Short abstract
Postpartum pneumomediastinum is a rare complication of labour and delivery, where air leaks into the mediastinum following rupture of marginal alveoli. It follows prolonged and forceful Valsalva manoeuvres that increase intra-thoracic pressure. Subcutaneous emphysema may also develop. A chest radiograph can confirm the diagnosis, however a computed tomography thorax maybe required. Treatment is conservative as it is usually self-limiting. We present a case of postpartum pneumomediastinum following a delay in the second stage of labour and subsequent instrumental delivery. She developed chest pain and dyspnea 40 min post-delivery, and subcutaneous emphysema was palpable. Supplementary nasal flow oxygen was administered for 24 h prior to discharge. There is sparse evidence or guidance as to the management of postpartum pneumomediastinum, but consensus appears to be supplemental oxygen for 24 h. More data are needed on the type and duration of oxygen therapy, need for repeat imaging and management of subsequent pregnancies.
Keywords: Pneumomediastinum, Hamman’s syndrome, delivery, postpartum, pregnancy, subcutaneous emphysema
Introduction
The differential diagnosis and management of sudden onset chest pain or shortness of breath immediately post-delivery can be challenging. Postpartum pneumomediastinum is a rare complication of labour and vaginal birth where air leaks into the mediastinum following rupture of marginal alveoli into perivascular tissue planes.1 It follows prolonged and forceful Valsalva manoeuvres that increase intra-thoracic pressure, forcing air to dissect into the mediastinum in susceptible individuals. The incidence is estimated to be around 1 in 100,000 vaginal deliveries.2–4 It is associated with a prolonged second stage and instrumental deliveries. Smoking and respiratory disease are thought to increase the risk.3 Patients typically present with sudden onset chest pain or shortness of breath. However, cough, dysphagia and dysphasia have all been described.4 It typically occurs in the second stage of labour but symptoms may not become apparent until the postpartum period. Subcutaneous emphysema may develop and in severe cases this can lead to compression of the larynx or jugular veins. A chest radiograph is used to confirm the diagnosis, however a computed tomography (CT) scan of the thorax may be required to characterize the pathology and rule out other diagnoses. Treatment remains conservative as it is usually self-limiting, with air being slowly reabsorbed. Recurrent pneumomediastinum is thought to be uncommon, however data are sparse.
Case report
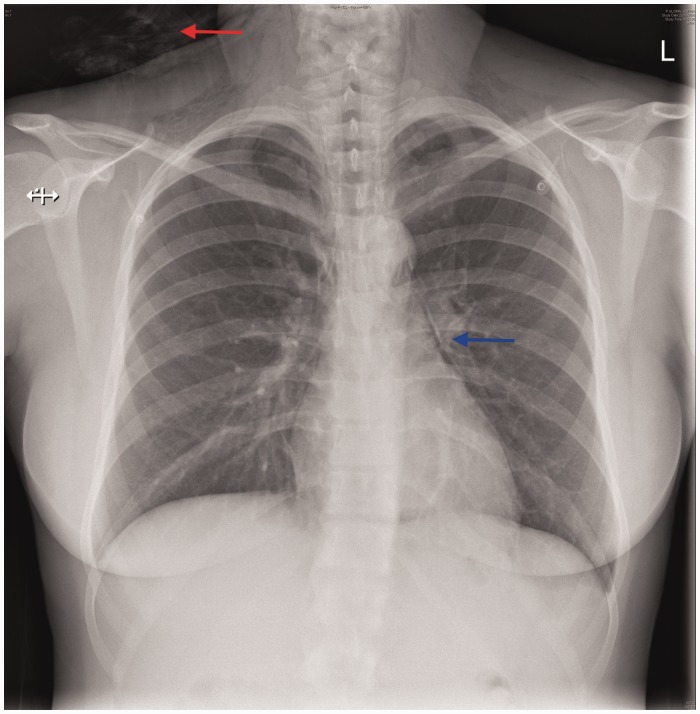
A 23-year-old nulliparous woman was in spontaneous labour at 38 + 2 weeks gestation. She was low-risk at booking, did not smoke and had a body mass index of 25 kg/m2. Pain relief during the first stage of labour consisted of Entonox® (oxygen/nitrous oxide) and diamorphine. At full dilatation after actively pushing for over 2 h, a delay in progress was suspected due to the baby being in an occiput-posterior position. After a failed Ventouse, Neville-Barnes forceps were applied which facilitated the delivery of a 3110 g baby boy, with AGPAR scores of 9 (1 min) and 10 (5 min). The third stage was managed actively and the placenta was delivered within 4 min. Estimated blood loss was 610 ml. Forty minutes postpartum she developed pleuritic chest pain and shortness of breath. Initial clinical observations revealed a heart rate 116 bpm, blood pressure 126/68 mmHg, respiratory rate 24 bpm and oxygen saturations 99% on air. Her chest was clear on auscultation with normal heart sounds. An arterial blood gas was within normal limits. An electrocardiogram showed sinus tachycardia with no evidence of right heart stain. Blood tests revealed a white blood cell count of 21 mmol/L (reference range: 4.0–11.0 × 109), haemoglobin 100 g/L (120–150 g/L) and C-reactive protein of 104 mg/L (0–4 mg/L). A baseline and 12-h troponin were 16 ng/L and 10 ng/L, respectively (0–13 ng/L). An erect chest X-ray demonstrated a pneumomediastinum with subcutaneous emphysema (Figure 1). On further direct examination, subcutaneous emphysema could be palpated around her neck and anterior chest. Nasal flow oxygen (2L/min) was administered for 24 h prior to discharge.
Figure 1.
Pneumomediastinum (blue arrows) with subcutaneous emphysema (red arrow).
Discussion
Pneumomediastinum together with subcutaneous emphysema is collectively called Hamman’s syndrome, described by Dr Louis Virgil Hamman at the John Hopkin’s Hospital, Baltimore, in 1939.5 On auscultation of the heart, Hamman’s crunch may be heard, which is described as a crunching sound synchronous with the cardiac cycle. Hamman’s syndrome can also rarely be associated with pneumopericardium or pneumothoraces.
We present a case of postpartum pneumomediastium following a delayed second stage of labour and subsequent instrumental delivery. The use of sequential instruments (Ventouse followed by forceps) can increase both maternal and fetal morbidity.6 This ultimately results in a higher number of prolonged and forceful Valsalva manoeuvres, which were possible contributing factors. It is the Valsalva manoeuvres that increase the intra-alveolar pressure causing rupture of marginal alveoli and air tracking into the peri-bronchial vascular sheaths and into the mediastinum. The differential diagnosis of acute chest pain postpartum would include pulmonary embolism, pericarditis, dissecting aortic aneurysm, pneumothorax (both simple and tension), oesophageal rupture and ischaemic heart disease.
Entonox®, a combination of oxygen and nitrous oxide, was used as analgesia during her labour. It has been reported that the use of nitrous oxide can increase the risk of gas trapping by diffusion, and therefore increase the risk of developing or expanding a pneumomediastinum.7 Furthermore, it may be prudent to avoid this in women with a history of pneumomediastium.
A repeat chest radiograph was not performed; however, it is unclear how this would change the management of these patients if clinically well. The pain resolved within 48 h and the patient was discharged on day 3 postpartum. Further radiological examinations should be reserved for patients who remain symptomatic or deteriorate during the recovery phase.
Data regarding recurrent pneumomediastinum is sparse, however, it is likely to be rare and therefore subsequent vaginal delivery should be encouraged. During her next pregnancy, it is important to be aware of the association between a prolonged second stage of labour and instrumental delivery with pneumomediastinum. Regional analgesia may be used but it is associated with a longer second stage of labour and increases the chances of requiring an instrumental delivery.8 The obstetric team should have a high index of suspicion for recurrent pneumomediastium if she develops chest pain or becomes hypoxic during the intra-partum or postpartum periods. If a pneumomediastinum does reoccur, delivery should be expedited using either forceps or vacuum extraction in the second stage.
Regarding the use of supplemental oxygen to aid resolution; The British Thoracic Society (BTS) guidelines for pneumothorax state ‘if a patient is hospitalised for observation, supplemental high-flow oxygen should be given where feasible’.9 This is based on a paper from, 1971 in which the rate of pneumothorax absorption increased fourfold during periods of supplemental oxygen therapy in 12 patients breathing air and in 10 patients breathing air and a high concentration of oxygen alternately.10 It is thought that the administration of high-flow supplementary oxygen reduces the partial pressure of nitrogen in the alveoli and blood compared to the pleural space, therefore increasing the diffusion gradient of nitrogen resulting in faster resolution.10 There is currently no such data to suggest this is the case for resolution of pneumomediastinum; however, it appears the consensus is to provide supplemental oxygen for 24 h. Interestingly, the patient described was given low-flow oxygen delivered by nasal cannula, not high-flow.
Conclusion
Postpartum pneumomediastinum is a rare complication of labour and delivery. The differential diagnosis of sudden onset chest pain and shortness of breath immediately post delivery is broad, but should be considered in women with a prolonged second stage, instrumental delivery or lung disease. The presence of subcutaneous emphysema should also be sought. A thorough examination and assessment of diagnostic imaging is essential to ensure this condition is not missed leading to inappropriate investigations being performed. There is sparse evidence or guidance as to the management of these patients, but consensus appears to be supplemental oxygen for 24 h. More data are needed on the type and duration of oxygen therapy, need for repeat imaging and management of subsequent pregnancies.
Declaration of conflicting interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: AB has received support for educational lectures from Janssen-Cilag Ltd, Novartis Ireland Ltd and AstraZenica. AJ and KK have none to declare.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Written consent from the patient was obtained for publication.
Guarantor
ADJ
Contributorship
All the authors were involved with the management of the patient on the ward and contributed to the writing of the case report. AJ obtained patient consent. ADJ, KK and AB approved the final draft.
References
- 1.Hamman LV. Mediastinal emphysema. JAMA 1945; 128: 1–6. [Google Scholar]
- 2.Heffner JE, Sahn SA. Pleural disease in pregnancy. Clin Chest Med 1992; 13: 667–678. [PubMed] [Google Scholar]
- 3.Kandiah S, Iswariah H, Elgey S. Postpartum pneumomediastinum and subcutaneous emphysema: two case reports. Case Rep Obstetr Gynecol 2013; 2013: 735154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Revicky V, Simpson P, Fraser D. Postpartum pneumomediastinum: An uncommon cause for chest pain. Obstetr Gynecol Int 2010; 2010: 956142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamman LV. Spontaneous mediastinal emphysema. Bull Johns Hopkins Hosp Baltimore 1939; 64: 1–21. [Google Scholar]
- 6.Gardella C, Taylor M, Benedetti T, et al. The effect of sequential use of vacuum and forceps for assisted vaginal delivery on neonatal and maternal outcomes. Am J Obstet Gynecol 2001; 185: 896–902. [DOI] [PubMed] [Google Scholar]
- 7.Jayran-Nejad Y. Subcutaneous emphysema in labour. Anaesthesia 1993; 48: 139–140. [DOI] [PubMed] [Google Scholar]
- 8.National Institute for Health and Care Excellence. Intrapartum care: care of healthy women and their babies during childbirth (Clinical Guideline 109). 2014. Available at: https://www.nice.org.uk/guidance/cg190 (accessed November 2017). [PubMed]
- 9.Northfield TC. Oxygen therapy for spontaneous pneumothorax. BMJ 1971; 4: 86–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacDuff A, Arnold A, Harvey J. Management of spontaneous pneumothorax: British Thoracic Society pleural disease guideline 2010. Thorax 2010; 65: ii18–ii31. [DOI] [PubMed] [Google Scholar]