Abstract
Context
In healthy females, oxytocin levels decrease postmeal, corresponding to increased satiety. The postprandial response of oxytocin in females with anorexia nervosa (AN)/atypical AN is unknown.
Objectives
To determine the pattern of postprandial serum oxytocin levels in females with AN/atypical AN, relationship with appetite, and effect of weight, eating behavior, and endogenous estrogen status.
Design
Cross-sectional.
Setting
Clinical research center.
Participants
67 women (36 with AN [<85% expected body weight (EBW)]; 31 with atypical AN [≥ 85% EBW)]), age 22.4 ± 0.9 (mean ± SEM) years, categorized by weight, restricting vs binge/purge behavior, and estrogen status.
Interventions
Standardized mixed meal.
Main Outcome Measurements
Blood sampling for oxytocin occurred fasting and 30, 60, and 120 minutes postmeal. Subjective appetite was assessed using visual analog scales.
Results
In females with AN/atypical AN, oxytocin levels decreased from fasting to 60 (P = 0.002) and 120 (P = 0.005) minutes postmeal. The decrease in oxytocin from fasting to 120 minutes was greater in females with atypical AN than AN (P = 0.027) and did not differ by restricting vs binge/purge behavior or estrogen status. Controlling for caloric intake, the decrease in oxytocin was inversely related to the decrease in hunger postmeal in females with atypical AN (P = 0.04).
Conclusions
In females with AN/atypical AN, oxytocin levels decrease postmeal, as established in healthy females. Weight, but not restricting vs binge/purging nor endogenous estrogen status, affects postprandial oxytocin levels. The postprandial change in serum oxytocin levels is related to appetite in females with atypical AN only, suggesting a disconnect between oxytocin secretion and appetite in the undernourished state.
Postprandial oxytocin (OT) decrease in anorexia nervosa (AN)/atypical AN. OT change is related to appetite in atypical AN only, suggesting a disconnect between OT and appetite in undernourished state.
Anorexia nervosa (AN), particularly prevalent among young females (1), is characterized by self-imposed caloric restriction with or without episodes of binge eating and/or purging and associated with intense fear of weight gain (2). A chronic course is common, more than one-third of patients never fully recover, and AN is associated with high rates of premature death (3–7). Despite advancements in the multidisciplinary therapeutic approach for AN in recent years, the treatment of AN remains challenging (4, 5, 7–9). Therefore, it is important to improve our understanding of the pathophysiological mechanisms involved in AN to identify potential therapeutic approaches.
The hypothalamic neurohormone oxytocin (OT), an anorexigenic peptide shown to reduce food intake in animal models and humans (10–17), has been implicated in the pathophysiology of AN (18, 19). In addition to modulation of appetite and metabolism, OT has other effects that are clinically relevant in AN, including anxiolytic, antidepressant, and prosocial properties, and anabolic effects on bone (20–22). Low basal levels of peripheral OT have been identified in low-weight as well as weight-restored individuals with AN, and low OT levels are associated with the severity of clinical features, including psychopathology (anxiety and depressive symptoms as well as social-emotional deficits) and bone loss (23–25). Furthermore, genetic polymorphisms of the OT receptor are associated with the severity of eating disorder psychopathology in AN (26).
Although OT is established as an anorexigenic hormone, data on the response of peripheral OT levels to food intake are limited. In a large group of healthy females, we recently demonstrated a significant decrease in peripheral OT levels in response to a standardized mixed meal with a balanced macronutrient content (27). Furthermore, the postprandial drop in OT was associated with subjective appetite independent of menstrual phase, age, and quantity of food consumed; in particular, the less pronounced the decrease of OT after a meal, the lower the postprandial ratings of hunger and the higher the ratings of fullness (27). The effects of food consumption on peripheral oxytocin levels and relationship to subjective appetite have not been established in females with AN.
In this study, we aimed to improve our understanding of the neurobiological role of postprandial OT secretion in AN in a large group of females and spanning across AN and atypical AN, who have similar psychopathology and behavior but higher weight than typical AN (2). The pathophysiology of AN and atypical AN is largely unknown and it is not clear why only some with restrictive eating and associated psychopathology will reach low weights. Because low weight predicts poor outcome and early weight gain is associated with a favorable prognosis (28, 29), divergent physiology between weight groups may help to inform susceptibility to low weight and therefore worse prognosis. We hypothesized a disruption in OT signaling in response to a meal in AN and atypical AN, marked by absence of the expected postprandial decrease in peripheral OT and lack of a relationship between OT secretion and appetite. In a large group of females with AN/atypical AN, we investigated (1) peripheral OT release in response to a mixed meal standardized for macronutrient content; and the effect of body weight, primary restricting vs binge/purge behaviors, and endogenous estrogen status on postprandial OT levels; and (2) relationships between postprandial OT levels and subjective appetite.
Subjects and Methods
We studied 67 females ages 10 to 50 years old with AN [n = 36; operationalized as full Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) criteria for AN and weight <85% of expected body weight (EBW)] and atypical AN [n = 31; operationalized as DSM-5 criteria for atypical AN, including individuals who had never been low weight (n = 16) and individuals who were formerly <85% EBW but at study entry were ≥85% EBW (n = 15)]. All study participants were recruited from the community through advertisements and referrals from health care providers, as well as from the Eating Disorders Clinical and Research Program and Massachusetts General Hospital (MGH)-affiliated and community centers. Women met criteria for AN or atypical AN by the DSM-5 criteria (2), evaluated by either the Structured Clinical Interview from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (SCID-IV) or the Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version. All individuals were categorized by eating behavior into a primary restricting group (n = 56 participants) if weight loss was achieved through dieting, fasting, and/or excessive exercise, and a binge/purge group (n = 14 participants) if the participant had greater than one episode/month of binge eating or purging behavior (i.e., self-induced vomiting, misuse of diuretics, laxatives or enemas) assessed by the SCID-IV.
Outpatient study visits took place at the Translational and Clinical Research Center at Massachusetts General Hospital (MGH) and the MGH Athinoula A. Martinos Center for Biomedical Imaging. Subjects were excluded for any history of psychosis by SCID-IV or Kiddie Schedule for Affective Disorders and Schizophrenia for School Age Children-Present and Lifetime Version, active substance or alcohol abuse within the past month, or suicidal ideation. Other exclusion criteria were diabetes mellitus, hematocrit <30% or potassium <3.0 mmol/L, untreated hypothyroidism, prior history of gastrointestinal tract surgery (including gastrectomy, gastric bypass surgery, and small or large bowel resection), any other medical explanation for low weight (e.g., brain tumor) or unstable medical illness involving cardiovascular, hepatic, renal, respiratory, endocrine, or neurologic systems, use of systemic hormones (oral/transdermal contraceptive) within 8 weeks or use of depot medroxyprogesterone acetate within 3 months, pregnancy, or breastfeeding. If participants were taking psychotropic medications, the dose had to be stable for 6 weeks before study visits.
The study was approved by the Partners Human Research Committee. Informed consent was obtained from subjects at least 18 years old (N = 55) and parents of subjects younger than 18 years (N = 12). Child assent was obtained from subjects younger than 18 years.
A screening visit was conducted by a member of the medical team to determine eligibility for the protocol. Following informed consent/assent, the following were performed: a complete medical history; physical examination, including height, weight, and Tanner staging for puberty (when necessary); and blood and urine collection (serum or urine human chorionic gonadotropin, blood for hematocrit, electrolytes, and TSH). In cases in which the family could provide records of the laboratory work required for study eligibility performed within 2 months of the screening visit, these laboratories were not repeated, except for urine human chorionic gonadotropin testing.
Qualifying participants returned for a main study visit and were asked to fast overnight before the morning blood draw. Medical history and physical examination findings since the screening visit were updated. Percentage of EBW was calculated for all subjects, body mass index [BMI, weight (kg)/height (m2)] was calculated for subjects ≥20 years, and both BMI and BMI z score were calculated for subjects <20 years. A mixed meal standardized for macronutrient content of ∼400 kcal was given to all participants. Participants could choose among menu options prepared by a dietitian with similar macronutrient content of approximately 20% protein, 20% fat, and 60% carbohydrates (e.g., cereal with whole milk and yogurt; bagel with peanut butter, dried cranberries, and nonfat milk; or toast with peanut butter, yogurt, dried cranberries, and lactose-free milk). A mixed meal of 400 kcal has previously been shown to induce endocrine and brain circuitry responses in humans (30, 31), including a decrease in serum OT in healthy females (27). Participants were asked to eat the entire meal over 15 minutes. Quantification of nutrient intake was performed by a dietitian. Quantity of food consumed was calculated as total kilocalories consumed per body weight. Subjective appetite was assessed immediately prior and immediately after the meal using visual analog scales, a reliable and widely used method to assess subjective appetite (32). Subjects were asked to answer questions about appetite by making a mark on a 10-cm line with extremes on either end indicating how they felt in terms of hunger and fullness at that moment. Total scores were calculated by measuring the distance from the left side of the line in cm, resulting in scores ranging from 0 to 10. An IV catheter was placed, and blood sampling for OT was performed fasting immediately before the meal and 30, 60, and 120 minutes after the start of the meal. Disordered eating psychopathology was assessed with the Eating Disorder Examination (EDE), which is a clinical interview. Symptoms of eating disorder psychopathology are assessed in a semistructured clinical interview, and from the obtained answers, scores for four subscales (Restraint, Eating Concern, Weight Concern, and Shape Concern) and a global score are calculated. Scores range from 0 to 6 with higher scores indicating increased symptom severity (33).
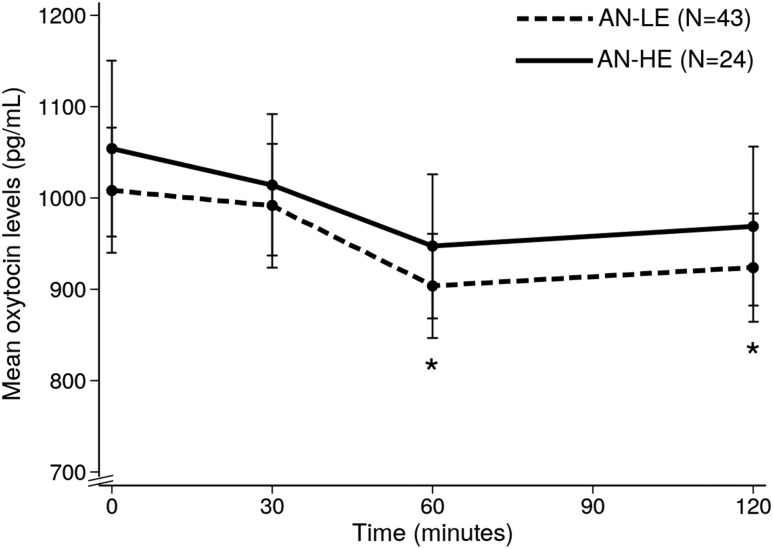
Participants were categorized by current estrogen status. If they were in the early to mid-follicular phase (day 1 to 10 of the menstrual cycle, when estrogen levels are lowest), amenorrheic for at least the last 3 consecutive months, or premenarcheal, they were considered to have lower estrogen levels (AN-LE, n = 43 participants). All other participants were classified as having higher estrogen levels (AN-HE, n = 24 participants). Results in postprandial OT levels did not differ when the three premenarcheal females were excluded from analysis.
Biochemical measurements
Serum samples were stored at −80°C. Samples from all study subjects were run in a single batch. OT concentration was measured in unextracted serum by an ELISA in the Brigham Research Assay Core Laboratory using reagents purchased from Enzo Life Sciences, Farmingdale, NY. We have previously demonstrated a robust correlation between extracted and unextracted serum OT levels (34). The assay has a detection limit of 15 pg/mL. In-house quality-control samples had a mean of 81 and 120 pg/mL, and low- and high-quality control pools between-assay coefficient of variation (CV) of 18 and 20%, respectively. The cross-reactivity of Lys8-vasopressin, Arg8-vasopressin, met-enkephalin, vasoactive intestinal polypeptide, somatostatin, Ser4, Ile8-OT, and alpha-atrial natriuretic polypeptide in the OT assay is <0.02%. Serum estradiol and progesterone were available in 47 (70%) of study participants. Serum estradiol was measured using liquid chromatography-tandem mass spectrometry. The assay had a detection limit of 1 pg/mL, intra-assay CV <5%, and interassay CV <12%. Serum progesterone was analyzed using liquid chromatography-tandem mass spectrometry. The assay had detection limit of 0.05 ng/mL, intra-assay CV <9.3%, and interassay CV <10.8%. Other biochemical measurements were obtained using standard techniques.
Statistical analysis
Nonnormally distributed variables were logarithmically transformed when possible to approximate a normal distribution. Spearman rank test was used to investigate correlations between OT levels and ratings of hunger and fullness. Comparisons between two groups were performed using Student t test or Mann-Whitney U test, and within group comparisons of hormone levels at different timepoints were performed using the 2-sided paired t test or Wilcoxon signed-rank test, as appropriate. Multivariate linear regression analysis was constructed to assess the relationship between ratings of subjective appetite and OT parameters after controlling for quantity of food consumed. Statistical significance was defined as a two-tailed P value <0.05. Quantitative data are reported as mean ± SEM (normal distribution) or as median and interquartile range (nonnormal distribution), and categorical data as percentages. STATA software, V.14.2 (StataCorp LLC, College Station, TX), was used for statistical analysis.
Results
Clinical characteristics of the participants
Clinical characteristics of all participants are summarized in Table 1. Mean age was 22.4 ± 0.9 years and mean %EBW was 85.4 ± 1.3. All three premenarcheal participants were prepubertal (Tanner stage 1). Thirty-eight subjects (56.7%) were on antidepressant medication, 23 (34.3%) on anxiolytic medication, 7 (10.5%) on antipsychotic medication, 2 (3.0%) on psychostimulant medication, and 1 (1.5%) was taking lithium. Use of psychiatric medication did not differ by weight (AN vs atypical AN; P ≥ 0.437), restricting vs binge/purge behavior group (P ≥ 0.210), or estrogen status (P ≥ 0.239). Four patients (5.9%) were on levothyroxine for primary hypothyroidism with normal TSH and 18 (26.9%) were not taking any medication.
Table 1.
Clinical Characteristics and Oxytocin Parameters of the Study Participants (n = 67)
| Variables | All Participants |
|---|---|
| Age, y | 22.4 ± 0.9 |
| BMI z score (<20 y, n = 28) | −1.3 ± 0.1 |
| BMI (≥20 y, n = 39), kg/m2 | 17.9 ± 0.2 |
| Expected body weight, % | 85.4 ± 1.3 |
| Restricting/binge-purge behavior, n (%) | 53 (79)/14 (21) |
| Premenarchal, n (%) | 3 (4.5) |
| Lower estrogen status, n (%)a | 43 (64.2) |
| Higher estrogen status, n (%) | 24 (35.8) |
| Kilocalories consumed | 376.9 ± 9.9 |
| EDE global score | 3.0 ± 0.2 |
| Oxytocin parameters | |
| OT T0, pg/mL | 1024.6 ± 55.4 |
| OT T30 pg/mL | 999.6 ± 51.0 |
| Difference in OT from T0 to T30, pg/mL | −24.9 ± 28.4 |
| OT T60, pg/mL | 919.5 ± 45.8 |
| Difference in OT from T0 to T60 (pg/mL) | −105.1 ± 32.6 (0.002)b |
| OT T120, pg/mL | 940.2 ± 48.7 |
| Difference in OT from T0 to T120 (pg/mL) | −84.4 ± 28.9 (0.005)b |
| OT nadir, pg/mL | 820.4 ± 42.6 |
| Difference in OT from T0 to nadir (pg/mL) | −204.3 ± 24.7 (<0.001)b |
| Change in OT from T0 to nadir, % | −17.8 ± 1.9 |
Data are reported as mean ± SEM.
Abbreviations: T0, time 0 (fasting); T30, time 30 min following the meal; T60, time 60 min following the meal; T120, time 120 min following the meal.
Lower estrogen status includes females who are in the early to mid-follicular phase of the menstrual cycle, amenorrheic, or premenarcheal.
P value compared with baseline oxytocin (paired t test).
Oxytocin response to mixed meal in full sample of AN and atypical AN participants
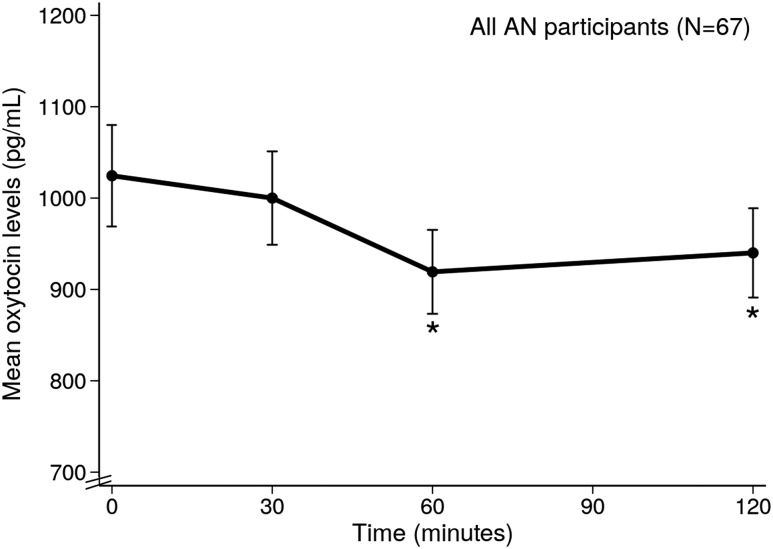
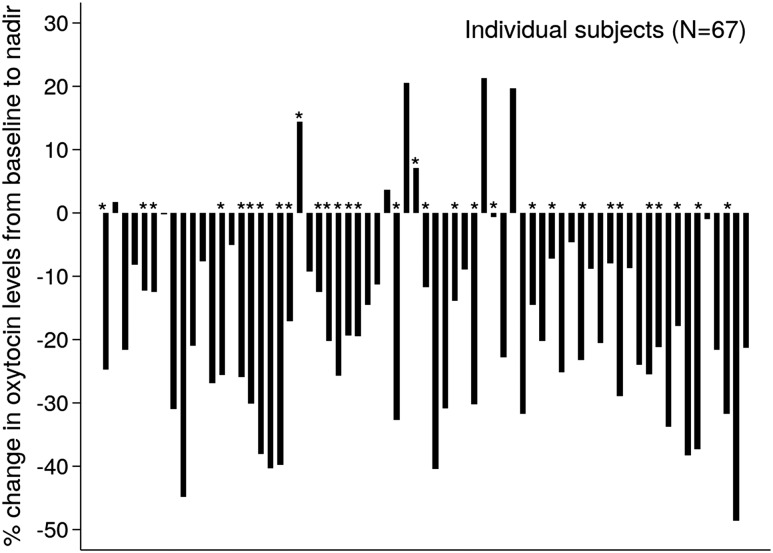
On average, AN and atypical AN participants consumed 376.9 ± 9.9 kcal (89.5 ± 0.02%) of the mean 420.5 ± 0.9 kcal standardized mixed meal provided. Table 1 reports OT levels while fasting and in response to the mixed meal in all participants. Mean OT levels significantly decreased from fasting (1024.6 ± 55.4 pg/mL) to 60 and 120 minutes after the standardized mixed meal across the full sample (Fig. 1). The lowest OT level in each subject at any of the three timepoints following the meal was considered to be the OT nadir; mean OT nadir levels were 820.4 ± 42.6 pg/mL. Mean percentage change in OT from baseline to nadir was −17.8 ± 1.9%. A numerical decrease in OT levels after the meal was consistently observed in 60 subjects (89.6% of the participants) (Fig. 2). Clinical characteristics (age, %EBW, restricting vs binge/purge behavior, estrogen status, quantity of food consumed, psychiatric medication) did not differ between patients who had a decrease in OT levels following the meal and those who did not. Nadir OT levels were observed 30 minutes after the meal in 15 (22.3%), at 60 minutes in 29 (43.4%) participants, and at 120 minutes in 25 (37.3%) participants.
Figure 1.
Mean oxytocin levels at all time points after meal in all AN/atypical AN participants. *P < 0.05 (compared with baseline oxytocin levels).
Figure 2.
Percentage change in oxytocin from baseline (fasting) to nadir in individual subjects. *Participants with atypical AN (N=31).
Oxytocin response in AN vs atypical AN
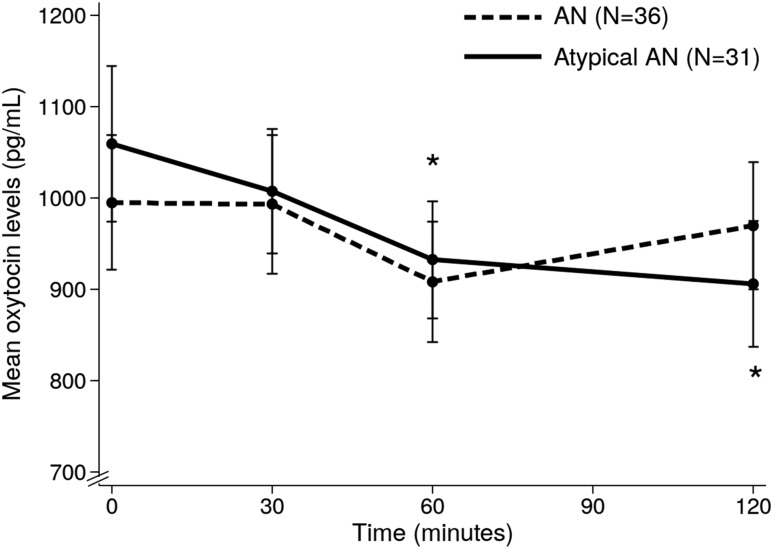
AN and atypical AN groups did not differ in mean age (23.6 ± 1.1 vs 21.1 ± 1.5 years, P = 0.165, respectively), restricting vs binge/purge behavior (P = 0.773), estrogen status (P = 0.444), severity of disordered eating psychopathology (by EDE global score: 3.31 ± 0.24 vs 2.71 ± 0.26, respectively, P = 0.101), or quantity of food consumed at the test meal (367.3 ± 16.2 vs 388.2 ± 9.9 kcal, respectively, P = 0.295). Per study design, %EBW was lower in AN compared with atypical AN (77.9 ± 0.8 vs 94.0 ± 1.4%, P < 0.0001). Mean OT levels did not differ between groups at any timepoint (Fig. 3). A numerical decrease in OT levels after the meal was consistently observed in 31 AN and in 29 atypical AN participants (86.1% vs 93.5%, respectively) (Fig. 2). A significant decrease in OT levels from baseline to 60 and 120 minutes after the meal was observed in atypical AN but not in AN participants. The absolute decrease and % change in OT from fasting to 120 minutes was greater in atypical AN compared with AN (−152.9 ± 35.7 vs −25.4.2 ± 42.2 pg/mL, P = 0.027; −11.1 ± 3.7 vs 2.4 ± 5.8%, P = 0.039).
Figure 3.
Postprandial oxytocin profile in AN/atypical AN participants according to body weight. AN, (%EBW <85); atypical AN, (%EBW ≥85). *P < 0.05 (compared with baseline oxytocin levels in atypical AN group).
Oxytocin response by restricting vs binge/purge behavior group
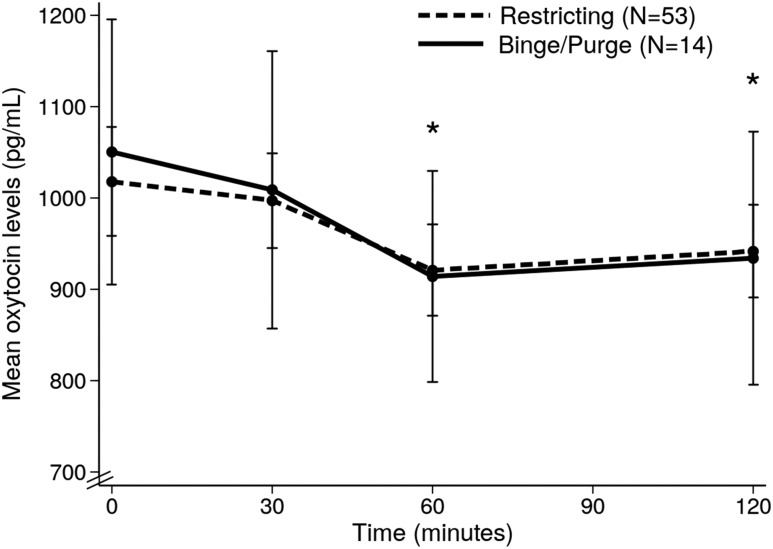
Restricting and binge/purge groups did not differ in mean age (P = 0.217), %EBW (P = 0.811), estrogen status (P = 0.547), severity of disordered eating psychopathology (by EDE global score; P = 0.487), or quantity of food consumed at the test meal (377.1 ± 11.7 vs 376.4 ± 17.2 kcal, respectively, P = 0.977). No differences in OT parameters were observed between restricting and binge/purge groups at any timepoint (Fig. 4).
Figure 4.
Postprandial oxytocin profile in AN/atypical AN participants according to restricting vs binge/purge behaviors groups. *P < 0.05 (compared with baseline oxytocin levels in restricting behavior group).
Effects of endogenous estrogen status on OT postprandial levels
AN-LE and AN-HE did not differ in mean age (P = 0.254), restricting vs binge/purge behavior groups (P = 0.546), %EBW (P = 0.698), severity of disordered eating psychopathology (by EDE global score; P = 0.699), or quantity of food consumed at the test meal (P = 0.545). Per study design, estradiol and progesterone levels were lower in AN-LE than in AN-HE participants (estradiol: 28.0 ±5.1 and 61.0 ± 15.2 pg/mL, respectively, P = 0.016; and progesterone: 0.2 ± 0.2 and 1.9 ± 0.9 ng/dL, respectively, P = 0.013). Mean OT levels did not differ according to estrogen status (Fig. 5) nor based on estradiol levels (less than vs greater than or equal to 60 pg/mL) at any timepoint (P ≥ 0.502). A significant decrease in OT levels from fasting to 60 and 120 minutes after the meal was observed in AN-LE participants. In AN-HE, which included a smaller number of participants than AN-LE, the OT decrease at 60 and 120 minutes was not statistically significant despite mean absolute decreases in OT levels that were similar to AN-LE.
Figure 5.
Postprandial oxytocin profile in AN/atypical AN participants according to estrogen status. *P < 0.05 (compared with baseline oxytocin levels in the AN-LE group).
Relationship between oxytocin levels and subjective appetite
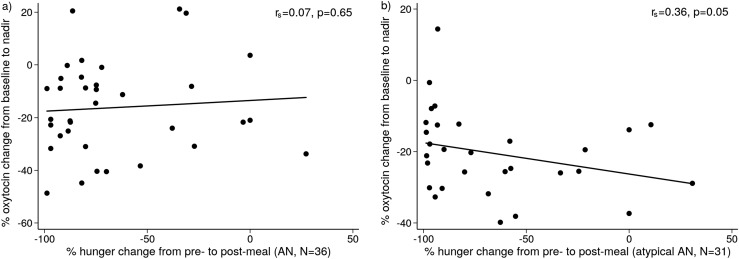
Ratings of subjective appetite did not differ between AN groups (AN vs atypical AN, restricting vs binge/purge, and AN-LE vs AN-HE; P ≥ 0.436). In atypical AN but not AN, there was an inverse association between the decrease in OT levels and the decrease in hunger ratings after the meal (rs = −0.36, P = 0.05) (Fig. 6); this association was significant after controlling for quantity of food consumed (P = 0.04). Specifically, the lesser the postprandial reduction in OT, the greater was the postprandial reduction in hunger ratings in atypical AN. There were no significant associations between postprandial change in OT levels and appetite in the other groups.
Figure 6.
Correlation between percentage change in oxytocin from baseline to nadir and percentage change in ratings of hunger from pre- to postmeal in (a) AN patients (rs = 0.07, P = 0.65) and in (b) atypical AN (rs = 0.36, P = 0.05).
Discussion
This investigation assesses endogenous postprandial OT secretion in a large group of females with AN and atypical AN. This study improves our understanding of OT physiology in response to caloric consumption in females with AN and atypical AN and suggests that low body weight in individuals with AN blunts the postprandial drop in peripheral OT levels and disrupts the relationship between OT and subjective appetite. Whether this disruption of the OT-appetite dynamic in response to food intake in AN is an adaptive response to chronic starvation and/or translates to reduced anorexigenic signaling is an important area for future research.
We recently demonstrated in a large group of healthy females an important decrease in peripheral OT levels at 30 and 60 minutes after a mixed meal followed by a restoration in OT levels at 120 minutes (27). In the current study of females with AN and atypical AN, we also observed a reduction in OT levels following the same mixed meal; this reduction from baseline was significant 60 and 120 minutes after the meal. This delay in the postprandial OT response in AN and atypical AN may be explained by slow gastric emptying and intestinal transit, which has been previously demonstrated in this population (35–37). Whether these differences in the dynamics of postprandial OT secretion between healthy controls and AN/atypical AN have a role in disease pathophysiology is unknown. Although a decrease in OT levels after a meal was consistent in the majority of the subjects, 10% showed a postprandial increase in OT levels despite similar clinical characteristics (age, %EBW, restricting vs binge-purge behavior, estrogen status, quantity of food consumed or use of psychiatric medications). Further studies examining the impact of genetics on OT response to caloric intake may improve our understanding of why some individuals respond differently. Peripheral OT secretion via the posterior pituitary can be synchronous, inversely related, or independent of central OT functioning (38, 39). Interestingly, in healthy females, we have demonstrated an association between OT changes after a meal and subjective appetite measures; here we observed a similar relationship between postprandial OT changes and appetite only in those with atypical AN (≥85% EBW). Our data support prior findings suggesting altered OT dynamics in females with AN (25, 30, 40–42). To our knowledge, prior studies examining postprandial OT patterns in AN are limited to only one study from our group (30). In this previous study, significant differences in OT levels were not detected from baseline to any postprandial time point in AN or healthy controls; however, statistical power was limited by small sample size. Furthermore, this earlier study did not evaluate postprandial OT levels according to AN subtype or the relationship between OT levels and appetite measures. Importantly, the current study evaluates postprandial OT secretion and provides a better understanding of the physiology and dynamics of OT in response to food intake in AN and atypical AN.
Few studies have examined OT levels according to %EBW in AN. Lower cerebrospinal fluid (CSF) OT levels have been found in low-weight AN (<60% EBW) compared with normal-weight women with bulimia nervosa or healthy females (43). Interestingly, low CSF OT levels in underweight AN in the active phase of the disease were followed with a rise in CSF OT levels after weight recovery (85% EBW) (43). In a prior small study, we reported lower fasting and postprandial serum OT levels in weight-recovered AN (at a stable weight with regular menses for at least a year) compared with underweight amenorrheic restrictive subtype AN between 18 and 28 years of age (30). In contrast, here we compared OT secretory patterns in AN (<85% EBW) vs higher weight females with atypical AN across subtypes with variable estrogen exposure and a broad age range (10 to 50 years). Importantly, subtype, estrogen status, and eating disorder psychopathology score were similar in AN vs atypical AN groups. Although there were no between-group differences in OT levels, the postprandial decrease in OT levels previously demonstrated in healthy females and seen here in those with atypical AN, was absent in those with AN. These data suggest that weight status might be a more important determinant of OT dynamics in response to a meal than AN subtype per se, because AN subtype frequency was similar in atypical AN vs AN groups. However, future prospective studies with larger samples also including weight-restored AN would be necessary to clarify if OT dysfunction is a trait marker in those patients with lifetime low-weight AN.
Although studies of OT in females with bulimia nervosa, who have similar (but often more frequent) behaviors to those with binge/purge type but maintain a normal or overweight, have not shown differences in baseline CSF or plasma OT levels compared with controls (41–44), there are limited data available regarding OT levels in AN by restricting vs binge/purge behavior (41). One small study reported low CSF OT concentrations in low-weight females with restricting AN but not those with binge/purge AN compared with controls, but only 5 restricting AN and 12 binge/purge AN were studied and peripheral OT levels were not assessed (43). Consistent with our findings, Monteleone et al. observed no differences in fasting plasma OT levels between AN subtypes in a study of 15 restricting AN and 8 binge/purge AN (41). We additionally did not observe any differences in postprandial OT levels or dynamic changes between restricting vs binge/purge groups. Importantly, our groups were comparable because estrogen status, %EBW, disordered eating psychopathology, use of psychiatric medication, and quantity of food consumed at the test meal did not differ between restricting and binge/purge groups. Although our study included a large number of participants with AN/atypical AN, the number of binge/purge participants was small, limiting our ability to detect differences between eating behavior groups. However, this report of postprandial OT patterns by subtype provides preliminary support for similar dynamics in between restricting and binge/purge AN groups.
In the current study, similar to our prior results in normal-weight healthy females, we show a significant relationship between postprandial change in OT levels and subjective appetite in atypical AN, specifically, the lesser the postprandial reduction in OT, the greater the postprandial reduction in hunger ratings (27). In contrast, this relationship was not observed in AN. Importantly, these differences cannot be attributed to differences in estrogen status or quantity of food consumed at the test meal because these were similar between groups. Our results suggest a dissociation between postprandial OT secretion and appetite in underweight AN, but not in those with atypical AN who are at a higher weight. This lack of a relationship between anorexigenic OT and hunger in females with low-weight AN may represent an adaptive response to chronic undernutrition. Future studies will be important to determine whether there is a resistance to OT effects on appetite in the undernourished state.
The consideration of estrogen status is important when examining OT dynamics in females because estradiol stimulates OT release and endogenous OT levels are affected by menstrual cycle phase, with lower levels in the early to mid-follicular phase (when estradiol levels are low), and a peak at ovulation (when estradiol levels are high) (27, 45–49). Although we previously demonstrated lower fasting and postprandial OT levels in healthy females in the early to mid-follicular phase compared with other menstrual cycle phases (27), we did not observe any differences in fasting or postprandial OT levels in females with AN/atypical AN according to estrogen status, despite significantly higher serum estradiol levels in AN-HE vs AN-LE. Consistent with our data, a prior study showed a failure of OT stimulation in response to oral contraceptive pill administration in low-weight AN compared with controls (42), and no associations were observed between menstrual status and CSF OT levels in patients with AN (43). Because estrogen induces OT gene transcription via the estrogen receptor β in the hypothalamic paraventricular nucleus (50), these prior clinical research studies (42, 43) and ours suggest that the hypothalamic interplay between estrogen and OT might be altered in AN, leading to OT resistance to estrogen stimulation. Interestingly, in the stimulation study, the response to oral contraceptive pill normalized after weight gain (42). In our study, both the lower and higher estrogen status groups included individuals with a range of body weights. Further research is needed specifically focusing on lower weight individuals with AN, weight-recovered AN, and atypical AN to determine whether endogenous estradiol affects OT secretory patterns. Given fluctuations in estradiol levels throughout the menstrual cycle, subtle changes may have been missed by dividing the analysis into only two groups. A future study might focus on more detailed assessments capturing each cycle phase and also including men. Based on our findings in females with AN/atypical AN that body weight, but not estrogen status, affects postprandial OT dynamics, we might expect similar findings in men.
In summary, females with AN and atypical AN showed a decrease in OT levels after a meal, as established in healthy females. Body weight but not endogenous estrogen status or restricting vs binge/purge behaviors impacted postprandial serum OT levels in females with AN/atypical AN, whereas none of these factors influenced fasting serum OT levels. Postprandial OT levels were related to subjective appetite in atypical AN but not low-weight AN, suggesting a disconnect between postprandial OT secretion and appetite in the undernourished state. Whether altered postprandial OT release and the dissociation between OT levels and appetite are a consequence of undernutrition and/or reflect a resistance to the anorexigenic properties of OT in low-weight AN are important areas for future research.
Acknowledgments
The authors thank the Massachusetts General Hospital Translational and Clinical Research Center staff, the MGH Athinoula A. Martinos Center for Biomedical Imaging staff, and study participants.
Financial Support: This work was supported by Fundación Alfonso Martín Escudero (to A.A.); Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustees (to F.P.); and National Institutes of Health Grants R01 MH103402 (to M.M., E.A.L., K.T.E.), R01 MH083657 (A.K.), K23 MH092560 (E.A.L.), P30 DK040561, 1 UL1 TR001102-01, and 8 ULI TR000170-05. Funding sources had no role in the design of the study, data analysis, or writing of the manuscript.
Disclosure Summary: E.A.L. has a financial interest in OXT Therapeutics, a company developing an intranasal oxytocin and long-acting analogs of oxytocin to treat obesity and metabolic disease. E.A.L.’s interests were reviewed and are managed by Massachusetts General Hospital and Partners HealthCare in accordance with their conflict of interest policies. The remaining authors have nothing to disclose.
Glossary
Abbreviations:
- AN
anorexia nervosa
- AN-HE
anorexia nervosa-higher estrogen
- AN-LE
anorexia nervosa-lower estrogen
- BMI
body mass index
- CSF
cerebrospinal fluid
- CV
coefficient of variation
- DSM
Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition
- EBW
expected body weight
- EDE
Eating Disorder Examination
- MGH
Massachusetts General Hospital
- OT
oxytocin
- SCID-IV
Structured Clinical Interview from the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition
References and Notes
- 1. Lindvall Dahlgren C, Wisting L, Rø Ø. Feeding and eating disorders in the DSM-5 era: a systematic review of prevalence rates in non-clinical male and female samples. J Eat Disord. 2017;5(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association. [Google Scholar]
- 3. Bulik C. One half of patients with anorexia nervosa fully recovered after 21 years but the other half had a chronic or lethal course. Evid Based Ment Health. 2002;5(2):59. [DOI] [PubMed] [Google Scholar]
- 4. Zipfel S, Löwe B, Reas DL, Deter HC, Herzog W. Long-term prognosis in anorexia nervosa: lessons from a 21-year follow-up study. Lancet. 2000;355(9205):721–722. [DOI] [PubMed] [Google Scholar]
- 5. Fichter MM, Quadflieg N. Six-year course and outcome of anorexia nervosa. Int J Eat Disord. 1999;26(4):359–385. [DOI] [PubMed] [Google Scholar]
- 6. Eddy KT, Tabri N, Thomas JJ, Murray HB, Keshaviah A, Hastings E, Edkins K, Krishna M, Herzog DB, Keel PK, Franko DL. Recovery from anorexia nervosa and bulimia nervosa at 22-year follow-up. J Clin Psychiatry. 2017;78(2):184–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sullivan PF. Mortality in anorexia nervosa. Am J Psychiatry. 1995;152(7):1073–1074. [DOI] [PubMed] [Google Scholar]
- 8. Zeeck A, Herpertz-Dahlmann B, Friederich HC, Brockmeyer T, Resmark G, Hagenah U, Ehrlich S, Cuntz U, Zipfel S, Hartmann A. Psychotherapeutic treatment for anorexia nervosa: a systematic review and network meta-analysis. Front Psychiatry. 2018;9:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hartmann A, Weber S, Herpertz S, Zeeck A; German Treatment Guideline Group for Anorexia Nervosa. Psychological treatment for anorexia nervosa: a meta-analysis of standardized mean change. Psychother Psychosom. 2011;80(4):216–226. [DOI] [PubMed] [Google Scholar]
- 10. Kublaoui BM, Gemelli T, Tolson KP, Wang Y, Zinn AR. Oxytocin deficiency mediates hyperphagic obesity of Sim1 haploinsufficient mice. Mol Endocrinol. 2008;22(7):1723–1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morton GJ, Thatcher BS, Reidelberger RD, Ogimoto K, Wolden-Hanson T, Baskin DG, Schwartz MW, Blevins JE. Peripheral oxytocin suppresses food intake and causes weight loss in diet-induced obese rats. Am J Physiol Endocrinol Metab. 2012;302(1):E134–E144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011;3(12):1169–1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Roberts ZS, Wolden-Hanson T, Matsen ME, Ryu V, Vaughan CH, Graham JL, Havel PJ, Chukri DW, Schwartz MW, Morton GJ, Blevins JE. Chronic hindbrain administration of oxytocin is sufficient to elicit weight loss in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol. 2017;313(4):R357–R371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blevins JE, Graham JL, Morton GJ, Bales KL, Schwartz MW, Baskin DG, Havel PJ. Chronic oxytocin administration inhibits food intake, increases energy expenditure, and produces weight loss in fructose-fed obese rhesus monkeys. Am J Physiol Regul Integr Comp Physiol. 2015;308(5):R431–R438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maejima Y, Rita RS, Santoso P, Aoyama M, Hiraoka Y, Nishimori K, Gantulga D, Shimomura K, Yada T. Nasal oxytocin administration reduces food intake without affecting locomotor activity and glycemia with c-Fos induction in limited brain areas. Neuroendocrinology. 2015;101(1):35–44. [DOI] [PubMed] [Google Scholar]
- 16. Blevins JE, Thompson BW, Anekonda VT, Ho JM, Graham JL, Roberts ZS, Hwang BH, Ogimoto K, Wolden-Hanson T, Nelson J, Kaiyala KJ, Havel PJ, Bales KL, Morton GJ, Schwartz MW, Baskin DG. Chronic CNS oxytocin signaling preferentially induces fat loss in high-fat diet-fed rats by enhancing satiety responses and increasing lipid utilization. Am J Physiol Regul Integr Comp Physiol. 2016;310(7):R640–R658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maejima Y, Aoyama M, Sakamoto K, Jojima T, Aso Y, Takasu K, Takenosihita S, Shimomura K. Impact of sex, fat distribution and initial body weight on oxytocin’s body weight regulation. Sci Rep. 2017;7(1):8599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Lawson EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol. 2017;13(12):700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Plessow F, Eddy KT, Lawson EA. The neuropeptide hormone oxytocin in eating disorders. Curr Psychiatry Rep. 2018;20(10):91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Scantamburlo G, Hansenne M, Fuchs S, Pitchot W, Maréchal P, Pequeux C, Ansseau M, Legros JJ. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology. 2007;32(4):407–410. [DOI] [PubMed] [Google Scholar]
- 21. Neumann ID, Slattery DA. Oxytocin in general anxiety and social fear: a translational approach. Biol Psychiatry. 2016;79(3):213–221. [DOI] [PubMed] [Google Scholar]
- 22. Tamma R, Colaianni G, Zhu LL, DiBenedetto A, Greco G, Montemurro G, Patano N, Strippoli M, Vergari R, Mancini L, Colucci S, Grano M, Faccio R, Liu X, Li J, Usmani S, Bachar M, Bab I, Nishimori K, Young LJ, Buettner C, Iqbal J, Sun L, Zaidi M, Zallone A. Oxytocin is an anabolic bone hormone. Proc Natl Acad Sci USA. 2009;106(17):7149–7154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Afinogenova Y, Schmelkin C, Plessow F, Thomas JJ, Pulumo R, Micali N, Miller KK, Eddy KT, Lawson EA. Low fasting oxytocin levels are associated with psychopathology in anorexia nervosa in partial recovery. J Clin Psychiatry. 2016;77(11):e1483–e1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schmelkin C, Plessow F, Thomas JJ, Gray EK, Marengi DA, Pulumo R, Silva L, Miller KK, Hadjikhani N, Franko DL, Eddy KT, Lawson EA. Low oxytocin levels are related to alexithymia in anorexia nervosa. Int J Eat Disord. 2017;50(11):1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lawson EA, Donoho DA, Blum JI, Meenaghan EM, Misra M, Herzog DB, Sluss PM, Miller KK, Klibanski A. Decreased nocturnal oxytocin levels in anorexia nervosa are associated with low bone mineral density and fat mass. J Clin Psychiatry. 2011;72(11):1546–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Acevedo SF, Valencia C, Lutter M, McAdams CJ. Severity of eating disorder symptoms related to oxytocin receptor polymorphisms in anorexia nervosa. Psychiatry Res. 2015;228(3):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aulinas A, Pulumo RL, Elisa A, Mancuso CC, Meghan S, Tolley C, Plessow F, Thomas JJ, Eddy KT, Miller KK, Klibanski A, Misra M, Lawson EA. Endogenous oxytocin levels in relation to food intake, menstrual phase, and age in females. J Clin Endocrinol Metab. 2019;104(4):1348–1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Madden S, Miskovic-Wheatley J, Wallis A, Kohn M, Hay P, Touyz S. Early weight gain in family-based treatment predicts greater weight gain and remission at the end of treatment and remission at 12-month follow-up in adolescent anorexia nervosa. Int J Eat Disord. 2015;48(7):919–922. [DOI] [PubMed] [Google Scholar]
- 29. Fichter MM, Quadflieg N, Crosby RD, Koch S. Long-term outcome of anorexia nervosa: Results from a large clinical longitudinal study. Int J Eat Disord. 2017;50(9):1018–1030. [DOI] [PubMed] [Google Scholar]
- 30. Lawson EA, Holsen LM, Santin M, Meenaghan E, Eddy KT, Becker AE, Herzog DB, Goldstein JM, Klibanski A. Oxytocin secretion is associated with severity of disordered eating psychopathology and insular cortex hypoactivation in anorexia nervosa. J Clin Endocrinol Metab. 2012;97(10):E1898–E1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hunt KF, Dunn JT, le Roux CW, Reed LJ, Marsden PK, Patel AG, Amiel SA. Differences in regional brain responses to food ingestion after Roux-en-Y gastric bypass and the role of gut peptides: a neuroimaging study. Diabetes Care. 2016;39(10):1787–1795. [DOI] [PubMed] [Google Scholar]
- 32. Flint A, Raben A, Blundell JE, Astrup A. Reproducibility, power and validity of visual analogue scales in assessment of appetite sensations in single test meal studies. Int J Obes Relat Metab Disord. 2000;24(1):38–48. [DOI] [PubMed] [Google Scholar]
- 33. Cooper Z, Fainburn C. The eating disorder examination: A semi‐structured interview for the assessment of the specific psychopathology of eating disorders. Int J Eat Disord. 1987;6(1):1–8. [Google Scholar]
- 34. Lawson EA, Ackerman KE, Estella NM, Guereca G, Pierce L, Sluss PM, Bouxsein ML, Klibanski A, Misra M. Nocturnal oxytocin secretion is lower in amenorrheic athletes than nonathletes and associated with bone microarchitecture and finite element analysis parameters. Eur J Endocrinol. 2013;168(3):457–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bluemel S, Menne D, Milos G, Goetze O, Fried M, Schwizer W, Fox M, Steingoetter A. Relationship of body weight with gastrointestinal motor and sensory function: studies in anorexia nervosa and obesity. BMC Gastroenterol. 2017;17(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Norris ML, Harrison ME, Isserlin L, Robinson A, Feder S, Sampson M. Gastrointestinal complications associated with anorexia nervosa: a systematic review. Int J Eat Disord. 2016;49(3):216–237. [DOI] [PubMed] [Google Scholar]
- 37. Hutson WR, Wald A. Gastric emptying in patients with bulimia nervosa and anorexia nervosa. Am J Gastroenterol. 1990;85(1):41–46. [PubMed] [Google Scholar]
- 38. Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. Alpha-melanocyte-stimulating hormone stimulates oxytocin release from the dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypophysis. J Neurosci. 2003;23(32):10351–10358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Amico JA, Challinor SM, Cameron JL. Pattern of oxytocin concentrations in the plasma and cerebrospinal fluid of lactating rhesus monkeys (Macaca mulatta): evidence for functionally independent oxytocinergic pathways in primates. J Clin Endocrinol Metab. 1990;71(6):1531–1535. [DOI] [PubMed] [Google Scholar]
- 40. Maguire S, O’Dell A, Touyz L, Russell J. Oxytocin and anorexia nervosa: a review of the emerging literature. Eur Eat Disord Rev. 2013;21(6):475–478. [DOI] [PubMed] [Google Scholar]
- 41. Monteleone AM, Scognamiglio P, Volpe U, Di Maso V, Monteleone P. Investigation of oxytocin secretion in anorexia nervosa and bulimia nervosa: relationships to temperament personality dimensions. Eur Eat Disord Rev. 2016;24(1):52–56. [DOI] [PubMed] [Google Scholar]
- 42. Chiodera P, Volpi R, Capretti L, Marchesi C, d’Amato L, De Ferri A, Bianconi L, Coiro V. Effect of estrogen or insulin-induced hypoglycemia on plasma oxytocin levels in bulimia and anorexia nervosa. Metabolism. 1991;40(11):1226–1230. [DOI] [PubMed] [Google Scholar]
- 43. Demitrack MA, Lesem MD, Listwak SJ, Brandt HA, Jimerson DC, Gold PW. CSF oxytocin in anorexia nervosa and bulimia nervosa: clinical and pathophysiologic considerations. Am J Psychiatry. 1990;147(7):882–886. [DOI] [PubMed] [Google Scholar]
- 44. Frank GK, Kaye WH, Altemus M, Greeno CG. CSF oxytocin and vasopressin levels after recovery from bulimia nervosa and anorexia nervosa, bulimic subtype. Biol Psychiatry. 2000;48(4):315–318. [DOI] [PubMed] [Google Scholar]
- 45. Engel S, Klusmann H, Ditzen B, Knaevelsrud C, Schumacher S. Menstrual cycle-related fluctuations in oxytocin concentrations: a systematic review and meta-analysis. Front Neuroendocrinol. 2019;52:144–155. [DOI] [PubMed] [Google Scholar]
- 46. Amico JA, Seif SM, Robinson AG. Elevation of oxytocin and the oxytocin-associated neurophysin in the plasma of normal women during midcycle. J Clin Endocrinol Metab. 1981;53(6):1229–1232. [DOI] [PubMed] [Google Scholar]
- 47. Kumaresan P, Kumaresan M, Hossini M, Arellano C, Vasicka A. Human ovulation and plasma oxytocin. Int J Gynaecol Obstet. 1983;21(5):413–418. [DOI] [PubMed] [Google Scholar]
- 48. Salonia A, Nappi RE, Pontillo M, Daverio R, Smeraldi A, Briganti A, Fabbri F, Zanni G, Rigatti P, Montorsi F. Menstrual cycle-related changes in plasma oxytocin are relevant to normal sexual function in healthy women. Horm Behav. 2005;47(2):164–169. [DOI] [PubMed] [Google Scholar]
- 49. Shukovski L, Healy DL, Findlay JK. Circulating immunoreactive oxytocin during the human menstrual cycle comes from the pituitary and is estradiol dependent. J Clin Endocrinol Metab. 1989;68(2):455–460. [DOI] [PubMed] [Google Scholar]
- 50. Patisaul HB, Scordalakes EM, Young LJ, Rissman EF. Oxytocin, but not oxytocin receptor, is regulated by oestrogen receptor beta in the female mouse hypothalamus. J Neuroendocrinol. 2003;15(8):787–793. [DOI] [PubMed] [Google Scholar]