Abstract
Objective:
Gestational diabetes mellitus is increasing worldwide, mainly in developing countries, and physical activity has not been studied in gestational diabetes mellitus prevention among low-income population. This prospective cross-sectional study assessed the gestational diabetes mellitus risk related to physical activity in early pregnancy among low-income women.
Methods:
A prospective cross-sectional study with 544 low-income pregnant women was conducted at the Instituto de Medicina Integral Prof. Fernando Figueira, Brazil. Gestational diabetes mellitus was diagnosed using the International Association of Diabetes and Pregnancy Study Groups criteria. Physical activity was assessed during early pregnancy using the Pregnancy Physical Activity Questionnaire and categorized as sedentary, light, moderate, or vigorous intensity.
Results:
Gestational diabetes mellitus occurred in 95 of 544 women (17.4%). Body mass index was higher in the gestational diabetes mellitus group. Nearly half of all pregnant women studied were physically inactive, and none of them were classified as vigorous physical active. Sedentary physical activity pattern was associated with a higher odds of gestational diabetes mellitus (odds ratio = 1.8, 95% confidence interval = 1.1–2.9), which did not change after adjusting for several covariates (odds ratio = 1.9, 95% confidence interval = 1.2–3.1).
Conclusion:
Physical inactivity in early pregnancy is associated with a higher risk of gestational diabetes mellitus among low-income women.
Keywords: Physical activity, gestational diabetes mellitus, low income, prospective study
Introduction
Gestational diabetes mellitus (GDM) is the most prevalent metabolic disease during pregnancy.1 GDM is associated with adverse pregnancy outcomes for both mothers and their offspring.2,3 Some of these adverse outcomes persist throughout life.4,5
The incidence of GDM is increasing worldwide, especially in developing countries, and is associated with the overweight/obesity epidemic.6 Populations living in poor food environments are at greater risk of inadequate diets and of developing diet-related chronic disease, such as type 2 diabetes mellitus;7,8 however, there are no studies on GDM in low-income populations. Some studies have recommended public health efforts to reduce pregnancy obesity and overweight by promoting physical activity and healthy eating among women of childbearing age.6,9 However, data regarding physical activity and GDM prevention are still conflicting. Some studies have shown an inverse association between physical activity and GDM10–15 but others have not.16–19 Some recent reviews have pointed out that scientific evidence is still required to make an informed decision regarding the role of physical activity in the prevention of GDM.20–22
This response is even more necessary for the low-income population because obesity is increasing faster in this population. The aim of this cohort study was to determine the incidence of GDM in association with the physical activity pattern in early pregnancy among low-income women.
Methods
Study population and setting
This prospective study followed up pregnant women from the first trimester to delivery. Pregnant women were recruited at the Instituto de Medicina Integral Prof. Fernando Figueira (IMIP), Recife, Brazil, between November 2012 and February 2014. IMIP is a referral hospital for maternal and child health care in northeastern Brazil. This study is a secondary research analysis of the database “Epidemiological, clinical, therapeutic, and preventive aspects of Gestational Diabetes Mellitus,” which was developed at IMIP. This Project had been previously approved by the IMIP Research Ethics Committee (n 2671-2011), and all participants had signed an informed consent form.
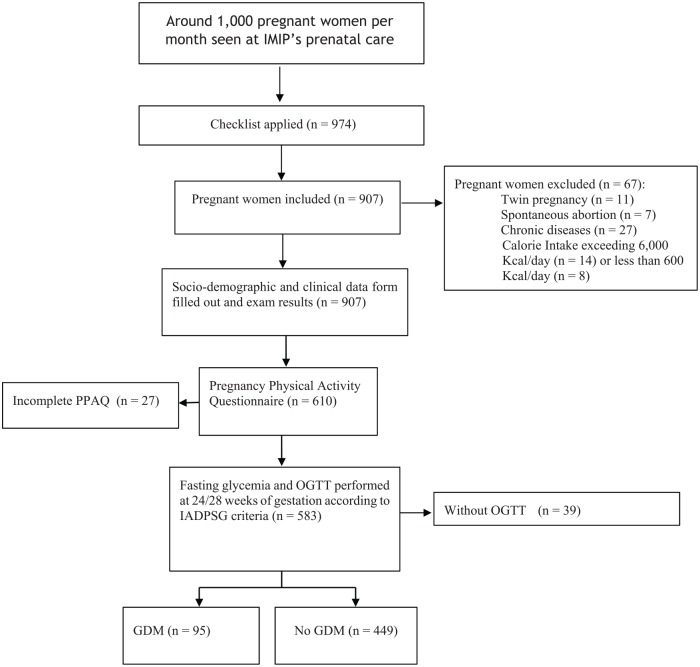
A convenience sample was recruited from IMIP outpatient prenatal unit. Eligibility criteria included women with low income, that is, annual per capita income of US$1025 or less, according to the World Bank,23 pregnancy of up to 20 weeks, age between 18 and 45 years, and a current resident of the Recife metropolitan area. Pregnant women who had developed diabetes mellitus before pregnancy, who had multiple gestations, who suffered from mental disorders, and who had congenital anomalies were excluded from the study. Research visits to the clinic happened in early (first trimester), mid- (second trimester), and late pregnancy (third trimester), and immediately after delivery. A flowchart of the participants is shown in Figure 1.
Figure 1.
Flowchart showing the enlisting and follow-up of study participants.
GDM diagnosis
GDM diagnosis was based on the International Association of Diabetes and Pregnancy Study Groups (IADPSG) criteria of at least one abnormal value of the 75-g oral glucose tolerance test (OGTT) at 24–28 weeks of gestation: fasting plasma glucose level ⩾92 mg/dL, 1-h glucose level ⩾180 mg/dL, or 2-h glucose level ⩾153 mg/dL.24 All women who screened positive for GDM were followed up by the hospital prenatal team made up of an obstetrician, a nurse, and a nutritionist; they also had frequent regular appointments depending on their clinical status and glucose readings.
Assessment of physical activity pattern
Physical activity pattern was assessed using the Pregnancy Physical Activity Questionnaire (PPAQ)25 in the week preceding the interview. PPAQ evaluates different levels of intensity, allowing for calculation of the average weekly energy expenditure for each area of activity. Activity levels for the different activity subgroups “housework/caregiving” (e.g. cooking, dressing children, household chores), “transportation” (e.g. walking routes, driving), “sports/exercise/recreation” (e.g. walking/hiking, swimming, sport instruction), and “employment” (e.g. occupation) are defined separately and assessed with 16 detailed questions each. The duration of each activity subgroup was determined and then multiplied by its intensity as defined by the compendium of physical activities.26 The unit MET (metabolic equivalent of task) describes metabolic states and energy expenditure. MET-h/week = intensity of activity (MET-value) × duration of activity (h/week). One MET corresponds to the rate of energy expenditure while seated at rest (0.9 METs are observed during sleep), and values up to 18 METs are attained during higher intensity physical activities such as sprinting. Physical activity pattern was classified according to its intensity: sedentary or physically inactive (<1.5 METs), light intensity (1.5 to <3.0 METs), moderate intensity (3.0–6.0 METs), or vigorous intensity (>6.0 METs).
Other assessments
The information collected during the interview was carried out by a previously trained researcher and included age, race/ethnicity, sociodemographic characteristics, and reproductive and medical history.
Pre-pregnancy body mass index (BMI) was calculated based on the information given by the mother and was then compared to the first weight measure taken at the first prenatal care visit by an electronic scale with a 0.1-kg degree of accuracy (Seca, Germany). The measured weight was the one used in case of a difference greater than 5 kg between what the mother informed and the actual weight measured. The height was measured on barefoot in centimeter using standard height measuring board and recorded to the nearest 1 cm. Nutritional status was considered as underweight (BMI < 18.5), normal weight (BMI = 18.5–24.9), overweight (BMI = 25.0–29.9), and obese (BMI ⩾ 30).
Statistical analysis
Sample size calculation was based on statistical power of 80% and a level of significance set at 5%. Considering GDM incidence of 18% and a risk reduction with physical activity at 40%, it was calculated that 509 women would be necessary. Considering a loss of 10%, the sample calculated was 559 participants.
Bivariate statistical analysis was performed using the chi-square test, Fisher’s exact test, and chi-square test for linear trend. The aim was to identify the set of variables that showed an association with GDM. Variables with a level of significance lower than 0.20 were selected to compose a multivariate model that was adjusted using logistic regression to quantify the adjusted effects of the variables on the occurrence of GDM. The odds ratios (ORs) and their respective 95% confidence intervals (CIs) were computed. A backward selection of the variables for the adjusted model was used with significance level of 0.05 as a criterion for the permanence of variables in the adjusted model. Statistical analysis was performed using the statistical software Stata, version 12 (StataCorp., College Station, TX, USA).
Results
A total of 544 pregnant women completed the study (Figure 1). Overall, most participants were young and primiparas. A total of 219 pregnant women (40.2%) were overweight or obese, 10 (1.8%) had previous GDM, and 311 (57.1%) had a family history of type 2 diabetes mellitus. Some characteristics of participants who developed GDM (95/544; 17.4%) and did not develop GDM (449/544; 82.6%) are shown in Table 1. The two groups were similar except for BMI, which was higher in the GDM group.
Table 1.
Some characteristics of pregnant women with and without GDM.
| GDM |
p value | ||
|---|---|---|---|
| Yes (%) n = 95 |
No (%) n = 449 |
||
| Age (Quartis) | 0.320 | ||
| 14.0–22.0 | 34 (35.4) | 125 (27.9) | |
| 23.0–26.0 | 23 (24.0) | 119 (26.6) | |
| 27.0–30.0 | 21 (21.9) | 93 (20.8) | |
| 31.0–45.0 | 17 (18.8) | 112 (24.8) | |
| Schooling (⩽9 years) | 15 (15.6) | 55 (12.3) | 0.991 |
| Married | 68 (70.8) | 355 (79.2) | 0.072 |
| Primipara | 45 (45.9) | 245 (54.5) | 0.163 |
| Number of living children (⩾2) | 32 (33.6) | 189 (42.0) | 0.109 |
| Employed | 43 (44.8) | 238 (53.1) | 0.138 |
| Student | 13 (13.5) | 83 (18.5) | 0.245 |
| Skin color | 0.345 | ||
| White | 35 (36.5) | 151(33.7) | |
| Black | 12 (12.5) | 84 (18.8) | |
| Mixed | 48 (51.0) | 214 (47.5) | |
| Prior abortion | 25 (26.0) | 100 (22.3) | 0.432 |
| Family history of diabetes | 50 (52.6) | 241 (53.6) | 0.790 |
| BMI (kg/m2) | 25.4 (±4.9) | 24.2 (±4.6) | 0.031 |
| Systolic blood pressure | 106.7 (±14.9) | 105.2 (±12.6) | 0.378 |
| Diastolic blood pressure | 67.3 (±9.3) | 66.4 (±9.6) | 0.426 |
GDM: gestational diabetes mellitus; BMI: body mass index.
Nearly half of all pregnant women studied were physically inactive, and none of them were classified as vigorous physical active (Table 2). Most women expended energy in housework/caregiving activities.
Table 2.
Physical activity pattern in low-income pregnant women with and without GDM.
| Physical activity pattern | Total | GDM | Non-GDM | p value |
|---|---|---|---|---|
| Sedentary (<1.5 METs) | 262 (48.2%) | 58 (61.1%) | 205 (45.7%) | 0.008 |
| Light (1.5 to <3.0 METs) | 38 (6.9%) | 7 (7.3%) | 31 (6.9%) | 0.897 |
| Moderate (3.0–6.0 METs) | 244 (44.9%) | 30 (31.6%) | 213 (47.4%) | 0.006 |
| Vigorous (>6.0 METs) | 0 | 0 | 0 | |
| Total | 544 | 95 | 449 |
GDM: gestational diabetes mellitus; MET: metabolic equivalent of task.
Of the 95 (17.4%) women diagnosed with GDM, 58 (61.0%) were physically inactive and 37 (39.0%) physically active (p = 0.008). This association between physical activity pattern and GDM was not linear; p values for physical activity of light intensity and moderate intensity were, respectively, 0.897 and 0.006.
GDM incidence was more common among overweight/obese pregnant women as compared with normal/underweight pregnant women: 59 (62.1%) versus 36 (37.9%), p = 0.001.
Multivariable analysis using logistic regression with variables with a value of p < 0.20 in the bivariate analyses was used for the initial model: marital status, work, number of births, age, live children, physical activity, and nutritional status. A significant association was observed for all variables with a value of p < 0.05. An OR = 1.8 was observed with a 95% CI (1.12–2.91) for the initial model regarding the association between GDM and physical inactivity. For the final model, an OR = 1.9 and 95% CI (1.19–3.05) was observed. Results of multivariate analysis using logistic regression also showed a strong association between overweight and obesity and the development of GDM, both in the initial model, OR = 3.1 and 95% CI (1.81–5.20), and in the final model, OR = 2.9 and 95% CI (1.74–4.95), p < 0.001 (Table 3)
Table 3.
Multivariable analysis of factors associated with gestational diabetes mellitus.
| Variables | Gestational diabetes Unadjusted OR |
Gestational diabetes Adjusted ORa |
||
|---|---|---|---|---|
| OR (95% CI) | p value | OR (95% CI) | p value | |
| Married | 0.064 | 0.049 | ||
| Yes | 1.0 | 1.0 | ||
| No | 1.7 (0.9–2.9) | 1.7 (1.0–3.0) | ||
| Employed | 0.088 | |||
| Yes | 1.0 | |||
| No | 1.5 (0.9–2.4) | |||
| Births | 0.044 | 0.047 | ||
| 0 | 1.0 | 1.0 | ||
| 1 | 0.1 (0.0–1.0) | 0.1 (0.0–1.0) | ||
| >2 | 0.4 (0.0–4.2) | 0.4 (0.0–4.4) | ||
| Number of living children | 0.025 | 0.030 | ||
| 0 | 0.2 (0.0–1.5) | 0.2 (0.0–1.5) | ||
| 1 | 1.0 | 1.0 | ||
| >2 | 0.2 (0.0–0.7) | 0.2 (0.0–0.7) | ||
| Physical activity pattern | 0.016 | 0.008 | ||
| Inactive | 1.8 (1.1–2.9) | 1.9 (1.1–3.0) | ||
| Active | 1.0 | 1.0 | ||
| Nutritional status | <0.001 | <0.001 | ||
| Underweight | 1.5 (0.6–3.5) | 1.5 (0.6–3.5) | ||
| Normal | 1.0 | 1.0 | ||
| Overweight/obesity | 3.1 (1.8–5.2) | (1.7–4.9) | ||
OR: odds ratio; CI: confidence interval.
All variables in the initial model, but employed.
Discussion
GDM incidence was found in 17.4% of pregnant women in the study, very similar to that reported by the International Diabetes Federation1 (16.8%) and to the numbers seen in other recent studies. A cross-sectional study was carried out in Pakistan, and an incidence of 17.2% was observed among 1210 pregnant women.27 In another study conducted in China, with 1683 pregnant women and in which IADPSG diagnostic criteria were also used, the authors found GDM incidence to be 12.4%.28 Cosson et al.,29 in Paris, detected a 14.6% GDM incidence in 9795 pregnant women.
More than half of the overweight or obese pregnant women (61.5%) developed GDM, and these women were three times (OR = 3.1, 95% CI = 1.81–5.20) more likely to develop GDM. Explanations for the increase in obesity, especially in low-income populations, include reduced physical activity and consumption of high-energy diets. They are exposed to high-fat, high-sugar, high-salt, energy-dense, and micronutrient-poor foods, which tend to be lower in cost and nutrient quality. Low-income pregnant women also are at risk of excessive gestational weight gain. Systematic reviews using a meta-analysis to evaluate risk factors for GDM regarded overweight/obesity to be an important risk factor for GDM.30–32 Torloni et al.,30 in a systematic review using a meta-analysis that included 70 studies (59 cohorts and 11 case–control studies) observed that the likelihood of an obese pregnant woman to develop GDM was three to five times higher. These data highlight nutritional status in early pregnancy as an important risk factor for GDM.
In our study, physically inactive pregnant women were twice as likely to develop GDM. However, the association between physical activity pattern and GDM was not linear, possibly because we had a low number of participants with light physical activity pattern; the physical activity pattern concentrated between sedentary and moderate. Researchers in China evaluated 11,450 pregnant women at the 12th gestational week and also found moderate physical activity during pregnancy to be a protective factor for GDM, with an OR of 0.81 and 95% CI (0.67–0.97).33 In India, physical inactivity was also associated with a fourfold increase in the risk of GDM and maternal and neonatal complications.34 A recent systematic review using a meta-analysis concluded that pre-pregnancy or early pregnancy physical activity was associated with 30% and 21% reduced odds of GDM, respectively.21 Sauder et al.35 found that physical activity, measured using PPAQ, was significantly associated with a reduced risk of dysglycemia (adjusted OR = 0.67, 95% CI = 0.44–1.00).
However, some results are conflicting and the association between physical activity pattern during pregnancy and risk of GDM has not been well established yet. A multicenter cohort study conducted in Central American countries and comprising 1241 pregnant women pointed out that physical activity in early pregnancy was not associated with GDM.36 Three meta-analyses provide further support for the hypothesis that physical activity decreases the risk of GDM.21,37,38 However, Yin et al.39 disagree with this idea based on their findings in another systematic review using a meta-analysis. Possibly, different study designs, different diagnostic criteria for GDM, shortfalls or statistical power, indirect or inaccurate physical activity evaluation, and the inability to control for confounding factors may explain divergent findings when determining the association between physical activity during pregnancy and risk of GDM.
Physical activity decreases during pregnancy, and pregnant women usually adopt a sedentary lifestyle. The American College of Obstetricians and Gynecologists (ACOG) recommends that pregnant women, in the absence of contraindications, engage in 30 min or more of physical activity of at least moderate intensity on most, if not all, days of the week.40
The following are the strengths of our study: a large sample size of low-income women was studied; the design was a prospective cohort population-based study including continuous evaluation throughout pregnancy; GDM was defined according to the recommended IADPSG’s diagnostic criteria; and data collection included many variables.
Our study also has some limitations. At first, we used self-reported physical activity data, which may imply recall bias. Nevertheless, the use of a prospectively administered and validated questionnaire might reduce potential recall bias. The PPAQ was originally developed in English by Chasan-Taber et al.,36 and Cronbach’s alpha assessed the reliability of the total scale as 0.78 and ranging from 0.78 to 0.93 for each subscale. Moreover, in a study conducted by Morkrid et al.,41 its reliability was confirmed by Cronbach’s alpha of 0.85%. PPAQ was translated and culturally adapted to Portuguese.42 It should also be emphasized that the pregnant women in this study were of low income and that heavy household chores may have been underestimated in this population.43
The association between calorie intake and incident GDM was not analyzed, which can be considered as another limitation of our study. Moreover, we report results in a specific population and hence they cannot be generalized.
Conclusion
Our findings suggest that in low-income women with a pattern of physical inactivity in early pregnancy, the risk of GDM increases. Overweight/obesity was also a risk factor for GDM. Furthermore, studies designed as randomized control trials and cohort studies are needed to conclusively establish the association between physical activity and GDM in low-income populations.
Acknowledgments
We thank to UNCISAL.
Footnotes
Data availability statement: The data that support the findings of this study are available from the corresponding author (J.G.A.) upon reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from the IMIP Research Ethics Committee (approval number: 2671-2011).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by CNPq, 401609/2013-8.
Informed consent: Written informed consent was obtained from all subjects before the study.
ORCID iD: João Guilherme Alves  https://orcid.org/0000-0002-9170-0808
https://orcid.org/0000-0002-9170-0808
References
- 1. International Diabetes Federation. IDF diabetes Atlas. 6th ed Brussels: IDF Executive Office, 2013, p. 44, www.idf.org/diabetesatlas [Google Scholar]
- 2. Boriboonhirunsarn D, Talungjit P, Sunsaneevithayakul P, et al. Adverse pregnancy outcomes in gestational diabetes mellitus. J Med Assoc Thai 2006; 89(Suppl. 4): S23–S28. [PubMed] [Google Scholar]
- 3. Wang Z, Kanguru L, Hussein J, et al. Incidence of adverse outcomes associated with gestational diabetes mellitus in low- and middle-income countries. Int J Gynaecol Obstet 2013; 121(1): 14–19. [DOI] [PubMed] [Google Scholar]
- 4. Sibai BM, Ross MG. Hypertension in gestational diabetes mellitus: pathophysiology and long-term consequences. J Matern Fetal Neonatal Med 2010; 23(3): 229–233. [DOI] [PubMed] [Google Scholar]
- 5. Damm P, Houshmand-Oeregaard A, Kelstrup L, et al. Gestational diabetes mellitus and long-term consequences for mother and offspring: a view from Denmark. Diabetologia 2016; 59(7): 1396–1399. [DOI] [PubMed] [Google Scholar]
- 6. Kim SY, England L, Wilson HG, et al. Percentage of gestational diabetes mellitus attributable to overweight and obesity. Am J Public Health 2010; 100(6): 1047–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gittelsohn J, Trude A. Diabetes and obesity prevention: changing the food environment in low-income settings. Nutr Rev 2017; 75(Suppl. 1): 62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cosson E, Bihan H, Reach G, et al. Psychosocial deprivation in women with gestational diabetes mellitus is associated with poor feto maternal prognoses: an observational study. BMJ Open 2015; 5(3): e007120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Badon SE, Enquobahrie DA, Wartko PD, et al. Healthy lifestyle during early pregnancy and risk of gestational diabetes mellitus. Am J Epidemiol 2017; 186: 326–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Cordero Y, Mottola MF, Vargas J, et al. Exercise is associated with a reduction in gestational diabetes mellitus. Med Sci Sports Exerc 2015; 47(7): 1328–1333. [DOI] [PubMed] [Google Scholar]
- 11. Oken E1, Ning Y, Rifas-Shiman SL, et al. Associations of physical activity and inactivity before and during pregnancy with glucose tolerance. Obstet Gynecol 2006; 108(5): 1200–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu J, Laditka JN, Mayer-Davis EJ, et al. Does physical activity during pregnancy reduce the risk of gestational diabetes among previously inactive women? Birth 2008; 35(3): 188–195. [DOI] [PubMed] [Google Scholar]
- 13. Redden SL, LaMonte MJ, Freudenheim JL, et al. The association between gestational diabetes mellitus and recreational physical activity. Matern Child Health J 2011; 15(4): 514–519. [DOI] [PubMed] [Google Scholar]
- 14. Ming WK, Ding W, Zhang CJP, et al. The effect of exercise during pregnancy on gestational diabetes mellitus in normal-weight women: a systematic review and meta-analysis. BMC Pregnancy Childbirth 2018; 18(1): 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nguyen CL, Pham NM, Lee AH, et al. Physical activity during pregnancy is associated with a lower prevalence of gestational diabetes mellitus in Vietnam. Acta Diabetol 2018; 55(9): 955–962. [DOI] [PubMed] [Google Scholar]
- 16. Oostdam N, van Poppel MN, Wouters MG, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. BJOG 2012; 119(9): 1098–1107. [DOI] [PubMed] [Google Scholar]
- 17. Stafne SN, Salvesen KÅ, Romundstad PR, et al. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstet Gynecol 2012; 119(1): 29–36. [DOI] [PubMed] [Google Scholar]
- 18. Nobles C1, Marcus BH, Stanek EJ, 3rd, et al. Effect of an exercise intervention on gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol 2015; 125(5): 1195–1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Van der Ploeg HP, van Poppel MN, Chey T, et al. The role of pre-pregnancy physical activity and sedentary behaviour in the development of gestational diabetes mellitus. J Sci Med Sport 2011; 14(2): 149–152. [DOI] [PubMed] [Google Scholar]
- 20. Morkrid K, Jenum AK, Berntsen S, et al. Objectively recorded physical activity and the association with gestational diabetes. Scand J Med Sci Sports 2014; 24(5): e389–e397. [DOI] [PubMed] [Google Scholar]
- 21. Mijatovic-Vukas J, Capling L, Cheng S, et al. Associations of diet and physical activity with risk for gestational diabetes mellitus: a systematic review and meta-analysis. Nutrients 2018; 10(6): E698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aune D, Sen A, Henriksen T, et al. Physical activity and the risk of gestational diabetes mellitus: a systematic review and dose-response meta-analysis of epidemiological studies. Eur J Epidemiol 2016; 31(10): 967–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. The World Bank. World Bank Country and lending groups 2016, http://data.worldbank.org/about/country-and-lending-groups (2016, accessed 4 April 2016).
- 24. International Association of Diabetes and Pregnancy Study Groups Consensus Panel. International Association of Diabetes and Pregnancy Study Groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010; 33: 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chasan-Taber L, Schmidt MD, Roberts DE, et al. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004; 36(10): 1750–1760. [DOI] [PubMed] [Google Scholar]
- 26. Ainsworth BE, Haskell WL, Whitt MC, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc 2000; 32(9 Suppl.): S498–S504. [DOI] [PubMed] [Google Scholar]
- 27. Fatima SS, Rehman R, Alam F, et al. Gestational diabetes mellitus and the predisposing factors. J Pak Med Assoc 2017; 67(2): 261–265. [PubMed] [Google Scholar]
- 28. Yan Y, Liu Z, Liu D. Heterogeneity of glycometabolism in patients with gestational diabetes mellitus : Retrospective study of 1,683 pregnant women. J Diabetes Investig 2017; 8(4): 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cosson E, Vicaut E, Sandre-Banon D, et al. Early screening for gestational diabetes mellitus is not associated with improved pregnancy outcomes: an observational study including 9795 women. Diabetes Metab. Epub ahead of print 28 November 2018. DOI: 10.1016/j.diabet.2018.11.006. [DOI] [PubMed] [Google Scholar]
- 30. Torloni MR, Betrán AP, Horta BL, et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes Rev 2009; 10(2): 194–203. [DOI] [PubMed] [Google Scholar]
- 31. Parnell AS, Correa A, Reece EA. Pre-pregnancy obesity as a modifier of gestational diabetes and birth defects associations: a systematic review. Matern Child Health J 2017; 21(5): 1105–1120. [DOI] [PubMed] [Google Scholar]
- 32. Lamminpää R, Vehviläinen-Julkunen K, Schwab U. A systematic review of dietary interventions for gestational weight gain and gestational diabetes in overweight and obese pregnant women. Eur J Nutr 2018; 57(5): 1721–1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leng J, Liu G, Zhang C, et al. Physical activity, sedentary behaviors and risk of gestational diabetes mellitus: a population-based cross-sectional study in Tianjin, China. Eur J Endocrinol 2016; 174(6): 763–773. [DOI] [PubMed] [Google Scholar]
- 34. Anjana RM, Sudha V, Lakshmipriya N, et al. Physical activity patterns and gestational diabetes outcomes—the wings project. Diabetes Res Clin Pract 2016; 116: 253–262. [DOI] [PubMed] [Google Scholar]
- 35. Sauder KA, Starling AP, Shapiro AL, et al. Diet, physical activity and mental health status are associated with dysglycaemia in pregnancy: the Healthy Start Study. Diabet Med 2016; 33(5): 663–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chasan-Taber L, Silveira M, Lynch KE, et al. Physical activity before and during pregnancy and risk of abnormal glucose tolerance among Hispanic women. Diabetes Metab 2014; 40(1): 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tobias DK, Zhang C, van Dam RM, et al. Physical activity before and during pregnancy and risk of gestational diabetes mellitus: a meta-analysis. Diabetes Care 2011; 34: 223–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Russo LM, Nobles C, Ertel KA, et al. Physical activity interventions in pregnancy and risk of gestational diabetes mellitus: a systematic review and meta-analysis. Obstet Gynecol 2015; 125(3): 576–582. [DOI] [PubMed] [Google Scholar]
- 39. Yin YN, Li XL, Tao TJ, et al. Physical activity during pregnancy and the risk of gestational diabetes mellitus: a systematic review and meta-analysis of randomised controlled trials. Br J Sports Med 2014; 48(4): 290–295. [DOI] [PubMed] [Google Scholar]
- 40. Committee on Obstetric Practice. ACOG committee opinion. Exercise during pregnancy and the postpartum period. Number 267, January 2002. American College of Obstetricians and Gynecologists. Int J Gynaecol Obstet 2002; 77(1): 79–81. [DOI] [PubMed] [Google Scholar]
- 41. Morkrid K, Jenum AK, Sletner L, et al. Failure to increase insulin secretory capacity during pregnancy-induced insulin resistance is associated with ethnicity and gestational diabetes. Eur J Endocrinol 2012; 167(4): 579–588. [DOI] [PubMed] [Google Scholar]
- 42. Silva FT, Costa FS. Transcultural adaptation of the pregnancy physical activity questionnaire-PPAQ to Portuguese: a tool for evaluation of physical activity in Brazilian pregnant. FIEP Bull 2009; 79(Special ed.): 318–322. [Google Scholar]
- 43. Coll CV, Domingues MR, Hallal PC, et al. Changes in leisure-time physical activity among Brazilian pregnant women: comparison between two birth cohort studies (2004 – 2015). BMC Public Health 2017; 17(1): 119. [DOI] [PMC free article] [PubMed] [Google Scholar]