Abstract
Objective: To compare the results of lateral versus medial‐sided augmentation techniques in single‐bundle posterior cruciate ligament (PCL) reconstruction with remnant preservation.
Methods: Forty‐two cases of isolated chronic PCL ruptures were reconstructed in a single‐bundle manner with remnant preservation. The patients were randomly separated into two groups: in the medial‐sided augmentation (MSA) group the graft passed through the medial side of the remnant and in the lateral‐sided augmentation (LSA) group it passed through the lateral side.
Results: Nineteen patients in the MSA group and 17 in the LSA group were followed up for a minimum of 2 years. At the final follow‐up, the average side‐to‐side differences in posterior laxity were 1.6 ± 1.2 mm and 1.5 ± 1.3 mm respectively in the MSA and LSA groups. According to the International Knee Documentation Committee (IKDC) scale, patient numbers graded as normal, nearly normal and abnormal were 14 (73.7%), 4 (21.1%), and 1 (5.3%) in the MSA group, and 13 (76.5%), 3 (17.6%), and 1 (5.9%) in the LSA group. The IKDC subjective scores were 93.1 ± 3.8 and 92.6 ± 4.1, the Lysholm scores were 95.0 ± 4.6 and 93.7 ± 4.2, and the Tegner scores were 5.4 ± 0.9 and 5.6 ± 0.7 respectively in the MSA and LSA groups. Statistical analysis showed no significant differences between the MSA and the LSA group regarding all subjective and objective results.
Conclusion: In single‐bundle PCL reconstruction with remnant preservation, similar subjective and objective results can be obtained with MSA and LSA techniques.
Keywords: Arthroscopy, Hamstring tendon, Posterior cruciate ligament, Reconstruction
Introduction
Various methods of posterior cruciate ligament (PCL) reconstruction have been reported, including single and double‐bundle reconstruction, with a trans‐tibial or tibial‐inlay approach, with different graft materials and fixation methods 1 , 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 , 16 , 17 . On the whole, the results, especially the objective results, of PCL reconstruction are not as predictable as that of anterior cruciate ligament (ACL) reconstruction. In recent years, PCL reconstruction with remnant preservation has been explored in an attempt to improve the final stability. Though some favorable results have been reported 13 , no interest has been shown in where to place the graft in relation to the remnant fibers. Technically, the graft can pass through the medial or lateral side of the remnant, which we have named medial‐sided augmentation (MSA) and lateral‐sided augmentation (LSA) respectively. The main differences in the two approaches are that after MSA only a small part of the graft is covered by the remnant and the posterior septum, however after LSA most of the graft is wrapped by the remnant and the posterior septum, which may imply better blood supply for the graft. So we assumed that in PCL reconstruction with remnant preservation, LSA might guarantee better biomechanical and functional results. The purpose of this study was to compare the results of the two augmentation techniques.
Materials and methods
Patient data
The indications for single‐bundle PCL reconstruction with remnant preservation were similar to other kinds of PCL reconstruction, symptomatic PCL rupture with at least two positive posterior drawer tests. However, a prerequisite for reconstructing PCL with remnant preservation is that remnant fibers connecting the PCL femoral and tibial insertion sites exist. Exclusion criteria included all cases of combined ligament injury, and those not having PCL reconstructed in exactly this way (including seven strands of hamstring tendons). Patients with Outerbridge 3 to 4 degree cartilage lesions were also excluded. The patients were randomly separated into two groups according to their admittance number. The patients with odd numbers fell into the MSA group, and those with even numbers into the LSA group. After exclusion according to the aforementioned criteria, patients with isolated chronic PCL injury, having single‐bundle PCL reconstruction without remnant preservation, were included in this study as controls. The indication for PCL reconstruction without remnant preservation was that there were no remnant fibers connecting the PCL femoral and tibial insertion sites.
From January 2003 to December 2004, 21 patients with similar basic data were allocated to each of the MSA and LSA groups, and 16 patients to the control group. Preoperatively, all patients were evaluated according to the International Knee Documentation Committee (IKDC) objective and subjective rating scale, as well as the Lysholm and Tegner rating scales. KT‐1000 examination was taken as a supplement to the manual posterior drawer test. There was no statistically significant difference between the two groups in regard to the preoperative subjective IKDC, Lysholm, and Tegner scores (P > 0.05, Table 1).
Table 1.
Patient preoperative data
| Groups | Male : Female | Age at surgery (range) | Injury Time (range) | Average laxity (x̄± SD) mm | IKDC | Lysholm | Tegner |
|---|---|---|---|---|---|---|---|
| MSA | 17:4 | 29 years (24–47) | 24 months (6–49) | 9.7 ± 3.5 | 54.6 ± 8.8 | 45.3 ± 6.6 | 3.9 ± 0.9 |
| LSA | 16:5 | 28 years (22–49) | 23 months (4–46) | 9.9 ± 4.3 | 52.9 ± 8.9 | 44.7 ± 8.1 | 4.1 ± 0.8 |
IKDC, International Knee Documentation Committee; LSA, lateral‐sided augmentation; MSA, medial‐sided augmentation.
Surgical technique
All the PCL reconstructions were performed arthroscopically by the senior surgeon. However, graft preparation was always done by two assistants while the senior surgeon was creating tunnels. The patient was supine, with the knee flexed 90°, and the leg leaning against a laterally positioned post.
Graft preparation
Graft harvest and preparation were performed as described by Zhao et al. for single‐bundle PCL reconstruction with seven strands of hamstring tendon 17 , 18 . Both the semitendinosus (ST) and gracilis tendons (GT) were harvested. When the ST after trimming was longer than 28 cm, and the GT was 21 to 27 cm long, a 7‐stranded graft, comprised of a 4‐stranded graft of ST and a 3‐stranded graft of GT, could be made with a length of more than 7 cm. The proximal end of the graft was tethered to or suspended on a polyester tape that was used for proximal suspension fixation. The distal end of the graft was to be suspended through the in‐braided or through‐passing sutures. The expected graft length in the femoral tunnel, the joint and the tibial tunnel were respectively 2 cm, 3 to 3.5 cm, and more than 1.5 cm. The diameter of the proximal end of the graft was 8 to 11 mm, with a mean of 9.5 mm. The graft was pre‐tensioned with 80–100 N force for a minimum of 5 min. A mark was made 25 mm from the proximal end by suturing the multi‐strands of tendon together with a colored absorbable suture.
Single‐bundle PCL reconstruction with seven strands of hamstring tendons without remnant preservation was performed as reported by Zhao and Huangfu 17 . Single‐bundle PCL reconstruction in the MSA and LSA manner was performed as follows.
Creation of posteromedial and posterolateral portals
Anterolateral and anteromedial arthroscopy portals were fashioned, and the knee inspected. Any meniscal lesions were treated. The arthroscope was placed into the posteromedial compartment through the anterolateral portal. Under visualization a posteromedial portal was created, and a switching stitch put in and passed through the posterior septum into the posterolateral compartment. Then the scope was placed into the posterolateral compartment from the posteromedial portal. Under visualization a posterolateral portal was created above the joint line, and about 3 cm proximal from the posterior edge of the lateral femoral condyle at more than 90° knee flexion (Fig. 1).
Figure 1.
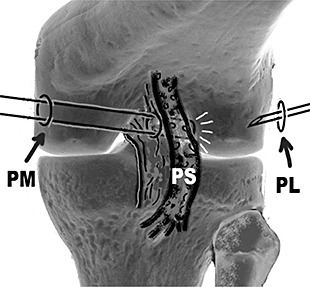
Creation of posterolateral portal. PL, posterolateral portal; PM, posteromedial portal; PS, posterior septum.
Exposure of the PCL tibial insertion site
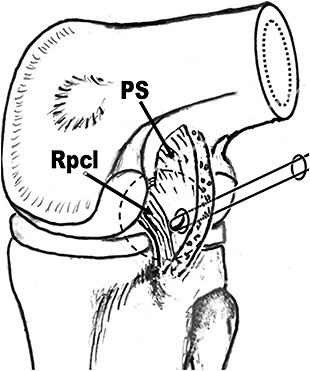
A shaver was placed into the posterolateral compartment through the posterolateral portal. Following retrieval of the scope back to the posteromedial compartment, the shaver was placed into the posterior septum. Then part of the posterior septum above and posterior to the PCL tibial insertion site was removed to expose the PCL at insertion (Fig. 2). To prevent injury to the posterior vascular structure passing nearby, the posterior septum tissue was firstly separated bluntly from any remnant PCL fibers with the shaver, and then removed with the shaver facing upwards. The un‐motorized shaver was used as a probe to define the posterior capsule to make sure the shaving maneuver was inside the joint. In the few cases in which the posterior capsule was too soft to be felt, all shaving maneuvers were performed with the shaver leaning against the remnant PCL fibers, which were always tight due to the automatic posterior sag of the tibia.
Figure 2.
Exposure of PCL insertion site (View from the posteromedial portal). PS, posterior septum; Rpcl, PCL remnant.
Creation of the tibial tunnels
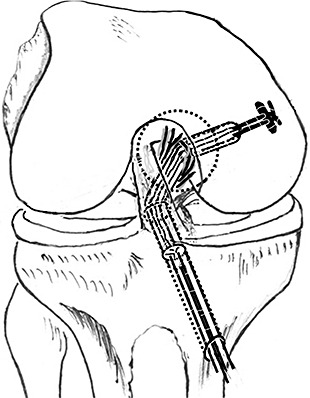
The scope was placed in through the posteromedial portal to visualize creation of the tibial tunnel. In the MSA group, the PCL tibial aiming device was placed from the anteromedial portal, through the interspace between PCL and medial femoral condyle, into the posterior compartment. In the LSA group, the PCL tibial aiming device was placed into the posterior compartment through the interspace between the PCL remnant and ACL. The centers of the inner openings of the tibial tunnels were 1 cm below the joint line in both groups, but located at the medial and lateral part of the PCL footprint respectively in the MSA and LSA group. A guide wire was drilled in from the medial side of the proximal tibia. The tibial tunnels angled the tibial axis by 45°. Then the guide wires were over‐drilled to create tibial tunnels to expected sizes (Fig. 3). No fluoroscopy was used. A curette was placed into the posterior compartment through the posterolateral portal to cover the tip of the guide wire when drilling the tibial tunnel, in order to prevent sudden protrusion of the drill and injury to the posterior vascular structure.
Figure 3.
Creation of tibial tunnel. LSA, lateral‐sided augmentation; MSA, medial‐sided augmentation; PS, posterior septum; Rpcl, PCL remnant.
Creation of the femoral tunnels
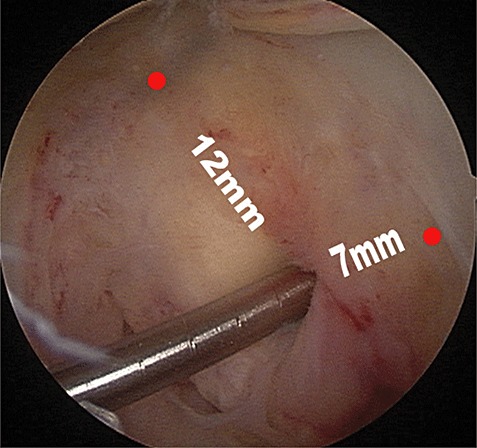
The femoral tunnels were created through an inside‐to‐outside approach. To locate the femoral tunnel as reproducibly as possible, we used the midpoint of the anterior edge of the femoral notch roof as a reference landmark (Fig. 4). The femoral tunnel was located 12 mm posterior to this landmark and 7 to 8 mm proximal to the distal cartilage edge. The inner part was equal to the graft in width and 25 mm long, the outer part was 4.5 mm wide. Bone debris left in the tunnels due to the remnant fibers overlying the inner opening was removed with the shaver, to avoid any hindrance to graft in‐placement.
Figure 4.
Location of the femoral tunnel: 12 mm from the midpoint of the anterior edge of the femoral notch roof and 7 mm from the distal cartilage.
Graft placement
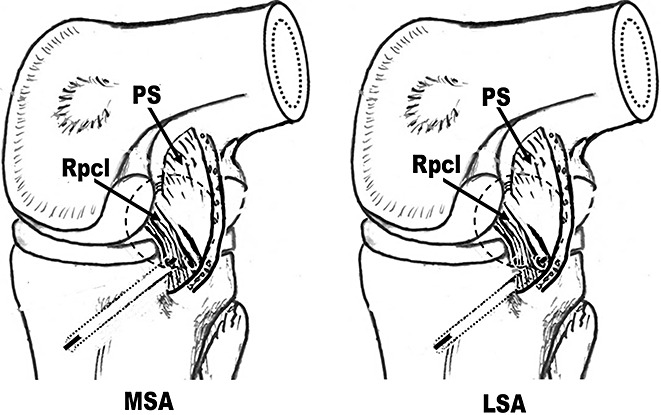
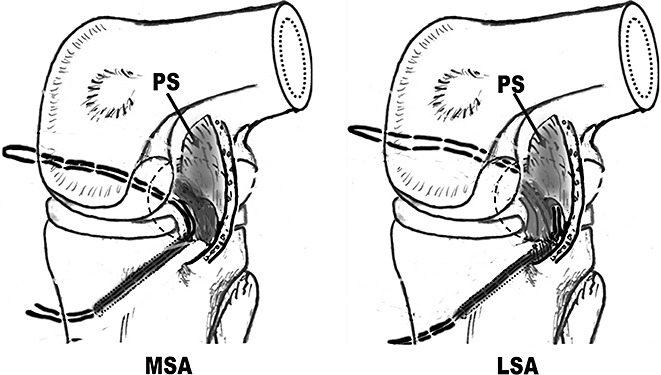
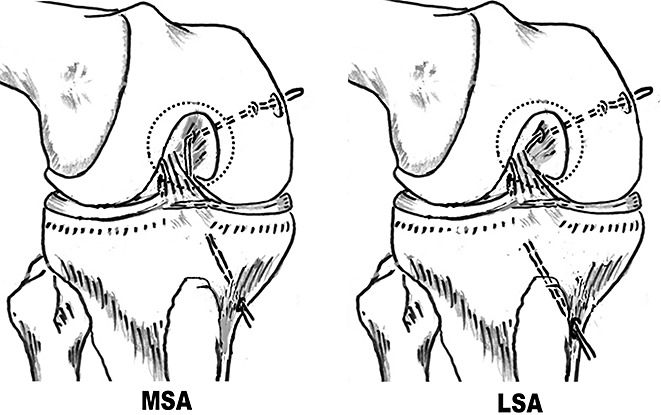
Guide threads were put into the posterior compartment through the tibial tunnel, passed through the medial or lateral side of the PCL respectively in the MSA and LSA groups (Fig. 5), and then through the femoral tunnels (Fig. 6). Then the tapes on the proximal side of the graft composite were passed through the tibial tunnels into the joint, and then outside the femoral tunnels.
Figure 5.
Placement of guide threads (View from the posteromedial portal). LSA, lateral‐sided augmentation; MSA, medial‐sided augmentation; PS, posterior septum.
Figure 6.
Placement of guide threads (View from the anterolateral portal). LSA, lateral‐sided augmentation; MSA, medial‐sided augmentation.
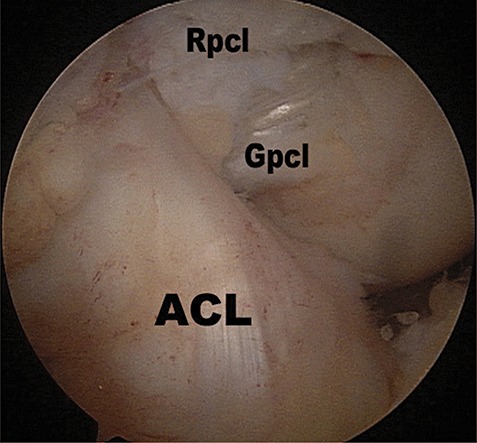
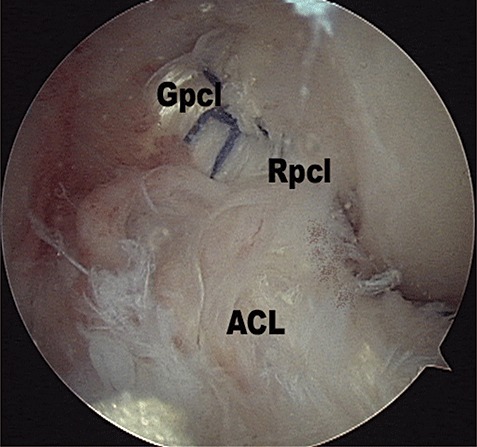
In the MSA group, a long hemostat was inserted into the posteromedial compartment through the posteromedial portal to the under side of the graft at the posterior curving point (the killer‐turn) of the graft. With the posterior side of the medial femoral condyle as a fulcrum, the hemostat was used as a lever to lift the graft into the joint, which was then pulled into the femoral tunnel (Fig. 7).
Figure 7.
Placement of graft in the MSA group. ACL, anterior cruciate ligament; Gpcl, PCL graft; Rpcl, PCL remnant.
In the LSA group, the scope was placed through the posteromedial portal. A hemostat was inserted through the posterolateral portal to the anterior side of the posterior curving of the graft composite, and used as a lever to lift the graft into the joint (Fig. 8). Finally the graft was pulled into the femoral tunnel (Fig. 9). Because the graft passed through the posterior septum, it was difficult, but not impossible, to finally pull it into the femoral tunnel.
Figure 8.
Placement of graft in LSA group (View from the posteromedial portal). PS, posterior septum.
Figure 9.
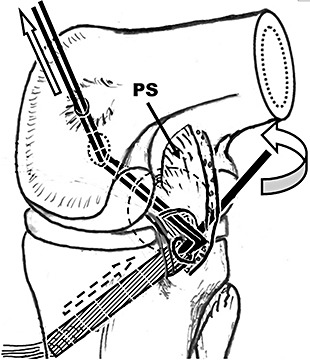
Placement of graft in LSA group (View from the anterolateral portal). ACL, anterior cruciate ligament; Gpcl, PCL graft; Rpcl, PCL remnant.
Graft fixation
The polyester tape was fixed on a mini‐plate (Aesculap, Tuttlingen, Germany) overlying the orifice of the femoral tunnel (Fig. 10). In 70° knee flexion and with maximal anterior drawing force against ACL imposed, the distal end of the anterolateral bundle was fixed on to a titanium button (Aesculap) over the outer opening. Then the knee was flexed several times to 70° to accommodate the graft composite. The tension of the graft was checked by pulling the distal threads. The buttons were rotated to increase graft tension to prevent them being pulled away from the orifice.
Figure 10.
Graft fixation in LSA group (View from the anterolateral portal).
Postoperative management
In all groups the patients followed the same rehabilitation protocol. The knee was braced in extension for the first 2 weeks. Isometric muscle contraction and patella manipulation were performed. Flexion and proprioception exercises began from the third week. Flexion to more than 120° was permitted at the end of the third month. The brace was used for the first 2 months at rest, locked in 0° to prevent extension limitation, and also used when bearing weight to prevent unexpected flexion. Three months post operation, the patients began running and other kinds of mobility training.
Post‐operative rehabilitation occurred at home, and was supervised by the senior author through outpatient consultation. At the last rehabilitation consultation two years after surgery, the patients were evaluated with IKDC, Lysholm, and Tegner rating scales. KT‐1000 examinations, with the neutral point determined by anterior drawing of the knee with maximal manual force, were done on all patients. The differences between the pre and post‐operative KT‐1000 examination results, subjective IKDC, Lysholm and Tegner scores in each group, and the differences in post‐operative KT‐1000 examination results, subjective IKDC, Lysholm and Tegner scores between each of the three groups were analyzed statistically through Student's t test and the χ2 test, with 0.05 as the significance level.
Results
Nineteen patients in the MSA group, 17 in the LSA group and all 16 patients in the control group were followed up for a minimum of two years. The mean length of surgery was 40 min (range, 30 to 55 min), 45 min (34 to 65 min) and 40 min (30 to 50 min) respectively in the MSA, LSA and control group. Partial meniscectomy was performed in two, three and three patients respectively in the MSA, LSA and control group. No neurovascular injury occurred during surgery. No infection or other complication occurred.
Range of motion
Before surgery, two patients in each of the MSA and LSA groups had 5° flexion limitation. All had normal extension. At the last follow‐up, one patient in each of the MSA and LSA group had 5° extension limitation; the other patients had normal knee extension. Two patients in each of the MSA and LSA groups had 5° flexion limitation, and the others had normal flexion.
Stability
At the latest follow‐up, KT‐1000 examination showed that in the MSA group the side‐to‐side differences in posterior laxity were 0 to 2 mm in 15 patients (78.9%), 3 to 5 mm in three (15.8%) and 6 to 10 mm in one (5.3%), with an average of 1.6 ± 1.2 mm. In the LSA group, the posterior laxities were 0 to 2 mm in 14 patients (82.3%), 3 to 5 mm in two (11.8%) and 6 to 10 mm in one (5.9%), with an average of 1.5 ± 1.3 mm. In the control group, they were 0 to 2 mm in 12 patients (75%), 3 to 5 mm in two (12.5%) and 6 to 10 mm in two (12.5%), with an average of 1.8 ± 1.5 mm (Table 2). The posterior drawer test showed negative in 14 patients, one plus in four and two plus in one in the MSA group; negative in 13 patients, one plus in one and two plus in two in the LSA group; and negative in 11, one plus in 2 and two plus in 3 in the control group.
Table 2.
Post‐operative results
| Groups | KT‐1000 results (side to side difference) | IKDC | Lysholm | Tegner | |||
|---|---|---|---|---|---|---|---|
| Patient numbers | Average laxity (x̄± SD)mm | ||||||
| <3 mm | 3–5 mm | >5 mm | |||||
| MSA (n = 19) | 15 | 3 | 1 | 1.6 ± 1.2 | 93.1 ± 3.8 | 95.0 ± 4.6 | 5.4 ± 0.9 |
| LSA (n = 17) | 14 | 2 | 1 | 1.5 ± 1.3 | 92.6 ± 4.1 | 93.7 ± 4.2 | 5.6 ± 0.7 |
| Control (n = 16) | 12 | 2 | 2 | 1.8 ± 1.5 | 90.1 ± 4.6 | 91.5 ± 4.0 | 5.2 ± 1.3 |
IKDC, International Knee Documentation Committee; LSA, lateral‐sided augmentation; MSA, medial‐sided augmentation.
Statistical analysis showed that there were no significant differences between the MSA and the LSA groups in regard to the KT‐1000 examination and posterior drawer test results in regard to posterior laxity. However, there were significant differences between each of the MSA and LSA groups and the control group.
The whole function of the knee
Before surgery, all patients were graded as abnormal or severely abnormal according to the IKDC knee examination form. At the final follow‐up, the patient numbers graded according to the IKDC scale as normal, nearly normal and abnormal were 14 (73.7%), four (21.1%), and one (5.3%) in the MSA group; 13 (76.5%), three (17.6%), and one (5.9%) in the LSA group; and 11 (68.8%), three (18.8%), and two (12.5%) in the control group. The IKDC subjective scores were 93.1 ± 3.8, 92.6 ± 4.1, and 90.1 ± 4.6; the Lysholm scores were 95.0 ± 4.6, 93.7 ± 4.2, and 91.5 ± 4.0; and the Tegner scores were 5.4 ± 0.9, 5.6 ± 0.7, and 5.2 ± 1.3 respectively in the MSA, LSA, and control groups (Table 2). The improvement in post‐operative functional scores in each group was statistically significant when compared with the preoperative results.
Statistical analysis showed that there were no significant differences between the MSA and LSA group regarding all subjective and objective results. However, there were statistically significant differences between each of the MSA and LSA groups and the control group in regard to the IKDC ligaments results, the IKDC subjective score, and the Lysholm score.
Discussion
Because so many variables influence the results of PCL reconstruction, the optimal techniques are still being pursued. Few clinical studies have been reported concerning the outcome of isolated PCL reconstructions. In studies of single‐bundle PCL reconstruction, with results evaluated according to the IKDC scale, 9%–23.8% of patients have been graded as abnormal or severely abnormal 5 , 9 , 10 , 13 . Analysis has shown that although almost the same graft materials have been used for PCL reconstruction as for ACL reconstruction, the results of PCL reconstruction are not comparable to those of ACL reconstruction. As for double‐bundle PCL reconstruction, although it has received interest in recent years, no advantage over single‐bundle PCL reconstruction has been established for it clinically 5 , 14 , 15 , 16 .
PCL reconstruction techniques with remnant preservation have been reported by several authors. Jung et al. have tensioned the PCL remnant and reconstructed the anterolateral bundle 13 . Wang et al. have performed single bundle PCL reconstruction using hamstring tendon grafts with remnant augmentation 9 . Yoon et al. have performed double‐bundle augmentation of the posterior cruciate ligament using split Achilles allografts 10 . We think that slack remnant fibers may contract during the healing of the new graft, and contribute to total failure load. In this study, stability and functional results in both the MSA and LSA groups, compared with those in the control group, showed that favorable results could be obtained through both of these remnant preservation techniques.
Through statistical analysis, we found no significant difference between the two augmentation techniques. The LSA technique is a little more difficult than the MSA technique to perform because the graft passes through the posterior septum. As no advantage of the LSA technique over the MSA technique was found through this study, we prefer the more simple MSA technique.
One obvious weakness of this study is that stress radiography, which has been reported to be more accurate than KT‐1000 examination for detecting posterior tibia displacement 19 , 20 , was not used to evaluate posterior knee laxity. This is due to the commercial unavailability of the Telos system in China. The other obvious weakness is that the patients in the control group were not in the same condition prior to surgery as those in the MSA and LSA groups, because PCL injury with no connecting remnant may indicate more severe injury than that with connecting remnant. Though patients with a connecting remnant before surgery who have the remnant removed during the operation may constitute better controls, we do not want to do such a study as we believe that remnant preservation is of benefit to the patients. Patient numbers are also limited. Tunnel creation inevitably releases some remnant fibers from the insertion, to what extent these unreleased remnant fibers play a role is still unknown. The tibial tunnels located at the medial and lateral part of the PCL footprint respectively in the MSA and LSA group, which means these were not normal PCL reconstruction techniques, may prevent meaningful interpretation of the results.
Arthroscopic single‐bundle PCL reconstruction with remnant preservation achieved 94.7% and 94.1% normal and nearly normal results respectively in the MSA and LSA group at a minimum of two years. There is no statistically significant difference between the two groups in regard to the subjective and objective results.
References
- 1. Clancy WG Jr, Shelbourne KD, Zoellner GB, et al. Treatment of knee joint instability secondary to rupture of the posterior cruciate ligament. Report of a new procedure. J Bone Joint Surg Am, 1983, 65: 310–322. [PubMed] [Google Scholar]
- 2. Berg EE. Posterior cruciate ligament tibial inlay reconstruction. Arthroscopy, 1995, 11: 69–76. [DOI] [PubMed] [Google Scholar]
- 3. Harner CD, Hoher J. Evaluation and treatment of posterior cruciate ligament injuries. Am J Sports Med, 1998, 26: 471–482. [DOI] [PubMed] [Google Scholar]
- 4. Mariani PP, Adriani E, Santori N, et al. Arthroscopic posterior cruciate ligament reconstruction with bone‐tendon‐bone patellar graft. Knee Surg Sports Traumatol Arthrosc, 1997, 5: 239–244. [DOI] [PubMed] [Google Scholar]
- 5. Chen CH, Chen WJ, Shih CH. Arthroscopic reconstruction of the posterior cruciate ligament: a comparison of quadriceps tendon autograft and quadruple hamstring tendon graft. Arthroscopy, 2002, 18: 603–612. [DOI] [PubMed] [Google Scholar]
- 6. Ahn JH, Chung YS, Oh I. Arthroscopic posterior cruciate ligament reconstruction using the posterior trans‐septal portal. Arthroscopy, 2003, 19: 101–107. [DOI] [PubMed] [Google Scholar]
- 7. Ahn JH, Yoo JC, Wang JH. Posterior cruciate ligament reconstruction: double‐loop hamstring tendon autograft versus Achilles tendon allograft—clinical results of a minimum 2‐year follow‐up. Arthroscopy, 2005, 21: 965–969. [DOI] [PubMed] [Google Scholar]
- 8. Sekiya JK, West RV, Ong BC, et al. Clinical outcomes after isolated arthroscopic single‐bundle posterior cruciate ligament reconstruction. Arthroscopy, 2005, 21: 1042–1050. [DOI] [PubMed] [Google Scholar]
- 9. Wang CJ, Chan YS, Weng LH. Posterior cruciate ligament reconstruction using hamstring tendon graft with remnant augmentation. Arthroscopy, 2005, 21: 1401. [DOI] [PubMed] [Google Scholar]
- 10. Yoon KH, Bae DK, Song SJ, et al. Arthroscopic double‐bundle augmentation of posterior cruciate ligament using split Achilles allograft. Arthroscopy, 2005, 21: 1436–1442. [DOI] [PubMed] [Google Scholar]
- 11. Seon JK, Song EK. Reconstruction of isolated posterior cruciate ligament injuries: a clinical comparison of the transtibial and tibial inlay techniques. Arthroscopy, 2006, 22: 27–32. [DOI] [PubMed] [Google Scholar]
- 12. MacGillivray JD, Stein BE, Park M, et al. Comparison of tibial inlay versus transtibial techniques for isolated posterior cruciate ligament reconstruction: minimum 2‐year follow‐up. Arthroscopy, 2006, 22: 320–328. [DOI] [PubMed] [Google Scholar]
- 13. Jung YB, Jung HJ, Tae SK, et al. Tensioning of remnant posterior cruciate ligament and reconstruction of anterolateral bundle in chronic posterior cruciate ligament injury. Arthroscopy, 2006, 22: 329–338. [DOI] [PubMed] [Google Scholar]
- 14. Makino A, Aponte Tinao L, Ayerza MA, et al. Anatomic double‐bundle posterior cruciate ligament reconstruction using double‐double tunnel with tibial anterior and posterior fresh‐frozen allograft. Arthroscopy, 2006, 22: 684–e1.‐5. [DOI] [PubMed] [Google Scholar]
- 15. Garofalo R, Jolles BM, Moretti B, et al. Double‐bundle transtibial posterior cruciate ligament reconstruction with a tendon‐patellar bone‐semitendinosus tendon autograft: clinical results with a minimum of 2 years’ follow‐up. Arthroscopy, 2006, 22: 1331–1338.e1. [DOI] [PubMed] [Google Scholar]
- 16. Hatayama K, Higuchi H, Kimura M, et al. A comparison of arthroscopic single‐ and double‐bundle posterior cruciate ligament reconstruction: review of 20 cases. Am J Orthop, 2006, 35: 568–571. [PubMed] [Google Scholar]
- 17. Zhao J, Huangfu X. Arthroscopic single‐bundle posterior cruciate ligament reconstruction: retrospective review of 4‐ versus 7‐strand hamstring tendon graft. Knee, 2007, 14: 301–305. [DOI] [PubMed] [Google Scholar]
- 18. Zhao J, He Y, Wang J. Simultaneous arthroscopic reconstruction of the anterior and posterior cruciate ligaments with autogenous hamstring tendons. Arthroscopy, 2006, 22: 497–504. [DOI] [PubMed] [Google Scholar]
- 19. Garavaglia G, Lubbeke A, Dubois‐Ferriere V, et al. Accuracy of stress radiography techniques in grading isolated and combined posterior knee injuries: A cadaveric study. Am J Sports Med 2007; 35: 2051–2056. [DOI] [PubMed] [Google Scholar]
- 20. Schulz MS, Russe K, Lampakis G, et al. Reliability of stress radiography for evaluation of posterior knee laxity. Am J Sports Med 2005; 33: 502‐506. [DOI] [PubMed] [Google Scholar]


