Abstract
When we grow older, understanding speech in noise becomes more challenging. Research has demonstrated the role of auditory temporal and cognitive deficits in these age-related speech-in-noise difficulties. To better understand the underlying neural mechanisms, we recruited young, middle-aged, and older normal-hearing adults and investigated the interplay between speech understanding, cognition, and neural tracking of the speech envelope using electroencephalography. The stimuli consisted of natural speech masked by speech-weighted noise or a competing talker and were presented at several subject-specific speech understanding levels. In addition to running speech, we recorded auditory steady-state responses at low modulation frequencies to assess the effect of age on nonspeech sounds. The results show that healthy aging resulted in a supralinear increase in the speech reception threshold, i.e., worse speech understanding, most pronounced for the competing talker. Similarly, advancing age was associated with a supralinear increase in envelope tracking, with a pronounced enhancement for older adults. Additionally, envelope tracking was found to increase with speech understanding, most apparent for older adults. Because we found that worse cognitive scores were associated with enhanced envelope tracking, our results support the hypothesis that enhanced envelope tracking in older adults is the result of a higher activation of brain regions for processing speech, compared with younger adults. From a cognitive perspective, this could reflect the inefficient use of cognitive resources, often observed in behavioral studies. Interestingly, the opposite effect of age was found for auditory steady-state responses, suggesting a complex interplay of different neural mechanisms with advancing age.
NEW & NOTEWORTHY We measured neural tracking of the speech envelope across the adult lifespan and found a supralinear increase in envelope tracking with age. Using a more ecologically valid approach than auditory steady-state responses, we found that young and older, as well as middle-aged, normal-hearing adults showed an increase in envelope tracking with increasing speech understanding and that this association is stronger for older adults.
Keywords: aging, electrophysiology, neural tracking of the speech envelope, speech understanding
INTRODUCTION
Although people live longer, the rapid aging of the world population also has major implications for the society, such as the increased need for specialized health care (United Nations 2017). One of the major burdens that adults over 60 yr old typically experience is the increased difficulty to communicate, especially in situations with background noise (World Health Organization 2015). Because speech-in-noise problems can result in social isolation and an increased risk for cognitive impairment, such as dementia (Goman and Lin 2018; Wayne and Johnsrude 2015), it is important to develop adequate auditory diagnostic tests and rehabilitation strategies to cope with this.
Currently, hearing aids are the most well-known rehabilitation devices to handle these speech-in-noise difficulties. Although they can compensate for the typical decreased audibility in the high frequencies by amplifying the signal, it has been demonstrated that this compensation cannot fully restore speech understanding (Humes and Coughlin 2009; Tremblay et al. 2003). Furthermore, it has been shown that older adults with clinically normal audiograms still report speech understanding problems and that these difficulties are more pronounced for speech embedded in a competing talker than for maskers such as stationary speech-weighted noise (Goossens et al. 2017; Helfer and Freyman 2014). Hence, a growing body of research supports the role of additional factors underlying speech understanding problems beyond age-related hearing loss (Guest et al. 2018; Humes et al. 2012; Martin and Jerger 2005; Working Group on Speech Understanding and Aging 1988).
Several studies investigating age-related temporal processing deficits have suggested that the elderly experience more difficulties in using silent gaps in competing speech to obtain a release from masking (Füllgrabe et al. 2015; Helfer and Vargo 2009; Hopkins and Moore 2011). Additionally, it is known that competing speech not only results in a spectrotemporal overlap, i.e., energetic masking, but also requires high-level cognitive processes to inhibit the masker. It is therefore not surprising that several studies have demonstrated an association between the age-related decline of cognitive functions and the increased difficulty on speech-in-noise tests (Cahana-Amitay et al. 2015; Desjardins and Doherty 2013; Füllgrabe et al. 2015; Gordon-Salant and Cole 2016; Janse 2012; Janse and Jesse 2014). Recently, speech-in-noise problems in patients with a clinically normal audiogram were also linked to cochlear synaptopathy, i.e., the loss of synapses between inner hair cells and auditory nerve fibers (Zheng and Guan 2018). Evidence for this association is based on the results of temporal-bone studies that show a degeneration of peripheral synapses with advancing age (Viana et al. 2015; Wu et al. 2019). Although this pathophysiology may underlie age-related speech-in-noise problems, currently the specific contribution cannot be defined because no consensus is yet achieved about which noninvasive test can be used for the diagnosis of cochlear synaptopathy in living humans (Guest et al. 2018).
In addition, it is difficult to determine whether the increased difficulty with informational maskers with advancing age is purely due to deficient temporal processing or age-related cognitive decline because these are closely intertwined (Humes et al. 2012; Martin and Jerger 2005; Working Group on Speech Understanding and Aging 1988). Although a combination of these factors and peripheral hearing loss are likely to underlie most speech understanding problems, more insight is still needed into the specific contribution of each factor. Different techniques can be used to disentangle these factors (reviews of Humes et al. 2012 and Pichora-Fuller and Souza (2003)). The most common and appropriate way is to recruit persons across the lifespan with audiometric thresholds within the normal range and screen them for cognitive impairment. Despite this design, it remains very difficult to disentangle these factors when only behavioral tests are administered (Humes et al. 2012; Schoof and Rosen 2014). Therefore, more researchers are using objective techniques to investigate the neural changes of aging related to speech processing, which cannot be predicted from the audiogram.
With advancing age, anatomical changes occur along the whole auditory pathway, from the spiral ganglion neurons, midbrain up to the auditory cortex (Cardin 2016; Kraus and Anderson 2013; Peelle and Wingfield 2016). Additionally, functional changes have been found, such as the loss of connectivity between cortical brain regions and increased bilateral activity in regions outside the core speech processing network (Cabeza 2002; Davis et al. 2008; Peelle et al. 2010; Wong et al. 2009). Because speech processing is a rapid, time-varying phenomenon, methods with a high time resolution, such as magneto- and electroencephalography (MEG and EEG), are required to accurately investigate this (Lopes da Silva 2013). In recent years, studies using MEG or EEG have consistently shown a decrease in the amplitude of responses that are mainly generated in the brainstem with advancing age (Anderson et al. 2012; Bidelman et al. 2014; Goossens et al. 2016; Leigh-Paffenroth and Fowler 2006; Presacco et al. 2016b). For the cortex on the other hand, no general consensus has been found yet. In most research, higher response amplitudes have been observed during the presentation of nonspeech sounds or short syllables (Bidelman et al. 2014; Sörös et al. 2009; Tlumak et al. 2015). Goossens et al. (2016) for example, found higher amplitudes for 4-Hz auditory steady-state responses (ASSRs) in normal-hearing (NH) older adults than in young and middle-aged adults. Similarly, increased neural speech envelope tracking has been found for NH older adults (Presacco et al. 2016b). In contrast, studies have also shown a decrease in neural tracking of frequency-modulated stimuli with advancing age (Henry et al. 2017) or no change for 20-Hz ASSRs (Goossens et al. 2016; Grose and Mamo 2010; Leigh-Paffenroth and Fowler 2006).
These controversial results are likely to be due to methodological differences. Researchers have been using different techniques but also diverse types of sounds such as clicks, frequency, or amplitude-modulated tones. Only recently, continuous speech stimuli were used to measure neural tracking of the envelope to study the processing of natural speech in noise (Das et al. 2016; Ding and Simon 2012; Kong et al. 2015; O’Sullivan et al. 2015). To our knowledge, only one study has used a similar approach to investigate the effect of age. Presacco et al. (2016b) presented two stories to a group of young (18–27 yr) and a group of older (61–73 yr) NH listeners and instructed them to attend to one talker and ignore the other. The stories were presented at different signal-to-noise ratios (SNRs: quiet, +3, 0, −3, and −6 dB). To measure the cortical tracking of the envelope, the envelope of the attended talker was reconstructed from MEG responses. Based on the correlation between the actual speech envelope and the reconstructed envelope, it was found that older adults had an enhanced cortical representation of the attended speech envelope compared with their younger counterparts. According to the authors, these results suggest a possible imbalance between inhibitory and excitatory processes (de Villers-Sidani et al. 2010) or the loss of connections between different brain regions (Peelle et al. 2010).
The benefit of the approach used by Presacco et al. (2016b) is that the stimulus closely resembles daily life speech. Therefore, it is more ecologically valid than objective measures based on the responses to repeated, short, artificial stimuli, such as auditory brainstem responses (ABRs), cortical evoked responses, or ASSRs. Additionally, envelope tracking measures can be used to objectively evaluate a person’s speech understanding, because there is ample evidence that the envelope is an important cue for speech perception (Drullman et al. 1994; Shannon et al. 1995), and studies have shown an increase in neural envelope tracking with increasing speech understanding for young NH participants (Ding and Simon 2013; Kong et al. 2015; Peelle et al. 2013; Vanthornhout et al. 2018).
The present study was designed to further investigate the effect of age on neural envelope tracking and speech understanding in young, middle-aged, and older NH adults by measuring envelope tracking using a single-trial and more ecologically valid method than ASSRs. First, we expect, based on the results of Presacco et al. (2016b), to find enhanced envelope tracking with advancing age. In contrast to Presacco et al. (2016b), who compared MEG responses of two extreme age groups, we also included middle-aged NH adults because it has been shown that speech understanding (Goossens et al. 2017; Helfer and Freyman 2014) and cognitive function (Singh-Manoux et al. 2012; Vercammen et al. 2017a) start to decrease from 45–50 yr on. Second, we further extend the findings of Presacco et al. (2016b) by directly investigating the association between speech understanding and envelope tracking. Similarly to a recent study of Vanthornhout et al. (2018) conducted in young NH adults, we hypothesize to also find an increase in envelope tracking with speech understanding for middle-aged and older NH adults. To investigate this, participants were instructed to recall standardized sentences out loud during the EEG recording. This way, a more direct association between envelope tracking and speech understanding is ensured compared with previous studies in which neural responses were related to ratings or behavioral scores obtained before or after the EEG recording (Anderson et al. 2011; Ding and Simon 2013; Goossens et al. 2018; Vanthornhout et al. 2018). Additionally, we not only presented sentences at highly intelligible fixed SNRs, as did Presacco et al. (2016b), but also presented the stimuli at several subject-specific SNRs to ensure a range of different speech understanding levels for each individual.
MATERIAL AND METHODS
Participants
To investigate the effect of age beyond age-related hearing loss and cognitive impairment, middle-aged and older adults were recruited through a screening across Flanders, Belgium. Adults with normal hearing in both ears, with no indication of cognitive impairment or learning disability, were recruited. From the 90 adults older than 44 yr of age, only 42 persons met the inclusion criteria. This was not unexpected because hearing declines from age 40 yr on (International Organization for Standardization 2000; Moore et al. 2014). Additionally, persons were also excluded when a score lower than 26/30 was obtained on the Montreal Cognitive Assessment (MoCA; Nasreddine et al. 2005). Last, the medical history and the presence of learning disabilities were questioned, because serious concussions, medication used to treat, for example, insomnia (van Lier et al. 2004), and learning disabilities such as dyslexia are known to affect brain responses (De Vos et al. 2017; Poelmans et al. 2012; Power et al. 2016).
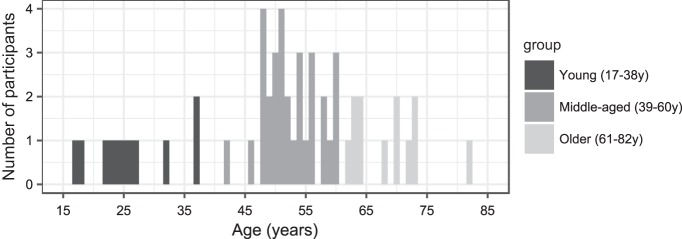
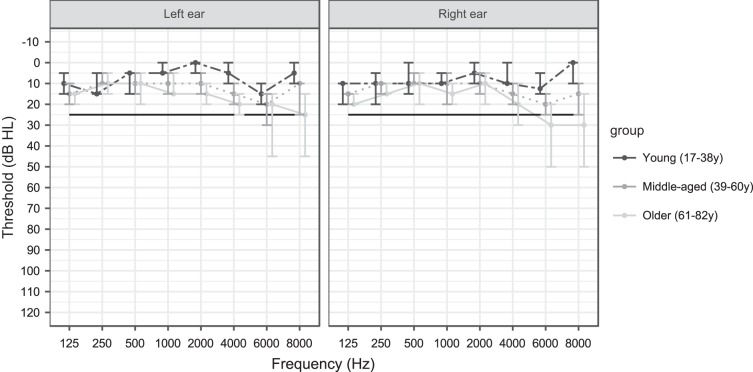
After screening, a total of 54 participants (13 men and 41 women) participated in the study. Their age ranged from 17 to 82 yr (see Fig. 1, histogram of ages). All participants had Flemish as their mother tongue and were normal hearing in both ears [thresholds from 125 to 4,000 Hz lower or equal to 30 dB HL (hearing level); Fig. 2]. Symmetrical hearing was ensured based on the criteria derived from the AMCLASS algorithm of Margolis and Saly (2008). As can be inferred from Fig. 2, elevated thresholds were typically observed at the higher frequencies (6,000 and 8,000 Hz) for the older participants. This, however, should not influence the outcomes of this study because we used ER-3A insert phones with a decreasing frequency response from 4,000 Hz and higher (Etymotic Research Inc. 2015). Last, we examined the handedness and ear preference of our participants using a Flemish, modified version of the laterality preference inventory of Coren (1993) to choose the stimulation ear. More specifically, the right ear was chosen as stimulation ear, unless the participant showed a clear left ear preference. This study was approved by the Medical Ethics Committee UZ KU Leuven/Research [reference no. S57102 (Belg. Regnr: B322201422186) and S58970 (Belg. Regnr: B322201629016)]. All participants gave written informed consent and were paid for their participation if they were older than 35 yr.
Fig. 1.
Distribution of the number of participants per age group.
Fig. 2.
Median air conduction thresholds [in dB hearing loss (HL)] of young, middle-aged, and older participants. Error bars indicate interquartile range.
Stimuli
For the behavioral and EEG experiment, we used the same target stimuli and maskers. Moreover, we presented matrix sentences as well as a story to the participants because the structure of the matrix sentences does not resemble daily life communication. Two maskers were chosen because previous studies have shown that the effect of age can be more detrimental for competing talkers than for purely energetic maskers. In addition to these stimuli, two other stimuli were used in the present study. First, a story was presented to train the linear decoder used to reconstruct the speech envelope from the EEG (see Signal processing). Second, tone pips were presented to evaluate the effect of age on the responses to nonspeech stimuli (see EEG experiment).
Target stimuli.
In this study, we used the Flemish matrix sentence test (Luts et al. 2014) to evaluate a participant’s speech understanding behaviorally. The matrix sentence test consists of 13 lists of 20 sentences where each sentence has a fixed syntax structure of 5 word categories: name, verb, numeral, color, and object [e.g., “Lucas draagt twaalf paarse boten” (“Lucas carries twelve purple boats”)]. During the test, participants are instructed to recall the heard sentence by using a 5 × 11 matrix containing 10 possibilities for each word of the sentence, as well as the option to give no answer. The percentage of correctly recalled words is used as a measure for speech understanding.
Although the matrix sentences are translated into different languages to evaluate a person’s speech understanding, the structure of these sentences does not resemble daily life communication. To get more insight into daily life speech understanding, we chose to also present commercial recordings of stories to our participants, similar to studies investigating the cocktail party phenomenon (e.g., Das et al. 2016; Ding and Simon 2012; O’Sullivan et al. 2015). The story that we used in this study is “De Wilde Zwanen” by Hans Christian Andersen, narrated by a female, Flemish talker, and was 28 min long. The story was set to the same root mean square level and spectrum as the matrix sentences, and silences were shortened to a maximum duration of 200 ms based on the results of a previous study (Decruy et al. 2018).
Maskers.
To investigate how envelope tracking is affected by speech understanding, several levels of speech understanding were created by adding background noise. As shown before by Goossens et al. (2017) and Helfer and Freyman (2014), competing speech can have a more detrimental effect on speech understanding with advancing age, compared with speech-weighted noise. Because competing speech contains silent gaps, the listener could reconstruct the target more easily and achieve better speech understanding compared with a stationary masker (Festen and Plomp 1990; Francart et al. 2011; Koelewijn et al. 2012). However, this potential benefit depends on the temporal processing ability of the listener, which appears to deteriorate with healthy aging (Desjardins and Doherty 2013; Füllgrabe et al. 2015). To investigate this, we examined the effect of both a stationary speech-weighted noise (SWN) and a competing talker (CT) on speech understanding in the present study. For the matrix sentences as well as the story, we created an SWN that had the same long-term-average spectrum as the target stimulus. This resulted in optimal spectral masking, also called energetic masking. For the informational masker, we used a second story, “Bianca en Nero” by Béatrice Deru-Renard, which was narrated by a male, Flemish talker, in contrast to our target stimulus. Similar to the target story, the silences of the CT were shortened to 200 ms, and the spectrum and the root mean square level were matched to those of the target stimulus.
Setup
Environment.
In a first session, the behavioral experiments were conducted at the research group ExpORL of the KU Leuven or at home. For all participants, the second session took place at the research group to record the EEG in a triple-walled, soundproof booth, equipped with a Faraday cage to avoid electromagnetic interference.
Auditory stimulation.
For the auditory stimulation, we used a laptop connected to a RME Hammerfall DSP Multiface II or RME Fireface UC soundcard (RME, Haimhausen, Germany), running the software platform APEX (Dept. of Neurosciences, KU Leuven) (Francart et al. 2008). The target speech stimuli were presented monaurally through ER-3A insert phones (Etymotic Research, Elk Grove Village, IL) at an intensity of 55 dB SPL (A weighted). For all participants except one, stimuli were presented to the right ear. The maskers, SWN or CT, were presented to the same ear as the target stimulus, and their levels were adjusted according to the chosen SNR. Before the experiments were administered, all stimuli were first calibrated with a type 2260 sound-level pressure meter, a type 4189 0.5-in. microphone, and a 2-cm3 coupler (Bruel & Kjaer, Copenhagen, Denmark).
EEG recording.
To record the EEG, we used a BioSemi ActiveTwo system (Amsterdam, The Netherlands), with 64 active Ag-AgCl electrodes and two extra electrodes, serving as the common electrode (CMS) and current return path (DRL). Electrodes were mounted in head caps containing electrode holders placed according to the 10–20 electrode system. The EEG recordings were digitized at a sampling rate of 8,192 Hz and stored on a hard disk using the BioSemi ActiView software.
Experimental Procedures
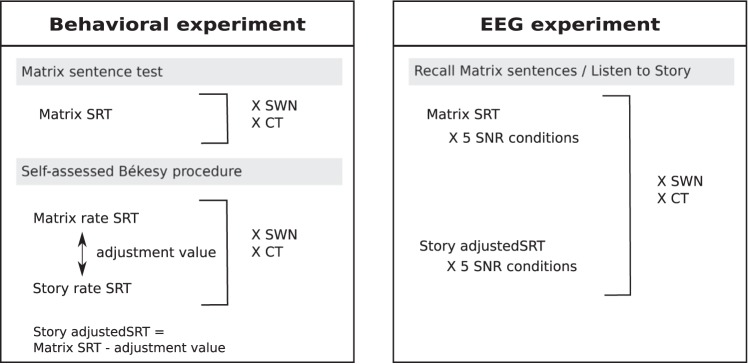
After screening, all participants first completed the behavioral experiment in which speech understanding in noise was evaluated for two speech materials, matrix and story, and two maskers, SWN and CT. The outcome of the speech-in-noise test was used not only to assess the effect of age on speech understanding but also to determine equivalent speech understanding levels across participants for the EEG experiment. In addition, two cognitive tasks were administered to investigate the contribution of working memory and inhibition. Finally, EEG was used to measure neural envelope tracking and get insight into the interplay between age, speech understanding, and envelope tracking. An overview of the main procedures is depicted in Fig. 3.
Fig. 3.
Overview of the main procedures conducted during the behavioral and electroencephalography (EEG) experiment. The speech reception thresholds (SRTs) of the different procedures are used to determine the signal-to-noise ratios (SNRs) for the EEG experiment. Two maskers, speech-weighted noise (SWN) and a competing talker (CT), are used to create the different conditions.
Speech understanding in noise: behavioral experiment.
During the matrix sentence test, participants were instructed to recall sentences. An adaptive procedure was chosen to converge as quickly as possible to the speech reception threshold (SRT; SNR at which 50% speech understanding is achieved). For this test, the procedure of Brand and Kollmeier (2002) was used to adapt the level of the masker (Luts et al. 2014). To avoid confounds of procedural learning, two training lists of 20 sentences each were first administered. The matrix sentences were presented in both SWN and CT, and the order of first presentation of SWN or CT was randomized across participants. The SRT was defined as the last SNR presented in a list of 20 sentences.
Following the matrix sentence test, we administered an adapted version of the self-assessed Békesy procedure (Decruy et al. 2018) to create equivalent speech understanding levels for the story used during the EEG experiment. During this procedure, participants were provided with a scale from 0 to 100% to rate their speech understanding. Based on these ratings, the level of the masker was adapted until the procedure converged to the SRT. More specifically, the SNR was decreased if the participant rated >50% or increased if the participant rated ≤50%. The procedure was administered at least twice, and the SRT was determined as the average of the last presented SNR of these two runs. When the outcome of a run differed more than 3 dB from the previous one, a third run was administered. In this case, the average of the last presented SNR of the second and third runs was used as the SRT to exclude procedural learning effects.
To ensure comparable understanding levels for matrix and story, as well as to avoid the confounds of rating biases, we used the difference in SRT between matrix and story on the self-assessed Békesy procedure (Decruy et al. 2018), further described as “Békesy procedure,” as an adjustment value. Consequently, the story-adjusted SRT per participant was calculated by subtracting this adjustment value from the SRT on the matrix test (Fig. 3). In the beginning of the study, the Békesy procedure was only administered in the presence of SWN. However, during the EEG recording of the first participants (13/54), we noticed that the difference in SRT between matrix and story was substantially larger when CT was used as the masker (i.e., more than 2 times the difference in SWN). Hence, for the remaining participants, the Békesy procedure was administered for both maskers. The story-adjusted SRT was first calculated on the basis of results in SWN, and if the difference between matrix and story was substantially larger for CT compared with SWN, the story-adjusted SRT was adapted. Although this led to differences across participants in the determination of the adjustment value, we do not believe that this influenced our results because we analyzed envelope tracking as a function of the exact speech understanding percentages calculated or rated during the EEG experiment.
Envelope tracking and cortical responses to tone pips: EEG experiment.
For each participant, we started the EEG experiment with the tone pips. After the tone pips, one block of the matrix and one block of the story were presented; within each block were all the different SNR conditions per masker (see Table 1). Next, the story “Milan,” which lasts 12 min and was written and narrated by Stijn Vranken, was presented without masker to provide an optimal condition to create a linear decoder (see Signal Processing), followed by the remaining two blocks of the matrix and story. The order of the matrix and story was quasi-randomized across participants, as well as the order of the maskers. For example, when the matrix sentences were presented first in SWN, CT was used to mask the story that preceded. Alternating the maskers kept the participants motivated.
Table 1.
Overview of the different matrix and story conditions for both maskers
| SWN | CT | |||
|---|---|---|---|---|
| SNR | Matrix | Story | Matrix | Story |
| 20% SU | SRT − 3 dB | Adjusted SRT − 3 dB | SRT − 4 dB | Adjusted SRT − 4 dB |
| 50% SU | SRT | Adjusted SRT | SRT | Adjusted SRT |
| 80% SU | SRT + 3 dB | Adjusted SRT + 3 dB | SRT + 4 dB | Adjusted SRT + 4 dB |
| 95% SU | SRT + 6 dB | Adjusted SRT + 6 dB | SRT + 8 dB | Adjusted SRT + 8 dB |
| Fixed SNR | No noise | No noise | 0 dB SNR | 0 dB SNR |
Different matrix and story conditions for the maskers speech-weighted noise (SWN) and competing talker (CT), presented during the electroencephalography (EEG) experiments [e.g., signal-to-noise ratio (SNR) at which a speech understanding (SU) score of 20% should be achieved: 20% SU]. To avoid rating bias, we created the story-adjusted speech reception threshold (SRT; see Fig. 3).
Procedure for ASSRs to tone pips.
During the EEG experiment, the participants were seated in a comfortable chair. First, the participants heard the tone pips, which were created using a sinusoid with a carrier frequency of 500 Hz and a total duration of 21 ms (4 ms on and off ramps). With an interstimulus interval of 500 ms, repeating these tone pips resulted in a repetition frequency of ~1.92 Hz. In total, 250 tone pips were presented to each participant at an intensity of 90 dB peak equivalent SPL.
Procedure for matrix and story.
During the matrix and story experiments, participants were asked to recall the matrix sentences out loud, similarly to the behavioral test. This way, their speech understanding score per condition could be determined and directly associated with the EEG responses. For the story, participants were asked to actively listen and answer a question about the content to keep them alert. In addition, participants were also asked to rate their speech understanding for both matrix and story using the same scale as for the Békesy procedure. Based on the percentage of correctly repeated words for the matrix sentences, a direct association between envelope tracking and speech understanding could be investigated. For the story, we adjusted the ratings of the participants by adding the difference score between the percentage of correctly repeated words and the rated percentage for the matrix sentences, per masker condition.
As shown in Table 1, we presented the matrix sentences and story at different specific SNRs to obtain a speech understanding score that could be directly associated with envelope tracking. We created four subject-specific SNR levels by lowering and raising the individual matrix and story-adjusted SRT by one or two times 3 dB. This way, we could create equivalent subject-specific speech understanding levels that covered the psychometric function of each individual (20%, 50%, 80%, 95% speech understanding). As can be inferred from Table 1, a larger step size of 4 dB was used for CT, because the use of fluctuating maskers results in less steeply sloping psychometric functions compared with stationary maskers (Francart et al. 2011; MacPherson and Akeroyd 2014). In addition to the subject-specific SNRs, two fixed SNRs across participants were presented to compare our results with related studies, to achieve highly intelligible SNR conditions as well as to investigate if the effect of age on envelope tracking remains when the stimulus is presented at a fixed SNR. In the block where CT was the masker, we presented the target and CT at the same level, i.e., 0 dB SNR. In the SWN block, the target talker was also presented without any masker (No noise). For the matrix sentences, a list of 20 sentences with a duration of 1.5–2.5 s per sentence was presented per SNR, whereas the story of 28 min was divided into two blocks, each with five equal parts of ~3 min. Altogether, each participant completed for both the matrix sentences and story, two blocks representing the two maskers, each consisting of five conditions (i.e., 4 subject-specific SNRs + 1 fixed SNR). The order of the SNRs within each block was randomized across participants.
Signal processing.
All signal processing analyses were done off-line, using MATLAB R2016b.
tone pips.
The ASSRs evoked by the tone pips were analyzed as follows (Goossens et al. 2016; Picton et al. 1987; Van Eeckhoutte et al. 2018; Vercammen et al. 2017b). First, the EEG signal was re-referenced to the average of the 64 channels, high-pass filtered with a cutoff frequency of 0.5 Hz, and segmented in epochs of 0.521 s, containing exactly one presentation of the tone pip. To remove artifacts, 5% of the epochs containing the largest peak-to-peak amplitudes were rejected. Next, denoising source separation (DSS) was applied using the epoched EEG data as input. DSS is an algorithm based on principal component analysis that designs a spatial filter that separates neural activity into stimulus-related and stimulus-unrelated components, based on a criterion of stimulus-evoked reproducibility (de Cheveigné and Simon 2008). After DSS was applied to the raw, filtered, unepoched data, the EEG data were segmented again, but now in epochs of ±2 s to ensure a good frequency resolution. Next, artifacts were removed again by rejecting 5% of the epochs containing the largest peak-to-peak amplitudes before the epochs were transformed into the frequency domain. A one-sample Hotelling T2 test (with α = 0.05; Hofmann and Wouters 2012; Hotelling 1931) was used to determine if the response amplitude differed significantly from the nonsynchronized neural background activity (EEG noise). For our statistical analysis, we calculated the size of the ASSR using the following formula:
with PN reflecting the power of the nonsynchronized neural activity and PS+N reflecting the total power of the synchronized neural response to the tone pip and EEG noise in the frequency bin of interest (1.92 Hz).
envelope reconstruction.
In this study, we measured neural tracking of the speech envelope by calculating the correlation between the actual acoustic speech envelope and the reconstructed envelope from the EEG response. First, the speech envelope was extracted according to Biesmans et al. (2017), i.e., filtering the target speech stimulus using a gammatone filter bank followed by a power law (Søndergaard et al. 2012; Søndergaard and Majdak 2013). To decrease processing time, the acoustic envelope was downsampled in a first step from 48,000 to 256 Hz. A type 2, zero-phase Chebyshev filter (with 80-dB attenuation at 10% outside the pass band) from 1 to 8 Hz was then applied to the envelope. Finally, after filtering, the speech envelope was further downsampled to 128 Hz.
Similarly to the acoustic envelope, the EEG data were first downsampled from 8,192 to 256 Hz. Next, a generic EEG artifact removal algorithm based on the multichannel Wiener filter (MWF) was applied to the EEG data (Somers et al. 2018). More specifically, the MWF was trained based on the data from the story “Milan” and then applied to the target stimuli, the matrix sentences and the story “De Wilde Zwanen.” After artifact removal, the EEG signals were re-referenced to the average of the 64 channels, and then the data were bandpass filtered using the Chebyshev filter, similarly to the acoustic envelope, and downsampled to 128 Hz.
To measure neural envelope tracking, we used the stimulus reconstruction approach described by Vanthornhout et al. (2018). More specifically, the reconstructed envelope ŝ(t) was obtained by applying a linear decoder to EEG signals. This decoder is a spatiotemporal filter that linearly combines the EEG signals of the different channels and their time-shifted versions to optimally reconstruct the envelope. Mathematically, this can be formulated as follows:
with time t ranging from 0 to T, where n is the index of the recording electrode ranging from 1 to 64, and τ is the poststimulus integration window length. We chose an integration window from 0 to 500 ms because it has been shown that older adults have delayed responses (Anderson et al. 2012; Presacco et al. 2016b; Tremblay et al. 2003). The decoder was created using the mTRF toolbox (Lalor et al. 2006, 2009). More specifically, the weights of the decoder were determined in a training phase by applying ridge regression to the inverse autocorrelation matrix: g = (RRT)−1(RST), where R is the time-lagged matrix of the EEG signal and S is the speech envelope. The decoder g was trained for each participant on the story “Milan” and contained a matrix of 64 (EEG channels) × 65 (time delays; 500 ms) elements.
After training, a subject-specific decoder was applied to the EEG data of the matrix sentences and the story “De Wilde Zwanen.” The reconstructed envelope was calculated for each condition, i.e., each SNR, and then correlated with the actual envelope using the bootstrapped Spearman correlation by conducting a Monte Carlo sampling (Vanthornhout et al. 2018). For the matrix sentences, the parts that contained silence or the participant’s answer were removed from the reconstructed envelope. A significance level of the correlation was calculated by correlating random permutations of the actual and reconstructed envelopes (1,000 times) and taking percentiles 2.5 and 97.5 to obtain a 95% confidence interval.
Cognitive tests.
The Flemish computerized version of the reading span test (RST; van den Noort et al. 2008; Vercammen et al. 2017a) and the paper version of the Stroop test (Hammes 1978) were used to evaluate working memory and inhibition. Although our participants were screened for cognitive impairment using the MoCA, studies have reported that even in a healthy aging population, cognitive function declines with age (Humes et al. 2012) and may be associated with increased cortical envelope tracking (Presacco et al. 2016a).
To assess working memory, participants were seated in front of a computer screen where a sentence was visually presented. Three sets of 20 sentences were administered, each containing 5 randomized subsets of 2, 3, 4, 5, or 6 sentences (Vercammen et al. 2017a). The participants were instructed to read the sentences out loud. After reading a subset, the participants were asked to recall as many as possible of the sentence-final words of the previous subset. Based on the correctly recalled sentence-final words, a final score of 60 was calculated for the RST (Vercammen et al. 2017a).
Last, a paper version of the Stroop test (Hammes 1978) was used to assess inhibition. Participants were presented three cards, each of which contained 10 rows of 10 elements each. On the first card, the color names “red, green, yellow and blue” were printed multiple times in black ink. Participants had to read these words as accurately and fast as they could while their response time was recorded using a manually operated stopwatch. On the second card, rectangles were presented in the same colors, and participants had to name the color. Because no inhibition was needed, this is called the congruent task. Finally, a third, incongruent, card was presented where the color names were printed in a different color than its meaning (e.g., the word “blue” colored red). The goal was to name the color of the words while inhibiting reading of the words. The score on this test was calculated as the difference in response time (in seconds) between the third and second cards. For means of visualization and interpretation of the results, the Stroop test results were analyzed by subtracting the difference score from the value “60,” which is also the maximum score that could be obtained on the RST.
Statistical Analysis
The statistical analyses were conducted using R software (version 3.4.4; nlme package version 3.1–131.1; Field et al. 2012; Pinheiro et al. 2017). The effect of age on the SRT and envelope tracking was analyzed using linear mixed-effect models (LMMs), because we collected multiple measurements per participant (e.g., 2 masker conditions), and these models can handle missing data well (13 data points are missing from the current study data; Baayen et al. 2008). The fixed-effect part of the LMMs consisted of the predictors of interest, whereas the random-effect part included the variable participant, nested in one of the repeated-measures predictors if this improved the model fit. Because no repeated measurements were conducted for the tone pips and cognitive tasks, the effect of age on these responses/scores was analyzed using linear fixed-effect models (LFMs). All models were fitted using the maximum likelihood estimation.
The best-fitting model was determined by first progressively introducing multiple fixed-effects and corresponding interactions. Next, the different models were compared using likelihood ratio tests and Akaike’s information criterion (AIC; Akaike 1974). The best-fitting model served as a starting point for the evaluation of the contribution of other predictors until the final best-fitting model was determined. Significant main and interaction effects of the final model are discussed in results by reporting the nonstandardized regression coefficient (β) with standard error (SE), degrees of freedom (df), t ratio, and P value per fixed-effect term. Using LMMs, we also compared the contribution of speech understanding vs. SNR as a predictor for neural envelope tracking. We evaluated the two models using AIC and the Pseudo-R2 for LMMs.
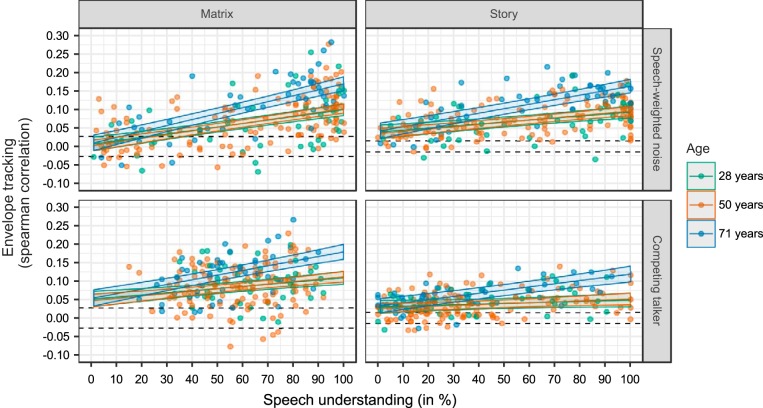
In all models, the predictors speech understanding and age were considered as continuous variables. To clearly visualize the interactions for envelope tracking at subject-specific SNRs, age was not plotted as a continuous variable in Fig. 5. Instead, three example regression lines representing a young (28 yr), middle-aged (50 yr), and older (71 yr) person were fitted on the data. Because the decline in auditory temporal processing is closely intertwined with the decrease in cognitive abilities with advancing age, we also used LMMs to assess if cognition has an additional effect on speech understanding in noise and envelope tracking, beyond age. A significance level of α = 0.05 was set for all the models unless otherwise stated (e.g., correction for multiple comparisons using the method of Holm 1979).
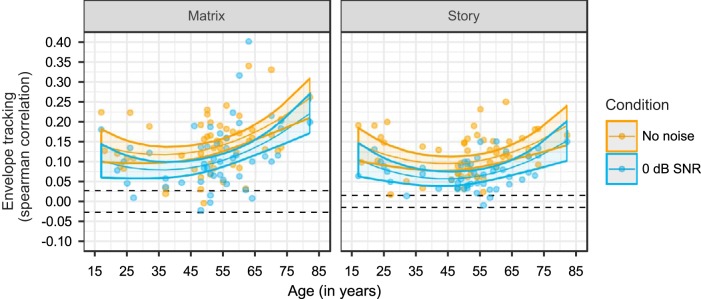
Fig. 5.
Neural tracking of the envelope as a function of speech understanding, measured in 54 normal-hearing adults using 2 speech materials (matrix and story) and 2 masker types (speech-weighted noise and a competing talker). Per speech material and masker, 4 data points are plotted per participant, representing the 4 subject-specific signal-to-noise ratio (SNR) conditions. In addition, 3 regression lines with confidence intervals (shaded areas) were fitted, representing the predicted data for an example person aged 28 (young), 50 (middle-aged), or 71 (older) yr (color-coded). Dashed black lines indicate the significance level of the measure for envelope tracking.
RESULTS
First, we determined the results of the behavioral speech understanding in noise experiment. Second, we analyzed the results regarding neural envelope tracking. More specifically, we investigated the association between speech understanding and envelope tracking. Next, we studied the effect of age on envelope tracking and tone pip responses. Finally, we analyzed the effect of age on cognition and the interplay between age, cognition, speech understanding in noise, and envelope tracking.
Speech Understanding in Noise
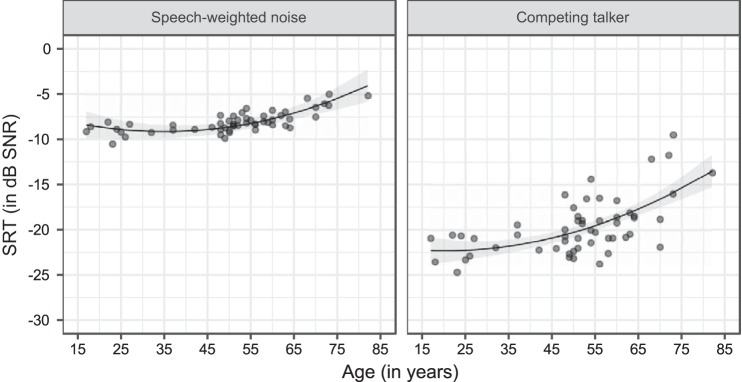
To assess the influence of age and masker type (SWN and CT) on speech understanding, we analyzed the matrix SRTs of all participants, obtained during the behavioral experiment in which a standard adaptive procedure was administered. We can infer from Fig. 4 and the best-fitting LMM that the matrix SRT increased supralinearly, i.e., quadratically, with advancing age (P = 0.002; Table 2). In addition to this, our participants achieved a significantly lower, better matrix SRT for CT compared with SWN (P < 0.001; Table 2). In addition to these main effects, we also detected a significant interaction between age and the type of masker, indicating a steeper increase in matrix SRT with advancing age when CT was used as masker compared with SWN (P = 0.001; Table 2). We noticed after data collection that 12 of 54 participants completed the behavioral experiment with stimuli (both masker and target) having maximal silence gaps of 300 ms instead of 200 ms. When the analyses were run with the exclusion of these participants, the same effects remained significant.
Fig. 4.
Matrix speech reception threshold (SRT) for the 2 masker types: speech-weighted noise (SWN) and a competing talker (CT) across the adult lifespan. Two regression lines with confidence intervals (shaded areas) were fitted to the data and indicate a supralinear increase of the matrix SRT with advancing age for both masker types. A significantly steeper slope was found when CT was used as masker compared with SWN (see Table 2). SNR, signal-to-noise ratio.
Table 2.
Linear mixed-effect model: effect of age and masker on speech reception threshold
| Fixed-Effect Terms | β Value | SE | df | t Ratio | P Value |
|---|---|---|---|---|---|
| Intercept (for SWN) | −6.3 | 1.62 | 52 | −3.89 | <0.001 |
| Age | −0.162 | 0.0676 | 51 | −2.39 | 0.02 |
| Age2 | 0.0023 | 7.04e-04 | 51 | 3.28 | 0.002 |
| Masker | −15.1 | 1.08 | 52 | −14 | <0.001 |
| Age:masker | 0.0689 | 0.0204 | 52 | 3.39 | 0.001 |
Regression coefficients (β values), standard errors (SE), degrees of freedom (df), t ratios, and P values per fixed-effect term. Age2, age squared; SWN, speech-weighted noise. Colon indicates interaction.
Envelope Tracking and Cortical ASSRs to Tone Pips
Below, the statistical analysis regarding the association between speech understanding and envelope tracking is first presented. Next, we focus on the effect of age on envelope tracking, measured at the subject-specific SNRs and highly intelligible fixed SNRs, as well as ASSRs to tone pips.
Effect of speech understanding on neural tracking of the speech envelope.
First, we investigated whether speech understanding or SNR is driving the differences in envelope tracking. To determine this, we used the speech understanding scores (SU), SNRs, and EEG data obtained during the four subject-specific conditions where each participant listened to stimuli at four SNRs situated around their SRT (e.g., SWN: SRT − 3 dB, SRT, SRT + 3 dB, SRT + 6 dB; Table 1). We built two LMMs with four predictors and their interactions [speech material, age, age2, and SU or SNR] and compared these models using AIC and Pseudo-R2. We did not include masker as a predictor in the models, because the interactions masker:SU and masker:SNR are highly correlated and consequently would not allow us to investigate the differential effect of SU and SNR on envelope tracking. For the comparison between the two LMMs with the four predictors, we found a smaller AIC and a higher Pseudo-R2 value for the model in which SU was included as a predictor (AIC =−2,850.19; Pseudo-R2 = 0.4594) compared with the model using SNR as a predictor (AIC = −2,714.4; Pseudo-R2 =0.3579). This indicates that envelope tracking is related to changes in SU that cannot be explained by changes in SNR. Therefore, and because the design of our experiments is focused on speech understanding, we will only include speech understanding as a predictor in the following analyses.
To investigate the effect of speech understanding on envelope tracking, we used an LMM with five predictors and their interactions [speech material (matrix and story), masker (SWN and CT), SU, age, and age2; Table 3 and Fig. 5]. First of all, we detected a significant main effect of speech understanding (P < 0.001). As shown in Fig. 5, we obtained a significant increase in envelope tracking with increasing speech understanding. Because we found a significant interaction between speech understanding and the type of speech material or masker (SU:speech material, P < 0.001; SU:masker, P = 0.002; Table 3), the steepness of this increase in envelope tracking depends on the presented speech material and masker. Because we did not find a significant three-way interaction, we can infer from the LMM and Fig. 5 that envelope tracking increases less steeply with speech understanding when CT is used as masker compared with SWN, and when the story is used as target stimulus instead of the matrix sentences across all ages (Table 3).
Table 3.
Linear mixed-effect model: effect of speech understanding, speech material, masker, and age on envelope tracking
| Fixed-Effect Terms | β Value | SE | df | t Ratio | P Value |
|---|---|---|---|---|---|
| Intercept (for Matrix/SWN) | 0.0274 | 0.0351 | 799 | 0.781 | 0.44 |
| SU | 0.00169 | 3.98e-04 | 799 | 4.24 | <0.001 |
| Speech material | 0.033 | 0.00756 | 799 | 4.37 | <0.001 |
| Masker | 0.0453 | 0.00777 | 799 | 5.82 | <0.001 |
| Age | −0.00107 | 0.00152 | 51 | −0.704 | 0.48 |
| Age2 | 1.13e-05 | 1.60e-05 | 51 | 0.708 | 0.48 |
| Speech material:masker | −0.0537 | 0.00606 | 799 | −8.85 | <0.001 |
| SU:age | −4.39e-05 | 1.74e-05 | 799 | −2.52 | 0.01 |
| SU:age2 | 6.03e-07 | 1.86e-07 | 799 | 3.25 | 0.001 |
| SU:speech material | −3.95e-04 | 1.06e-04 | 799 | −3.72 | <0.001 |
| SU:masker | −3.57e-04 | 1.12e-04 | 799 | −3.18 | 0.002 |
Regression coefficients (β values), standard errors (SE), degrees of freedom (df), t ratios, and P values per fixed-effect term. Age2, age squared; SU, speech understanding; SWN, speech-weighted noise. Colon indicates interaction.
Last, we found a significant interaction between the type of masker and speech material (P < 0.001; Table 3). Holm-adjusted post hoc tests indicate a significantly lower envelope tracking for the story compared with matrix when CT is used as masker (mean difference between matrix CT vs. story CT = 0.042, P < 0.001), whereas the opposite effect is found when SWN is used as masker (mean difference between matrix SWN vs. story SWN = −0.0117, P = 0.004). Accordingly, we detected a significantly higher envelope tracking for the matrix when CT was used as masker compared with SWN (mean difference between matrix SWN vs. matrix CT = −0.026, P < 0.001) and a significantly lower envelope tracking for the story when CT was used compared with SWN (mean difference between story SWN vs. story CT = 0.0277, P < 0.001).
Effect of age.
To investigate the neural changes in speech processing with advancing age, we first evaluated the effect of age on envelope tracking for the subject-specific and fixed SNR conditions. Next, we investigated the effect of age on nonspeech sounds by analyzing the ASSRs to tone pips.
speech envelope tracking at subject-specific snrs.
With regard to the effect of age, we first evaluated the EEG data for the four subject-specific SNR conditions (see LMM reported in Table 3). We detected a significant interaction between speech understanding and age, indicating that the slope of envelope tracking in function of speech understanding becomes quadratically, i.e., supralinearly, steeper with advancing age (P = 0.001; Table 3). This is illustrated in Fig. 5 where three example regression lines are depicted reflecting envelope tracking in function of speech understanding for three ages: 17, 50, and 71 yr old. The regression lines for the young and middle-aged participants are very similar, whereas the LMM predicts a significantly higher and steeper increase in envelope tracking with speech understanding for the older NH participant (71 yr old). To assess whether outliers have an influence on the interpretation on our data, we have run all analyses without the data of the participant who was 82 yr old. The same conclusions could be drawn from these analyses.
speech envelope tracking at fixed, highly intelligible snrs.
In addition to the subject-specific SNRs, we also investigated the influence of age on envelope tracking for matrix and story presented at two fixed, highly intelligible SNRs (No noise and 0 dB SNR; Table 4). The LMM, summarized in Table 4, showed that envelope tracking significantly increases with age in a supralinear way (P = 0.002). As can be inferred from Fig. 6, it seems that envelope tracking is stable from 17 until ±50 yr and then starts to gradually increase with advancing age. In addition, we detected a significant interaction between speech material and age (P = 0.02), indicating a less steeply sloping increase in envelope tracking with age for the story than for the matrix sentences (Table 4). Last, we found significantly lower envelope tracking when the matrix sentences and the story were presented at 0 dB SNR compared with the condition without noise (P < 0.001; Table 4). The same conclusions could be drawn from the analyses in which the data of the participant who was 82 yr old were removed.
Table 4.
Linear mixed-effect model: effect of No noise vs. 0 dB SNR, speech material, and age on envelope tracking
| Fixed-Effect Terms | β Value | SE | df | t Ratio | P Value |
|---|---|---|---|---|---|
| Intercept (for Matrix/No noise) | 0.202 | 0.0461 | 149 | 4.38 | <0.001 |
| 0 dB SNR | −0.0384 | 0.00691 | 149 | −5.55 | <0.001 |
| Speech material | 0.0218 | 0.0249 | 149 | 0.875 | 0.38 |
| Age | −0.00473 | 0.00194 | 51 | −2.43 | 0.02 |
| Age2 | 6.63e-05 | 2.02e-05 | 51 | 3.29 | 0.002 |
| Speech material:age | −0.00111 | 4.66e-04 | 149 | −2.37 | 0.02 |
Regression coefficients (β values), standard errors (SE), degrees of freedom (df), t ratios, and P values per fixed-effect term. SNR, signal-to-noise ratio. Colon indicates interaction.
Fig. 6.
Neural tracking of the envelope as a function of age for 2 fixed signal-to-noise ratio (SNR) conditions (No noise and 0 dB SNR) and 2 speech materials (matrix and story). Per fixed SNR condition (color-coded) and speech material, 1 data point is plotted per participant and 1 regression line with confidence intervals (shaded areas) is fitted, representing envelope tracking as a function of age. Dashed black lines indicate the significance level of the measure for envelope tracking.
cortical responses to nonspeech sounds: tone pips.
In addition to continuous speech, we evaluated the effect of age on the responses to nonspeech sounds. Moreover, we assessed the effect of age on the SNRs of the ASSRs to tone pips with a repetition frequency of 1.92 Hz (see materials and methods). As depicted in Fig. 7 and detected by the LFM, we found that the SNR of the tone pip responses significantly decreases with age in a linear way, in contrast to speech envelope tracking (β = −0.0606, SE = 0.0246, P = 0.02). Adding age as a quadratic, i.e., supralinear, fixed-effect term in the LFM did not improve the fit. To exclude the possibility that this significant decrease is due to a general decrease in neural activity with advancing age, we also analyzed the effect of age on the total power of the synchronized neural response and EEG noise together (ASSR) and the power of the EEG noise alone. Similarly to evaluating SNR as an outcome measure, we found a significant decrease in total power of the ASSR with advancing age (β = −0.00105, SE = 2.51e-04, P < 0.001) but no significant effect on the power of the EEG noise (β = −6.13e-06, SE = 4.68e-06, P = 0.2).
Fig. 7.
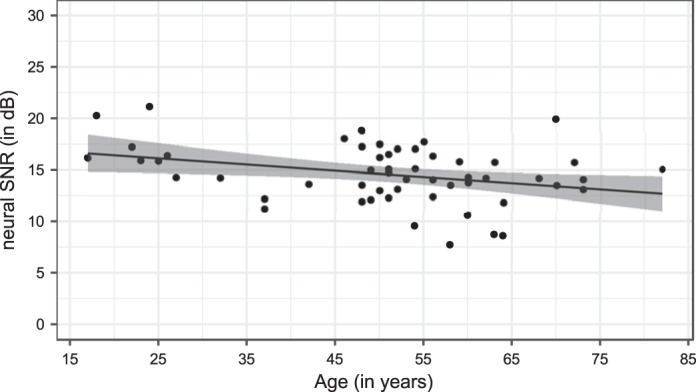
Neural signal-to-noise ratio (SNR) of the auditory steady-state responses to a tone pip, with a repetition frequency of 1.92 Hz, as a function of age. A regression line with confidence intervals (shaded areas) was fitted, representing a linear decrease of the tone pip response with advancing age.
Cognition
In addition to the neural age-related changes in response to frequencies important for speech understanding, it is also known that with advancing age, cognitive functions deteriorate. Moreover, studies have demonstrated a strong association between the decline in cognitive functions, such as working memory and inhibition, and increased difficulties with understanding speech in noise. Below, we first describe the relation between age and the results on two cognitive tasks. Second, we assess the specific contribution of working memory (i.e., RST scores) and inhibition (i.e., Stroop scores) on the matrix SRTs and on envelope tracking, beyond the effect of age.
Cognition in function of age.
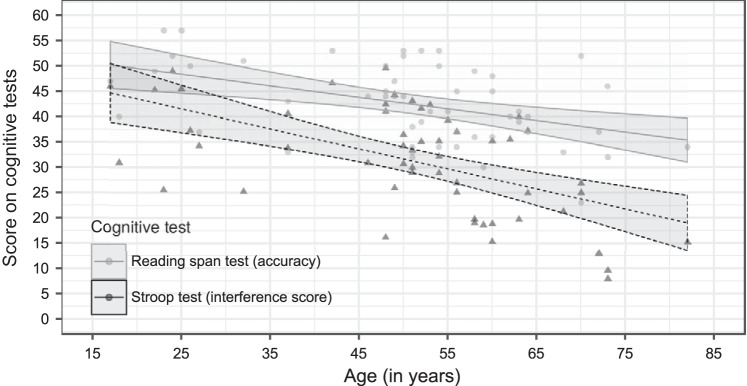
As shown in Fig. 8 and confirmed by the LFM with age as predictor, advancing age resulted in a decrease in cognitive abilities. We found a significant, linear decrease in both the RST scores (β = −0.228, SE = 0.0637, P = 0.001) and Stroop interference scores with advancing age (β = −0.395, SE = 0.0801, p < 0.001). Adding age as a quadratic, i.e., supralinear, fixed-effect term to the LFMs did not significantly improve the fit.
Fig. 8.
Results of the reading span test and the Stroop test as a function of age. The interference score is calculated as 60, subtracted by the difference score between the incongruent and congruent tasks. Regression lines with confidence intervals (shaded areas) show a linear decline in cognitive performance with advancing age for both cognitive tests (color-coded).
Association between cognition, age, the SRT, and envelope tracking.
speech understanding in noise.
To better understand the interplay between cognition, age, and speech understanding in noise, we used likelihood ratio tests and the AIC to evaluate if adding the Stroop or RST scores to the LMM (with age, age2, and masker as the 3 predictors, and their interactions; Table 2) improved the model fit. Adding the Stroop or the RST scores as a main, fixed-effect term did not significantly improve the model fit, but the interaction between age and the Stroop scores resulted in a significant improvement (β = −0.00385, SE = 0.00156, P = 0.02). Moreover, we found that worse matrix SRTs are associated with worse inhibition Stroop scores for older NH adults, whereas young adults show the opposite trend, i.e., better matrix SRTs are associated with worse Stroop scores. Despite this significant interaction effect, only a small extra part of the intersubject variability was explained by adding the Stroop scores to the model (Stroop improvement in Pseudo-R2 = 6.68e-04). In contrast to the Stroop, adding a main fixed-effect term for the RST scores or interaction with age or masker did not result in a significantly improvement of the model fit.
envelope tracking.
Last, we looked into the relation between envelope tracking and the scores on the cognitive tasks by adding the Stroop and RST scores as fixed-effect terms to the best-fitting model (with SU, speech material, masker, age, and age2 as the 5 predictors, and their interactions; Table 3). Although adding the Stroop or RST scores as main fixed-effect terms did not further improve the model fit (P > 0.05), likelihood ratio tests and the AIC showed an improvement when the interaction between the Stroop scores and the type of speech material (Stroop:speech material; Stroop improvement in Pseudo-R2 = 6.24e-03) or the interaction between the RST scores and the type of masker (RST:masker; RST improvement in Pseudo-R2 = 0.0041) was added. More specifically, the interaction between the Stroop scores and speech material indicates that enhanced envelope tracking is associated with worse scores on the Stroop test, especially when the matrix was the target talker (β = 7.73e-04, SE = 2.72e-04, P = 0.005). The interaction between the RST and the type of masker indicates that the association between envelope tracking and the RST scores has a different direction depending on the type of masker (β = 9.96e-04, SE = 3.73e-04, P = 0.008). Moreover, when SWN is the masker, worse RST scores are associated with enhanced envelope tracking, whereas for CT, better RST scores are related to enhanced envelope tracking. However, we have to note that plotting the interaction effect revealed large confidence bounds and that adding the RST scores to the model only explained a small extra amount of the variance.
DISCUSSION
The results of the present study demonstrate that age affects speech understanding in noise as well as neural tracking of the envelope in a supralinear way. More specifically, we found that speech understanding worsens with advancing age and that this effect is more detrimental when speech is embedded in a competing talker than in speech-weighted noise. Furthermore, we found that envelope tracking increases with speech understanding but that the degree of increase depends on the age of the participant as well as the type of speech material and masker that are presented. In contrast to this, the cortical responses to the tone pips decrease linearly with advancing age.
Age Affects Speech Understanding in Noise in a Supralinear Way
Our results are in line with previous studies showing that the matrix SRT worsens with advancing age, despite having audiometric thresholds within the normal range (Füllgrabe et al. 2015; Goossens et al. 2017; Helfer and Freyman 2014). Similarly to the review of Moore et al. (2014), we demonstrated a supralinear increase in the SRT with advancing age. More specifically, our results suggest a similar performance for young and middle-aged NH adults and a decreasing speech-in-noise performance starting from 50 yr of age. These results show the importance of the choice of age range when a study is performed. Moreover, the difference in SRT will be substantially larger when groups of young and older NH adults are compared when the ages of the older group range from 60 to 70 yr instead of 50 to 60 yr. Taken together, our data show that age should be used as a continuous variable in future studies to gain more detailed insight into the effect of age on speech understanding in noise.
In addition, we found, in line with the literature (Desjardins and Doherty 2013; Festen and Plomp 1990; Goossens et al. 2017; Helfer and Freyman 2014), that the supralinear increase in matrix SRT with age was substantially less steep for SWN compared with CT. This more detrimental age effect for CT could be due to age-related temporal deficits or a decline in cognition with advancing age (Humes et al. 2012; Pichora-Fuller et al. 2016; Working Group on Speech Understanding and Aging 1988). We assessed the specific contribution of these factors by relating the results on two cognitive tasks with speech understanding in noise. We found for the matrix SRTs no significant association between the type of masker and the results on the cognitive tests. Therefore, our results suggest that age-related temporal processing deficits, i.e., deterioration in release from masking when listening in the gaps, rather than cognitive decline, may underlie the differential age effect on the SRT in CT compared with SWN. This is plausible, because previous studies also found evidence for the decreased benefit of listening in the gaps with advancing age (Desjardins and Doherty 2013; Festen and Plomp 1990; Goossens et al. 2017; Helfer and Freyman 2014). Nevertheless, the contribution of cognition cannot entirely be excluded. One explanation can be the possible lack of sensitivity of the cognitive measures used in this study. More specifically, the inhibitory processes activated for the Stroop test may be mainly visually orientated in contrast to the auditory speech-in-noise task. This could be a problem, because the age-related decline of inhibitory processes is likely to be modality dependent (Guerreiro et al. 2010). Another explanation can be the strict inclusion criteria used in the present study. Our participants represent the best performers of a NH population across the adult lifespan, which could lead to nonsignificant effects.
Neural Tracking of the Speech Envelope and Modulations of Nonspeech Sounds Across the Adult Lifespan
Envelope tracking increases in a supralinear way with advancing age.
To our knowledge, this is the first study investigating the effect of age on neural tracking of the envelope of running speech across the adult lifespan, i.e., including young and older but also middle-aged NH adults. Our results are in agreement with a part of the literature that demonstrates an increase in envelope tracking with advancing age (nonspeech sounds: Bidelman et al. 2014; Goossens et al. 2016; Rufener et al. 2016; Sörös et al. 2009; Tlumak et al. 2015; running speech: Presacco et al. 2016a, 2016b). It should be pointed out, however, that we found a supralinear relation between envelope tracking and age that, to our knowledge, has not been demonstrated before. Moreover, a similar envelope tracking was observed for young and middle-aged NH adults and a substantially enhanced envelope tracking for NH adults from 50 yr on (Fig. 5). Thus, in line with the studies including middle-aged participants (Goossens et al. 2016; Tlumak et al. 2015), our results suggest that aging results in a gradual enhancement in envelope tracking, most apparent at an older age. Our data corroborate the findings of previous research that future studies should include middle-aged participants to determine the precise starting point of enhanced envelope tracking with aging.
Envelope tracking increases more with speech understanding for older than young and middle-aged adults.
In line with the results for young adults (Ding and Simon 2012; Vanthornhout et al. 2018), we found an increase in envelope tracking with increasing speech understanding for middle-aged and older NH adults. Thus, on an individual level, envelope tracking increases when the person understands the speech stimulus better. In addition, our results also demonstrate that envelope tracking for older adults increases more steeply with speech understanding than for younger and middle-aged NH adults. In other words, it seems that the effect of age is less apparent at low speech understanding levels (e.g., at 20% SU). On a group level, we found enhanced envelope tracking for older adults even when they achieved a similar speech understanding score or the stimulus was presented at the same SNR as their younger counterparts. Therefore, it seems that higher envelope tracking between individuals does not result from better speech understanding or higher SNRs, but rather is associated with the degree of difficulty that persons experience when processing speech. Goossens et al. (2018) investigated the relation between speech understanding and cortical encoding of the envelope across the lifespan and found that enhanced cortical envelope encoding is related to poorer speech understanding for NH adults. Although enhanced envelope tracking is likely to reflect the speech-in-noise difficulties in older adults, it is still unknown which factors contribute to this enhancement.
A first possible explanation includes the temporal deficits that adults develop with advancing age. In other words, to compensate for the speech-in-noise difficulties, older NH adults rely more on the low modulation frequencies important for speech understanding, i.e., the envelope, resulting in an enhanced envelope tracking. To date, there is evidence for this hypothesis for hearing impaired persons, because studies have shown an increased sensitivity for envelope modulations in persons with sensorineural hearing loss (Millman et al. 2017; Wallaert et al. 2017). However, to our knowledge, no study has found a decreased speech understanding in noise in NH older adults that was associated with increased envelope sensitivity. Second, enhanced enveloped tracking in older NH adults might also be the result of central compensatory gain mechanisms that increase the cortical activity of the same brain region to restore the degraded peripheral input (Chambers et al. 2016). Finally, it is also shown that with advancing age, the connection between brain regions deteriorates and the activity in regions outside the core speech processing network increases (Cabeza 2002; Davis et al. 2008; Peelle et al. 2010; Wong et al. 2009). Hence, decreased connectivity in older adults could result in activating more brain regions (e.g., frontal brain regions; Peelle et al. 2010) or in a higher activation of one specific region (e.g., lateral and inferior to primary left auditory cortex; Brodbeck et al. 2018) to process speech in the same way as their younger counterparts.
A similar explanation can be inferred from a cognitive perspective, where enhanced envelope tracking could reflect the inefficient use of cognitive resources (Presacco et al. 2016a) or the increased effort that older adults experience (e.g., Anderson Gosselin and Gagné 2011; Degeest et al. 2015; Lemke and Besser 2016). In line with Presacco et al. (2016a), we found that enhanced envelope tracking was associated with lower scores on the Stroop test, especially when the matrix sentences were presented. Additionally, the less apparent effect of age at lower understanding levels (e.g., 20% SU) also supports the role of cognition or effort in enhanced envelope tracking. When it is too difficult to understand the target talker, participants are not motivated anymore and are likely to give up (Pichora-Fuller et al. 2016; Wu et al. 2016). As a result, a minimal amount of brain regions similar to younger adults will be active to process the stimulus. In contrast, at a higher level of speech understanding (e.g., 50 or 60% SU), older adults are more motivated and will spend more effort, which could result in a higher envelope tracking. Although this is plausible, we have to note that it is also possible that the subject-specific SNR reflecting 20% SU was too low to reconstruct the envelope from the EEG.
A differential effect of age for running speech vs. tone pips.
In contrast to the enhanced envelope tracking for running speech, we found a linear decrease in cortical ASSRs with advancing age. This is in line with the study of Henry et al. (2017), who found weaker entrainment to frequency-modulated stimuli in older vs. young NH adults. Conversely, studies also showed increases in cortical ASSRs to nonspeech sounds (Goossens et al. 2016; Tlumak et al. 2015). Altogether, it is not likely that the differential age effect on envelope tracking vs. ASSRs to tone pips can be fully attributed to the difference between speech versus nonspeech sounds. Another, more likely, reason could involve the repetition frequency of the presented stimulus. The study of Tlumak et al. (2015) supports this explanation as their results suggest a turning point around 2.5 Hz from larger ASSRs in young compared with middle-aged and older adults to smaller ASSRs for higher modulation frequencies. To confirm this hypothesis, it would be interesting to include multiple modulation frequencies when evaluating the effect of age on nonspeech sounds at a particular modulation frequency.
Future Work
Using running speech, which is a more ecologically valid stimulus than the modulated or repeated sounds used for ABRs or ASSRs, we have demonstrated a supralinear increase in neural envelope tracking with advancing age that can be associated with the decreased speech understanding in noise in older NH adults. Hence, measurement of envelope tracking may have the potential to complement current behavioral speech-in-noise tests in the clinic. However, more studies are needed to understand the underlying reasons and neural mechanisms behind this enhanced envelope tracking. First of all, it would be interesting to also administer cognitive tasks specific for the auditory modality. Second, including listening effort measures such as ratings, EEG-based measures, or dual-task paradigms could be valuable to unravel the different reasons (for review, see McGarrigle et al. 2014; Pichora-Fuller et al. 2016). Last, conducting neural source analysis or using fMRI could also contribute in the search for the underlying mechanisms.
Conclusion
The present study provides new insights into the changes in envelope tracking when we grow older. Envelope tracking increases gradually with advancing age, whereas the tone pip responses linearly decrease. In addition, we find an association between speech understanding and envelope tracking, with a stronger association for older adults. With the cognitive results taken into account, enhanced envelope tracking may be the result of a higher activation of brain regions when older adults process speech. Hence, this could reflect the inefficient use of cognitive resources or increased listening effort, often observed in behavioral research. The relation between speech understanding and envelope tracking across the lifespan supports the use of envelope tracking measures in clinical tests, such as an objective test of speech understanding (Vanthornhout et al. 2018).
GRANTS
This research has received funding from the Europe project and Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Grant Agreement No. 637424). Furthermore, financial support is also provided by KU Leuven Special Research Fund Grant OT/14/119 (to T. Francart), and research of J. Vanthornhout is funded by a PhD grant from the Research Foundation Flanders (FWO).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
L.D., J.V., and T.F. conceived and designed research; L.D. performed experiments; L.D. analyzed data; L.D., J.V., and T.F. interpreted results of experiments; L.D. prepared figures; L.D. drafted manuscript; L.D., J.V., and T.F. edited and revised manuscript; L.D., J.V., and T.F. approved final version of manuscript.
ACKNOWLEDGMENTS
We are very grateful to all participants for help in this study, as well as to master student Elien Van den Borre for help in data acquisition and Annelies Devesse and Sam Denys for help during the screening. Furthermore, we thank Tine Goossens and Charlotte Vercammen for sharing knowledge about conducting research in an aging population, as well as Prof. Astrid van Wieringen for comments on the manuscript.
REFERENCES
- Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr 19: 716–723, 1974. doi: 10.1109/TAC.1974.1100705. [DOI] [Google Scholar]
- Anderson S, Parbery-Clark A, White-Schwoch T, Kraus N. Aging affects neural precision of speech encoding. J Neurosci 32: 14156–14164, 2012. doi: 10.1523/JNEUROSCI.2176-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S, Parbery-Clark A, Yi HG, Kraus N. A neural basis of speech-in-noise perception in older adults. Ear Hear 32: 750–757, 2011. doi: 10.1097/AUD.0b013e31822229d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson Gosselin P, Gagné JP. Older adults expend more listening effort than young adults recognizing speech in noise. J Speech Lang Hear Res 54: 944–958, 2011. doi: 10.1044/1092-4388(2010/10-0069). [DOI] [PubMed] [Google Scholar]
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. J Mem Lang 59: 390–412, 2008. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Bidelman GM, Villafuerte JW, Moreno S, Alain C. Age-related changes in the subcortical-cortical encoding and categorical perception of speech. Neurobiol Aging 35: 2526–2540, 2014. doi: 10.1016/j.neurobiolaging.2014.05.006. [DOI] [PubMed] [Google Scholar]
- Biesmans W, Das N, Francart T, Bertrand A. Auditory-inspired speech envelope extraction methods for improved EEG-based auditory attention detection in a cocktail party scenario. IEEE Trans Neural Syst Rehabil Eng 25: 402–412, 2017. doi: 10.1109/TNSRE.2016.2571900. [DOI] [PubMed] [Google Scholar]
- Brand T, Kollmeier B. Efficient adaptive procedures for threshold and concurrent slope estimates for psychophysics and speech intelligibility tests. J Acoust Soc Am 111: 2801–2810, 2002. doi: 10.1121/1.1479152. [DOI] [PubMed] [Google Scholar]
- Brodbeck C, Presacco A, Anderson S, Simon JZ. Over-representation of speech in older adults originates from early response in higher order auditory cortex. Acta Acust United Acust 104: 774–777, 2018. doi: 10.3813/AAA.919221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol Aging 17: 85–100, 2002. doi: 10.1037/0882-7974.17.1.85. [DOI] [PubMed] [Google Scholar]
- 3rd,Cahana-Amitay D, Spiro A. Sayers JT, Oveis AC, Higby E, Ojo EA, Duncan S, Goral M, Hyun J, Albert ML, Obler LK. How older adults use cognition in sentence-final word recognition. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 5585: 1–27, 2015. doi: 10.1080/13825585.2015.1111291. [DOI] [PubMed] [Google Scholar]
- Cardin V. Effects of aging and adult-onset hearing loss on cortical auditory regions. Front Neurosci 10: 199, 2016. doi: 10.3389/fnins.2016.00199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AR, Resnik J, Yuan Y, Whitton JP, Edge AS, Liberman MC, Polley DB. Central gain restores auditory processing following near-complete cochlear denervation. Neuron 89: 867–879, 2016. doi: 10.1016/j.neuron.2015.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coren S. The lateral preference inventory for measurement of handedness, footedness, eyedness, and earedness: norms for young adults. Bull Psychon Soc 31: 1–3, 1993. doi: 10.3758/BF03334122. [DOI] [Google Scholar]
- Das N, Biesmans W, Bertrand A, Francart T. The effect of head-related filtering and ear-specific decoding bias on auditory attention detection. J Neural Eng 13: 056014, 2016. doi: 10.1088/1741-2560/13/5/056014. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R. Que PASA? The posterior-anterior shift in aging. Cereb Cortex 18: 1201–1209, 2008. doi: 10.1093/cercor/bhm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cheveigné A, Simon JZ. Denoising based on spatial filtering. J Neurosci Methods 171: 331–339, 2008. doi: 10.1016/j.jneumeth.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villers-Sidani E, Alzghoul L, Zhou X, Simpson KL, Lin RCS, Merzenich MM. Recovery of functional and structural age-related changes in the rat primary auditory cortex with operant training. Proc Natl Acad Sci USA 107: 13900–13905, 2010. doi: 10.1073/pnas.1007885107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vos A, Vanvooren S, Vanderauwera J, Ghesquière P, Wouters J. Atypical neural synchronization to speech envelope modulations in dyslexia. Brain Lang 164: 106–117, 2017. doi: 10.1016/j.bandl.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Decruy L, Das N, Verschueren E, Francart T. The self-assessed Békesy procedure: validation of a method to measure intelligibility of connected discourse. Trends Hear 22: 2331216518802702, 2018. doi: 10.1177/2331216518802702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degeest S, Keppler H, Corthals P. The effect of age on listening effort. J Speech Lang Hear Res 58: 1592–1600, 2015. doi: 10.1044/2015_JSLHR-H-14-0288. [DOI] [PubMed] [Google Scholar]
- Desjardins JL, Doherty KA. Age-related changes in listening effort for various types of masker noises. Ear Hear 34: 261–272, 2013. doi: 10.1097/AUD.0b013e31826d0ba4. [DOI] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Emergence of neural encoding of auditory objects while listening to competing speakers. Proc Natl Acad Sci USA 109: 11854–11859, 2012. doi: 10.1073/pnas.1205381109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Simon JZ. Adaptive temporal encoding leads to a background-insensitive cortical representation of speech. J Neurosci 33: 5728–5735, 2013. doi: 10.1523/JNEUROSCI.5297-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drullman R, Festen JM, Plomp R. Effect of temporal envelope smearing on speech reception. J Acoust Soc Am 95: 1053–1064, 1994. doi: 10.1121/1.408467. [DOI] [PubMed] [Google Scholar]
- Etymotic Research Inc ER-3A Insert Earphones for Audiometry (Data sheet). Elk Grove Village, IL: Etymotic Research, 2015. [Google Scholar]
- Festen JM, Plomp R. Effects of fluctuating noise and interfering speech on the speech-reception threshold for impaired and normal hearing. J Acoust Soc Am 88: 1725–1736, 1990. doi: 10.1121/1.400247. [DOI] [PubMed] [Google Scholar]
- Field A, Miles J, Field Z. Mixed designs (GLM 5). In: Discovering Statistics Using R. London: Sage, 2012, p. 604–652. [Google Scholar]
- Francart T, van Wieringen A, Wouters J. APEX 3: a multi-purpose test platform for auditory psychophysical experiments. J Neurosci Methods 172: 283–293, 2008. doi: 10.1016/j.jneumeth.2008.04.020. [DOI] [PubMed] [Google Scholar]
- Francart T, van Wieringen A, Wouters J. Comparison of fluctuating maskers for speech recognition tests. Int J Audiol 50: 2–13, 2011. doi: 10.3109/14992027.2010.505582. [DOI] [PubMed] [Google Scholar]
- Füllgrabe C, Moore BC, Stone MA. Age-group differences in speech identification despite matched audiometrically normal hearing: contributions from auditory temporal processing and cognition. Front Aging Neurosci 6: 347, 2015. doi: 10.3389/fnagi.2014.00347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goman AM, Lin FR. Hearing loss in older adults – from epidemiological insights to national initiatives. Hear Res 369: 29–32, 2018. doi: 10.1016/j.heares.2018.03.031. [DOI] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A. Aging affects neural synchronization to speech-related acoustic modulations. Front Aging Neurosci 8: 133, 2016. doi: 10.3389/fnagi.2016.00133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A. Masked speech perception across the adult lifespan: Impact of age and hearing impairment. Hear Res 344: 109–124, 2017. doi: 10.1016/j.heares.2016.11.004. [DOI] [PubMed] [Google Scholar]
- Goossens T, Vercammen C, Wouters J, van Wieringen A. Neural envelope encoding predicts speech perception performance for normal-hearing and hearing-impaired adults. Hear Res 370: 189–200, 2018. doi: 10.1016/j.heares.2018.07.012. [DOI] [PubMed] [Google Scholar]
- Gordon-Salant S, Cole SS. Effects of age and working memory capacity on speech recognition performance in noise among listeners with normal hearing. Ear Hear 37: 593–602, 2016. doi: 10.1097/AUD.0000000000000316. [DOI] [PubMed] [Google Scholar]
- Grose JH, Mamo SK. Processing of temporal fine structure as a function of age. Ear Hear 31: 755–760, 2010. doi: 10.1097/AUD.0b013e3181e627e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerreiro MJ, Murphy DR, Van Gerven PW. The role of sensory modality in age-related distraction: a critical review and a renewed view. Psychol Bull 136: 975–1022, 2010. doi: 10.1037/a0020731. [DOI] [PubMed] [Google Scholar]
- Guest H, Munro KJ, Prendergast G, Millman RE, Plack CJ. Impaired speech perception in noise with a normal audiogram: no evidence for cochlear synaptopathy and no relation to lifetime noise exposure. Hear Res 364: 142–151, 2018. doi: 10.1016/j.heares.2018.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes J. De Stroop kleur-woord Test (2nd ed.). Lisse, The Netherlands: Swets & Zeitlinger, 1978. [Google Scholar]
- Helfer KS, Freyman RL. Stimulus and listener factors affecting age-related changes in competing speech perception. J Acoust Soc Am 136: 748–759, 2014. doi: 10.1121/1.4887463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helfer KS, Vargo M. Speech recognition and temporal processing in middle-aged women. J Am Acad Audiol 20: 264–271, 2009. doi: 10.3766/jaaa.20.4.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry MJ, Herrmann B, Kunke D, Obleser J. Aging affects the balance of neural entrainment and top-down neural modulation in the listening brain. Nat Commun 8: 15801, 2017. doi: 10.1038/ncomms15801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann M, Wouters J. Improved electrically evoked auditory steady-state response thresholds in humans. J Assoc Res Otolaryngol 13: 573–589, 2012. doi: 10.1007/s10162-012-0321-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm S. A simple sequentially rejective multiple test procedure. Scand J Stat 6: 65–70, 1979. [Google Scholar]
- Hopkins K, Moore BC. The effects of age and cochlear hearing loss on temporal fine structure sensitivity, frequency selectivity, and speech reception in noise. J Acoust Soc Am 130: 334–349, 2011. doi: 10.1121/1.3585848. [DOI] [PubMed] [Google Scholar]
- Hotelling H. The generalization of the student’s ratio. Ann Math Stat 2: 360–378, 1931. doi: 10.1214/aoms/1177732979. [DOI] [Google Scholar]
- Humes LE, Coughlin M. Aided speech-identification performance in single-talker competition by older adults with impaired hearing. Scand J Psychol 50: 485–494, 2009. doi: 10.1111/j.1467-9450.2009.00740.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humes LE, Dubno JR, Gordon-Salant S, Lister JJ, Cacace AT, Cruickshanks KJ, Gates GA, Wilson RH, Wingfield A. Central presbycusis: a review and evaluation of the evidence. J Am Acad Audiol 23: 635–666, 2012. doi: 10.3766/jaaa.23.8.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Organization for Standardization ISO-7029: Acoustics-Statistical Distribution of Hearing Thresholds as a Function of Age (Technical report). Geneva: International Organization for Standardization, 2000. [Google Scholar]
- Janse E. A non-auditory measure of interference predicts distraction by competing speech in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 19: 741–758, 2012. doi: 10.1080/13825585.2011.652590. [DOI] [PubMed] [Google Scholar]
- Janse E, Jesse A. Working memory affects older adults’ use of context in spoken-word recognition. Q J Exp Psychol (Hove) 67: 1842–1862, 2014. doi: 10.1080/17470218.2013.879391. [DOI] [PubMed] [Google Scholar]
- Koelewijn T, Zekveld AA, Festen JM, Kramer SE. Pupil dilation uncovers extra listening effort in the presence of a single-talker masker. Ear Hear 33: 291–300, 2012. doi: 10.1097/AUD.0b013e3182310019. [DOI] [PubMed] [Google Scholar]
- Kong YY, Somarowthu A, Ding N. Effects of spectral degradation on attentional modulation of cortical auditory responses to continuous speech. J Assoc Res Otolaryngol 16: 783–796, 2015. doi: 10.1007/s10162-015-0540-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus N, Anderson S. The effects of aging on auditory processing. Hear J 66: 36, 2013. doi: 10.1097/01.HJ.0000425774.80002.ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalor EC, Pearlmutter BA, Reilly RB, McDarby G, Foxe JJ. The VESPA: a method for the rapid estimation of a visual evoked potential. Neuroimage 32: 1549–1561, 2006. doi: 10.1016/j.neuroimage.2006.05.054. [DOI] [PubMed] [Google Scholar]
- Lalor EC, Power AJ, Reilly RB, Foxe JJ. Resolving precise temporal processing properties of the auditory system using continuous stimuli. J Neurophysiol 102: 349–359, 2009. doi: 10.1152/jn.90896.2008. [DOI] [PubMed] [Google Scholar]
- Leigh-Paffenroth ED, Fowler CG. Amplitude-modulated auditory steady-state responses in younger and older listeners. J Am Acad Audiol 17: 582–597, 2006. doi: 10.3766/jaaa.17.8.5. [DOI] [PubMed] [Google Scholar]
- Lemke U, Besser J. Cognitive load and listening effort: concepts and age-related considerations. Ear Hear 37, Suppl 1: 77S–84S, 2016. doi: 10.1097/AUD.0000000000000304. [DOI] [PubMed] [Google Scholar]
- Lopes da Silva F. EEG and MEG: relevance to neuroscience. Neuron 80: 1112–1128, 2013. doi: 10.1016/j.neuron.2013.10.017. [DOI] [PubMed] [Google Scholar]
- Luts H, Jansen S, Dreschler W, Wouters J. Development and normative data for the Flemish/Dutch Matrix test (Technical report). Leuven, Belgium: ExpORL, KU Leuven, 2014. https://lirias.kuleuven.be/bitstream/123456789/474335/1/Documentation+Flemish-Dutch+Matrix_December2014.pdf. [Google Scholar]
- MacPherson A, Akeroyd MA. Variations in the slope of the psychometric functions for speech intelligibility: a systematic survey. Trends Hear 18: 10–16, 2014. doi: 10.1177/2331216514537722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis RH, Saly GL. Asymmetric hearing loss: definition, validation, and prevalence. Otol Neurotol 29: 422–431, 2008. doi: 10.1097/MAO.0b013e31816c7c09. [DOI] [PubMed] [Google Scholar]
- Martin JS, Jerger JF. Some effects of aging on central auditory processing. J Rehabil Res Dev 42, Suppl 2: 25–44, 2005. doi: 10.1682/JRRD.2004.12.0164. [DOI] [PubMed] [Google Scholar]
- McGarrigle R, Munro KJ, Dawes P, Stewart AJ, Moore DR, Barry JG, Amitay S. Listening effort and fatigue: what exactly are we measuring? A British Society of Audiology Cognition in Hearing Special Interest Group ‘white paper’. Int J Audiol 53: 433–445, 2014. doi: 10.3109/14992027.2014.890296. [DOI] [PubMed] [Google Scholar]
- Millman RE, Mattys SL, Gouws AD, Prendergast G. Magnified neural envelope coding predicts deficits in speech perception in noise. J Neurosci 37: 7727–7736, 2017. doi: 10.1523/JNEUROSCI.2722-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DR, Edmondson-Jones M, Dawes P, Fortnum H, McCormack A, Pierzycki RH, Munro KJ. Relation between speech-in-noise threshold, hearing loss and cognition from 40-69 years of age. PLoS One 9: e107720, 2014. doi: 10.1371/journal.pone.0107720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, Cummings JL, Chertkow H. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 53: 695–699, 2005. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan JA, Power AJ, Mesgarani N, Rajaram S, Foxe JJ, Shinn-Cunningham BG, Slaney M, Shamma SA, Lalor EC. Attentional selection in a cocktail party environment can be decoded from single-trial EEG. Cereb Cortex 25: 1697–1706, 2015. doi: 10.1093/cercor/bht355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Gross J, Davis MH. Phase-locked responses to speech in human auditory cortex are enhanced during comprehension. Cereb Cortex 23: 1378–1387, 2013. doi: 10.1093/cercor/bhs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Troiani V, Wingfield A, Grossman M. Neural processing during older adults’ comprehension of spoken sentences: age differences in resource allocation and connectivity. Cereb Cortex 20: 773–782, 2010. doi: 10.1093/cercor/bhp142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peelle JE, Wingfield A. The neural consequences of age-related hearing loss. Trends Neurosci 39: 486–497, 2016. doi: 10.1016/j.tins.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Kramer SE, Eckert MA, Edwards B, Hornsby BW, Humes LE, Lemke U, Lunner T, Matthen M, Mackersie CL, Naylor G, Phillips NA, Richter M, Rudner M, Sommers MS, Tremblay KL, Wingfield A. Hearing impairment and cognitive energy: the Framework for Understanding Effortful Listening (FUEL). Ear Hear 37, Suppl 1: 5S–27S, 2016. doi: 10.1097/AUD.0000000000000312. [DOI] [PubMed] [Google Scholar]
- Pichora-Fuller MK, Souza PE. Effects of aging on auditory processing of speech. Int J Audiol 42, Suppl 2: S11–S16, 2003. doi: 10.3109/14992020309074638. [DOI] [PubMed] [Google Scholar]
- Picton TW, Vajsar J, Rodriguez R, Campbell KB. Reliability estimates for steady-state evoked potentials. Electroencephalogr Clin Neurophysiol 68: 119–131, 1987. doi: 10.1016/0168-5597(87)90039-6. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D, DebRoy S, Sarkar D; R Core Team . nlme: Linear and Nonlinear Mixed Effects Models (Online). R package version 3.1–131.1, 2017. https://CRAN.R-project.org/package=nlme.
- Poelmans H, Luts H, Vandermosten M, Boets B, Ghesquière P, Wouters J. Auditory steady state cortical responses indicate deviant phonemic-rate processing in adults with dyslexia. Ear Hear 33: 134–143, 2012. doi: 10.1097/AUD.0b013e31822c26b9. [DOI] [PubMed] [Google Scholar]
- Power AJ, Colling LJ, Mead N, Barnes L, Goswami U. Neural encoding of the speech envelope by children with developmental dyslexia. Brain Lang 160: 1–10, 2016. doi: 10.1016/j.bandl.2016.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S. Effect of informational content of noise on speech representation in the aging midbrain and cortex. J Neurophysiol 116: 2356–2367, 2016a. doi: 10.1152/jn.00373.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presacco A, Simon JZ, Anderson S. Evidence of degraded representation of speech in noise, in the aging midbrain and cortex. J Neurophysiol 116: 2346–2355, 2016b. doi: 10.1152/jn.00372.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rufener KS, Oechslin MS, Wöstmann M, Dellwo V, Meyer M. Age-related neural oscillation patterns during the processing of temporally manipulated speech. Brain Topogr 29: 440–458, 2016. doi: 10.1007/s10548-015-0464-0. [DOI] [PubMed] [Google Scholar]
- Schoof T, Rosen S. The role of auditory and cognitive factors in understanding speech in noise by normal-hearing older listeners. Front Aging Neurosci 6: 307, 2014. doi: 10.3389/fnagi.2014.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon RV, Zeng FG, Kamath V, Wygonski J, Ekelid M. Speech recognition with primarily temporal cues. Science 270: 303–304, 1995. doi: 10.1126/science.270.5234.303. [DOI] [PubMed] [Google Scholar]
- Singh-Manoux A, Kivimaki M, Glymour MM, Elbaz A, Berr C, Ebmeier KP, Ferrie JE, Dugravot A. Timing of onset of cognitive decline: results from Whitehall II prospective cohort study. BMJ 344: d7622–d7622, 2012. doi: 10.1136/bmj.d7622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers B, Francart T, Bertrand A. A generic EEG artifact removal algorithm based on the multi-channel Wiener filter. J Neural Eng 15: 036007, 2018. doi: 10.1088/1741-2552/aaac92. [DOI] [PubMed] [Google Scholar]
- Søndergaard P, Torrésani B, Balazs P. The Linear Time Frequency Analysis Toolbox. Int J Wavelets Multiresolution Inf Process 10: 1250032, 2012. doi: 10.1142/S0219691312500324. [DOI] [Google Scholar]
- Søndergaard PL, Majdak P. The auditory modeling toolbox. In: The Technology of Binaural Listening, edited by Blauert J. Berlin: Springer, 2013, p. 33–56. [Google Scholar]
- Sörös P, Teismann IK, Manemann E, Lütkenhöner B. Auditory temporal processing in healthy aging: a magnetoencephalographic study. BMC Neurosci 10: 34, 2009. doi: 10.1186/1471-2202-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tlumak AI, Durrant JD, Delgado RE. The effect of advancing age on auditory middle- and long-latency evoked potentials using a steady-state-response approach. Am J Audiol 24: 494–507, 2015. doi: 10.1044/2015_AJA-15-0036. [DOI] [PubMed] [Google Scholar]
- Tremblay KL, Piskosz M, Souza P. Effects of age and age-related hearing loss on the neural representation of speech cues. Clin Neurophysiol 114: 1332–1343, 2003. doi: 10.1016/S1388-2457(03)00114-7. [DOI] [PubMed] [Google Scholar]
- United Nations World Population Prospects: The 2017 Revision, Key Findings and Advance Tables (ESA/P/WP/248). New York: United Nations, Department of Economic and Social Affairs, Population Division, 2017. https://population.un.org/wpp/Publications/. [Google Scholar]
- van den Noort M, Bosch P, Haverkort M, Hugdahl K. A standard computerized version of the reading span test in different languages. Eur J Psychol Assess 24: 35–42, 2008. doi: 10.1027/1015-5759.24.1.35. [DOI] [Google Scholar]
- Van Eeckhoutte M, Luke R, Wouters J, Francart T. Stability of auditory steady state responses over time. Ear Hear 39: 260–268, 2018. doi: 10.1097/AUD.0000000000000483. [DOI] [PubMed] [Google Scholar]
- van Lier H, Drinkenburg WH, van Eeten YJ, Coenen AM. Effects of diazepam and zolpidem on EEG beta frequencies are behavior-specific in rats. Neuropharmacology 47: 163–174, 2004. doi: 10.1016/j.neuropharm.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Vanthornhout J, Decruy L, Wouters J, Simon JZ, Francart T. Speech intelligibility predicted from neural entrainment of the speech envelope. J Assoc Res Otolaryngol 19: 181–191, 2018. doi: 10.1007/s10162-018-0654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen C, Goossens T, Wouters J, van Wieringen A. How age affects memory task performance in clinically normal hearing persons. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 5585: 264–280, 2017a. doi: 10.1080/13825585.2016.1200005. [DOI] [PubMed] [Google Scholar]
- Vercammen C, van Wieringen A, Wouters J, Francart T. Desynchronisation of auditory steady-state responses related to changes in interaural phase differences: an objective measure of binaural hearing. Int J Audiol 56: 464–471, 2017b. doi: 10.1080/14992027.2017.1288304. [DOI] [PubMed] [Google Scholar]
- Viana LM, O’Malley JT, Burgess BJ, Jones DD, Oliveira CACP, Santos F, Merchant SN, Liberman LD, Liberman MC. Cochlear neuropathy in human presbycusis: Confocal analysis of hidden hearing loss in post-mortem tissue. Hear Res 327: 78–88, 2015. doi: 10.1016/j.heares.2015.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallaert N, Moore BCJ, Ewert SD, Lorenzi C. Sensorineural hearing loss enhances auditory sensitivity and temporal integration for amplitude modulation. J Acoust Soc Am 141: 971–980, 2017. doi: 10.1121/1.4976080. [DOI] [PubMed] [Google Scholar]
- Wayne RV, Johnsrude IS. A review of causal mechanisms underlying the link between age-related hearing loss and cognitive decline. Ageing Res Rev 23, Pt B: 154–166, 2015. doi: 10.1016/j.arr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- Wong PC, Jin JX, Gunasekera GM, Abel R, Lee ER, Dhar S. Aging and cortical mechanisms of speech perception in noise. Neuropsychologia 47: 693–703, 2009. doi: 10.1016/j.neuropsychologia.2008.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization World Report on Ageing and Health Technical report. Geneva: World Health Organization, 2015. [Google Scholar]
- Working Group on Speech Understanding and Aging. Committee on Hearing, Bioacoustics, and Biomechanics, Commission on Behavioral and Social Sciences and Education, National Research Council Speech understanding and aging. J Acoust Soc Am 83: 859–895, 1988. doi: 10.1121/1.395965. [DOI] [PubMed] [Google Scholar]
- Wu PZ, Liberman LD, Bennett K, de Gruttola V, O’Malley JT, Liberman MC. Primary neural degeneration in the human cochlea: evidence for hidden hearing loss in the aging ear. Neuroscience 407: 8–20, 2019. doi: 10.1016/j.neuroscience.2018.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YH, Stangl E, Zhang X, Perkins J, Eilers E. Psychometric functions of dual-task paradigms for measuring listening effort. Ear Hear 37: 660–670, 2016. doi: 10.1097/AUD.0000000000000335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Y, Guan J. Cochlear synaptopathy: a review of hidden hearing loss. J Otorhinolaryngol Disord Treat 1: 1–11, 2018. [Google Scholar]