Abstract
Objectives:
Being the most common type of oral malignancies, oral squamous cell carcinoma is initiated by epithelial dysplasia, which can be marked by the expression of nuclear factor kappa B and cyclooxygenase 2. Curcumin has been known for its anti-cancer potential. The objective of this study was to evaluate the anti-cancer potential of curcumin on oral squamous cell carcinoma based on the expression of the nuclear factor kappa B and cyclooxygenase 2 during epithelial dysplasia stage.
Methods:
This experimental study was performed on 35 Sprague Dawley rats at the Veterinary Medicine Faculty, Bogor Agricultural Institute, Indonesia. At the beginning of the experiments, all rats were induced by 100 µg 0.5% 7,12-dimethylbenz(a)anthracene every 2 days for the duration of 28 days. Once epithelial dysplasia stage was reached, all rats were then randomly divided into control group (that did not receive curcumin) or the experimental group (the group that received curcumin for the next 4 weeks). After 4 weeks, the histopathological examination of haematoxylin and eosin and immunohistochemistry examination were conducted. Data were gathered and analyzed by using the Wilcoxon–Mann–Whitney test.
Results:
The results of the current study revealed that the experimental group showed significantly less nuclear factor kappa B (p < 0.01) and cyclooxygenase 2 (p = 0.03) expressions compared to the control group.
Conclusion:
The results of the study suggested that curcumin was effective in suppressing nuclear factor kappa B and cyclooxygenase 2 expression in experimentally induced oral squamous cell carcinoma. Future studies investigating curcumin anti-cancer potential in a further stage of oral squamous cell carcinoma, as well as the involvement of other components that might improve curcumin anti-cancer potential, are of importance.
Keywords: Oral squamous cell carcinoma, curcumin, anti-cancer, NFκB, COX-2
Introduction
Oral squamous cell carcinoma (OSCC) is one of the most common malignancies found in the oral cavity,1 and the number eight among most common cancers.2,3 This particular type of oral malignancy is known for its low survival rate with less than 60% of those who experienced this type of cancer survived more than 5 years.2,4 There are several factors that have been identified as the risk factors for OSCC, namely tobacco use, alcohol consumption, betel nut chewing, and smoking.1,4,5 Aside from its high prevalence, OSCC has also been known for its impact on quality of life of its sufferer, as reported in previous studies.6–8 Due to its detrimental impact on the sufferer, more lines of possible therapy are explored, including those of alternative medicine with the involvement of natural herbs.
One of the most explored natural herbs for the purpose of (oral) cancer treatment is curcumin.9–11 Curcumin, a polyphenol derived from Curcuma longa which also known as turmeric, has been known for its therapeutic potentials, including anti-inflammatory, anti-oxidant, analgesic, anti-cancer, anti-microbial, as well as anti-septic potential.9,12 The anti-cancer effect of curcumin for several types of cancer was proven by its ability to inhibit the pathways as well as products of several proteins and enzymes involved in cancer development, such as nuclear factor kappa B (NFκB) and cyclooxygenase 2 (COX-2).9,13 NFκB and COX-2 are the two factors that have been known for their increased expression in oral pre-cancer lesion as well as OSCC.14–16
The NFκB is a transcription factor that is involved in the induction of several genes that will eventually lead to an inflammatory process. The NFκB plays a major role in the malignant behavior of a tumor, including OSCC. The constitutive activation of NFκB or increased activity of IκB kinase (IKK) in tumor cells protects the cells from induction of apoptosis caused by chemotherapy. This particular mechanism among any other mechanisms acts as the scientific explanation of the survival and development the tumor cells.17 As for COX-2, this particular enzyme is known as the isoform of the cyclooxygenase (COX) enzyme that is induced by mitogens, cytokines, growth factors, and tumor promoter.18 It has been revealed that COX-2 enhances cancer invasion as well as metastasis by decreasing the expression of E-cadherin which eventually will lead to phenotypic changes in the epithelial cells, which then enhances the carcinogenic potential.19 These are the reasons of the increased expression of COX-2 and NFκB in several types of cancer.
Based on the above explanation, a study that aimed on evaluating the anti-cancer potential of curcumin on OSCC through the analysis of NFκB and COX-2 expression at epithelial dysplasia stage was designed. This in vivo study used histopathological examination with hematoxylin and eosin (HE) staining method to confirm the epithelial dysplasia stage, and immunohistochemistry analysis to evaluate the NFκB and COX-2 expression.
Methods
This current experimental study was conducted at the Animal Teaching Hospital of the Faculty of Veterinary Medicine, Bogor Agricultural Institute, Bogor, Indonesia, by using 40 Sprague Dawley rats. Prior to the start of the study, an ethical clearance from the Health Research Ethical Committee of the Bogor Agricultural Institute was obtained. All procedures performed were in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals and Basel Declaration. All authors declared that all legal and ethical aspect of the current study has been fulfilled prior to and during the study period. Ethical clearance (number: 34-2017 ACUC RSHP FKH-IPB) was given by the Animal Medical Research Ethics Committee of Bogor Agricultural University, prior to the start of the study. Sample size calculation was performed by using the G Power formula.20 Based on the study literature, the effect size between the two groups was set at 48%, the type 1 error was fixed at 5% (p = 0.05), the power of a study was set at 80%, and the direction of effect was two tailed. Based on this calculation, the number of sample needed for each group was 16 animals. Expected attrition or death of animals was then set at 20%, and therefore, the numbers of animal per group was set at 20 animals.
Once ethical clearance was gained, 40 healthy male Sprague Dawley rats that aged about 8 weeks old, weighted about 200–300 mg and presented no anatomical abnormalities, went through PES vaccination, as well as internal and external parasite elimination procedure were recruited for the study. All rats were then quarantined for 7 days for adaptation purposes. Once the quarantined period was completed, 20 customized cages were prepared, whereas each cage was occupied by two rats.
7,12-Dimethylbenz(a)anthracene (DMBA)
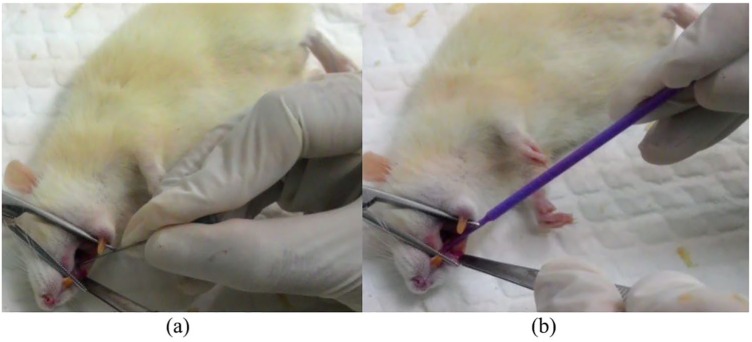
7,12-Dimethylbenz(a)anthracene, which is also known as DMBA and classified as phenanthrene derivative, is known as potent carcinogen.21 The use of DMBA to induce OSCC has been documented in previous studies.22,23 In the current study, prior to the application of DMBA, each rat was anesthetized by administering ketamine hydrochloride and xylazin on abdominal muscle. The dose was 45 mg per kilogram body weight for ketamine hydrochloride and 0.35 mg per kilogram body weight for xylazin. Once the rat was anesthetized, a slight scratch was then made by using a 27-gauge needle in the buccal region followed by the application of 0.5% DMBA (≈100 µg), topically to the wounded tissue (Figure 1). DMBA application was repeated every 2 days for the period of 28 days. On the 29th day, curcumin consumption in the experimental group was initiated. In order to have a random allocation, each rat was given a number and each number was randomly assigned to a group by using a random sequence generator. All rats were then randomly assigned to one of the following: the control group that did not have curcumin consumption; or the experimental group, the group that had 80 mg of curcumin intake per kilogram body weight, thrice per day for 28 days.
Figure 1.
(a) A slight scratch on the buccal mucous was made by using a 27-gauge needle. (b) DMBA was being applied on the scratched buccal mucous.
Curcumin
Curcumin used in the current study was produced by a national natural herb company named Sidomuncul. Each curcumin package contains 50 curcumin capsules (100 mg). Based on previous studies, the suggested dose for curcumin was 80 mg per kilogram body weight.24,25 Therefore, the dose for every rat was customized based on their bodyweight. Curcumin was then mixed into a special food mixture that comprised of 4% crude fat, 5% crude fiber, 12% crude ash, 12% moisture, 30% crude protein, niacin, vitamin (A, B1, B2, B5, B12, D3, E), pantothenic, biotin, and choline. This customized curcumin food mixture weighted about 100 mg (curcumin composition varied between 16 and 24 mg per rat, added with 76–84 mg food components) and was administered orally (Figure 2) to each rat in the experimental group thrice a day. Those in the control group received the same food mixture with the exact consumption time, with the absence of curcumin (100 mg of food components). Curcumin consumption was carried on for 28 days. During curcumin consumption period, five rats (four from the control group and one from the experimental group) were declared dead by our field veterinarian. Therefore, only 35 rats were euthanized and included in the final analysis.
Figure 2.
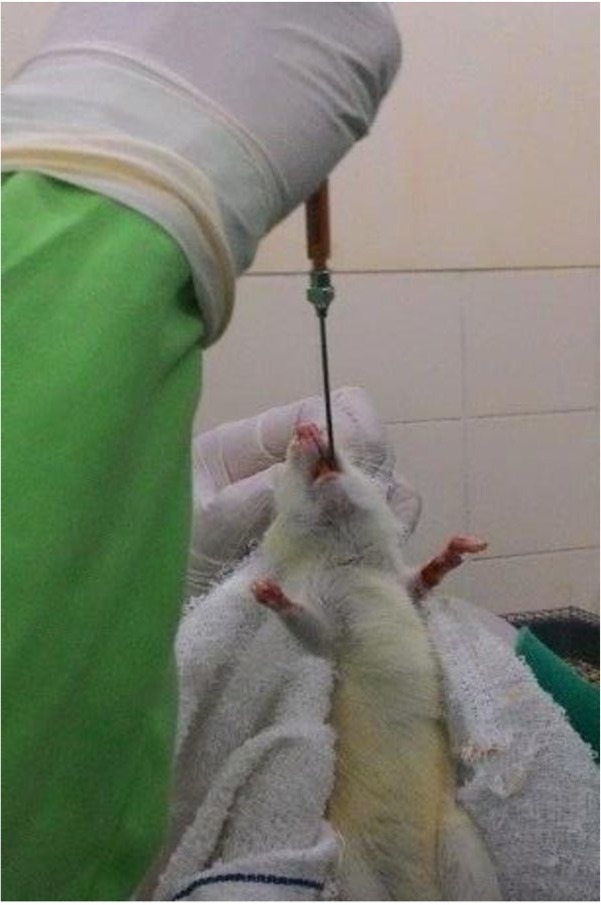
Oral administration of special curcumin mixture.
Euthanasia procedure
Euthanasia procedure for every rat was performed on the 63rd day by injecting sodium pentobarbital as much as 180 mg per kilogram body weight by using 26-gauge syringe. Intraperitoneal sodium pentobarbital has been recommended by the Canadian Council on Animal Care (CCAC) as well as American Veterinary Medical Association (AVMA) as the acceptable euthanasia method in rats and has been tested for its efficacy in previous studies.26–28 The intraperitoneal injection was performed by a certified veterinarian. Once the injection was performed, the veterinarian closely observed the rat for 3 min and then placed the index finger at the common carotid artery for 1 min to detect a pulse. If a pulse was still detected, the veterinarian would continue to place the index finger on the common carotid artery for another minute until no pulse was detected. Once a pulse was no longer detected, the veterinarian declared the time of death.
Histopathological examination
Once the rat was declared dead by the veterinarian, an incision of the buccal mucosa was made for the purpose of biopsy. A sample of excised buccal mucosa was then inserted into a formalin buffer solution to be fixated. The sample was then embedded into a paraffin block. This procedure was repeated for all 35 rats until all paraffin blocks were ready. The cutting of the paraffin blocks was performed by using a rotary microtome for the thickness of 5 μm. Prepared paraffin block was placed on the object glass prior to HE staining. This particular staining was performed in order to confirm the epithelial dysplasia stage of OSCC.
Immunohistochemistry examination
A Starr Trek Universal HRP Detection system (Biocare Medical, USA) with the Labeled Streptavidin-Biotin (LSAB) immunoperoxide complex method was used for the immunohistochemistry protocol. Another paraffin blocks with the same thickness (5 μm) as the ones used in the HE staining were prepared in object glasses prior to the examination. The primary antibody used in immunohistochemistry protocol was the rabbit polyclonal antibody NFκB-p105/p50 (GeneTex, USA) for NFκB, and rabbit polyclonal antibody COX-2 (Abcam, USA) for COX-2.
Data interpretation and grading system
As data interpretation of NFκB and COX-2 expression was performed semi-quantitatively based on cell distribution and color intensity criteria (Table 1), a Histoscore (H-score) was calculated and the data were converted according to the H-score (see also Table 1). H-score was calculated by using the following formula29
Table 1.
Grading system criteria of NFκB and COX-2 immunohistochemistry examination.
| Classification | |||||
|---|---|---|---|---|---|
| Cell distribution | 0 | 1 | 2 | 3 | 4 |
| No IRC detected | IRC < 20% | IRC 20%–50% | IRC 51%–80% | IRC > 80% | |
| Color intensity | 0 | 1 | 2 | 3 | |
| No color | Positive—weak | Positive—moderate | Positive—strong | ||
| Histoscore | 0–1 | 2–3 | 4–8 | 9–12 | |
| Negative | Weak | Moderate | Strong | ||
NFκB: nuclear factor kappa B; COX-2: cyclooxygenase 2; IRC: immunoreactive cells.
Data analysis
In order to analyze the expression differences of NFκB and COX-2 between the control group and the experimental group, the Wilcoxon–Mann–Whitney test was used. The analysis was performed by using SPSS version 23 by IBM.
Results
In the current study, DMBA was used to induce epithelial dysplasia stage of OSCC in 40 male Sprague Dawley rats. Twenty healthy rats were randomly assigned to each group, yet, at the end of the experimental period, four samples in the control group were dead, and one sample from the experimental group was dead. Therefore, there were only 16 rats in the control group and 19 rats in the experimental group. Mild to moderate degree of epithelial dysplasia in all experimental animals in this study was confirmed through histopathological examination. For comparison purposes, the histology image of a healthy rat oral mucosa can be viewed in an article by Kondo et al.30 and Scrobota et al.31 Mild epithelial dysplasia (Figure 3(a)) was confirmed through the histopathological changes shown in basal or parabasal layer while moderate epithelial dysplasia was confirmed through the thickening of the squamous epithelium as well as the basal cell membrane, pigmentation of the nucleus, the appearance of mitotic cells, cells maturation, and stratification (Figure 3(b)).
Figure 3.
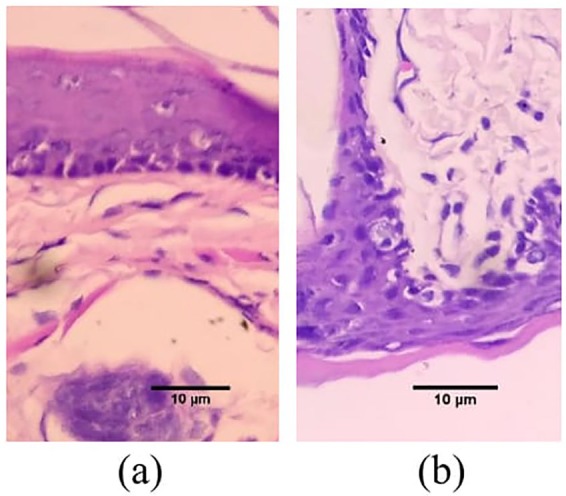
Histopathological image of epithelial dysplasia in OSCC: (a) Initial level of epithelial dysplasia in OSCC and (b) moderate level of epithelial dysplasia in OSCC.
As for NFκB and COX-2 expression, analysis was performed based on the expression in the cytoplasm and in the nucleus of the epithelial cells. When the expression between control group and experimental group was compared, it was revealed that the experimental group had more samples that showed mild (26.3%) and moderate (63.1%) expression of NFκB compared to the control group of which all samples showed strong expression of NFκB (Table 2). As for COX-2 expression, the experimental group had six (31.6%) samples with no sign of COX-2 expression. The difference of NFκB immunoexpression between control group and experimental group was proven to be highly significant (p < 0.01), and the difference of COX-2 immunoexpression between control group and experimental group was also proven to be significant (p = 0.03).
Table 2.
Comparison of the immunoexpression of NFκB and COX-2 in control group and experimental group.
| Variable | No. | Number of cells | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control |
Experimental | ||||||||
| IRC | HS | IRC | HS | ||||||
| NFκB | 1 | 4 | 12 | 2 | 6 | ||||
| 2 | 3 | 9 | 2 | 4 | |||||
| 3 | 4 | 12 | 2 | 6 | |||||
| 4 | 4 | 12 | 2 | 4 | |||||
| 5 | 3 | 9 | 2 | 4 | |||||
| 6 | 4 | 12 | 2 | 6 | |||||
| 7 | 3 | 12 | 2 | 4 | |||||
| 8 | 4 | 9 | 2 | 4 | |||||
| 9 | 4 | 12 | 1 | 2 | |||||
| 10 | 3 | 9 | 3 | 9 | |||||
| 11 | 4 | 12 | 1 | 2 | |||||
| 12 | 4 | 12 | 2 | 6 | |||||
| 13 | 3 | 9 | 2 | 4 | |||||
| 14 | 3 | 9 | 2 | 4 | |||||
| 15 | 4 | 12 | 1 | 2 | |||||
| 16 | 3 | 9 | 1 | 1 | |||||
| 17 | Total cells: 57 | 2 | 2 | ||||||
| 18 | 2 | 4 | |||||||
| 19 | 1 | 2 | |||||||
| Total cells: 34 | |||||||||
| H-score | H-score | ||||||||
| 0–1 | 2–3 | 4–8 | 9–12 | 0–1 | 2–3 | 4–8 | 9–12 | ||
| 0 (0%) |
0 (0%) |
0 (0%) |
16 (100%) |
1 (5.3%) |
5 (26.3%) |
12 (63.1%) |
1 (5.3%) |
||
| p value < 0.01 | |||||||||
| No. | IRC | HS | IRC | HS | |||||
| COX-2 | 1 | 2 | 4 | 1 | 1 | ||||
| 2 | 2 | 4 | 2 | 4 | |||||
| 3 | 1 | 2 | 2 | 2 | |||||
| 4 | 2 | 4 | 1 | 1 | |||||
| 5 | 1 | 2 | 2 | 2 | |||||
| 6 | 2 | 4 | 2 | 2 | |||||
| 7 | 2 | 4 | 1 | 1 | |||||
| 8 | 1 | 2 | 2 | 2 | |||||
| 9 | 2 | 4 | 1 | 1 | |||||
| 10 | 2 | 4 | 2 | 4 | |||||
| 11 | 3 | 6 | 1 | 2 | |||||
| 12 | 2 | 4 | 2 | 4 | |||||
| 13 | 1 | 2 | 1 | 2 | |||||
| 14 | 2 | 4 | 2 | 4 | |||||
| 15 | 1 | 2 | 2 | 4 | |||||
| 16 | 1 | 2 | 2 | 4 | |||||
| 17 | Total cells: 27 | 1 | 1 | ||||||
| 18 | 2 | 4 | |||||||
| 19 | 1 | 1 | |||||||
| Total cells: 30 | |||||||||
| H-score | H-score | ||||||||
| 0–1 | 2–3 | 4–8 | 9–12 | 0–1 | 2–3 | 4–8 | 9–12 | ||
| 0 (0%) |
6 (37.5%) |
10 (62.5%) |
0 (0%) |
6 (31.6%) |
5 (26.3%) |
8 (42.1%) |
0 (0)% |
||
| p value = 0.03 | |||||||||
NFκB: nuclear factor kappa B; COX-2: cyclooxygenase 2; IRC: immune-reactive cells; HS: Histoscore; H-score: 0–1 (no sign of expression), 2–3 (mild expression), 4–8 (moderate expression), and 9–12 (strong expression).
Significance level at p < 0.05.
The number of cells evaluated in the current study can also be viewed in Table 2. The difference in the number of cells being evaluated was due to expression difference in each group. The highlights of the histopathological findings of NFκB and COX-2 expression at epithelial dysplasia level of OSCC between control group and experimental group can be seen in Figure 4. Furthermore, the analysis of the current study suggested that the effective dose of curcumin to suppress the expression of NFκB and COX-2 was an oral dose of 80 mg per kilogram body weight, administered thrice a day.
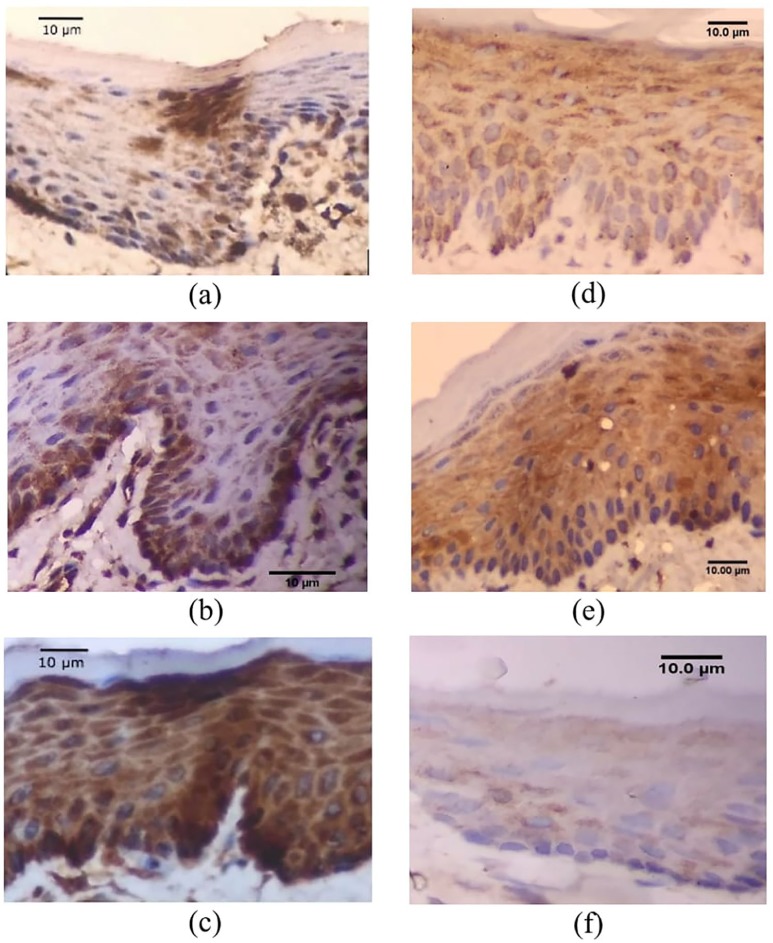
Figure 4.
Histopathological image of NFκB and COX-2 expression: (a) Mild expression of NFκB in experimental group, (b) moderate expression of NFκB in experimental group, (c) strong expression of NFκB in control group, (d) mild expression of COX-2 in experimental group, (e) moderate expression of COX-2 in control group, and (f) negative COX-2 expression in experimental group.
Discussion
Curcumin has been known for its medicinal potential, one of which is its anti-cancer potential. The results of the current study were in support of results provided by previous studies.11,32,33 A study by Anggarwal et al.33 about the efficacy of curcumin on human head and neck squamous cell carcinoma (HNSCC) showed how curcumin effectively suppressed the growth of the human HNSCC by interfering with the NFκB signaling pathway. NFκB, an inducible transcription factor that has been well acknowledged for its involvement in several types of cancer, is activated by a wide variety of factors, including carcinogens, tumor promoters, tumor necrosis factor (TNF) family, smoking habit, as well as UV, x-rays, and γ-radiation. The activation of NFκB has been strongly associated with the invasion and metastasis of cancer.33,34
There are several hypotheses proposed on how NFκB involved in the development of cancer. It has been shown that highly metastatic human HNSCC actively produces pro-inflammatory cytokines, namely TNF-α, several types of interleukin (IL), and granulocyte-macrophage colony stimulating, which have been known for their roles in the activation of NFκB.35 Upon activation, IκBα protein will then go through phosphorylation, ubiquitination, and degradation, which then lead to the release of p65 and p50, a subunit of NFκB. The p65 and p50 were then translocated to the nucleus to bind specific DNA sequences in the promoter of various genes, ready to initiate the transcription process.36 Based on this above mentioned, the activation of NFκB is predicted to have a major role in tumor metastasis.35 Therefore, inhibition of this particular factor is hypothesized to have an effect on tumor development as well.
In relation to the anti-cancer potential of curcumin, several mechanisms that might explain how curcumin inhibits NFκB activation have been proposed, including inhibition of cell proliferation, suppression of the cell cycle, and induction of apoptosis.32 A study by Anggarwal et al. revealed that the inhibition of NFκB activation was performed through the abrogation of IκBα kinase (IKK), which then leads to the suppression of several cell survival gene products as well as several cell proliferative gene products, such as B-cell lymphoma 2 (Bcl-2), cyclin D1, IL-6, COX-2, and matrix metallopeptidase 9 (MMP-9). Furthermore, the NFκB inhibition by cell-permeable P65-based peptide also suppressed the proliferation of HNSCC cells. And as curcumin completely inhibited the kinase activity, it was suggested that the type of inhibition provided by curcumin on NFκB activation is a direct inhibition.33
As for COX-2 expression in OSCC, previous studies have revealed that COX-2 overexpression has been suspected to be the cause of tumorigenesis promotion through pro-carcinogen activation, cancer cell proliferation stimulation, as well as apoptosis induction.14,37 In a study conducted by Sawhney et al.,14 about the expression of NFκB and COX-2 in oral precancerous lesion (OPL) and OSCC, it was shown that both factors were found to be overexpressed and that they were positively correlated. The significant parallel-increase of both factors from normal condition OPL to OSCC indicates their involvement with the development and progression of the cancer.14 Therefore, it is only natural that an inhibition of the NFκB activation will be followed by the suppressed expression of COX-2 as shown in the results of the current study.
Aside from the indirect effect of curcumin on COX-2 through the inhibition of NFκB activation, a more direct mechanism of COX-2 inhibition by curcumin was revealed by Zhang et al.38 In their study about the inhibition of curcumin on COX-2, it was shown that curcumin inhibited the chenodeoxycholate (CD)-mediated induction of COX-2 regardless of the presence of exogenous prostaglandin E2 (PGE2). This particular mechanism showed that the inhibitory effect of curcumin on COX-2 transcription is unrelated to its inhibitory effect of COX-2 enzyme activity, indicating the direct effect of curcumin inhibition on COX-2 transcription activity.38 This direct effect of curcumin on COX-2 might also responsible for the results gained in the current study, whereas a difference between NFκB and COX-2 expression was observed and that more cells showed no sign of COX-2 expression in the experimental group (Table 2).
Another point to note was the method of delivery of curcumin used in the current study. In regard to the delivery method, curcumin in the current study was given through a food mixture that contained several components (see the “Method” section), namely crude fat, crude fiber, crude ash, moisture, crude protein, niacin, vitamin, pantothenic, biotin, and choline. In regard to these components, a study conducted by Mirzaee et al.39 about the diverse effects of different protein-based “vehicles” on curcumin stability as well as bioavailability has emphasized the increase of curcumin anti-cancer activity when a certain type of protein is present. Yet, as there was no evaluation regarding the role of proteins or vitamins included in the food mixture of the current study, no further explanation about the role of the proteins, vitamins, or mineral on curcumin stability as well as bioavailability can be provided. Therefore, future study that incorporates this particular evaluation is highly suggested.
In relation to the involvement of additional component to enhance curcumin anti-cancer potential, another component that has been highly related to better curcumin bioavailability is piperine. Previous studies as well as literatures have indicated the importance of piperine to increase the systemic bioavailability of curcumin.40,41 Yet, curcumin used in the current study managed to show its efficacy in suppressing the expression of NFκB and COX-2 without the involvement of piperine, which indicates the need for future study regarding the involvement of piperine in bettering curcumin anti-cancer activity. Another study conducted by Naksuriya et al.42 about kinetic degradation of curcumin suggested that when curcumin was loaded in curcumin mixture such as polymeric micelles, it tended to be more stable. Based on these previous studies, further investigations about the involvement of piperine and other components regarding their effect on curcumin bioavailability as well as curcumin anti-cancer potential on OSCC patients are in the agenda of future study.
Study limitations
In regard to the methodology and the promising results of the current study, several study limitations that should be identified and improved in future study are as follows: (1) the current study did not evaluate nor involve other components that might interact with curcumin and improve curcumin anti-cancer potential; (2) the current study did not conduct the western blot analysis that might reinforce the results of the study; and (3) the current study was performed on animal, therefore the implementation of results on human requires further study and evaluation.
Conclusion
In summary, the results of the current study suggested that curcumin was highly effective in inhibiting the epithelial dysplasia stage of OSCC induced by DMBA. In regard to the results of the current study, future study that
Investigates the efficacy of curcumin anti-cancer potential on OSCC with the involvement of piperine is suggested.
Investigates curcumin anti-cancer potential with the involvement of other components as its “vehicles” is considered to be of important.
Investigates curcumin anti-cancer potential on human participants with OSCC should be conducted in the near future.
Acknowledgments
We would like to acknowledge and thank the staffs of the Pathology Anatomy Laboratory of the Animal Teaching Hospital of the Faculty of Veterinary Medicine, Bogor Agricultural Institute, Bogor, Indonesia, for their valuable and kind assistance during the research period.
Footnotes
Animal welfare: The present study followed international, national, and/or institutional guidelines for humane animal treatment and complied with relevant legislation. All procedures performed were in accordance with the standards set forth in the Guide for the Care and Use of Laboratory Animals and Basel Declaration.
Author contributions: All authors equally contributed to the completion of the current manuscript, whereas I.H. and E.S. acted as the field researcher and contributed in the data analysis process. A.H. performed the statistical analysis and contributed in manuscript writing; T.M. contributed significantly on research planning, manuscript writing as well as data analysis process; while B.P. and H.Y.Y. contributed equally on research planning and data analysis.
Availability of data and materials: All data gained in the current study are available from the corresponding author on reasonable request.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical approval: Ethical approval for this study was obtained from Animal Care and Use Committee (ACUS) of Rumah Sakit Hewan Pendidikan (Animal Hospital), Veterinary Faculty, Bogor Agricultural Institute, Bogor, Indonesia (34-2017 ACUC RSHP FKH-IPB).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: All authors would like to thank Padjadjaran University, Bandung, Indonesia, for funding the current study through the Academic Leadership Grant (ALG).
ORCID iD: Tantry Maulina  https://orcid.org/0000-0002-6975-990X
https://orcid.org/0000-0002-6975-990X
References
- 1. Salian V, Dinakar C, Shetty P, et al. Etiological trends in oral squamous cell carcinoma: a retrospective institutional study. Cancer Transl Med 2016; 2(2): 33–36. [Google Scholar]
- 2. Ng JH, Iyer NG, Tan M-H, et al. Changing epidemiology of oral squamous cell carcinoma of the tongue: a global study. Head Neck 2016; 39(2): 297–304. [DOI] [PubMed] [Google Scholar]
- 3. Gul H, Asif F, Ghaffar I, et al. Epidemiology and pathological trends in oral squamous cell carcinoma in a local tertiary care hospital. Int J Community Med Public Health 2017; 4(12): 4440–4444. [Google Scholar]
- 4. Feller L, Lemmer J. Oral squamous cell carcinoma: epidemiology, clinical presentation and treatment. J Cancer Ther 2012; 3: 263–268. [Google Scholar]
- 5. Tandon P, Dadhich A, Saluja H, et al. The prevalence of squamous cell carcinoma in different sites of oral cavity at our Rural Health Care Centre in Loni, Maharashtra—a retrospective 10 year study. Contemp Oncol (Pozn) 2017; 21(2): 178–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nicolatou-Galitis O, Psyrri A. Trismus and reduced quality of life in patients with oral squamous cell carcinoma who received postoperative radiotherapy alone or combined with chemotherapy. J Clin Oncol 2017; 35(Suppl. 31): 222–222. [Google Scholar]
- 7. Kessler PA, Bloch-Birkholz A, Leher A, et al. Evaluation of quality of life of patients with oral squamous cell carcinoma. Comparison of two treatment protocols in a prospective study. Radiother Oncol 2004; 70(3): 275–282. [DOI] [PubMed] [Google Scholar]
- 8. Rehman B, ud Din Q, Khan M, et al. Evaluation of the quality of life in patients with oral squamous cell carcinoma. J Khyber Coll Dent 2012; 2(2): 67–73. [Google Scholar]
- 9. Wilken R, Veen MS, Wang MB, et al. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer 2011; 10: 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ardito F, Perrone D, Giuliani M, et al. Effects of curcumin on squamous cell carcinoma of tongue: an in vitro study. Curr Top Med Chem 2018; 18(3): 233–243. [DOI] [PubMed] [Google Scholar]
- 11. Zhen L, Fan D, Yi X, et al. Curcumin inhibits oral squamous cell carcinoma proliferation and invasion via EFGR signalling pathways. Int J Clin Exp Pathol 2014; 7(10): 6438–6446. [PMC free article] [PubMed] [Google Scholar]
- 12. Perrone D, Ardito F, Giannatempo G, et al. Biological and therapeutic activities, and anticancer properties of curcumin. Exp Ther Med 2015; 10(5): 1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shanmugam MK, Rane G, Kanchi MM, et al. The multifaceted role of curcumin in cancer prevention and treatment. Molecules 2015; 20(2): 2728–2769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sawhney M, Rohatgi N, Kaur J, et al. Expression of NF-jB parallels COX-2 expression in oral precancer and cancer: association with smokeless tobacco. Int J Cancer 2007; 120: 2545–2556. [DOI] [PubMed] [Google Scholar]
- 15. Mohammad S, Ram H, Gupta PN, et al. Overexpression of COX-2 in oral squamous cell carcinoma patients undergoing chemoradiotherapy. Natl J Maxillofac Surg 2011; 2(1): 17–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alok A, Singh ID, Singh S, et al. Curcumin: pharmacological actions and its role in head and neck squamous cell carcinoma—a review. Indian J Acad Oral Med Radiol 2017; 29(2): 115–118. [Google Scholar]
- 17. Nakayama H, Ikebe T, Beppu M, et al. High expression levels of nuclear factor κB, IκB kinase α and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer 2001; 92(12): 3037–3044. [DOI] [PubMed] [Google Scholar]
- 18. Urade M. Cyclooxygenase (COX)-2 as a potent molecular target for prevention and therapy of oral cancer. Japan Dent Sci Rev 2008; 44: 57–65. [Google Scholar]
- 19. Santoro A, Bufo P, Russo G, et al. Expression and clinical implication of cyclooxygenase-2 and e-cadherin in oral squamous cell carcinomas. Cancer Biol Ther. Epub ahead of print 28 July 2015. DOI: 10.1080/15384047.2015.1071741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Charan J, Kantharia ND. How to calculate sample size in animal studies. J Pharmacol Pharmacother 2013; 4(4): 303–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. National-Center-for-Biotechnology-Information. 7,12-Dimethylbenzanthracene. https://pubchem.ncbi.nlm.nih.gov/compound/6001 (2018, accessed 20 October, 2018).
- 22. Selvasundaram R, Manoharan S, Buddhan R, et al. Chemopreventive potential of esculetin in 7,12-dimethylbenz(a)anthracene-induced hamster buccal pouch carcinogenesis. Mol Cell Biochem 2018; 448(1–2): 145–153. [DOI] [PubMed] [Google Scholar]
- 23. Manimaran A, Rajamanickam B, Shanmugam M. Emodin downregulates cell proliferation markers during DMBA induced oral carcinogenesis in golden Syrian hamster. Afr J Tradit Complement Altern Med 2017; 14(2): 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Gupta SC, Patchva S, Aggarwal BB. Therapeutic roles of curcumin: lessons learned from clinical trials. AAPS J 2013; 15(1): 195–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manoharan S, Balakrishnan S, Menon VP, et al. Chemopreventive efficacy of curcumin and piperine during 7,12-dimethylbenz[a]anthracene-induced hamster buccal pouch carcinogenesis. Singapore Med J 2009; 50(2): 139–146. [PubMed] [Google Scholar]
- 26. Zatroch KK, Knight CG, Reimer JN, et al. Refinement of intraperitoneal injection of sodium pentobarbital for euthanasia in laboratory rats (Rattus norvegicus). BMC Vet Res 2017; 13(1): 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. American Veterinary Medical Association (AVMA). AVMA guidelines for the euthanasia procedure of animals: 2013 edition. https://www.avma.org/KB/Policies/Documents/euthanasia.pdf (2013, accessed 20 October 2018).
- 28. Boivin GP, Bottomley MA, Schiml PA, et al. Physiologic, behavioral, and histologic responses to various euthanasia methods in C57BL/6NTac male mice. J Am Assoc Lab Anim Sci 2017; 56(1): 69–78. [PMC free article] [PubMed] [Google Scholar]
- 29. Stenger M. Calculatinh H-score. http://www.ascopost.com/issues/april-10-2015/calculating-h-score/ (2015, accessed 20 October, 2018).
- 30. Kondo M, Yamato M, Takagi R, et al. Significantly different proliferative potential of oral mucosal epithelial cells between six animal species. J Biomed Mater Res A 2014; 102(6): 1829–1837. [DOI] [PubMed] [Google Scholar]
- 31. Scrobota I, Bolfa P, Filip AG, et al. Natural chemopreventive alternatives in oral cancer chemoprevention. J Physiol Pharmacol 2016; 67(1): 161–172. [PubMed] [Google Scholar]
- 32. LoTempio MM, Veena MS, Steele HL, et al. Curcumin suppresses growth of head and neck squamous cell carcinoma. Clin Cancer Res 2005; 11(19): 6994–7002. [DOI] [PubMed] [Google Scholar]
- 33. Anggarwal S, Takada Y, Singh S, et al. Inhibition of growth and survival of human head and neck squamous cell carcinoma cells by curcumin via modulation of nuclear factor κB signaling. Int J Cancer 2004; 111: 679–692. [DOI] [PubMed] [Google Scholar]
- 34. Wang D, Veena MS, Stevenson K, et al. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor κB by an AKT-independent pathway. Clin Cancer Res 2008; 14(19): 6228–6236. [DOI] [PubMed] [Google Scholar]
- 35. Yan M, Xu Q, Zhang P, et al. Correlation of NF-kappaB signal pathway with tumor metastasis of human head and neck squamous cell carcinoma. BMC Cancer 2010; 10: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gupta SC, Kim JH, Prasad S, et al. Regulation of survival, proliferation, invasion, angiogenesis, and metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev 2010; 29(3): 405–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sobolewski C, Cerella C, Dicato M, et al. The role of cyclooxygenase-2 in cell proliferation and cell death in human malignancies. Int J Cell Biol 2010; 2010: 215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang F, Altorki NK, Mestre JR, et al. Curcumin inhibits cyclooxygenase-2 transcription in bile acid- and phorbol ester-treated human gastrointestinal epithelial cells. Carcinogenesis 1999; 20(3): 445–451. [DOI] [PubMed] [Google Scholar]
- 39. Mirzaee F, Hosseinzadeh L, Ashrafi-Kooshk MR, et al. Diverse effects of different “protein-based” vehicles on the stability and bioavailability of curcumin: spectroscopic evaluation of the antioxidant activity and cytotoxicity in vitro. Protein Pept Lett 2019; 26(2): 132–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fadus MC, Lau C, Bikhchandani J, et al. Curcumin: an age-old anti-inflammatory and anti-neoplastic agent. J Tradit Complement Med 2017; 7(3): 339–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hewlings SJ, Kalman DS. Curcumin: a review of its’ effects on human health. Foods 2017; 6(10): 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Naksuriya O, van Steenbergen MJ, Torano JS, et al. A kinetic degradation study of curcumin in its free form and loaded in polymeric micelles. AAPS J 2016; 18(3): 777–787. [DOI] [PMC free article] [PubMed] [Google Scholar]