Abstract
Objective: The aim of the present study was to evaluate the effect of tissue‐engineered constructs on repair of large segmental bone defects in goats.
Methods: Allogenic demineralized bone matrix (aDBM) was seeded with autologous marrow stromal cells (aMSC) for seven days to construct DBM–MSC grafts prior to implantation. 24 goats were randomly divided into three groups (eight in each). In each group, 3 cm diaphyseal femoral defects were created unilaterally, and subsequently filled with the DBM‐MSC grafts, DBM alone and an untreated control, respectively. Radiological analysis and biomechanical evaluation were performed at 12 and 24 weeks after operation.
Results: Obvious increases in radiological scoring and biomechanical strength were found in the DBM‐MSC group when compared to the DBM group. X‐ray examination showed excellent bone healing in the DBM‐MSC group, whereas only partial bone repair was seen in the DBM group, and no healing in untreated controls. Histologically, a tendency to bone regeneration and remodeling was far more obvious for the DBM‐MSC group than the DBM only and untreated controls.
Conclusion: Our results strongly suggest that transplantation of bone MSC within a DBM could have advantages for the bone repair of large segmental defects.
Keywords: Bone matrix, Stem cells, Tissue engineering
Introduction
Large segmental bone defects are still a very serious problem in clinical practice, for when a significant quantity of bone is lost the periosteum, (the source of bone progenitors), is often severely damaged. To achieve the mechanical strength required by load bearing bone is then rather difficult. Autologous bone grafts are currently considered the gold standard for bone tissue repair, but their availability is limited. Moreover, there are often complications of morbidity at the donor sites. Furthermore, devitalization of the transplant due to resorption processes usually leads to reduced mechanical stability 1 . In order to overcome the problems associated with autologous bone grafting extensive research has been conducted, with continued interest in the field of synthetic bone grafts (such as ceramics and biopolymers) in recent decades. However synthetic materials lack osteoinductivity.
Bone tissue engineering, which combines osteogenic cells with osteoconductive scaffolds to enhance the process of bone reconstitution and remodeling, provides an innovative approach to the regeneration of bone. In this field, attempts have been made to focus primarily on seeded cells, suitable scaffolds, and the synergistic interaction of these with each other.
Autologous marrow stromal cells (aMSC) have a high proliferation and osteochondral differentiation potential 2 , are non‐immunogenic and easy to obtain and handle, and are therefore the best choice as seeded cells. It has been demonstrated that pro‐seeding of bone marrow stromal cells onto suitable matrices prior to implantation can improve the healing process of large bone defects 3 .
For bone tissue engineering, ideal scaffolds must be biocompatible, osteoconductive, resorbable, and provide temporary mechanical strength and design flexibility for defined indications as well as a bio‐signal for cell differentiation 4 .
A demineralized bone matrix (DBM), which can induce bone formation while gradually being degraded and finally replaced by new bone, has been applied as a good bone substitute in clinical practice since 1967 5 . In addition, as a three‐dimensional structure, DBM provides an excellent environment for cell attachment, proliferation, osteogenic differentiation, and bone regeneration 6 . Although DBM has insufficient strength for the repair of bearing bone, a suitable internal or external fixator can counteract this shortage.
DBM seeded with bone marrow cells have been reported to accelerate the healing of critical size bone defects in a small animal model (rat) 7 , but its effect remains unclear for the healing of large segmental bone defects in a large mammalian animal model (such as goat, horse, pig). Such testing is a necessary step in transferring experimental research results to clinical application. Undoubtedly, tests in a large mammalian animal model (such as goat) can mimic the clinical situation better. Meanwhile, we have also found that extra culture of the tissue‐engineered constructs in vitro can dramatically increase the survival rate of MSC after the tissue‐engineered constructs are transplanted into models, which is beneficial to the repair of bone defect.
In the present study, segmental bone defects in goat femurs were repaired by tissue‐engineered bone in order to determine the effect and feasibility of such a tissue engineering technique. First, autologous goat MSC were seeded into an allogenic DBM which was cultured for seven days in vitro to become mature prior to implantation in vivo. Then 3 cm defects in the goat femurs were made and filled with the complex of cells/scaffolds. Samples were examined at 12 and 24 weeks postoperatively. Our results show that transplantation of bone MSC within demineralized bone matrix strongly encourages the repair of large segmental bone defects.
Materials and methods
Isolation and cultivation of bone MSC
Marrow aspirates of goats harvested from the iliac crest, as described by Pittenger et al. 2 , were washed in DMEM/ F12 (Hyclone, Logan, UT, USA) with heparin (approx. 200 units/ml final) and centrifuged for 5 min at 900 g. The fat layer and supernatant were removed and the washed cells centrifuged over a Percoll gradient of 1.073 g/ml (Phamacia Biotech, Uppsala, Sweden) for 30 min at 900 g. The isolated mononuclear cells from the density interface were plated at 5 × 105 cells/cm2 in culture flasks with primary media Dulbecco's modified Eagle's medium (DMEM) Ham's F‐12 supplemented by 15% fetal bovine serum (Hyclone), 100 IU/ml penicillin and 100 µg/ml streptomycin). The cell number and viability were determined by cell counts using a hemocytometer and Trypan blue exclusion. The cell viability was above 90%. The medium was changed after the first three days to remove nonadherent cells: most adherent cells should be marrow stromal cells (MSC) 2 . Subsequently, the medium was replaced every two days. Cells were harvested at the confluence with 0.25% trypsin (Gibco, Karlsruhe, Germany), counted, and replanted at 1 × 104 cells/cm2 in culture flasks with osteogenic conditional media (DMEM/F‐12 supplemented by 10% fetal bovine serum, 100 IU/ml penicillin, 100 µg/ml streptomycin, 10 mM of sodium β‐glycerophosphate, 50 mg/L of vitamin C and 1 × 10−8 M of dexamethasone [Sigma‐Aldrich, St Louis, MO, USA]). The cell cultures were maintained in a humidified atmosphere of 95% air with 5% CO2 at 37°C. The passage‐2 cells were analyzed and seeded on the matrices.
MSC were then cultured on glass sections in osteogenic media for two weeks, after which sections were prepared for alkaline phosphatase and von Kossa staining.
Matrix loading
A goat DBM was fabricated according to the method described by Glowacki & Mulliken 8 . Totally demineralized bone matrix were harvested and shaped into a sheet (10 mm × 10 mm × 2 mm) by a section cutter. The passage‐2 MSC were concentrated by a centrifuge at 900 g for 5 min at room temperature, resulting in 1 × 106 cells per milliliter. The DBM sheets were soaked in cell suspension for 4 h in a CO2 incubator and then transferred into a 6‐well plate for subculturing. Each sheet was subcultured in one well with 4 ml of the culture medium. All carriers were cultured in conditional media for 7 days before implantation.
Surgical procedure
This study was approved by the Animal Experiment Committee of The Third Army Medical University. Twenty‐four adult goats, aged 11.5 to 13.6 months with weights ranging from 19.6 to 25.2 kg, were included in this study, and were randomly divided into three groups (eight in each group). Animals were operated on under general anesthesia with 0.1 ml/kg Sumianxin (Changchun Munition University, Changchun, China), which was given intramuscularly 5 min preoperatively. Under aseptic conditions, one femur of each goat was exposed through a straight incision. A 3 cm (approx. 20% of the mean femur length) segmental defect together with periosteum, centered between the innermost interlocking holes of the intramedullary nail, was created by performing two osteotomies with an oscillating saw under constant cooling with saline. An intramedullary nail was introduced from the trochanteric fossa of the femur. Two screws locked the nail proximally, and two distally. The defects were filled with DBM‐MSC complex in group I, with DBM only in group II, and were not treated in group III (n= 8 in each group). The wound was closed in layers. Eight × 105 IU penicillin was injected intramuscularly twice daily postoperatively for three days. During this study, one goat which sustained a deep infection was excluded from further analysis and replaced by a new one. All other animals were handled according to the standard procedures for laboratory animals. Four goats from each group were killed for examination at 12 and 24 weeks, respectively.
Scanning Electron Microscopy (SEM) of the DBM/MSC complex
After seven days of co‐culturing, the complex of cells and scaffolds was fixed with 2.5% glutaraldehyde in a 0.1 M cacodylate buffer for 1 h at 41°C and post‐fixed with 1% osmium tetroxide in veronal buffer. They were then dehydrated in a graded ethanol series, critical point dried with CO2, and gold sputter‐coated for examination using an AMRAY100B SEM (Amray, Bedford, MA, USA).
X‐ray analysis of bone regeneration
Anteroposterior and lateral radiographs of the femur were taken immediately after surgery and at 12 and 24 week postoperatively in order to evaluate the position of the nail, locking screws, and graft material. The exposure conditions were 63 kV, 16 ms, 6.3 mAs. The exposure distance was 1 m. Contact radiographs were taken of the explanted femurs, which were stripped of all soft tissue, for a semi‐quantitative evaluation of radiographs with a modified score according to Salkeld et al. 9 (Table 1).
Table 1.
Radiographic grading score according to Salkeld et al. 9
| Description | Score |
|---|---|
| No change from immediate postoperative appearance | 0 |
| A slight increase in radiodensity | 1 |
| Recognizable increase in radiodensity | 2 |
| Bridging of at least one cortex with material of nonuniform radiodensity | 3 |
| Defect bridged on both medial and lateral sides with bone of uniform radiodensity, cut ends of the cortex still visible | 4 |
| Defect bridged by uniform new bone, with at least one of four cortices obscured by new bone | 5 |
| Defect replaced by uniform new cortex completely | 6 |
Biomechanical testing
Immediately after explantation, a torsional test to failure was performed on both femurs of each animal, using a multifunctional mechanical testing machine to torque the proximal bone end at 0.5 mm/s to failure. Torque was continuously measured at the fixed distal bone end. Torque‐angular displacement curves were generated, from which the torsional strength (torque at failure) was calculated. The end of each experimental femur was embedded in dental plaster in such a way that the non‐inserted portion comprised the 3 cm defect and 1 cm of the diaphysis proximal and distal to it. All holes of the locking bolts were embedded to prevent failure through these holes during the test. The non‐inserted portion in the contralateral intact femur consisted of an identical 5 cm segment of shaft, located at the same level. To reduce errors associated with geometric or size differences among animals in different groups, torsional strength was expressed as a percentage of the value of the contralateral intact femur. Nonunion was defined as a bone defect with a torsional strength of less than 20% of that of the contralateral intact bone 10 . Below the 20% level, the torque–angular displacement curves showed a slow nonlinear increase in torque, indicating plastic deformation, which is consistent with the presence of a fibrous nonunion.
Histological analysis of newly formed bone
After the biomechanical test, the newly formed bone tissue in the area of the defect was sectioned in a small bone block, and the central surface analyzed for histology and histomorphometry according to the procedures described below. Samples were fixed in 10% neutral buffered formalin for 48 h, subjected to decalcification, dehydrated in graded alcohols, and embedded in paraffin. Then samples were sectioned to 5 µm thickness, and stained with hematoxylin and eosin as well as Masson staining. Some specimens were used for the assay of Indian ink perfusion. Photomicrographs of sections were taken with an Olympus (Tokyo, Japan) BX‐60 light microscope and an Olympus 3CCD color video camera.
Statistical analysis
All data were presented as mean ± 1 SD, and was subjected to an Analysis of Variables (ANOVA) test using SSPS 10.1 software. The level of significance was considered to be P < 0.05.
Results
MSC culture and osteogenic differentiation in vitro
After 4 to 6 days of in vitro culture of the bone MSC, the morphology of fibroblastoid cells was displayed with a spindle structure and arrayed in colony forming units‐fibroblastoid (CFU‐F) (Fig. 1). The presence of CFU‐F suggests that in vitro cultured MSC may contain stem‐like cells 11 .
Figure 1.
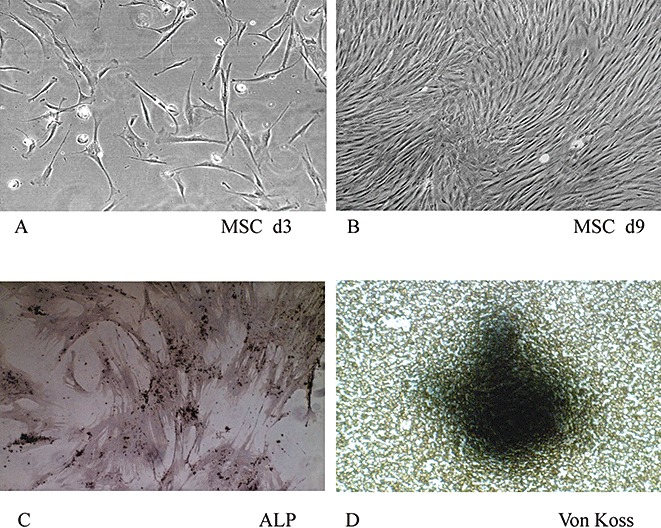
Culture and osteogenic differentiation of MSC in vitro. MSC were cultured for 6 days (A) and 9 days (B) in vitro (original magnification × 100). Alkaline phosphatase staining (C) and Von Kossa staining (D) of MSC were assessed after MSC had been cultured in osteogenic medium for two weeks (original magnification ×200).
Osteogenic differentiation of MSC was induced by an osteogenic medium for 2 weeks. The induced cells formed alkaline phosphatase‐positive aggregates and Von Kossa stain‐positive nodules (Fig. 1). Both of these results indicated that MSC could be induced to differentiate into osteoblasts in vitro.
To examine the cell‐scaffold interaction, SEM was performed. It showed a high percentage of open pores with connections to each pore in the DBM scaffold and that MSC had grown and spread a collagenous‐like extracellular matrix over the surface and inner pores of the DBM blocks (Fig. 2).
Figure 2.
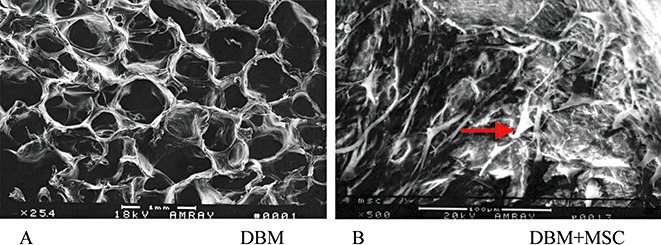
SEM photomicrograph of a cross‐section of DBM only (A) (original magnification ×25.4) shows the porous structure. After MSC were seeded on a DBM scaffold for further culture for five days in vitro, an SEM image of tissue‐engineered bone (B) (original magnification ×500) shows there are considerable numbers of cells (arrow) in the pores of the DBM scaffold.
Gross examination of goat bone defects
On gross examination 12 weeks postoperatively the femurs in group I (DBM/MSC) and group II (DBM alone) appeared to be mechanically stable and to consist of a bone‐like tissue, which was rather irregularly shaped with large callus formation. Comparatively speaking, there was poorer new bone formation in group II. Twenty‐four weeks postoperatively the defects had healed in all cases in groups I and II, but in group I the contour of the femur had almost completely repaired, with only moderate callus formation, while in the DBM only group it remained irregular. In contrast, the untreated defects were filled with fibrous tissue and appeared mechanically unstable at both check times, which demonstrated that such large bone defects could not be repaired without some intervention (Fig. 3).
Figure 3.
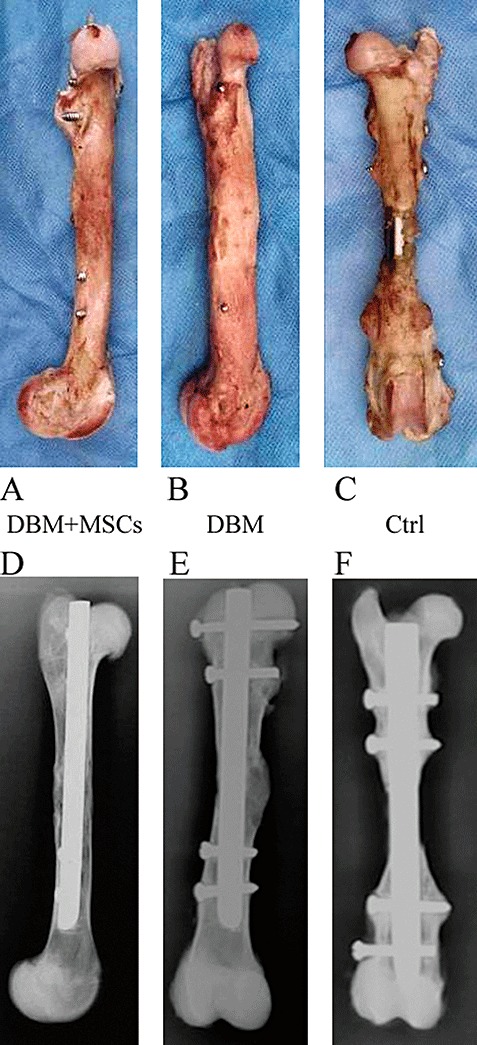
Photos of reconstructed goat femurs after removal of soft tissue in the DBM‐MSC group (A), DBM group (B), and untreated control group (C) taken 24 weeks post surgery. Contact radiographs of reconstructed goat femurs taken 24 weeks post surgery (D, E, F).
X‐ray analysis of bone regeneration
Radiological investigation revealed no radiographic evidence of bone formation immediately after surgery and a correct position in all cases. In the experimental group with cell transplants, radiograph analysis after 12 weeks documented a complete bone bridge in the defect area following bone formation and calcification. In DBM transplants without cells, the grade of bone formation was significantly inferior to those with cell transplants, whereas untreated control defects demonstrated only inappropriate or no radiographic evidence of bone formation (Fig. 3). The median radiographic score of healing after 12 and 24 weeks is shown in Table 2. There were significant differences in the radiographic score among the groups (P < 0.05).
Table 2.
Results of radiographic grading score
| Time | DBM–MSCs | DBM | Untreated |
|---|---|---|---|
| 12 weeks | 4.06 ± 0.94 | 3.05 ± 0.76 | 1.05 ± 0.42 |
| 24 weeks | 5.25 ± 0.66 | 4.66 ± 0.64 | 1.48 ± 0.82 |
Biomechanical testing of newly formed bone
The biomechanical properties of the new bone was examined by a torsional strength test, during which the bones were loaded in torsion until failure. The torsional strength in group I was significantly higher than in group II and group III (P < 0.05). The results from group III showed nonunion. At 24 weeks post surgery the strength of the reconstructed femur in group I (DBM plus MSC) was increased to 85% of the intact contralateral femur (Fig. 4).
Figure 4.
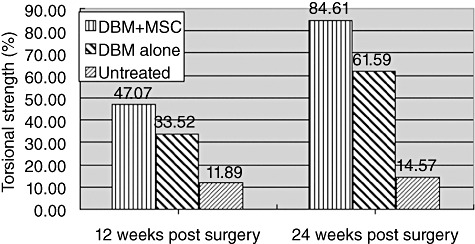
Torsional strength of reconstructed goat femur at 12 weeks and 24 weeks post surgery. The results are normalized as a relative percentage between the reconstructed femur and the normal intact contralateral femur (100%, n= 4).
Histological analysis of newly formed bone
At 12 weeks post surgery histological results from the DBM plus MSC group showed that an endochondral ossification pattern had occurred, and abundant hypertrophied cells were embedded in a cartilage‐like matrix that contained osteoid tissue elements and invading blood vessels (Fig. 5). New bone had formed on the surface of the scaffolds. This finding confirmed that the natural porosity and interconnected pore network of DBM was beneficial to the osteogenesis of loaded MSC. Newly formed woven bone was close to the host lamellar bone, and bony bridging was observed between them. Meanwhile, after the DBM scaffold had mostly been degraded, the newly formed woven bone began to enter the lamellar bone. Interestingly, no infiltration of mononuclear‐macrophages or lymphocytes was found around the graft, which suggested that the tissue‐engineered bone did not induce a strong immune reaction. Similar findings were detected in the DBM alone group, but the amount of newly formed bone was smaller and fibrous tissue formation greater (Fig. 5B). On the contrary, in the untreated control group fibrous tissue filled the whole defect (Fig. 5C).
Figure 5.
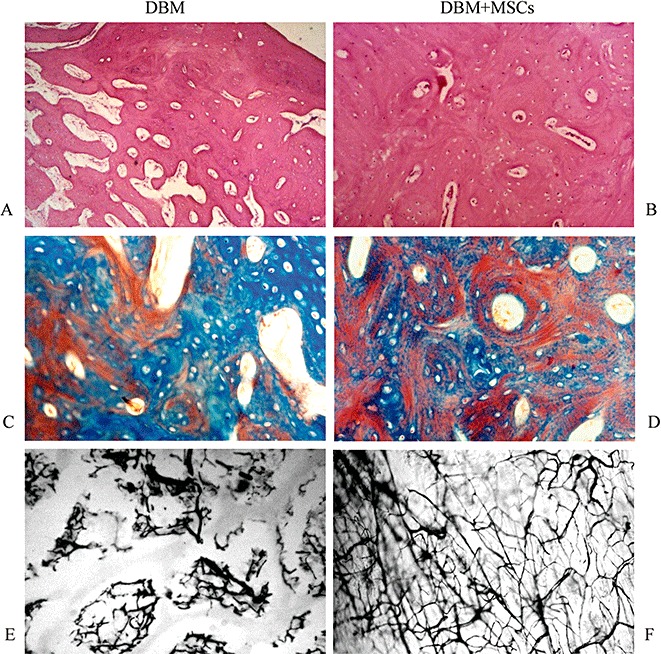
Histological analysis of newly formed bone by HE staining (A, B), Masson staining (C, D), and Indian ink staining (E, F) at 12 weeks post surgery. The amount of newly formed bone in the DBM group (A) was less than in the DBM plus MSC group (B). However, the formation of fibrous tissue in the DBM group was more. An endochondral ossification pattern was observed in both DBM plus MSC and DBM groups, and cortical bone had formed around newly formed woven bone in the DBM plus MSC group. Compared with the DBM group, the more newly formed vessels were observed in the DBM plus MSC group by methods of Masson staining (D) and Indian ink staining (F).
More newly formed bone was observed in the defect space at 24 weeks post surgery. In both the DBM/MSC and DBM alone groups, the defects had been filled by newly formed bone with a structure typical of mature bone tissue, while the DBM had been completely reabsorbed. Formation of continuous cortex and recanalization of the medullary cavity had occurred in the area of the bone defect. However, the quantity and quality of newly formed bone in the DBM/MSC group was superior to that in the DBM alone group. There was little bone formation in the bone defect area in the untreated control group. Moreover, there was no bone bridge formation between the ends of the defect in this group.
Discussion
The repair of large segmental bone defects is still a serious clinical problem in orthopaedic surgery. Bone tissue engineering may provide an alternative approach to repairing large segmental bone defects. In this study, autologous MSC were seeded onto an allogenic DBM scaffold, and co‐cultured for seven days in vitro to increase secretion of matrix. Then the tissue‐engineered graft was transplanted into large segmental bone defects in goats. At 12 weeks post surgery abundant new bone tissue had formed in the area of bone defect, which suggested that a tissue‐engineered graft is a practical substitute for the repair of bone defects in vivo.
The technology of bone tissue engineering includes three elements, these being seeding cells, biologically active factors, and reabsorbable scaffolds 12 , 13 , 14 . Ideal seeding cells, one of the keys to tissue engineering, should be able to proliferate expeditiously, be capable of osteogenesis, and be compatible with scaffold materials without inciting a strong immune reaction when implanted in vivo. Though periosteal cells have been shown to form bone tissue in vivo, the procedure for harvesting periosteum is invasive and can cause donor site morbidity. Moreover, the proliferative capability of periosteal cells is limited, which means they do not match the capability of tissue‐engineered bone. However, MSC can be obtained from bone marrow by a less invasive procedure and can easily be made to proliferate and be induced into osteoblasts in vitro. Therefore they may be a promising source of seeding cells for bone tissue engineering 15 . In this study, CFU‐F of MSC were observed in vitro, which suggests they have the potential for rapid proliferation 10 . It was also revealed by assay of alkaline phosphatase‐positive aggregates and Von Kossa stain‐positive nodules, and expression of type I collagen detected by immunocytochemistry staining, that MSC had an osteogenic phenotype in vitro. We also confirmed the osteogenic capability of MSC in vivo studies. Bone defects of 3 cm were made in goat femurs and filled with autologous MSC plus allogenic DBM, which is a key pre‐step towards clinical application. At the 12th week post surgery, abundant new bone tissue had bridged between the ends of the bone defects (Fig. 5). These results suggest that MSC are a promising cell source for bone tissue engineering.
A three‐dimensional scaffold is another key element of tissue‐engineered bone. An appropriate scaffold is biocompatible and porous, and has the required mechanical strength. A biocompatible scaffold material seldom affects the proliferation and differentiation of seeding cells, and induces hardly any immune reaction in vivo. Currently, several materials have been used as scaffolds, including demineralized bone matrix, collagen composites, fibrin, calcium phosphate, polylactide, poly(lactide‐co‐glycolide), polylactide‐polyethylene glycol, hydroxyapatite, dental plaster, and titanium. More extensive animal and clinical research has been conducted on DBM. Many reports have shown encouraging evidence that DBM can promote the repair of bone defects in clinical and experimental settings 16 , 17 . In this study DBM, with its porous three‐dimensional structure, provides an excellent environment for the attachment and proliferation of MSC in vitro and the repair of bone defects in vivo. The process of degradation of DBM induced a few mononuclear cells around the DBM, but inflammatory reaction and cellular infiltration around the defect area were not pronounced. All of this confirms that DBM is a biocompatible scaffold for bone tissue engineering. However, the strength of DBM is not enough to meet the needs of load‐bearing bone. Fortunately, internal or external fixation should solve this problem.
It is important to repair large segmental bone defects, especially those in load‐bearing bone, because repair can not only rapidly increase the quantity of newly formed bone, but also quickly increase the biomechanical strength of the restructured bone. This study was designed to closely mimic a clinical situation. A 3 cm defect, which is more than 20% of the whole length and twice the diameter of the femur, was created in the femurs of goats. It has been proved that such a defect cannot possibly heal without intervention 18 . Our study showed that an inter‐locking medullary nail can supply sufficient mechanical stability and is an effective means of inter‐fixation in this femur fracture animal model. In this study, mature bone tissue had bridged the defects by the 12th week post surgery in the DBM/MCS group (Fig. 5). In addition, the regenerated bone was well remodeled and the DBM had been completely degraded and absorbed by 24 weeks post surgery. Although there were individual variations in the osteogenic process in the DBM/MSC group, the quantity and quality of newly formed bone was superior to that in both the DBM alone and the untreated control group.
Our results strongly indicate that aMSC are an alternative cell source for bone tissue engineering, that DBM is a promising scaffold material for bone tissue engineering, and that the tissue‐engineered graft fabricated by MSC and DBM is an effective approach for repairing large segmental bone defects of mammals. Based on the broad clinical application of aMSC alone and allogenic DBM alone, a tissue‐engineered graft fabricated by MSC and DBM could now be applied clinically for treatment of bone defects.
Acknowledgments
This work was supported by The National High Technology Research and Development (863) Program of China (2006AA02A122), Chongqing Key Scientific and Technologic Program (2006AB5008), and the Military Fund.
Grant Sources: This work was supported by The National High Technology Research and Development (863) Program of China (2006AA02A122), Chongqing Key Scientific and Technologic Program (2006AB5008), and the Military Fund.
References
- 1. Younger EM, Chapman MW. Morbidity at bone graft donor sites. J Orthop Trauma, 1989, 3: 192 – 195. [DOI] [PubMed] [Google Scholar]
- 2. Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science, 1999, 284: 143 – 147. [DOI] [PubMed] [Google Scholar]
- 3. Kon E, Muraglia A, Corsi A, et al. Autologous bone marrow stromal cells loaded onto porous hydroxyapatite ceramic accelerate bone repair in critical‐size defects of sheep long bones. J Biomed Mater Res, 2000, 49: 328 – 337. [DOI] [PubMed] [Google Scholar]
- 4. Kim BS, Mooney DJ. Development of biocompatible synthetic extracellular matrices for tissue engineering. Trends Biotechnol, 1998, 16: 224 – 230. [DOI] [PubMed] [Google Scholar]
- 5. Urist MR, Silverman BF, Buring K, et al. The bone induction principle. Clin Orthop Relat Res, 1967, 53: 243 – 283. [PubMed] [Google Scholar]
- 6. Perka C, Schultz O, Spitzer RS, et al. Segmental bone repair by tissue‐engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials, 2000, 21: 1145 – 1153. [DOI] [PubMed] [Google Scholar]
- 7. Gebhart M, Lane J. A radiographical and biomechanical study of demineralized bone matrix implanted into a bone defect of rat femurs with and without bone marrow. Acta Orthop Belg, 1991, 57: 130 – 143. [PubMed] [Google Scholar]
- 8. Glowacki J, Mulliken JB. Demineralized bone implants. Clin Plast Surg, 1985, 12: 233 – 241. [PubMed] [Google Scholar]
- 9. Salkeld SL, Patron LP, Barrack RL, et al. The effect of osteogenic protein‐1 on the healing of segmental bone defects treated with autograft or allograft bone. J Bone joint Surg Am, 2001, 83: 803 – 816. [DOI] [PubMed] [Google Scholar]
- 10. Den Boer FC, Patka P, Bakker FC, et al. New segmental long bone defect model in sheep: quantitative analysis of healing with dual energy X‐ray absorptiometry. J Orthop Res, 1999, 17: 654 – 660. [DOI] [PubMed] [Google Scholar]
- 11. Shang Q, Wang Z, Liu W. Tissue‐engineered bone repair of sheep cranial defects with autologous bone marrow stromal cells. J Craniofac Surg, 2001, 12: 586 – 593. [DOI] [PubMed] [Google Scholar]
- 12. Service RF. Tissue engineers build new bone. Science, 2000, 289: 1498 – 1500. [DOI] [PubMed] [Google Scholar]
- 13. Reddi AH. Morphogenesis and tissue engineering of bone and cartilage: inductive signals, stem cells, and biomimetic biomaterials. Tissue Eng, 2000, 6: 351 – 359. [DOI] [PubMed] [Google Scholar]
- 14. Bruder SP, Fox BS. Tissue engineering of bone. Cell based strategies. Clin Orthop Relat Res, 1999, 367: S68 – 83. [DOI] [PubMed] [Google Scholar]
- 15. Maniatopoulos C, Sodek J, Melcher AH. Bone formation in vitro by stromal cells obtained from bone marrow of young adult rats. Cell Tissue Res, 1998, 254: 317 – 330. [DOI] [PubMed] [Google Scholar]
- 16. Russell JL, Block JE. Clinical utility of demineralized bone matrix for osseous defects, arthrodesis, and reconstruction: impact of processing techniques and study methodology. Orthopedics, 1999, 22: 524 – 531. [PubMed] [Google Scholar]
- 17. Kastena R, Luginbühl M, Van Griensven M, et al. Comparison of human bone marrow stromal cells seeded on calcium‐deficient hydroxyapatite, beta‐tricalcium phosphate and demineralized bone matrix. Biomaterials, 2003, 24: 2593 – 2603. [DOI] [PubMed] [Google Scholar]
- 18. Qin H, Xu JZ, Wang XQ, et al. Preparation of animal model of long bone defect used in tissue engineering. Chinese Journal of Clinical Rehabilitation, 2004, 20: 3974 – 3975. [Google Scholar]


