Short abstract
The use of diagnostic imaging involving ionising radiation may be necessary in pregnancy and requires an assessment of the most appropriate and safest imaging modality which will provide the necessary information balanced with the potential risks to the mother and fetus. In most cases, this will involve a potential fetal radiation dose well below 50 mGy. At these doses, there is no risk of lethality, genetic damage/epigenetic change, teratogenicity, growth impairment or sterility. Older epidemiological data indicating a potential increased cancer risk have been contradicted by newer data and better understanding of the biology of low dose radiation. The linear no-threshold rule has been challenged by many and more realistic estimates of oncogenicity risk along with the potential risks of contrast agents are summarised in this review. Imaging in the pregnant population is increasing in both the number of examinations performed and the number of patients being imaged, with the greatest increase being computed tomography scans. Counselling and obtaining informed consent for imaging that involves radiation requires the clinician to communicate with the woman and her family a realistic estimate of the potential radiation dose to herself and her fetus, to describe and quantitate the risks of this estimated dose, to outline the benefits of the imaging procedure and to respond to any questions or concerns. As almost all diagnostic imaging involves doses below the 50 mGy threshold, clinically indicated investigations should not be withheld during pregnancy. All allied staff must also be well informed to ensure the patient receives a consistent message about the risks and benefits of the proposed test.
Keywords: Counselling, contrast agents, diagnostic imaging, oncogenicity, pregnancy risks, radiation
Introduction
The use of diagnostic imaging in pregnancy requires an assessment of the most appropriate and safest imaging modality which will provide maximal information balanced with the potential risks to the mother and fetus. Imaging techniques that do not involve electromagnetic radiation, i.e. ultrasound and magnetic resonance imaging (MRI) are considered safe in pregnancy. Various guidelines have been published expressing confidence in the use of MRI throughout pregnancy, including the first trimester, with no evidence of adverse outcomes on pregnancy or the fetus/neonate.1–6
X-rays, computerised tomography (CT) with and without iodinated contrast, nuclear scanning and positron emission tomography scanning all involve potential exposure of both mother and fetus to ionising radiation. Such imaging may occur inadvertently or by intention during pregnancy, necessitating appropriate counselling. In addition, CT, MRI or even ultrasound may require administration of a contrast agent to improve image definition or other additional information.
The major radiation source for most women during pregnancy is environmental, arising from a number of sources including cosmic, gamma and radon. The magnitude of the exposure varies (see Table 1) with a global average estimated radiation exposure over the nine months of pregnancy being 2.3 mSv.7,8 For a fetus, the dose received is much smaller than that of the mother, as a result of attenuation through the maternal tissue.9
Table 1.
Sources of environmental radiation.7
| Source | Exposure (mSv) |
|---|---|
| Airport scannerAir travel: | 0.005 per exposure0.005 per short flight*0.03 per long flight* |
| Aircrew/frequent flyers | 1–5 per year |
| Background radiation | |
| • UK or Australia | 1.8 per year |
| • USA | 6.2 per year |
| • Kerala, India | 30 per year |
| • Some areas of Brazil and Sudan | 40 per year |
| • Ramsar in Iran | Up to 260 per year |
| Uranium miners/ nuclear industry workers | Additional to background: 1.5–2.5 per year |
The unit mSv refer to exposure whilst the absorbed dose is measured in mGy.*At higher latitudes, multiply by a factor of 2--3.
When a pregnancy is declared, by a female working in the presence of radiation, the embryo or fetus is afforded the same level of protection as the general public, i.e. 1 mSv per year. The governance of the radiation industry requires that all operators adhere to an ‘as low as (is) reasonably achievable’ (ALARA) principle, which means making every reasonable effort to maintain exposures to ionising radiation as far below the dose limits as practical.
Radiation dose and pregnancy
The calculation of radiation dose, both maternal whole body and organ specific as well as the estimated fetal absorbed dose, is calculated based on established dosimetry tables or specific calculations made by a medical physicist on a case-by-case basis. Published dosimetry tables do vary but for counselling purposes, diagnostic imaging procedures can be divided into negligible (<0.1 mGy), low-moderate (0.1–10 mGy) and higher dose (10–50 mGy).4 All radiation doses are potentially additive and where multiple procedures are performed, cumulative dosage should be calculated by a qualified medical physicist.
For most standard radiography, the fetal dose is very low (Table 2) although adjustments are often made to procedures to lower the fetal absorbed dose even further.9 This should only be done if image quality is not compromised. In addition to technical aspects of the radiation procedure, e.g. type of procedure, the site and radiation dose administered, maternal factors will influence the fetal absorbed dose. During X-rays and CT scanning, the ‘thickness’ of the mother, which alters as pregnancy progresses, will influence the penetration of the dose and hence the fetal absorbed dose. The use of appropriate shielding will significantly reduce the fetal absorbed dose as well as the dose to adjacent maternal tissues.
Table 2.
Estimated fetal dose from common imaging procedures.
| Fetal dose range (mGy) | Examples of procedures |
|---|---|
| 0.001–1.0 | X-rays: beyond 10 cm of uterus including head, chest, breast, teeth, extremitiesX-rays: abdomen, pelvis, hippulmonary angiogramCT: head or neck, pelvimetry, chest, computed tomography pulmonary angiogram (CTPA)Nuclear scan: Lung ventilation, liver/spleen, ventilation/perfusion (V/Q) |
| 1.1–10 | X-rays: Barium enema, intravenous pyelography, lumbar spineCT: lumbar spine, abdomen, coronary artery angiographyNuclear scan: renal, white cell, bone, cardiac. |
| 11–50 | CT: pelvis, pelvis and abdomen, pelvis and abdomen and chestNuclear scan: PET/CT whole body scan, myocardial perfusion |
In the case of nuclear medicine studies, fetal absorbed dose will represent the cumulative effect of external irradiation from the maternal tissues as well as placental transfer and fetal uptake of radiopharmaceuticals. For radioisotopes that are excreted in urine, radiation from bladder contents or the placenta will have a greater impact in early pregnancy compared with later pregnancy. In general, the whole body fetal absorbed dose from nuclear scanning studies tends to decrease throughout pregnancy.11 By using smaller administered doses and longer imaging times, the fetal absorbed dose may be further reduced, but there is concern that this may compromise the value of the study. Although a reduction of the injected dose can be compensated for by increasing the scanning time, if the acquisition time is longer patients are more likely to move or ask to interrupt the scan to void the bladder (not a rare occurrence in pregnant women).12 Strategies to aid excretion of radiopharmaceuticals, e.g. ensuring prompt and complete bladder emptying will reduce the fetal absorbed dose without compromising image quality and should be encouraged.
Nuclear scanning has been enhanced by the addition of hybrid single photon emission computed tomography (SPECT) which allows simultaneous acquisition of combined multi-modality imaging, with seamless fusion of three-dimensional volume datasets. The application of SPECT provides enhanced information with simultaneous imaging of function and anatomical localisation. The fetal radiation dose from such procedures will be greater than from any single modality but the additional information may be critical.
Risks of ionising radiation
A number of national and international bodies have issued guidelines or policy documents which indicate a negligible risk to the fetus for radiation doses below 50 mGy, well below the dose from any single diagnostic imaging event although cumulative studies could lead to such a dose.4–6 The effects of radiation exposure in pregnancy depend on the time of exposure as well as the fetal absorbed dose. Until the placenta implants, the cells of the conceptus are hypoxic and therefore less radiosensitive. In the very early embryo, the effect of radiation is more likely to be failure to implant or undetectable death of the embryo, i.e. an all or none phenomenon.13
Radiation effects may be classified as either deterministic, also called tissue reactions, or stochastic. Cell killing, leading to fetal death, gross malformation, developmental abnormalities or growth retardation is a deterministic event, i.e. there is a threshold dose below which no effect is seen and the higher the dose the greater the effect. In comparison, malignancy and hereditary abnormalities are stochastic effects, i.e. the absorbed dose influences the probability but not the severity of the effect. Both may be relevant when imaging in pregnancy although the evidence for stochastic effects is scant and inconsistent.14
Experimental assessment of very high dose radiation has identified seven specific areas of potential concern to the pregnant woman and her fetus.
Lethality
Genetic damage/epigenetic change
Teratogenicity
Growth impairment
Sterility
Oncogenicity
With regard to potential fetal dosage from individual diagnostic radiation procedures, there is no evidence for any increase in lethality (miscarriage or stillbirth), teratogenicity, genetic damage/epigenetic changes, growth impairment, mental retardation or sterility (Table 3).
Table 3.
| Gestation (weeks) | Effect | Estimated threshold (mGy) |
|---|---|---|
| 2–4 | Miscarriage or no effect | >50–100 |
| 4–10 | Congenital anomalies: skeleton, eyes, genitals Intrauterine growth restriction | 200 |
| 10–17 | Microcephaly Intellectual impairment | >200 |
| >18 | Intellectual impairment | >250 |
Oncogenicity
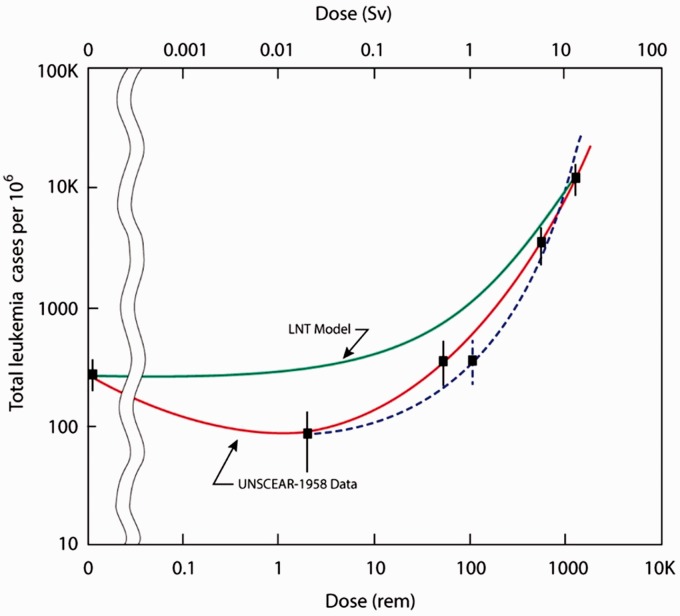
It has generally been assumed that the relationships observed between radiation dose and adverse effects at high levels of radiation exposure also apply (proportionally) to low levels: the linear no-threshold (LNT) hypothesis. This concept remains a source of controversy amongst experts with the International Commission on Radiological Protection and the World Health Organisation supporting the theory whilst the World Nuclear Association and the United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) argue that there is compelling biologic and epidemiologic data to refute the LNT modelling of radiation risk. The LNT model does not account for proven biological adaptive responses that change the shape of the LNT dose–response line in the low dose range (Figure 1) including antioxidant production, apoptosis, immune system-mediated effects and repair of DNA double-strand breaks. All biologic and experimental data suggest the existence of practical thresholds for carcinogenesis. This concept means that below the dose threshold, the carcinogenic risk, if it exists, is so small that it is without clinical importance. Cuttler18 has used the leukaemia incidence data in Hiroshima survivors to compare the estimated (based on LNT) versus actual incidence (gathered by UNSCEAR) to illustrate the discrepancies (Figure 1).
Figure 1.
Actual leukaemia incidence (UNSCEAR data) in the Hiroshima survivors for 1950–57. Graphic extrapolation based on estimated dose (±standard error) compared with LNT model. The dotted line represents a re-estimate of the doses because many of these individuals had features of radiation complications indicating a potential underestimation of their dose based on distance alone. Source: Reproduced from Cuttler18 under CC-BY-NC license.
A large number of epidemiological studies have been performed to assess the possible effects of prenatal radiation on the incidence of malignant disease. All are flawed by problems such as retrospectivity, small study size, inadequate or inappropriate case and control selection, and variability in the determination of radiation exposure and measurement of outcome parameters. The studies in which prenatal radiation exposure have been associated with an increased incidence of malignancy have found a relative risk ratio of all cancer of 1.5–2.4.19–21 However, a number of other epidemiological studies have failed to establish any statistically significant association between prenatal exposure to radiation and childhood malignancy.22–24 At least part of the increased risk associated with irradiation could be accounted for by the fact that mothers with a higher incidence of illness during pregnancy (a susceptibility which might be associated with an increased risk of tumour in their offspring) had a greater incidence of exposure to diagnostic radiation. The National Radiological Protection Board has adopted an estimated additional risk (EAR) co-efficient for cancer incidence under 15 years of age following low-dose irradiation in utero of 0.006% per mGy compared with a risk of 0.0018% per mGy for a dose received just after birth. Estimates for the extra risk of childhood cancer from a fetal radiation dose of 1000 mGy range from 0.022 (Oxford Survey Childhood Cancer) through to 0.060.25,26 Given a baseline risk of childhood cancer of between 1.0 and 2.5 per 1000, Table 4 gives estimates of risk comparing a low risk versus the high risk modelling based on available data.27
Table 4.
Estimates of cancer risk from fetal radiation in pregnancy – comparing a low risk versus high risk model.27
| Fetal dose (mGy) | Low risk model | High risk model |
|---|---|---|
| 10 | 1 in 4545 | 1 in 1667 |
| 20 | 1 in 2272 | 1 in 834 |
| 30 | 1 in 1515 | 1 in 556 |
| 40 | 1 in 1136 | 1 in 417 |
| 50 | 1 in 909 | 1 in 334 |
Perhaps, a simpler statistic is that quoted by Tirada et al.9 of an EAR of cancer following a 50 mGy in utero exposure of 1.1–3.0 events per 1000 exposures. The data regarding the risk at different gestations and on various systems and organs are imprecise with wide confidence intervals (CIs) owing to the low doses and the limited sample sizes in available studies.17
Radioiodine and its effects on the fetal thyroid
Nuclear scanning of the thyroid is generally avoided in pregnancy; however, inadvertent scans do occur in early pregnancy and the following data are helpful when counselling such women.28 Table 5 describes the estimated fetal radiation dose from thyroid nuclear scans based on current dosimetry assessment.11
Table 5.
Estimated fetal radiation dose from thyroid scanning at different stages of pregnancy.11
| Radiopharmaceutical | Fetal dose (mGy) at three months | Fetal dose (mGy) at six months | Fetal dose (mGy) at nine months |
|---|---|---|---|
| 99mTc | 5.52 | 1.17 | 0.66 |
| 131I | 0.31a | 0.7a | 0.51a |
aIn the case of 131I, the fetal thyroid dose is significantly larger than this tabulated fetal dose.
The special case of radioiodine administered for treatment of thyrotoxicosis or thyroid cancer requires specific mention. 131I given for therapeutic rather than diagnostic purposes crosses the placenta readily and the fetal thyroid begins to accumulate iodine from about the end of the first trimester. In early pregnancy, the major risk is from external gamma radiation from the maternal bladder whilst after 12 weeks, fetal and/or placental uptake increases and the fetal thyroid becomes functional leading to accumulation of tracer. Hence, the cumulative fetal thyroid dose in later pregnancy is much greater than the dose to the rest of the fetus.10,29 If therapeutic doses of radio-iodine are inadvertently given to a pregnant woman with thyrotoxicosis, there is a significant risk of fetal thyroid damage after 12 weeks’ gestation. Inadvertent use of radioiodine prior to 12 weeks is unlikely to damage the fetal thyroid.30 If pregnancy is confirmed shortly after dose administration, maternal hydration and frequent voiding should be encouraged and potassium iodide may be given as a thyroid-blocking agent. If therapeutic 131I is administered to a woman of childbearing age, a minimum period of six months before conceiving is recommended by some experts.28 However, this is based on an intention to achieve stable thyroid function post thyroid ablation, rather than any risk of radiation or iodine damage to the fetal thyroid. From a radiation safety point of view, conception should be delayed until the potential fetal dose from residual radioiodine is less than 1 mGy. A number of observational studies have demonstrated no impact on fertility, pregnancy or neonatal outcome after radioiodine therapy,31–33 and termination should not be recommended even if the period to conception was less than six months as the risk of harm is low.
Maternal risks of radiation in pregnancy and postpartum
The main area of concern both during pregnancy and lactation has been the effects of radiation on breast tissue. This is particularly relevant with regard to investigation of suspected pulmonary embolism where there is a great deal of debate about CTPA versus V/Q scanning.34–40 The fetal radiation dose varies with gestation but is below 1 mGy with both CTPA and V/Q, but the maternal breast dose with CTPA is 10–70 mGy, significantly greater than with V/Q (<1.5). In a recent paper, Isidoro et al.41 published estimations of radiation dose to both the fetus and maternal breast at varying gestations (Table 6). The use of bismuth breast shields or modulation of the CT technique can reduce this dose but may impair image quality. The lung dose from either study is similar at 7–14 mGy.
Table 6.
Estimated absorbed dose (mGy) for maternal breast and fetus from CT and nuclear imaging for investigation of suspected pulmonary embolism.41
| Organ | CTPA non-contrast | CTPA contrast enhanced | Perfusion (Q) SPECT scan only | V/Q SPECT | |
|---|---|---|---|---|---|
| Estimated maternal breast dose | 12 | 22 | 0.25 | 0.50 | |
| Estimated fetal absorbed dose by gestation | |||||
| Early | 0.16 | 0.21 | 0.14 | 0.18 | |
| Three months | 0.20 | 0.28 | 0.20 | 0.25 | |
| Six months | 0.43 | 0.73 | 0.25 | 0.31 | |
| Nine months | 0.42 | 0.57 | 0.20 | 0.25 | |
CTPA: computed tomography pulmonary angiogram; SPECT: single photon emission computed tomography.
Technical factors caused by pregnancy, such as hyperdynamic circulation and hemodilution, can affect contrast opacification in CTPA. Poor lung expansion and breath-holding secondary to the gravid uterus can also affect the CTPA. Other imaging options include CXR with perfusion scanning (Q) only or magnetic resonance angiography (MRA). The latter has lower diagnostic yield but no radiation.
Previous assessments have estimated the lifetime risk of breast cancer from a dose of 20 mGy to the breast is approximately 1/1200 at age 20, 1/2000 at age 30 and 1/3500 at age 40.40,42 From these estimates, the additional breast cancer risk from CTPA in a 30-year-old woman is estimated as 1/2000 compared with 1/40,000 for a V/Q scan. This figure is not very high, but studies have suggested that this rate is seven times higher in the pregnant woman and hence should be considered in the diagnostic algorithm.43,44 In breastfeeding women, only tiny amounts of iodinated or gadolinium-based contrast medium given to a lactating mother are excreted into breast milk, and only a minute proportion enters the baby’s gut. Therefore, after CTPA or MRA it is not necessary to discard breast milk.
After a V/Q scan, only very tiny amounts of radioactivity can be detected in breast milk.45 Interruption of breastfeeding for 12 hours is recommended but milk pumped during the waiting period can usually be stored and used later, with the storage duration dependent on the radioactive isotope’s half-life.46,47
In addition to the fetal radiation dose, a number of other factors may determine the choice between CTPA and V/Q scanning including sensitivity, specificity, rate of inconclusive studies, the availability of each investigation, the hemodynamic stability of the woman, the presence or absence of a likely alternative diagnosis and the presence or absence of deep venous thrombosis. If multiple investigations are performed, a cumulative fetal and maternal breast radiation dose should be calculated.
Contrast agents
A number of X-ray and CT procedures require the administration of iodine-containing contrast agents. There are no reports of teratogenesis from iodinated contrast agents but the amount of inorganic iodine available to interfere with thyroid metabolism is about 0.1% of the dose administered. Non-ionic contrast agents have been shown to cross the placenta and inhibit Type II and III deiodinases which can reduce intracellular triiodothyronine, the levels of which directly affects transcription of many genes that are important in fetal development.48 In addition, depending on the dose of iodine, there is a theoretical risk of fetal thyroid blockade although to date this has not been reported.49
The use of gadolinium, although not teratogenic, has generated concern as gadolinium has been shown to cross the placental barrier and will remain in the amniotic fluid indefinitely. There are concerns that the gadolinium ion may dissociate from its chelate molecule leading to possible adverse effects such as neurotoxicity.50 A recent large retrospective study of first trimester exposure with gadolinium (n = 397) identified a slight increase in stillbirths or neonatal death with an adjusted relative risk of 3.70 (95% CI 1.55–8.85) although this may be related to the condition necessitating the MRI rather than the procedure.3 There was a significant increase in a composite outcome of rheumatological/inflammatory/infiltrative skin conditions (adjusted hazard ratio for all MRI exposure in first trimester 1.36 95% CI 1.09–1.69) but there was no increase in connective tissue or skin disease resembling nephrogenic systemic fibrosis, a syndrome associated with gadolinium exposure outside of pregnancy. These outcomes were not increased in MRI without gadolinium but adverse events were all rare. Expert bodies have concluded that the effects of MRI contrast agents remain unknown and may be harmful. There is no requirement to cease breastfeeding following the administration of gadolinium to a lactating woman as it has low lipid solubility with less than 0.04% measurable in breast milk.4
Contrast-enhanced ultrasound is a technique combining traditional ultrasound with contrast agents such as small air bubbles or gas-filled microbubbles to enhance echogenicity in order to more accurately image blood flow, e.g. for assessment of septal defects in the heart. Although the European Federation of Societies for Ultrasound in Medicine and Biology have recommended against its use because of limited experience, small studies in human pregnancy have demonstrated no harm.51,52 After a stroke or transient ischaemic event, the presence or absence of a right–left cardiac shunt may influence management and should not be withheld because of pregnancy.
Counselling and informed consent
Counselling and obtaining informed consent for imaging that involves radiation requires the clinician to communicate with the woman and her family a realistic estimate of the potential radiation dose to herself and her fetus, to describe and quantitate the risks of this estimated dose, to outline the benefits of the imaging procedure and to respond to any questions or concerns. All allied staff must also be well informed to ensure the patient receives a consistent message about the risks and benefits of the proposed test(s).
For practical purposes, no specific counselling is required for women undergoing diagnostic imaging with a predicted fetal absorbed dose of less than 10 mGy. This includes all X-ray and CT scanning not involving the abdomen and most nuclear scans. For direct exposures or nuclear scanning with a potential exposure greater than 10 mGy, the women should be counselled on a risk/benefit basis. Whilst there is an ongoing debate in the literature regarding the appropriateness of applying a LNT model for counselling, all current guidelines use such a model in their recommendations. If there is any specific risk, it is limited to childhood malignancy, but, as described above, for each 10 mGy exposure, theoretical projections based on a LNT assumption indicate a likely maximum risk of one additional case of childhood cancer (not death) per 1700 exposures of 10 mGy.17 This must be balanced against the benefit of the imaging or treatment in terms of management of the maternal condition.
Conclusion
Audit data from the United States have shown that imaging in the pregnant population is increasing in both the number of examinations performed and the number of patients being imaged, with the greatest increase being CT scans.53 When the mother’s condition necessitates diagnostic radiation it is necessary to balance the risks of the procedure with the benefits to be gained. As almost all diagnostic imaging involves doses below the 50 mGy threshold, clinically indicated investigations should not be withheld because of concerns regarding fetal radiation exposure. In all cases it is essential that the radiologist, radiographer or nuclear physician be informed that the woman is pregnant so that they may make appropriate provisions. If direct pelvic or abdominal radiation is considered necessary during pregnancy, the woman should be counselled regarding the benefits and risks of the procedure stressing the very low incidence of complications and the importance of the information to be derived. The ‘as low as reasonably possible’ principle should apply to both patients and occupational exposures to minimise radiation exposure at all times.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical approval
Not applicable
Informed consent
Not applicable
Guarantor
SL
Contributorship
SL is the sole author.
References
- 1.Webb JA, Thomsen HS, Morcos SK. The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 2005; 15: 1234–1240. [DOI] [PubMed] [Google Scholar]
- 2.Kanal E, Barkovich AJ, Bell C, et al. ACR guidance document for safe MR practices: 2007. AJR Am J Roentgenol 2007; 188: 1447–1474. [DOI] [PubMed] [Google Scholar]
- 3.Ray JG, Vermeulen MJ, Bharatha A, et al. Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 2016; 316: 952–961. [DOI] [PubMed] [Google Scholar]
- 4.ACOG. Committee Opinion No. 723 summary: guidelines for diagnostic imaging during pregnancy and lactation. Obstet Gynecol 2017; 130: 933–934. [DOI] [PubMed] [Google Scholar]
- 5.Wall BF, Meara JR, Muirhead CR, et al. Protection of pregnant patients during diagnostic medical exposures to ionising radiation. London: Health Protection Agency, The Royal College of Radiologists and the College of Radiographers, 2009, pp.1–24, https://www.rcr.ac.uk/system/files/publication/field_publication_files/HPA_preg_2nd.pdf (accessed 1 September 2018).
- 6.Rowan M. A new national pregnancy policy template. Phys Med 2018; 52: 169. [Google Scholar]
- 7.World Nuclear Association. Nuclear radiation and health effects, 2016, http://www.world-nuclear.org/information-library/safety-and-security/radiation-and-health/nuclear-radiation-and-health-effects.aspx (accessed 1 September 2018).
- 8.Environmental Protection Agency. Radiation sources and doses, 2018, https://www.epa.gov/radiation/radiation-sources-and-doses (accessed 6 February 2018).
- 9.Tirada N, Dreizin D, Khati NJ, et al. Imaging pregnant and lactating patients. Radiographics 2015; 35: 1751–1765. [DOI] [PubMed] [Google Scholar]
- 10.Stabin MG. Doses from medical radiation sources, 2013, http://hps.org/hpspublications/articles/dosesfrommedicalradiation.html (accessed 1 September 2018).
- 11.Motavalli LR, Hakimabad HM, Azghadi EH. Fetal and maternal dose assessment for diagnostic scans during pregnancy. Phys Med Biol 2016; 61: 3596–3608. [DOI] [PubMed] [Google Scholar]
- 12.Zanotti-Fregonara P, Hindie E. Performing nuclear medicine examinations in pregnant women. Phys Med 2017; 43: 159–164. [DOI] [PubMed] [Google Scholar]
- 13.American College of Radiology. ACR-SPR practice parameter for imaging pregnant or potentially pregnant adolescents and women with ionizing radiation, 2013, https://www.acr.org/-/media/ACR/Files/Practice-Parameters/Pregnant-Pts.pdf (accessed 1 September 2018).
- 14.National Research Council. Health risks from exposure to low levels of ionizing radiation: BEIR VII phase 2. Washington, DC: National Academies Press, 2006. [PubMed] [Google Scholar]
- 15.Valentin J. The 2007 recommendations of the International Commission on Radiological Protection. Oxford: Elsevier, 2007, pp.1–333. [Google Scholar]
- 16.Tharmalingam S, Sreetharan S, Kulesza AV, et al. Low-dose ionizing radiation exposure, oxidative stress and epigenetic programing of health and disease. Radiat Res 2017; 188: 525–538. [DOI] [PubMed] [Google Scholar]
- 17.Valentin J. Biological effects after prenatal irradiation (embryo and fetus): ICRP Publication 90 approved by the Commission in October 2002. Ann ICRP 2003; 33: 1–206. [PubMed] [Google Scholar]
- 18.Cuttler J. Remedy for radiation fear – discard the politicized science. Dose Response 2014; 12: 170–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stewart A, Webb J, Giles D, et al. Malignant disease in childhood and diagnostic irradiation in utero. Lancet 1956; 268: 447. [DOI] [PubMed] [Google Scholar]
- 20.Stewart A, Webb J, Hewitt DJ. A survey of childhood malignancies. Br Med J 1958; 1: 1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacMahon B. Prenatal X-ray exposure and childhood cancer. J Natl Cancer Inst 1962; 28: 1173–1191. [PubMed] [Google Scholar]
- 22.Court Brown W, Doll R, Hill AB. Incidence of Leukaemia after to diagnostic radiation in utero. Br Med J 1960; 2: 1539–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Polhemus DW, Koch R. Leukemia exposure and medical radiation. Paediatrics 1959; 23: 453–461. [PubMed] [Google Scholar]
- 24.Naumburg E, Bellocco R, Cnattingius S, et al. Intrauterine exposure to diagnostic X rays and risk of childhood leukemia subtypes. Radiat Res 2001; 156: 718–723. [DOI] [PubMed] [Google Scholar]
- 25.Doll R, Wakeford R. Risk of childhood cancer from fetal irradiation. Br J Radiol 1997; 70: 130–139. [DOI] [PubMed] [Google Scholar]
- 26.Ratnapalan S, Bentur Y, Koren G. Doctor, will that x-ray harm my unborn child? Can Med Assoc J 2008; 179: 1293–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coakley FV, Cody DD, Mahesh M. The pregnant patient: alternatives to CT and dose-saving modifications to CT technique, https://www.imagewisely.org/imaging-modalities/computed-tomography/imaging-physicians/articles/the-pregnant-patient (2010, accessed 1 September 2018).
- 28.Alexander EK, Pearce EN, Brent GA, et al. 2017 Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and the postpartum. Thyroid 2017; 27: 315–389. [DOI] [PubMed] [Google Scholar]
- 29.Watson E. Radiation absorbed dose to the human fetal thyroid. In: Fifth international radiopharmaceutical dosimetry symposium, 1992. Oak Ridge, TN: Oak Ridge Associated Universities.
- 30.Stagnaro-Green A, Abalovich M, Alexander E, et al. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum. Thyroid 2011; 21: 1081–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sawka AM, Lakra DC, Lea J, et al. A systematic review examining the effects of therapeutic radioactive iodine on ovarian function and future pregnancy in female thyroid cancer survivors. Clin Endocrinol 2008; 69: 479–490. [DOI] [PubMed] [Google Scholar]
- 32.Garsi JP, Schlumberger M, Rubino C, et al. Therapeutic administration of 131I for differentiated thyroid cancer: radiation dose to ovaries and outcome of pregnancies. J Nucl Med 2008; 49: 845–852. [DOI] [PubMed] [Google Scholar]
- 33.Anderson C, Engel SM, Weaver MA, et al. Birth rates after radioactive iodine treatment for differentiated thyroid cancer. Int J Cancer 2017; 141: 2291–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zamorano J, Achenbach S, Baumgartner H, et al. ESC guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; 35: 3033–3073.25173341 [Google Scholar]
- 35.Wan T, Skeith L, Karovitch A. Guidance for the diagnosis of pulmonary embolism during pregnancy: consensus and controversies. Thromb Res 2017; 157: 23–28. [DOI] [PubMed] [Google Scholar]
- 36.Tromeur C, van der Pol LM, Klok FA, et al. Pitfalls in the diagnostic management of pulmonary embolism in pregnancy. Thromb Res 2017; 151: S86–S91. [DOI] [PubMed] [Google Scholar]
- 37.Papadakis GZ, Karantanas AH, Perisinakis K. Pulmonary embolism diagnostics of pregnant patients: what is the recommended clinical pathway considering the clinical value and associated radiation risks of available imaging tests? Phys Med 2017; 43: 178–185. [DOI] [PubMed] [Google Scholar]
- 38.McLintock C, Brighton T, Chunilal S, et al. Recommendations for the diagnosis and treatment of deep venous thrombosis and pulmonary embolism in pregnancy and the postpartum period. ANZJOG 2012; 52: 14–22. [DOI] [PubMed] [Google Scholar]
- 39.Leung AN, Bull TM, Jaeschke R, et al. An official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline: evaluation of suspected pulmonary embolism in pregnancy. Am J Respir Crit Care Med 2011; 184: 1200–1208. [DOI] [PubMed] [Google Scholar]
- 40.Schembri GP, Miller AE, Smart R. Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin Nucl Med 2010; 40: 442–454. [DOI] [PubMed] [Google Scholar]
- 41.Isidoro J, Gil P, Costa G, et al. Radiation dose comparison between V/P-SPECT and CT-angiography in the diagnosis of pulmonary embolism. Phys Med 2017; 41: 93–96. [DOI] [PubMed] [Google Scholar]
- 42.US National Academy of Sciences, National Research Council, Committee to Assess Health Risks: from exposure to low levels of ionizing radiation. Health risks from exposure to low levels of ionizing radiation: BEIR VII Phase 2 Washington, DC: National Academies Press, 2006. [PubMed]
- 43.Chen J, Lee RJ, Tsodikov A, et al. Does radiotherapy around the time of pregnancy for Hodgkin’s disease modify the risk of breast cancer? Int J Radiat Oncol Biol Phys 2004; 58: 1474–1479. [DOI] [PubMed] [Google Scholar]
- 44.de Gelder R, Draisma G, Heijnsdijk EA, et al. Population-based mammography screening below age 50: balancing radiation-induced vs prevented breast cancer deaths. Br J Cancer 2011; 104: 1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leide-Svegborn S, Ahlgren L, Johansson L, et al. Excretion of radionuclides in human breast milk after nuclear medicine examinations. Biokinetic and dosimetric data and recommendations on breastfeeding interruption. Eur J Nucl Med Mol Imaging 2016; 43: 808–821. [DOI] [PubMed] [Google Scholar]
- 46.Administration of Radioactive Substances Advisory Committee. Notes for guidance on the clinical administration of radiopharmaceuticals and the use of sealed radioactive sources Oxfordshire: Health Protection Agency, 2006. [PubMed]
- 47.Anderson PO. Radiopharmaceuticals. Breastfeed Med 2016; 11: 216–217. [DOI] [PubMed] [Google Scholar]
- 48.Agrawal NK. Maternal-fetal thyroid interactions. Thyroid hormone. Chapter 5. London: InTech Open Access Publisher, 2012, pp.125–156.
- 49.Atwell TD, Lteif AN, Brown DL, et al. Neonatal thyroid function after administration of IV iodinated contrast agent to 21 pregnant patients. AJR Am J Roentgenol 2008; 191: 268–271. [DOI] [PubMed] [Google Scholar]
- 50.Hui FK, Mullins M. Persistence of gadolinium contrast enhancement in CSF: a possible harbinger of gadolinium neurotoxicity? AJNR Am J Neuroradiol 2009; 30: E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piscaglia F, Nolsøe C, Dietrich CA, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012; 33: 33–59. [DOI] [PubMed] [Google Scholar]
- 52.Murotsuki J. Contrast-enhanced ultrasound in obstetrics and gynecology. Donald School J Ultrasound Obstet Gynecol 2007; 1: 19 [Google Scholar]
- 53.Lazarus E, DeBenedectis C, North D, et al. Utilization of imaging in pregnant patients: 10-year review of 5270 examinations in 3285 patients – 1997–2006. Radiology 2009; 251: 517–524. [DOI] [PubMed] [Google Scholar]