Abstract
Aim
To investigate the genetic factors involved in the development of non-alcoholic fatty liver disease (NAFLD) and its sequelae in a Middle Eastern population.
Methods
This genetic case-control association study, conducted in 2018, enrolled 30 patients with NAFLD and 30 control individuals matched for age, sex, and body mass index. After quality control measures, entire exonic regions of 3654 genes associated with human diseases were sequenced. Allelic association test and enrichment analysis of the significant genetic variants were performed.
Results
The association analysis was conducted on 27 NAFLD patients and 28 controls. When Bonferroni correction was applied, NAFLD was significantly associated with rs2303861, a variant located in the CD82 gene (P = 2.49 × 10−7, adjusted P = 0.0059). When we used Benjamini-Hochberg adjustment for correction, NAFLD was significantly associated with six more variants. Enrichment analysis of the genes corresponding to all the seven variants showed significant enrichment for miR-193b-5p (P = 0.00004, adjusted P = 0.00922).
Conclusion
A variant on CD82 gene and a miR-193b expression dysregulation may have a role in the development and progression of NAFLD and its sequelae.
Non-alcoholic fatty liver disease (NAFLD) is the most common liver disease in many parts of the world and an important global health concern (1-4). As a hepatic manifestation of metabolic syndrome, it is closely associated with obesity, insulin resistance, and dyslipidemia (5). NAFLD prevalence is steadily on the rise due to a global increasing trend in obesity incidence (5,6) and within the next decade NAFLD is predicted to replace hepatitis C as the leading indication for liver transplantation in the United States (1).
The main causes of mortality among patients with NAFLD are malignancies and cardiovascular diseases (7,8). The condition is an independent risk factor for hepatocellular carcinoma (HCC), which was previously thought to require liver cirrhosis as its precursor but has been recently described in patients with simple hepatic steatosis and no sign of inflammation or fibrosis (9,10). NAFLD is also closely associated with an increased risk of colorectal cancer (11,12), as well as with myocardial remodeling, thus playing an important role in the development of heart failure (13,14). The observed association between NAFLD, HCC, colorectal cancer, and heart failure implies that there is a possible underpinning defect in cellular processes that link cell metabolism to cell division and metabolite trafficking.
The underlying risk factors for NAFLD (eg, obesity and diabetes mellitus) are associated with numerous genetic polymorphisms (15-17). Genetic factors could also in part explain the extreme variation in the worldwide NAFLD prevalence (6) and considerable inter-individual variability in disease severity, morbidity, and mortality (18). NAFLD was found to be significantly associated with SNP rs738409 (I148M) in PNPLA3 gene on chromosome 22, encoding an enzyme responsible for the hydrolysis of triacylglycerols in adipocytes; individuals homozygous for this allele had more than twice as much hepatic fat content as non-carriers (19). Variants in other genes (eg, MBOAT7, TM6SF2, etc) (20), as well as dysregulated expression of several micro-RNAs (miRNAs), were also found to contribute to NAFLD pathogenesis (21). For instance, miR-33a/b modulates the risk of metabolic syndrome by regulating various metabolic pathways (22).
To the best of our knowledge, no genetic case-control association study has so far been conducted on NAFLD in the Middle Eastern population, despite the fact that the underlying genetic factors of NAFLD and their relative contributions might differ from those in other populations. Therefore, after controlling for traditional risk factors, we investigated the underlying genetic factors involved in the development of NAFLD and its sequelae in an Iranian population.
Materials and methods
Study population
The study included 30 patients with NAFLD (17 female) and 30 healthy controls (17 female) matched for age, sex, and body mass index (BMI), who were randomly selected (with a random number generator) from participants of a cross-sectional population-based study previously conducted in Shiraz (23). Briefly, the previous study had enrolled 542 adult unrelated participants randomly selected from the general population through a proportional cluster random sampling. They had been interviewed to obtain demographic information and physically examined by a medical doctor. Participants with alcohol consumption, participants serologically positive for hepatitis B or hepatitis C, and those identified through the interview to have hepatic steatosis due to other competing etiologies (eg, drugs, bariatric surgery, etc) had been excluded. NAFLD had been ultrasonographically diagnosed by an experienced radiologist unaware of the patients’ clinical information according to a validated protocol (24).
The current study included only patients with confirmed moderate-to-severe hepatic steatosis. The absence of hepatic steatosis in the control group was also documented by ultrasonography.
This study was conducted in compliance with the Declaration of Helsinki. Written informed consent was obtained from all study participants who had been assured that their data would be kept confidential. The study was approved by the Research Ethics Committee of Health Policy Research Center affiliated to Shiraz University of Medical Sciences Ethics Committee.
Clinical and laboratory evaluation
Weight, height, waist circumference, hip circumference, and blood pressure had been measured and BMI was calculated (23). Fasting venous blood samples were obtained, serum was separated, and fasting glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), triacylglycerols, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, hepatitis B surface antigen, and hepatitis C virus antibodies were measured with an automated analyzer (CS-1200 Auto-Chemistry Analyzer, DIRUI Industrial Co, Changchun, China).
DNA preparation, genotyping, and quality control
Genomic DNA was extracted from EDTA-anticoagulated peripheral whole blood collected from each individual with QIAamp DNA isolation mini kit (QIAGEN, Hilden, Germany) by salting out technique. The quality of isolated DNA specimens was assessed by QUBIT 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA). The entire exonic regions of 3654 genes associated with human diseases (inherited disease panel, Agilent Technologies, Santa Clara, CS, USA) were sequenced above 70 × coverage on an Illumina NextSeq 500 platform (Illumina, San Diego, CA, USA). A total of 54 100 variants located in genomic loci of these genes were identified. Target Enrichment for Illumina Multiplexed sequencing protocol (v. D0, November 2015) with SureSelectQXT (Agilent Technologies) was used for individual library preparation and sample pooling. The following quality control measures were performed in PLINK, v. 1.07 (Free Software Foundation Inc., Boston, MA, USA) (25): single nucleotide polymorphisms (SNPs) with minor allele frequency of <0.05, those with significant deviation from the Hardy-Weinberg equilibrium (P < 10−5), and those genotyped for less than half of the studied population were excluded from analysis. All samples were screened for known damaging variants in the ATP7B, HFE, and SERPINA1 genes corresponding to Wilson’s disease, hemochromatosis, and α1-antitrypsin deficiency, respectively. Samples with damaging mutations were excluded from the study.
Statistical analysis
The normality of distribution of continuous variables was tested with the one-sample Kolmogorov-Smirnov test. The variables are presented as mean ± standard deviation. Significance of differences between two groups was tested with the t test for independent samples. The analyses were conducted in IBM SPSS, version 20 (IBM, Armonk, NY, USA).
The association between SNP genotype and NAFLD was assessed with the allelic association test in PLINK, which is a simple χ2 test for association based on a 2 × 3 case-control genotype counts contingency table (25,26). The obtained P values were adjusted with Bonferroni correction for multiple comparisons, with the level of significance set at P < 2.1 × 10−6. Manhattan plot was drawn in Haploview (27). Logistic regression analysis was performed in PLINK using an additive genetic model adjusting for age, sex, and BMI. Genotypes for SNPs with significant P values were recoded as 0, 1, or 2, according to the number of alternative alleles present. Multiple linear regression analysis, performed in SPSS, assessed the effect of the SNP’s alternative alleles that were found significant in allelic association test on clinical and biochemical parameters after adjustment for age, sex, and BMI. False discovery rate for each association was calculated with the Benjamini-Hochberg method (28). SNPs with a false discovery rate <0.05 were included in the enrichment analysis. Information about overlapping or nearest gene corresponding to the associated SNPs was obtained by an SNP annotation tool (29). Enrichment analysis was carried out with Enrichr on miRTarBase database (30,31). Significantly enriched miRNAs were identified from the list of genes corresponding to the SNPs that were found to be significantly different between patients and controls with the Fisher exact test in Enrichr.
Results
After quality control, 55 individuals – 27 patients with NAFLD and 28 controls – and 23 732 SNPs were included in the association analysis. Patients with NAFLD had significantly higher mean fasting blood glucose and serum ALT (P < 0.05) (Table 1). The groups did not differ in any other parameter.
Table 1.
Clinical and biochemical characteristics of cases and controls
| Parameter, mean and standard deviation | NAFLD group (n = 27) | Control group (n = 28) | P |
|---|---|---|---|
| Age (years) |
52.2 (10.1) |
51.0 (9.0) |
0.638 |
| Body mass index (kg/m2) |
28.0 (5.3) |
27.2 (5.1) |
0.569 |
| Fasting blood glucose (mg/dL) |
109.6 (40.4) |
90.4 (14.7) |
0.026 |
| Triacylglycerols (mg/dL) |
159.8 (71.0) |
158.29 (75.6) |
0.940 |
| Low-density lipoprotein cholesterol (mg/dL) |
106.8 (35.6) |
117.7 (27.3) |
0.208 |
| High-density lipoprotein cholesterol (mg/dL) |
45.2 (8.5) |
49.7 (11.0) |
0.093 |
| Aspartate aminotransferase (IU/L) |
28.0 (12.1) |
24.1 (13.6) |
0.273 |
| Alanine aminotransferase (IU/L) |
34.0 (18.5) |
20.2 (8.7) |
0.001 |
| Systolic blood pressure (mm Hg) |
120.6 (16.8) |
120.1 (22.1) |
0.933 |
| Diastolic blood pressure (mm Hg) |
77.9 (8.1) |
78.5 (8.2) |
0.807 |
| Waist circumference (cm) |
96.2 (18.6) |
95.0 (11.5) |
0.763 |
| Hip circumference (cm) | 103.7 (18.9) | 104.1 (8.5) | 0.911 |
When Bonferroni correction was applied, NAFLD was significantly associated with rs2303861, located on CD82 gene (Figure 1, Table 2). After adjusting for age, sex, and BMI, the alternative allele of ‘G’ was associated with a 9-fold increase in the risk of developing NAFLD compared with the wild type ‘A’ (odds ratio 9.07, 95% confidence interval 1.92-42.81). When Benjamini-Hochberg method was applied, six more SNPs were found to have false discovery rates of <0.05 (Table 2), meaning that they were also associated with NAFLD development.
Figure 1.
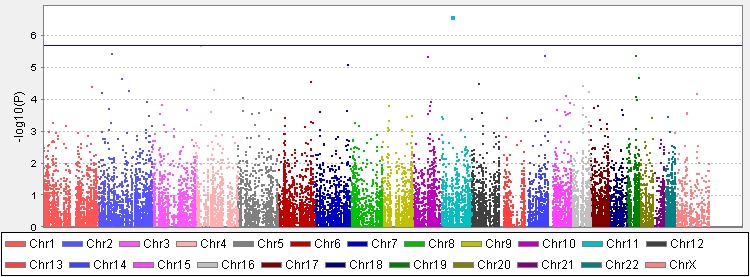
The Manhattan plot based on the data obtained from the allelic association test. The x-axis represents the chromosomal location of the studied single nucleotide polymorphisms (SNPs). The y-axis is –log10(P value). The solid horizontal line corresponds to the Bonferroni-adjusted significance threshold, a P value of 2.1 × 10−6.
Table 2.
Leading single nucleotide polymorphisms (SNPs) associated with non-alcoholic fatty liver disease (NAFLD) identified by the allelic association test. The adjusted odds ratio (OR) and confidence interval (CI) were obtained in logistic regression analysis with an additive genetic model adjusting for age, sex, and body mass index
| Chromosome | SNP ID | Position | Gene | Wild/alternative allele | P (unadjusted) | P (Bonferroni correction) | FDR-BH* | Adjusted OR (95% CI) |
|---|---|---|---|---|---|---|---|---|
| 11 |
rs2303861 |
44618466 |
CD82 |
A/G |
2.49 × 10−7 |
0.0059 |
0.0059 |
9.07 (1.92-42.81) |
| 4 |
rs13116873 |
26362678 |
RBPJ |
C/G |
2.12 × 10−6 |
0.0502 |
0.0181 |
13.91 (2.38-81.45) |
| 2 |
rs4671501 |
63404754 |
WDPCP |
A/C |
3.69 × 10−6 |
0.0875 |
0.0181 |
0.10 (0.02-0.58) |
| 14 |
rs1742546 |
91417155 |
CCDC88C |
G/A |
4.15 × 10−6 |
0.0984 |
0.0181 |
0.25 (0.10-0.61) |
| 19 |
rs2075754 |
41871176 |
RPS19 |
T/C |
4.39 × 10−6 |
0.1043 |
0.0181 |
4.96 (1.57-15.66) |
| 10 |
rs3752752 |
71695444 |
CDH23 |
T/C |
4.57 × 10−6 |
0.1085 |
0.0181 |
4.52 (1.71-11.92) |
| 7 | rs740949 | 148808972 | EZH2 | A/G | 8.37 × 10−6 | 0.1987 | 0.0284 | 0.19 (0.06-0.57) |
*False discovery rate obtained by Benjamini-Hochberg method.
Multiple linear regression analysis did not show any significant association between the seven SNP alleles and clinical and biochemical parameters, taking into account the multiple statistical tests performed (Table 3). Enrichment analysis of the genes corresponding to the seven SNPs found in the association study showed significant enrichment for miR-193b-5p (P = 0.00004, adjusted P = 0.00922).
Table 3.
Association between significant single nucleotide polymorphisms (SNPs) and clinical and biochemical variables in non-alcoholic fatty liver disease (NAFLD) patients and controls. Coefficients (B) and their standard errors (SE) and P values were obtained from multiple linear regression analysis adjusted for age, sex, and body mass index
| SNP |
Subject |
Aspartate aminotransferase |
Alanine aminotransferase |
Fasting blood glucose |
Triacylglycerols |
Low-density lipoprotein |
High-density lipoprotein |
Systolic blood pressure |
Diastolic blood pressure |
Waist circumference |
Hip circumference |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
B (SE) |
P |
||
| rs2303861 |
Control |
0.09 (3.89) |
0.982 |
-1.71 (2.51) |
0.502 |
-1.89 (3.82) |
0.625 |
-2.22 (22.78) |
0.923 |
-2.99 (8.18) |
0.718 |
2.28 (3.00) |
0.454 |
12.80(4.88) |
0.015 |
1.90(2.22) |
0.401 |
3.15 (2.31) |
0.185 |
-0.48(1.55) |
0.761 |
| NAFLD |
-2.63 (2.82) |
0.362 |
-2.76 (3.69) |
0.463 |
-2.15 (8.52) |
0.803 |
-7.94 (16.76) |
0.64 |
-6.14 (7.07) |
0.395 |
0.78 (1.90) |
0.687 |
1.97 (3.17) |
0.542 |
0.52 (1.87) |
0.782 |
-1.12 (3.06) |
0.716 |
-1.07 (3.05) |
0.729 |
|
| rs13116873 |
Control |
7.37 (8.55) |
0.397 |
10.78 (5.20) |
0.049 |
-0.64 (8.58) |
0.941 |
17.39 (50.81) |
0.735 |
-2.86 (18.33) |
0.877 |
-0.80 (6.78) |
0.907 |
21.55 (11.60) |
0.076 |
2.41 (5.03) |
0.636 |
-6.58 (5.19) |
0.218 |
-5.86 (3.25) |
0.084 |
| NAFLD |
0.16 (3.18) |
0.96 |
2.08 (4.11) |
0.618 |
17.28 (8.71) |
0.06 |
5.06 (18.63) |
0.788 |
3.01 (7.94) |
0.709 |
1.39 (2.09) |
0.512 |
4.45 (3.42) |
0.206 |
2.35 (2.02) |
0.256 |
4.49 (3.26) |
0.182 |
5.17 (3.20) |
0.12 |
|
| rs4671501 |
Control |
-0.09 (2.32) |
0.971 |
-2.00 (2.93) |
0.505 |
0.94 (3.78) |
0.808 |
-4.01 (17.37) |
0.817 |
-8.75 (7.15) |
0.241 |
0.44 (3.42) |
0.899 |
11.66 (5.99) |
0.072 |
3.59 (2.65) |
0.197 |
3.42 (2.05) |
0.117 |
1.43 (1.45) |
0.338 |
| NAFLD |
-10.33 (14.69) |
0.49 |
-26.23 (18.44) |
0.171 |
-60.50 (41.90) |
0.165 |
-75.54 (86.53) |
0.394 |
-62.85 (35.00) |
0.088 |
22.68 (7.87) |
0.01 |
-6.05 (16.48) |
0.718 |
-1.08 (9.66) |
0.912 |
6.53 (8.40) |
0.447 |
-2.91 (4.97) |
0.566 |
|
| rs1742546 |
Control |
-15.89 (4.27) |
0.003 |
-6.04 (3.56) |
0.118 |
-3.88 (4.90) |
0.446 |
54.14 (27.09) |
0.071 |
-22.89 (8.47) |
0.021 |
-1.48 (3.66) |
0.695 |
3.05 (7.21) |
0.68 |
-1.51 (2.66) |
0.581 |
-5.47 (2.38) |
0.042 |
0.90 (1.72) |
0.612 |
| NAFLD |
-1.73 (3.43) |
0.622 |
-3.51 (4.15) |
0.413 |
-19.42 (12.12) |
0.133 |
-9.93 (24.47) |
0.692 |
-7.70 (10.42) |
0.473 |
1.47 (3.01) |
0.634 |
-4.66 (3.59) |
0.217 |
-2.27 (2.19) |
0.319 |
7.50 (4.21) |
0.098 |
-8.24 (4.23) |
0.073 |
|
| rs2075754 |
Control |
-6.94 (5.16) |
0.208 |
-6.38 (6.42) |
0.344 |
11.28 (7.94) |
0.186 |
80.10 (31.87) |
0.031 |
11.31 (13.70) |
0.429 |
-3.25 (6.59) |
0.633 |
-15.23 (9.35) |
0.135 |
-10.00 (4.19) |
0.038 |
-0.173 (5.90) |
0.977 |
-2.28 (3.82) |
0.564 |
| NAFLD |
-2.65 (2.34) |
0.281 |
-1.41 (4.48) |
0.759 |
-1.64 (5.29) |
0.762 |
-10.54 (16.29) |
0.531 |
-16.28 (6.36) |
0.027 |
-3.20 (1.48) |
0.053 |
4.04 (4.60) |
0.399 |
2.80 (2.49) |
0.286 |
2.51 (2.15) |
0.267 |
1.42 (1.02) |
0.192 |
|
| rs3752752 |
Control |
-1.93 (5.82) |
0.746 |
0.67 (2.19) |
0.766 |
2.56 (4.07) |
0.541 |
-12.42 (29.84) |
0.684 |
-8.50 (10.86) |
0.448 |
7.85 (3.56) |
0.046 |
-0.40 (5.88) |
0.946 |
1.02 (1.87) |
0.594 |
2.44 (3.18) |
0.457 |
-0.811 (1.83) |
0.665 |
| NAFLD |
-2.69 (3.72) |
0.48 |
-4.10 (4.13) |
0.335 |
-1.75 (11.09) |
0.876 |
18.09 (22.93) |
0.442 |
11.74 (7.90) |
0.157 |
1.24 (2.25) |
0.589 |
-0.21 (4.33) |
0.962 |
1.41 (2.59) |
0.595 |
0.54 (4.33) |
0.903 |
-1.50 (4.14) |
0.722 |
|
| rs740949 | Control |
1.13 (4.11) |
0.786 |
0.91 (2.84) |
0.753 |
0.82 (3.33) |
0.81 |
0.07 (23.93) |
0.998 |
-9.37 (7.61) |
0.237 |
-0.35 (3.30) |
0.917 |
0.28 (6.29) |
0.965 |
1.97 (2.38) |
0.421 |
0.11 (2.51) |
0.965 |
1.73 (1.62) |
0.302 |
| NAFLD | 1.16 (4.23) | 0.789 | 4.42 (7.78) | 0.581 | 21.80 (16.93) | 0.222 | 65.19 (37.44) | 0.107 | -22.96 (14.77) | 0.146 | -3.78 (3.85) | 0.345 | -2.35 (5.44) | 0.673 | -3.95 (2.88) | 0.195 | 0.77 (2.23) | 0.735 | -0.42 (2.04) | 0.841 | |
Discussion
Our study showed a significant association between NAFLD and a variant located in CD82 gene, as well as significant enrichment for miR-193b-5p, meaning that the identified genes had significantly more targets of miR-193b-5p than expected.
One of the basic mechanisms leading to NAFLD development and progression is insulin resistance. Therefore, it is not surprising that NAFLD patients in our study had a higher fasting blood glucose level and ALT, the liver enzyme most closely associated with NAFLD (32). Since the cases and controls were matched for BMI, the differences observed between them could mainly be attributed to the processes involved in NAFLD development rather than to obesity.
CD82, or KAI1, expression is decreased or abolished in various malignant tumors (33). CD82 protein was present in about one-third of colorectal cancer tissues compared with more than half of normal mucosal tissues (34). In addition, its expression was lower in hepatocellular malignant cells compared with healthy tissue (35) and in HCC patients compared with patients with liver cirrhosis or hepatitis, or control individuals (35). Taking all this into account, our finding that a variant located in CD82 gene was significantly associated with NAFLD might explain the increased risk of several malignancies in patients with NAFLD. However, further functional studies are required to assess the effect of this variant on CD82 gene expression.
CD82 null mice demonstrated enhanced bone marrow adipogenic potential, evidenced by augmented differentiation into adipocytes and enhanced expression of adipocyte differentiation markers (36). Our findings suggest that a similar mechanism may also take place in hepatocytes, leading to hepatic steatosis observed in patients with NAFLD. Furthermore, rs2303861, found in this study to be associated with NAFLD, is in linkage disequilibrium with another variant, rs7942159, located on PNPLA2 gene, which is known to be involved in fat mobilization in adipose tissue (37,38). This might explain the effects of rs2303861 polymorphism in CD82 observed in our study.
Expression of miR-193b is directly correlated with the secretion of adiponectin (39), a secretory protein exclusively produced by adipocytes, which increases hepatic insulin sensitivity and is inversely correlated with the presence of NAFLD and body fat content (40,41). One study also reported an inverse correlation between miR-193b-5p expression level and BMI, glucose levels, and insulin responses to a 75-g oral glucose tolerance test (42). miR-193b expression level was significantly lower in liver cancer exosome and whole tissue compared with control tissues (43). Furthermore, miR-193b down-regulation was associated with metastasis and depth of invasion in patients with liver cancer (43). Down-regulation of miR-193b was also demonstrated in colorectal cancer tissues (44) and in patients with heart failure (45).
In summary, decreased miR-193b expression and the presence of a variant in CD82 gene might result in insulin resistance, dysregulation of adipokines, and an increase in hepatic fat content, which might all cause NAFLD and activate biological pathways leading to HCC, colorectal cancer, and heart failure. Our findings link miRNA expression to higher risk of cancer and heart disease in NAFLD patients, thus opening avenues for the treatment of NAFLD complications using miRNA blockers and miRNA mimics.
The strength of this study is the homogeneity of the studied population, use of next generation sequencing technology instead of array-based techniques, and the focus on the genetic loci associated with human diseases. The random selection of the studied sample from a population-based study can be considered an additional strength, as the studied individuals may be considered representative of the general population residing in our region.
On the other hand, the small number of participants might have decreased the statistical power of our study to detect a small effect size. Therefore, our findings require further replication studies in larger cohorts from other populations to corroborate their potential significance.
In conclusion, our study provides an insight into a possible association between decreased miR-193b expression and NAFLD development, and subsequent increased incidence of colorectal cancer, HCC, and heart failure. The existence of such association could allow the use of this miRNA in NAFLD management as a therapeutic target, disease biomarker, or method for treatment response assessment. Further studies, preferably performed in an independent cohort, should validate the associations found in this study, as well as unravel the underlying biological mechanisms involved.
Received: December 5, 2018
Accepted: June 28, 2019
Acknowledgments
Funding This study was partly supported by a grant from National Institute for Medical Research Development (NIMAD) of Islamic Republic of Iran to KBL and the NIMAD research grant awarded to MAF. The funding agency had no role in the study design, data collection and analysis, and interpretation of results.
Ethical approval given by Research Ethics Committee of Health Policy Research Center affiliated to Shiraz University of Medical Sciences Ethics Committee.
Declaration of authorship MAF and KBL conceived or designed the study. BH, MS, SB, and AK acquired the data. PH, MAF, and KBL analyzed or interpreted the data. PH drafted the manuscript. PH, BH, MS, SB, AK, MAF, and KBL critically revised the manuscript for important intellectual content. All authors gave final approval of the version to be submitted and agree to be accountable for all aspects of the work.
Competing interests All authors have completed the Unified Competing Interest form at www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Abdelmalek MF, Diehl AM. Nonalcoholic fatty liver diseases and nonalcoholic steatohepatitis. In: Kasper DL, Hauser SL, Jameson JL, Fauci AS, Longo DL, Loscalzo J, editors. Harrison's principles of internal medicine. 2. 19 ed. New York: McGraw Hill; 2015. p. 2054-7. [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol. 2013;10:686–90. doi: 10.1038/nrgastro.2013.171. [DOI] [PubMed] [Google Scholar]
- 3.Lankarani KB, Ghaffarpasand F, Mahmoodi M, Lotfi M, Zamiri N, Heydari ST, et al. Non alcoholic fatty liver disease in southern Iran: a population based study. Hepat Mon. 2013;13:e9248. doi: 10.5812/hepatmon.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moghaddasifar I, Lankarani KB, Moosazadeh M, Afshari M, Ghaemi A, Aliramezany M, et al. Prevalence of non-alcoholic fatty liver disease and its related factors in Iran. Int J Organ Transplant Med. 2016;7:149–60. [PMC free article] [PubMed] [Google Scholar]
- 5.Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice Guideline by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology. 2012;55:2005–23. doi: 10.1002/hep.25762. [DOI] [PubMed] [Google Scholar]
- 6.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 7.EASL-EASD-EASO Clinical Practice Guidelines for the management of non-alcoholic fatty liver disease. J Hepatol. 2016;64:1388–402. doi: 10.1016/j.jhep.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 8.Kim D, Kim WR, Kim HJ, Therneau TM. Association between noninvasive fibrosis markers and mortality among adults with nonalcoholic fatty liver disease in the United States. Hepatology. 2013;57:1357–65. doi: 10.1002/hep.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kahali B, Halligan B, Speliotes EK. Insights from genome-wide association analyses of nonalcoholic fatty liver disease. Semin Liver Dis. 2015;35:375–91. doi: 10.1055/s-0035-1567870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paradis V, Zalinski S, Chelbi E, Guedj N, Degos F, Vilgrain V, et al. Hepatocellular carcinomas in patients with metabolic syndrome often develop without significant liver fibrosis: a pathological analysis. Hepatology. 2009;49:851–9. doi: 10.1002/hep.22734. [DOI] [PubMed] [Google Scholar]
- 11.Kim GA, Lee HC, Choe J, Kim MJ, Lee MJ, Chang HS, et al. Association between non-alcoholic fatty liver disease and cancer incidence rate. J Hepatol 2017. S0168-8278(17)32294-8 [DOI] [PubMed] [Google Scholar]
- 12.Ahn JS, Sinn DH, Min YW, Hong SN, Kim HS, Jung SH, et al. Non-alcoholic fatty liver diseases and risk of colorectal neoplasia. Aliment Pharmacol Ther. 2017;45:345–53. doi: 10.1111/apt.13866. [DOI] [PubMed] [Google Scholar]
- 13.VanWagner LB, Wilcox JE, Colangelo LA, Lloyd-Jones DM, Carr JJ, Lima JA, et al. Association of nonalcoholic fatty liver disease with subclinical myocardial remodeling and dysfunction: a population-based study. Hepatology. 2015;62:773–83. doi: 10.1002/hep.27869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Simon TG, Bamira DG, Chung RT, Weiner RB, Corey KE. Nonalcoholic steatohepatitis is associated with cardiac remodeling and dysfunction. Obesity (Silver Spring) 2017;25:1313–6. doi: 10.1002/oby.21879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan DZ, Garske KM, Alvarez M, Bhagat YV, Boocock J, Nikkola E, et al. Integration of human adipocyte chromosomal interactions with adipose gene expression prioritizes obesity-related genes from GWAS. Nat Commun. 2018;9:1512. doi: 10.1038/s41467-018-03554-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cirillo E, Kutmon M, Gonzalez Hernandez M, Hooimeijer T, Adriaens ME, Eijssen LMT, et al. From SNPs to pathways: Biological interpretation of type 2 diabetes (T2DM) genome wide association study (GWAS) results. PLoS One. 2018;13:e0193515. doi: 10.1371/journal.pone.0193515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loomba R, Schork N, Chen CH, Bettencourt R, Bhatt A, Ang B, et al. Heritability of hepatic fibrosis and steatosis based on a prospective twin study. Gastroenterology. 2015;149:1784–93. doi: 10.1053/j.gastro.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anstee QM, Day CP. The genetics of NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:645–55. doi: 10.1038/nrgastro.2013.182. [DOI] [PubMed] [Google Scholar]
- 19.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennacchio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–5. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Del Campo JA, Gallego-Duran R, Gallego P, Grande L. Genetic and epigenetic regulation in nonalcoholic fatty liver disease (NAFLD). Int J Mol Sci. 2018;19 doi: 10.3390/ijms19030911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.He Z, Hu C, Jia W. miRNAs in non-alcoholic fatty liver disease. Front Med. 2016;10:389–96. doi: 10.1007/s11684-016-0468-5. [DOI] [PubMed] [Google Scholar]
- 22.Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108:9232–7. doi: 10.1073/pnas.1102281108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honarvar B, Lankarani KB, Kashani P, Rafiee T. Dietary determinants of non-alcoholic fatty liver disease in lean and non-lean adult patients: a population-based study in shiraz, southern Iran. Hepat Mon. 2017;17 doi: 10.5812/hepatmon.44962. [DOI] [Google Scholar]
- 24.Hamaguchi M, Kojima T, Itoh Y, Harano Y, Fujii K, Nakajima T, et al. The severity of ultrasonographic findings in nonalcoholic fatty liver disease reflects the metabolic syndrome and visceral fat accumulation. Am J Gastroenterol. 2007;102:2708–15. doi: 10.1111/j.1572-0241.2007.01526.x. [DOI] [PubMed] [Google Scholar]
- 25.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–75. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Clarke GM, Anderson CA, Pettersson FH, Cardon LR, Morris AP, Zondervan KT. Basic statistical analysis in genetic case-control studies. Nat Protoc. 2011;6:121–33. doi: 10.1038/nprot.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 28.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society Series B. 1995;57:289–300. [Google Scholar]
- 29.Dayem Ullah AZ, Lemoine NR, Chelala C. SNPnexus: a web server for functional annotation of novel and publicly known genetic variants (2012 update) Nucleic Acids Res. 2012;40:W65–70. doi: 10.1093/nar/gks364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, et al. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinformatics. 2013;14:128. doi: 10.1186/1471-2105-14-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chou CH, Shrestha S, Yang CD, Chang NW, Lin YL, Liao KW, et al. miRTarBase update 2018: a resource for experimentally validated microRNA-target interactions. Nucleic Acids Res. 2018;46(D1):D296–D302. doi: 10.1093/nar/gkx1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Westerbacka J, Corner A, Tiikkainen M, Tamminen M, Vehkavaara S, Hakkinen AM, et al. Women and men have similar amounts of liver and intra-abdominal fat, despite more subcutaneous fat in women: implications for sex differences in markers of cardiovascular risk. Diabetologia. 2004;47:1360–9. doi: 10.1007/s00125-004-1460-1. [DOI] [PubMed] [Google Scholar]
- 33.Malik FA, Sanders AJ, Jiang WG. KAI-1/CD82, the molecule and clinical implication in cancer and cancer metastasis. Histol Histopathol. 2009;24:519–30. doi: 10.14670/HH-24.519. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q, Yang Y, Wu S, Li W, Zhang N, Dong X, et al. Evaluation of the correlation of KAI1/CD82, CD44, MMP7 and beta-catenin in the prediction of prognosis and metastasis in colorectal carcinoma. Diagn Pathol. 2015;10:176. doi: 10.1186/s13000-015-0411-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang W, Zhao CG, Sun HY, Zheng WE, Chen H. Expression characteristics of KAI1 and vascular endothelial growth factor and their diagnostic value for hepatocellular carcinoma. Gut Liver. 2014;8:536–42. doi: 10.5009/gnl13331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bergsma A, Ganguly SS, Dick D, Williams BO, Miranti CK. Global deletion of tetraspanin CD82 attenuates bone growth and enhances bone marrow adipogenesis. Bone. 2018;113:105–13. doi: 10.1016/j.bone.2018.05.020. [DOI] [PubMed] [Google Scholar]
- 37.Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–6. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 38.Machiela MJ, Chanock SJ. LDlink: a web-based application for exploring population-specific haplotype structure and linking correlated alleles of possible functional variants. Bioinformatics. 2015;31:3555–7. doi: 10.1093/bioinformatics/btv402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Belarbi Y, Mejhert N, Lorente-Cebrian S, Dahlman I, Arner P, Ryden M, et al. MicroRNA-193b controls adiponectin production in human white adipose tissue. J Clin Endocrinol Metab. 2015;100:E1084–8. doi: 10.1210/jc.2015-1530. [DOI] [PubMed] [Google Scholar]
- 40.Whitehead JP, Richards AA, Hickman IJ, Macdonald GA, Prins JB. Adiponectin–a key adipokine in the metabolic syndrome. Diabetes Obes Metab. 2006;8:264–80. doi: 10.1111/j.1463-1326.2005.00510.x. [DOI] [PubMed] [Google Scholar]
- 41.Machado MV, Diehl AM. Pathogenesis of nonalcoholic fatty liver disease. In: Sanyal AJ, Boyer TD, Terrault NA, Lindor KD, editors. Zakim and Boyer’s hepatology: a textbook of liver disease. 7th ed. Philadelphia: Elsevier; 2018. p. 369-90. [Google Scholar]
- 42.Meerson A, Traurig M, Ossowski V, Fleming JM, Mullins M, Baier LJ. Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-alpha. Diabetologia. 2013;56:1971–9. doi: 10.1007/s00125-013-2950-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yin W, Nie Y, Chen L, Wang Q, Liu S, He X, et al. Deregulation of microRNA-193b affects the proliferation of liver cancer via myeloid cell leukemia-1. Oncol Lett. 2018;15:2781–8. doi: 10.3892/ol.2017.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo F, Luo Y, Mu YF, Qin SL, Qi Y, Qiu YE, et al. miR-193b directly targets STMN1 and inhibits the malignant phenotype in colorectal cancer. Am J Cancer Res. 2016;6:2463–75. [PMC free article] [PubMed] [Google Scholar]
- 45.Wong LL, Armugam A, Sepramaniam S, Karolina DS, Lim KY, Lim JY, et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur J Heart Fail. 2015;17:393–404. doi: 10.1002/ejhf.223. [DOI] [PubMed] [Google Scholar]


