Abstract
The mammalian olfactory bulb displays a prominent respiratory rhythm, which is linked to the sniff cycle and is driven by sensory input from olfactory receptors in the nasal sensory epithelium. In rats and mice, respiratory frequencies occupy the same band as the hippocampal θ-rhythm, which has been shown to be a key player in memory processes. Hippocampal and olfactory bulb rhythms were previously found to be uncorrelated except in specific odor-contingency learning circumstances. However, many recent electrophysiological studies in both rodents and humans reveal a surprising cycle-by-cycle influence of nasal respiration on neuronal activity throughout much of the cerebral cortex beyond the olfactory system, including the prefrontal cortex, hippocampus, and subcortical structures. In addition, respiratory phase has been shown to influence higher-frequency oscillations associated with cognitive functions, including attention and memory, such as the power of γ-rhythms and the timing of hippocampal sharp wave ripples. These new findings support respiration’s role in cognitive function, which is supported by studies in human subjects, in which nasal respiration has been linked to memory processes. Here, we review recent reports from human and rodent experiments that link respiration to the modulation of memory function and the neurophysiological processes involved in memory in rodents and humans. We argue that respiratory influence on the neuronal activity of two key memory structures, the hippocampus and prefrontal cortex, provides a potential neuronal mechanism behind respiratory modulation of memory.
Keywords: cortical oscillations, memory, olfactory bulb, respiratory rhythm, cognition
INTRODUCTION
Over the past several years, neuroscientists studying mammalian cortical activity of many types have rediscovered breathing as a cortical organizing principle [summarized by Tort et al. (2018)]. While we never forgot that mammals breathe, outside of olfactory research, respiratory activity was primarily considered an important brain stem process that sometimes produces artifacts in intracellular recordings in waking animals and in fMRI and EEG recordings. Olfactory research has been less agnostic to respiration; many researchers over several decades have found that olfactory system activity is organized around the sniff cycle in more than a superficial way (Bhalla and Bower 1997; Fukunaga et al. 2012; Macrides and Chorover 1972; Pager 1983; Shusterman et al. 2011; Verhagen et al. 2007). Several recent studies examining respiratory activity in the hippocampus, medial prefrontal cortex (mPFC), and other neocortical areas have begun to show that, far from an annoyance, respiration may play a key role in organizing cortical activity in memory processes. To understand how breathing may be involved in memory, we first examine its role in central olfactory processes and then discuss how this plays out in neocortical systems in several mammalian species.
AN OLFACTORY PERSPECTIVE OF NEURONAL OSCILLATIONS AND MEMORY
During nasal breathing, olfactory receptor neurons fire rhythmically in phase with respiration and drive activity in olfactory bulb (OB) mitral and tufted cells. The receptor neurons are sensitive to both odors and mechanical stimulation from nasal airflow (Grosmaitre et al. 2007), which means that entrainment of OB neuronal activity with respiration can also be independent of odor perception. The result of both types of input is that the entire OB becomes entrained to the respiratory rhythm, and the signal is readily apparent in the local field potential (LFP) (Adrian 1950; Phillips et al. 2012). Early studies that addressed the OB respiratory rhythm began with the assumption that the signal was an artifact from breathing and determined that the signal was, in fact, produced within the OB (Ottoson 1959). Importantly, the dipole field produced by the cortical network shows a reversal of the OB LFP respiratory rhythm from superficial to deep layers, which means that the OB rhythm is produced within the OB circuit and not referred via volume conduction from elsewhere or due to artifact.
Respiration-locked oscillations have been the subject of intensive-investigations in the context of olfactory processing (Kay 2015; Kepecs et al. 2006) and serve as the basis for the development of a more general concept of perception, resulting from the cooperative activity of large, distributed populations of neurons. Population activity in the olfactory systems of rats, cats, and rabbits have been studied since the 1960s using LFP/electrocorticogram array measurements, including the OB, anterior olfactory nucleus, pyriform cortex (PC), and hippocampus (Freeman 1975; Kay and Freeman 1998), reviewed by Kay (2015) and Rojas-Líbano and Kay (2008). The respiratory frequency band in rabbits, cats, rats, and mice is strongly represented in the OB LFP (Rojas-Líbano et al. 2014), and this frequency (2–12 Hz) also overlaps with hippocampal θ-band oscillations (4–10 Hz). Although it has been tempting to assume that the two oscillations are related, they are usually not coherent (Buonviso et al. 2006; Kay and Freeman 1998; Macrides et al. 1982; Tsanov et al. 2014; Yanovsky et al. 2014).
Neuronal oscillations as a fundamental mechanism for dynamical organization of populations of excitatory neurons were first described and formalized using the olfactory system, albeit initially for higher frequencies (Freeman 1975). Interacting excitatory and inhibitory neuron populations produce what are now known as gamma band oscillations (40–100 Hz) and are amplified when the OB is isolated from top-down input (Gray and Skinner 1988). The OB is a relatively simple cortex that receives direct sensory drive from the nose and has bidirectional interactions with many other cortical areas, strongly resembling the circuit comprising first-order thalamic sensory nuclei and the GABAergic network of neurons in the thalamic reticular nucleus (Kay and Sherman 2007).
In the olfactory system, memory has been argued to be a dynamic state represented by spatiotemporal oscillations of high-frequency γ-band (20–100 Hz) coherent waveforms in the OB and PC, gated by respiratory activity (Barrie et al. 1996; Freeman and Schneider 1982). During a respiratory cycle, meaning-dependent metastable activity patterns are formed and maintained over mesoscopic areas of cortex (Barrie et al. 1996), labeled amplitude-modulated (AM) patterns (Freeman and Schneider 1982). These AM patterns occur during each sniff of a learned odor and evolve with new experience as more odors are learned. Models suggest that initiation of synaptic modification is episodic and depends on the input signal and the phase of the respiratory gate at the time of the input, corresponding to action of norepinephrine (Gray et al. 1986; Kozma and Freeman 2003). Similar sniff-locked meaning-based patterns at the level of the BOLD signal have also been observed in posterior pyriform cortex (Bensafi et al. 2003; Howard et al. 2009; Sobel et al. 1998).
Similarly, spatially distributed meaning-dependent cortical activity patterns associated with learning and memory were later shown in other sensory systems: visual, auditory, and somatosensory primary sensory areas (Barrie et al. 1996; Freeman and Rogers 2002). In these cortical areas, AM patterns in the γ-band occur at rates in the high-θ band; those with carrier frequencies in the β-band occur at rates in the low-θ band (Freeman 2005). Together, they suggest what has been called a “cinematographic” mechanism in support of cognitive operations, by which successive AM patterns or states are formed in sequence, reminiscent of the sequences of pictures in cinematography (Freeman 2004). These types of sequential patterns have been observed in many other circumstances, including attention (Tallon-Baudry 2004), coordination dynamics (Tognoli and Kelso 2014), and the intentional action-perception cycle (Kozma and Freeman 2017). Arguably, θ-band clocking of these perceptual recreations of remembered events can be linked to respiration, as we discuss below.
RESPIRATION AFFECTS OSCILLATIONS IN KEY MEMORY STRUCTURES AT FREQUENCIES IMPLICATED IN MEMORY FUNCTION
Although it was long known that OB respiratory oscillations could be observed in PC LFP signals (Freeman 1960), Boeijinga and Lopes da Silva first described coherence between the two structures linked to respiration frequency (Boeijinga and Lopes da Silva 1988). Fontanini and Bower further quantified the coupling of respiration-locked oscillations in the OB and PC of anesthetized rats (Fontanini and Bower 2005), and Kay verified coherence at respiratory frequency in waking rats between OB and PC with a phase delay that suggests that the OB drives the rhythm in the PC and downstream areas (Kay 2005). Studies addressing respiratory and θ-band coupling between the OB and the hippocampus have been scarce until recently. Early observations by Macrides in hamsters suggested that hippocampal and nasal respiratory coupling was relatively strong during exploratory behavior (Macrides 1975), but a later study suggested this was relatively rare in an odor discrimination context (Macrides et al. 1982). In the latter study, respiratory behavior was phase locked with hippocampal θ-band activity only during early reversal learning of responses to odors, returning to an uncoupled state once the reversal was learned. This implies a “negative” correlation between respiration-hippocampal θ-coherence and performance. In a later study, Kay reported the presence of OB respiratory system-locked oscillations in the hippocampus of behaving rats (Kay 2005), showing that hippocampal θ-oscillations were coherent with OB respiratory oscillations, while rats performed an odor discrimination task that required them to keep track of timing between trials. The findings also revealed that coherence magnitude was “positively” correlated with performance in the task and suggested a potential role of OB-hippocampus coherence in cognitive aspects of olfactory sensorimotor processing. These opposite relationships between respiration and hippocampal activity during initial and reversal learning of odor associations suggest that respiratory activity may play important and perhaps different roles in learning and memory. These effects suggest that respiration-locked rhythms could be of more general importance and are possibly present throughout cerebral cortex, causing respiration-locked modulations of cortical functions.
Despite the potential significance of these findings, the influence of respiration on cortical activity did not receive much attention until more recently, when a series of independent studies reported respiration-locked events in a variety of cortical areas and some subcortical structures in rodents and humans (Tort et al. 2018). Ito et al. were the first to report respiration-locked neuronal oscillations in the neocortex (somatosensory cortex) of awake, head-fixed mice and showed that these oscillations were driven almost entirely by OB activity (Ito et al. 2014). The same study also showed that γ-oscillations in these neocortical areas were power-modulated in phase with breathing, which added phase-amplitude coupling as a new aspect of respiratory influence on cortical activity. A recent report links respiratory rhythms to cortical γ-oscillations in motor, somatosensory, and visual cortices during whisking behavior (Rojas-Líbano et al. 2018). Because neocortical γ-oscillations are strongly implicated in cognitive functions, these findings suggest a potential link between respiratory phase and cognitive processes (Bosman et al. 2014), including memory (Colgin 2016). Later that year, Yanovsky et al. showed that the hippocampus in anesthetized mice produces respiration-locked oscillations, which are close in frequency (2–4 Hz) to, but distinct from, intrinsic hippocampal θ-oscillations (Lockmann et al. 2016; Yanovsky et al. 2014). A similar effect was later shown in anesthetized rats (Lockmann et al. 2016) and waking, head-fixed mice (Nguyen Chi et al. 2016). This rhythm, prominent in the dentate gyrus of the hippocampus, was dubbed the hippocampal respiratory rhythm.
These reports were followed by studies in the prefrontal cortex of mice, which also showed clear distinctions between respiration-locked and intrinsic θ-oscillations (Biskamp et al. 2017; Karalis and Sirota 2018; Lockmann and Tort 2018), with one distinction being that γ-amplitude was modulated with greater efficacy by the phase of respiration compared with that of intrinsic θ (Biskamp et al. 2017). Subsequent studies in rodents and humans confirmed these findings and showed that respiratory modulation of neuronal oscillations occurs in many cerebral cortical areas, including the prefrontal cortex and hippocampus (Biskamp et al. 2017; Heck et al. 2016, 2017; Herrero et al. 2018; Karalis and Sirota 2018; Liu et al. 2017; Lockmann et al. 2016; Lockmann and Tort 2018; Zelano et al. 2016), as well as during sleep (Perl et al. 2016).
RESPIRATION MODULATES MEMORY AND MEMORY-RELATED BEHAVIOR
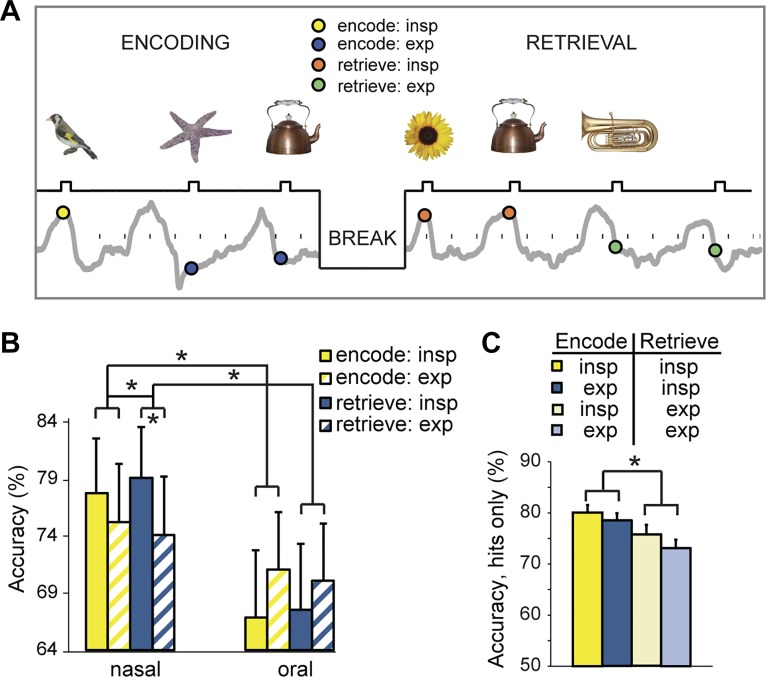
The most direct demonstrations that respiration modulates cognitive function come from studies of memory, and evidence comes from studies in both humans and mice. Zelano et al. presented a group of healthy human subjects with a sequence of pictures of everyday objects (Fig. 1), while they monitored each subject’s respiration (Zelano et al. 2016). They found that recall was more reliable for pictures presented during the inspiratory compared with expiratory phase of respiration. Importantly, this was only true during nasal respiration trials. The effects of respiratory phase on memory encoding or retrieval were not present when subjects breathed through the mouth. They also found that identification of emotion in faces was faster when these were presented during nasal breathing, and fear identification differed most significantly from oral breathing reaction times during inspiration. Results from Nakamura et al. suggest that the transition from expiration to inspiration might influence reaction time and recall accuracy in a delayed match-to-sample visual recognition task (Nakamura et al. 2018). If a transition from expiration to inspiration occurred between image presentation and response, subjects had significantly longer response times and decreased recall accuracy compared with trials without this transition (Nakamura et al. 2018). Somewhat puzzling, however, was the finding that the presence of the transition seemed to matter only when image presentations were phase locked to respiration and not when the presentations were randomly timed relative to respiratory phase (Nakamura et al. 2018).
Fig. 1.
Respiratory phase modulates episodic memory performance. A: in a recognition memory task, subjects viewed a series of different visual objects that occurred at different times within the breathing cycle. Interstimulus interval: 3–6 s. After a 20-min break, subjects were presented with the pictures previously presented in the encoding session plus an equal number of new pictures. B: memory performance was more accurate during inspiration than during expiration, with effects more pronounced for nasal than oral breathing, both for encoding and retrieval. C: an analysis of all “hit” trials revealed that recognition memory was significantly enhanced for pictures that had appeared during the inspiratory (vs. expiratory) phase of retrieval, but it made no difference whether those same pictures had been encountered in the same phase during encoding. *P < 0.05 in all panels. [From Zelano et al. 2016.]
While the above studies focused on memory encoding and recall, Arshamian et al. provided evidence that respiration also affects memory consolidation (Arshamian et al. 2018). In a test of episodic odor memory, participants were asked to memorize 12 target odors. One hour later, odor memory recall was tested by asking participants to identify target odors out of a random sequence of 12 target and 12 new odors. The key manipulation occurred during the 1-h consolidation phase, during which participants would breathe exclusively through their mouth or nose. The results show that odor memory recall was significantly increased after 1 h of nose breathing compared with mouth breathing (Arshamian et al. 2018).
Finally, when investigating a possible link between respiration and fear memory-related behavior in mice, Moberly et al. found evidence for a role of respiration-locked OB oscillations in controlling fear-related freezing behavior (Moberly et al. 2018). Mice who have learned to associate mild electrical foot shocks with a tone stimulus show a characteristic fear-related freezing behavior when exposed to the tone alone (Crawley 1999). During freezing, mice breathe at a very regular rate of ~4 Hz. Moberly et al. observed a significant increase in peak coherence at ~4 Hz between prelimbic prefrontal cortex (plPFC) LFP oscillations and breathing during freezing bouts, compared with nonfreezing periods (Moberly et al. 2018). Disrupting respiratory influence on the plPFC by disrupting OB activity had two effects: it reduced the coherence between plPFC activity and breathing to close to zero, and it significantly increased the duration of freezing bouts. Even though freezing behavior is induced by fear-memory, these results do not link respiration to memory in mice, but they suggest that respiration-locked rhythmic activity in the mouse OB modulates behavior that is controlled by association of cortical areas and not otherwise related to olfaction.
Taken together, the results summarized above are consistent with the view that OB activity is the main driving force behind respiration-locked brain activity (Ito et al. 2014; Lockmann and Tort 2018) and that this activity is causally involved in the influence of respiration on cognitive function and behavior. However, respiratory modulation of cortical rhythms may also have an extrabulbar source, perhaps from the brain stem. A recent study used ablation of the sensory neurons in the nasal epithelium and verified that OB coherence with higher-order areas at the respiratory rhythm was no longer evident (Karalis and Sirota 2018). In this condition, respiratory entrainment of the hippocampus and medial prefrontal cortex was reduced but still evident. This study complements a much older one in which top-down respiratory modulation of the rat OB was seen in tracheotomized rats (without any nasal respiratory drive) (Ravel and Pager 1990).
It is also important to point out that OB activity has not yet been directly monitored in any of the human studies. Thus, at this point, it is not clear whether the OB in humans plays the same causal role in driving respiration-locked brain activity as the OB in rodents is currently believed to do. Three human studies (Arshamian et al. 2018; Nakamura et al. 2018; Zelano et al. 2016) report effects of respiration on brain activity and cognitive functions that depended on nasal breathing, consistent with the assumption that OB activation is crucial. However, a recent study by Perl et al. (2019) tested three different cognitive functions: visuospatial perception, mathematical problem solving, and lexical decision making. They showed that performance in each one of these tasks was modulated with respiratory phase, but that the modulation of lexical problem solving (differentiating between words and random letter sequences) occurred during nasal and oral breathing. Even though mathematical and visuospatial perception tasks were modulated by nasal breathing only, their findings indicate that some cognitive functions may be modulated by respiratory phase independent of nasal airflow and, thus, possibly independent of OB bulb activation. How then do respiration-locked oscillations modulate memory function? In the following section, we review new findings that provide important clues as to potential neuronal mechanisms.
WHAT ASPECTS OF HIPPOCAMPAL AND PREFRONTAL NEURONAL ACTIVITY DOES RESPIRATION INFLUENCE THAT COULD LINK RESPIRATION TO MEMORY?
The hippocampus and prefrontal cortex are both essential but have distinct roles in the encoding and retrieval of memories (Preston and Eichenbaum 2013). There are three main types of oscillations in the hippocampus that are linked to memory function: θ-oscillations, γ-oscillations, and sharp wave ripples, and all three are involved in hippocampal-prefrontal communication during memory processes. In this section, we review results showing that all three types of rhythms are also modulated by respiration.
Theta rhythms play a central role in hippocampal activity (Buzsáki 2002; Gordon 2011), and θ-band coherence may be the default way for the hippocampus to establish functional connectivity with other structures, such as the amygdala (Popa et al. 2010), striatum (DeCoteau et al. 2007), and prefrontal cortex (Adhikari et al. 2010; Jones and Wilson 2005). Multiple lines of evidence point to θ-oscillations in the hippocampus and connected structures as essential components of the neuronal processes underlying memory encoding and retrieval. Theta oscillations play a key role in the temporal organization of neuronal activity within the hippocampal network (Gordon 2011; Hasselmo et al. 2002; Manns et al. 2007) and in the temporal coordination of memory-related neuronal activity between the mPFC and hippocampus (Backus et al. 2016; Benchenane et al. 2010; Colgin 2011; Siapas et al. 2005). Zelano et al. also analyzed intracranial EEG (iEEG) data from epilepsy patients to determine whether neuronal activity in memory-related areas, such as the hippocampus, was modulated by respiration. Their results show that human hippocampal θ-rhythms are amplitude modulated in phase with respiration (Zelano et al. 2016).
If θ-rhythms play a crucial role in the temporal organization of neuronal activity and functional connectivity within and between the hippocampus and mPFC, any influence of respiration on θ-rhythms is likely to influence memory function. In rodents, there is a direct link between respiration and neuronal θ-rhythms, because the respiratory frequency (2–12 Hz) is close to and overlapping with the θ-band, and respiration-locked rhythms have been found in the hippocampus (Kay 2005; Lockmann et al. 2016; Lockmann and Tort 2018; Macrides et al. 1982; Nguyen Chi et al. 2016) and the prefrontal cortex (Biskamp et al. 2017; Lockmann and Tort 2018). In humans, the respiratory frequency is much slower (0.16–0.33 Hz) than the θ-rhythm, but θ-power increases preferentially during inspiration (Zelano et al. 2016).
Gamma oscillations (25–120 Hz) in rodents are shown to be linked to respiration by phase-amplitude-coupling analysis. Biskamp et al. showed respiration-locked modulation of γ-power in the prefrontal cortex of mice, and γ-power was more strongly modulated by the respiration-locked rhythm than by intrinsic θ-oscillations (Biskamp et al. 2017). In humans, Herrero et al., using recordings from implanted electrodes in presurgical epilepsy patients, described respiration-locked modulation of γ-power in multiple cortical structures, including frontal cortex and hippocampus (Herrero et al. 2018). A related phase-based analysis also supports linking of γ to respiratory oscillations in multiple neocortical structures in rats during active whisking behavior (Rojas-Líbano et al. 2018).
Hippocampal γ-oscillations co-occur with θ-oscillations, and γ-power is modulated with the θ-phase (Bragin et al. 1995). There is evidence of a functional distinction between γ-oscillations in the low (25–55 Hz)- and high (>55 Hz)-frequency range, in that they seem to facilitate hippocampal communication along different pathways with different structures (Colgin et al. 2009), similar to slow and fast γ in the OB (Kay 2003). Fast γ oscillations in CA1 are coherent with fast γ in medial entorhinal cortex, an area through which sensory information reaches the hippocampus (Hafting et al. 2005). Slow γ oscillations are coherent between CA1 and CA3 (Colgin et al. 2009), a pathway implicated in memory retrieval (Steffenach et al. 2002). In the prefrontal cortex, the power of both slow and fast γ-oscillations was found to be phase-locked to hippocampal θ-oscillations (Dzirasa et al. 2009; Sirota et al. 2008), which may imply that fast and slow γ-frequencies correspond to mPFC directing attention to either entorhinally encoded environmental stimuli or the retrieval of memory content, respectively (Colgin 2011).
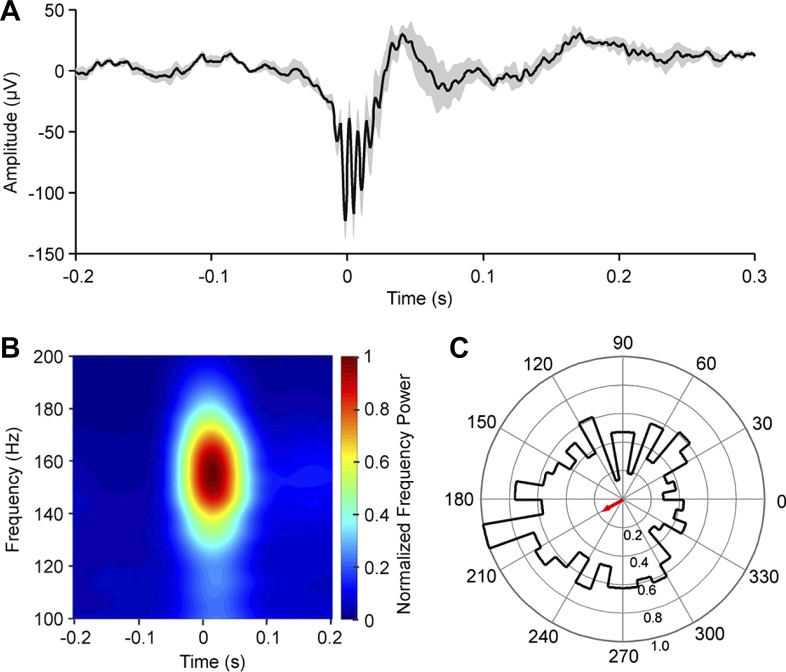
Hippocampal sharp wave ripples (SWRs) are brief, high-frequency (150–300 Hz) neuronal oscillations that co-occur with a sharp negativity in the hippocampal LFP and have been implicated in transfer of memories from the hippocampus to neocortex (Buzsáki 2015). Memories that initially depend on the hippocampus can be gradually transferred to the neocortex, a consolidation process linked to a replay of events during SWRs, which occur during slow wave sleep and during wakefulness in rats learning a spatial alternation task (Jadhav et al. 2012; Wilson and McNaughton 1994). Liu and colleagues recently showed that hippocampal SWRs are phase-locked to respiration (Fig. 2) (Liu et al. 2017). This finding, which has recently been replicated by Karalis and Sirota (Karalis and Sirota 2018), might provide a neuronal mechanism explaining why memory consolidation was improved by nasal versus oral breathing (Arshamian et al. 2018). Given what we know about the role of SWRs in memory consolidation, the phase locking of SWR to respiration might be necessary to create a proper temporal alignment between hippocampal SWR and neocortical excitability, to optimize the transfer of memory information. Taken together, substantial evidence strongly links respiration to major neuronal activity patterns associated with memory function.
Fig. 2.
Hippocampal sharp-wave ripple (SWR) activity in relation to the respiratory cycle in mice. A: average local field potential (LFP) aligned on hippocampal SWRs (means ± SE). Data are aligned on the onset of ripple-activity (at time 0 s). B: time-frequency mapping of LFPs around CA1 ripples. Color represents normalized frequency power. C: polar plot reflecting the distribution of SWR events relative to respiratory phase. Red arrow represents the mean vector determined by circular statistics (Rayleigh test: n = 382; r = 0.14; z = 7.35; P = 0.02). 0° represents the end of expiration, and 180° corresponds to the end of inspiration. Concentric circles mark r values, as indicated in the lower half of the circle. [From Liu et al. 2017.]
SNIFFING VERSUS BREATHING IN CORTICAL CIRCUITS
It is widely accepted that sniffing engages somewhat different motor circuits than the breathing that maintains oxygen levels. Furthermore, it has been noted since the time of Aristotle that a sniff to obtain an odor stimulus produces different perceptual effects than normal breathing behavior (Aristotle ca. 350 BCE), and modern modeling results quantify this effect (Zhao et al. 2006). Humans sniff by producing active prolonged inhalations, and small mammals, such as rats and cats, sniff by increasing the frequency of their nasal inhalations. Studies across many species have noted that cortical activity is significantly altered when individuals transition from breathing to sniffing (Freeman 1960; Rojas-Líbano et al. 2014; Sobel et al. 1998). It is now common to study mice in head-fixed protocols to allow very precise imaging and stimulation protocols. However, mice in these preparations tend to sniff at much slower frequencies than freely moving mice (Nguyen Chi et al. 2016). In humans, volitional sniffing activates the hippocampus and piriform cortex (Simonyan et al. 2007). Humans sniff when imaging odors and sniff longer for more pleasant odors, which suggests that the motor act of sniffing is linked with the percept (Bensafi et al. 2003). Does the change in sniff frequency (for rats, rabbits, cats, and mice) or duration (for humans) alter the cortical circuit by driving the OB differently via the olfactory nerve, or do the internal events that accompany the active sensing alter the cortical circuit in concert with the change in respiratory behavior? Answers to these questions will undoubtedly inform our understanding of the role of breathing in memory of these sensory events.
WHY RESPIRATION-LOCKED BRAIN ACTIVITY WAS IGNORED OUTSIDE OF OLFACTION RESEARCH
Investigations into brain functions not related to olfaction traditionally disregarded respiratory activity as a potential driving force behind neuronal oscillations. If respiration-locked activity patterns were observed, they were, often rightfully, treated as undesirable artifacts caused by brain tissue movement. Early electrophysiological recordings were mostly performed in anesthetized animals, where movements of animal’s chest against the supporting surface cause a visible movement of the exposed surface of the brain. Neuronal signals are sensitive to mechanical pressure exerted by the tip of the electrode against the membrane of a cell, but also change when the electrode tip moves slightly relative to a current source. These brain movements can, thus, result in rhythmic voltage fluctuations phase-locked to respiration. Also, anesthesia results in a very slow, regular breathing pattern, which, in turn, may prevent the detection of respiratory influence on oscillations at the normal, awake respiratory frequencies, which typically fall into the δ-/θ-range. This may explain why even decades of research on the olfactory system have not discovered the true extent of respiratory influence on brain activity (Rojas-Líbano et al. 2014).
It is remarkable that the sincere desire and development of techniques to avoid respiratory artifacts lead to conclusions that respiration does impact olfaction, but it was not detected in other cortices. Even in a study designed partially to examine the ways in which olfactory processing may impact neocortical activity, no evidence of respiratory modulation was seen at first (Barrie et al. 1996). Later developments in analysis methods required revisiting these conclusions. Just a few years later, the concept of “visual sniffing” was introduced, pointing to the potential link between respiration and visual processing (Kozma and Freeman 2001). A systematic Hilbert transform-based reanalysis of the earlier data, and many other experiments, did confirm the presence of bursts at θ rates but did not determine whether these were related to respiration (Freeman 2006; Freeman and Rogers 2002; Kozma and Freeman 2008).
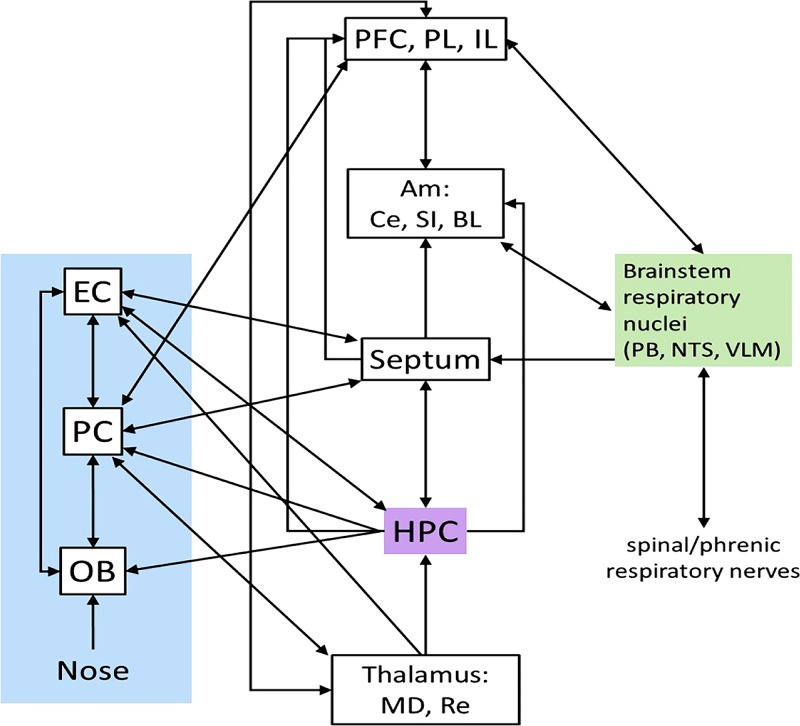
From our current knowledge, OB activity is likely to be the main driving force behind respiration-locked rhythms, at least in rodents. It is possible that in humans, other respiration-locked inputs, either via sensory inputs from the viscera and other nonnasal somatosensory efference copies of the respiratory process or from the brain stem, may also substantially contribute to driving respiration-locked activity. There are, in fact, many points of entry for the respiratory signal to enter cortical circuits (summarized in Fig. 3). To differentiate artifactual from neuronal causes of respiration locked neuronal activity, OB activity needs to be interrupted. That is straightforward in humans who can switch between nasal and oral breathing, but it requires invasive techniques in mice, such as olfactory bulbectomy (for long-term ablation), sensory neuron ablation, or optogenetic or pharmacological manipulations of OB activity for medium- to short-term ablation, because rats and mice are obligate nasal breathers.
Fig. 3.
Pathways by which respiratory drive may enter the cortical circuit. Left: olfactory system. Middle: regions connected with olfactory and respiratory systems, including the hippocampus. Right: respiratory brain stem connections (not detailed here). Not all connections are illustrated. Thalamic targets from the pyriform cortex are to the mediodorsal nucleus; thalamic efferents to the PC come from nucleus reuniens. Am, amygdala; BL, basolateral nucleus; Ce, central nucleus; EC, entorhinal cortex; HPC, hippocampus; IL, infralimbic; MD, mediodorsal nucleus; mPFC, medial prefrontal cortex; OB, olfactory bulb; PB, parabrachial nucleus; PC, pyriform cortex; PL, paralimbic; Re, nucleus reunions; SI, substantia innominata; NTS, nucleus tractus solitarius; VLM, ventrolateral medulla (Carlsen et al. 1982; Castle et al. 2005; Dobbins and Feldman 1994; Ellenberger and Feldman 1990; Ezure 2004; Gerrits and Holstege 1996; Hurley et al. 1991; Insausti et al. 2002; Künzle and Radtke-Schuller 2001; McKenna and Vertes 2004; Moga et al. 1990; Schwerdtfeger et al. 1990; Shipley and Adamek 1984; Van Groen et al. 1987; Van Groen and Wyss 1990; Vertes 2004; Wouterlood 1991).
OB respiratory activity accesses the hippocampus via a synapse in the entorhinal cortex, but otherwise, there are few known direct projections from olfactory areas to neocortical sensory areas, where respiration-locked oscillations and respiratory modulation of higher-frequency oscillations are found. How respiration modulates activity in those areas is still unknown, but modeling approaches suggest an answer. Simple graph-theoretical models of γ-oscillations modulated by respiration indicate the potential benefit of input-induced phase transitions in the interpretation of experimental findings (Heck et al. 2017). This means that modulated input from the hippocampus, for example, could suffice.
Provided the considerable influence of respiration on neuronal activity in cortical and subcortical structures and the fact that respiration influences memory, it is rather likely that future research will identify additional aspects of cognition that are modulated with respiratory phase. Studies of neuronal activity and timed behavior should, thus, routinely include monitoring of respiratory behavior, including differentiating nasal and oral breathing in humans.
GRANTS
D.H.H. was supported by National Institutes of Health Grant R01MH112143. R.K. was supported by Defense Advanced Research Projects Agency/Microsystems Technology Office (DARPA/MTO) Grant HR0011-16-1-0006 Superior Artificial Intelligence Grant. L.M.K. was supported by the Lifelong Learning Machines Program from DARPA/MTO Grant HR0011-18-2-0024 and National Institute on Deafness and Other Communication Disorders Grant R01 DC014367.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
D.H.H., R.K. and L.M.K. drafted the original manuscript, edited and revised the manuscript, and approved the final version.
REFERENCES
- Adhikari A, Topiwala MA, Gordon JA. Synchronized activity between the ventral hippocampus and the medial prefrontal cortex during anxiety. Neuron 65: 257–269, 2010. doi: 10.1016/j.neuron.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian ED. The electrical activity of the mammalian olfactory bulb. Electroencephalogr Clin Neurophysiol 2: 377–388, 1950. doi: 10.1016/0013-4694(50)90075-7. [DOI] [PubMed] [Google Scholar]
- Aristotle De Anima. ca. 350 BCE.
- Arshamian A, Iravani B, Majid A, Lundström JN. Respiration modulates olfactory memory consolidation in humans. J Neurosci 38: 10286–10294, 2018. doi: 10.1523/JNEUROSCI.3360-17.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backus AR, Schoffelen JM, Szebényi S, Hanslmayr S, Doeller CF. Hippocampal-prefrontal theta oscillations support memory integration. Curr Biol 26: 450–457, 2016. doi: 10.1016/j.cub.2015.12.048. [DOI] [PubMed] [Google Scholar]
- Barrie JM, Freeman WJ, Lenhart MD. Spatiotemporal analysis of prepyriform, visual, auditory, and somesthetic surface EEGs in trained rabbits. J Neurophysiol 76: 520–539, 1996. doi: 10.1152/jn.1996.76.1.520. [DOI] [PubMed] [Google Scholar]
- Benchenane K, Peyrache A, Khamassi M, Tierney PL, Gioanni Y, Battaglia FP, Wiener SI. Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66: 921–936, 2010. doi: 10.1016/j.neuron.2010.05.013. [DOI] [PubMed] [Google Scholar]
- Bensafi M, Porter J, Pouliot S, Mainland J, Johnson B, Zelano C, Young N, Bremner E, Aframian D, Khan R, Sobel N. Olfactomotor activity during imagery mimics that during perception. Nat Neurosci 6: 1142–1144, 2003. doi: 10.1038/nn1145. [DOI] [PubMed] [Google Scholar]
- Bhalla US, Bower JM. Multiday recordings from olfactory bulb neurons in awake freely moving rats: spatially and temporally organized variability in odorant response properties. J Comput Neurosci 4: 221–256, 1997. doi: 10.1023/A:1008819818970. [DOI] [PubMed] [Google Scholar]
- Biskamp J, Bartos M, Sauer JF. Organization of prefrontal network activity by respiration-related oscillations. Sci Rep 7: 45508, 2017. doi: 10.1038/srep45508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeijinga PH, Lopes da Silva FH. Differential distribution of beta and theta EEG activity in the entorhinal cortex of the cat. Brain Res 448: 272–286, 1988. doi: 10.1016/0006-8993(88)91264-4. [DOI] [PubMed] [Google Scholar]
- Bosman CA, Lansink CS, Pennartz CM. Functions of gamma-band synchronization in cognition: from single circuits to functional diversity across cortical and subcortical systems. Eur J Neurosci 39: 1982–1999, 2014. doi: 10.1111/ejn.12606. [DOI] [PubMed] [Google Scholar]
- Bragin A, Jandó G, Nádasdy Z, Hetke J, Wise K, Buzsáki G. Gamma (40-100 Hz) oscillation in the hippocampus of the behaving rat. J Neurosci 15: 47–60, 1995. doi: 10.1523/JNEUROSCI.15-01-00047.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonviso N, Amat C, Litaudon P. Respiratory modulation of olfactory neurons in the rodent brain. Chem Senses 31: 145–154, 2006. doi: 10.1093/chemse/bjj010. [DOI] [PubMed] [Google Scholar]
- Buzsáki G. Hippocampal sharp wave-ripple: a cognitive biomarker for episodic memory and planning. Hippocampus 25: 1073–1188, 2015. doi: 10.1002/hipo.22488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buzsáki G. Theta oscillations in the hippocampus. Neuron 33: 325–340, 2002. doi: 10.1016/S0896-6273(02)00586-X. [DOI] [PubMed] [Google Scholar]
- Carlsen J, De Olmos J, Heimer L. Tracing of two-neuron pathways in the olfactory system by the aid of transneuronal degeneration: projections to the amygdaloid body and hippocampal formation. J Comp Neurol 208: 196–208, 1982. doi: 10.1002/cne.902080208. [DOI] [PubMed] [Google Scholar]
- Castle M, Comoli E, Loewy AD. Autonomic brainstem nuclei are linked to the hippocampus. Neuroscience 134: 657–669, 2005. doi: 10.1016/j.neuroscience.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Colgin LL. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol 21: 467–474, 2011. doi: 10.1016/j.conb.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL. Rhythms of the hippocampal network. Nat Rev Neurosci 17: 239–249, 2016. doi: 10.1038/nrn.2016.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgin LL, Denninger T, Fyhn M, Hafting T, Bonnevie T, Jensen O, Moser MB, Moser EI. Frequency of gamma oscillations routes flow of information in the hippocampus. Nature 462: 353–357, 2009. doi: 10.1038/nature08573. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res 835: 18–26, 1999. doi: 10.1016/S0006-8993(98)01258-X. [DOI] [PubMed] [Google Scholar]
- DeCoteau WE, Thorn C, Gibson DJ, Courtemanche R, Mitra P, Kubota Y, Graybiel AM. Learning-related coordination of striatal and hippocampal theta rhythms during acquisition of a procedural maze task. Proc Natl Acad Sci USA 104: 5644–5649, 2007. doi: 10.1073/pnas.0700818104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86, 1994. doi: 10.1002/cne.903470106. [DOI] [PubMed] [Google Scholar]
- Dzirasa K, Ramsey AJ, Takahashi DY, Stapleton J, Potes JM, Williams JK, Gainetdinov RR, Sameshima K, Caron MG, Nicolelis MA. Hyperdopaminergia and NMDA receptor hypofunction disrupt neural phase signaling. J Neurosci 29: 8215–8224, 2009. doi: 10.1523/JNEUROSCI.1773-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res 513: 35–42, 1990. doi: 10.1016/0006-8993(90)91086-V. [DOI] [PubMed] [Google Scholar]
- Ezure K. Respiration-related afferents to parabrachial pontine regions. Respir Physiol Neurobiol 143: 167–175, 2004. doi: 10.1016/j.resp.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Fontanini A, Bower JM. Variable coupling between olfactory system activity and respiration in ketamine/xylazine anesthetized rats. J Neurophysiol 93: 3573–3581, 2005. doi: 10.1152/jn.01320.2004. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. A cinematographic hypothesis of cortical dynamics in perception. Int J Psychophysiol 60: 149–161, 2006. doi: 10.1016/j.ijpsycho.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Correlation of elctrical activity of prepyriform cortex and behavior in cat. J Neurophysiol 23: 111–131, 1960. doi: 10.1152/jn.1960.23.2.111. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Mass Action in the Nervous System. New York: Academic, 1975. [Google Scholar]
- Freeman WJ. Origin, structure, and role of background EEG activity. Part 2. Analytic phase. Clin Neurophysiol 115: 2089–2107, 2004. doi: 10.1016/j.clinph.2004.02.028. [DOI] [PubMed] [Google Scholar]
- Freeman WJ. Origin, structure, and role of background EEG activity. Part 3. Neural frame classification. Clin Neurophysiol 116: 1118–1129, 2005. doi: 10.1016/j.clinph.2004.12.023. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Rogers LJ. Fine temporal resolution of analytic phase reveals episodic synchronization by state transitions in gamma EEGs. J Neurophysiol 87: 937–945, 2002. doi: 10.1152/jn.00254.2001. [DOI] [PubMed] [Google Scholar]
- Freeman WJ, Schneider W. Changes in spatial patterns of rabbit olfactory EEG with conditioning to odors. Psychophysiology 19: 44–56, 1982. doi: 10.1111/j.1469-8986.1982.tb02598.x. [DOI] [PubMed] [Google Scholar]
- Fukunaga I, Berning M, Kollo M, Schmaltz A, Schaefer AT. Two distinct channels of olfactory bulb output. Neuron 75: 320–329, 2012. doi: 10.1016/j.neuron.2012.05.017. [DOI] [PubMed] [Google Scholar]
- Gerrits PO, Holstege G. Pontine and medullary projections to the nucleus retroambiguus: a wheat germ agglutinin-horseradish peroxidase and autoradiographic tracing study in the cat. J Comp Neurol 373: 173–185, 1996. doi:. [DOI] [PubMed] [Google Scholar]
- Gordon JA. Oscillations and hippocampal-prefrontal synchrony. Curr Opin Neurobiol 21: 486–491, 2011. doi: 10.1016/j.conb.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray CM, Freeman WJ, Skinner JE. Chemical dependencies of learning in the rabbit olfactory bulb: acquisition of the transient spatial pattern change depends on norepinephrine. Behav Neurosci 100: 585–596, 1986. doi: 10.1037/0735-7044.100.4.585. [DOI] [PubMed] [Google Scholar]
- Gray CM, Skinner JE. Centrifugal regulation of neuronal activity in the olfactory bulb of the waking rabbit as revealed by reversible cryogenic blockade. Exp Brain Res 69: 378–386, 1988. doi: 10.1007/BF00247583. [DOI] [PubMed] [Google Scholar]
- Grosmaitre X, Santarelli LC, Tan J, Luo M, Ma M. Dual functions of mammalian olfactory sensory neurons as odor detectors and mechanical sensors. Nat Neurosci 10: 348–354, 2007. doi: 10.1038/nn1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafting T, Fyhn M, Molden S, Moser MB, Moser EI. Microstructure of a spatial map in the entorhinal cortex. Nature 436: 801–806, 2005. doi: 10.1038/nature03721. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bodelón C, Wyble BP. A proposed function for hippocampal theta rhythm: separate phases of encoding and retrieval enhance reversal of prior learning. Neural Comput 14: 793–817, 2002. doi: 10.1162/089976602317318965. [DOI] [PubMed] [Google Scholar]
- Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Rezaie R, Freeman WJ, Wheless JW, Papanicolaou AC, Ruszinkó M, Sokolov Y, Kozma R. Breathing as a fundamental rhythm of brain function. Front Neural Circuits 10: 115, 2017. doi: 10.3389/fncir.2016.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck DH, McAfee SS, Liu Y, Babajani-Feremi A, Wheless JW, Papanicolaou AC, Ruszinkó M, Kozma R. Cortical rhythms are modulated by respiration (Preprint). bioRxiv 49007, 2016. doi: 10.1101/049007. [DOI]
- Herrero JL, Khuvis S, Yeagle E, Cerf M, Mehta AD. Breathing above the brain stem: volitional control and attentional modulation in humans. J Neurophysiol 119: 145–159, 2018. doi: 10.1152/jn.00551.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard JD, Plailly J, Grueschow M, Haynes JD, Gottfried JA. Odor quality coding and categorization in human posterior piriform cortex. Nat Neurosci 12: 932–938, 2009. doi: 10.1038/nn.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 308: 249–276, 1991. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Insausti R, Marcos P, Arroyo-Jiménez MM, Blaizot X, Martínez-Marcos A. Comparative aspects of the olfactory portion of the entorhinal cortex and its projection to the hippocampus in rodents, nonhuman primates, and the human brain. Brain Res Bull 57: 557–560, 2002. doi: 10.1016/S0361-9230(01)00684-0. [DOI] [PubMed] [Google Scholar]
- Ito J, Roy S, Liu Y, Cao Y, Fletcher M, Lu L, Boughter JD, Grün S, Heck DH. Whisker barrel cortex delta oscillations and gamma power in the awake mouse are linked to respiration. Nat Commun 5: 3572, 2014. doi: 10.1038/ncomms4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav SP, Kemere C, German PW, Frank LM. Awake hippocampal sharp-wave ripples support spatial memory. Science 336: 1454–1458, 2012. doi: 10.1126/science.1217230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MW, Wilson MA. Theta rhythms coordinate hippocampal-prefrontal interactions in a spatial memory task. PLoS Biol 3: e402, 2005. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karalis N, Sirota A. Breathing coordinates limbic network dynamics underlying memory consolidation (Preprint). bioRxiv 392530, 2018.
- Kay LM. Olfactory system oscillations across phyla. Curr Opin Neurobiol 31: 141–147, 2015. doi: 10.1016/j.conb.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Theta oscillations and sensorimotor performance. Proc Natl Acad Sci USA 102: 3863–3868, 2005. doi: 10.1073/pnas.0407920102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay LM. Two species of gamma oscillations in the olfactory bulb: dependence on behavioral state and synaptic interactions. J Integr Neurosci 2: 31–44, 2003. doi: 10.1142/S0219635203000196. [DOI] [PubMed] [Google Scholar]
- Kay LM, Freeman WJ. Bidirectional processing in the olfactory-limbic axis during olfactory behavior. Behav Neurosci 112: 541–553, 1998. doi: 10.1037/0735-7044.112.3.541. [DOI] [PubMed] [Google Scholar]
- Kay LM, Sherman SM. An argument for an olfactory thalamus. Trends Neurosci 30: 47–53, 2007. doi: 10.1016/j.tins.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Kepecs A, Uchida N, Mainen ZF. The sniff as a unit of olfactory processing. Chem Senses 31: 167–179, 2006. doi: 10.1093/chemse/bjj016. [DOI] [PubMed] [Google Scholar]
- Kozma R, Freeman WJ. Analysis of visual theta rhythm-experimental and theoretical evidence of visual sniffing. Int Joint Conf Neur Netw 2: 1118–1121, 2001. doi: 10.1109/IJCNN.2001.939517. [DOI] [Google Scholar]
- Kozma R, Freeman WJ. Basic principles of the KIV model and its application to the navigation problem. J Integr Neurosci 2: 125–145, 2003. doi: 10.1142/S0219635203000159. [DOI] [PubMed] [Google Scholar]
- Kozma R, Freeman WJ. Cinematic operation of the cerebral cortex interpreted via critical transitions in self-crganized dynamic systems. Front Syst Neurosci 11: 10, 2017. doi: 10.3389/fnsys.2017.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Freeman WJ. Intermittent spatio-temporal desynchronization and sequenced synchrony in ECoG signals. Chaos 18: 037131, 2008. doi: 10.1063/1.2979694. [DOI] [PubMed] [Google Scholar]
- Künzle H, Radtke-Schuller S. Oligosynaptic pathways possibly relaying visceral and/or gustatory information to the olfactory bulb in the hedgehog tenrec. Neurosci Lett 303: 53–56, 2001. doi: 10.1016/S0304-3940(01)01714-1. [DOI] [PubMed] [Google Scholar]
- Liu Y, McAfee SS, Heck DH. Hippocampal sharp-wave ripples in awake mice are entrained by respiration. Sci Rep 7: 8950, 2017. doi: 10.1038/s41598-017-09511-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockmann AL, Laplagne DA, Leão RN, Tort AB. A respiration-coupled rhythm in the rat hippocampus independent of theta and slow oscillations. J Neurosci 36: 5338–5352, 2016. doi: 10.1523/JNEUROSCI.3452-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockmann ALV, Tort ABL. Nasal respiration entrains delta-frequency oscillations in the prefrontal cortex and hippocampus of rodents. Brain Struct Funct 223: 1–3, 2018. doi: 10.1007/s00429-017-1573-1. [DOI] [PubMed] [Google Scholar]
- Macrides F. Temporal relationships between hippocampal slow waves and exploratory sniffing in hamsters. Behav Biol 14: 295–308, 1975. doi: 10.1016/S0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Macrides F, Chorover SL. Olfactory bulb units: activity correlated with inhalation cycles and odor quality. Science 175: 84–87, 1972. doi: 10.1126/science.175.4017.84. [DOI] [PubMed] [Google Scholar]
- Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J Neurosci 2: 1705–1717, 1982. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manns JR, Zilli EA, Ong KC, Hasselmo ME, Eichenbaum H. Hippocampal CA1 spiking during encoding and retrieval: relation to theta phase. Neurobiol Learn Mem 87: 9–20, 2007. doi: 10.1016/j.nlm.2006.05.007. [DOI] [PubMed] [Google Scholar]
- McKenna JT, Vertes RP. Afferent projections to nucleus reuniens of the thalamus. J Comp Neurol 480: 115–142, 2004. doi: 10.1002/cne.20342. [DOI] [PubMed] [Google Scholar]
- Moberly AH, Schreck M, Bhattarai JP, Zweifel LS, Luo W, Ma M. Olfactory inputs modulate respiration-related rhythmic activity in the prefrontal cortex and freezing behavior. Nat Commun 9: 1528, 2018. doi: 10.1038/s41467-018-03988-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moga MM, Herbert H, Hurley KM, Yasui Y, Gray TS, Saper CB. Organization of cortical, basal forebrain, and hypothalamic afferents to the parabrachial nucleus in the rat. J Comp Neurol 295: 624–661, 1990. doi: 10.1002/cne.902950408. [DOI] [PubMed] [Google Scholar]
- Nakamura NH, Fukunaga M, Oku Y. Respiratory modulation of cognitive performance during the retrieval process. PLoS One 13: e0204021, 2018. doi: 10.1371/journal.pone.0204021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen Chi V, Müller C, Wolfenstetter T, Yanovsky Y, Draguhn A, Tort AB, Brankačk J. Hippocampal respiration-driven rhythm distinct from theta oscillations in awake mice. J Neurosci 36: 162–177, 2016. doi: 10.1523/JNEUROSCI.2848-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottoson D. Studies on slow potentials in the rabbit’s olfactory bulb and nasal mucosa. Acta Physiol Scand 47: 136–148, 1959. doi: 10.1111/j.1748-1716.1960.tb00064.x. [DOI] [PubMed] [Google Scholar]
- Pager J. Unit responses changing with behavioral outcome in the olfactory bulb of unrestrained rats. Brain Res 289: 87–98, 1983. doi: 10.1016/0006-8993(83)90009-4. [DOI] [PubMed] [Google Scholar]
- Perl O, Arzi A, Sela L, Secundo L, Holtzman Y, Samnon P, Oksenberg A, Sobel N, Hairston IS. Odors enhance slow-wave activity in non-rapid eye movement sleep. J Neurophysiol 115: 2294–2302, 2016. doi: 10.1152/jn.01001.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perl O, Ravia A, Rubinson M, Eisen A, Soroka T, Mor N, Secundo L, Sobel N. Human non-olfactory cognition phase-locked with inhalation. Nat Hum Behav 3: 501–512, 2019. doi: 10.1038/s41562-019-0556-z. [DOI] [PubMed] [Google Scholar]
- Phillips ME, Sachdev RN, Willhite DC, Shepherd GM. Respiration drives network activity and modulates synaptic and circuit processing of lateral inhibition in the olfactory bulb. J Neurosci 32: 85–98, 2012. doi: 10.1523/JNEUROSCI.4278-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popa D, Duvarci S, Popescu AT, Léna C, Paré D. Coherent amygdalocortical theta promotes fear memory consolidation during paradoxical sleep. Proc Natl Acad Sci USA 107: 6516–6519, 2010. doi: 10.1073/pnas.0913016107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol 23: R764–R773, 2013. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravel N, Pager J. Respiratory patterning of the rat olfactory bulb unit activity: nasal versus tracheal breathing. Neurosci Lett 115: 213–218, 1990. doi: 10.1016/0304-3940(90)90457-K. [DOI] [PubMed] [Google Scholar]
- Rojas-Líbano D, Frederick DE, Egaña JI, Kay LM. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Front Behav Neurosci 8: 214, 2014. doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Kay LM. Olfactory system gamma oscillations: the physiological dissection of a cognitive neural system. Cogn Neurodyn 2: 179–194, 2008. doi: 10.1007/s11571-008-9053-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Líbano D, Wimmer Del Solar J, Aguilar-Rivera M, Montefusco-Siegmund R, Maldonado PE. Local cortical activity of distant brain areas can phase-lock to the olfactory bulb’s respiratory rhythm in the freely behaving rat. J Neurophysiol 120: 960–972, 2018. doi: 10.1152/jn.00088.2018. [DOI] [PubMed] [Google Scholar]
- Schwerdtfeger WK, Buhl EH, Germroth P. Disynaptic olfactory input to the hippocampus mediated by stellate cells in the entorhinal cortex. J Comp Neurol 292: 163–177, 1990. doi: 10.1002/cne.902920202. [DOI] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull 12: 669–688, 1984. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Shusterman R, Smear MC, Koulakov AA, Rinberg D. Precise olfactory responses tile the sniff cycle. Nat Neurosci 14: 1039–1044, 2011. doi: 10.1038/nn.2877. [DOI] [PubMed] [Google Scholar]
- Siapas AG, Lubenov EV, Wilson MA. Prefrontal phase locking to hippocampal theta oscillations. Neuron 46: 141–151, 2005. doi: 10.1016/j.neuron.2005.02.028. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Saad ZS, Loucks TM, Poletto CJ, Ludlow CL. Functional neuroanatomy of human voluntary cough and sniff production. Neuroimage 37: 401–409, 2007. doi: 10.1016/j.neuroimage.2007.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirota A, Montgomery S, Fujisawa S, Isomura Y, Zugaro M, Buzsáki G. Entrainment of neocortical neurons and gamma oscillations by the hippocampal theta rhythm. Neuron 60: 683–697, 2008. doi: 10.1016/j.neuron.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobel N, Prabhakaran V, Desmond JE, Glover GH, Goode RL, Sullivan EV, Gabrieli JD. Sniffing and smelling: separate subsystems in the human olfactory cortex. Nature 392: 282–286, 1998. doi: 10.1038/32654. [DOI] [PubMed] [Google Scholar]
- Steffenach HA, Sloviter RS, Moser EI, Moser MB. Impaired retention of spatial memory after transection of longitudinally oriented axons of hippocampal CA3 pyramidal cells. Proc Natl Acad Sci USA 99: 3194–3198, 2002. doi: 10.1073/pnas.042700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallon-Baudry C. Attention and awareness in synchrony. Trends Cogn Sci 8: 523–525, 2004. doi: 10.1016/j.tics.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Tognoli E, Kelso JA. The metastable brain. Neuron 81: 35–48, 2014. doi: 10.1016/j.neuron.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tort ABL, Brankačk J, Draguhn A. Respiration-entrained brain rhythms are global but often overlooked. Trends Neurosci 41: 186–197, 2018. doi: 10.1016/j.tins.2018.01.007. [DOI] [PubMed] [Google Scholar]
- Tsanov M, Chah E, Reilly R, O’Mara SM. Respiratory cycle entrainment of septal neurons mediates the fast coupling of sniffing rate and hippocampal theta rhythm. Eur J Neurosci 39: 957–974, 2014. doi: 10.1111/ejn.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Groen T, Lopes da Silva FH, Wadman WJ. Synaptic organization of olfactory inputs and local circuits in the entorhinal cortex: a current source density analysis in the cat. Exp Brain Res 67: 615–622, 1987. doi: 10.1007/BF00247292. [DOI] [PubMed] [Google Scholar]
- Van Groen T, Wyss JM. Extrinsic projections from area CA1 of the rat hippocampus: olfactory, cortical, subcortical, and bilateral hippocampal formation projections. J Comp Neurol 302: 515–528, 1990. doi: 10.1002/cne.903020308. [DOI] [PubMed] [Google Scholar]
- Verhagen JV, Wesson DW, Netoff TI, White JA, Wachowiak M. Sniffing controls an adaptive filter of sensory input to the olfactory bulb. Nat Neurosci 10: 631–639, 2007. doi: 10.1038/nn1892. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Differential projections of the infralimbic and prelimbic cortex in the rat. Synapse 51: 32–58, 2004. doi: 10.1002/syn.10279. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science 265: 676–679, 1994. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Wouterlood FG. Innervation of entorhinal principal cells by neurons of the nucleus reuniens thalami. anterograde PHA-L tracing combined with retrograde fluorescent tracing and intracellular injection with lucifer yellow in the rat. Eur J Neurosci 3: 641–647, 1991. doi: 10.1111/j.1460-9568.1991.tb00850.x. [DOI] [PubMed] [Google Scholar]
- Yanovsky Y, Ciatipis M, Draguhn A, Tort AB, Brankačk J. Slow oscillations in the mouse hippocampus entrained by nasal respiration. J Neurosci 34: 5949–5964, 2014. doi: 10.1523/JNEUROSCI.5287-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelano C, Jiang H, Zhou G, Arora N, Schuele S, Rosenow J, Gottfried JA. Nasal respiration entrains human limbic oscillations and modulates cognitive function. J Neurosci 36: 12448–12467, 2016. doi: 10.1523/JNEUROSCI.2586-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Dalton P, Yang GC, Scherer PW. Numerical modeling of turbulent and laminar airflow and odorant transport during sniffing in the human and rat nose. Chem Senses 31: 107–118, 2006. doi: 10.1093/chemse/bjj008. [DOI] [PubMed] [Google Scholar]