Abstract
Cardiac aspergillosis is a rare fungal infection that affects the heart and/or pericardium of immunocompromised patients. Here, the authors report a rare case of a 36-year-old female with aspergillus pericarditis. The patient was diagnosed with infective endocarditis and splenic infarction and treated with emergency splenectomy and double-valve replacement surgery. During the surgery, a fibropurulent pericardial tissue was found and excised. The culture report of the tissue confirmed the diagnosis of aspergillus pericarditis. The patient was successfully managed with intravenous voriconazole. Aspergillus do not usually infect the pericardium and such infections are rarely detected premortem, especially during a cardiac surgery. In this report, the infection was accidentally detected during the double-valve surgery. The authors conclude that because of its nonspecific clinical manifestations, a high degree of clinical suspicion is required for the early diagnosis and treatment of aspergillus pericarditis.
Keywords: Aspergillus pericarditis, corticosteroid, double-valve replacement surgery, infective endocarditis, voriconazole
INTRODUCTION
Cardiac aspergillosis is a rare fungal infection that affects the heart and/or pericardium of immunocompromised patients due to disseminated fungemia or invasive aspergillosis.[1] The risk factors for Aspergillus infection include prolonged use of antibiotics, immunosuppressive agents and parenteral nutrition.[1] Specifically, aspergillus pericarditis is the infection of the pericardium by Aspergillus, accounting for about 17% of all cardiac aspergillosis cases.[2] However, aspergillus pericarditis has a poor prognostic outcome, and thus its premortem diagnosis is challenging. For example, in a study that analyzed 29 aspergillus pericarditis cases, it was found that premortem diagnosis was made in only 10 cases.[3]
Here, the authors report a case of aspergillus pericarditis in a patient with infective endocarditis, splenic infarction and antiphospholipid syndrome. Considering the rarity of this infection, the authors describe this case with the objective of adding evidence to the limited existing literature, and thus improve its understanding and management.
CASE REPORT
A 36-year-old female recently diagnosed with infective endocarditis was referred to the Cardiac Surgery Unit at King Fahd Hospital of the University, Al Khobar, Saudi Arabia, for further evaluation. The patient presented with severe abdominal pain that had recently exacerbated, making her homebound. She also experienced orthopnea, exertional dyspnea, paroxysmal nocturnal dyspnea, cough and fatigue. Other comorbidities of the patient included rheumatic heart disease (since the age of 17 years), antiphospholipid syndrome with multiple miscarriages, transient ischemic attacks and deep venous thrombosis. Her rheumatic heart disease was characterized by moderate mitral regurgitation associated with thickened, fibrotic mitral valve leaflet and dilated annulus in the echocardiography, with follow-up at the cardiology unit of King Khalid Hospital, Hafr Al-Batin, Saudi Arabia. Further, the patient had mild left atrial dilatation, and diuretic therapy had been initiated shortly before infective endocarditis. In addition, she had been taking rivaroxaban (20 mg) to prevent the recurrence of venous thromboembolism and stroke as well as she was on chronic prednisolone treatment for her connective tissue disease (antiphospholipid antibody syndrome).
On physical examination, the patient was conscious, alert and oriented. Her blood pressure was normal, but she had tachycardia and tachypnea. She also had an oxygen saturation of 88% and bilateral coarse crepitation. Precordial examination revealed a pansystolic murmur in the mitral area. There were no signs of infective endocarditis, jugular venous pressure distension and peripheral edema. To treat pulmonary congestion, furosemide (40 mg) was administered intravenously, following which her condition improved. A chest X-ray revealed bilateral opacification in the lower zones, suggesting bilateral pleural effusion. The fluid was drained with a pigtail catheter under ultrasound guidance and was confirmed as exudate through laboratory investigations. The fluid was also sent for further microbiological analysis. A computed tomography (CT) scan of the chest and abdomen was requested to investigate her abdominal pain, and it revealed a large area of fluid density affecting the anterior aspect of the spleen and resulting in a bulging contour, likely representing splenic abscess. An echocardiographic study showed severe mitral regurgitation with a mass (measuring 7 mm × 7 mm; likely a vegetation of endocarditis) attached to the tip of the anterior mitral leaflet [Figure 1a and b]. In addition, the echocardiographic study showed a large mass (measuring 15 mm × 11 mm) (likely another vegetation) attached to the ventricular aspect of posterior cusp of the aortic valve associated with aortic regurgitation [Figure 2]. Further, a moderate-sized pericardial effusion was also noted in the echocardiogram and the left ventricular ejection fraction was 55%–60%. The blood culture was positive for Enterococcus faecalis. Based on these findings, her condition was diagnosed as infective endocarditis complicated by splenic infarction. Subsequently, the patient was admitted to the coronary care unit (CCU) for emergency splenectomy and double-valve replacement with tissue valve. Meanwhile, for the management of infective endocarditis, the infectious disease team initiated intravenous ampicillin 1 g and ceftriaxone 1 g at 12-hour intervals for 3 weeks, with a daily follow-up.
Figure 1.
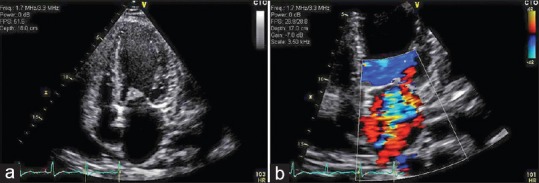
(a) An apical view of the echocardiogram showing vegetation in the anterior mitral leaflet; (b) An apical view of the echocardiogram with Doppler showing regurgitation
Figure 2.
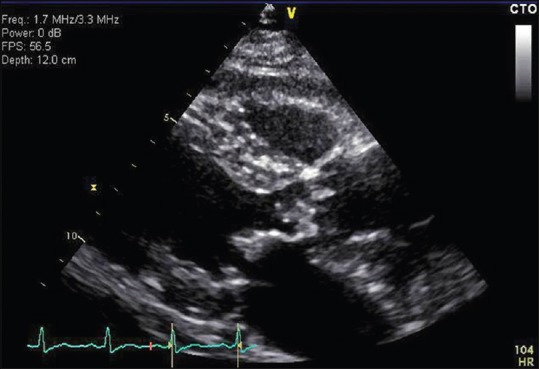
A parasternal view of the echocardiogram showing vegetation in the aortic valve
Open splenectomy was performed through a midline incision, and an abscess was observed and drained. The double-valve replacement surgery was then carried out under hypothermic cardioplegic arrest with an extracorporeal circulation. A fibropurulent tissue was observed in the pericardium and was accordingly removed. Finally, samples were taken from the splenic abscess, valves vegetation and pericardial tissue for further analysis. The culture results of the valve vegetation confirmed E. faecalis infection. Accordingly, the patient continued receiving the ampicillin and ceftriaxone regimen for infective endocarditis. However, the postoperative course was not smooth. On the 4th postoperative day, her hemoglobin decreased significantly. An ultrasound scan revealed free fluid in the abdomen, and a CT scan showed an intra-abdominal hematoma. On the 5th postoperative day, an exploratory laparotomy and blood evacuation were performed. On the 6th postoperative day, microscopic and culture results of the pericardial tissue revealed significant growth of Aspergillus [Figure 3a and b]. The culture of the valve vegetation was negative for any fungal growth, and the pleural fluid culture was positive for Aspergillus. To treat aspergillus pericarditis, intravenous voriconazole (360 mg) was administered twice daily for 1 day, after which the dose was changed to 240 mg twice daily for 1 week, with daily follow-up. On the 9th postoperative day, the course was smooth, and she was transferred from the CCU to the surgical ward and her antifungal treatment was continued under the supervision of the infectious disease team. The patient was followed up daily for 1 week by the infectious disease team, and there were no further complications. Consequently, the patient was discharged with a prescription of oral voriconazole 200 mg/day for 8 weeks. The patient remained symptom free when seen at the infectious disease clinic after 2 months.
Figure 3.
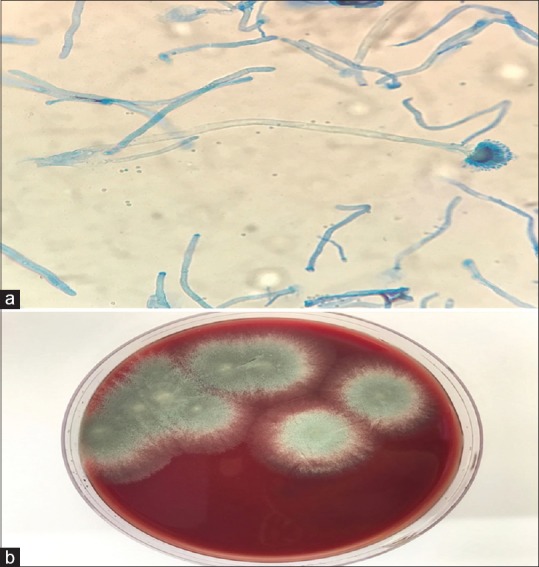
(a) A microscopic view of the pericardial tissue showing Aspergillus; (b) Culture of the pericardial tissue showing Aspergillus
DISCUSSION
Fungal infection of the pericardium is rare.[4] In an autopsy study that analyzed 60 patients with fungal infections of the heart, only 2 cases (3.5%) were found to have had pericardial infection.[5] Similarly, Aspergillus are known to infect the heart but not the pericardium and are rarely isolated premortem.[4,5] In the case described here, an occurrence of aspergillus pericarditis was accidentally detected during the double-valve surgery to treat the initial diagnosis. Subsequently, the patient was successfully managed with voriconazole.
The diagnosis of aspergillus pericarditis is very challenging owing to its lack of characteristic signs and symptoms. According to Biso et al.,[4] aspergillus pericarditis most commonly presents as chest pain, hypotension, cardiac tamponade and pericardial friction rub.[4] However, Le Moing et al.[3] found that of the 30 cases with aspergillus pericarditis, only 8 presented with cardiac tamponade. Our patient presented with abdominal pain, tachypnea, tachycardia, pansystolic murmurs, bilateral course crepitation and decreased oxygen saturation, which were unusual presentations.
In our case, the diagnosis of aspergillus pericarditis was confirmed premortem when the pericardial tissue samples obtained during the surgery returned positive for Aspergillus growth. Blood culture and valve vegetation tests also confirmed that the patient had E. faecalis infection.
The risk factors of invasive aspergillosis include granulocytopenia in leukemic patients, neutropenia following bone marrow or organ transplantation and extensive corticosteroid use or cytotoxic drug therapy.[6] Similarly, in our case, the patient had a history of prolonged use of corticosteroids. It should be noted that pericardial aspergillus is lethal in immunocompromised patients.[4]
Pericardial aspergillus can be due to either the mycotic emboli spreading hematogenously or directly from an infected endocardium.[7] Therefore, culture of the valve vegetation and blood are required to detect the source of infection. Besides, infective endocarditis with valve regurgitation is more likely due to a known rheumatic heart disease. Our case also had a splenic abscess, which suggests a possibility of Aspergillus disseminating and infecting the spleen; however, this hypothesis requires further investigation. Electro- and echocardiographic changes of pericarditis in a patient with pulmonary or disseminated Aspergillus infection may help to predict the cause. Patients with pancytopenia require a diagnostic and therapeutic pericardiocentesis to diagnose aspergillus pericarditis. Further, pericardial fluid examination can assist in diagnosis owing to its distinct physical features such as clear and straw-colored serosanguinous and grayish-green purulent.[8] Immunologically, recent studies revealed that serum antigens using galactomannan and 1,3-beta-D-glucan in the appropriate clinical setting are sufficient to make a presumptive diagnosis and initiate treatment.[6]
The initial therapy for invasive aspergillosis is voriconazole. This broad-spectrum antifungal agent has been shown to be effective and is associated with lower mortality rates than amphotericin B.[9] Other treatment strategies include combination therapy with voriconazole and echinocandins. Combination therapy can be used both as a primary treatment or as a salvage therapy.[10] In our case, voriconazole administered alone was sufficient.
In summary, this case report describes the diagnosis and management of a rare aspergillus pericarditis in a patient with bacterial infective endocarditis and splenic infarction. The authors concur with other authors who found that long-term use of corticosteroids is likely a risk factor of aspergillus pericarditis. Finally, because aspergillus pericarditis has nonspecific clinical manifestations, a high degree of clinical suspicion is required for early detection and diagnosis. Once diagnosed, an aggressive treatment is required.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient's brother has given his consent for his sister's images and other clinical information to be reported in the Journal. The patient's brother understands that his sister's name and initial would not be published and due efforts will be made to conceal her identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Cishek MB, Yost B, Schaefer S. Cardiac aspergillosis presenting as myocardial infarction. Clin Cardiol. 1996;19:824–7. doi: 10.1002/clc.4960191012. [DOI] [PubMed] [Google Scholar]
- 2.Comaru Pasqualotto AC, editor. Aspergillosis: From Diagnosis to Prevention. Netherlands: Springer; 2010. [Google Scholar]
- 3.Le Moing V, Lortholary O, Timsit JF, Couvelard A, Bouges-Michel C, Wolff M, et al. Aspergillus pericarditis with tamponade: Report of a successfully treated case and review. Clin Infect Dis. 1998;26:451–60. doi: 10.1086/516326. [DOI] [PubMed] [Google Scholar]
- 4.Biso S, Lekkham R, Climaco A. Aspergillus pericarditis with tamponade in a renal transplant patient. Case Rep Cardiol. 2017;2017:7134586. doi: 10.1155/2017/7134586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Atkinson JB, Connor DH, Robinowitz M, McAllister HA, Virmani R. Cardiac fungal infections: Review of autopsy findings in 60 patients. Hum Pathol. 1984;15:935–42. doi: 10.1016/s0046-8177(84)80123-9. [DOI] [PubMed] [Google Scholar]
- 6.Kupsky DF, Alaswad K, Rabbani BT. A rare case of aspergillus pericarditis with associated myocardial abscess and echocardiographic response to therapy. Echocardiography. 2016;33:1085–8. doi: 10.1111/echo.13214. [DOI] [PubMed] [Google Scholar]
- 7.Dimopoulos G, Frantzeskaki F, Kosmopoulos M, Taccone FS, van den Abeele AM, Bulpa P, et al. Endomyocardial and pericardial aspergillosis in critically ill patients. Mycoses. 2017;60:576–80. doi: 10.1111/myc.12630. [DOI] [PubMed] [Google Scholar]
- 8.Walsh TJ, Bulkley BH. Aspergillus pericarditis: Clinical and pathologic features in the immunocompromised patient. Cancer. 1982;49:48–54. doi: 10.1002/1097-0142(19820101)49:1<48::aid-cncr2820490112>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Segal BH, Walsh TJ. Current approaches to diagnosis and treatment of invasive aspergillosis. Am J Respir Crit Care Med. 2006;173:707–17. doi: 10.1164/rccm.200505-727SO. [DOI] [PubMed] [Google Scholar]
- 10.Marr KA, Schlamm HT, Herbrecht R, Rottinghaus ST, Bow EJ, Cornely OA, et al. Combination antifungal therapy for invasive aspergillosis: A randomized trial. Ann Intern Med. 2015;162:81–9. doi: 10.7326/M13-2508. [DOI] [PubMed] [Google Scholar]


