Abstract
Objective: To investigate whether: (i) rs12885713 (−16C > T) and rs5871 polymorphisms in the Calmodulin1 (CALM1) gene are predisposing factors for adolescent idiopathic scoliosis (AIS); and (ii) different single nucleotide polymorphisms (SNP) correlate with different subtypes of AIS.
Methods: A total of 100 AIS patients with Cobb angle above 30° were recruited for this study together with 100 healthy controls. Curve pattern, Cobb angle, and Risser sign were recorded. Two polymorphic loci, rs12885713 (−16C > T) and rs5871 loci, of the CALM1 gene were analyzed. All patients were grouped according to the Peking Union Medical College (PUMC) classification, the apical location of the major curve, and the Cobb angle.
Results: There was a statistically significant difference in the distribution of rs12885713 site polymorphism (P = 0.034) between PUMC type II (double curve) patients and controls, in the distribution of rs12885713 site polymorphism (P = 0.009) between lumbar curve cases and controls and in the distribution of rs5871 site polymorphism (P = 0.035) between thoracic curve patients and controls.
Conclusion: Different subtypes of AIS might be related to different SNP. The susceptibility of PUMC type II (double curve) AIS and lumbar curve might be related to CALM1 rs12885713 site polymorphism, while rs5871 site polymorphism might be a risk indicator for thoracic curve cases.
Keywords: Calmodulin, Classification, Polymorphism, Scoliosis, Single nucleotide
Introduction
Adolescent idiopathic scoliosis (AIS) is a common spinal deformity found in 1.5%–3% of the general population 1 . The etiology of AIS is poorly understood. Many hypotheses have been proposed, including genetic predisposition, abnormal growth and hormonal disturbances, and developmental neuromuscular dysfunction 2 , 3 , 4 , 5 , 6 . Several familial surveys of idiopathic scoliosis have provided strong evidence that genetic factors play a role in this condition, and AIS likely represents a complex genetic trait under the influence of multiple predisposition genes 7 , 8 , 9 , 10 , 11 . Although there are several reports researching the predisposition gene in AIS, current results remain uncertain or equivocal 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 .
While reviewing the literature we noticed an interesting phenomenon: all the previous studies had stratified subjects according to curve severity, such as Cobb angle > 10°, 20°, 30°, 40° 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 . However, in the immature AIS patient, risk of progression is related primarily to curve‐specific factors and growth potential. The curve‐specific factors include two aspects: double curves have a greater tendency to progress than single curve patterns, and the larger the curve at detection, the greater the risk of progression 20 . Since environmental factors have effects on curve severity, it seemed unreasonable to stratify the subjects with Cobb angle. So, we hypothesized that different single nucleotide polymorphisms (SNP) might be related to the occurrence or progression of single, double and triple curves, or thoracic, thoracolumbar and lumbar curves. The key to the hypothesis was to choose several appropriate SNP and test them with large sample sizes.
Calmodulin, a calcium‐binding receptor protein, is a critical mediator of cellular calcium ion concentration, and a regulator of many important enzymatic systems 21 . Previous studies have shown that an increased calmodulin concentration in platelets is associated with progression of AIS 22 , 23 , 24 . On the other hand, Mototani et al. have identified a functional SNP (T/C polymorphism, dbSNP#: rs12885713) in the promoter region of Calmodulin1 (CALM1), which affects transcription of the gene 25 . Otherwise, rs5871 (T/C polymorphism), with an average heterozygosity 0.201 ± 0.245, in the 3′‐UTR of CALM1, might be closely related to the stability and transcription of mRNA 26 (http://genome.ucsc.edu/cgi‐bin/hgc?hgsid=97296345&o=89941230&t=89941231&g=snp126&i=rs5871).
On the basis of the above‐mentioned information, we aimed to investigate: (i) the association between CALM1 gene polymorphisms and AIS; and (ii) the association between different SNP and different subtypes of AIS.
Materials and methods
Subjects
This study included 100 patients with AIS and 100 healthy controls. All patients had accepted deformity correction surgery at the Spinal Center of Peking Union Medical College Hospital from October 2005 to April 2007. The patients, 19 of whom were male and 81 female, with an average age of 15.11 years (range, 10–20), were diagnosed by clinical examination and radiographs. Spinal cord deformities were excluded by magnetic resonance imaging. The Cobb angle of the major curvature of AIS ranged from 30° to 90°.
The healthy controls, 25 of whom were male and 75 female, with an average age of 15.55 years (range, 10–19), were recruited from a trauma ward. All controls were examined with the forward bending test by an experienced spinal surgeon to rule out any scoliosis. In the case of any uncertainty, the clinician would refer the subject for a radiograph to ensure the absence of any scoliosis. Both patients and controls were excluded from the study if they had suffered from congenital deformities, neuromuscular diseases, endocrine diseases, skeletal dysplasia, connective tissue abnormalities, or mental retardation.
Informed consent was obtained from parents or subjects in both groups. The study was approved by the Clinical Research Ethics Committee of the university.
Blood sampling
Blood samples were taken from each subject by venipuncture. DNA was extracted from ethylenediaminetetraacetic acid anticoagulated blood with genomic DNA Mini Blood kits (QIAGEN NV, Hilden, Germany).
Molecular methods
The rs12885713 (−16C > T) allele is located in the promoter region. The oligonucleotide primers used to determine CALM1 gene rs12885713 (−16C > T) site polymorphism were:
forward, 5′‐GGGTAGGTAGCGGAGTGAACGG‐3′;
reverse, 5′‐TGGTGCGAGCGAAGGGAGGA‐3′.
Polymerase chain reaction was performed in 35 cycles of 94°C for 30 s, 63°C for 30 s, and 72°C for 30 s, with a final extension for 10 min at 72°C.
The rs5871 allele is located in the 3′‐UTR region that might be associated with stability and transcription of mRNA. The oligonucleotide primers used to determine the CALM1 gene rs5871 site polymorphism were:
forward, 5′‐AAGATCAAGCTACACATCAG‐3′;
reverse, 5′‐TCGGAGCACACGAAGTACAAG‐3′.
Polymerase chain reaction was performed in 35 cycles of 94°C for 40 s, 61°C for 40 s, and 72°C for 60 s, with a final extension for 5 min at 72°C.
DNA sequencing was carried out by ABI3730 genetic analyzer (AME Bioscience Ltd, Norwalk, CT, USA) and sequencing results of these samples were read with the GeneTool Lite program (BioTools, Edmonton, Canada). To ensure sequencing results, we did re‐sequence DNA in all samples from the opposite direction and confirmed the previous results.
Statistical analysis
The χ2 test was used to determine the significance of differences in the distribution of SNP between patients with AIS and controls, and to test if the genotype frequencies deviated from the Hardy‐Weinberg equilibrium. Then, all AIS patients were grouped according to different methods, including the Peking Union Medical College (PUMC)classification 27 , the apical location of the major curve, and the Cobb angle, and the distribution of SNP in different subtypes of AIS patients and the controls compared using the χ2 test. P < 0.05 was considered statistically significant.
Results
Demographic and anthropometric data
The age distribution of the 100 patients and 100 healthy controls were 15.11 years (range, 10–20) and 15.55 years (range, 10–19), respectively. The ages of the healthy controls did not have a normal distribution, so were compared with the patients using the nonparametric Wilcoxon test (Z =−1.903, P = 0.057). The gender distribution in the two groups was compared using the χ2 test (χ2= 0.741, P = 0.390). As a result, the subjects in the two groups were age‐ and gender‐ matched.
According to the PUMC classification, there were 23 patients with single curve (type I), 67 patients with double curve (type II), and 10 patients with triple curve (type III). According to the apical location of the major curve, there were 57 patients with thoracic curve (apex between T2 and T11‐12 disc), 23 patients with thoracolumbar curve (apex between T12 and L1), and 20 patients with lumbar curve (apex between L1‐2 disc and L4‐5 disc). Of the 100 patients, 86 had a Cobb angle above 40°, and 47 a Cobb angle above 50°.
Genotypes of CALM1 gene
The genotypes in our study are shown in the following figures: rs12885713 (−16C > T) allele polymorphisms are composed of CC, TT homozygosis, and C/T heterozygosis (Fig. 1); rs5871 allele polymorphisms are composed of CC, TT homozygosis, and C/T heterozygosis (Fig. 2). All six genotypes were detected in our current studies. The gene polymorphic distributions did not significantly deviate from the Hardy‐Weinberg equilibrium in the two groups (Table 1).
Figure 1.
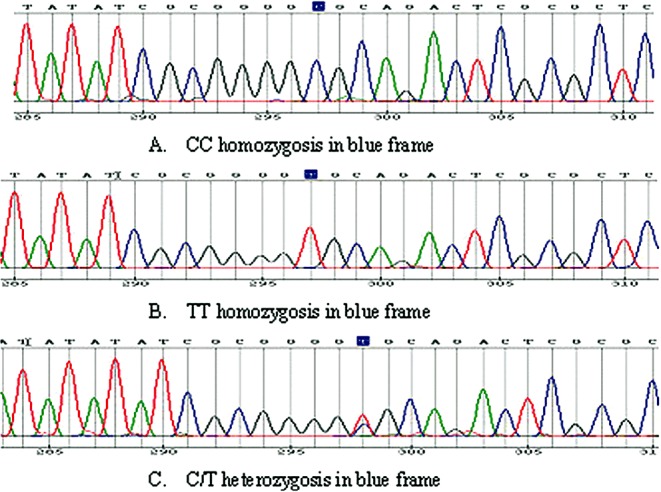
Results of genetic analysis for CALM1 gene rs12885713 (−16C > T). (A)Type CC, (B)Type TT, (C)Type C/T. Different color means different nucleotide: green‐A, blue‐C, black‐G, red‐T.
Figure 2.
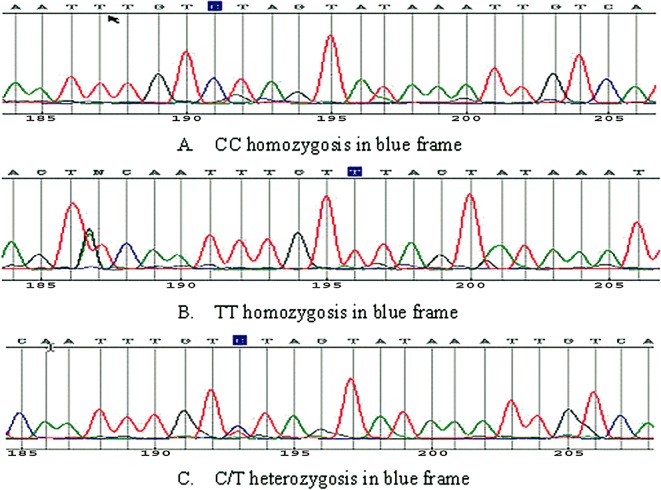
Results of genetic analysis for CALM1 gene rs5871. (A)Type CC, (B)Type TT, (C)Type C/T. Different color means different nucleotide: green‐A, blue‐C, black‐G, red‐T.
Table 1.
HWE* analysis of the two SNP in the two groups
| Analysis factors | AIS | Controls | ||
|---|---|---|---|---|
| χ2‐value | P‐value | χ2‐value | P‐value | |
| rs12885713 | 0.342 | >0.05 | 0.077 | >0.05 |
| rs5871 | 0.160 | >0.05 | 1.780 | >0.05 |
All the genotype frequencies followed HWE.
HWE, Hardy‐Weinberg Equilibrium.
Association between CALM1 gene polymorphisms and AIS
The distribution of CALM1 gene rs12885713 and rs5871 site polymorphisms in the case and control groups is shown in Table 2. There were no differences in the polymorphic distribution of both genotypes and alleles in the two groups.
Table 2.
Association between CALM1 rs12885713 and rs5871 polymorphisms and AIS
| Genotypes | Alleles | ||||
|---|---|---|---|---|---|
| CC | CT | TT | C | T | |
| rs12885713 | |||||
| AIS patients (n = 100) | 73 | 24 | 3 | 170 (85%) † | 30 (15%) ‡ |
| Controls (n = 100) | 66 | 31 | 3 | 163 (81.5%) † | 37 (18.5%) ‡ |
| χ2‐value = 11.560 | χ2‐value = 0.879 | ||||
| P‐value = 0.282 | P‐value = 0.349 | ||||
| rs5871 | |||||
| AIS patients (n = 100) | 40 | 45 | 15 | 125 (62.5%) † | 75 (37.5%) ‡ |
| Controls (n = 100) | 33 | 43 | 24 | 109 (55.5%) † | 91 (45.5%) ‡ |
| χ2‐value = 1.505 | χ2‐value = 3.610 | ||||
| P‐value = 0.471 | P‐value = 0.057 | ||||
Over‐represented alleles.
Under‐represented alleles.
Association between CALM1 gene polymorphisms and PUMC classification
Table 3 demonstrates the association between PUMC classification and two SNP in the CALM1 gene. It was shown that the distribution of rs12885713 allele polymorphisms was significantly different between type II (double curve) patients and controls, but no statistically significant differences were found in the other patient groups compared with controls. Otherwise, there was no statistically significant difference between AIS patients with different PUMC classification and controls in the distribution of rs5871 allele polymorphisms. In addition, no significant differences in the distribution of two SNP in the three subtypes of AIS patients were found.
Table 3.
Analysis of different PUMC classification AIS patients and two SPN
| AIS | Controls | χ2‐value | P‐value | |||
|---|---|---|---|---|---|---|
| C | T | C | T | |||
| rs12885713 allele | ||||||
| PUMC I † | 37 | 9 | 163 | 37 | 0.028 | 0.867 |
| PUMC II ‡ | 96 | 38 | 163 | 37 | 4.478 | 0.034 |
| PUMC III § | 16 | 4 | 163 | 37 | 0.000 | 1.000 |
| χ2‐value = 1.739 | ||||||
| P‐value = 0.419* | ||||||
| rs5871 allele | ||||||
| PUMC I † | 24 | 22 | 109 | 91 | 0.082 | 0.775 |
| PUMC II ‡ | 59 | 75 | 109 | 91 | 3.519 | 0.061 |
| PUMC III § | 12 | 8 | 109 | 91 | 0.222 | 0.637 |
| χ2‐value = 2.292 | ||||||
| P‐value = 0.318* | ||||||
Comparison among three subtypes.
23 patients.
67 patients.
10 patients.
Association between CALM1 gene polymorphisms and apical location of the major curve
Table 4 illustrates the association between apical location of the major curve and two SNP in the CALM1 gene. It was shown that the distribution of rs12885713 allele polymorphisms was significantly different in lumbar curve patients compared with controls. The difference in distribution of rs5871 allele polymorphisms between thoracic curve patients and controls was also statistically significant.
Table 4.
Analysis of different apical location of the major curve and two SPN
| AIS | Controls | χ2‐value | P‐value | |||
|---|---|---|---|---|---|---|
| C | T | C | T | |||
| rs12885713 allele | ||||||
| Thoracic curve † | 86 | 28 | 163 | 37 | 1.625 | 0.602 |
| Thoracolumbar curve ‡ | 35 | 11 | 163 | 37 | 0.964 | 0.326 |
| Lumbar curve § | 28 | 12 | 163 | 0.009 | 37 | 2.713 |
| χ2‐value = 0.473 | ||||||
| P‐value = 0.790* | ||||||
| rs5871 allele | ||||||
| Thoracic curve † | 48 | 66 | 109 | 91 | 4.462 | 0.035 |
| Thoracolumbar curve ‡ | 27 | 19 | 109 | 91 | 0.078 | 0.779 |
| Lumbar curve § | 20 | 20 | 109 | 91 | 0.272 | 0.602 |
| χ2‐value = 2.929 | ||||||
| P‐value = 0.231* | ||||||
Comparison among three subtypes.
57 patients (apex between T2 and T11‐12 disc).
23 patients (apex between T12 and L1).
20 patients (apex between L1‐2 disc and L4‐5 disc).
Association between CALM1 gene polymorphisms and Cobb angle
Table 5 indicates the association between Cobb angle and two SNP in the CALM1 gene. In regard to both rs12885713 and rs5871 allele polymorphisms, no significant difference in distribution was found between the two subgroups of AIS patients and the controls. We also found no significant difference in regard to the two SNP between the two groups of patients with Cobb angle ≥40° and ≥50°.
Table 5.
Analysis of the Cobb angle and two SPN
| AIS | Controls | χ2‐value | P‐value | |||
|---|---|---|---|---|---|---|
| C | T | C | T | |||
| rs12885713 allele | ||||||
| ≥40° † | 146 | 26 | 163 | 37 | 0.753 | 0.386 |
| ≥50° ‡ | 82 | 12 | 163 | 37 | 1.514 | 0.219 |
| χ2‐value = 0.274 | ||||||
| P‐value = 0.601* | ||||||
| rs5871 allele | ||||||
| ≥40° † | 110 | 62 | 109 | 91 | 3.43 | 0.065 |
| ≥50° ‡ | 59 | 35 | 109 | 91 | 1.784 | 0.182 |
| χ2‐value = 0.037 | ||||||
| P‐value = 0.848* | ||||||
Comparison among three subtypes.
86 patients.
47 patients.
Discussion
Although a strong inherited component has been reported in familial studies in AIS, the predisposing genes remain unclear 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19 .
The risk of progression is related primarily to curve‐specific factors and growth potential. The double curve pattern has a greater tendency to progress than single curve patterns, and the larger the curve is, the greater the risk of progression 20 . Although a great deal of studies have usually stratified the subjects by the severity of curve in the previous reports, the nosogenesis of AIS is still controversial. 12 , 13 , 14 , 15 , 16 , 17 , 18 , 19
So far, there have been many kinds of classification to guide treatment of the disease, such as the King, Lenke and PUMC classifications. 27 , 28 , 29 . Because of the simple and clear definitions of single, double and triple curves in the PUMC classification, we chose this over other methods for the present study 27 . According to the apical location of the major curve, there are three main curve patterns in AIS: thoracic, thoracolumbar and lumbar 27 . Each has its own particular characteristics, predicted course, and outcome.
Calmodulin, a calcium‐binding receptor protein, is a critical mediator of control of cellular calcium ion concentration and a regulator of many important enzymatic systems 21 . It can regulate the contractile properties of muscles and platelets through its interaction with actin and myosin, and also regulates cellular calcium ion concentration through transport across the cell membrane.
In past studies, it has been shown that increased calmodulin levels in platelets might be associated with progression of AIS. In 1985 Cohen et al. found a 2.5–3 fold increase in the level of calmodulin in platelets of the patients who had idiopathic scoliosis 22 . They also found that the concentration of calmodulin correlated with the severity of the spinal curve. Kindsfater et al. compared 17 patients who had AIS with varying degrees of curve, and 10 age‐ and gender‐matched controls 23 . They found that platelet calmodulin concentrations in the skeletally immature patient with progressive curves (>10° progression per year) were considerably higher than the concentrations in stable curves (3.8 vs. 0.7 nm/µm of protein). These data were based on a single calmodulin determination for each patient during the growth period. Conversely, calmodulin concentrations in the stable (non‐progressive) and control groups were very similar. In 2002 Lowe et al. reported the results of follow‐up of 55 patients with AIS for 1–3 years during their growth period 24 . They found that calmodulin concentrations increased in all patients with progressive curves (13/13), remained stable in 73% of patients with non‐progressive curves (11/15), and were higher generally in curves greater than 30° and double structural curves. Calmodulin concentrations usually decreased in patients undergoing brace treatment (14/17) or spinal fusion (9/10).
In addition, several reports have suggested that calmodulin might interact with melatonin or estrogen. Benítez‐King et al. have proposed that melatonin might bind to calmodulin and is an antagonist to calmodulin function 30 . Increased calmodulin in patients with AIS reported by others, and decreased melatonin in experimental and clinical studies by Machida, imply that there may be an association between calmodulin and melatonin metabolism in idiopathic scoliosis 31 .
Furthermore, it has been reported that calmodulin has an affinity for the estrogen receptor and decreases its estrogen‐binding capacity 32 . The dissociation constant of the estrogen‐binding receptor increased 2–3 fold when calmodulin was bound to it. So, there is a hypothesis that the effect of calmodulin on curve progression might be induced by loss of estrogen‐binding affinity for the estrogen receptor, and that estrogen metabolism might be one of the risk factors for curve progression 17 .
In all, although there are so many previous studies, the mechanism of the influence of melatonin and estrogen in the occurrence of AIS is still controversial. So, it might be the appropriate time to pay attention to their common functional bridge: calmodulin 17 , 18 , 19 , 33 , 34 , 35 , 36 .
Previous studies have shown that calmodulin concentration might play a role in curve progression. Two polymorphic loci (rs12885713, rs5871) might affect the transcription, expression and stability of the calmodulin gene 21 , 22 , 23 , 24 , 25 , 26 .
To investigate rs12885713 allele polymorphisms in vitro, Mototani et al. generated fusion constructs containing the −16C or −16T allele, and transfected these in parallel into OUMS‐27 cells or Huh‐7 cells 25 . In OUMS‐27 cells, the construct containing the −16C allele showed >2‐fold luciferase activity as compared to that containing the −16T allele. Similar results were observed in Huh‐7 cells. In addition, to investigate the effects of −16C > T on CALM1 transcription in vivo, they measured the amount of CALM1 transcripts under the control of each allele using RNA difference plot analysis 25 . The results suggested that the suppressor(s) of CALM1 transcription bind more tightly to the −16T allele. Finally, they found that the susceptibility −16C allele increases CALM1 transcription in vitro and in vivo.
The CALM 1 gene rs5871 site, heterozygosis 20.1%, is located in the region of 3′‐UTR. Previous reports have demonstrated that the 3′‐UTR of mRNA is an important regulatory site controlling interactions with mRNA degradation machinery. Three′‐UTR RNA‐binding proteins that recognize specific mRNA sequence elements and secondary structure dictate the fate of mRNA transcripts. SNP in the 3′‐UTR could disrupt RNA‐protein interactions, resulting in altered mRNA stability 26 , 37 , 38 , 39 , 40 .
The present study indicates that the polymorphisms of rs12885713 or rs5871 alleles were not the primary allele in all patients with AIS. However, in the following analysis, we found suggestive evidence of an association between CALM1 gene polymorphisms and different subtypes of AIS. According to our current case‐control study, we found a statistically significant difference between double curve (type II) patients and controls, and between lumbar curve patients and controls, in the distribution of rs12885713 (−16C > T) allele polymorphism. There was also a statistically significant difference between thoracic curve patients and controls in the distribution of rs5871 allele polymorphisms.
However, the present study showed no association between CALM1 gene polymorphisms and the severity of AIS. We consider that, being a complex disease, genetic factors maybe primarily determine the occurrence of single, double or triple curves, while environmental factors may determine or affect the severity of the curves. On the basis of the results of the present study, if a further study could confirm the association between CALM 1 gene polymorphisms and double curve, this would be of great clinical value for prediction and treatment of the disease in the future.
In conclusion, different subtypes of AIS might be associated with different SNP: (i) double curve (PUMC type II) AIS might be related to the CALM1 gene rs12885713 site polymorphism; (ii) lumbar curve might be associated with rs12885713 site polymorphism; and (iii) thoracic curve might be correlated with rs5871 site polymorphism. Further study of the distribution of SNP in the CALM 1 gene in different subtypes of AIS is necessary.
Grant Sources: This work was supported by National Natural Science Foundation of China (NSFC 30672137).
References
- 1. Lonstein JE. Adolescent idiopathic scoliosis. Lancet, 1994, 344: 1407–1412. [DOI] [PubMed] [Google Scholar]
- 2. Barrack RL, Whitecloud TS 3rd, Burke SW, et al. Proprioception in idiopathic scoliosis. Spine, 1984, 9: 681–685. [DOI] [PubMed] [Google Scholar]
- 3. Dickson RA. The aetiology of spinal deformities. Lancet, 1988, 1: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 4. Miller NH. Genetics of familial idiopathic scoliosis. Clin Orthop Relat Res, 2002, 401: 60–64. [DOI] [PubMed] [Google Scholar]
- 5. Burwell RG. Aetiology of idiopathic scoliosis: current concepts. Pediatr Rehabil, 2003, 6: 137–170. [DOI] [PubMed] [Google Scholar]
- 6. Lowe TG, Edgar M, Margulies JY, et al. Etiology of idiopathic scoliosis: current trends in research. J Bone Joint Surg Am, 2000, 82: 1157–1168. [DOI] [PubMed] [Google Scholar]
- 7. Kelly PJ, Eisman JA, Sambrook PN. Interaction of genetic and environmental influences on peak bone density. Osteoporos Int, 1990, 1: 56–60. [DOI] [PubMed] [Google Scholar]
- 8. Cowell HR, Hall JN, MacEwen GD. Genetic aspects of idiopathic scoliosis. Clin Orthop Relat Res, 1972, 86: 121–131. [DOI] [PubMed] [Google Scholar]
- 9. Wynne‐Davies R. Familial (idiopathic) scoliosis: a family survey. J Bone Joint Surg Br, 1968, 50: 24–30. [PubMed] [Google Scholar]
- 10. Czeizel A, Bellyei A, Barta O, et al. Genetics of adolescent idiopathic scoliosis. J Med Genet, 1978, 15: 424–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Riseborough EJ, Wynne‐Davies R. A genetic survey of idiopathic scoliosis in Boston, Massachusetts. J Bone Joint Surg Am, 1973, 55: 974–982. [PubMed] [Google Scholar]
- 12. Wise CA, Barnes R, Gillum J, et al. Localization of susceptibility to familial idiopathic scoliosis. Spine, 2000, 25: 2372–2380. [DOI] [PubMed] [Google Scholar]
- 13. Salehi LB, Mangino M, De Serio S, et al. Assignment of a locus for autosomal dominant idiopathic scoliosis (IS) to human chromosome 17p11. Hum Genet, 2002, 111: 401–404. [DOI] [PubMed] [Google Scholar]
- 14. Chan V, Fong GC, Luk KD, et al. A genetic locus for adolescent idiopathic scoliosis linked to chromosome 19p13.3. Am J Hum Genet, 2002, 71: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Justice CM, Miller NH, Marosy B, et al. Familial idiopathic scoliosis: evidence of an X‐linked susceptibility locus. Spine, 2003, 28: 589–594. [DOI] [PubMed] [Google Scholar]
- 16. Miller NH, Justice CM, Marosy B, et al. Identification of candidate regions for familial idiopathic scoliosis. Spine, 2005, 30: 1181–1187. [DOI] [PubMed] [Google Scholar]
- 17. Inoue M, Minami S, Nakata Y, et al. Association between estrogen receptor gene polymorphisms and curve severity of idiopathic scoliosis. Spine, 2002, 27: 2357–2362. [DOI] [PubMed] [Google Scholar]
- 18. Wu J, Qiu Y, Zhang L, et al. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine, 2006, 31: 1131–1136. [DOI] [PubMed] [Google Scholar]
- 19. Tang NL, Yeung HY, Lee KM, et al. A relook into the association of the estrogen receptor [alpha] gene (PvuII, XbaI) and adolescent idiopathic scoliosis: a study of 540 Chinese cases. Spine, 2006, 31: 2463–2468. [DOI] [PubMed] [Google Scholar]
- 20. Weinstein SL. Natural history. Spine, 1999, 24: 2592–2600. [DOI] [PubMed] [Google Scholar]
- 21. Cheung WY. Calmodulin plays a pivotal role in cellular regulation. Science, 1980, 207: 19–27. [DOI] [PubMed] [Google Scholar]
- 22. Cohen DS, Solomons CS, Lowe TG. Altered platelet calmodulin activity in idiopathic scoliosis. Orthop Trans 1985; 9: 106. [Google Scholar]
- 23. Kindsfater K, Lowe T, Lawellin D, et al. Levels of platelet calmodulin for the prediction of progression and severity of adolescent idiopathic scoliosis. J Bone Joint Surg Am, 1994, 76: 1186–1192. [DOI] [PubMed] [Google Scholar]
- 24. Lowe T, Lawellin D, Smith D, et al. Platelet calmodulin levels in adolescent idiopathic scoliosis: do the levels correlate with curve progression and severity? Spine, 2002, 27: 768–775. [DOI] [PubMed] [Google Scholar]
- 25. Mototani H, Mabuchi A, Saito S, et al. A functional single nucleotide polymorphism in the core promoter region of CALM1 is associated with hip osteoarthritis in Japanese. Hum Mol Genet, 2005, 14: 1009–1017. [DOI] [PubMed] [Google Scholar]
- 26. Hollams EM, Giles KM, Thomson AM, et al. MRNA stability and the control of gene expression: implications for human disease. Neurochem Res, 2002, 27: 957–980. [DOI] [PubMed] [Google Scholar]
- 27. Qiu G, Zhang J, Wang Y, et al. A new operative classification of idiopathic scoliosis: a Peking union medical college method. Spine, 2005, 30: 1419–1426. [DOI] [PubMed] [Google Scholar]
- 28. King HA, Moe JH, Bradford DS, et al. The selection of fusion levels in thoracic idiopathic scoliosis. J Bone Joint Surg Am, 1983, 65: 1302–1313. [PubMed] [Google Scholar]
- 29. Lenke LG, Betz RR, Harms J, et al. Adolescent idiopathic scoliosis: a new classification to determine extent of spinal arthrodesis. J Bone Joint Surg Am, 2001, 83: 1169–1181. [PubMed] [Google Scholar]
- 30. Benítez‐King G, Ríos A, Martínez A, et al. In vitro inhibition of Ca2+/calmodulin‐dependent kinase II activity by melatonin. Biochim Biophys Acta, 1996, 1290: 191–196. [DOI] [PubMed] [Google Scholar]
- 31. Machida M. Cause of idiopathic scoliosis. Spine, 1999, 24: 2576–2583. [DOI] [PubMed] [Google Scholar]
- 32. Bouhoute A, Leclercq G. Calmodulin decreases the estrogen binding capacity of the estrogen receptor. Biochem Biophys Res Commun, 1996, 227: 651–657. [DOI] [PubMed] [Google Scholar]
- 33. Sadat‐Ali M, Al‐Habdan I, Al‐Othman A. Adolescent idiopathic scoliosis. Is low melatonin a cause? Joint Bone Spine, 2000, 67: 62–64. [PubMed] [Google Scholar]
- 34. Brodner W, Krepler P, Nicolakis M, et al. Melatonin and adolescent idiopathic scoliosis. J Bone Joint Surg Br, 2000, 82: 399–403. [DOI] [PubMed] [Google Scholar]
- 35. Qiu XS, Tang NL, Yeung HY, et al. Melatonin receptor 1B (MTNR1B) gene polymorphism is associated with the occurrence of adolescent idiopathic scoliosis. Spine, 2007, 32: 1748–1753. [DOI] [PubMed] [Google Scholar]
- 36. Azeddine B, Letellier K, Wang da S, et al. Molecular determinants of melatonin signaling dysfunction in adolescent idiopathic scoliosis. Clin Orthop Relat Res, 2007, 462: 45–52. [DOI] [PubMed] [Google Scholar]
- 37. Tourrière H, Chebli K, Tazi J. mRNA degradation machines in eukaryotic cells. Biochimie, 2002, 84: 821–837. [DOI] [PubMed] [Google Scholar]
- 38. Mangus DA, Evans MC, Jacobson A. Poly(A)‐binding proteins: multifunctional scaffolds for the post‐transcriptional control of gene expression. Genome Biol, 2003, 4: 223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wilkie GS, Dickson KS, Gray NK. Regulation of mRNA translation by 5′‐ and 3′‐UTR‐binding factors. Trends Biochem Sci, 2003, 28: 182–188. [DOI] [PubMed] [Google Scholar]
- 40. Gow JM, Chinn LW, Kroetz DL. The effects of ABCB1 3′‐untranslated region variants on mRNA stability. Drug Metab Dispos, 2008, 36: 10–15. Epub 2007 Oct 16. [DOI] [PubMed] [Google Scholar]


