Abstract
Human leukocyte antigen (HLA) alleles have been implicated as risk factors for immune-mediated adverse drug reactions. The authors recently reported a strong association between HLA-A*32:01 and vancomycin-induced drug reaction with eosinophilia and systemic symptoms. Identification of individuals with the risk allele before or shortly after the initiation of vancomycin therapy is of great clinical importance to prevent morbidity and mortality, and improve drug safety and antibiotic treatment options. A prerequisite to the success of pharmacogenetic screening tests is the development of simple, robust, cost-effective single HLA allele test that can be implemented in routine diagnostic laboratories. In this study, the authors developed a simple, real-time allele-specific PCR for typing the HLA-A*32:01 allele. Four-hundred and fifty-eight DNA samples including 30 HLA-A*32:01–positive samples were typed by allele-specific PCR. Compared with American Society for Histocompatibility and Immunogenetics–accredited, sequence-based, high-resolution, full-allelic HLA typing, this assay demonstrates 100% accuracy, 100% sensitivity (95% CI, 88.43% to 100%), and 100% specificity (95% CI, 99.14% to 100%). The lowest limit of detection of this assay using PowerUp SYBR Green is 10 ng of template DNA. The assay demonstrates a sensitivity and specificity to differentiate the HLA-A*32:01 allele from closely related non–HLA-A*32 alleles and may be used in clinical settings to identify individuals with the risk allele before or during the course of vancomycin therapy.
Human leukocyte antigens (HLA) play a key role in the development of severe immune-mediated adverse drug reactions.1 Multiple phenotypically distinct immune-mediated adverse drug reactions have been associated in particular with the carriage of specific HLA class I risk alleles.2 HLA alleles are highly polymorphic genes and are mainly involved in antigen recognition as a trimolecular complex of MHC–peptide–T-cell receptor. Thus, the presence of a specific HLA allele determines the repertoire of epitopes that can be presented, restricting the specificity of reacting T cells.3
The authors recently reported a strong association between HLA-A*32:01 and vancomycin-induced drug reaction with eosinophilia and systemic symptoms (DRESS) in patients of European ancestry and showed that approximately 20% of subjects exposed to vancomycin for more than 2 weeks can be expected to develop DRESS.4 Vancomycin is a widely used antibiotic for the treatment of frequently encountered serious Gram-positive bacterial infections, including methicillin-resistant Staphylococcus aureus. In reports of life-threatening T-cell–mediated reactions, such as DRESS, vancomycin features prominently in causality, and recent studies suggest that vancomycin is the most common cause of antibiotic-related DRESS.5, 6 DRESS is a rare, but potentially life-threatening, T-cell–mediated and multisystem disorder characterized by the delayed onset of fever, a widespread rash, white cell abnormalities, and the involvement of internal organs following administration of a drug.7, 8 The mortality rate of DRESS is up to 10%, and long-term sequelae have been described up to 4 years following acute disease.9 Given that vancomycin is often initiated empirically as part of combination therapy in an emergent setting of severe infection and that DRESS typically takes 2 or more weeks of drug exposure to manifest clinically, it is proposed that the antibiotic should not be delayed but that HLA-A*32:01 testing be ordered immediately and that consideration be given to substituting vancomycin for an alternative antibiotic if the test result returns positive. HLA-A*32:01 testing may also have diagnostic utility and assist in drug causality assignment in subjects who have symptoms in keeping with DRESS, improving the safety of future antibiotic therapy. A single-allele assay for HLA-A*32:01 with a turnaround time of <2 days would hence be of clinical utility and a potentially cost-effective option to improve the safety of this antibiotic that is in widespread global use.
Conventional HLA typing is done by serological or sequence-specific typing methods such as PCR amplification with use of sequence-specific oligonucleotide probes or sequence-based typing (SBT) techniques. Standard serological approaches lack specificity, because commercially available monoclonal antibodies cross-react with different HLA alleles.10 HLA typing using sequence-specific oligonucleotide probes sometimes results in low-resolution products that are unable to resolve some HLA alleles.11 HLA typing by SBT is able to resolve HLA alleles with high resolution but is comparatively expensive, requires specialized DNA sequencing equipment and a skilled operator for analysis, and is time consuming to prepare and analyze results. This study tested the potential of using a simple and fast PCR assay that utilizes allele-specific PCR (AS-PCR). This AS-PCR method should be less susceptible to laboratory or analysis errors, and be easier and less expensive to implement as a clinical test for the presence and absence of carriage of HLA-A*32:01.
Materials and Methods
DNA Samples
DNA samples were drawn from the Vanderbilt BioVU biorepository, which represents DNA linked to a deidentified electronic health record. Genomic DNA is extracted from discarded EDTA blood samples using the Qiagen automated DNA purification kit (Qiagen, Valencia, CA).
The study population was selected from the Vanderbilt Electronic Systems for Pharmacogenomic Assessment (VESPA) cohort.12 For this study, 458 DNA samples from this databank were analyzed by AS-PCR/melting curve. These DNA samples from the VESPA cohort had previously undergone high-resolution, full-allelic HLA typing by next-generation sequencing and in-depth genotyping with structured race assignment.13 DNA sample identity was blinded to the operator (F.X.R.) at the time of the validation of the assay. The DNA samples were of good quality with a mean DNA concentration of 50 ng/μL and a 260/280 ratio over 1.7. For this assay, the DNA concentration of samples were normalized to a concentration of 25 ng/μL with sterile deionized water (Cat# W3500; Sigma-Aldrich, Castle Hill, NSW, Australia). The study sample contained a good representation of the HLA-A*32:01 allele (n = 30) with a broad range of HLA-A*32 closely related alleles as listed in Table 1.
Table 1.
HLA Genotypes of Samples Eliminated by the Present Assay (HLA-A*32:01 AS-PCR)
| HLA-A specificities | n |
|---|---|
| A*01:01 | 123 |
| A*02:01, A*02:05 | 202 |
| A*11:01 | 45 |
| A*23:01 | 9 |
| A*24:02 | 41 |
| A*25:01 | 12 |
| A*26:01 | 18 |
| A*29:02 | 28 |
| A*30:01 | 21 |
| A*31:01 | 31 |
| A*33:01 | 7 |
| A*66:01 | 2 |
| A*68:01, A*68:02 | 49 |
| A*74:01:01 | 3 |
| A*74:11 | 1 |
HLA, human leucocyte antigen.
Primers
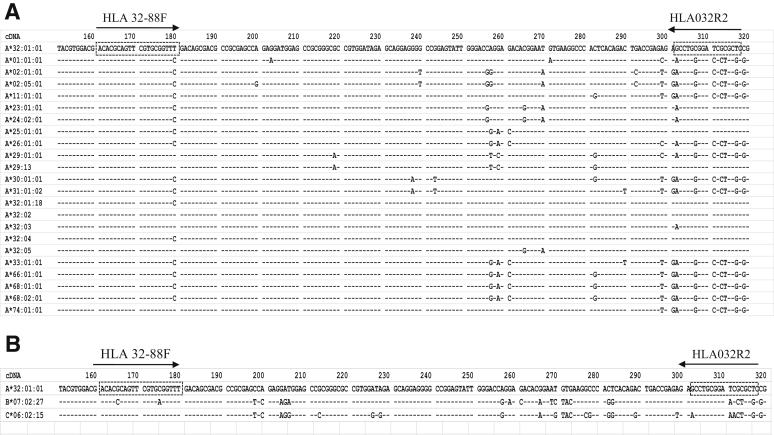
Primers (Table 2) were designed within the exon 2 of the HLA-A locus. Exon 2 DNA sequences of HLA-A*32 group and other closest alleles (Table 1) from the IMGT/HLA database were aligned with HLA-A*32:01:01 using the IMGT/HLA alignment tool (IMGT/HLA database, https://www.ebi.ac.uk/ipd/imgt/hla/align.html, last accessed March 8, 2019). The forward primer is a locked nucleic acid primer (LNA) and contains the sequence unique to the allele groups HLA-A*32 (except A*32:04 and A*32:01:18), A*29, and A*74. The reverse primer contains the sequence of the HLA-A*32 group (except A*32:03) and of the HLA-A*25 allele group. This primer set combination specifically amplifies all HLA-A*32 alleles, except A*32:03, A*32:04, and A*32:01:18, yielding a 157-bp product. HLA-A*29:13 allele is the only non–HLA-A*32 group allele that can be amplified with this primer set combination (Figure 1A). Alignments of exon 2 of all HLA-B and HLA-C alleles (Figure 1B) show that no other class I alleles are targeted by this primer combination, except for HLA-B*07:27 and HLA-C*06:02:15, that could potentially be amplified. However, there are quite a number of mismatches in the reverse primer, and the frequency of these alleles is very low in the global population. Internal control primers were designed to amplify the highly conserved housekeeping gene galactosylceramidase (GALC) (HGNC:4115), yielding a 352-bp product.
Table 2.
Primer Sequences for HLA Typing of HLA-A*32:01 Allele
| Primer | Description | Sequence | Target |
|---|---|---|---|
| HLA 32-88F | HLA-A*32 forward primer | 5′-GACGACACGCAGTTCGTGCGGTT+T-3′ | HLA-A*32 |
| HLA 032R2 | HLA-A*32 reverse primer | 5′-GAGCGCGATCCGCAGGC-3′ | HLA-A*32 |
| GALC-F: | GALC forward primer | 5′-TTACCCAGAGCCCTATCGTTCT-3′ | GALC |
| GALC-R: | GALC reverse primer | 5′-GTCTGCCCATCACCACCTATT-3′ | GALC |
Plus sign marks the location of a locked nucleic acid in the primer sequence.
HLA, human leucocyte antigen.
Figure 1.
Binding sites of the set of primers and comparison of the different allele sequences of the target region. Exon 2 DNA sequences of HLA-A*32 group and other closest alleles from IMGT/HLA database were aligned with HLA-A*32:01:01 using the IMGT/HLA alignment tool (IMGT/HLA database, https://www.ebi.ac.uk/ipd/imgt/hla/align.html, last accessed March 8, 2019). Primer positions are marked by dashed boxes. The arrows show the direction of the forward primer: HLA 32-88F and reverse primer: HLA032R2. A: The forward primer: HLA 32-88F: 5′-ACACGCAGTTCGTGCGGTT+T-3′ is a locked nucleic acid primer (locked at position 180) and contains the sequence unique to the allele groups HLA-A*32 (except A*32:04 and A*32:01:18), A*29, and A*74 (plus sign marks the location of a locked nucleic acid in the primer sequence). The reverse primer: HLA032R2: 5′-GAGCGCGATCCGCAGGC-3′ contains the sequence of the HLA-A*32 group (except A*32:03) and of the HLA-A*25 allele group. The combination of forward and reverse primers is specific for and amplify all HLA-A*32 alleles, except A*32:03, A*32:04, and A*32:01:18. It is worth noting that HLA-A*29:13 allele is the only non–HLA-A*32 group allele that can be amplified with this primer combination. B: Alignment of exon 2 of HLA-B and HLA-C alleles (IMGT/HLA database) shows that only HLA-B*07:02:27 and HLA-C*06:15 may be amplified by this primer set combination; however, there are quite a number of mismatches within the reverse primer that may prevent the amplification.
Allele-Specific PCR for Detection of HLA-A*32:01
The real-time PCR reaction contained 2 μL (50 ng) of total DNA, 1× PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Scoresby, VIC, Australia), 250 nmol/L of each HLA-A*32–specific primer, and 50 nmol/L of each GALC primer in a 10-μL final volume. The master mix was dispensed on a 96- or 384-well real-time quantitative PCR (qPCR) plate using the Mantis Liquid Handler (Formulatrix, Bedford, MA) by using a high-volume chip. DNA samples were stamped on a 96- or 384-well qPCR plate straight from the DNA storage plates using the Biomek FXP liquid handler (Beckman Coulter, Lane Cove West, NSW, Australia).
The real-time PCR was performed in 96- or 384-well optical plates on a Bio-Rad CFX96/384 qPCR machine (Bio-Rad Laboratories, Gladesville, NSW, Australia) using the following cycling conditions: initial denaturation at 96°C for 6 minutes to allow polymerase activation, followed by 35 cycles at 96°C for 30 seconds and at 62°C for 1 minute. This was followed by a melting curve cycle from 65°C to 95°C with 0.5°C increments for 5 seconds. The conditions of the PCR, such as primer concentrations and cycling conditions, were optimized to enable a clear separation of both the HLA-A*32–specific melting point (Tm) peak compared with the internal control Tm peak during melt curve analysis.
A standard AS-PCR was performed using the same reaction conditions as for the real-time PCR. The standard AS-PCR was performed in 96-well half-skirt PCR plates (AXYGEN Scientific, Corning, NY) on the Bio-Rad C1000 thermocycler (Bio-Rad Laboratories). PCR products were analyzed by electrophoresis on a 1% agarose gel containing 0.2 μg/mL ethidium bromide and run at 115 V for 30 minutes at room temperature. The gel was visualized by a transilluminator (ChemiDoc XRS+; Bio-Rad Laboratories).
Data Analysis
Raw real-time PCR data were analyzed using CFX Manager Software version 3.0 (Bio-Rad Laboratories). Statistical analyses for validation studies to calculate sensitivity, specificity, and confidence intervals were performed using GraphPad Prism software version 5.02 for Windows (GraphPad Software, San Diego, CA).
Results
To validate this HLA-A*32:01 typing assay, 458 samples previously typed using American Society for Histocompatibility and Immunogenetics–accredited, sequence-based, high-resolution, full-allelic HLA typing were analyzed using real-time PCR with PowerUp SYBR Green Master Mix.13 All 30 positive samples of the 458 samples were accurately identified as positive for the HLA-A*32:01 allele.
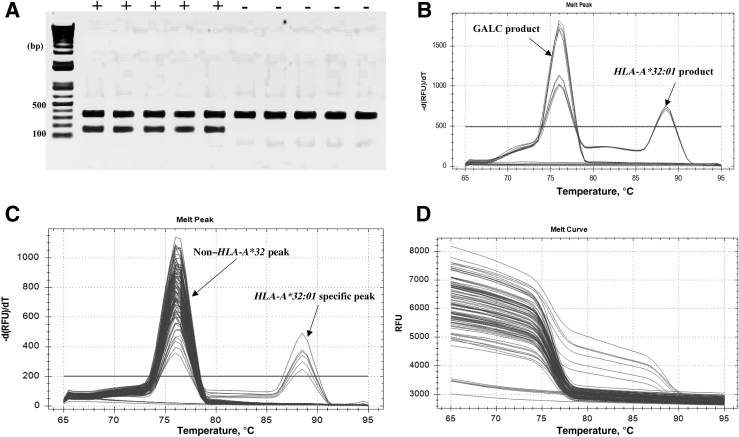
Samples were called positive or negative for HLA-A*32:01 based on the presence or absence of HLA-A*32:01–specific melt peaks. HLA-A*32:01–positive samples (n = 30) showed two peaks at 88.5°C ± 0.0°C (means ± SEM; range: 88.50°C to 88.50°C) for the HLA-A*32:01 allele and 76.05°C ± 0.03°C (means ± SEM; range: 76.00°C to 76.50°C) for GALC. The other 428 non–HLA-A*32 samples showed a single peak at 76.07°C ± 0.01°C (means ± SEM; range: 75.50°C to 76.50°C) for GALC (Figure 2). No melting curves were detectable for the nontemplate negative controls (Table 3).
Figure 2.
AS-PCR results. A:HLA-A*32:01–positive (+) and non–HLA-A*32 (−) samples were amplified by PCR using the following validated primers: HLA 32-88F forward primer and HLA032R2 reverse primer to amplify the HLA-A*32 allele. GALC-F and GALC-R primers were used to amplify the internal control housekeeping gene in a multiplexed reaction. PCR products were run on a 1% agarose gel containing 0.2 μg/mL ethidium bromide at 115 V for 30 minutes. The gel was visualized by a transilluminator (ChemiDoc XRS+; Bio-Rad Laboratories). HLA-A*32:01–positive samples show two bands of 157 bp (HLA-A*32:01 product) and 352 bp (GALC product). Non–HLA-A*32 samples show only one band of 352 bp (GALC product). B: Tm peaks of both HLA-A*32:01 and GALC housekeeping gene are shown by an arrow. Tm peaks for the HLA-A*32:01 allele were clearly separate from the GALC Tm peak following melt curve analysis. C: Melting peaks for a subset of the 458 samples tested in a real-time PCR with PowerUp SYBR Green are shown. Tm peaks of HLA-A*32:01 and non–HLA-A*32:01 alleles are shown by arrows. HLA-A*32:01–positive samples show double Tm peaks at 88.5°C ± 0.0°C (range: 88.50°C to 88.50°C) for the HLA-A*32:01 allele and 76.05°C ± 0.03°C (range: 76.00°C to 76.50°C) for GALC. Non–HLA-A*32 allele samples show a single Tm peak at 76.07°C ± 0.01°C (range 75.50°C to 76.50°C) for GALC. D: Melting curves for a subset of the 458 samples tested in real-time PCR. Data are expressed as means ± SEM. n = 30 (C, HLA-A*32:01–positive samples). RFU, relative fluorescence units; Tm, melting point.
Table 3.
Melting Curve Analysis of HLA-A*32:01 and GALC Amplicons
| HLA alleles | Tm peaks |
Validation | ||
|---|---|---|---|---|
| HLA-A*32:01 | GALC | Amplicon length, bp | ||
| HLA-A*32:01 | 88.5 | 76 | 157 | Positive |
| Non–HLA-A*32 | None | 76 | 352 | Negative |
GALC, galactosylceramidase; Tm, melting point.
For the standard AS-PCR, samples were called positive or negative based on the presence or absence of the HLA-A*32:01–specific PCR product after agarose gel electrophoresis. HLA-A*32:01–positive samples showed two bands of 157 bp (HLA-A*32:01) and 352 bp (GALC). Non–HLA-A*32 samples showed only one band of 352-bp (GALC) (Figure 2). No product was detectable for the nontemplate negative controls. Thus, the sensitivity and specificity of this assay for the HLA-A*32:01 allele in these 458 DNA samples were 100% (95% CI, 88.43% to 100%) and 100% (95% CI, 99.14% to 100%), respectively (Table 4).
Table 4.
Comparison of the Present Assay with SBT
| HLA-A*32:01 qPCR | HLA SBT, n |
||
|---|---|---|---|
| HLA-A*32 | Non–HLA-A*32 | Total | |
| Positive | 30 | 0 | 30 |
| Negative | 0 | 428 | 428 |
| Total | 30 | 428 | 458 |
qPCR, real-time quantitative PCR; SBT, sequence-based typing.
Discussion
This study describes a simple, fast, and inexpensive PCR assay that utilizes AS-PCR with melt curve analysis for the detection of the HLA-A*32:01 allele. By using a combination of primers that included a LNA forward primer, the HLA-A*32:01 allele was specifically amplified in a real-time PCR. The assay was both 100% sensitive and specific, making it safe and appropriate for clinical use. The assay was able to exclude all non–HLA-A*32:01 alleles included in the validation of this study. It is worth mentioning that the primer set combination could amplify the HLA-A*29:13 allele. However, the frequency of this allele is very low, at around 0% in both Caucasian and African American populations (Allele Frequency Net Database, http://www.allelefrequencies.net, last accessed March 8, 2019).
In addition, alignments of exon 2 of all HLA-B and HLA-C alleles (Figure 1B) show that no other class I alleles are targeted by this primer combination, except for HLA-B*07:02:27 and HLA-C*06:02:15, that may be amplified. However, there are quite a number of mismatches in the reverse primer that may prevent their amplification. Furthermore, the frequency of these alleles is very low within the global population (Allele Frequency Net Database, http://www.allelefrequencies.net, last accessed January 15, 2019). Samples were not found in our biorepository for the validation of these two non–HLA-A alleles.
Amplification of low-level nonspecific products in real-time PCR has been previously reported.15 The formation of these nonspecific products can be influenced by annealing temperature, primer concentration, magnesium concentration, and DNA inputs.14 In this optimized assay, nonspecific amplification was not detected. Also, a LNA primer that specifically target HLA-A*32 alleles was used. The incorporation of a LNA primer into oligonucleotide primers provides an increase of specific binding strength for target DNA amplification.15 In addition, a commercial, optimized real-time PCR master mix (PowerUp SYBR Green Master Mix), which is reported to be formulated for maximum specificity and reproducibility, was used. The detection range for template DNA in this assay was between 10 ng and 100 ng. However, the assay optimal DNA concentration was between 25 ng and 50 ng.
The specificity of the assay was assessed for only the HLA-A*32:01 allele. The primer set combination was designed to specifically amplify all HLA-A*32 alleles, except A*32:03, A*32:04, and A*32:01:18. Given the low frequency of other HLA-A*32 alleles (eg, A*32:02, A*32:03, A*32:04, and A*32:01:18), especially in those of European ancestry16 (Allele Frequency Net Database, http://www.allelefrequencies.net, last accessed January 15, 2019), other non–HLA-A*32:01 alleles were not found in our biorepository of samples primarily from those of European ancestry. Notably, across the entire Vanderbilt BioVU cohort with imputed HLA typing (N = 65,638), the only HLA-A*32 allele imputed was HLA-A*32:01. European ancestry represented 85% of this cohort. Furthermore, it is currently unknown whether HLA-A*32 alleles other than HLA-A*32:01 are associated with vancomycin DRESS or whether there are associations between other HLA alleles and vancomycin DRESS in those of non-European ancestry. It is known for instance that the carriage rate of HLA-A*32:01 is approximately 2% to 3% in African Americans, which is half that carried in European Americans. This would mean that if the positive predictive value is equivalent to the almost 20% in European Americans, approximately twice as many African Americans would need to be tested to prevent or pre-empt one case of vancomycin DRESS, which is still a cost-effective ratio. It will be important to determine whether alleles other than HLA-A*32:01 are associated with vancomycin DRESS in non-European races.
As for previous associations between drug hypersensitivity and HLA alleles such as between abacavir and HLA-B*57:01, an allele also primarily represented in those of European ancestry, the association was specific for HLA-B*57:01, and to-date, HLA-B*57:01 screening has a 100% negative predictive value for abacavir hypersensitivity.17 It has been shown that HLA-B*58:01, HLA-B*57:03, and HLA-B*57:02, which differ by as few as 2 amino acids in the antigen-binding cleft, are not associated with abacavir hypersensitivity.18 HLA-A*32:01 has a leucine at position 156 that is a key peptide binding residue that differs from the glutamine at 156 for HLA-A*32:02 and HLA-A*32:03 (IPD-IMGT/HLA database, https://www.ebi.ac.uk/cgi-bin/ipd/imgt/hla/align.cgi, last accessed January 4, 2019). On the other hand, it has been demonstrated that in some drug hypersensitivity phenotypes, different alleles of the same family confer susceptibility to an adverse drug reaction. This is the case of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in which in addition to the HLA-B*15:02 risk allele, members of the same HLA-B*75 serotype could also present carbamazepine to activate CD8+ T cells.19 Wei et al19 found that three amino acid residues (Asn63, Ile95, and Leu156) in the peptide-binding groove of HLA-B*15:02 were involved in the presentation of carbamazepine to CD8+ T cells. Asn63, which is shared by members of the B*75 family, was the key residue to confer this specificity.
High-resolution HLA typing is performed by direct sequencing of HLA class I and class II sequences. Although SBT remains the gold standard for HLA typing, it is significantly more expensive, requires significant expertise and labor, and has a longer turnaround time compared with this assay in terms of time, cost, and labor, and remains the domain of specialty immunogenetics and transplant laboratories. SBT also requires sophisticated equipment, highly trained staff, and a robust informatics and quality assurance infrastructure that might not be available in most of the clinical settings. HLA typing by hybridization of the PCR amplicon with sequence-specific oligonucleotide probes is an alternative method, but this method shows low-resolution typing.20
In comparison with current HLA typing methods in terms of time and cost, this HLA-A*32:01 screening assay seems to be appropriate for use in clinical settings. On the cost of reagents alone, calculations show that it costs around 20-fold less using this real-time PCR assay in comparison to HLA typing by SBT. Time for operator setup and analysis also favors this rapid real-time assay due to the higher number of steps required for SBT. A single sample can be typed for HLA-A*32:01 with a turnaround time of <3 hours, with <1 hour of operator hands-on time with real-time PCR, whereas SBT such as Sanger sequencing has a turnaround of at least 8 to 10 hours with 3 hours of hands-on time. Ideal batch scale for SBT is approximately 90 samples and adds 10 hours of data analysis, whereas for real-time PCR, the reaction can scale to approximately 384 sample batches with minimal addition of operator time when robotic setup is available. In addition, the level of operator expertise and sophisticated sequencing equipment needed for next-generation sequencing might not be available in most of the clinical settings.
The assay described here appears to give both a specificity and sensitivity of 100%, with the advantage of being very fast and cheaper compared with other HLA typing methods. The flexible methodology means that this could be set up in a variety of specialty immunogenetics or nonspecialty laboratory settings that have access to a PCR platform that can perform melting curve analysis. Those settings without a qPCR platform can use the standard AS-PCR followed by agarose gel electrophoresis. Although limited testing was conducted on a standard PCR machine with the PowerUp SYBR Green Master Mix followed by agarose gel electrophoresis analysis and gel visualization, this assay was found to successfully adapt in settings that do not have a qPCR platform.
The considerable reduction of operator manual handling of post-PCR amplicon and manipulation of results also reduces the potential risk of sample mix-up as well as contamination. Where testing a large number of samples is needed, the use of a robotic liquid handling system for transferring master mixes and DNA samples could provide some benefits in reducing turnaround time and human errors. In cases where unusual melt curves are found due to poor purity of DNA or operator technical error, samples should be further evaluated by using conventional PCR genotyping or SBT, or by obtaining another sample. However, such cases were not encountered during this study's validation experiments.
Currently, single-allele assays exist or have been published for HLA-B*57:01, HLA-B*15:02, HLA-B*58:01, HLA-B*13:01, and HLA-A*31:01.21, 22, 23, 24, 25, 26 Most of these single-allele assays have been advocated as a pre-prescription screening strategy with specific drugs and specific populations.27, 28 The association of HLA-A*32:01 and vancomycin DRESS has only been recently reported. Currently, the authors are unaware of the existence of other available PCR-based methods specifically for the detection of HLA-A*32:01.
This study is therefore novel in both reporting a new single-allele test specific for HLA-A*32:01 and also in proposing a new use approach to pharmacogenetic testing that is practical and convenient for both the clinician and the laboratory. Based on a prevalence of HLA-A*32:01 in a population of predominant European ancestry of 6.8% and estimates from that, that approximately 20% of those carrying HLA-A*32:01 and exposed to vancomycin for at least 2 weeks will develop vancomycin DRESS, it can be estimated that 75 patients would need to be tested for HLA-A*32:01 to prevent one case of DRESS. Given the low cost and relative convenience of testing in this clinical context and the severe implications of DRESS, this number needed to test should be sufficient to justify testing from a cost-effectiveness standpoint in most populations with carriage frequencies of HLA-A*32:01 similar to the population tested in this study. Furthermore, it would also be feasible to use this rapid-turnaround single-allele assay as an ancillary diagnostic test in patients who have developed DRESS on multiple antibiotics including vancomycin.
Conclusions
In conclusion, this AS-PCR is a fast and reliable method for typing the HLA-A*32:01 allele. This assay demonstrates the sensitivity and specificity needed for the assignment of the HLA-A*32:01 allele, but the authors caution that these assay characteristics may not be maintained with any modification to the method. In addition as per the authors' experience with HLA-B*57:01, the development of an allele-specific international quality assurance program will help minimize false positive and negative errors that may significantly impact patient safety.29
Acknowledgments
We thank the staff of the core laboratory (Institute for Immunology and Infectious Diseases, Murdoch University) for laboratory assistance, and Dr. Rakesh Veedu (Centre for Comparative Genomic, Perth, Australia) for synthesizing the LNA primer.
Footnotes
Supported by NIH grants 1P50GM115305-01 (E.J.P., S.A.M.), R21AI139021 (E.J.P., S.A.M.), R34AI136815 (E.J.P., S.A.M.), F30 AI131780 (K.C.K.), P50 GM115305 (K.C.K.), and T32 GM7347 (K.C.K.); and NHMRC grant APP1123499 (E.J.P.).
F.X.R. and A.C. contributed equally to this work.
Disclosures: S.A.M. has royalties from UpToDate and has equity in IIID Pty Ltd that holds a patent for HLA-B*57:01 testing for abacavir hypersensitivity; E.J.P. has received consulting fees from Biocryst, royalties from UpToDate, and has equity in IIID Pty Ltd, which holds a patent for HLA-B*57:01 testing for abacavir hypersensitivity.
References
- 1.Redwood A.J., Pavlos R.K., White K.D., Phillips E.J. HLAs: key regulators of T-cell-mediated drug hypersensitivity. HLA. 2018;91:3–16. doi: 10.1111/tan.13183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.White K.D., Chung W.H., Hung S.I., Mallal S., Phillips E.J. Evolving models of the immunopathogenesis of T cell-mediated drug allergy: the role of host, pathogens, and drug response. J Allergy Clin Immunol. 2015;136:219–234. doi: 10.1016/j.jaci.2015.05.050. quiz 235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paul S., Weiskopf D., Angelo M.A., Sidney J., Peters B., Sette A. HLA class I alleles are associated with peptide-binding repertoires of different size, affinity, and immunogenicity. J Immunol. 2013;191:5831–5839. doi: 10.4049/jimmunol.1302101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konvinse K.C., Trubiano J.A., Pavlos R., James I., Shaffer C.M., Bejan C.A., Pilkinton M.A., Rosenbach M., Zwerner J.P., Williams K.B., Jack Bourke J., Martinez P., Rwandamuriye F., Chopra A., Watson M., Mallal S.A., Redwood A., White K.D., Phillips E.J. HLA-A*32:01 is strongly associated with vancomycin-induced drug reaction with eosinophilia and systemic symptoms. J Allergy Clin Immunol. 2018;144:183–192. doi: 10.1016/j.jaci.2019.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin Y.F., Yang C.H., Sindy H., Lin J.Y., Rosaline Hui C.Y., Tsai Y.C., Wu T.S., Huang C.T., Kao K.C., Hu H.C., Chiu C.H., Hung S.I., Chung W.H. Severe cutaneous adverse reactions related to systemic antibiotics. Clin Infect Dis. 2014;58:1377–1385. doi: 10.1093/cid/ciu126. [DOI] [PubMed] [Google Scholar]
- 6.Blumenthal K.G., Peter J.G., Trubiano J.A., Phillips E.J. Antibiotic allergy. Lancet. 2019;393:183–198. doi: 10.1016/S0140-6736(18)32218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Husain Z., Reddy B.Y., Schwartz R.A. DRESS syndrome: part I. clinical perspectives. J Am Acad Dermatol. 2013;68:693.e1–693.e14. doi: 10.1016/j.jaad.2013.01.033. quiz 706-708. [DOI] [PubMed] [Google Scholar]
- 8.Pavlos R., Mallal S., Ostrov D., Buus S., Metushi I., Peters B., Phillips E. T cell-mediated hypersensitivity reactions to drugs. Annu Rev Med. 2015;66:439–454. doi: 10.1146/annurev-med-050913-022745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cacoub P., Musette P., Descamps V., Meyer O., Speirs C., Finzi L., Roujeau J.C. The DRESS syndrome: a literature review. Am J Med. 2011;124:588–597. doi: 10.1016/j.amjmed.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 10.Carapito R., Radosavljevic M., Bahram S. Next-generation sequencing of the HLA locus: methods and impacts on HLA typing, population genetics and disease association studies. Hum Immunol. 2016;77:1016–1023. doi: 10.1016/j.humimm.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Sayer D., Whidborne R., Brestovac B., Trimboli F., Witt C., Christiansen F. HLA-DRB1 DNA sequencing based typing: an approach suitable for high throughput typing including unrelated bone marrow registry donors. Tissue Antigens. 2001;57:46–54. doi: 10.1034/j.1399-0039.2001.057001046.x. [DOI] [PubMed] [Google Scholar]
- 12.Roden D.M., Pulley J.M., Basford M.A., Bernard G.R., Clayton E.W., Balser J.R., Masys D.R. Development of a large-scale de-identified DNA biobank to enable personalized medicine. Clin Pharmacol Ther. 2008;84:362–369. doi: 10.1038/clpt.2008.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karnes J.H., Shaffer C.M., Bastarache L., Gaudieri S., Glazer A.M., Steiner H.E., Mosley J.D., Mallal S., Denny J.C., Phillips E.J., Roden D.M. Comparison of HLA allelic imputation programs. PLoS One. 2017;12:e0172444. doi: 10.1371/journal.pone.0172444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz-Villalba A., van Pelt-Verkuil E., Gunst Q.D., Ruijter J.M., van den Hoff M.J. Amplification of nonspecific products in quantitative polymerase chain reactions (qPCR) Biomol Detect Quantif. 2017;14:7–18. doi: 10.1016/j.bdq.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ballantyne K.N., van Oorschot R.A., Mitchell R.J. Locked nucleic acids in PCR primers increase sensitivity and performance. Genomics. 2008;91:301–305. doi: 10.1016/j.ygeno.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Cao K., Hollenbach J., Shi X., Shi W., Chopek M., Fernández-Viña M.A. Analysis of the frequencies of HLA-A, B, and C alleles and haplotypes in the five major ethnic groups of the United States reveals high levels of diversity in these loci and contrasting distribution patterns in these populations. Hum Immunol. 2001;62:1009–1030. doi: 10.1016/s0198-8859(01)00298-1. [DOI] [PubMed] [Google Scholar]
- 17.Mallal S., Nolan D., Witt C., MAsel G., MArtin A., Moore C., Sayer D., Castley A., Mamotte C., Maxwell D., James I., Christiansen F. Association between HLA-B*57:01, HLA-DR7 and HLA-DQ3 and hypersensitivity to HIV-1 reverse transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 18.Mallal S., Phillips E., Carosi G., Molina J.M., Workman C., Tomazic J., Jagel-Guedes E., Rugina S., Kozyrev O., Cid J.F., Hay P., Nolan D., Hughes S., Hughes A., Ryan S., Fitch N., Thorborn D., Benbow A., PREDICT-1 Study Team HLA-B*5701 screening for hypersensitivity to abacavir. N Engl J Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 19.Wei C.Y., Chung W.H., Huang H.W., Chen Y.T., Hung S.I. Direct interaction between HLA-B and carbamazepine activates T cells in patients with Stevens-Johnson syndrome. J Allergy Clin Immunol. 2012;129:1562–1569.e5. doi: 10.1016/j.jaci.2011.12.990. [DOI] [PubMed] [Google Scholar]
- 20.Dunn P.P. Human leucocyte antigen typing: techniques and technology, a critical appraisal. Int J Immunogenet. 2011;38:463–473. doi: 10.1111/j.1744-313X.2011.01040.x. [DOI] [PubMed] [Google Scholar]
- 21.Nguyen D.V., Vida C., Chu H.C., Fulton R., Li J., Fernando S.L. Validation of a rapid, robust, inexpensive screening method for detecting the HLA-B*58:01 allele in the prevention of allopurinol-induced severe cutaneous adverse reactions. Allergy Asthma Immunol Res. 2017;9:79–84. doi: 10.4168/aair.2017.9.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen D.V., Vidal C., Chu H.C., Do N.T., Tran T.T., Le H.T., Fulton R.B., Li J., Fernando S.L. Validation of a novel real-time PCR assay for detection of HLA-B*15:02 allele for prevention of carbamazepine-induced Stevens-Johnson syndrome/toxic epidermal necrolysis in individuals of Asian ancestry. Hum Immunol. 2016;77:1140–1146. doi: 10.1016/j.humimm.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 23.Uchiyama K., Kubota F., Ariyoshi N., Matsumoto J., Ishii I., Kitada M. Development of a simple method for detection of the HLA-A*31:01 allele. Drug Metab Pharmacokinet. 2013;28:435–438. doi: 10.2133/dmpk.dmpk-12-nt-136. [DOI] [PubMed] [Google Scholar]
- 24.Chen P., Lin J.J., Lu C.S., Ong C.T., Hsieh P.F., Yang C.C., Tai C.T., Wu S.L., Lu C.H., Hsu Y.C., Yu H.Y., Ro L.S., Lu C.T., Chu C.C., Tsai J.J., Su Y.H., Lan S.H., Sung S.F., Lin S.Y., Chuang H.P., Huang L.C., Chen Y.J., Tsai P.J., Liao H.T., Lin Y.H., Chen C.H., Chung W.H., Hung S.I., Wu J.Y., Chang C.F., Chen L., Chen Y.T., Shen C.Y., Taiwan SJS Consortium Carbamazepine-induced toxic effects and HLA-B*1502 screening in Taiwan. N Engl J Med. 2011;364:1126–1133. doi: 10.1056/NEJMoa1009717. [DOI] [PubMed] [Google Scholar]
- 25.Hammond E., Mamotte C., Nolan D., Mallal S. HLA-B*5701 typing: evaluation of an allele-specific polymerase chain reaction melting assay. Tissue Antigens. 2007;70:58–61. doi: 10.1111/j.1399-0039.2007.00840.x. [DOI] [PubMed] [Google Scholar]
- 26.Liu Z., Chen G., Kang X., Han M., Chen R., Chen C., Wang H. A multiplex allele-specific real-time polymerase chain reaction assay for HLA-B*13:01 genotyping in four Chinese populations. HLA. 2016;88:164–171. doi: 10.1111/tan.12863. [DOI] [PubMed] [Google Scholar]
- 27.Phillips E.J., Mallal S.A. Pharmacogenetics of drug hypersensitivity. Pharmacogenomics. 2010;11:973–987. doi: 10.2217/pgs.10.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ferrell P.B., Jr., McLeod H.L. Carbamazepine, HLA-B*1502 and risk of Stevens-Johnson syndrome and toxic epidermal necrolysis: US FDA recommendations. Pharmacogenomics. 2008;9:1543–1546. doi: 10.2217/14622416.9.10.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hammond E., Almeida C.A., Mamotte C., Nolan D., Phillips E., Schollaardt T.A., Gill M.J., Angel J.B., Neurath D., Li J., Giulivi T., McIntyre C., Koultchitski G., Wong B., Reis M., Rachlis A., Cole D.E., Chew C.B., Neifer S., Lalonde R., Roger M., Jeanneau A., Mallal S. External quality assessment of HLA-B*5701 reporting: an international multicentre survey. Antivir Ther. 2007;12:1027–1032. [PubMed] [Google Scholar]