Abstract
The CHRNA7 gene that encodes the α7-subunit of the nicotinic acetylcholine receptor (α7-nAChR) has been associated with some autism spectrum disorders and other neurodevelopmental conditions characterized, in part, by auditory and language impairment. These conditions may include auditory processing disorders that represent impaired timing of neural activity, often accompanied by problems understanding speech. Here, we measure timing properties of sound-evoked activity via the auditory brainstem response (ABR) of α7-nAChR knockout mice of both sexes and wild-type colony controls. We find a significant timing delay in evoked ABR signals that represents midbrain activity in knockouts. We also examine spike-timing properties of neurons in the inferior colliculus, a midbrain nucleus that exhibits high levels of α7-nAChR during development. We find delays of evoked responses along with degraded spiking precision in knockout animals. We find similar timing deficits in responses of neurons in the superior paraolivary nucleus and ventral nucleus of the lateral lemniscus, which are brainstem nuclei thought to shape temporal precision in the midbrain. In addition, we find that other measures of temporal acuity including forward masking and gap detection are impaired for knockout animals. We conclude that altered temporal processing at the level of the brainstem in α7-nAChR-deficient mice may contribute to degraded spike timing in the midbrain, which may underlie the observed timing delay in the ABR signals. Our findings are consistent with a role for the α7-nAChR in types of neurodevelopmental and auditory processing disorders and we identify potential neural targets for intervention.
NEW & NOTEWORTHY Disrupted signaling via the α7-nicotinic acetylcholine receptor (α7-nAChR) is associated with neurodevelopmental disorders that include impaired auditory processing. The underlying causes of dysfunction are not known but a common feature is abnormal timing of neural activity. We examined temporal processing of α7-nAChR knockout mice and wild-type controls. We found degraded spike timing of neurons in knockout animals, which manifests at the level of the auditory brainstem and midbrain.
Keywords: auditory disorders, autism, forward masking, gap detection, inferior colliculus, superior paraolivary nucleus, ventral nucleus of the lateral lemniscus
INTRODUCTION
As many as 10% of preschool children exhibit some form of developmental speech processing disorder that cannot be attributed to known causes such as peripheral hearing deficits or identifiable neurological problems (Beitchman et al. 1986; Tallal et al. 1993). The occurrence of auditory processing disorders in children is of great concern due to potential life-long consequences including poor academic and social development (Bailey 2010). Because these disorders emerge in individuals that pass clinical hearing tests, detection and diagnosis have been challenging and therapeutic options remain limited.
Such listening disorders are accompanied by normal audiometric hearing thresholds that assess inner ear function, indicating that these processing deficits are located within the brain (Eggermont 2015b). Studies of central auditory processing disorders have identified degraded timing of midbrain activity as a predictor of listening difficulties in adults and children (Johnson et al. 2005; Mehraei et al. 2016). However, due to limitations in the available tools used to examine neural function in humans, the source of dysfunction remains unresolved. Animal models have provided a wealth of information about neural pathways early in the ascending auditory system that mediate temporal processing and that may contribute to central processing disorders (Felix et al. 2018).
Of particular interest are disorders that are associated with known genetic abnormalities because the underlying changes in structure and/or function in the brain can be explored using genetically engineered mice. In particular, for central auditory processing disorders, knockout (KO) mouse models can be used to elucidate how the processing of timing information may be altered in the developing auditory system and ultimately how degraded temporal processing impacts listening in humans.
One promising mouse model of degraded temporal processing involves disrupted cholinergic signaling during development. Copy number variation in the CHRNA7 gene that encodes the α7-nicotinic acetylcholine receptor (α7-nAChR) is considered to be a primary candidate gene causing the wide spectrum of neurodevelopmental disorders associated with the 15q13.3 microdeletion syndrome (Bacchelli et al. 2015; Gillentine and Schaaf 2015; Gillentine et al. 2017; Hoppman-Chaney et al. 2013; Lepichon et al. 2010; Liao et al. 2011; Lowther et al. 2015; Popovici et al. 2013; Shinawi et al. 2009). The functional consequence of both deletion (loss) and duplication (gain) of CHRNA7 is decreased calcium flux and therefore a similar clinical phenotype (Gillentine et al. 2017). It is probable that CHRNA7 copy number variants do not cause all of the symptoms associated with the 15q13.3 microdeletion syndrome.
The 15q.13.3 region encompasses seven protein-encoding genes, one microRNA, and two putative pseudogenes (Uddin et al. 2018). The phenotype specific to a single gene deletion or duplication in humans is difficult to determine because they rarely occur. Poor language development is a common feature in autism diagnosis, but all cases involve more than CHRNA7 deletion (Miller et al. 2009). Genome-wide studies, however, have found that CHRNA7 is one of a few genes associated with reading disability and specific language impairment (Gialluisi et al. 2016; Pettigrew et al. 2015; Simpson et al. 2015), supporting the hypothesis that CHRNA7 is an important gene for the development of language.
In rodent brainstem and midbrain nuclei associated with auditory temporal processing, the α7-nAChR is highly expressed and developmentally regulated in normal hearing animals (Happe and Morley 1998, 2004; Morley and Happe 2000). Manipulating expression of the mouse α7-nAChR and measuring the resulting effects on auditory processing can provide insight into the potential causes of deficits in humans.
Robust and transient expression of the α7-nAChR is observed in the posterovental cochlear nucleus (PVCN) early in development followed by high-peak expression in the two major targets of the PVCN, the superior paraolivary nucleus (SPON) and ventral nucleus of the lateral lemniscus (VNLL), as well as more moderate expression in other brainstem nuclei (Happe and Morley 1998, 2004). The SPON and VNLL in turn provide prominent inputs to the inferior colliculus (IC) in the auditory midbrain, which also exhibits high α7-nAChR expression during development (Happe and Morley 2004) and is thought to be an important early site for processing for temporal features of vocalizations (Portfors and Perkel 2014; Portfors and Sinex 2005).
Dysregulation of the α7-nAChR degrades the normally strong and reliable glutamatergic synapses of developing rodent auditory brainstem pathways (Baumann and Koch 2017; Morley and Felix 2017). In other systems, it has been demonstrated that α7-nAChR is obligatory for innervation; permanent synaptic deficits occur in its absence (Lozada et al. 2012). Therefore, disruptions in cholinergic signaling during early critical periods of low-level auditory processing could have wide-ranging consequences.
In the studies reported here, we investigated whether α7-nAChR loss (deletion) in the mouse is associated with abnormal auditory temporal processing. Understanding the contribution of α7-nAChR to auditory processing may increase our understanding of language impairment and could assist in the development or refinement of noninvasive diagnostic tests, α7-specific pharmacological treatments (Deutsch et al. 2010, 2011; Hashimoto 2015; Olincy et al. 2016) and behavioral therapeutic strategies (Russo et al. 2010).
MATERIALS AND METHODS
Animals
Auditory brainstem responses (ABR) and single-unit recordings were obtained in 20 α7-nAChR KO mice (12 female) and 20 age-matched colony controls (10 female), both groups on a C57Bl/6 background strain. Mice were aged 8–14 wk. Animals were housed with same-sex littermates on a reversed 12-h light-dark schedule and had free access to food and water. All animal care and use procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Boys Town National Research Hospital and Washington State University, an Association for Assessment and Accreditation of Laboratory Animal Care-accredited institution.
Surgical Procedures
Subcutaneous injections of buprenorphine (0.1 mg/kg) and meloxicam (2 mg/kg) were given before surgery. Anesthesia was maintained with isoflurane (0.5–2.5%) inhalation while the mouse was placed in a stereotaxic frame and was maintained throughout the surgery. A subcutaneous injection of lidocaine (4 mg/kg) was given immediately before incising and reflecting the tissue overlying the skull. Once the skull was exposed and cleaned, a lightweight metal post was attached using dental cement (Charisma). A tungsten ground pin was placed in the subcranial space of the frontal lobe (Felix et al. 2017a). Three holes were hand drilled part way through the skull to accommodate active and reference electrodes for ABR recordings. For single-unit recordings, access to the auditory midbrain and brainstem was achieved by performing a craniotomy ~1 mm2 using coordinates from a mouse brain atlas (Paxinos and Franklin 2001). The craniotomy was covered with bone wax, and the exposed skull and surrounding tissue were treated with topical lidocaine gel (2%), as well as a triple antibiotic ointment that contained neomycin, polymyxin B, and bacitracin. The animal was returned to its home cage for a recovery period lasting at least 1 day. The bone wax overlying the craniotomy was removed before each recording session and reapplied at the conclusion of the session, along with topical lidocaine and antibiotic application (Muniak et al. 2012).
Acoustic Stimulation
Stimulus generation and data acquisition were controlled by custom-written software (SSHF, Amy Boyle, Washington State University Vancouver). Acoustic stimuli were output through a 16-bit digital-to-analog converter (500,000 samples/s; National Instruments), sent to a programmable attenuator (PA-5; Tucker Davis Technologies), and routed to a speaker (LCY K100; Ying Tai) placed 10 cm from the ear. The speaker output was calibrated before each recording session over a range of 3–100 kHz using a one-fourth-inch calibrated microphone (model 4135; Brüel & Kjær) positioned at the location normally occupied by the animal’s ear. The gradual roll-off of intensity measured at high frequencies was corrected online so that the sound pressure level for each frequency at a given intensity varied by <2 dB SPL. Distortion components of tonal stimuli were buried in the noise floor at ≤50 dB below the signal level as determined by analyzing fast Fourier transforms of the digitized microphone signals.
Recording Procedures
Animals were lightly dosed with acepromazine maleate (2 mg/kg ip) at the beginning of each session to aid in the placement of the animal in a foam body restraint that was housed in a sound-attenuating chamber. The use of the sedative has little effect on fundamental auditory response properties in the brainstem (Felix et al. 2012). The head post was secured to a stereotaxic device to render the head immobile and maintain a consistent position during recordings. The animal was monitored frequently, and the experiment was terminated if the animal appeared distressed. Experimental sessions lasted no more than 4 h, and each animal was used for one to three recording sessions separated by at least 1 day.
ABR Recordings
To determine whether KO animals had abnormal audiometric thresholds compared with wild-type (WT) controls (n = 20 for each group), ABRs were recorded immediately before single-unit recordings during the same session. Low-impedance needle electrodes were placed with the positive electrode at the intersection of midline and bregma suture marks, connected to the positive input of a differential amplifier (Dagan 2400). The reference electrodes were placed in respective left and right occipital bones overlying the cerebellum and connected to the negative amplifier input (van Looij et al. 2004). We used pure tone stimuli (5-ms duration, 1-ms cos2 rise-fall, 10/s) presented to the right ear at 4, 8, 16, 32, and 64 kHz to determine hearing thresholds. For each frequency, sound levels were varied pseudorandomly from 0 to 90 dB SPL in steps of 5 dB. We sampled averaged responses (n = 500) from a 20-ms window. Signals were amplified by a factor of 2,000, bandpass filtered (100–3,000 Hz; Krohn-Hite 3550), and digitized at 25 kHz (National Instruments DAq). Recordings were stored using custom software (SSHF) for offline analysis. Masked ABRs were recorded using a 16-kHz tone as the probe, presented at 60 dB SPL (5-ms duration). The probe was preceded by a 16-kHz tone masker (50-ms duration), with a silent gap between stimuli (10-ms duration). The level of the masker was varied randomly from 0 to 90 dB SPL in 5-dB steps.
Single-Unit Recordings
Micropipette glass electrodes filled with 1 M NaCl were advanced in the brain using a hydraulic micropositioner (David Kopf Instruments), and neural activity was amplified, bandpass filtered (300–10,000 Hz; Krohn-Hite), and digitized with a 16-bit analog-to-digital converter (25,000 samples/s; National Instruments). Individual raw waveforms were viewed online, recorded, and then stored for offline analysis. Single-unit responses were isolated by advancing the electrode slowly into the brain while presenting a broadband noise search stimulus (70 dB SPL). Upon isolation of a unit, the characteristic frequency (CF) was determined, defined as the frequency that required the lowest sound level to elicit an evoked response to at least half of the stimulus presentations. Thresholds were determined audiovisually for each neuron before data collection and were defined as the lowest intensity to evoke a response at CF to at least half of the stimulus presentations. The experimental protocol consisted of the recording of activity in the absence of sound (spontaneous spiking) before each test, followed by the generation of a peristimulus time histogram (PSTH) of responses to 100 presentations of CF pure tones presented 20 dB above threshold (evoked spiking), and a rate-level test that varied the intensity of the CF tone (10 presentations) from 10 dB below threshold to 40 dB above threshold.
Recording Site Deposits
Recording sites were labeled by ejecting either Fluorogold (1% in sodium acetate; Fisher) or dextran rhodamine (1%; Fisher) from the recording pipette via current (4 μA, 5-min, duration and 50% duty cycle). To recover the tracer deposits, animals were deeply anesthetized with isoflurane and perfused with 10% phosphate-buffered formalin. The brains were placed in a 20% sucrose solution overnight and sectioned coronally at 40 μm with a freezing microtome. Tissue sections were mounted on Superfrost Plus slides (Fisher Scientific), dehydrated, cleared, and coverslipped with DPX (Electron Microscopy Sciences). Images were taken with a confocal microscope (TCS SP8; Leica).
Data Analysis
Recorded waveforms were analyzed using custom software written in Python (SSHF). ABR thresholds were defined as the lowest sound level that evoked a consistent waveform with identifiable peaks. We measured properties of peaks I and IV, which are thought to represent respective auditory nerve and midbrain activity (Zheng et al. 1999). These peaks were chosen because they were the most prominent and reliable, and they have been implicated in cochlear synaptopathy-induced hearing deficits in rodents (Mehraei et al. 2016). Thresholds were determined subjectively by two independent observers. Response amplitudes were defined as the difference between the baseline voltage and the maximum peak voltage. Latencies were defined as the time of the maximum peak amplitude from the sound onset.
Spiking events were detected by a threshold feature that reliably separated single unit activity with consistent waveforms from the noise floor, with at least a 4:1 signal to noise ratio. Spiking rate (spikes per stimulus), first-spike latency from the sound onset, as well as the standard deviation of first-spike latency (jitter) were calculated from response spike times. Response duration was classified as either onset, intermediate or sustained if evoked spiking persisted for 0–40, 40–80, or 80–120 ms from the start of a 100-ms CF pure tone presented 20 dB above threshold, respectively. Spiking in each time window that occurred in response to over half of the stimulus presentations was considered evoked, whereas spiking that did not meet this criterion was classified as spontaneous. Before statistical analyses, tests were performed to determine whether each data set fit a normal distribution (D’Agostino and Stephens 1986). Statistics were applied using either SSHF or graphing software used to generate data plots (Prism; Graph Pad). The Student’s t-test (two-tailed) or the Mann-Whitney test was used to determine whether spiking differed between neurons of KO and WT animals. The Pearson correlation coefficient was used to quantify relationships between normalized values of measured parameters (Sheskin 2011), and the F-test was used to determine whether the variance of first spike latencies was different between conditions (Snedecor and Cochran 1983). We used the Fisher’s exact test with the Freeman-Halton extension to determine differences in proportions of subsets of data (Freeman and Halton 1951). The critical significance level for each test was set at α = 0.05, and values in the text represent the sample means ± SD.
RESULTS
Timing Is Altered in the Auditory Midbrain of α7-nAChR KO Animals
We tested whether the α7-nAChR KO had abnormal hearing thresholds and whether ABR activity was differentially affected by deficient cholinergic signaling. We measured averaged peak amplitudes and latencies in KO and age-matched WT control mice. We focused on ABR waves I and IV to measure peripheral and central auditory function, respectively. Wave I is generated by the synchronous activation of large numbers of auditory nerve fibers, whereas wave IV is generated by synchronous pre- and postsynaptic activity in the midbrain of the mouse (Markand 1994). We examined wave IV in the mouse because it is thought to be equivalent to ABR wave V in humans, which has been used to study processing in the central auditory system (Mehraei et al. 2016).
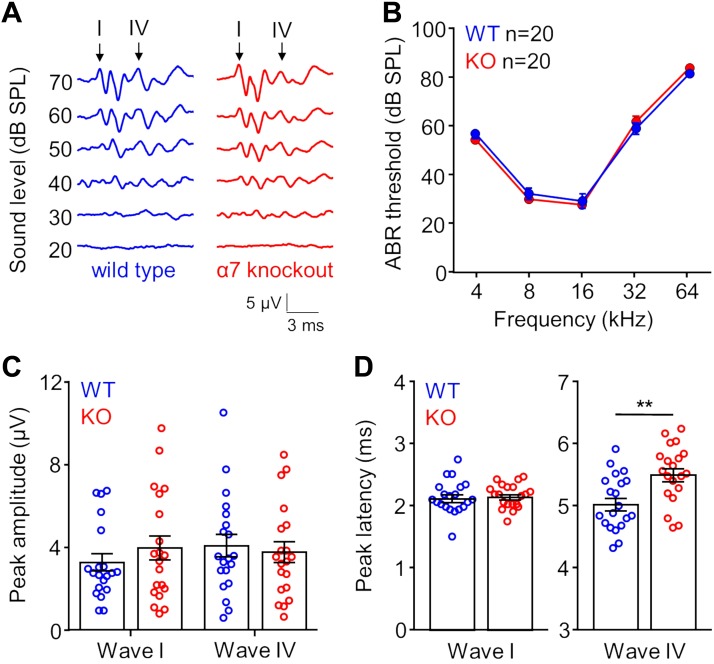
We reliably detected waves I and IV in KO and WT animals (Fig. 1A), and ABR thresholds across all pure tone frequencies tested were not significantly different between groups (P > 0.85 for all frequencies, ANOVA/Sidak’s multiple comparisons test; Fig. 1B). In addition, we measured no differences in maximum peak amplitude between KO and WT groups for both wave I (KO: 3.97 ± 0.59 µV; WT: 3.27 ± 0.41 µV; P = 0.34, Student’s t-test) and wave IV (KO: 3.78 ± 0.51 µV; WT: 4.08 ± 0.54 µV; P = 0.68, Student’s t-test) to 16-kHz stimulation presented 20 dB above the ABR threshold (Fig. 1C). We also measured the timing of wave peaks and found no difference in wave I latency between the KO (2.13 ± 0.04 ms) and WT group (2.12 ± 0.06 ms; P = 0.83, Student’s t-test; Fig. 1D, left). However, the timing of ABR wave IV was significantly different between groups. Specifically, wave IV peak latency was delayed for the KO group (5.50 ± 0.1 ms) compared with the WT group (5.02 ± 0.1 ms; P = 0.002, Student’s t-test; Fig. 1D, right).
Fig. 1.
α7-Knockout (KO) mice show differences in the timing of auditory brainstem response (ABR) wave IV. A: representative traces of wild-type (WT; left) and KO (right) ABR signals to 16 kHz pure tone stimulation presented at different intensities. Arrows indicate ABR waves I and IV, which were used for additional measurements. B: ABR thresholds for WT and KO mice were similar for frequencies of pure tones that encompass most of the mouse hearing range (P > 0.85 for all frequencies, ANOVA/Sidak’s multiple comparisons test). C: maximum peak amplitudes for waves I and IV to 16-kHz tone stimulation presented 20 dB above the ABR threshold were not different between WT and KO animals (wave I: P = 0.34; wave IV: P = 0.68, Student’s t-test). D: wave I peak latencies to 16-kHz stimulation (+20dB re: threshold) were not different between WT and KO groups (left; P = 0.83, Student’s t-test). In contrast, wave IV latencies for the KO group were longer, overall (right; P = 0.002, Student’s t-test). Error bars represent the SE. **P < 0.01.
Responses Are Altered for a Subset of IC Neurons in α7-nAChR KO Animals
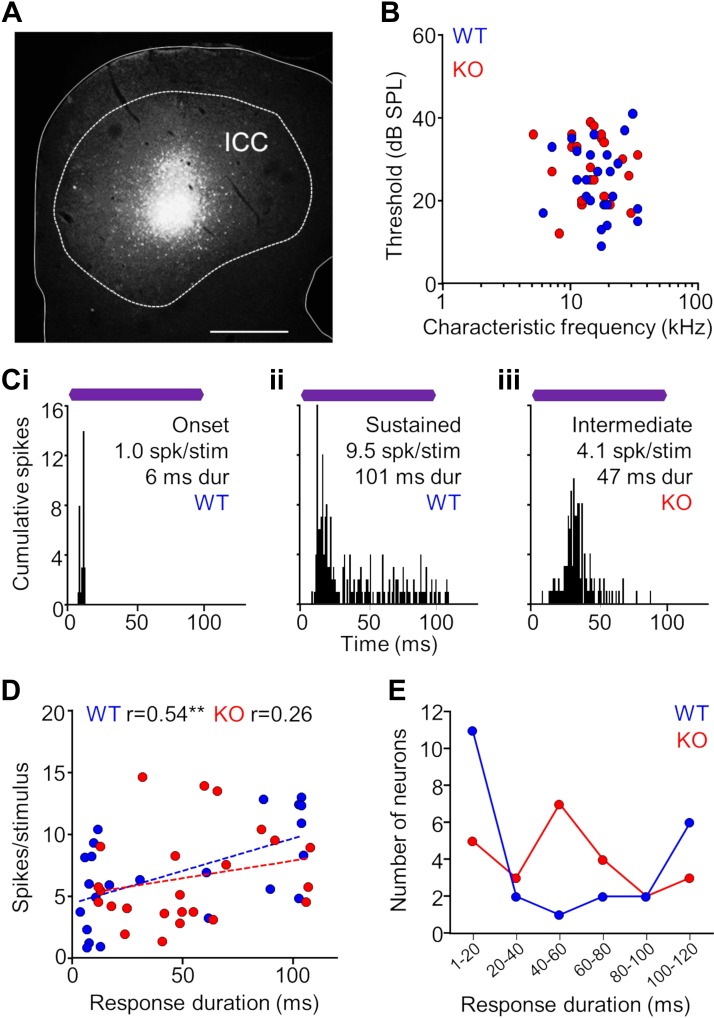
Because wave IV of the ABR is attributed to auditory midbrain processing, we recorded responses of single units in the inferior colliculus of α7-KO and -WT mice to more fully explore whether deficits in cholinergic signaling disrupt temporal processing in the auditory midbrain. We confirmed that our recording sites were from the central nucleus of the IC (ICC) by marking each recording site with a fluorescent tracer (Fig. 2A). The distribution of characteristic frequencies and minimum thresholds were similar for KO and WT single-unit responses in the ICC (n = 24 for both groups; Fig. 2B). In addition, the width of frequency tuning measured by the Q value was not different between KO and WT neurons when measured at 10 dB (P = 0.55, Mann-Whitney test) and 30 dB (P = 0.66) above the minimum threshold. For neurons in WT animals, common spiking patterns to repeated CF tone stimulation included transient responses evoked by the sound onset and sustained responses evoked for the entire sound duration (Fig. 2, Ci and Cii). An additional response type with an intermediate duration was observed qualitatively for a subset of neurons in KO animals (Fig. 2Ciii). The distributions of evoked response duration and spiking rate were positively correlated for neurons in WT and KO groups, although this relationship was not significant for the latter (WT: r = 0.54, P = 0.01; KO: r = 0.26, P = 0.22, Spearman correlation; Fig. 2D). Furthermore, there were no differences between groups with respect to mean response duration (P = 0.15, Mann-Whitney test) and spiking rate (P = 0.69, Mann-Whitney test), and the ranges for these measurements between groups were similar. However, there was a difference in the proportions of onset, intermediate, and sustained responses between the groups (P = 0.04, Fisher-Freeman-Halton test). The majority of WT responses were either transient (1- to 40-ms duration, 54% of units) or sustained (80- to 120-ms duration, 33%), with far fewer intermediate responses (40- to 80-ms duration; 13%). For the KO group, transient (33%) and sustained (21%) were represented, but there was a greater occurrence (46%) of intermediate responses (Fig. 2E).
Fig. 2.
Single-unit properties of auditory midbrain neurons in α7-knockout (KO) and wild-type (WT) mice. A: midbrain recording site in the central nucleus of the inferior colliculus (ICC; dashed outline) marked by a Fluorogold tracer deposit delivered from the electrode. Scale bar = 250 μm. B: distribution of characteristic frequencies and minimum thresholds for KO and WT single-unit responses in the ICC (n = 24 for both groups). C: representative examples of spiking patterns in the ICC. For neurons in WT animals, common spiking patterns to repeated characteristic frequency (CF) tone stimulation included transient responses evoked by the sound onset (i) and sustained responses evoked for the entire sound duration (ii). An additional response type with an intermediate duration was qualitatively observed for a subset of neurons in KO animals (iii). Purple bars represent the 100-ms CF tone stimulus presented at 20 dB above threshold. D: the distribution of evoked response duration and spiking rate was positively correlated for WT and KO units, although less so for the latter (Spearman correlation). There were no differences between groups with respect to mean duration (P = 0.15, Mann-Whitney test) and spiking rate (P = 0.69, Mann-Whitney test), and the ranges for each measurement were similar. However, there was a qualitative difference in the distribution of durations of the groups. E: the majority of WT responses were either transient (1- to 40-ms duration, 54%) or sustained (80- to 120-ms duration, 33%), with far fewer intermediate responses (40- to 80-ms duration; 13%). For the KO group, transient (33%) and sustained (21%) were represented, but there was a greater occurrence (46%) of intermediate responses.
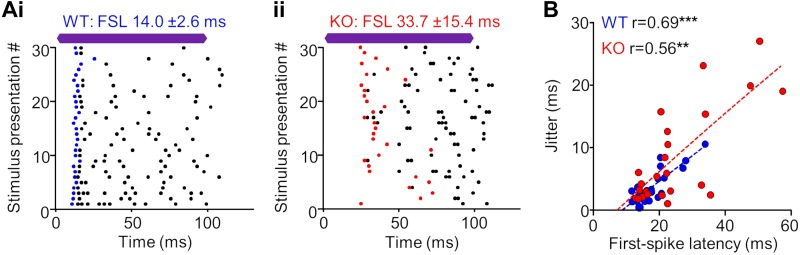
Neurons in the α7-KO group appeared to have degraded spike-timing compared with WT units, including delayed first-spike latency (FSL) to the onset of sound stimulation (Fig. 2Ciii). Nearly all cells (7/8) with a mean FSL >30 ms were recorded in KO animals. In particular, intermediate duration units represented the majority (5/7) of long-latency KO cells (Fig. 3A). To determine whether spike-timing was altered in the KO group compared with the WT control group, we measured the FSL and the standard deviation of the FSL (jitter) when stimuli were presented at 20 dB above threshold. There was a positive correlation between FSL and jitter for both KO (r = 0.69, P = 0.0002, Spearman correlation) and WT (r = 0.56, P = 0.004) groups (Fig. 3B). However, a subset of neurons in the KO group had substantially higher FSLs compared with neurons in the WT group, which resulted in an overall difference between groups (P = 0.01, Mann-Whitney test).
Fig. 3.
A subset of neurons in the auditory midbrain of α7-knockout (KO) mice exhibit responses with altered temporal properties. A: raster plots of representative wild-type (WT; i) and KO (ii) responses depicting evoked spiking events to repeated sound stimulation. The first evoked spike to each stimulus presentation is highlighted in blue and red for respective WT and KO examples. The mean first-spike latency (FSL) and the SD of the FSL were determined and used to assess spike timing. Purple bars represent the 100-ms characteristic frequency (CF) tone stimulus presented at 20 dB above threshold. B: there was a positive correlation for FSL and the SD of the FSL, known as jitter, for both groups (**P < 0.01, ***P < 0.001, Spearman correlation). A subset of neurons in the KO group had substantially higher FSLs compared with the WT neurons, which resulted in an overall difference between groups (P = 0.01, Mann-Whitney test).
In addition to measuring the FSL and jitter of responses to stimuli presented at 20 dB above minimum threshold, we also assessed spiking at 10 and 30 dB above threshold. We found that at each sound level tested, the FSL of responses in KO animals was significantly higher compared with the WT group (10 dB above threshold: P = 0.02; 20 dB above threshold: P = 0.01; 30 dB above threshold: P = 0.01, Mann-Whitney U-test). Response jitter also was significantly higher for neurons in KO animals at 10 dB (P = 0.002) and 20 dB (P = 0.02) above threshold. At 30 dB above threshold, jitter was higher for units in the KO group (12.7 ± 3.3 ms) compared with the WT group (5.3 ± 0.8 ms), but this difference was not significant (P = 0.17).
Our findings suggest that a subset of neurons in the auditory midbrain of KO mice exhibited altered temporal properties. Deficient timing of responses in the KO group occur despite no differences between groups for CF, threshold, and frequency tuning as measured by Q values. The degraded spike timing of KO units also was present at three different stimulus sound levels, despite no significant differences in spiking rate at those levels compared with the WT group (10 dB above threshold: P = 0.23; 20 dB above threshold: P = 0.69; 30 dB above threshold: P = 0.30, Mann-Whitney U-test).
Forward Masking Effects Are Suppressed in α7-nAChR KO Mice
The presence of altered spike timing for ICC neurons in KO mice led us to further investigate temporal processing by measuring forward masking properties at the level of the midbrain. Forward masking is a phenomenon whereby the presence of a leading sound increases the detection threshold of a trailing sound, often when the two sounds do not overlap in time (Plomp 1964). We chose to examine this type of masking because it is thought to be important for recognizing and understanding speech and it relies in large part on the precise temporal processing of brainstem and midbrain pathways (Frisina 2001; Nelson et al. 2009).
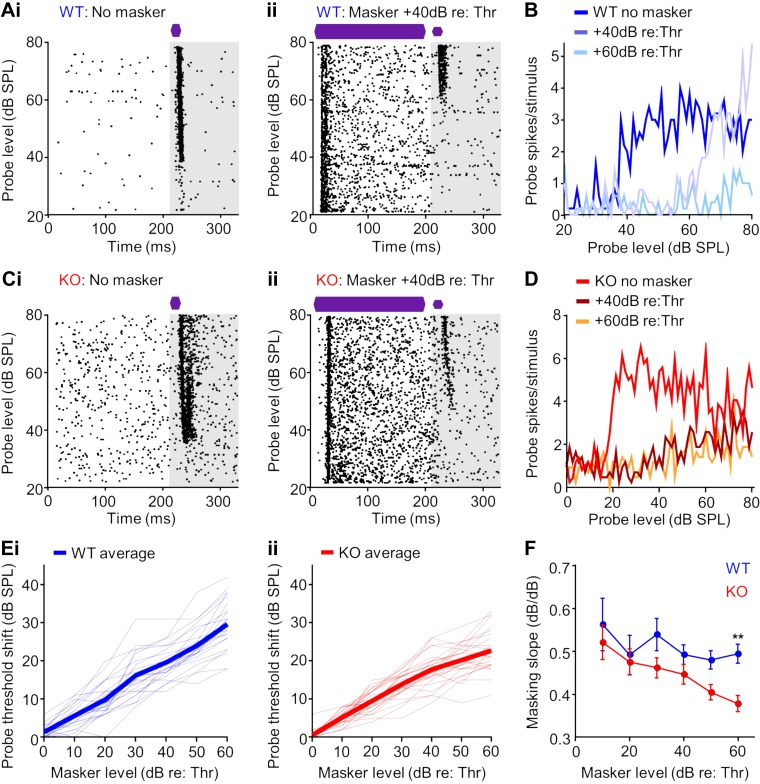
Forward masking effects were assessed by first detecting a unit’s threshold to a short (20 ms) CF pure tone probe sound in the absence of a preceding masker sound (Fig. 4A). We then conducted a series of tests in which the masker was held at a fixed intensity while the probe’s intensity was systematically varied and the resulting evoked spiking threshold to the probe was measured (Fig. 4A). By repeating this paradigm at different masker intensities, we obtained forward masking relationships for ICC single units in WT (Fig. 4, A and B) and KO (Fig. 4, C and D) groups. In the WT example in Fig. 4A, an increase in threshold to the masked probe response compared with no masker was evident, and the masked threshold further increased when the masker level increased from 40 to 60 dB above the unit’s threshold (Fig. 4A). For KO units, a second pattern was also observed where increases in masked probe threshold saturated at high masker levels. For example, the unit shown in Fig. 4, C and D, had no change in probe response when the masker level was changed from 40 to 60 dB above the unit’s threshold. Overall for the WT group, for every 10-dB increase in masker level above the unit’s threshold to the probe alone, the masked probe threshold increased by ~5 dB (Fig. 4Ei). This forward masking slope of 0.5 has been reported previously for midbrain neurons in normal hearing animals (Nelson et al. 2009), as well as for behavioral tests in humans (Plack and Oxenham 1998). For KO mice, units approximated a 0.5 masking slope at low masker levels, but slopes decreased at high masker levels (Fig. 4Eii). There were differences in mean masking slopes between groups at high masker levels, with a significant difference at the highest level tested (+60 dB re threshold: P = 0.0095, Kruskal-Wallis test/Dunn’s multiple comparison’s test; Fig. 4F), suggesting abnormal forward masking properties in the midbrain of α7-KO mice.
Fig. 4.
Growth of forward masking is degraded for neurons in the midbrain of α7-knockout (KO) mice. A: raster plot of a representative wild-type (WT) neuronal response in the inferior colliculus (ICC) to a 20-ms characteristic frequency (CF) probe stimulus presented alone (i) and preceded by 200-ms CF masker tone with a 10-ms interstimulus gap (ii). The analysis window of the probe response to assess forward masking is depicted in gray. B: spiking rate functions determined from the probe analysis window for the no masker condition and for the masker presented at 40 and 60 dB above the threshold from the neuron shown in A. C: raster plots for no masker (i) and masker presented 40 dB above threshold (ii) conditions for a representative KO neuron. D: spiking rate functions for the neuron in C exhibited forward masking suppression of the probe response but to a lesser degree than the WT example shown in A. E: threshold increased as a function of masker level (relative to minimum threshold) for neurons in the ICC of WT (i) and KO (ii) animals. Thin dashed lines depict data from individual neurons. Thick lines represent the average for each group. F: threshold shift (dB) per increase in masker relative to threshold (dB) was consistent across masker levels for WT animals. In contrast, this masking slope decreased at the highest masker levels for neurons in KO animals (masker 60 dB above threshold: P = 0.0095, Kruskal-Wallis test/Dunn’s multiple comparison’s test), suggesting impaired growth of masking. Error bars represent the SE. **P < 0.01.
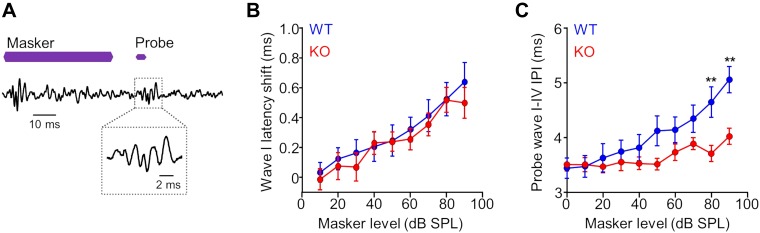
Our single-unit data set contained a subset of KO neurons with properties that deviated greatly from WT units, whereas the remainder of KO units had similar properties as their control counterparts. Because we sampled only a small fraction of neurons in the ICC, we used the ABR to determine whether subpopulations of abnormal single units could substantially impact temporal processing on a more global scale. Therefore, we assessed forward masking effects by measuring masker-induced latency shifts of probe-evoked ABR waves (Fig. 5A).
Fig. 5.
Forward masking effects are reduced in the ABR of α7-knockout (KO) mice at high masker levels. A: forward masking effects were measured as masker-induced shifts in the peak latencies of the auditory brainstem response (ABR) to the probe stimulus (inset). Purple bars represent the 16-kHz tone masker followed by a 16-kHz tone probe separated by a 10-ms silent gap. B: wave I peak latencies to the probe increased with increasing masker level (0–90 dB SPL) for both KO and WT groups, with no difference between groups (P > 0.9 for all masker levels, ANOVA/Bonferroni’s multiple comparisons test). C: the shift in wave IV latency relative to the shift in wave I latency increased with increasing masker level for the WT group. This relationship was reduced for the KO group, particularly at the highest masker levels of 80 and 90 dB (P = 0.007 and 0.002, respectively. For B and C, error bars represent the SE. **P < 0.01.
Forward masking was observed for the probe-evoked wave I, in which the wave latency increased systematically with increasing masker level (0–90 dB SPL) for both KO and WT groups with no difference between groups (P > 0.9 for all masker levels, ANOVA/Bonferroni’s multiple comparisons test; Fig. 5B). To assess masking effects on wave IV latency, we measured the absolute difference in latency between waves I and IV. For WT animals, there was a steady increase in the wave I–IV interpeak latency with increasing masker level, which indicated a growth of masking effect at the level of the midbrain compared the auditory nerve (Fig. 5C). In contrast, the increased interpeak interval with masker level was greatly reduced for KO compared with WT mice, particularly at the highest masker levels of 80 and 90 dB (P = 0.007 and 0.002, respectively, ANOVA/Bonferroni’s multiple comparisons test; Fig. 5C). Thus impaired masking properties were observed at the single-unit level and for ABR signals generated by synchronous activity of thousands of neurons, particularly at high masker levels.
Spike Timing Is Disrupted in Brainstem Nuclei in α7-nAChR KO Animals
For α7-KO animals, temporal abnormalities were observed for centrally, but not for peripherally, mediated auditory processing (Fig. 1D). This finding suggests that neural disruption first occurs either at the level of the midbrain or in brainstem pathways that provide input to the midbrain. We examined temporal properties of single-units in the SPON and VNLL of KO and WT mice to determine if deficits were present in the brainstem. Among the myriad of brainstem nuclei that send inputs to the IC, we focused on the SPON and VNLL for several reasons. These nuclei are the primary targets of octopus cells, the most temporally precise neurons in the auditory system (Felix et al. 2017b), and they are thought to shape temporal response properties in the midbrain via robust synaptic inhibition (Felix et al. 2015; Recio-Spinoso and Joris 2014). In addition, both nuclei have been implicated in increasing forward masking effects at high masker levels in the midbrain in modeling studies (Gai 2016; Salimi et al. 2017). These models are of interest to the present study because we observed decreased masking specifically at high masker levels in the ICC of α7-KO mice (Fig. 4E).
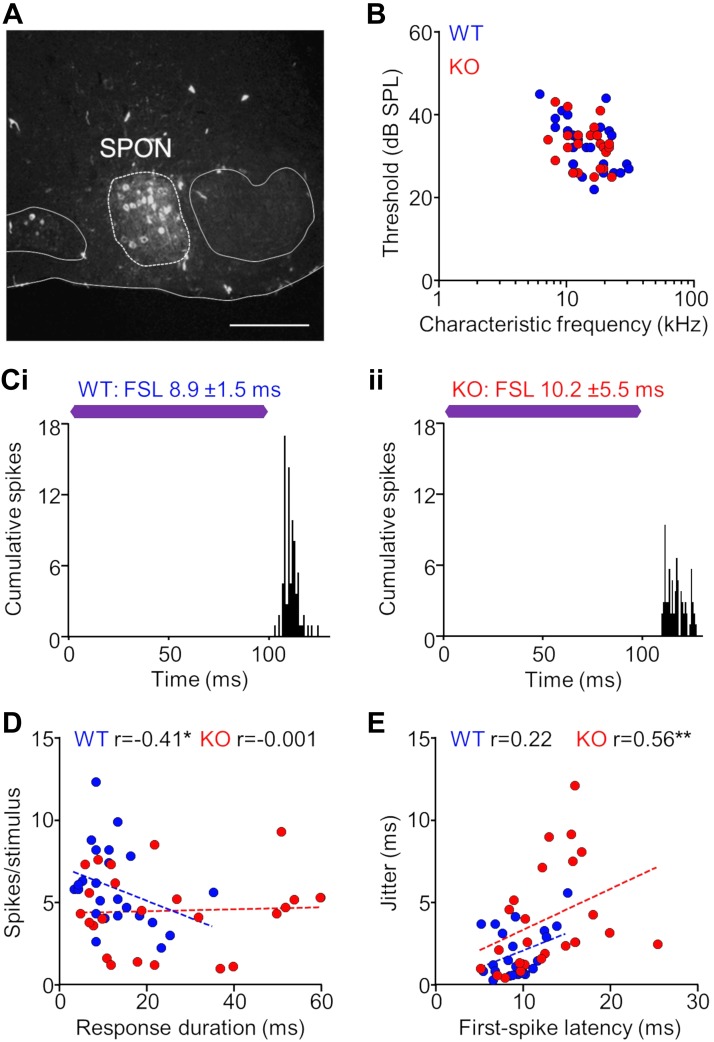
To ensure that we were always recording in the SPON, we marked all recording sites with tracer deposits delivered from the electrode (Fig. 6A). The distribution of characteristic frequencies and minimum thresholds of single-unit responses in the SPON were similar for KO and WT groups (Fig. 6B). Spiking patterns in the SPON for responses in both WT and KO animals exhibited transient spiking evoked by the sound offset (Fig. 6C). We found no difference in spike rate between groups (KO: 4.5 ± 0.5 spikes/stim; WT: 5.9 ± 0.5 spikes/stim; P = 0.073, Mann-Whitney test). However, we did find longer response durations for KO units (24.3 ± 3.7 ms) compared with WT units (12.3 ± 1.6 ms; P = 0.022, Mann-Whitney test). For WT units, transient responses exhibited robust spiking compared with more sustained responses, resulting in a negative correlation between spiking rate and duration (r = −0.41, P = 0.046, Spearman correlation). There was no such relationship for responses in the SPON of KO animals (r = −0.001, P = 0.996; Fig. 6D). Overall, we measured degraded spike timing properties in the SPON of KO mice. Both first-spike latencies and spiking jitter were greater for KO (FSL: 12.5 ± 0.9 ms; jit: 3.9 ± 0.7 ms) compared with WT (FSL: 9.2 ± 0.5; jit: 1.9 ± 0.3 ms) units (FSL: P = 0.008, Mann-Whitney test; jit: P = 0.007). Overall, jitter increased with increasing FSL for SPON neurons in both KO and WT groups (Fig. 6E). This relationship was highly significant for KO (r = 0.56, P = 0.004, Spearman correlation) but was not significant for WT units (r = 0.22, P = 0.31).
Fig. 6.
Neurons in the superior paraolivary nucleus of α7-knockout (KO) mice exhibit impaired temporal properties. A: recording sites in the superior paraolivary nucleus (SPON; dashed line) were marked with Fluororuby tracer deposits delivered from the electrode. The locations of the medial nucleus of the trapezoid body to the left of the SPON and the lateral superior olive to the right are outlined for reference. Scale bar = 150 μm. B: distribution of characteristic frequencies and minimum thresholds for KO and wild-type (WT) single-unit responses in the SPON (n = 24 for both groups). C: representative examples of spiking patterns in the SPON. For responses in both WT (i) and KO (ii) animals, transient spiking was evoked by the sound offset. First-spike latency (FSL) and jitter (FSL SD) were determined to assess temporal properties of each response. Purple bars represent the 100-ms characteristic frequency (CF) tone stimulus presented at 20 dB above threshold. D: transient, short-duration responses exhibited robust spiking compared with more sustained responses for WT neurons, resulting in a negative correlation (*P < 0.05, Spearman correlation). There was no such relationship for responses in the SPON of KO animals. E: jitter increased with increasing FSL for SPON neurons in both KO and WT groups (**P < 0.01, Spearman correlation). A subset of KO responses had highly elevated FSLs and corresponding jitter compared with WT responses, resulting in an overall difference between groups (P = 0.008 for FSL, Mann-Whitney test).
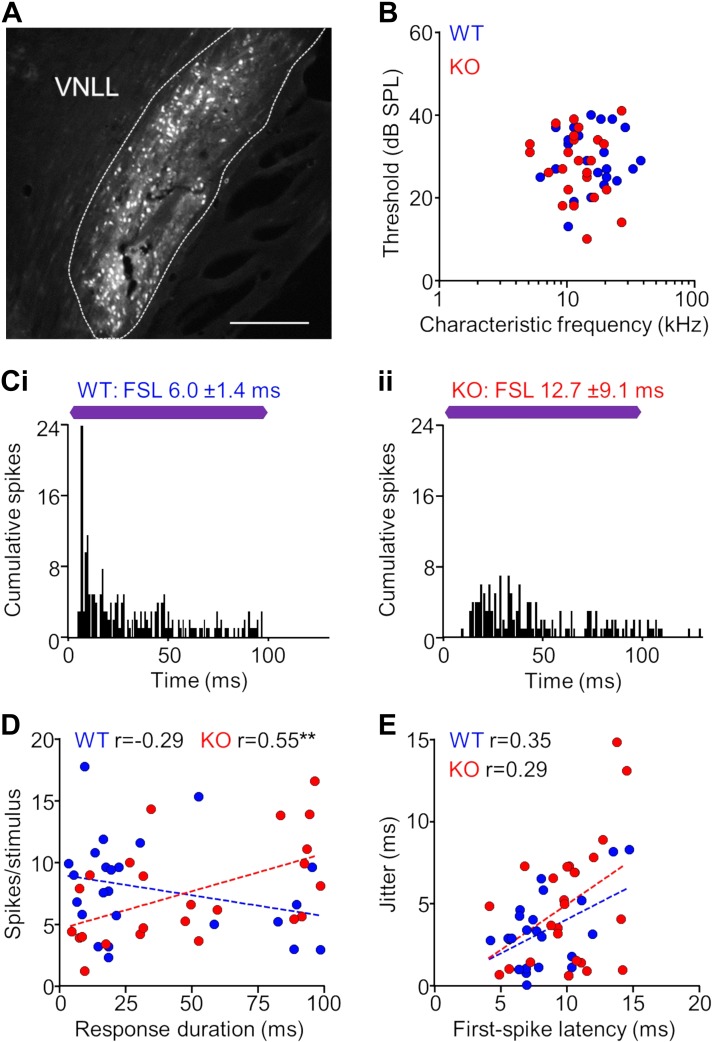
Abnormalities that were observed in the SPON of KO mice were also found in the VNLL. Again, to confirm that we were recording in the VNLL, we marked recording sites with tracer deposits (Fig. 7A). Properties of units recorded in the VNLL of KO and WT mice had a high degree of overlap (Fig. 7B), and both populations exhibited robust spiking evoked by the sound onset, either with or without a weaker sustained response component (Fig. 7C). We found no differences in the spiking rate and duration between KO (spikes: 7.6 ± 0.8; duration: 48.2 ± 7.1 ms) and WT (spikes: 7.9 ± 0.8 spikes/stim; duration: 34.0 ± 6.6 ms) units (spikes: P = 0.77, duration: P = 0.15, Mann-Whitney test). In contrast to the SPON, we found no relationship between response durations and spiking rate for WT neurons (r = −0.29, P = 0.17, Spearman correlation). Conversely, spiking magnitude increased overall with increasing duration for KO neurons in the VNLL (r = 0.55, P = 0.005; Fig. 7D). Although we found no difference in spiking rate between groups, we measured significantly longer first-spike latencies for KO units (9.9 ± 0.6 ms) compared with WT units (8.2 ± 0.5 ms, P = 0.03, Mann-Whitney test). However, there was no significant difference in jitter between groups (KO: 4.9 ± 0.8 ms, WT: 3.3 ± 0.5 ms, P = 0.14). Similar to the SPON, jitter increased with increasing FSL, but this relationship was not significant for KO (r = 0.29, P = 0.17, Spearman correlation) or WT units (r = 0.35, P = 0.09; Fig. 7E). Overall, degraded spike timing was observed for neurons at the level of the brainstem in the SPON and VNLL of α7-KO mice, which indicates that temporal processing deficits are present early in the ascending central auditory pathway.
Fig. 7.
Neurons in the ventral nucleus of the lateral lemniscus of α7-knockout (KO) mice exhibit impaired temporal properties. A: recording sites in the ventral nucleus of the lateral lemniscus (VNLL; dashed line) were marked with Fluororuby tracer deposits delivered from the electrode. Scale bar = 100 μm. B: distribution of characteristic frequencies and minimum thresholds for KO and wild-type (WT) single-unit responses in the VNLL (n = 24 for both groups). C: representative examples of spiking patterns in the VNLL. For responses in both WT (i) and KO (ii) animals, robust spiking was evoked by the sound onset, either with or without a weaker sustained response component. First-spike latency (FSL) and jitter (FSL SD) were determined to assess temporal properties of each response. Purple bars represent the 100-ms characteristic frequency (CF) tone stimulus presented at 20 dB above threshold. D: no relationship was found between response duration and spiking rate for WT neurons (**P < 0.01, Spearman correlation). In contrast, spiking magnitude increased with increasing duration for KO neurons in the VNLL. E: jitter increased with increasing FSL for VNLL neurons in both KO and WT groups (Spearman correlation). Overall, FSL was elevated in the KO group compared with the WT neurons (P = 0.03, Mann-Whitney test).
Additional Measures of Temporal Acuity Are Degraded in α7-nAChR KO Animals
Gap detection.
Resolving short silent gaps between sounds is critical for normal speech perception, and spiking activity on the single-unit level is thought to be a neural correlate of behavioral performance on gap detection tasks (Glasberg and Moore 1989; Snell and Frisina 2000). For instance, the failure of auditory neurons to recognize short gaps in ongoing stimuli may underlie problems with understanding speech in which the listener has difficulty differentiating speech elements that sound similar (Eggermont 2015a). We tested gap detection properties in auditory midbrain and brainstem neurons to further gauge differences in temporal acuity between α7-KO and WT mice.
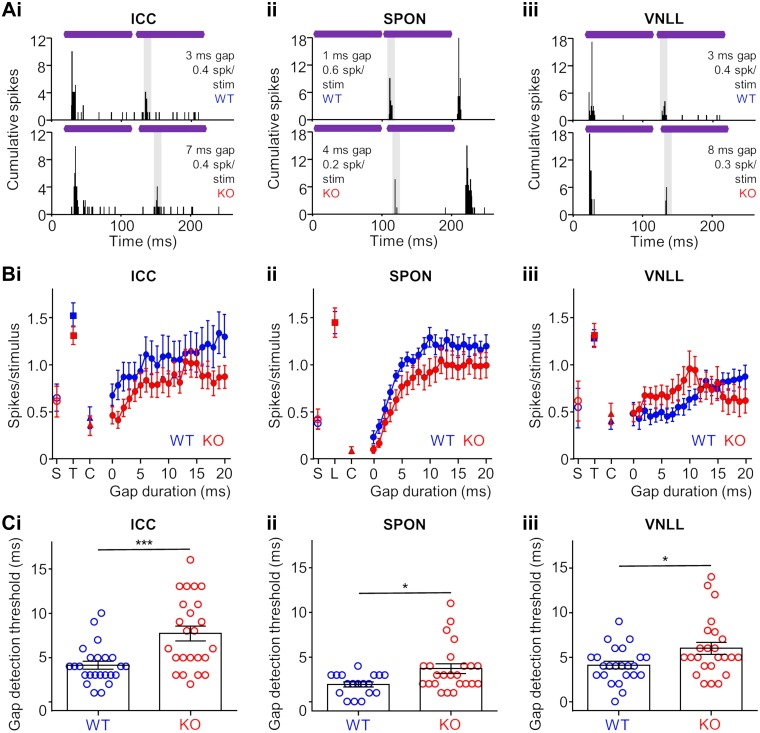
When two identical tones were presented sequentially but with no gap between them, ICC neurons failed to respond to the trailing tone in both KO and WT animals. The presence of distinct spiking evoked by both the leading and trailing tones signaled detection of a gap between stimuli. The majority of neurons in the ICC of WT mice could detect gaps that were 5 ms or shorter, whereas most neurons from KO animals had gap detection thresholds greater than 5 ms. Representative examples from WT and KO mice are shown in Fig. 8Ai, top and bottom, respectively. Overall, mean spiking rates were higher for units in WT compared with KO mice at all gap durations tested, but differences were significant for only the low gap durations (P = 0.0001–0.025 for 2–8 ms, except P = 0.17 for 4 ms gap, Kruskal-Wallis test/Dunn’s multiple comparisons test; Fig. 8Bi). For short gaps, the reduced spiking rates for units in KO mice reflected an absence or severely degraded response to the trailing tone and an inability to robustly detect a gap. For each unit, we measured the shortest gap that was reliably detected, termed the gap detection threshold (GDT). In the ICC, neurons in KO mice collectively had a significantly higher GDT (7.7 ± 0.8 ms) compared with units in WT animals (4.1 ± 0.4 ms; P = 0.0008, Mann-Whitney test), suggesting impaired temporal acuity for the KO group (Fig. 8Ci).
Fig. 8.
Temporal acuity measured by gap detection is impaired in the auditory brainstem and midbrain of α7-knockout (KO) mice. A: representative wild-type (WT) and KO responses in the inferior colliculus (ICC; i), superior paraolivary nucleus (SPON; ii), and ventral nucleus of the lateral lemniscus (VNLL; iii) that recognized short gaps between sound stimuli. Purple bars represent identical 100-ms characteristic frequency (CF) tones (respective leading and trailing tones) presented at 20 dB above threshold with a variable silent gap between stimuli. For each example, the presence of a second bout of evoked spiking indicates that the gap was detected and signals two distinct sounds. The shaded area in each plot represents the analysis window used for analyses in B and C (see materials and methods). B: spiking rates in the gap analysis window for various gap durations for ICC (i), SPON (ii), and VNLL (iii) WT and KO neurons. The spontaneous spiking rate in the absence of sound stimulation (S), as well as spiking to either the trailing (T) or leading (L) sound presented alone. Spiking to the continuous sound (C) used to assess spiking in the analysis window is also shown for each group (see materials and methods). C: threshold for the minimum gap duration detected by neurons in the ICC (i), SPON (ii), and VNLL (iii). For each nucleus, gap detection thresholds were higher for KO responses (P = 0.001, 0.018, 0.036 for the ICC, SPON, and VNLL, respectively, Mann-Whitney test). *P < 0.05; ***P = 0.001.
Neurons in the brainstem of KO mice also exhibited deficits in gap detection ability. For the SPON, we did not measure significant differences in spiking rate at any of gap durations tested (Fig. 8Aii), but we observed an overall increase in GDT for units in the KO group (3.7 ± 0.5 ms) compared with those in the WT control group (1.9 ± 0.3 ms; P = 0.02, Mann-Whitney test; Fig. 8Cii). Responses for VNLL neurons produced similar results as those for the SPON (Fig. 8Aiii). We found no significant differences in spiking rate between units in KO and WT mice to any gap durations tested (Fig. 8Biii), but KO units had significantly elevated GDTs (6.0 ± 0.7 ms) compared with the WT group (4.2 ± 0.5 ms; P = 0.4, Mann-Whitney test; Fig. 8Ciii). In each nucleus, neurons in KO mice had degraded gap detection abilities compared with units in the WT group.
Amplitude modulation.
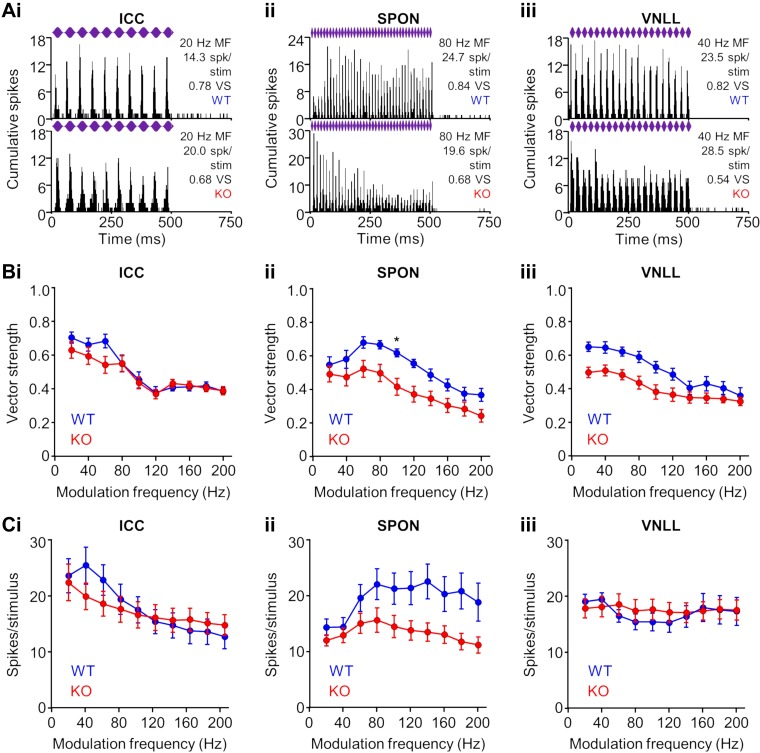
Natural sounds contain periodic fluctuations in amplitude. For speech, amplitude modulations occur at several frequency ranges, some of which are low (<200 Hz) and require coarse temporal processing (Joris et al. 2004). At the level of the midbrain, neurons respond with highly synchronous activity to low- and midmodulation frequency stimuli (Krishna and Semple 2000). We recorded responses to sinusoidally amplitude-modulated (SAM) tones in the midbrain and brainstem of KO and WT mice. Neurons in the ICC of both groups responded to SAM tones with high fidelity, as measured by the vector strength, where a vector strength of one indicates perfect synchronization of spiking to each cycle of modulation (Fig. 9Ai). Mean vector strengths (Fig. 9Bi) and spiking rates (Fig. 9Ci) were lower for low modulation frequency SAM tones for units in KO compared with WT mice, but these differences were not statistically significant (P > 0.1 for each case, Kruskal-Wallis test/Dunn’s multiple comparisons test).
Fig. 9.
Temporal acuity to envelope modulation responses of neurons in α7-knockout (KO) mice. A: representative wild-type (WT) and KO responses in the inferior colliculus (ICC; i), superior paraolivary nucleus (SPON; ii), and ventral nucleus of the lateral lemniscus (VNLL; iii) to sinusoidal amplitude modulation (SAM) of the sound envelope. Purple bars represent 500-ms characteristic frequency (CF) tones presented at 20 dB above threshold with 100% modulation depth at various modulation frequencies (MF). Response fidelity to individual cycles of modulation was determined by the vector strength (VS; see materials and methods). B: lower vector strengths for KO neurons in the ICC (i), SPON (ii), and VNLL (iii), particularly at low modulation frequencies, were not significant in most cases (except SPON 100 Hz: P = 0.03, Kruskal-Wallis test/Dunn’s multiple comparisons test). C: no difference was found between spiking rates of WT and KO neurons in the ICC (i) and VNLL (ii). Spiking rates were consistently lower to most modulation frequencies for KO neurons in the SPON, but the differences did not reach statistical significance (P > 0.1 for each condition, Kruskal-Wallis test/Dunn’s multiple comparisons test). *P < 0.05.
Like the ICC, differences in the temporal acuity of brainstem neurons to envelope modulation were largely absent among KO and WT groups (Fig. 9, Aii and Aiii). Lower vector strengths were measured for KO neurons in the SPON and VNLL, particularly at low-modulation frequencies but were not significant in most cases (except SPON 100 Hz: P = 0.03, Kruskal-Wallis test/Dunn’s multiple comparisons test; Fig. 9, Bii and Biii). In addition, spiking rates were consistently lower to most modulation frequencies for KO neurons in the SPON, but the differences did not reach statistical significance (P > 0.05 for each condition, Kruskal-Wallis test/Dunn’s multiple comparisons test; Fig. 9Cii). Furthermore, no differences in spiking rates were measured between groups for VNLL units (Fig. 9Ciii). Generally, we did not find clear impairments in responses to SAM stimuli for KO mice, which was in contrast to other measures of temporal acuity that were tested.
DISCUSSION
Our results demonstrate that α7-nAChR KO mice have temporal processing deficits in the auditory brainstem and midbrain. These deficits are present despite normal audiometric thresholds and normal timing of auditory nerve activity. Taken together, the abnormalities in α7-KO mice are similar to key features of central auditory processing disorders in humans, which can affect a wide range of individuals.
Temporal Deficits in α7-KO Mice Arise Early in the Central Auditory Pathway
Auditory disorders involving the processing of complex sounds such as speech are often viewed as higher order deficits in the cortex. However, cortical processing of speech relies on the extraction of critical temporal information that occurs subcortically (Frisina 2001). The central nucleus of the ICC in the midbrain occupies a critical position in the central auditory pathway. The ICC integrates inputs from nearly all afferent parallel pathways from the brainstem, including the extraction of temporal information from specialized neuronal circuits. Our results indicate that ICC neurons in α7-KO mice had impaired spike timing. In addition, we found deficits for masking properties, gap detection thresholds, and the fidelity of responses to modulation of the sound envelope. We tested these measures because they are neural correlates of behavioral tests of temporal acuity that are impaired in humans with speech processing and language problems (Eggermont 2015b).
Cholinergic signaling, particularly during development, is important for establishing precise temporal properties of auditory neurons (Baumann and Koch 2017). In other neural systems apart from audition, the α7-nAChR is critical for establishing glutamatergic synapses during development (Halff et al. 2014; Lin et al. 2010, 2014; Lozada et al. 2012; Molas and Dierssen 2014). Faithful synaptic connections are especially important in the auditory system, where timing differences of approximately several milliseconds can have clear perceptual consequences (Eggermont 2001). In normal hearing WT animals, the ICC exhibits high α7-nAChR expression compared with most brain regions, including other auditory nuclei, especially during development (Happe and Morley 2004; Morley and Happe 2000; Morley et al. 1983). Therefore, it is not surprising that ICC neurons in α7-KO mice had changes in temporal properties. Studies of neural activity in humans suggest that the midbrain may be a site of origin for auditory deficits associated with listening problems (Johnson et al. 2008). However, our findings indicate that problems might also occur earlier in the brainstem.
In addition to the ICC, we examined properties of the SPON and VNLL in α7-KO and WT groups. These prominent auditory brainstem nuclei are of interest to the present study for several reasons. Like the ICC, the SPON and VNLL exhibit higher levels of α7-nAChR expression relative to other auditory nuclei (Baumann and Koch 2017; Felix and Magnusson 2016; Happe and Morley 2004). The formation of strong, reliable synapses early in SPON and VNLL development occurs during a period that overlaps with peak α7-expression (Happe and Morley 2004). Thus cholinergic input likely aids in establishing robust connections. Consistent with this notion, a recent study demonstrated that disruption of α7-nAChR signaling during VNLL development leads to delays of the maturation of temporal processing (Baumann and Koch 2017). We found that α7-receptor KO mice contained SPON and VNLL neurons that exhibited degraded temporal processing. In normal hearing mice, there is evidence that precisely timed synaptic inhibition from the SPON shapes temporal properties of neurons in the ICC (Felix et al. 2015). Therefore, the midbrain deficits in KO mice that we observed in the present study may be inherited partly from brainstem inputs. It is likely that changes in the ICC of KO animal are likely a combination of degraded properties inherited from the brainstem and of response features created de novo at the level of the midbrain.
KO Mice Exhibit Features Similar to Human Auditory Processing Disorders
The behavioral manifestations of auditory processing disorders, particularly among children, have been an area of growing interest (Bellis and Bellis 2015). The urgency to better understand such disorders stems from the realization that difficulty understanding speech even when sounds are loud enough to hear can lead to problems with listening and learning and can hinder normal social interaction (Banai et al. 2007, 2009). The fact that listening problems are commonly diagnosed in children suggests that abnormal development of neural pathways is a potential contributor to auditory deficits, perhaps in combination with cognitive and attentional factors (Billiet and Bellis 2011). In addition, studies in children and adults have shown that degraded timing of sound-evoked midbrain activity is associated with auditory processing disorders, which indicates a distinct precortical auditory component (Johnson et al. 2005; Mehraei et al. 2016).
Although we have clues for when and where deficits associated with auditory processing disorders may arise, the mechanisms of dysfunction remain largely unknown. Our α7-KO animals had impaired cholinergic signaling from birth and showed signs of temporal processing deficits. These features are similar to cases in which individuals with genetically based reduction of α7-receptor expression during development are diagnosed with neurodevelopmental disorders and experience difficulty processing temporal information and have problems with speech and language (Brandwein et al. 2015). Because precise temporal processing is critical for speech perception and strong auditory synapses are needed for precise signaling, any disruption of the formation and strengthening of synapses during development can have negative consequences for speech perception. The current study provides one example of cholinergic deficiency during development that can lead to delays in midbrain activity that is in line with studies of humans with auditory processing disorders (Johnson et al. 2005; Mehraei et al. 2016). Using animal models that reproduce features of known human genetic conditions can provide broader insight into the potential causes of complex disorder phenotypes.
Potential Role of the α7-nAChR in Neurodevelopmental Disorders
Neurodevelopmental disorders, including autism spectrum disorders (ASDs), schizophrenia, and epilepsy exhibit complex phenotypes, which include abnormal auditory processing. Shared features among these disorders provide clues for dissecting the underlying mechanisms of dysfunction and for identifying potential therapeutic targets. For instance, ASD is characterized by a heterogeneous and highly variable phenotype that includes a delay in language acquisition and an inability to adequately understand attended speech in noisy listening conditions (Alcántara et al. 2004). In real-life social situations, this type of auditory difficulty may contribute to impaired verbal and nonverbal communication (Lin et al. 2014). Deficits in the discrimination of complex sounds, such as speech syllables embedded in background noise, are evident in adults with ASD compared with control groups (Groen et al. 2009). This observation is notable in light of reports of enhanced processing of simple acoustic stimuli associated with ASD (Mottron et al. 2006; Samson et al. 2006). Behavioral evidence suggests that ASD-related problems with complex sound discrimination may be due in part to temporal processing deficits (Groen et al. 2009; Kwakye et al. 2011).
The α7-KO mouse model does not exhibit the severe phenotype usually found in the human15q.13.3 microdeletion syndrome. In humans, CHRNA7 deletions are usually associated with a partial deletion of the adjacent gene, OTUD7A (OTU deubiquitinase 7A) (Uddin et al. 2018; Yin et al. 2018). Consistent with those findings, the Otud7A mouse KO is more severely impaired than the α7-KO (Uddin et al. 2018; Yin et al. 2018), suggesting that CHRNA7 has more selective roles in developmental disorders. The underlying neural changes associated with auditory processing impairment in the Chrna7 deletion are not known, but structural changes in dendritic spines in hippocampus and cortical regions has been reported (Morley and Mervis 2013). Similar spine change s are also found in the Otud7A mouse KO (Uddin et al. 2018).
Mouse models with the hemizygous and homozygous deletion of the orthologous region for the 15q.13.3 microdeletion (Df[h15q13]/+) recapitulate the cardinal features of the microdeletion syndrome (Fejgin et al. 2014). CHRNA7 deletion alone cannot account for many of these features, but it is clearly a relevant gene. For example, the Df[h15q13]/+ mouse exhibits a predominant a hyperactivity brain pattern that is normalized by treatment with the α7-nAChR allosteric modulator LU AF58801 (Gass et al. 2016). Also, transduction of α7 DNA into primary cultures of cortical neurons from Df[h15q13]/+ embryonic brains rescues dendritic spine abnormalities. These studies support the conclusion that CHRNA7 has an important function in human brain development and function.
Changes in the temporal properties of neurons in KO mice are likely driven by deficient cholinergic signaling during development due to deletion of the Chrna7 gene. In humans, reduced expression of the CHRNA7 gene can lead to auditory processing and language difficulties. The use of animal models of genetically based human disorders, such as the α7-KO mouse, represents a powerful tool for understanding the causes and mechanisms of neural dysfunction.
GRANTS
R. A. Felix 2nd was supported by an Emerging Research Grant from the Hearing Health Foundation, C. V. Portfors was supported by National Institute of Deafness and Other Communications Disorders Grant R01-DC-013102, and B. J. Morley was supported by the Nebraska Tobacco Settlement Biomedical Research Foundation.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
R.A.F., B.J.M., and C.V.P. conceived and designed research; R.A.F., V.A.C., and D.M.N. performed experiments; R.A.F. and V.A.C. analyzed data; R.A.F. and C.V.P. interpreted results of experiments; R.A.F. prepared figures; R.A.F. drafted manuscript; R.A.F., B.J.M., and C.V.P. edited and revised manuscript; R.A.F., B.J.M., and C.V.P. approved final version of manuscript.
REFERENCES
- Alcántara JI, Weisblatt EJ, Moore BC, Bolton PF. Speech-in-noise perception in high-functioning individuals with autism or Asperger’s syndrome. J Child Psychol Psychiatry 45: 1107–1114, 2004. doi: 10.1111/j.1469-7610.2004.t01-1-00303.x. [DOI] [PubMed] [Google Scholar]
- Bacchelli E, Battaglia A, Cameli C, Lomartire S, Tancredi R, Thomson S, Sutcliffe JS, Maestrini E. Analysis of CHRNA7 rare variants in autism spectrum disorder susceptibility. Am J Med Genet A 167: 715–723, 2015. doi: 10.1002/ajmg.a.36847. [DOI] [PubMed] [Google Scholar]
- Bailey T. Auditory pathways and processes: implications for neuropsychological assessment and diagnosis of children and adolescents. Child Neuropsychol 16: 521–548, 2010. doi: 10.1080/09297041003783310. [DOI] [PubMed] [Google Scholar]
- Banai K, Abrams D, Kraus N. Sensory-based learning disability: Insights from brainstem processing of speech sounds. Int J Audiol 46: 524–532, 2007. doi: 10.1080/14992020701383035. [DOI] [PubMed] [Google Scholar]
- Banai K, Hornickel J, Skoe E, Nicol T, Zecker S, Kraus N. Reading and subcortical auditory function. Cereb Cortex 19: 2699–2707, 2009. doi: 10.1093/cercor/bhp024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann VJ, Koch U. Perinatal nicotine exposure impairs the maturation of glutamatergic inputs in the auditory brainstem. J Physiol 595: 3573–3590, 2017. doi: 10.1113/JP274059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beitchman JH, Nair R, Clegg M, Patel PG, Ferguson B, Pressman E, Smith A. Prevalence of speech and language disorders in 5-year-old kindergarten children in the Ottawa-Carleton region. J Speech Hear Disord 51: 98–110, 1986. doi: 10.1044/jshd.5102.98. [DOI] [PubMed] [Google Scholar]
- Bellis TJ, Bellis JD. Central auditory processing disorders in children and adults. Handb Clin Neurol 129: 537–556, 2015. doi: 10.1016/B978-0-444-62630-1.00030-5. [DOI] [PubMed] [Google Scholar]
- Billiet CR, Bellis TJ. The relationship between brainstem temporal processing and performance on tests of central auditory function in children with reading disorders. J Speech Lang Hear Res 54: 228–242, 2011. doi: 10.1044/1092-4388(2010/09-0239). [DOI] [PubMed] [Google Scholar]
- Brandwein AB, Foxe JJ, Butler JS, Frey HP, Bates JC, Shulman LH, Molholm S. Neurophysiological indices of atypical auditory processing and multisensory integration are associated with symptom severity in autism. J Autism Dev Disord 45: 230–244, 2015. doi: 10.1007/s10803-014-2212-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Agostino RB, Stephens MA. Goodness-of-Fit Techniques. New York: Dekker, 1986. [Google Scholar]
- Deutsch SI, Urbano MR, Burket JA, Herndon AL, Winebarger EE. Pharmacotherapeutic implications of the association between genomic instability at chromosome 15q13.3 and autism spectrum disorders. Clin Neuropharmacol 34: 203–205, 2011. doi: 10.1097/WNF.0b013e31823a1247. [DOI] [PubMed] [Google Scholar]
- Deutsch SI, Urbano MR, Neumann SA, Burket JA, Katz E. Cholinergic abnormalities in autism: is there a rationale for selective nicotinic agonist interventions? Clin Neuropharmacol 33: 114–120, 2010. doi: 10.1097/WNF.0b013e3181d6f7ad. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Between sound and perception: reviewing the search for a neural code. Hear Res 157: 1–42, 2001. doi: 10.1016/S0378-5955(01)00259-3. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Animal models of auditory temporal processing. Int J Psychophysiol 95: 202–215, 2015a. doi: 10.1016/j.ijpsycho.2014.03.011. [DOI] [PubMed] [Google Scholar]
- Eggermont JJ. Auditory Temporal Processing and Its Disorders. Oxford, UK: Oxford University Press, 2015b. [Google Scholar]
- Fejgin K, Nielsen J, Birknow MR, Bastlund JF, Nielsen V, Lauridsen JB, Stefansson H, Steinberg S, Sorensen HB, Mortensen TE, Larsen PH, Klewe IV, Rasmussen SV, Stefansson K, Werge TM, Kallunki P, Christensen KV, Didriksen M. A mouse model that recapitulates cardinal features of the 15q13.3 microdeletion syndrome including schizophrenia- and epilepsy-related alterations. Biol Psychiatry 76: 128–137, 2014. doi: 10.1016/j.biopsych.2013.08.014. [DOI] [PubMed] [Google Scholar]
- Felix RA 2nd, Elde CJ, Nevue AA, Portfors CV. Serotonin modulates response properties of neurons in the dorsal cochlear nucleus of the mouse. Hear Res 344: 13–23, 2017a. doi: 10.1016/j.heares.2016.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Gourévitch B, Portfors CV. Subcortical pathways: Towards a better understanding of auditory disorders. Hear Res 362: 48–60, 2018. doi: 10.1016/j.heares.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Kadner A, Berrebi AS. Effects of ketamine on response properties of neurons in the superior paraolivary nucleus of the mouse. Neuroscience 201: 307–319, 2012. doi: 10.1016/j.neuroscience.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Magnusson AK. Development of excitatory synaptic transmission to the superior paraolivary and lateral superior olivary nuclei optimizes differential decoding strategies. Neuroscience 334: 1–12, 2016. doi: 10.1016/j.neuroscience.2016.07.039. [DOI] [PubMed] [Google Scholar]
- Felix RA 2nd, Magnusson AK, Berrebi AS. The superior paraolivary nucleus shapes temporal response properties of neurons in the inferior colliculus. Brain Struct Funct 220: 2639–2652, 2015. doi: 10.1007/s00429-014-0815-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix RA 2nd, Gourévitch B, Gómez-Álvarez M, Leijon SC, Saldaña E, Magnusson AK. Octopus cells in the posteroventral cochlear nucleus provide the main excitatory input to the superior paraolivary nucleus. Front Neural Circuits 11: 37, 2017b. doi: 10.3389/fncir.2017.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman GH, Halton JH. Note on an exact treatment of contingency, goodness of fit and other problems of significance. Biometrika 38: 141–149, 1951. doi: 10.1093/biomet/38.1-2.141. [DOI] [PubMed] [Google Scholar]
- Frisina RD. Subcortical neural coding mechanisms for auditory temporal processing. Hear Res 158: 1–27, 2001. doi: 10.1016/S0378-5955(01)00296-9. [DOI] [PubMed] [Google Scholar]
- Gai Y. ON and OFF inhibition as mechanisms for forward masking in the inferior colliculus: a modeling study. J Neurophysiol 115: 2485–2500, 2016. doi: 10.1152/jn.00892.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gass N, Weber-Fahr W, Sartorius A, Becker R, Didriksen M, Stensbøl TB, Bastlund JF, Meyer-Lindenberg A, Schwarz AJ. An acetylcholine alpha7 positive allosteric modulator rescues a schizophrenia-associated brain endophenotype in the 15q13.3 microdeletion, encompassing CHRNA7. Eur Neuropsychopharmacol 26: 1150–1160, 2016. doi: 10.1016/j.euroneuro.2016.03.013. [DOI] [PubMed] [Google Scholar]
- Gialluisi A, Visconti A, Willcutt EG, Smith SD, Pennington BF, Falchi M, DeFries JC, Olson RK, Francks C, Fisher SE. Investigating the effects of copy number variants on reading and language performance. J Neurodev Disord 15: 8–17, 2016. doi: 10.1186/s11689-016-9147-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillentine MA, Berry LN, Goin-Kochel RP, Ali MA, Ge J, Guffey D, Rosenfeld JA, Hannig V, Bader P, Proud M, Shinawi M, Graham BH, Lin A, Lalani SR, Reynolds J, Chen M, Grebe T, Minard CG, Stankiewicz P, Beaudet AL, Schaaf CP. The cognitive and behavioral phenotypes of individuals with CHRNA7 duplications. J Autism Dev Disord 47: 549–562, 2017. doi: 10.1007/s10803-016-2961-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillentine MA, Schaaf CP. The human clinical phenotypes of altered CHRNA7 copy number. Biochem Pharmacol 97: 352–362, 2015. doi: 10.1016/j.bcp.2015.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasberg BR, Moore BC. Psychoacoustic abilities of subjects with unilateral and bilateral cochlear hearing impairments and their relationship to the ability to understand speech. Scand Audiol Suppl 32: 1–25, 1989. [PubMed] [Google Scholar]
- Groen WB, van Orsouw L, Huurne N, Swinkels S, van der Gaag RJ, Buitelaar JK, Zwiers MP. Intact spectral but abnormal temporal processing of auditory stimuli in autism. J Autism Dev Disord 39: 742–750, 2009. doi: 10.1007/s10803-008-0682-3. [DOI] [PubMed] [Google Scholar]
- Halff AW, Gómez-Varela D, John D, Berg DK. A novel mechanism for nicotinic potentiation of glutamatergic synapses. J Neurosci 34: 2051–2064, 2014. doi: 10.1523/JNEUROSCI.2795-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Happe HK, Morley BJ. Nicotinic acetylcholine receptors in rat cochlear nucleus: [125I]-α-bungarotoxin receptor autoradiography and in situ hybridization of α 7 nAChR subunit mRNA. J Comp Neurol 397: 163–180, 1998. doi:. [DOI] [PubMed] [Google Scholar]
- Happe HK, Morley BJ. Distribution and postnatal development of α 7 nicotinic acetylcholine receptors in the rodent lower auditory brainstem. Brain Res Dev Brain Res 153: 29–37, 2004. doi: 10.1016/j.devbrainres.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Hashimoto K. Targeting of α7 nicotinic acetylcholine receptors in the treatment of schizophrenia and the use of auditory sensory gating as a translational biomarker. Curr Pharm Des 21: 3797–3806, 2015. doi: 10.2174/1381612821666150605111345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoppman-Chaney N, Wain K, Seger PR, Superneau DW, Hodge JC. Identification of single gene deletions at 15q13.3: further evidence that CHRNA7 causes the 15q13.3 microdeletion syndrome phenotype. Clin Genet 83: 345–351, 2013. doi: 10.1111/j.1399-0004.2012.01925.x. [DOI] [PubMed] [Google Scholar]
- Johnson KL, Nicol T, Zecker SG, Kraus N. Developmental plasticity in the human auditory brainstem. J Neurosci 28: 4000–4007, 2008. doi: 10.1523/JNEUROSCI.0012-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KL, Nicol TG, Kraus N. Brain stem response to speech: a biological marker of auditory processing. Ear Hear 26: 424–434, 2005. doi: 10.1097/01.aud.0000179687.71662.6e. [DOI] [PubMed] [Google Scholar]
- Joris PX, Schreiner CE, Rees A. Neural processing of amplitude-modulated sounds. Physiol Rev 84: 541–577, 2004. doi: 10.1152/physrev.00029.2003. [DOI] [PubMed] [Google Scholar]
- Krishna BS, Semple MN. Auditory temporal processing: responses to sinusoidally amplitude-modulated tones in the inferior colliculus. J Neurophysiol 84: 255–273, 2000. doi: 10.1152/jn.2000.84.1.255. [DOI] [PubMed] [Google Scholar]
- Kwakye LD, Foss-Feig JH, Cascio CJ, Stone WL, Wallace MT. Altered auditory and multisensory temporal processing in autism spectrum disorders. Front Integr Nuerosci 4: 129, 2011. doi: 10.3389/fnint.2010.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lepichon JB, Bittel DC, Graf WD, Yu S. A 15q13.3 homozygous microdeletion associated with a severe neurodevelopmental disorder suggests putative functions of the TRPM1, CHRNA7, and other homozygously deleted genes. Am J Med Genet A 152A: 1300–1304, 2010. doi: 10.1002/ajmg.a.33374. [DOI] [PubMed] [Google Scholar]
- Liao J, DeWard SJ, Madan-Khetarpal S, Surti U, Hu J. A small homozygous microdeletion of 15q13.3 including the CHRNA7 gene in a girl with a spectrum of severe neurodevelopmental features. Am J Med Genet A 155A: 2795–2800, 2011. doi: 10.1002/ajmg.a.34237. [DOI] [PubMed] [Google Scholar]
- Lin H, Hsu FC, Baumann BH, Coulter DA, Lynch DR. Cortical synaptic NMDA receptor deficits in α7 nicotinic acetylcholine receptor gene deletion models: implications for neuropsychiatric diseases. Neurobiol Dis 63: 129–140, 2014. doi: 10.1016/j.nbd.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Vicini S, Hsu FC, Doshi S, Takano H, Coulter DA, Lynch DR. Axonal α7 nicotinic ACh receptors modulate presynaptic NMDA receptor expression and structural plasticity of glutamatergic presynaptic boutons. Proc Natl Acad Sci USA 107: 16661–16666, 2010. doi: 10.1073/pnas.1007397107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowther C, Costain G, Stavropoulos DJ, Melvin R, Silversides CK, Andrade DM, So J, Faghfoury H, Lionel AC, Marshall CR, Scherer SW, Bassett AS. Delineating the 15q13.3 microdeletion phenotype: a case series and comprehensive review of the literature. Genet Med 17: 149–157, 2015. doi: 10.1038/gim.2014.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozada AF, Wang X, Gounko NV, Massey KA, Duan J, Liu Z, Berg DK. Glutamatergic synapse formation is promoted by α7-containing nicotinic acetylcholine receptors. J Neurosci 32: 7651–7661, 2012. doi: 10.1523/JNEUROSCI.6246-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markand ON. Brainstem auditory evoked potentials. J Clin Neurophysiol 11: 319–342, 1994. doi: 10.1097/00004691-199405000-00004. [DOI] [PubMed] [Google Scholar]
- Mehraei G, Hickox AE, Bharadwaj HM, Goldberg H, Verhulst S, Liberman MC, Shinn-Cunningham BG. Auditory brainstem response latency in noise as a marker of cochlear synaptopathy. J Neurosci 36: 3755–3764, 2016. doi: 10.1523/JNEUROSCI.4460-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller DT, Shen Y, Weiss LA, Korn J, Anselm I, Bridgemohan C, Cox GF, Dickinson H, Gentile J, Harris DJ, Hegde V, Hundley R, Khwaja O, Kothare S, Luedke C, Nasir R, Poduri A, Prasad K, Raffalli P, Reinhard A, Smith SE, Sobeih MM, Soul JS, Stoler J, Takeoka M, Tan WH, Thakuria J, Wolff R, Yusupov R, Gusella JF, Daly MJ, Wu BL. Microdeletion/duplication at 15q13.2q13.3 among individuals with features of autism and other neuropsychiatric disorders. J Med Genet 46: 242–248, 2009. doi: 10.1136/jmg.2008.059907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molas S, Dierssen M. The role of nicotinic receptors in shaping and functioning of the glutamatergic system: a window into cognitive pathology. Neurosci Biobehav Rev 46: 315–325, 2014. doi: 10.1016/j.neubiorev.2014.05.012. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Farley GR, Javel E. Nicotinic acetylcholine receptors in mammalian brain. Trends Pharmacol Sci 4: 225–227, 1983. doi: 10.1016/0165-6147(83)90373-5. [DOI] [Google Scholar]
- Morley BJ, Felix RA. A time to listen: perinatal smoking affects the development of temporal sound processing. J Physiol 595: 3241–3242, 2017. doi: 10.1113/JP274097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley BJ, Happe HK. Cholinergic receptors: dual roles in transduction and plasticity. Hear Res 147: 104–112, 2000. doi: 10.1016/S0378-5955(00)00124-6. [DOI] [PubMed] [Google Scholar]
- Morley BJ, Mervis RF. Dendritic spine alterations in the hippocampus and parietal cortex of alpha7 nicotinic acetylcholine receptor knockout mice. Neuroscience 233: 54–63, 2013. doi: 10.1016/j.neuroscience.2012.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mottron L, Dawson M, Soulières I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord 36: 27–43, 2006. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- Muniak MA, Mayko ZM, Ryugo DK, Portfors CV. Preparation of an awake mouse for recording neural responses and injecting tracers. J Vis Exp 64: 3755, 2012. doi: 10.3791/3755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PC, Smith ZM, Young ED. Wide-dynamic-range forward suppression in marmoset inferior colliculus neurons is generated centrally and accounts for perceptual masking. J Neurosci 29: 2553–2562, 2009. doi: 10.1523/JNEUROSCI.5359-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olincy A, Blakeley-Smith A, Johnson L, Kem WR, Freedman R. Brief report: initial trial of alpha7-nicotinic receptor stimulation in two adult patients with autism spectrum disorder. J Autism Dev Disord 46: 3812–3817, 2016. doi: 10.1007/s10803-016-2890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The Mouse Brain in Stereotaxic Coordinates. New York: Elsevier, 2001. [Google Scholar]
- Pettigrew KA, Reeves E, Leavett R, Hayiou-Thomas ME, Sharma A, Simpson NH, Martinelli A, Thompson P, Hulme C, Snowling MJ, Newbury DF, Paracchini S. Copy number variation screen identifies a rare de novo deletion at chromosome 15q13.1–13.3 in a child with language impairment. PLoS One 10: e0134997, 2015. doi: 10.1371/journal.pone.0134997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plack CJ, Oxenham AJ. Basilar-membrane nonlinearity and the growth of forward masking. J Acoust Soc Am 103: 1598–1608, 1998. doi: 10.1121/1.421294. [DOI] [PubMed] [Google Scholar]
- Plomp R. Rate of decay of auditory sensation. J Acoust Soc Am 36: 277–282, 1964. doi: 10.1121/1.1918946. [DOI] [Google Scholar]
- Popovici C, Busa T, Missirian C, Milh M, Moncla A, Philip N. Mosaic 15q13.3 deletion including CHRNA7 gene in monozygotic twins. Eur J Med Genet 56: 274–277, 2013. doi: 10.1016/j.ejmg.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Portfors CV, Perkel DJ. The role of ultrasonic vocalizations in mouse communication. Curr Opin Neurobiol 28: 115–120, 2014. doi: 10.1016/j.conb.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portfors CV, Sinex DG. Coding of communication sounds in the inferior colliculus. In: The Inferior Colliculus, edited by Weiner JA, Schreiner CE. New York: Springer, 2005. [Google Scholar]
- Recio-Spinoso A, Joris PX. Temporal properties of responses to sound in the ventral nucleus of the lateral lemniscus. J Neurophysiol 111: 817–835, 2014. doi: 10.1152/jn.00971.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo NM, Hornickel J, Nicol T, Zecker S, Kraus N. Biological changes in auditory function following training in children with autism spectrum disorders. Behav Brain Funct 6: 60, 2010. doi: 10.1186/1744-9081-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi N, Zilany MS, Carney LH. Modeling responses in the superior paraolivary nucleus: implications for forward masking in the inferior colliculus. J Assoc Res Otolaryngol 18: 441–456, 2017. doi: 10.1007/s10162-016-0612-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samson F, Mottron L, Jemel B, Belin P, Ciocca V. Can spectro-temporal complexity explain the autistic pattern of performance on auditory tasks? J Autism Dev Disord 36: 65–76, 2006. doi: 10.1007/s10803-005-0043-4. [DOI] [PubMed] [Google Scholar]
- Sheskin DJ. Handbook of Parametric and Nonparametric Statistical Procedures. New York: Taylor and Francis, 2011. [Google Scholar]
- Shinawi M, Schaaf CP, Bhatt SS, Xia Z, Patel A, Cheung SW, Lanpher B, Nagl S, Herding HS, Nevinny-Stickel C, Immken LL, Patel GS, German JR, Beaudet AL, Stankiewicz P. A small recurrent deletion within 15q13.3 is associated with a range of neurodevelopmental phenotypes. Nat Genet 41: 1269–1271, 2009. doi: 10.1038/ng.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson NH, Ceroni F, Reader RH, Covill LE, Knight JC, Hennessy ER, Bolton PF, Conti-Ramsden G, O’Hare A, Baird G, Fisher SE, Newbury DF; SLI Consortium . Genome-wide analysis identifies a role for common copy number variants in specific language impairment. Eur J Hum Genet 23: 1370–1377, 2015. doi: 10.1038/ejhg.2014.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snedecor GW, Cochran WG. Statistical Methods. Ames, IA: Iowa State University Press, 1983. [Google Scholar]
- Snell KB, Frisina DR. Relationships among age-related differences in gap detection and word recognition. J Acoust Soc Am 107: 1615–1626, 2000. doi: 10.1121/1.428446. [DOI] [PubMed] [Google Scholar]
- Tallal P, Miller S, Fitch RH. Neurobiological basis of speech: a case for the preeminence of temporal processing. Ann N Y Acad Sci 682: 27–47, 1993. doi: 10.1111/j.1749-6632.1993.tb22957.x. [DOI] [PubMed] [Google Scholar]
- Uddin M, Unda BK, Kwan V, Holzapfel NT, White SH, Chal il L, Woodbury-Smith M, Ho KS, Harward E, Murtaza N, Dave B, Pellecchia G, D’Abate L, Nalpathamkalam T, Lamoureux S, Wei J, Speevak M, Stavropoulos J, Hope KJ, Doble BW, Nielsen J, Wassman ER, Scherer SW, Singh KK. OTUD7A regulates neurodevelopmental phenotypes in the 15q13.3 microdeletion syndrome. Am J Hum Genet 102: 278–295, 2018. doi: 10.1016/j.ajhg.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Looij MA, Liem SS, van der Burg H, van der Wees J, De Zeeuw CI, van Zanten BG. Impact of conventional anesthesia on auditory brainstem responses in mice. Hear Res 193: 75–82, 2004. doi: 10.1016/j.heares.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Yin J, Chen W, Chao ES, Soriano S, Wang L, Wang W, Cummock SE, Tao H, Pang K, Liu Z, Pereira FA, Samaco RC, Zoghbi HY, Xue M, Schaaf CP. Otud7α knockout mice recapitulate many neurological features of 15q13.3 microdeletion syndrome. Am J Hum Genet 102: 296–308, 2018. doi: 10.1016/j.ajhg.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng QY, Johnson KR, Erway LC. Assessment of hearing in 80 inbred strains of mice by ABR threshold analyses. Hear Res 130: 94–107, 1999. doi: 10.1016/S0378-5955(99)00003-9. [DOI] [PMC free article] [PubMed] [Google Scholar]