Abstract
Polycystic ovary syndrome (PCOS) and hyperprolactinemia (HPRL) are the two most common etiologies of anovulation in women.
Since the 1950s, some authors think that there is a pathophysiological link between PCOS and HPRL. Since then, many authors have speculated about the link between these two endocrine entities, but no hypothesis proposed so far could ever be confirmed. Furthermore, PCOS and HPRL are frequent endocrine diseases and a fortuitous association cannot be excluded.
The evolution of knowledge about PCOS and HPRL shows that studies conducted before the 2000s are obsolete given current knowledge. Indeed, most of the studies were conducted before consensual diagnosis criteria of PCOS and included small numbers of patients. In addition, the investigation of HPRL in these studies relied on obsolete methods and did not look for the presence of macroprolactinemia. It is therefore possible that HPRL that has been attributed to PCOS corresponded in fact to macroprolactinemia or to pituitary microadenomas of small sizes that could not be detected with the imaging methods of the time.
Recent studies that have conducted a rigorous etiological investigation show that HPRL found in PCOS correspond either to non-permanent increase of prolactin levels, to macroprolactinemia or to other etiologies. None of this recent study found HPRL related to PCOS in these patients.
Thus, the link between PCOS and HPRL seems to be more of a myth than a well-established medical reality and we believe that the discovery of an HPRL in a PCOS patient needs a standard etiological investigation of HPRL.
Keywords: PCOS, Hyperprolactinemia, macroprolactinemia, hyperandrogenism, prolactinoma
Introduction
Polycystic Ovarian Syndrome (PCOS) and hyperprolactinemia (HPRL) are the two most common endocrine disorders in women of reproductive age. Indeed, the prevalence of PCOS is estimated at about 4 to 21% when the PCOS is diagnosed according to the Rotterdam criteria1 and the prevalence of HPRL was estimated at 4% in a cohort of female blood donors2, and with an estimated incidence rate of 49 per 100 000 persons-years3.
So, the concomitant discovery of hyperprolactinemia (HPRL) and polycystic ovarian syndrome (PCOS) is not a rare situation in women being investigated for menstrual disorders. The association between HPRL and PCOS has been described since the 1950s and has suggested the existence of a pathophysiological link between these two entities but data from the literature on this subject are unclear. Therefore, the question persists as to whether a moderate hyperprolactinemia can be attributed to PCOS or, given the high prevalence of both diseases, whether it is merely a fortuitous association.
The aim of this review was to clarify the hypothetical epidemiological and physiopathological links between PCOS and HPRL. To answer this question, we first recalled the complex history of the PCOS diagnosis as well as the management of the HPRL. Then, in a second part of this study, we performed a critical and updated review of the available literature on the subject.
A short history of PCOS: from the discovery of the disease to recent guidelines
Polycystic ovarian syndrome (PCOS) was first described in 1935 by Irving Stein and Michael Leventhal who reported 7 cases of patients presented with amenorrhea, infertility and enlarged multicystic ovaries4.
That same year, Laquer and Butenandt discovered the testosterone and the world of biochemistry was upset down with the development of the first androgens assay and the better understanding of their physiological and pathological origins5,6. Subsequently, it was discovered that androgens were synthesized by the adrenal gland but also by the ovaries, and that excess of androgens was responsible for hyperandrogenism in women7–11. This knowledge has provided a better understanding of PCOS and these symptoms.
Subsequently, other authors described other patients with PCOS, which confirms the reality of this syndrome and allows to complete the phenotypic spectrum of the disease12–17. Thus, the presence of clinical and/or biological hyperandrogenism or obesity was added to the PCOS entity.
It also became clear that many other endocrine disorders could mimic the PCOS phenotype and that it was necessary to eliminate these diseases before the diagnosis of PCOS: non classic congenital adrenal hyperplasia, hypercorticism, ovarian or adrenal virilizing tumors, hypothyroidism, iatrogenic androgen excess and especially hyperprolactinemia18,19.
Thus, the definition of the PCOS remained rather confused until 1990 when the first international diagnostic criteria of the PCOS were established by the National Health Institute (NIH), nearly 50 years after the first description of the syndrome20. These first diagnostic criteria defined PCOS by the presence of chronic oligoanovulation associated with clinical and/or biological hyperandrogenism. Morphological descriptions of the ovaries were excluded from these early guidelines20.
The development of ultrasound technologies in the 1970s and 1980s gave notable changes in the diagnosis of PCOS21,22. Ultrasound technologies were rapidly improved and ultrasonography was soon validated for ovarian exploration of PCOS23–27. The use of ultrasound has become common in clinical practice since the 80s and old imaging techniques to assess the size of the ovaries (pneumography, culdoscopy, etc.) have quickly become obsolete.
With the improvement of hormonal assays and ultrasound techniques, it has become necessary to modify the PCOS diagnostic criteria.
The Rotterdam conference in 2003 integrated the ultrasound criteria for the diagnosis of PCOS28,29. These ultrasound criteria, although imperfect and subject to recurring revisions30,31, have at least clarified the definition of PCOS up to the latest recent European Society of Human Reproduction and Embryology (ESHRE) recommendations32,33 (Table 1).
Table I.
Evolution of PCOS diagnosis criteria according to different guidelines.
| NIH 1990 (the 2 criterias are required)* |
|---|
| 1. Chronic anovulation |
| 2. Clinical and/or biological hyperandrogenism |
| Rotterdam 2003 (2 out of 3 criteria are required)* |
| 1. Oligo-anovulation |
| 2. Clinical and/or biological hyperandrogenism |
| 3. Ultrasound criteria: ovarian volume>10cm3, AFC>12 |
| Androgen excess (AE) Society 2006 and AE-PCOS Society 2009 (the 2 criterias are required)* |
| 1. Clinical and/or biological hyperandrogenism |
| 2. Oligo-anovulation or ultrasound criteria |
| NIH 2012 (2 out of 3 criteria are required)* |
| 1. Clinical and/or biological hyperandrogenism |
| 2. Oligo-anovulation |
| 3. Ultrasound criteria: ovarian volume>10cm3, AFC>12 |
| Phenotypes: A 1+2+3, B 1+2, C 1+3, D 2+3 |
| ESHRE 2018 (2 out of 3 criteria are required)* |
| 1. Clinical and/or biological hyperandrogenism |
| 2. Oligo-anovulation |
| 3. Ultrasound criteria: ovarian volume>10cm3 (AFC>20 only with endovaginal ultrasound transducers with a frequency bandwidth that includes 8MHz) |
After exclusion of non classic congenital adrenal hyperplasia, hypercorticism, ovarian or adrenal virilizing tumors, hypothyroidism and hyperprolactinemia.
Abreviations: AE, Androgen Excess; AFC, antral follicular count; ESHRE, European Society of Human Reproduction and Embryology; NIH, National Health Institute; PCOS, Polycystic ovary syndrome.
Moreover, it has also become clear that there are different types of PCOS depending on whether the patient had all the symptoms of PCOS (phenotype A) or only 2 out of 3 criteria (phenotype B, C and D). This is why the Androgen Excess and PCOS Society (AE-PCOS) in 2009 and the NIH in 2012 proposed separating PCOS into different phenotypes, in order to clarify the phenotypic heterogeneity of PCOS34,35 (Table 1).
To conclude this first part of our work, PCOS has experienced a complex history punctuated by a constant improvement of its understanding and accompanied by an evolution of diagnostic criteria (table I)20,29,33,35–37. Most studies conducted prior to 1990 (NIH guidelines) include patients labeled PCOS, according to non-consensual diagnostic criteria. In addition, the evolution of ultrasound techniques as well as hormonal assays makes it difficult to compare PCOS in the 1980s with current PCOS women. Then, this historical retrospective highlight that old studies are not applicable to our current practice.
Management of the hyperprolactinemia through the decades
Human prolactin was first assayed in New York in 1970 by Andrew Frantz and David Kleinberg, who successfully developed an assay which was able to separate prolactin and growth hormone (GH)38.
This discovery helped to improve the knowledge on this hormone and the pulsatile secretion of prolactin was quickly described in the following years. It was then admitted that high serum prolactin level must be systematically controlled to ensure that it is a permanent hyperprolactinemia.
The first case of macroprolactinemia was published in 1981 by Whittaker39 and the existence of macroprolactin was subsequently confirmed by other authors who published similar observations and developed methods for its detection40–42. Briefly, the majority of prolactin in the bloodstream is monomeric but dimeric and polymeric (bind to immunoglobulin G) forms may also coexist. These forms of prolactin is unable to bind to prolactin receptors and exhibits no systemic response. Macroprolactinemia can cause artificially elevated serum prolactin value associated with a lack of symptoms of hyperprolactinemia and each kit of prolactin assays has a different sensitivity for the detection of macroprolactin43,44.
To differentiate these different forms of circulating prolactin, the reference method is gel filtration chromatography. However, this exam is expensive and time consuming. It has thus been demonstrated that the detection of these inactive forms of prolactin is possible by carrying out a precipitation of the serum with polyethylene glycol (PEG)45.
However, although macroprolactin has been known for a long time, its involvement in the misdiagnosis of hyperprolactinemia has not been seriously emphasized in different studies until the 2000s46–49. Currently, guidelines do not yet recommend a systematic screening of macroprolactinemia.
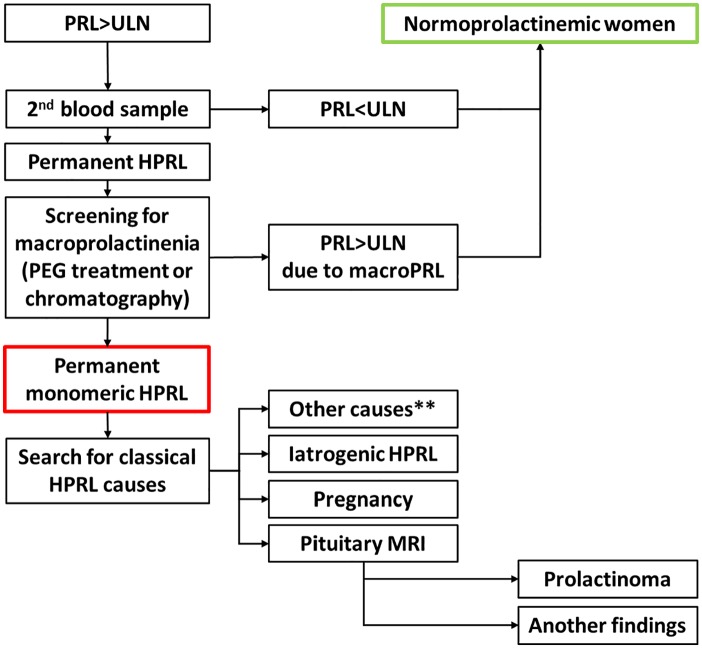
In 2005, the international guidelines for the management of HPRL clearly recommended that moderate hyperprolactinemia should be systematically monitored on a second assay and investigated for macroprolactin, especially in the absence of symptoms of hyperprolactinemia50,51 (Fig. 1).
Fig 1.
Diagnostic algorithm for the management of hyperprolactinemia.
Abbreviations: PRL, prolactin; HPRL, hyperprolactinemia; ULN, Upper limit of normal; MRI, magnetic resonance imaging.
** Others causes: hypothyroidism, chronic renal failure, cirrhosis, chest wall lesions, breast stimulation, etc.
Obviously, studies conducted until the 2000s did not screen macroprolactinemia, which leads to an interpretation bias. Indeed, macroprolactinemia is a common cause of elevated prolactin and is present in approximately 4% to 40% of hyperprolactinemic patients depending on the referral population52,53. It is therefore possible that some idiopathic HPRL patient that have been described in older studies are elevated prolactin levels related to the presence of macroprolactin.
The other breakthrough in the management of HPRL was the development of MRI. Lanteburg and Mansfield received the Nobel Prize in 2003 for their work that led to the development of the first MRIs in the 1970s54. The first pituitary MRIs were described in the 1980s55,56 and this modern and powerful imaging method has quickly replaced the radiographs of sella turcica, by improving the detection of pituitary adenomas. The gradual improvement of MRI techniques has allowed the detection of smaller adenoma, up to the current MRI allowing the detection of adenoma of about 3 mm57.
Once again, this historical retrospective highlights the rapid progression of knowledge concerning the management of hyperprolactinemia over the past 30 years. Most studies conducted prior to the 2000s did not performed pituitary MRI but radiographs of sella turcica or did not search for macroprolactin and so it is likely that many cases of HPRL were misdiagnosed as idiopathic. Then, this historical retrospective highlight again that old studies are not applicable to our current practice.
Link between HPRL and PCOS: what the literature analysis reveals?
In 1954, Forbes described 6 patients with prolactin adenoma associated with clinical hyperandrogenism58. Subsequently, other authors have published cases of patients with PCOS and hyperprolactinemia59–62. These observations have suggested the possibility of a common pathophysiological link between hyperprolactinemia and PCOS and many hypotheses were proposed to explain this association.
Prevalence and Causes of HPRL in PCOS Women
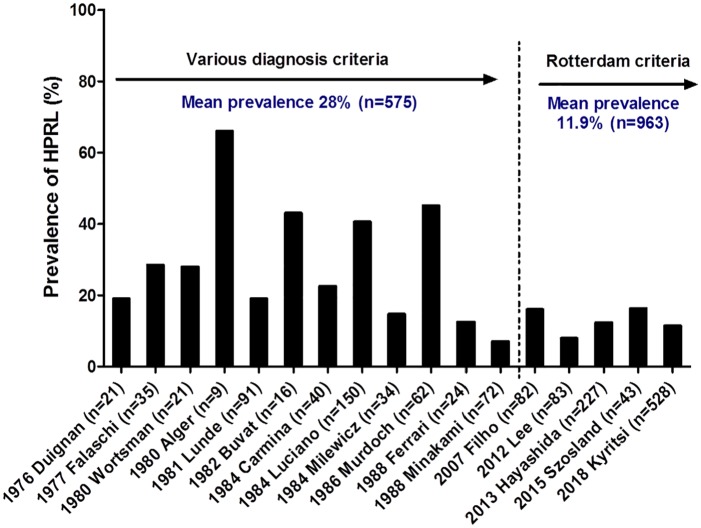
The prevalence of hyperprolactinemia in PCOS women is very variable in the literature, ranging from 3% to 67% (Fig. 2). The majority of this studies was conducted before the first diagnostic criteria of PCOS published in 1990 by the NIH59,60,63–72. When analyzing all of these studies according to their years of publication, we note that the prevalence of hyperprolactinemia is more homogeneous since the PCOS was diagnosed with consensus criteria73–77 (Fig. 2).
Fig 2.
Prevalence of hyperprolactinemia in PCOS women in the literature over time.
The names of the first authors and the date of publication are given for each study as well as the number of PCOS women studied (indicated in parenthesis).
In addition, these studies were conducted before the consensus for the management of HPRL and were methodologically very unequal. The existence of permanent and monomeric hyperprolactinemia (i.e., confirmed on a second independent sample and not explained by the presence of macroprolactin) has not been confirmed in the vast majority of these studies. Finally, these studies have focused on a small number of PCOS patients, except Kyritsi& al in 2018 75. Thus, the prevalence of hyperprolactinemia in women with PCOS is still unclear.
Concerning the causes of hyperprolactinemia found in PCOS women, the data in the recent literature are concordant and seem to invalidate the hypothesis that hyperprolactinemia is part of the PCOS. Indeed, Filho et al. analyzed a population of 82 women with PCOS and found that 16% (n = 13) had a pathological elevation of circulating prolactin levels. A rigorous etiological approach found a classical cause of hyperprolactinemia for each of these 13 women (prolactin adenomas (n = 9), hyperprolactinemic drugs (n = 3), macroprolactin (n = 1))73.
Hayashida et al. in 2014 studied a larger population of 227 PCOS women. 6% of PCOS women had elevated prolactin (n = 16), which was consistently explained by the presence of macroprolactin74.
These two studies are in contradiction with the results of Kyritsi et al. in 2018 that indicate a prevalence of idiopathic HPRL of 23% in 76 PCOS women with HPRL75. However, in this study, the exploration of HPRL was incomplete because the search for macroprolactin was not performed in most patients and pituitary MRI was not performed in all patients.
More recently, there have been reported cases of twin sisters presenting with features of PCOS associated with idiopathic hyperprolactinemia. In both of these sisters, normalization of prolactin with cabergoline treatment led to the normalization of menstrual cycles and plasma androgen measurements78. This cases convey a message that any prolactin elevation in patients presenting with features suggestive of PCOS should be rigorously evaluated, especially once the prolactin is normalized in order to confirm the reality of the PCOS .
Thus, data from recent literature do not seem to confirm the presence of hyperprolactinemia in women with PCOS when a rigorous etiological investigation was conducted. However, data from the literature is still insufficient to be conclusive on the subject, and a rigorous study on a larger cohort of PCOS women is needed to confirm these findings.
Hypotheses to explain a physiopathological link between HPRL and PCOS
The most common hypothesis to explain the link between HPRL and PCOS is a possibly common hypothalamic-pituitary abnormality that can explain both PCOS and hyperprolactinemia.
Indeed, studies have shown a synchronization between the prolactin and LH secretion peaks in women with PCOS71,79,80. In addition, some studies have suggested that dopamine can also slow down the secretion of LH81–83. It has thus been hypothesized that the high levels of LH found in PCOS women would be secondary to a decrease in dopaminergic tone that would also be responsible for an increase in prolactin82,84. However, there is conflicting results regarding the effect of dopamine inhibitor or agonist therapy on LH levels in PCOS women85–88.
Another hypothesis suggests that PCOS causes hyperprolactinemia because it induces relative hyperestrogenemia89,90. Indeed, various experimental studies have shown an increase in the secretion of prolactin under the action of estrogen91,92. However, different arguments oppose this hypothesis. First, various studies have shown that combined oral contraceptive (containing estrogens) does not result in an increase in prolactinoma size93,94. In addition, the few older studies that have studied the effect of wedge ovarian resection or ovarian drilling on prolactin values are conflicting95,96. Unfortunately, no recent study has evaluated the impact of combined oral contraceptive pills on prolactin levels.
It also has been suggested the possibility of an acceleration of GnRH pulsatility in PCOS women. This phenomena would be involved in the increase of LH and in the decrease of dopaminergic tone (which induce hyperprolactinemia)90. However, there was no evidence of decreased prolactin levels in PCOS women who benefit from pituitary desensitization with GnRH-agonists97.
Thus, studies on the subject are rare and none has convincingly demonstrated a real physiopathological link between PCOS and hyperprolactinemia.
Conclusion
To conclude, we have shown here that the link between hyperprolactinemia and PCOS comes from old studies in which PCOS were diagnosed according to non-consensual criteria and in which hyperprolactinemia was insufficiently explored in the light of recent knowledge. In addition, data from the literature suggest that there is no hyperprolactinemia related to PCOS once HPRL was rigorously explored in these women.
The recent study of Hayashida& al. demonstrates that the elevation of prolactin linked to the presence of a macroprolactin is not uncommon in PCOS women and that it is therefore essential to screen it74. This first step is essential to limit the misdiagnosis and thus avoid the unnecessary prescription of a pituitary MRI or even dopaminergic agonist treatment, as already pointed out by Escobar-Morreale years ago98.
In case of excessive prolactin level, it is recommended to confirm the reality of hyperprolactinemia by performing a second independent sample and eliminating an excess related to the presence of macroprolactin51 (Fig. 1).
Finally, in case of confirmation of hyperprolactinemia, it is necessary to carry out a thorough etiological investigation in search of classical etiologies of HPRL before concluding that HPRL is secondary to PCOS (prolactinoma, drug induced HRPL, pregnancy, hypothyroidism, chronic renal failure, cirrhosis, chest wall lesions, breast stimulation, etc..).
Filho in 2007 and already Luciano in 1984 clearly demonstrated that when the etiological investigation of hyperprolactinemia was rigorous, there was no hyperprolactinemia related to PCOS67,73 (Fig. 1).
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contribution: CD and GR performed the literature review and co-wrote the first draft, JY and DD took care of draft revision.
ORCID iD: Didier Dewailly  https://orcid.org/0000-0001-8521-5163
https://orcid.org/0000-0001-8521-5163
References
- 1. Lizneva D, Suturina L, Walker W, Brakta S, Gavrilova-Jordan L, Azziz R. Criteria, prevalence, and phenotypes of polycystic ovary syndrome. FertilSteril. 2016;106(1):6-15. doi:10.1016/j.fertnstert.2016.05.003 [DOI] [PubMed] [Google Scholar]
- 2. Alpañés M, Sanchón R, Martínez-García MÁ, Martínez-Bermejo E, Escobar-Morreale HF. Prevalence of hyperprolactinaemia in female premenopausal blood donors. Clinical Endocrinology. 2013;79(4):545-549. doi:10.1111/cen.12182 [DOI] [PubMed] [Google Scholar]
- 3. Soto-Pedre E, Newey PJ, Bevan JS, Greig N, Leese GP. The epidemiology of hyperprolactinaemia over 20 years in the Tayside region of Scotland: the Prolactin Epidemiology, Audit and Research Study (PROLEARS). Clinical Endocrinology. 2017;86(1):60-67. doi:10.1111/cen.13156 [DOI] [PubMed] [Google Scholar]
- 4. Stein IF, Leventhal ML. Amenorrhea associated with bilateral polycystic ovaries. Am J Obstet Gynecol. 1935;29:181-191. [Google Scholar]
- 5. Butenandt A, Testosterone Hanisch G. The transformation of dehydroandrosterone into androstendiol and testosterone; a method for producing testosterone from cholesterin. Hoppe-Seyler’s Z Physiol Chem. 1935;237:89-98. [Google Scholar]
- 6. David K, Dingemanse E, Freud J, Laquer E. Crystalline male hormone from the testes (Testosterone) is more effective than androsterone derived from urine or cholesterin. Hoppe-Seyler’s Z physiol Chem. 1935;233:281-282. [Google Scholar]
- 7. Butenandt A, Tscherning K. ÜberAndrosteron, ein krystallisiertes männliches Sexual hormon. I. Isolierung und Reindarstellung aus Männerharn. Hoppe-Seyler’s Z Physiol Chem. 1934;229:167. [Google Scholar]
- 8. Callow NH, Callow RK. The isolation of androsterone and transdehydroandrosterone from the urine of normal women. Biochem J. 1938;32(10):1759-1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dignam WJ, Pion RJ, Lamb EJ, Simmer HH. Plasma androgens in women. II. Patients with polycystic ovaries and hirsutism. Acta Endocrinol. 1964;45:254-271. [PubMed] [Google Scholar]
- 10. Finkelstein M, Forchielli E, Dorfman RI. Estimation of testosterone in human plasma. J Clin Endocrinol Metab. 1961;21:98-101. doi:10.1210/jcem-21-1-98 [DOI] [PubMed] [Google Scholar]
- 11. Ponse K. La FonctionAndrogène de l’ovaire Chez l’animal. Rapports de La IIIème Réunion DesEndocrinologues de Langue Française (Bruxelles).Doin, masson et Cie Edit; Paris; 1955. [Google Scholar]
- 12. Culiner A, Shippel S. Virilism and theca-cell hyperplasia of the ovary; a syndrome. J ObstetGynaecol Br Emp. 1949;56(3):439-445. [DOI] [PubMed] [Google Scholar]
- 13. Ginsburg J, Havard CW. Polycystic ovary syndrome. Br Med J. 1976;2(6038): 737-740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Goldzieher JW, Axelrod LR. Clinical and biochemical features of polycystic ovarian disease. FertilSteril. 1963;14:631-653. [DOI] [PubMed] [Google Scholar]
- 15. Stein IF, Cohen MR. Surgical treatment of bilateral polycystic ovaries- amenorrhea and sterility. Am J Obstet Gynecol. 1939;38:465-480. [Google Scholar]
- 16. Stein IF, Cohen MR, Elson R. Results of bilateral ovarian wedge resection in 47 cases of sterility; 20 year end results; 75 cases of bilateral polycystic ovaries. Am J Obstet Gynecol. 1949;58(2):267-274. [DOI] [PubMed] [Google Scholar]
- 17. Vara P, Niemineva K. The Stein-Leventhal syndrome. Ann ChirGynaecol Fenn. 1951;40(1):23-33. [PubMed] [Google Scholar]
- 18. Netter A., Le syndrome de, Stein et, Leventhal en. 1977. In: Actualités Gynécologiques. Vol 1 Masson; 8. Paris; 1977:229-239. [Google Scholar]
- 19. Yen SS. The polycystic ovary syndrome. Clin Endocrinol (Oxf). 1980;12(2):177-207. [DOI] [PubMed] [Google Scholar]
- 20. Zawadski JK, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Polycystic Ovary Syndrome. Blackwell Scientific Publications; Boston; 1992:377-384. [Google Scholar]
- 21. Donald I, Macvicar J, Brown TG. Investigation of abdominal masses by pulsed ultrasound. Lancet. 1958;1(7032):1188-1195. [DOI] [PubMed] [Google Scholar]
- 22. von Micsky LI. Ultrasonics in obstetrics and gynecology. Pbstet Gynecol. 1965;25: 420-421. [Google Scholar]
- 23. Orsini LF, Venturoli S, Lorusso R, Pluchinotta V, Paradisi R, Bovicelli L. Ultrasonic findings in polycystic ovarian disease. FertilSteril. 1985;43(5):709-714. [DOI] [PubMed] [Google Scholar]
- 24. Parisi L, Tramonti M, Casciano S, Zurli A, Gazzarrini O. The role of ultrasound in the study of polycystic ovarian disease. J Clin Ultrasound. 1982;10(4):167-172. [DOI] [PubMed] [Google Scholar]
- 25. Swanson M, Sauerbrei EE, Cooperberg PL. Medical implications of ultrasonically detected polycystic ovaries. J Clin Ultrasound. 1981;9(5):219-222. [DOI] [PubMed] [Google Scholar]
- 26. Venturoli S, Paradisi R, Saviotti E, et al. Ultrasound study of ovarian morphology in women with polycystic ovary syndrome before and during treatment with an oestrogen/progestogen preparation. Arch Gynecol. 1983;234(2):87-93. [DOI] [PubMed] [Google Scholar]
- 27. Zemlyn S. Comparison of pelvic ultrasonography and pneumography for ovarian size. J Clin Ultrasound. 1974;2(4):331-338. [DOI] [PubMed] [Google Scholar]
- 28. Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. FertilSteril. 2004;81(1):19-25. [DOI] [PubMed] [Google Scholar]
- 29. Rotterdam ESHRE/ASRM-Sponsored PCOS consensus workshop group. Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS). Hum Reprod. 2004;19(1):41-47. [DOI] [PubMed] [Google Scholar]
- 30. Dewailly D. Diagnostic criteria for PCOS: Is there a need for a rethink? Best Pract Res Clin ObstetGynaecol. 2016;37:5-11. doi:10.1016/j.bpobgyn.2016.03.009 [DOI] [PubMed] [Google Scholar]
- 31. Lie Fong S, Laven JSE, Duhamel A, Dewailly D. Polycystic ovarian morphology and the diagnosis of polycystic ovary syndrome: redefining threshold levels for follicle count and serum anti-Müllerian hormone using cluster analysis. Hum Reprod. 2017;32(8):1723-1731. doi:10.1093/humrep/dex226 [DOI] [PubMed] [Google Scholar]
- 32. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. FertilSteril. 2018;110(3):364-379. doi:10.1016/j.fertnstert.2018.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Teede HJ, Misso ML, Costello MF, et al. Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Hum Reprod. 2018;33(9):1602-1618. doi:10.1093/humrep/dey256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. Fertility and Sterility. 2009;91(2):456-488. doi:10.1016/j.fertnstert.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 35. National Institutes of Health. Evidence-based methodology workshop on polycystic ovary syndrome,. December 2012. https://prevention.nih.gov/docs/programs/pcos/FinalReport.pdf.
- 36. Azziz R, Carmina E, Dewailly D, et al. The Androgen Excess and PCOS Society criteria for the polycystic ovary syndrome: the complete task force report. FertilSteril. 2009;91(2):456-488. doi:10.1016/j.fertnstert.2008.06.035 [DOI] [PubMed] [Google Scholar]
- 37. Orio F, Azziz R. Androgen Excess Society Annual Meeting Committee, Androgen Excess Society. Report on the Third Annual Meeting of the Androgen Excess Society, San Diego, California, June 3, 2005 FertilSteril. 2006;86(5):1318-1320. doi:10.1016/j.fertnstert.2006.06.049 [DOI] [PubMed] [Google Scholar]
- 38. Frantz AG, Kleinberg DL. Prolactin: evidence that it is separate from growth hormone in human blood. Science. 1970;170(3959):745-747. [DOI] [PubMed] [Google Scholar]
- 39. Whittaker PG, Wilcox T, Lind T. Maintained fertility in a patient with hyperprolactinemia due to big, big prolactin. J Clin Endocrinol Metab. 1981;53(4):863-866. doi:10.1210/jcem-53-4-863 [DOI] [PubMed] [Google Scholar]
- 40. Jackson RD, Wortsman J, Malarkey WB. Characterization of a large molecular weight prolactin in women with idiopathic hyperprolactinemia and normal menses. J Clin Endocrinol Metab. 1985;61(2):258-264. doi:10.1210/jcem-61-2-258 [DOI] [PubMed] [Google Scholar]
- 41. Jackson RD, Wortsman J, Malarkey WB. Macroprolactinemia presenting like a pituitary tumor. Am J Med. 1985;78(2):346-350. [DOI] [PubMed] [Google Scholar]
- 42. Larrea F, Escorza A, Granados J, et al. Familial occurrence of big-big prolactin as the predominant immunoreactive human prolactin species in blood. FertilSteril. 1987;47(6):956-963. [PubMed] [Google Scholar]
- 43. Fahie-Wilson M, Smith TP. Determination of prolactin: the macroprolactin problem. Best Pract Res Clin Endocrinol Metab. 2013;27(5):725-742. doi:10.1016/j.beem.2013.07.002 [DOI] [PubMed] [Google Scholar]
- 44. Overgaard M, Pedersen SM. Serum prolactin revisited: parametric reference intervals and cross platform evaluation of polyethylene glycol precipitation-based methods for discrimination between hyperprolactinemia and macroprolactinemia. Clin Chem Lab Med. 2017;55(11):1744-1753. doi:10.1515/cclm-2016-0902 [DOI] [PubMed] [Google Scholar]
- 45. Smith TP, Fahie-Wilson MN. Reporting of post-PEG prolactin concentrations: time to change. Clin Chem. 2010;56(3):484-485. doi:10.1373/clinchem.2009.135210 [DOI] [PubMed] [Google Scholar]
- 46. Gibney J, Smith TP, McKenna TJ. The impact on clinical practice of routine screening for macroprolactin. J Clin Endocrinol Metab. 2005;90(7):3927-3932. doi:10.1210/jc.2004-2234 [DOI] [PubMed] [Google Scholar]
- 47. Hattori N. Macroprolactinemia: a new cause of hyperprolactinemia. J Pharmacol Sci. 2003;92(3):171-177. [DOI] [PubMed] [Google Scholar]
- 48. Hauache OM, Rocha AJ, Maia ACM, Maciel RMB, Vieira JGH. Screening for macroprolactinaemia and pituitary imaging studies. Clin Endocrinol (Oxf). 2002;57(3):327-331. [DOI] [PubMed] [Google Scholar]
- 49. Vallette-Kasic S, Morange-Ramos I, Selim A, et al. Macroprolactinemia Revisited: A Study on 106 Patients. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):581-588. doi:10.1210/jcem.87.2.8272 [DOI] [PubMed] [Google Scholar]
- 50. Casanueva FF, Molitch ME, Schlechte JA, et al. Guidelines of the Pituitary Society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf). 2006;65(2):265-273. doi:10.1111/j.1365-2265.2006.02562.x [DOI] [PubMed] [Google Scholar]
- 51. Melmed S, Casanueva FF, Hoffman AR, et al. Diagnosis and treatment of hyperprolactinemia: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96(2):273-288. doi:10.1210/jc.2010-1692 [DOI] [PubMed] [Google Scholar]
- 52. Kalsi AK, Halder A, Jain M, Chaturvedi PK, Sharma JB. Prevalence and reproductive manifestations of macroprolactinemia. Endocrine. 2019;63(2):332-340. doi:10.1007/s12020-018-1770-6 [DOI] [PubMed] [Google Scholar]
- 53. Samson SL, Hamrahian AH, Ezzat S. American association of clinical endocrinologists, american college of endocrinology disease state clinical review : clinical relevance of macroprolactin in the absence of true hyperprolactinemia. Endocrine Practice. 2015;21(12):1427-1435. doi:10.4158/EP15938.DSC [DOI] [PubMed] [Google Scholar]
- 54. Pohost GM. The History of Cardiovascular Magnetic Resonance. JACC: Cardiovascular Imaging. 2008;1(5):672-678. doi:10.1016/j.jcmg.2008.07.009 [DOI] [PubMed] [Google Scholar]
- 55. Bilaniuk LT, Zimmerman RA, Wehrli FW, et al. Magnetic resonance imaging of pituitary lesions using 1.0 to 1.5 T field strength. Radiology. 1984;153(2):415-418. doi:10.1148/radiology.153.2.6484173 [DOI] [PubMed] [Google Scholar]
- 56. Kaufman B. Magnetic resonance imaging of the pituitary gland. Radiol Clin North Am. 1984;22(4):795-803. [PubMed] [Google Scholar]
- 57. Takahashi M, Uematsu H, Hatabu H. MR imaging at high magnetic fields. Eur J Radiol. 2003;46(1):45-52. [DOI] [PubMed] [Google Scholar]
- 58. Forbes AP, Henneman PH, Griswold GC, Albright F. Syndrome characterized by galactorrhea, amenorrhea and low urinary FSH: comparison with acromegaly and normal lactation. J Clin Endocrinol Metab. 1954;14(3):265-271. doi:10.1210/jcem-14-3-265 [DOI] [PubMed] [Google Scholar]
- 59. Duignan NM. Polycystic ovarian disease. Br J ObstetGynaecol. 1976;83(8):593-602. [DOI] [PubMed] [Google Scholar]
- 60. Falaschi P, Rocco A, Toscano V, Sciarra F. Polycystic ovary syndrome and hyperprolactinemia. J Steroid Biochem. 1977;8:13. [Google Scholar]
- 61. Lavric MV. Galactorrhea and amenorrhea with polycystic ovaries. Del Castillo syndrome or polycystic ovarian syndrome. Am J Obstet Gynecol. 1969;104(6):814-817. [PubMed] [Google Scholar]
- 62. Thorner MO, McNeilly AS, Hagan C, Besser GM. Long-term treatment of galactorrhoea and hypogonadism with bromocriptine. Br Med J. 1974;2(5916):419-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Alger M, Vazquez-Matute L, Mason M, Canales ES, Zárate A. Polycystic ovarian disease associated with hyperprolactinemia and defective metoclopramide response. FertilSteril. 1980;34(1):70-71. [DOI] [PubMed] [Google Scholar]
- 64. Buvat J, Siame-Mourot C, Fourlinnie JC, Lemaire A, Buvat-Herbaut M, Hermand E. Androgens and prolactin levels in hirsute women with either polycystic ovaries or “borderline ovaries.” FertilSteril. 1982;38(6):695-700. [DOI] [PubMed] [Google Scholar]
- 65. Carmina E, Rosato F, Maggiore M, Gagliano AM, Indovina D, Jannì A. Prolactin secretion in polycystic ovary syndrome (PCO): correlation with the steroid pattern. Acta Endocrinol. 1984;105(1):99-104. [DOI] [PubMed] [Google Scholar]
- 66. Ferrari E, Bossolo PA, Foppa S, et al. Prolactin secretion in polycystic ovary syndrome: circadian rhythmicity and dynamic aspects. Gynecol Endocrinol. 1988; 2(2):101-111. [DOI] [PubMed] [Google Scholar]
- 67. Luciano AA, Chapler FK, Sherman BM. Hyperprolactinemia in polycystic ovary syndrome. FertilSteril. 1984;41(5):719-725. [PubMed] [Google Scholar]
- 68. Lunde O. Hyperprolactinaemia in polycystic ovary syndrome. Ann ChirGynaecol. 1981;70(4):197-201. [PubMed] [Google Scholar]
- 69. Milewicz A. Prolactin levels in the polycystic ovary syndrome. J Reprod Med. 1984;29(3):193-196. [PubMed] [Google Scholar]
- 70. Minakami H, Abe N, Oka N, Kimura K, Tamura T, Tamada T. Prolactin release in polycystic ovarian syndrome. Endocrinol Jpn. 1988;35(2):303-310. [DOI] [PubMed] [Google Scholar]
- 71. Murdoch AP, Dunlop W, Kendall-Taylor P. Studies of prolactin secretion in polycystic ovary syndrome. Clin Endocrinol (Oxf). 1986;24(2):165-175. [DOI] [PubMed] [Google Scholar]
- 72. Wortsman J, Hirschowitz JS. Galactorrhea and hyperprolactinemia during treatment of polycystic ovary syndrome. Obstet Gynecol. 1980;55(4):460-463. [PubMed] [Google Scholar]
- 73. Filho RB, Domingues L, Naves L, Ferraz E, Alves A, Casulari LA. Polycystic ovary syndrome and hyperprolactinemia are distinct entities. Gynecol Endocrinol. 2007;23(5):267-272. doi:10.1080/09513590701297708 [DOI] [PubMed] [Google Scholar]
- 74. Hayashida SAY, Marcondes JAM, Soares JM, et al. Evaluation of macroprolactinemia in 259 women under investigation for polycystic ovary syndrome. Clin Endocrinol (Oxf). 2014;80(4):616-618. doi:10.1111/cen.12266 [DOI] [PubMed] [Google Scholar]
- 75. Kyritsi EM, Dimitriadis GK, Angelousi A, et al. The value of prolactin in predicting prolactinoma in hyperprolactinaemic polycystic ovarian syndrome. Eur J Clin Invest. 2018;48(7):e12961. doi:10.1111/eci.12961 [DOI] [PubMed] [Google Scholar]
- 76. Lee D-Y, Oh Y-K, Yoon B-K, Choi D. Prevalence of hyperprolactinemia in adolescents and young women with menstruation-related problems. Am J Obstet Gynecol. 2012;206(3):213.e1–5. doi:10.1016/j.ajog.2011.12.010 [DOI] [PubMed] [Google Scholar]
- 77. Szosland K, Pawlowicz P, Lewiński A. Prolactin secretion in polycystic ovary syndrome (PCOS). Neuro Endocrinol Lett. 2015;36(1):53-58. [PubMed] [Google Scholar]
- 78. Goyal A, Ganie MA. Idiopathic Hyperprolactinemia Presenting as Polycystic Ovary Syndrome in Identical Twin Sisters: A Case Report and Literature Review. Cureus. July 2018. doi:10.7759/cureus.3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Laatikainen T, Tulenheimo A. Prolactin pulsatility in polycystic ovarian disease. J Endocrinol Invest. 1985;8(2):157-161. doi:10.1007/BF03350674 [DOI] [PubMed] [Google Scholar]
- 80. Venturoli S, Porcu E, Fabbri R, et al. Episodic pulsatile secretion of FSH, LH, prolactin, oestradiol, oestrone, and LH circadian variations in polycystic ovary syndrome. Clin Endocrinol (Oxf). 1988;28(1):93-107. [DOI] [PubMed] [Google Scholar]
- 81. Ferrari C, Rampini P, Malinverni A, et al. Inhibition of luteinizing hormone release by dopamine infusion in healthy women and in various pathophysiological conditions. Acta Endocrinol. 1981;97(4):436-440. [DOI] [PubMed] [Google Scholar]
- 82. Leblanc H, Lachelin GC, Abu-Fadil S, Yen SS. Effects of dopamine infusion on pituitary hormone secretion in humans. J Clin Endocrinol Metab. 1976;43(3):668-674. doi:10.1210/jcem-43-3-668 [DOI] [PubMed] [Google Scholar]
- 83. Pehrson JJ, Jaffee WL, Vaitukaitis JL. Effect of dopamine on gonadotropin-releasing hormone-induced gonadotropin secretion in postmenopausal women. J Clin Endocrinol Metab. 1983;56(5):889-892. doi:10.1210/jcem-56-5-889 [DOI] [PubMed] [Google Scholar]
- 84. Quigley ME, Rakoff JS, Yen SS. Increased luteinizing hormone sensitivity to dopamine inhibition in polycystic ovary syndrome. J Clin Endocrinol Metab. 1981;52(2):231-234. doi:10.1210/jcem-52-2-231 [DOI] [PubMed] [Google Scholar]
- 85. Barnes RB, Lobo RA. Central opioid activity in polycystic ovary syndrome with and without dopaminergic modulation. J Clin Endocrinol Metab. 1985;61(4):779-782. doi:10.1210/jcem-61-4-779 [DOI] [PubMed] [Google Scholar]
- 86. Barnes RB, Mileikowsky GN, Cha KY, Spencer CA, Lobo RA. Effects of dopamine and metoclopramide in polycystic ovary syndrome. J Clin Endocrinol Metab. 1986;63(2):506-509. doi:10.1210/jcem-63-2-506 [DOI] [PubMed] [Google Scholar]
- 87. Buvat J, Buvat-Herbaut M, Marcolin G, et al. A double blind controlled study of the hormonal and clinical effects of bromocriptine in the polycystic ovary syndrome. J Clin Endocrinol Metab. 1986;63(1):119-124. doi:10.1210/jcem-63-1-119 [DOI] [PubMed] [Google Scholar]
- 88. Steingold KA, Lobo RA, Judd HL, Lu JK, Chang RJ. The effect of bromocriptine on gonadotropin and steroid secretion in polycystic ovarian disease. J Clin Endocrinol Metab. 1986;62(5):1048-1051. doi:10.1210/jcem-62-5-1048 [DOI] [PubMed] [Google Scholar]
- 89. Azziz R, Carmina E, Chen Z, et al. Polycystic ovary syndrome. Nat Rev Dis Primers. 2016;2:16057. doi:10.1038/nrdp.2016.57 [DOI] [PubMed] [Google Scholar]
- 90. Robin G, Catteau-Jonard S, Young J, Dewailly D. Physiopathological link between polycystic ovary syndrome and hyperprolactinemia: myth or reality?. GynecolObstetFertil. 2011;39(3):141-145. doi:10.1016/j.gyobfe.2010.11.002 [DOI] [PubMed] [Google Scholar]
- 91. Christin-Maître S, Delemer B, Touraine P, Young J. Prolactinoma and estrogens: pregnancy, contraception and hormonal replacement therapy. Ann Endocrinol (Paris). 2007;68(2-3):106-112. doi:10.1016/j.ando.2007.03.008 [DOI] [PubMed] [Google Scholar]
- 92. Touraine P, Goffin V. Physiologie de la prolactine. EMC - Gynécologie. 2015; 11(1):1-13. [Google Scholar]
- 93. Corenblum B, Donovan L. The safety of physiological estrogen plus progestin replacement therapy and with oral contraceptive therapy in women with pathological hyperprolactinemia. FertilSteril. 1993;59(3):671-673. [DOI] [PubMed] [Google Scholar]
- 94. Testa G, Vegetti W, Motta T, et al. Two-year treatment with oral contraceptives in hyperprolactinemic patients. Contraception. 1998;58(2):69-73. [DOI] [PubMed] [Google Scholar]
- 95. Dahlgren E, Johansson S, Lindstedt G, et al. Women with polycystic ovary syndrome wedge resected in 1956 to 1965: a long-term follow-up focusing on natural history and circulating hormones. FertilSteril. 1992;57(3):505-513. [DOI] [PubMed] [Google Scholar]
- 96. Szilágyi A, Hole R, Keckstein J, Rossmanith WG. Effects of ovarian surgery on the dopaminergic and opioidergic control of gonadotropin and prolactin secretion in women with polycystic ovarian disease. Gynecol Endocrinol. 1993;7(3):159-166. [DOI] [PubMed] [Google Scholar]
- 97. Acién P, Mauri M, Gutierrez M. Clinical and hormonal effects of the combination gonadotrophin-releasing hormone agonist plus oral contraceptive pills containing ethinyl-oestradiol (EE) and cyproterone acetate (CPA) versus the EE-CPA pill alone on polycystic ovarian disease-related hyperandrogenisms. Hum Reprod. 1997;12(3):423-429. [DOI] [PubMed] [Google Scholar]
- 98. Escobar-Morreale HF. Macroprolactinemia in women presenting with hyperandrogenic symptoms: Implications for the management of polycystic ovary syndrome. FertilSteril. 2004;82(6):1697-1699. doi:10.1016/j.fertnstert.2004.06.045 [DOI] [PubMed] [Google Scholar]