increases in inflammation and altered pulmonary function. Leptin levels are elevated in the context of obesity and in the offspring in models of maternal obesity (12). Leptin receptors have been identified in several lung-specific cell types, and higher plasma leptin levels are associated with lower lung function (forced expiratory volume) in healthy children (8).
In our mouse model, we observed increases in body weight and serum leptin levels and decreases in right lung/body weight ratio in the offspring at PN7 (Fig. 2). These data are similar to the findings observed previously in rat models of maternal HFD (3, 28). Using a rat model, Baack et al. (3) reported lower lung weights, and decreased von Wildebrand-positive vessels, in the lungs of the offspring born to dams fed HFDs, and Song et al. (28) reported similar findings in a rat model with increased leptin and inflammation in the offspring. Given that leptin signaling is highly associated with angiogenesis and that leptin-deficient mice have decreased lung volume and alveolar surface area (17) and increased airway diameter (2), we hypothesized that mice born to obese dams would also have deficits in lung development.
Chronic systemic inflammation is also associated with obesity (33). Inflammation leads to cytokine release, specifically TNFα, which subsequently activates of NFκB-related pathways (20, 34). In our model, we previously reported increases in several serum cytokines at PN7 and PN21 (14). In the current study, we analyzed TNFα, IL-6, and IL-5 in lung tissue homogenates. We observed no differences in cytokine levels in lung tissues from pups that were from CD-fed dams versus HFD-fed dams. However, investigations revealed increases in IκBα, IκKβ, and p65 protein levels at PN21 in those same mice (Fig. 3B). To assess inflammation further, macrophage numbers were measured in lung tissues at PN90. We observed an effect of adult diet with increased numbers of lung resident macrophages at this time indicating that chronic inflammation was likely occurring because of the HFD ingested by the adult animals (Fig. 3C). Collectively, these observations suggest that NFκB pathways are activated and that NFκB-mediated genes are likely upregulated. However, we cannot rule out that the changes we observe in NFκB-related proteins are not because of the influence of the increased number of macrophages rather than from lung tissues. Our data agree with that reported by Song et al. (28) in rat pups exposed to maternal HFD with greater numbers of inflammatory cells within the lung tissues and with MacDonald et al. (22) in Balb/cByJ mice exposed to maternal HFD with elevated lL-6 levels and increased numbers of neutrophils in the bronchoalveolar lavage fluid. Furthermore, Zabolotny et al. (34) reported that increases in TNFα expression activates the NFκB p65 subunit, which in turn, binds to the promoter of PTP1B and induces its expression both in vitro and in vivo.
PTP1B was first identified as a regulator of the insulin receptor and is expressed in a wide range of tissues, but the liver and white adipose tissues appear to have the greatest effect on metabolism (5, 19). Furthermore, PTP1B has been identified as a key negative regulator of JAK2/STAT3 phosphorylation, which is a significant regulator of angiogenesis (5). Whole-body deletion of PTP1B causes no discernable health deficits in mice, but when placed on an HFD, knockout mice are resistant to weight gain, have decreased adipocyte cells mass, have enhanced insulin sensitivity, and have significantly lower leptin levels compared with control mice (10, 18). PTP1B has been found to be a direct regulator of VEGF signaling likely through dephosphorylation of VEGFR2 (19). Our data indicate that PTP1B protein levels were elevated and that the ratios of phospho-/total VEGFR2 were lower in the lungs of offspring of HFD-fed dams compared with offspring of CD-fed dams at PN21; however, these changes were no longer evident at PN90 (Fig. 4).
Angiogenic activation of the leptin receptor, Ob-R, occurs through phosphorylation of tyrosine 1175 and subsequent autophosphorylation of the JAK/STAT pathway, specifically through JAK2. Phosphorylation of JAK2 leads to further phosphorylation of STAT3, AKT, and VEGFR2. At PN21 we observed decreases in phospho-/total STAT3 and decreases in phospho-/total VEGFR2, which are driven largely by the increases in tVEGFR2. Conversely, we did not observe differences in phospho-AKT (Fig. 4B), but the levels of total AKT were decreased making the ratio of phospho-/total greater in the pups fed by HFD dams (Fig. 4B). This net decrease in AKT because of maternal HFD was also observed in the rat model and was attributed to maladaptation of the pulmonary vascular bed (3). By PN90 we no longer observed decreases in phospho-/total STAT3 (Fig. 4E) similar to the rat model, indicating this decrease was an early response to either maternal factors or only relevant to a developing lung. PECAM and VCAM levels are often used as a marker of endothelial cells. At PN21 we observed less PECAM and VCAM protein in lung homogenates and at PN90 less PECAM staining (Fig. 5). Collectively, these data indicate that there are fewer endothelial cells, which suggests that there are fewer vessels in the lung of mice from HFD-fed dams compared with controls. Our PN21 data correspond with the findings of Baack et al. (3) in that fewer vessels were present at birth in the lungs of rat pups born to dams fed HFD (40% fat).
Angiogenesis is a key player in the formation of alveoli and the capillary network required for gas exchange. The number of alveoli was lower and the size or area of the alveoli was larger in mice that were exposed to either maternal or adult HFD with additive effects in the mice exposed to both, HFD/HFD (Fig. 6, A and B). This is contrary to the effects observed by Baack et al. (3) in a rat model of maternal HFD in which no differences in alveolarization were noted. These differences may be because of the species or that the diet used in the rat studies was 40% fat, and the diet used in our studies was 60% fat which may have yielded a greater metabolic imbalance. Our data would imply that the decrease in vessel formation we observed at PN21 impairs the formation of alveoli in the lung. Pulmonary function tests (PFTs) were performed as a marker of lung disease. PFTs indicated that adult HFD had the greatest effect on resistance, elastance, and compliance in the lung but that maternal HFD affected the airway resistance and inspiratory capacity (Fig. 6C). Again, this is different than the PFTs reported in rat pups exposed to maternal obesity and the studies in the Balb/cByj mice (3, 22). In rats, PFT increases in pressure volume loop area and decreases in compliance were observed in response to streptozotocin treatment, but no differences were observed because of diet alone. However, in the Balb/cByj mouse model, increases in resistance were observed with maternal HFD using methacholine challenge.
Collectively, these data suggest that maternal HFD induces expression of pulmonary PTP1B in the offspring and that signaling pathways are suppressed leading to alterations in lung development and function. These data will contribute to our understanding of the pulmonary morbidities observed in infants born to mothers with obesity and provide new avenues for future investigations and potential therapeutic interventions.
GRANTS
The authors acknowledge funding support from NIH, National Center for Complementary and Alternative Medicine/Office of Dietary Supplements Grant R01AT006880, to L. K. Rogers) and National Institute of Child Health and Development (Grant R01HD088033, to L. K. Rogers).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
S.S.S., A.E.G., R.D.B., and L.K.R. conceived and designed research; K.M.H., S.M., S.S.S., A.E.G., S.W.L., and L.K.R. performed experiments; K.M.H., S.M., S.S.S., A.E.G., S.W.L., R.D.B., and L.K.R. analyzed data; S.W.L., R.D.B., and L.K.R. interpreted results of experiments; K.M.H., R.D.B., and L.K.R. prepared figures; R.D.B. and L.K.R. drafted manuscript; K.M.H., S.M., S.S.S., A.E.G., S.W.L., R.D.B., and L.K.R. edited and revised manuscript; K.M.H., S.M., S.S.S., A.E.G., S.W.L., R.D.B., and L.K.R. approved final version of manuscript.
REFERENCES
- 1.Adya R, Tan BK, Randeva HS. Differential effects of leptin and adiponectin in endothelial angiogenesis. J Diabetes Res 2015: 648239, 2015. doi: 10.1155/2015/648239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arteaga-Solis E, Zee T, Emala CW, Vinson C, Wess J, Karsenty G. Inhibition of leptin regulation of parasympathetic signaling as a cause of extreme body weight-associated asthma. Cell Metab 17: 35–48, 2013. [Erratum in Cell Metab 17: 463–464, 2013]. doi: 10.1016/j.cmet.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baack ML, Forred BJ, Larsen TD, Jensen DN, Wachal AL, Khan MA, Vitiello PF. Consequences of a maternal high-fat diet and late gestation diabetes on the developing rat lung. PLoS One 11: e0160818, 2016. doi: 10.1371/journal.pone.0160818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buist AS, McBurnie MA, Vollmer WM, Gillespie S, Burney P, Mannino DM, Menezes AM, Sullivan SD, Lee TA, Weiss KB, Jensen RL, Marks GB, Gulsvik A, Nizankowska-Mogilnicka E; BOLD Collaborative Research Group . International variation in the prevalence of COPD (the BOLD Study): a population-based prevalence study. Lancet 370: 741–750, 2007. doi: 10.1016/S0140-6736(07)61377-4. [DOI] [PubMed] [Google Scholar]
- 5.Cho H. Protein tyrosine phosphatase 1B (PTP1B) and obesity. Vitam Horm 91: 405–424, 2013. doi: 10.1016/B978-0-12-407766-9.00017-1. [DOI] [PubMed] [Google Scholar]
- 6.Duijts L, Reiss IK, Brusselle G, de Jongste JC. Early origins of chronic obstructive lung diseases across the life course. Eur J Epidemiol 29: 871–885, 2014. doi: 10.1007/s10654-014-9981-5. [DOI] [PubMed] [Google Scholar]
- 7.Durrani-Kolarik S, Pool CA, Gray A, Heyob KM, Cismowski MJ, Pryhuber G, Lee LJ, Yang Z, Tipple TE, Rogers LK. miR-29b supplementation decreases expression of matrix proteins and improves alveolarization in mice exposed to maternal inflammation and neonatal hyperoxia. Am J Physiol Lung Cell Mol Physiol 313: L339–L349, 2017. doi: 10.1152/ajplung.00273.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eising JB, Uiterwaal CS, Evelein AM, Visseren FL, van der Ent CK. Relationship between leptin and lung function in young healthy children. Eur Respir J 43: 1189–1192, 2014. doi: 10.1183/09031936.00149613. [DOI] [PubMed] [Google Scholar]
- 9.Eising JB, Uiterwaal CS, van der Ent CK. Maternal body mass index, neonatal lung function and respiratory symptoms in childhood. Eur Respir J 46: 1342–1349, 2015. doi: 10.1183/13993003.00784-2014. [DOI] [PubMed] [Google Scholar]
- 10.Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science 283: 1544–1548, 1999. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- 11.Elshenawy S, Simmons R. Maternal obesity and prenatal programming. Mol Cell Endocrinol 435: 2–6, 2016. doi: 10.1016/j.mce.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 12.Frühbeck G. Intracellular signalling pathways activated by leptin. Biochem J 393: 7–20, 2006. doi: 10.1042/BJ20051578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ghosh R, Gilda JE, Gomes AV. The necessity of and strategies for improving confidence in the accuracy of western blots. Expert Rev Proteomics 11: 549–560, 2014. doi: 10.1586/14789450.2014.939635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Graf AE, Lallier SW, Waidyaratne G, Thompson MD, Tipple TE, Hester ME, Trask AJ, Rogers LK. Maternal high fat diet exposure is associated with increased hepcidin levels, decreased myelination, and neurobehavioral changes in male offspring. Brain Behav Immun 58: 369–378, 2016. doi: 10.1016/j.bbi.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grasemann H. Metabolic origins of childhood asthma. Mol Cell Pediatr 2: 6, 2015. doi: 10.1186/s40348-015-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heida NM, Leifheit-Nestler M, Schroeter MR, Müller JP, Cheng IF, Henkel S, Limbourg A, Limbourg FP, Alves F, Quigley JP, Ruggeri ZM, Hasenfuss G, Konstantinides S, Schäfer K. Leptin enhances the potency of circulating angiogenic cells via src kinase and integrin (alpha)vbeta5: implications for angiogenesis in human obesity. Arterioscler Thromb Vasc Biol 30: 200–206, 2010. doi: 10.1161/ATVBAHA.109.192807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang K, Rabold R, Abston E, Schofield B, Misra V, Galdzicka E, Lee H, Biswal S, Mitzner W, Tankersley CG. Effects of leptin deficiency on postnatal lung development in mice. J Appl Physiol (1985) 105: 249–259, 2008. doi: 10.1152/japplphysiol.00052.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol 20: 5479–5489, 2000. doi: 10.1128/MCB.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lanahan AA, Lech D, Dubrac A, Zhang J, Zhuang ZW, Eichmann A, Simons M. PTP1b is a physiologic regulator of vascular endothelial growth factor signaling in endothelial cells. Circulation 130: 902–909, 2014. doi: 10.1161/CIRCULATIONAHA.114.009683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Lin R, Zhao B, Guan R, Li T, Jin R. Correlation between oxidative stress and the NF-κB signaling pathway in the pulmonary tissues of obese asthmatic mice. Mol Med Rep 13: 1127–1134, 2016. doi: 10.3892/mmr.2015.4663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lock M, McGillick EV, Orgeig S, McMillen IC, Morrison JL. Regulation of fetal lung development in response to maternal overnutrition. Clin Exp Pharmacol Physiol 40: 803–816, 2013. doi: 10.1111/1440-1681.12166. [DOI] [PubMed] [Google Scholar]
- 22.MacDonald KD, Moran AR, Scherman AJ, McEvoy CT, Platteau AS. Maternal high-fat diet in mice leads to innate airway hyperresponsiveness in the adult offspring. Physiol Rep 5: e13082, 2017. doi: 10.14814/phy2.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacDonald KD, Vesco KK, Funk KL, Donovan J, Nguyen T, Chen Z, Lapidus JA, Stevens VJ, McEvoy CT. Maternal body mass index before pregnancy is associated with increased bronchodilator dispensing in early childhood: A cross-sectional study. Pediatr Pulmonol 51: 803–811, 2016. doi: 10.1002/ppul.23384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGillick EV, Lock MC, Orgeig S, Morrison JL. Maternal obesity mediated predisposition to respiratory complications at birth and in later life: understanding the implications of the obesogenic intrauterine environment. Paediatr Respir Rev 21: 11–18, 2017. doi: 10.1016/j.prrv.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 25.Nie X, Li C, Hu S, Xue F, Kang YJ, Zhang W. An appropriate loading control for western blot analysis in animal models of myocardial ischemic infarction. Biochem Biophys Rep 12: 108–113, 2017. doi: 10.1016/j.bbrep.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park MS, Rieger-Fackeldey E, Schanbacher BL, Cook AC, Bauer JA, Rogers LK, Hansen TN, Welty SE, Smith CV. Altered expressions of fibroblast growth factor receptors and alveolarization in neonatal mice exposed to 85% oxygen. Pediatr Res 62: 652–657, 2007. doi: 10.1203/PDR.0b013e318159af61. [DOI] [PubMed] [Google Scholar]
- 27.Scholtens S, Wijga AH, Brunekreef B, Kerkhof M, Postma DS, Oldenwening M, de Jongste JC, Smit HA. Maternal overweight before pregnancy and asthma in offspring followed for 8 years. Int J Obes 34: 606–613, 2010. doi: 10.1038/ijo.2009.194. [DOI] [PubMed] [Google Scholar]
- 28.Song Y, Yu Y, Wang D, Chai S, Liu D, Xiao X, Huang Y. Maternal high-fat diet feeding during pregnancy and lactation augments lung inflammation and remodeling in the offspring. Respir Physiol Neurobiol 207: 1–6, 2015. doi: 10.1016/j.resp.2014.12.003. [DOI] [PubMed] [Google Scholar]
- 29.Stocks J, Sonnappa S. Early life influences on the development of chronic obstructive pulmonary disease. Ther Adv Respir Dis 7: 161–173, 2013. doi: 10.1177/1753465813479428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thacker JS, Yeung DH, Staines WR, Mielke JG. Total protein or high-abundance protein: Which offers the best loading control for Western blotting? Anal Biochem 496: 76–78, 2016. doi: 10.1016/j.ab.2015.11.022. [DOI] [PubMed] [Google Scholar]
- 31.Wankhade UD, Thakali KM, Shankar K. Persistent influence of maternal obesity on offspring health: Mechanisms from animal models and clinical studies. Mol Cell Endocrinol 435: 7–19, 2016. doi: 10.1016/j.mce.2016.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Warburton D, Schwarz M, Tefft D, Flores-Delgado G, Anderson KD, Cardoso WV. The molecular basis of lung morphogenesis. Mech Dev 92: 55–81, 2000. doi: 10.1016/S0925-4773(99)00325-1. [DOI] [PubMed] [Google Scholar]
- 33.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112: 1821–1830, 2003. doi: 10.1172/JCI200319451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem 283: 14230–14241, 2008. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]