Abstract
Objective:
Uterus transplantation is rapidly becoming a viable clinical option for women with uterine-factor infertility and a desire for parenthood. Radiological imaging plays a central role in selecting the optimal living donors for uterus transplantation and serves to exclude any pathology and evaluate the uterine vasculature. The latter is the most important variable in the ultimate technical success of the uterus transplant. In this first report of imaging in the setting of uterus transplantation, we report our experience with living-donor selection, and the evolution of the imaging techniques that ultimately allowed a significant improvement in donor selection and transplant outcome. We also suggest a framework for preoperative imaging in uterus transplantation.
Methods:
Between 2016 and 2018, 27 potential living donors were evaluated by imaging prior to uterine donation for uterus transplantation. Predonation imaging included a screening chest radiograph, dual-phase computed tomography (CT) angiograms of the abdomen and pelvis in the arterial and venous phases and pelvic sonography with Doppler. Seventeen potential donors additionally underwent multiphasic pelvic MR angiograms. The imaging performed was meant to display features of the vascular anatomy relevant for uterus transplantation.
Results:
Out of the 27 potential live donors who were evaluated by imaging, 9 eventually donated their uterus for transplantation. The most frequent reason for exclusion was suboptimal quality of the vessels (33%), including small uterine arteries, the presence of atherosclerosis or small size/poor quality of the uterine or utero-ovarian veins, or both. The next most common reason was voluntary patient withdrawal or failure to complete the evaluation process (28%). Three potential donors (16.6%) were rejected for uterine factors, fibroids, and/or adenomyosis. Other reasons for donor rejection included ABO incompatibility and unfavorable psychological evaluation.
Conclusion:
Diagnostic imaging plays a crucial role in selecting appropriate potential donors, screening prospective recipients, planning the graft procedure, and following up on any graft or nongraft-related complications in both the donor and recipient after the transplantation procedure is performed. Contrast-enhanced CT and MR angiographies have complementary roles, especially when evaluating the donor for adequacy of the arterial and venous supply to the uterine graft and the experience gained from our series indicates that the inclusion of both modalities contributed directly to successful uterus transplant graft survival by selecting patients with favorable arterial and venous vasculature.
Keywords: Uterus, transplantation, imaging, evaluation, donor, recipient computed tomography angiography–magnetic resonance angiography
Introduction
In 2014, the first successful birth after a uterus transplant was reported in Sweden,1 followed in 2017 by the first birth in the United States.2 The main indication for uterus transplant is infertility due to uterine factors, which may be congenital, functional, or acquired. It is estimated that uterine-factor infertility affects 3%–5% of women worldwide.3 Uterus transplant is still considered experimental, with approximately 40 cases performed worldwide at the time of this writing. The procedure is unique in several ways: it can be performed with living or deceased donors; it is a temporary transplant removed after successful delivery; and unlike other living-donor transplants, like kidney and liver, the uterus has exhausted its function in the living donor while still being perfectly functional for the recipient. To date, most uterus transplants have been performed with living-donor grafts.1,2,4–9
Radiological imaging is an essential part of the living-donor evaluation. As for any organ transplant, the graft needs blood inflow and outflow. Arterial inflow is provided by the uterine arteries, branches of the internal iliac artery, and outflow is via the uterine veins, which are tributaries of the internal iliac veins. The ovarian veins may contribute to the drainage via the utero-ovarian segment. Preserving ovarian venous drainage in premenopausal donors is paramount to avoid surgery-induced menopause. The satisfactory visualization of these vessels using currently available imaging modalities has been challenging, even though our experience showed such visualization to be very important for determining appropriateness for uterus transplantation. Three of the transplanted grafts in our initial series failed due to vascular issues, and this served as a trigger to refine preoperative imaging and add the routine use of magnetic resonance (MR) angiography. Despite significant improvements in vascular evaluation, more experience and imaging technique refinements are needed to achieve the level of comfort and confidence obtained with living kidney and liver donor preoperative imaging evaluation.
With increasing experience of uterus transplantation, the standard pelvic/uterine imaging modalities, comprising mainly ultrasound and MR imaging, are being adapted to address the issues raised by uterine transplantation, particularly in terms of evaluating arterial inflow and adequacy and anatomy of the venous outflow.
This study reports our experience with living-donor selection, focusing on lessons learned in preoperative imaging based on the first 27 potential and actual donors. To our knowledge, this is the first report that aims to suggest a framework for imaging in the setting of uterus transplantation.
Materials and methods
Between 2016 and 2018, 27 potential living donors were evaluated by imaging prior to uterine donation for uterus transplantation. Predonation imaging included a screening chest radiograph, dual-phase computed tomography (CT) angiograms of the abdomen and pelvis in the arterial and venous phases and pelvic sonography with Doppler. Seventeen potential donors also underwent multiphasic pelvic MR angiograms. Table 1 summarizes the predonation imaging protocol recommendations for living uterus donor candidates. The imaging performed for each of our potential donors and the transplantation outcomes are listed in Table 2.
Table 1.
Imaging parameters and protocol recommendations in live donor candidate evaluation.
| Imaging | Parameters used | Protocol recommendation |
|---|---|---|
| Ultrasound | • Scanner: LOGIQE9 (GE Medical Systems, Chicago, IL, USA)
• Transducers: 4 MHz curvilinear transabdominal and 8 MHz multifrequency transvaginal transducers • Scan parameters: • Transabdominal images: 100% acoustic output; grayscale images at 4 MHz; color Doppler images at 2.5 MHz, PRF: 0.8–1; pulsed-wave Doppler images at 2.1 MHz, PRF: 2.1–4.4 • Transvaginal images: 100% acoustic output; grayscale images at 8 MHz; color Doppler images at 4.2 MHz, PRF: 1.4–1.7; pulsed-wave Doppler at 4.2 MHz, PRF: 1.7; power Doppler at 4.2 MHz, PRF: 1.7 |
• Transabdominal and transvaginal grayscale images of the uterus in axial and sagittal orientation to the uterus
• Longitudinal and transverse images through the ovaries and adnexa, documenting the uterine and ovarian size, uterine orientation and version status, endometrial thickness and homogeneity, any uterine masses, fibroids, or evidence of adenomyosis • Color and spectral Doppler images of the proximal, mid, and distal portions of the uterine arteries and veins and the bilateral iliac vessels as well as color and/or power Doppler images of myometrial perfusion |
| CT angiogram | • Scanner: 32-slice GE LightSpeed Pro (GE Medical Systems, Chicago, IL, USA)
• Intravenous contrast: weight-based low-osmolarity nonionic intravenous iodinated contrast (Omnipaque; GE Healthcare, Chicago, IL, USA) |
• Source images in the axial plane using automatic modulation at 0.625 mm thickness
• Scan in the arterial phase using bolus triggering • Repeat axial images in the venous phase • Axial reformats at 2.5 mm slice thickness • Sagittal and coronal reformats at 5 mm slab thickness • Axial, sagittal, and coronal maximum-intensity projection images at 10 mm slab thickness • Reconstructed images displayed utilizing soft tissue algorithm |
| MR pelvis and angiogram | • Scanner: 1.5T Discovery MR 750W or Signa HD XT (GE Medical Systems, Chicago, IL, USA)
• Contrast: weight-based double-dose intravenous Gadobutrol (Gadavist; Bayer Healthcare Pharmaceuticals, Montville, NJ, USA) |
• Axial FIESTA (optional axial FIESTA fat saturation) 4 mm slice thickness with 4 mm gaps through the pelvis
• Axial two-dimensional time of flight FSPGR angiogram at 1.4 and 0.9 mm slice gaps • Axial precontrast VIBE images at 2 mm thickness and 1 mm gaps • Axial postcontrast images in the arterial and venous phases • Delayed images at 4–6 min postinjection and reconstructed maximum-intensity projection rotation and tumble images • All postcontrast images obtained with 4 mm slice thickness and 2 mm gaps • Additional sagittal and coronal postcontrast VIBE images recommended especially in the venous phase • Optional: axial HASTE (5 mm thickness and 5 mm gap) |
CT: computed tomography; MR: magnetic resonance; FIESTA: fast imaging employing steady-state acquisition; FSPGR: fast spoiled gradient; HASTE: half-Fourier acquisition single-shot turbo spin echo; PRF: pulse repetition frequency; VIBE: volumetric interpolated breath-hold sequence.
Table 2.
Imaging performed in the donors and case outcomes.
| Donor | Uterus donated | Pelvic US | Pelvic CT/CTA | Pelvic MRI/MRA | Outcome |
|---|---|---|---|---|---|
| 1 | − | + | + | − | |
| 2 | + | + | + | − | Uterine necrosis in immediate postoperative period |
| 3 | + | + | + | − | Uterine necrosis in immediate postoperative period |
| 4 | + | + | + | − | Uterine necrosis in immediate postoperative period |
| 5 | + | + | + | − | Delivered 14 months after surgery |
| 6 | − | + | + | − | |
| 7 | − | + | + | + | |
| 8 | − | + | + | − | |
| 9 | − | + | − | − | |
| 10 | + | + | + | + | Delivered 16 months after surgery. Attempting second pregnancy. |
| 11 | − | + | − | − | |
| 12 | − | + | + | + | |
| 13 | + | + | + | + | Pregnant awaiting delivery |
| 14 | − | + | + | + | |
| 15 | − | + | + | + | |
| 16 | − | + | + | + | |
| 17 | − | + | + | + | |
| 18 | − | + | + | + | |
| 19 | + | + | + | + | Graft failure due to bilateral UA thrombosis |
| 20 | − | + | + | + | |
| 21 | + | + | + | + | Pregnant awaiting delivery |
| 22 | − | + | + | + | |
| 23 | + | + | + | + | Graft failure due to bleeding |
| 24 | − | + | − | − | |
| 25 | − | + | + | + | |
| 26 | + | + | + | + | Pregnant awaiting delivery |
| 27 | − | + | + | + | |
| Deceased donor | + | − | − | − | Graft remains viable, early pregnancy ended in miscarriage. Attempting pregnancy. |
CT/CTA: computed tomography/CT angiography; IVF: in vitro fertilization; MRI/MRA: magnetic resonance imaging/magnetic resonance angiography; UA: uterine artery; US: ultrasound.
The imaging performed was meant to display features of the vascular anatomy relevant for uterus transplantation. The venous drainage of the uterus is via the bilateral uterine veins draining to the internal iliac veins, augmented by or supplanted by uterogonadal veins draining venous blood from the uterus and ipsilateral ovary to the inferior vena cava or renal vein.10–13 In our initial transplant series, the uterogonadal veins (without harm caused to the donor ovaries) and/or the uterine veins were anastomosed to the recipient external iliac veins (Figure 1). Other studies report use of gonadal veins as well.14 The uterus is supplied by the uterine arteries, a branch of the anterior division of the internal iliac arteries. Similar to the venous drainage, the vascular supply to the uterus may be contributed to by branches of the ipsilateral gonadal artery. In our initial transplant series, the uterine arteries were anastomosed bilaterally to the external iliac arteries.
Figure 1.
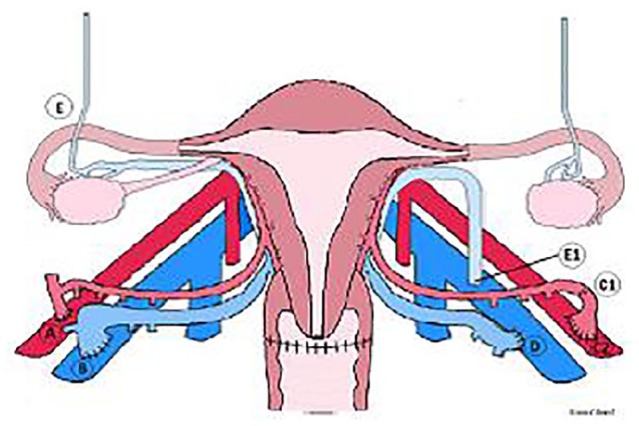
Uterine veins and uterogonadal veins. In our series, the uterine veins were anastomosed to the anterior or medial aspects of the iliac veins (B and D, respectively). The uterogonadal veins were anastomosed to the anterior aspect of the iliac vein where applicable.
Source: Reproduced with permission from the study by Testa et al.8
Results
Out of the 27 potential live donors who were evaluated by imaging, 9 donated their uterus for transplantation. Table 3 lists the reasons why some of the donors were found unsuitable. The most frequent reason for exclusion was suboptimal quality of the vessels (33%), including small uterine arteries, the presence of atherosclerosis or small size/poor quality of the uterine or utero-ovarian veins, or both. The next most common reason was voluntary patient withdrawal or failure to complete the evaluation process (28%). Three potential donors (16.6%) were rejected for uterine factors, fibroids, and/or adenomyosis. Other reasons for donor rejection included ABO incompatibility and unfavorable psychological evaluation.
Table 3.
Reasons for donor rejection among 27 potential living uterus donors.
| Reasons | Case, n (%) |
|---|---|
| Suboptimal uterine arterial supply or venous drainage | 6 (33.3) |
| ABO incompatibility | 1 (5.5) |
| Voluntary withdrawal or patient did not complete evaluation | 5 (27.7) |
| Uterine factors, fibroids, and/or adenomyosis | 3 (16.6) |
| Psychological evaluation criteria not met | 1 (5.5) |
| Other reasons | 2 (11.1) |
Ultrasound
Grayscale ultrasound demonstrated no evidence of abnormality that would have precluded uterus donation in most of the 27 potential donors (Figure 2). Four patients had uterine masses consistent with leiomyomas, which were multiple in three cases. Three of these patients were excluded; the other donated her uterus (Case 4). Two patients had lower-segment cesarean section scars, but this finding did not preclude the selection of these patients. Two patients had findings consistent with focal or diffuse adenomyosis. At least two patients were suspected to have endometrial polyps. Simple-appearing and hemorrhagic cysts were identified in four cases.
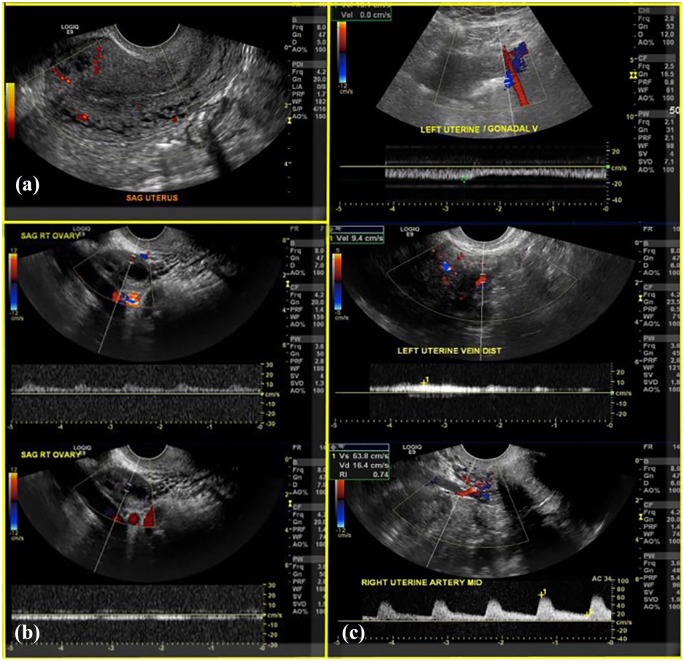
Figure 2.
Grayscale ultrasound performed in the potential uterus donors to assess uterine size and morphology and endometrial appearance: (a) normal uterine size and morphology and endometrial stripe. Intramyometrial flow shown using power Doppler. (b) Normal ovarian perfusion and arterial and venous spectral waveforms. (c) Spectral waveforms of the uterine artery and vein and the uterogonadal vein.
Doppler ultrasound was used to show uterine and ovarian color Doppler perfusion and spectral waveforms within the intramyometrial vessels, the uterine arteries/veins, and intraovarian flow (Figure 2). In most cases, it was not possible to distinguish between uterine and uterogonadal veins.
CT
CT was used to evaluate the vascular anatomy of the graft, predominantly the patency and caliber of the uterine and iliac arteries, and to screen for any vascular abnormalities (Figure 3). Visualization of the uterine venous drainage was suboptimal on CT in the initial four patients whose workup did not include pelvic MR/MR venogram, due to poor opacification of the veins. The vascular diameters ranged from 0.9 to 5.0 mm for the uterine arteries, 2.1 to 8.4 mm for the uterine veins, and 2.0 to 8.0 mm for the uterogonadal veins; the mean diameters were 2.1, 5.2, and 5.2 mm, respectively (Table 4). Maximum-intensity projection images were included in postprocessing and facilitated visualization of the vasculature, as well as aiding the visualization of calcific atherosclerotic changes. The inclusion of the entire abdomen and pelvis allowed us to screen for evidence of calcified or noncalcified atherosclerotic arterial disease elsewhere as a marker of the health of the donor arterial vasculature. No specific urographic sequences were performed to evaluate the ureteric courses.
Figure 3.
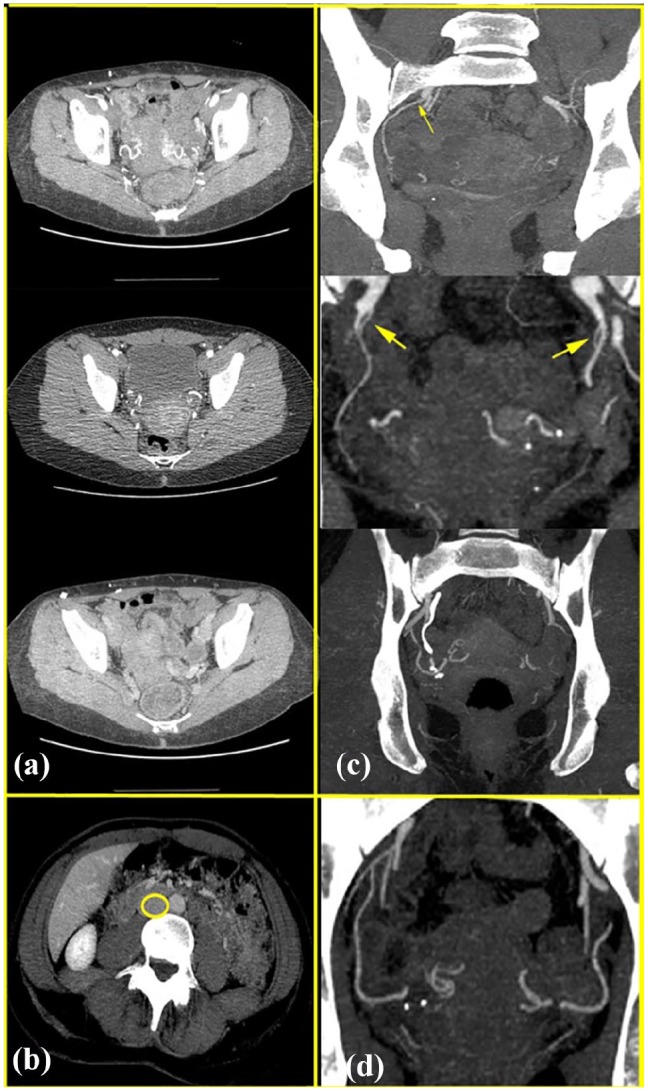
(a) CT demonstrating patency of the uterine arteries. (b) Suboptimal venous phase opacification, with the inferior vena cava outlined in yellow. (c) High origin of right uterine artery from the internal iliac artery (thin arrow), diminutive uterine arteries (large arrows), and coarse uterine artery calcification. (d) Maximum-intensity projection images also facilitated visualization of the entire bilateral uterine artery courses to advantage.
Table 4.
Uterine arterial supply and venous drainage evaluation by CT and/or MR.
| Case | CT angiography |
MRA |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| UA diameters (mm) |
Uterine veins (mm) |
Utero-ovarian veins (mm) |
Uterine arteries (mm) |
Uterine veins (mm) |
Utero-ovarian veins (mm) |
|||||||
| R | L | R | L | R | L | R | L | R | L | R | L | |
| 1 | 2.9 | 2 | 4 | 2.1 | ||||||||
| 2 | 1.7 | 1.6 | 7.1 | 6.4 | ||||||||
| 3 | 2.2 | 2.1 | 7.1 | 8 | ||||||||
| 4 | 2.1 | 1.9 | 7.9 | 8.4 | ||||||||
| 5 | 2.2 | 2.1 | 8 | 4.5 | ||||||||
| 6 | 2.1 | 2.5 | 5.8 | |||||||||
| 7 | 2.5 | 2.6 | 8 | 7.5 | MRA field of view not optimized to pelvis | |||||||
| 8 | Diminutive UAs | |||||||||||
| 9 | ||||||||||||
| 10 | 2 | Comment | n/a | n/a | n/a | 11 | 2 | 1.5–2 | n/a | n/a | 6 | 8–9 |
| 11 | ||||||||||||
| 12 | 2.5 | 2.5 | n/a | 4 | 3 | 6 | 2 | 2 | n/a | 4 | 3 | 6 |
| 13 | 1.4 | 1.8 | Not seen well | Not seen well | n/a | n/a | 3 | 3 | n/a | n/a | ||
| 14 | 1 | 0.9 | Poorly seen vessels | |||||||||
| 15 | 1.9 | 1.8 | n/a | n/a | 4 | n/a | n/a | n/a | ||||
| 16 | 2 | 1.8 | 5.5 | 4 | n/a | n/a | n/a | n/a | n/a | n/a | ||
| 17 | 2.3 | 2.3 | n/a | n/a | 4 | 3–5 | 3–5 | n/a | ||||
| 18 | 2.2 | 2 | 2 | 2 | 4 | 3 | 5 | 4 | ||||
| 19 | 2.1 | 2 | n/a | n/a | 3.5 | 2 | 6 | n/a | ||||
| 20 | 1.6 | 1.6 | n/a | n/a | 4 | 2 | n/a | n/a | ||||
| 21 | 3 | 5 | 5 | 8 | 2 | 4 | 3 | 3 | 5 | 8 | n/a | 4 |
| 22 | 1.5 | 1.5 | 6 | 6 | n/a | n/a | n/a | n/a | 6.7 | 6 | n/a | n/a |
| 23 | 2 | 1.8 | n/a | n/a | 4.6 | 3 | n/a | n/a | ||||
| 24 | ||||||||||||
| 25 | 2.5 | 2.3 | 4.5 | 5.2 | 3.3 | n/a | n/a | n/a | 4 | 4.7 | 3.4 | 3.1 |
| 26 | 1 | 1 | n/a | n/a | n/a | n/a | 1–2 | 1–2 | 1–2.5 | 2–3 | 3 | 2 |
| 27 | 2 | 1 | 4 | 4 | n/a | 4 | 2 | n/a | 4 | 4 | n/a | 5 |
| Mean in mm | 2.0 | 2.2 | 4.9 | 5.4 | 2.8 | 6.3 | 2.16 | 2.2 | 4.1 | 3.9 | 4.4 | 4.7 |
L: left; n/a: not available; R: right; CT: computed tomography; MR: magnetic resonance; MRA: magnetic resonance angiography; UA: uterine artery.
MR imaging
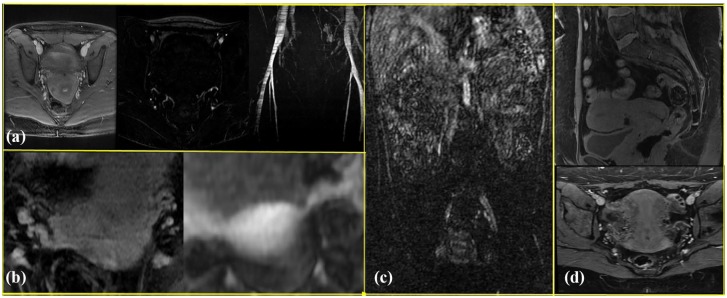
The addition of MR angiographic imaging improved the visualization of the primary uterine drainage by uterine versus uterogonadal veins and their relative size and courses (Figure 4). Performance of the Valsalva maneuver was helpful in increasing the venous conspicuity. Evaluation of the uterine arteries (Figure 5) was felt to be best accomplished with CT angiography (CTA). The field of view was limited to the pelvis for optimal assessment of the pelvic viscera and vasculature.
Figure 4.
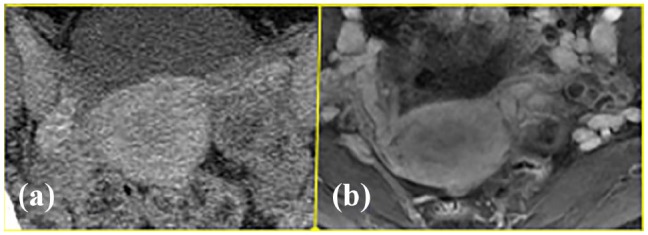
Venographic phase images on (a) CT and (b) MR, showing better uterine vein depiction on MR venography.
Figure 5.
(a) Arterial phase subtraction images demonstrating the uterine arteries and time-of-flight maximum-intensity projection image showing the bilateral common, external, and internal iliac arteries. (b) The Valsalva maneuver helped increase the venous conspicuity (right image) when compared to images obtained without the maneuver (left image). A small field of view was crucial; (c) shows a large field of view precluding evaluation of the pelvic viscera or vasculature on a coronal postcontrast subtracted image compared with (d) where an appropriately tailored field of view shows normal uterine appearance in the oblique-axial and sagittal planes. Note susceptibility artifact from prior lower-segment uterine cesarean section scar.
Concordance of vessels between imaging and intraoperative assessment
Table 5 lists the vessels utilized for the transplantation procedure based on intraoperative assessment of the quality and suitability of the vessels versus the angiographic findings of vessel size on CTA or MR angiography. Overall, there was good concordance between imaging and intraoperative findings, although some of the veins found suitable by intraoperative assessment were not adequately visualized on pretransplant MR venography.
Table 5.
Vessels used for transplantation compared with imaging measurements of the vessels.
| Transplant case | CT angiography |
MR angiography |
Vessels used for transplantation | Concordant with the imaging findings? (Yes, No, NA) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| UV |
UOV |
UV |
UOV |
|||||||
| R | L | R | L | R | L | R | L | |||
| 1 | 7.1 | 6.4 | NA | NA | NA | NA | NA | NA | R UV/L UOV | Y/NA |
| 2 | NA | 7.1 | 8 | NA | NA | NA | NA | NA | R UOV/L UV | Y/NA |
| 3 | 7.9 | 8.4 | NA | NA | NA | NA | NA | NA | R UOV/L UV | NA/Y |
| 4 | 8 | 4.5 | NA | NA | NA | NA | NA | NA | R UOV/L UOV | NA/NA |
| 5 | NA | NA | NA | 11 | NA | NA | 6 | 8–9 | R UOV/L UOV | Y/Y |
| 6 | NA | NA | NA | NA | 3 | 3 | NA | NA | R UV/L UV | Y/Y |
| 7 | NA | NA | NA | NA | 3.5 | 2 | 6 | NA | R UOV/L UOV | Y/NA |
| 8 | NA | NA | NA | NA | 4.6 | 3 | NA | NA | R UV/L UV + UOV | Y/Y |
| 9 | NA | NA | NA | NA | 1–2.5 | 2–3 | 3 | 2 | R UOV/L UV | Y/Y |
CT: computed tomography; MR: magnetic resonance; R: right; L: left; NA: not available; UV: uterine vein; UOV: utero-ovarian vein.
Discussion
The novelty of uterus transplantation makes every case pivotal from a learning perspective. The intraoperative findings and case outcomes from our initial 10 cases of uterus transplantation helped modify our selection protocols for both recipients and donors, as well as for uterine grafts. The imaging protocol was under continuous improvement during the initial cases, and the changes made improved the quality of the uterine grafts selected and subsequently the clinical outcomes. Most importantly, close coordination between the transplant team and an experienced radiologist was valuable to identify patients with relative or absolute contraindications to transplantation (e.g. significant uterine arterial atherosclerosis, uterine anomalies in the donors, adenomyosis, etc.) and to plan the graft procedures appropriately. In an attempt to minimize both the risks to the living donor and the costs the ultrasound examination was done first. This procedure is typically much less invasive and much less costly than a CT or MR angiography. The pelvic ultrasound examination allowed for assessment of the endometrial lining for any focal lesions, in addition to assessing the spectral and color Doppler patterns of the myometrium and uterine and ovarian vasculature. Only a few of the donors were excluded due to findings on ultrasound.
The size and quality of the uterine venous drainage via uterine or utero-ovarian veins are crucial to the outcome, that is, graft survival. Our experience suggests that CT angiography was suboptimal for evaluation of the donor uterine venous drainage. We believe that adding pelvic MR angiography in the later cases directly contributed to the technical success and graft survival in those cases.
The presence of occult atherosclerosis, not detected by imaging, may prove to be a significant factor in graft failure, as was the case in at least one patient in our series (Case 4), even in the absence of any known predisposing factors. One possible strategy would be the selection of uterine donors who are relatively younger and thus at lower statistical risk of harboring occult atherosclerosis. The other factor that contributed to graft failure in the other two initial cases was related to challenges in establishing an adequate venous anastomosis. The inclusion of MR angiographic imaging can significantly increase the diagnostic confidence in evaluating the uterine venous outflow. Contrast-enhanced and noncontrast MR techniques are complementary and have been used extensively in plastic and transplant surgery.15–17 A small field of view, especially on the contrast-enhanced images, is key to optimize the spatial resolution. An antiperistaltic agent (Glucagon) was used to combat physiological bowel motion degradation of the image quality. Prone positioning may be of value to address the issue of respiratory motion.
CT angiography appears to afford better assessment of the uterine arterial vasculature, as well as enabling assessment of macroscopic evidence of atherosclerosis. However, noncontrast images were not obtained, limiting evaluation for subtle calcific atherosclerotic disease. The use of CT angiography is already widely established in preoperative planning.18 Adding precontrast images may be helpful, as may the use of dual-energy CT when available to enable evaluation of calcification and angiographic findings on a single-phase examination.19 We did not utilize either in our current case series.
Deceased uterine donors are becoming more common across the world, and many centers have institutional review board approval to use both living and deceased donors. To date, our center has done two cases of deceased donor uterus transplantation, and they posed a significant clinical and imaging challenge, as there was frequently a lack of comprehensive past medical history, requisite laboratory data, and dedicated imaging.
Conclusion
Diagnostic imaging plays a crucial role in selecting appropriate potential donors, screening prospective recipients, planning the graft procedure, and following up on any graft or nongraft-related complications in both the donor and recipient after the transplantation procedure is performed. Contrast-enhanced CT and MR angiographies have complementary roles, especially when evaluating the donor for adequacy of the arterial and venous supply to the uterine graft.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Brännström M, Johannesson L, Bokström H, et al. Livebirth after uterus transplantation. Lancet 2015; 385: 607–616. [DOI] [PubMed] [Google Scholar]
- 2. Testa G, McKenna GJ, Gunby RT, Jr, et al. First live birth after uterus transplantation in the United States. Am J Transplant 2018; 18(5): 1270–1274. [DOI] [PubMed] [Google Scholar]
- 3. Allyse M, Amer H, Coutifaris C, et al. American Society for Reproductive Medicine position statement on uterus transplantation: a committee opinion. Fertil Steril 2018; 110: 605–610. [DOI] [PubMed] [Google Scholar]
- 4. Brannstrom M, Johannesson L, Dahm-Kahler P, et al. First clinical uterus transplantation trial: a six-month report. Fertil Steril 2014; 101(5): 1228–1236. [DOI] [PubMed] [Google Scholar]
- 5. Brännström M, Bokström H, Dahm-Kähler P, et al. One uterus bridging three generations: first live birth after mother-to-daughter uterus transplantation. Fertil Steril 2016; 106: 261–266. [DOI] [PubMed] [Google Scholar]
- 6. Flyckt RL, Farrell RM, Perni UC, et al. Deceased donor uterine transplantation: innovation and adaptation. Obstet Gynecol 2016; 128(4): 837–842. [DOI] [PubMed] [Google Scholar]
- 7. Erman Akar M, Ozkan O, Aydinuraz B, et al. Clinical pregnancy after uterus transplantation. Fertil Steril 2013; 100: 1358–1363. [DOI] [PubMed] [Google Scholar]
- 8. Testa G, Koon EC, Johannesson L, et al. Living donor uterus transplantation: a single center’s observations and lessons learned from early setbacks to technical success. Am J Transplant 2017; 17(11): 2901–2910. [DOI] [PubMed] [Google Scholar]
- 9. Fageeh W, Raffa H, Jabbad H, et al. Transplantation of the human uterus. Int J Gynaecol Obstet 2002; 76: 245–251. [DOI] [PubMed] [Google Scholar]
- 10. Karaosmanoglu D, Karcaaltincaba M, Karcaaltincaba D, et al. MDCT of the ovarian vein: normal anatomy and pathology. AJR Am J Roentgenol 2009; 192(1): 295–299. [DOI] [PubMed] [Google Scholar]
- 11. Saksouk FA, Johnson SC. Recognition of the ovaries and ovarian origin of pelvic masses with CT. Radiographics 2004; 24(Suppl. 1): S133–S146. [DOI] [PubMed] [Google Scholar]
- 12. Pavkov ML, Koebke J, Notermans HP, et al. Quantitative evaluation of the utero-ovarian venous pattern in the adult human female cadaver with plastination. World J Surg 2004; 28(2): 201–205. [DOI] [PubMed] [Google Scholar]
- 13. Asayama Y, Yoshimitsu K, Aibe H, et al. MDCT of the gonadal veins in females with large pelvic masses: value in differentiating ovarian versus uterine origin. AJR Am J Roentgenol 2006; 186(2): 440–448. [DOI] [PubMed] [Google Scholar]
- 14. Wei L, Xue T, Tao KS, et al. Modified human uterus transplantation using ovarian veins for venous drainage: the first report of surgically successful robotic-assisted uterus procurement and follow-up for 12 months. Fertil Steril 2017; 108(2): 346.e–356.e1. [DOI] [PubMed] [Google Scholar]
- 15. Kagen AC, Hossain R, Dayan E, et al. Modern perforator flap imaging with high-resolution blood pool MR angiography. Radiographics 2015; 35(3): 901–915. [DOI] [PubMed] [Google Scholar]
- 16. Katsumori T, Kasahara T, Kin Y, et al. Magnetic resonance angiography of uterine artery: changes with embolization using gelatin sponge particles alone for fibroids. Cardiovasc Intervent Radiol 2007; 30(3): 398–404. [DOI] [PubMed] [Google Scholar]
- 17. Takahashi N, Yoshino O, Hiraike O, et al. The assessment of myometrium perfusion in patients with uterine fibroid by arterial spin labeling MRI. Springerplus 2016; 5(1): 1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujioka H, Kato T, Sone M, et al. Study of preoperative 3D-CT angiography of uterine artery in patients with uterine cervical cancer. Nihon Hoshasen Gijutsu Gakkai Zasshi 2017; 73(2): 112–119. [DOI] [PubMed] [Google Scholar]
- 19. Brockmann C, Jochum S, Sadick M, et al. Dual-energy CT angiography in peripheral arterial occlusive disease. Cardiovasc Intervent Radiol 2009; 32: 630–637. [DOI] [PubMed] [Google Scholar]