Abstract
Background:
Empirical therapy of Helicobacter pylori frequently results in treatment failure due to unrecognized antimicrobial resistance. The aim of this study was to investigate the effectiveness of susceptibility-guided therapy for rescue treatment of H. pylori infection in China.
Methods:
This was a prospective study of consecutive 200 patients infected with H. pylori with one or more treatment failures. The therapy chosen was susceptibility based using the most effective, best-tolerated regimens first and a locally proven, reliably effective regimen for multidrug-resistant infections. All patients received 14-day triple therapy, i.e. esomeprazole 20 mg and amoxicillin 1 g twice a day plus clarithromycin 500 mg twice a day, metronidazole 400 mg twice a day, or levofloxacin 500 mg daily, or, for multidrug-resistant infections, amoxicillin-containing bismuth quadruple therapy with esomeprazole 20 mg twice a day, bismuth 220 mg twice a day, amoxicillin 1 g three times a day, and metronidazole 400 mg four times a day. Antibiotic resistance was determined by agar dilution.
Results:
The eradication rate of susceptibility-guided therapy overall was 94.5% (189/200, 95% confidence interval: 90.4–97.2%). Around 28% (56/200) of patients carried strains susceptible to one of the tested antibiotics and were prescribed the triple therapy. A total of 144 multidrug-resistant patients received bismuth quadruple therapy. The eradication rates were all greater than 90%, i.e. 91.7% (11/12), 92.3% (12/13), and 93.5% (29/31) in those who received clarithromycin, metronidazole, and levofloxacin-containing triple therapy and 95.1% (137/144) for the bismuth quadruple therapy. There were no differences in eradication rates between the subgroups.
Conclusions:
Although susceptibility-guided therapy proved high efficacious despite the high proportion of multidrug-resistant strains, the strategy suggested the best approach for this population would be empirical amoxicillin-containing bismuth quadruple therapy. ClinicalTrials.gov identifier: NCT03413020.
Keywords: Helicobacter pylori, rescue treatment, susceptibility-guided therapy
Introduction
Empirical therapy of Helicobacter pylori frequently results in treatment failure due to unrecognized antimicrobial resistance. In the era of increasing antibiotic resistance, the clinical management of patients infected with H. pylori who failed prior treatments remains a challenge. bismuth quadruple therapy has been recommended as an empiric rescue therapy in current guidelines.1,2 However, tetracycline is not universally available in many areas including China.2
As with other infectious diseases, the general rule is that the results of antimicrobial therapy are best when therapy is susceptibility guided. Although susceptibility-guided therapy is recommended for rescue treatment for H. pylori by the Maastricht V/Florence Consensus Report,1 no guidance has been provided regarding a reliable strategy for how to utilize the results of testing. Evidence on the effectiveness of susceptibility-guided therapy for rescue therapy is very limited. A systemic review reported an eradication rate of susceptibility-guided therapy in third-line treatment of less than 80%, providing no guidance.3
We used a strategy based on susceptibility testing to prioritize therapy and to identify the preferred approach within a population. Prioritization was based on the concept that among susceptible infections, the most effective and best-tolerated regimen should be used first (i.e. among clarithromycin, metronidazole, and levofloxacin triple therapy).4 We designed this pattern according to antibiotic pharmacologic characteristics and safety. Clarithromycin with the lowest minimal inhibitory concentrations (MICs) was listed first and levofloxacin was last based on the US Food and Drug Administration’s concerns and warnings about toxicities.4 We only recommended locally reliable effective therapies, which consisted of 14-day triple therapies for susceptible infections. Finally, for multidrug-resistant infections, a locally proven, effective, amoxicillin-containing modified bismuth quadruple therapy that substituted amoxicillin for tetracycline was used.5,6 The study population comprised patients infected with H. pylori with at least one prior treatment failure residing in China where resistance was common.
Materials and methods
Study design and participants
This was a prospective, single-center, open-label, single-arm interventional trial conducted from January 2018 to July 2018 at Renji Hospital, School of Medicine, Shanghai Jiao Tong University, China. Informed consent was obtained from all subjects before enrollment. The study was approved by the Ethics Committee of Renji Hospital, School of Medicine, Shanghai Jiao Tong University, number (2017)195. The study was registered with ClinicalTrials.gov on January 2018 (NCT03413020).
Consecutive subjects with persistent H. pylori infection who had previously failed at least one course of treatment were enrolled. H. pylori infection was determined by all three positive tests, i.e. 13C-urea breath test (13C-UBT), rapid urease testing, and culture. Exclusion criteria included subjects younger than 18 years of age, näive to H. pylori treatment, active peptic ulcer, pregnancy or lactation, history of surgery of the upper gastrointestinal tract, presence of significant clinical diseases or malignancy, use of antibiotics or proton pump inhibitors (PPIs) within the previous 8 weeks, or allergy to any drugs given in this study.
H. pylori isolation and antimicrobial susceptibility testing
At endoscopy, two biopsy specimens were collected from the gastric antrum and corpus to isolate H. pylori strains. The specimens were transported in saline-containing tubes on ice to the H. pylori culture laboratory at the Shanghai Institute of Digestive Disease. The specimens were cultured and maintained on brain heart infusion agar medium (Oxoid, Basingstoke, UK) containing 5% defibrinated sheep blood under microaerophilic conditions (85% N2, 10% CO2, and 5% O2) at 37°C. Strains were identified if they were Gram-negative, positive for urease, oxidase, and catalase, and had spiral or curved rods in morphology. MICs of clarithromycin, metronidazole, and levofloxacin were determined by the agar-dilution method and incubated for 3 days under microaerobic conditions. ATCC43504 was chosen as the quality-control strain. The subject was considered to have a resistant infection when any isolate was resistant to an antibiotic. The resistance break points for clarithromycin, metronidazole, and levofloxacin resistance were defined as 0.5 mg/L, 8 mg/L, and 1 mg/L, according to the European Committee on Antimicrobial Susceptibility Testing, respectively.7
Interventions
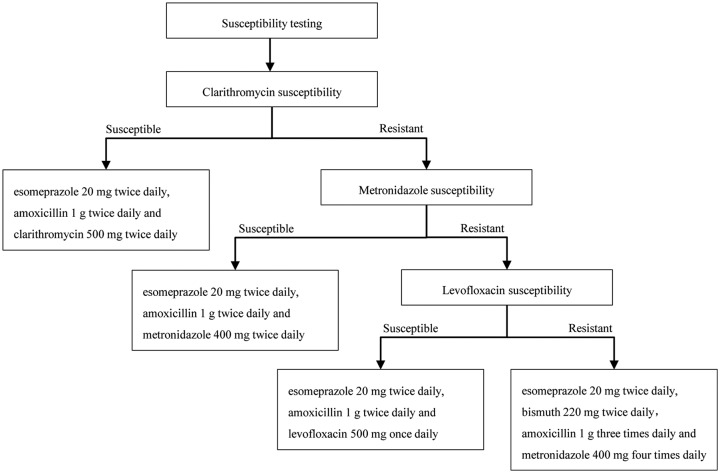
Eligible subjects were assigned to 14 days of susceptibility-guided therapy based on the susceptibility pattern of clarithromycin, metronidazole, and levofloxacin (Figure 1). The choice was made only according to the susceptible/resistant status, regardless of the value of MIC. If a strain was susceptible to clarithromycin, the combination of esomeprazole (AstraZeneca , Cambridge, UK) 20 mg twice daily, amoxicillin (Reyoung Pharmaceutical, Shandong, China) 1 g twice daily, and clarithromycin (Abbott Laboratories, Chicago, USA) 500 mg twice daily (EAC) was given. If a strain was resistant to clarithromycin, but susceptible to metronidazole, the combination of esomeprazole 20 mg twice daily, amoxicillin 1 g twice daily, and metronidazole (Shanghai Xinyi Wanxiang Pharmaceutical Industry, Shanghai, China) 400 mg twice daily (EAM) was given. If a strain was resistant to both clarithromycin and metronidazole but susceptible to levofloxacin, the combination of esomeprazole 20 mg twice daily, amoxicillin 1 g twice daily, and levofloxacin (Daiichi Sankyo, Tokyo, Japan) 500 mg once daily was given (EAL). If a strain was resistant to all three tested antibiotics, subjects would receive a combination of esomeprazole 20 mg twice daily, bismuth potassium citrate (Dawnrays Pharma, Suzhou, China) 600 mg (220 mg elemental bismuth) twice daily, amoxicillin 1 g three times daily, and metronidazole 400 mg four times daily (EBAM). Esomeprazole and bismuth were given 30 min before meals and antibiotics were given 30 min after meals. Esomeprazole 20 mg is equivalent to 32 mg of omeprazole and is equivalent to a double-dose PPI.8
Figure 1.
Design of susceptibility-guided therapy.
All subjects received pretreatment instructions about drug administration and possible adverse events and were asked to keep a diary to record symptoms and adherence. Adverse events were graded according to their influence on daily life and classified as ‘mild’ (discomfort but did not interfere with daily life), ‘moderate’ (discomfort that partially interfered with daily life), or ‘severe’ (discomfort that seriously interfered with daily life). Adherence was defined as good when more than 80% of the total pills were taken.9 The medical cost was calculated according to published reports of the National Medical Products Administration of China. All costs were measured in US dollars.
Outcomes
The primary outcome of this study was the eradication rate. H. pylori eradication was determined by ¹³C-UBT at least 6 weeks after the completion of treatment. Each subject was given, orally, a capsule containing 75 mg ¹³C-urea. Baseline and 30-min breath samples were assayed following the manufacturer’s guidelines. Eradication was defined as negative from ¹³C-UBT < 4% (4% was the cut-off value). Secondary outcomes were the prevalence of adverse events, adherence, cost, and antibiotic-resistance rate and its effect on eradication rate.
Sample size estimation and statistical analysis
For this study, the definition of an effective therapy was that the therapy would reliably achieve an eradication rate of 90% or greater in adherent patients.10 The sample size estimation was based on previous trials. We assumed the eradication rate of susceptibility-guided therapy to be 96.2% according to previous studies.5,11 Assuming the target value was 90%, with 96.2% point estimation of eradication rate of susceptibility-guided therapy, a power of 90%, and an alpha of 0.025 (one-sided), 180 subjects would be required for this prospective single-center trial. Taking into consideration 10% lost to follow up, at least 198 subjects were expected to be recruited for the study.
Continuous variables were described by mean with standard deviation and categorical variables by percentages. Eradication rates were evaluated by intention-to treat (ITT) and per-protocol (PP) analysis. All subjects were included in the ITT analysis. Subjects who did not return for a follow-up 13C-UBT were recorded as treatment failures in the ITT analysis. Subjects who violated the study protocol, such as not taking at least 80% of treatment drugs or those without post-treatment H. pylori status, were excluded from the PP analysis. The confidence limits for eradication rates were calculated by the Clopper–Pearson method with SAS 9.4 (SAS Institute Inc., Cary, NC, USA). If the lower bound of the 95% confidence interval (CI) was greater than 90%, good effectiveness of susceptibility-guided therapy could be concluded. Differences between groups were evaluated by chi-square test or Fisher’s exact test for categorical variables. p < 0.05 was considered statistically significant.
Results
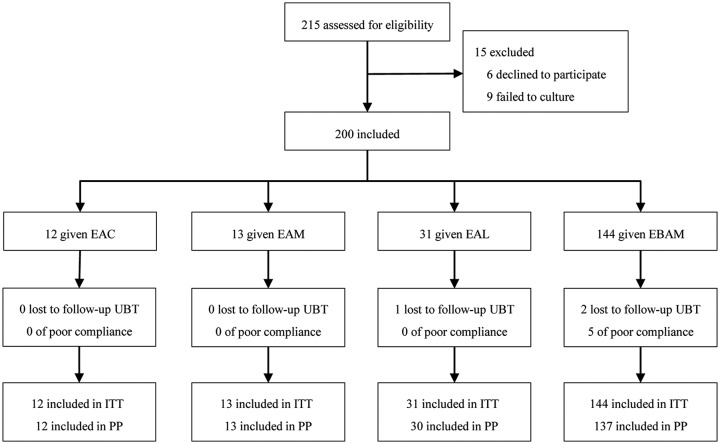
Figure 2 shows the trial profile. A total of 215 subjects were assessed for eligibility in the study. H. pylori was successfully isolated in 95.8% (206/215) of subjects and 200 subjects were enrolled in this study. Table 1 shows the demographic and clinical characteristics of the patients. Patients had a median of 2.4 previous H. pylori treatments (range 1–6 treatments). A total of 40 patients had one previous treatment failure, 79 patients had two treatment failures, and 81 patients had experienced at least three treatment failures. Prior treatments included various combinations of antibiotics (Table S1).
Figure 2.
Flow diagram of this study.
EAC, esomeprazole, amoxicillin, and clarithromycin; EAM, esomeprazole, amoxicillin, and metronidazole; EAL, esomeprazole, amoxicillin, and levofloxacin; EBAM, esomeprazole, bisMUTh, amoxicillin, and metronidazole; ITT, intention-to-treat; PP, per-protocol; 13C-UBT, 13C-urea breath test.
Table 1.
Demographic and clinical data of patients.
| Variables | EAC group (n = 12) | EAM group (n = 13) | EAL group (n = 31) | EBAM group (n = 144) | Total (n = 200) |
|---|---|---|---|---|---|
| Sex | |||||
| Female | 3 (25%) | 8 (61.5%) | 15 (48.4%) | 98 (68.1%) | 124 (62%) |
| Male | 9 (75%) | 5 (38.5%) | 16 (51.6%) | 46 (31.9%) | 76 (38%) |
| Age (years) | 40.9 (11.7) | 52.4 (15.3) | 43.1 (15.3) | 49.1 (12.1) | 47.9 (13.1) |
| Diagnosis | |||||
| Dyspepsia | 9 (75%) | 13 (100%) | 26 (83.9%) | 131 (91%) | 179 (89.5%) |
| Peptic ulcer | 3 (25%) | 0 | 5 (16.1%) | 13 (9%) | 21 (10.5%) |
| Treatment failure | |||||
| 1 | 6 (50%) | 3 (23.1%) | 8 (25.8%) | 23 (16%) | 40 (20%) |
| 2 | 5 (41.7%) | 7 (53.8%) | 18 (58.1%) | 49 (34%) | 79 (39.5%) |
| ⩾ 3 | 1 (8.3%) | 3 (23.1%) | 5 (16.1%) | 72 (50%) | 81 (40.5%) |
| Antibiotic resistance | |||||
| Clarithromycin | 0 | 13 (100%) | 31 (100%) | 144 (100%) | 188 (94%) |
| Metronidazole | 12 (100%) | 0 | 31 (100%) | 144 (100%) | 187 (93.5%) |
| Levofloxacin | 9 (75%) | 9 (69.2%) | 0 | 144 (100%) | 162 (81%) |
| Loss of follow up | 0 | 0 | 1 (3.2%) | 2 (1.4%) | 3 (1.5%) |
| Adherence * | 12 (100%) | 13 (100%) | 31 (100%) | 139 (96.5%) | 195 (97.5%) |
Data are n (%), or mean (standard deviation). *Taken > 80% of tablets. EAC, esomeprazole, amoxicillin, and clarithromycin; EAM, esomeprazole, amoxicillin, and metronidazole; EAL, esomeprazole, amoxicillin, and levofloxacin; EBAM, esomeprazole, bisMUTh, amoxicillin, and metronidazole.
Susceptibility results
The resistance rates of the strains to clarithromycin, metronidazole, and levofloxacin were 94% (188/200), 93.5% (187/200), and 81% (162/200) (Table 1). Only 28% (56/200) of patients carried strains susceptible to one of the tested antibiotics and were prescribed triple therapy. In our study population, the resistance rates of clarithromycin, metronidazole, and levofloxacin were 98.3% (169/172), 99.2% (122/123), and 93.9% (92/98), respectively, in patients who had received these antibiotics in their prior therapies.
Eradication rates
As shown in Table 2, the ITT eradication rate for the entire population was 94.5% (189/200, 95% CI: 90.4–97.2%) and 96.9% (186/192, 95% CI: 93.3–98.8%) in the PP analysis. In both the ITT and PP analysis, the lower bound of the 95% CI was greater than the pre-established target value (90%). Subgroup analysis was also performed to determine the effectiveness of each regimen. In the ITT analysis, the eradication rates were 91.7% (11/12, 95% CI: 61.5–99.8%), 92.3% (12/13, 95% CI: 64.0–99.8%), 93.5% (29/31, 95% CI: 78.6–99.2%), and 95.1% (137/144, 95% CI: 90.2–98.0%) in patients who received EAC, EAM, EAL, and EBAM, respectively. In the PP analysis, they were 91.7% (11/12, 95% CI: 61.5–99.8%), 92.3% (12/13, 95% CI: 64.0–99.8%), 96.7% (29/30, 95% CI: 82.8–99.9%), and 97.8% (134/137, 95% CI: 93.7–99.5%). There were no statistically significant differences in eradication rates between subgroups (p = 0.554 in ITT analysis and p = 0.189 in PP analysis). One patient in the EAL group and two patients in the EBAM group were lost to follow up and did not return for the ¹³C-UBT so were scored as treatment failures in the ITT analysis. Five patients in the EBAM group violated protocol (discontinuation of medication due to adverse events) and three of them received follow-up ¹³C-UBT and showed successful eradication. These eradicated patients had followed the therapy for 4 days, 7 days, and 10 days, respectively.
Table 2.
Eradication rate in each regimen.
| Eradication rate | Total (n = 200) | EAC group (n = 12) | EAM group (n = 13) | EAL group (n = 31) | EBAM group (n = 144) | p value |
|---|---|---|---|---|---|---|
| ITT | 189/200 (94.5%) | 11/12 (91.7%) | 12/13 (92.3%) | 29/31 (93.5%) | 137/144 (95.1%) | 0.554 |
| 95% CI | 90.4–97.2% | 61.5–99.8% | 64.0–99.8% | 78.6–99.2% | 90.2–98.0% | |
| PP | 186/192 (96.9%) | 11/12 (91.7%) | 12/13 (92.3%) | 29/30 (96.7%) | 134/137 (97.8%) | 0.189 |
| 95% CI | 93.3–98.8% | 61.5–99.8% | 64.0–99.8% | 82.8–99.9% | 93.7–99.5% |
Data are n/N (%), 95% CI. CI, confidence interval; EAC, esomeprazole, amoxicillin, and clarithromycin; EAM, esomeprazole, amoxicillin, and metronidazole; EAL, esomeprazole, amoxicillin, and levofloxacin; EBAM, esomeprazole, bisMUTh, amoxicillin, and metronidazole; ITT, intention-to-treat; PP, per-protocol.
Adverse effects, adherence, and costs
The frequencies of adverse events were 16.7% (2/12), 0% (0/13), 6.5% (2/31), and 37.5% (54/144) in EAC, EAM, EAL, and EBAM groups, respectively (p < 0.001 triple therapies versus bismuth quadruple therapy). The adverse events were all mild in the patients who had received triple therapy. In the EBAM group, 19.4% (28/144) of patients reported mild adverse events, 14.6% (21/144) of patients reported moderate adverse events, and 3.5% (5/144) of patients reported severe adverse events. Adherence was good except for five patients in the EBAM group who failed to take at least 80% of the drugs due to nausea, vomiting, dizziness, and skin rash. All the adverse events disappeared after stopping the treatment (Table 3).
Table 3.
Adverse events and adherence in each regimen.
|
Variables |
EAC group (n = 12) | EAM group (n = 13) | EAL group (n = 31) | EBAM group (n = 144) |
|---|---|---|---|---|
| Total | 2 (16.7%) | 0 | 2 (6.5%) | 54 (37.5%) |
| AE grade | ||||
| Mild | 2 (16.7%) | 0 | 2 (6.5%) | 28 (19.4%) |
| Moderate | 0 | 0 | 0 | 21 (14.6%) |
| Severe | 0 | 0 | 0 | 5 (3.5%) |
| AE variety | ||||
| Bad taste | 2 (16.7%) | 0 | 0 | 1 (0.7%) |
| Dyspepsia | 0 | 0 | 1 (3.2%) | 4 (2.8%) |
| Nausea | 0 | 0 | 0 | 30 (20.8%) |
| Vomiting | 0 | 0 | 0 | 5 (3.5%) |
| Dizziness | 0 | 0 | 0 | 15 (10.4%) |
| Headache | 0 | 0 | 0 | 1 (0.7%) |
| Abdominal pain | 0 | 0 | 1 (3.2%) | 0 |
| Fatigue | 0 | 0 | 0 | 5 (3.5%) |
| Bloating | 0 | 0 | 0 | 6 (4.2%) |
| Diarrhea | 0 | 0 | 0 | 2 (1.4%) |
| Skin rash | 0 | 0 | 0 | 4 (2.8%) |
| Fever | 0 | 0 | 0 | 1 (0.7%) |
| Poor adherence due to AEs | 0 | 0 | 0 | 5 (3.5%) |
Data are n (%). AE, adverse event; EAC, esomeprazole, amoxicillin, and clarithromycin; EAM, esomeprazole, amoxicillin, and metronidazole; EAL, esomeprazole, amoxicillin, and levofloxacin; EBAM, esomeprazole, bismuth, amoxicillin, and metronidazole.
Medical costs were US$96.1, US$40.6, and US$65.5 for the EAC, EAM, and EAL triple therapy and US$49 for the EBAM quadruple therapy. An additional US$130.8 was required for endoscopy and the susceptibility test performance.
Discussion
With any infectious disease, susceptibility-guided therapy, whether based on data from the individual infection or from local or regional data, should be the best choice.4 In this prospective study, we confirmed that susceptibility-based therapy overall was efficient with 94.5% ITT cure rate despite very high rates of antibiotic resistance.
The current guideline recommends susceptibility-guided therapy for patients who fail two courses of treatment.1 It has been previously demonstrated that susceptibility-guided triple therapies were more effective and cost saving than empiric triple therapy for first-line treatment.12,13 However, which rescue treatment is best is unclear as results from previous studies have been extremely heterogeneous.14–22 Studies from Spain, USA, and Germany reported surprisingly low cure rates (< 70%) for those with multiple treatment failures even after rescue therapies applied with susceptibility testing.14–19 Short duration, weak therapy, and lack of uniformity in selecting PPIs and antibiotics likely contributed to the low effectiveness. Recently, Liou and colleagues reported a multicenter study comparing the effectiveness of 14-day genotypic resistance-guided and empirical therapy for rescue treatment.20 Sequential therapies based on levofloxacin and clarithromycin susceptibility were given as tailored therapy and empirical therapy was determined according to medication history. Both achieved low effectiveness (78% and 72% ITT cure rates), possibly because of the use of low effective sequential therapies, low dosages, and short duration of metronidazole and tetracycline. On the other hand, Fiorini and colleagues21 and Kwon and colleagues22 achieved higher cure rates (89.4% and 87.8%) by using quinolone therapy for susceptible strains, and rifabutin triple therapy or bismuth quadruple therapy for resistant strains. Locally highly effective regimens are required to obtain high local success rates.
Our approach of not limiting antibiotic susceptibility testing to one antibiotic (e.g. clarithromycin) allowed us to provide reliable advice for clinicians. In our population, we have repeatedly shown that amoxicillin and tetracycline resistance are rare despite repeated use and thus they were not included in our panel.5 In other regions such as Iran or Pakistan,23 it may be necessary to include them. In addition to confirming the power of combining susceptibility testing with locally reliable effective therapies, we also show how data about the population can further modify suggestions for therapy.
Antibiotic resistance can rapidly emerge after prior failure of eradication therapy. Therefore, multiple treatment failures should be avoided because they can increase the rate of multidrug- resistant strains. In our population, multidrug-resistant strains were predominant suggesting that the recommendation for initial therapy with the locally proven effective empirical regimen would likely be the best overall approach. This study also confirmed the importance of regular assessment of local, regional, and national H. pylori susceptibility data as stressed in the recent Houston Consensus Conference and is in line with the principles of antimicrobial stewardship.24–26 We also confirmed that even in the presence of a very high prevalence of antimicrobial resistance, triple therapies can still be successful if given at the optimum doses and durations and based on antibiotic susceptibility testing.
Standard methods of susceptibility testing need invasive procedures (endoscopy), experienced bacterial culture, additional expense, and time consumption (10–20 days in our laboratory). Owing to the high prevalence of upper digestive tract diseases such as peptic ulcer and cancer, endoscopy-and-treat policy has been recommended in a few countries in East Asia, which allows for individualized diagnosis and treatment.27 Although culture is still not widely available for H. pylori, molecular-based susceptibility testing is a promising technology as it is convenient and fast, and allows for culture-free and even noninvasive testing if stool testing proves accurate and reliable. However, currently molecular testing appears promising only for clarithromycin, and possibly fluoroquinolones.28,29 The limit of molecular approaches is that they do not detect resistance caused by other unknown mutations or mechanisms and they cannot be used for all antibiotics.
In addition to antibiotic resistance, adverse events and adherence are also important factors influencing eradication results. We paid special attention to patient education about adverse events and the importance of adherence. Adverse events were infrequent among patients treated with triple therapy. All were mild and associated with good adherence to therapy. Adherence was also high (96.5%) despite the prevalence of adverse events in 37.5% with nearly half of them being moderate or severe in patients who received the modified bismuth quadruple therapy. Susceptibility-guided therapy allowed us to identify patients with susceptible strains and thus be able to cure the infections using high effectiveness, less complex regimens with fewer drugs and a low number of adverse events.
Our study had limitations. First, this trial was not a randomized trial comparing a highly effective empirical therapy with susceptibility-guided therapy. However, the goal was not to be a comparative trial but to test whether susceptibility-guided therapy could provide excellent results in a difficult to treat population and to identify the best strategy for a population. Meanwhile, currently recommended effective empirical regimens such as bismuth quadruple therapy, rifabutin-containing therapy, and furazolidone quadruple therapy are often unavailable in many areas of the world and may be contraindicated by some patients. Second, all the regimens used contained amoxicillin such that our findings do not apply to patients allergic to penicillin. Third, the modified bismuth quadruple therapy used for triple-resistant infections does not apply to the areas where bismuth is not available.
In conclusion, susceptibility-guided therapy proved highly effective despite multiple prior treatment failures for H. pylori, and it enabled avoiding the administration of unnecessary antibiotics by identifying patients likely to benefit from triple therapies and thus achieved very high success rates and good adherence. However, the availability of endoscopic examination and success rate of H. pylori culture should be taken into consideration before implementing the susceptibility-guided strategy. The high prevalence of multidrug-resistant infections suggests that in this population of prior treatment failures, empirical use of a proven highly effective regimen such as the amoxicillin-containing modified bismuth quadruple would likely be the best strategy.
Supplemental Material
Supplemental material, Supplementary_Data for Susceptibility-guided therapy for Helicobacter pylori infection treatment failures by Lou Yu, Laisheng Luo, Xiaohua Long, Xiao Liang, Yingjie Ji, Qi Chen, Yanyan Song, Xiaobo Li, David Y. Graham and Hong Lu in Therapeutic Advances in Gastroenterology
Acknowledgments
HL designed the study and critically revised the manuscript. LY wrote the protocol and drafted the manuscript. LY, LL, X-Long, X-Liang, YJ, QC, X-Li, and HL recruited patients to the study. YS led the statistical analyses of the data. DYG supervised the study and critically revised the manuscript. All authors reviewed the draft and approved the final version of this report.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: this study was supported by a grant from the Clinical Research Center, Shanghai Jiao Tong University School of Medicine, China, grant number DLY201608.
Conflict of interest statement: DYG is a consultant for RedHill Biopharma regarding novel H. pylori therapies. He has received research support for the culture of Helicobacter pylori and is the PI of an international study of the use of antimycobacterial therapy for Crohn’s disease. He is also a consultant for BioGaia in relation to probiotic therapy for H. pylori infection and for Takeda in relation to H. pylori therapies.
ORCID iD: Hong Lu  https://orcid.org/0000-0002-3127-6048
https://orcid.org/0000-0002-3127-6048
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lou Yu, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Laisheng Luo, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Xiaohua Long, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Xiao Liang, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Yingjie Ji, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Qi Chen, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
Yanyan Song, Department of Biostatistics, Institute of Medical Sciences, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
Xiaobo Li, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai, China.
David Y. Graham, Department of Medicine, Michael E DeBakey Veterans Affairs Medical Center and Baylor College of Medicine, Houston, TX, USA
Hong Lu, Division of Gastroenterology and Hepatology, Key Laboratory of Gastroenterology & Hepatology, Ministry of Health, Shanghai Institute of Digestive Disease, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, 145 Middle Shandong Road, Shanghai 200001, China; Clinical Research Center, Shanghai Jiao Tong University School of Medicine, 555 Zhongshan South 2nd Rd, Xuhui Qu, Shanghai Shi, China.
References
- 1. Malfertheiner P, Megraud F, O’Morain CA, et al. Management of Helicobacter pylori infection – the Maastricht V/Florence consensus report. Gut 2017; 66: 6–30. [DOI] [PubMed] [Google Scholar]
- 2. Liu WZ, Xie Y, Lu H, et al. Fifth Chinese national consensus report on the management of Helicobacter pylori infection. Helicobacter 2018; 23: e12475. [DOI] [PubMed] [Google Scholar]
- 3. Puig I, López-Góngora S, Calvet X, et al. Systematic review: third-line susceptibility-guided treatment for Helicobacter pylori infection. Therap Adv Gastroenterol 2016; 9: 437–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shiotani A, Lu H, Dore MP, et al. Treating Helicobacter pylori effectively while minimizing misuse of antibiotics. Cleve Clin J Med 2017; 84: 310–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen Q, Zhang W, Fu Q, et al. Rescue therapy for Helicobacter pylori eradication: a randomized non-inferiority trial of amoxicillin or tetracycline in bismuth quadruple therapy. Am J Gastroenterol 2016; 111: 1736–1742. [DOI] [PubMed] [Google Scholar]
- 6. Choe JW, Jung SW, Kim SY, et al. Comparative study of Helicobacter pylori eradication rates of concomitant therapy vs modified quadruple therapy comprising proton-pump inhibitor, bismuth, amoxicillin, and metronidazole in Korea. Helicobacter 2018; 23: e12466. [DOI] [PubMed] [Google Scholar]
- 7. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters. Version 8.0. 2018, http://www.eucast.org. (2018, accessed 3 January 2018).
- 8. Graham DY, Lu H, Dore MP. Relative potency of proton-pump inhibitors, Helicobacter pylori therapy cure rates, and meaning of double-dose PPI. Helicobacter 2019; 24: e12554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Liou JM, Fang YJ, Chen CC, et al. Concomitant, bismuth quadruple, and 14-day triple therapy in the first-line treatment of Helicobacter pylori: a multicentre, open-label, randomised trial. Lancet 2016; 388: 2355–2365. [DOI] [PubMed] [Google Scholar]
- 10. Graham DY. Efficient identification and evaluation of effective Helicobacter pylori therapies. Clin Gastroenterol Hepatol 2009; 7: 145–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu JY, Liou JM, Graham DY. Evidence-based recommendations for successful Helicobacter pylori treatment. Expert Rev Gastroenterol Hepatol 2014; 8: 21–28. [DOI] [PubMed] [Google Scholar]
- 12. Wenzhen Y, Yumin L, Quanlin G, et al. Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med 2010; 49: 1103–1109. [DOI] [PubMed] [Google Scholar]
- 13. López-Góngora S, Puig I, Calvet X, et al. Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother 2015; 70: 2447–2455. [DOI] [PubMed] [Google Scholar]
- 14. Gomollón F, Sicilia B, Ducóns JA, et al. Third line treatment for Helicobacter pylori: a prospective, culture-guided study in peptic ulcer patients. Aliment Pharmacol Ther 2000; 14: 1335–1338. [DOI] [PubMed] [Google Scholar]
- 15. Vicente R, Sicilia B, Gallego S, et al. Helicobacter pylori eradication in patients with peptic ulcer after two treatment failures: a prospective culture-guided study. Gastroenterol Hepatol 2002; 25: 438–442. [DOI] [PubMed] [Google Scholar]
- 16. Tan B, Yang JC, Young CL, et al. Helicobacter pylori antimicrobial susceptibility testing-guided salvage therapy in the USA: a real life experience. Dig Dis Sci 2018; 63: 437–445. [DOI] [PubMed] [Google Scholar]
- 17. Bhakta D, Graham DY, Chan J, et al. Lessons from using culture-guided treatment after referral for multiple treatment failures for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2018; 16: 1531–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Draeger S, Wuppenhorst N, Kist M, et al. Outcome of second- and third-line Helicobacter pylori eradication therapies based on antimicrobial susceptibility testing. J Antimicrob Chemother 2015; 70: 3141–3145. [DOI] [PubMed] [Google Scholar]
- 19. Blumel B, Goelz H, Kist M, et al. Retrospective study on outcome of salvage Helicobacter pylori eradication therapies based on molecular genetic susceptibility testing. Helicobacter 2018; 23: e12494. [DOI] [PubMed] [Google Scholar]
- 20. Liou JM, Chen PY, Luo JC, et al. Efficacies of genotypic resistance-guided vs empirical therapy for refractory Helicobacter pylori infection. Gastroenterology 2018; 155: 1109–1119. [DOI] [PubMed] [Google Scholar]
- 21. Fiorini G, Vakil N, Zullo A, et al. Culture-based selection therapy for patients who did not respond to previous treatment for Helicobacter pylori infection. Clin Gastroenterol Hepatol 2013; 11: 507–510. [DOI] [PubMed] [Google Scholar]
- 22. Kwon YH, Kim N, Lee JY, et al. Comparison of the efficacy of culture-based tailored therapy for Helicobacter pylori eradication with that of the traditional second-line rescue therapy in Korean patients: a prospective single tertiary center study. Scand J Gastroenterol 2016; 51: 270–276. [DOI] [PubMed] [Google Scholar]
- 23. Savoldi A, Carrara E, Graham DY, et al. Prevalence of antibiotic resistance in Helicobacter pylori: a systematic review and meta-analysis in World Health Organization Regions. Gastroenterology 2018; 155: 1372–1382.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. El-Serag HB, Kao JY, Kanwal F, et al. Houston consensus conference on testing for Helicobacter pylori infection in the United States. Clin Gastroenterol Hepatol 2018; 16: 992–1002.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dyar OJ, Huttner B, Schouten J, et al. What is antimicrobial stewardship? Clin Microbiol Infect 2017; 23: 793–798. [DOI] [PubMed] [Google Scholar]
- 26. Hulscher M, Prins JM. Antibiotic stewardship: does it work in hospital practice? A review of the evidence base. Clin Microbiol Infect. 2017; 23: 799–805. [DOI] [PubMed] [Google Scholar]
- 27. Asaka M. A new approach for elimination of gastric cancer deaths in Japan. Int J Cancer 2013; 132: 1272–1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mégraud F, Bénéjat L, Ontsira Ngoyi EN, et al. Molecular approaches to identify Helicobacter pylori antimicrobial resistance. Gastroenterol Clin North Am 2015; 44: 577–596. [DOI] [PubMed] [Google Scholar]
- 29. Arslan N, Yilmaz O, Demiray-Gurbuz E. Importance of antimicrobial susceptibility testing for the management of eradication in Helicobacter pylori infection. World J Gastroenterol 2017; 23: 2854–2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Data for Susceptibility-guided therapy for Helicobacter pylori infection treatment failures by Lou Yu, Laisheng Luo, Xiaohua Long, Xiao Liang, Yingjie Ji, Qi Chen, Yanyan Song, Xiaobo Li, David Y. Graham and Hong Lu in Therapeutic Advances in Gastroenterology