Abstract
Septic arthritis and sepsis are common and feared complications of staphylococcal infections, and the increasing antibiotic resistance among staphylococci urge the extended research for virulence factors involved in these diseases. Staphylcoccus aureus produces a number of virulence factors controlled by several global regulatory genes including agr and sarA. MgrA is a recently identified global regulator, belonging to the SarA subfamily, which upregulates expression of several virulence factors including capsule and sortase. In addition, MgrA has been shown to regulate antibiotic resistance and decrease bacterial autolysis.
In this study we have assessed the role of mgrA gene expression on induction and progression of septic arthritis and sepsis. Mice inoculated with the mgrA mutant displayed significantly less severe arthritis and showed a significantly better weight development, than wild-type inoculated mice. Importantly, all 10 mice inoculated with the mgrA mutant survived as compared to 70% mortality in the wild-type inoculated mice (p = 0.003). In addition, the mgrA mutant showed significantly less bacterial persistence in kidneys as compared to the wild-type strain. We conclude that mgrA regulates virulence factors important for establishment and progression of septic arthritis and sepsis.
Keywords: Staphylococcus aureus, Arthritis, Sepsis, Virulence factor, mgrA
1. Introduction
The spectrum of diseases caused by Staphylococcus aureus is extremely wide, ranging from superficial skin infections to severe and life threatening infections like, infectious endocarditis, septic arthritis, and sepsis [1].
Septic arthritis mediated by S. aureus is a medical emergency because of its potential for causing mortality in addition to rapid joint destruction, in particular in patients with rheumatoid arthritis, and immunocompromized subjects. The pathogenecity of S. aureus is a very complex process involving coordinated activity of several enzymes and toxins like proteases, hemolysins, expression of capsular polysaccharides (CP) as well as adhesins like collagen adhesin, clumping factor and fibronectin-binding proteins [1]. The virulence factors are coordinately regulated by a network of regulatory genes [2]. The accessory gene regulator (agr) and staphylococcal accessory regulator (sarA) loci are the most well characterized global regulatory elements that coordinate synthesis of virulence factors like adhesins and exoproteins during the infection [2,3]. These two regulatory elements have been proven to regulate virulence factors important for development and progression of septic arthritis and sepsis [4,5].
MgrA is a multiple gene regulator belonging to the SarA family, which has been shown to directly regulate several virulence genes by binding to the promotor regions [6,7]. Several virulence factors have been shown to be upregulated by mgrA, e.g. CPs, sortase A, serineproteases, leukotoxins, and α-hemolysins. In contrast, several cell wall associated or surface associated proteins including protein A, are down-regulated by MgrA [8,9]. MgrA can also act as a repressor or activator of genes involved in bacterial metabolism [8]. In addition, MgrA has also been shown to affect antibiotic resistance and to decrease bacterial autolysis [6,7].
However, the role of MgrA for bacterial virulence during in vivo staphylococcal infection is not known. Therefore we analyzed the role of mgrA gene expression on induction and progression of disease in a well established murine model of septic arthritis and sepsis [10].
2. Materials and methods
2.1. Mice
Female NMRI mice 6–8 weeks old were obtained from B&K universal AB (Sollentuna, Sweden) and maintained in the animal facility of the Department of Rheumatology and Inflammation Research; Göteborg University, Sweden. They were housed up to 10 animals in each cage under standard conditions of light and temperature and were fed standard laboratory food and water ad libitum.
2.2. Bacterial strains
In this study we used the wild-type strain Newman [11] and its isogenic mgrA negative mutant CYL1050 [8].
The bacterial strains were cultured on blood agar plates for 24 h and then streaked on another agar plate and reincubated for another 24 h. Thereafter the bacteria were harvested, aliquoted in 1 ml volumes, and kept frozen at −20 °C in phosphate-buffered saline (PBS) containing 5% bovine serum albumin w/v and 10% v/v dimethyl sulphoxide until use. Viable counts were made from six of these aliquots at different days and the number of bacteria/ml was determined as the average from the CFU obtained from all viable counts. Before i.v. administration to the mice, the bacterial suspension was thawed, washed in PBS and adjusted to appropriate concentration. After each inoculation procedure, viable counts were performed in the leftover suspension to confirm the actual number of administered bacteria.
2.3. Experimental protocols
Four separate in vivo experiments were performed (n = 10–15/group for each experiment). In all experiments the mice were inoculated i.v. into the tail vein with a suspension of staphylococci in a volume of 0.2 ml. During the course of experiments the mice were regularly weighed and examined for arthritis by an observer blinded to inoculation groups (I-M.J.) until being sacrificed by cervical dislocation. In the first experiment we assessed the impact of mgrA mutation on the mortality. Ten mice per group were inoculated with a calculated dose of 6 × 106 S. aureus per mouse, a bacterial dose known to induce sepsis. The obtained bacterial doses were 5.9 × 106 CFU of the wild-type Newman strain and 5.7 × 106 CFU of the isogenic mgrA mutant strain, as determined by viable counts of the inocula. This experiment was terminated 9 days after inoculation.
In the second experiment 10 mice per group were inoculated with an estimated dose of 4.5 × 106 staphylococci, the obtained bacterial doses being 4.3 × 106 of the Newman strain and 4.6 × 106 of the mgrA mutant strain, as determined by viable counts. After sacrifice at day 14 the spleens were weighted, kidneys were obtained for assessment of bacterial persistence and sera were analyzed for IL-6 levels. In the third experiment we wanted to assess the kinetics of the infection as a consequence of mgrA mutation. Fifteen mice per group were inoculated with a calculated dose of 3 × 106 staphylococci each, the obtained doses being 2.7 × 106 of the Newman strain and 3.2 × 106 of the mutant strain, as determined by viable counts of the inocula. Mice were sacrificed after 4 days (n = per group) and 11 days (n = 7 and 8 mice), respectively. Kidneys were examined for bacterial load by determination of colony forming units (CFUs) in serial dilutions of homogenate, and paws were microscopically evaluated for expression of synovitis and destruction of cartilage and bone. In the last in vivo experiment the preferential persistence of the staphylococci was assessed by co-inoculation of both isogenic strains. Ten mice were inoculated with a bacterial suspension conaining 1.1 × 106 CFU of the wild-type strain and 1.1 × 106 CFU of the mgrA mutant. After the sacrifice 11 days following the inoculation, bacterial cultures from the kidneys and joints were enumerated, colonies were screened for bacterial strain phenotype.
2.4. Clinical evaluation of arthritis
All four limbs of each mouse were inspected visually. Arthritis was defined as erythema and/or swelling of the joints. To evaluate the severity of arthritis, a clinical scoring system of 0–3 for each limb was used; score 0 representing normal, 1 mild swelling and/or erythema, 2 moderate swelling, and 3 marked swelling. The arthritis index was constructed by adding the scores from all limbs for each mouse [4].
2.5. Histopathological examination
Following the third in vivo experiment, all four limbs of each mouse were collected and fixed in paraformaldehyde, decalcificated and paraffin embedded. Tissue sections were stained with hematoxylin and eosin. All sections were coded before microscopical evaluation. The joints were studied microscopically with respect to synovial hypertrophy, defined as synovial membrane thickness of more than two cell layers, and cartilage/bone destruction. Histopathological scoring for both synovitis and erosion; was 0 = normal appearance, 1 = mild synovitis and/or erosion of cartilage and bone, 2 = moderate synovits and erosion of cartilage and bone, 3 = severe synovitis and erosion of cartilage and bone. The histological index was constructed by adding the scores from the relevant limbs in each animal [4,12].
2.6. Bacteriological examination of infected animals
The kidneys were asceptically removed, homogenized, diluted serially in PBS and transferred to agar plates containing 5% v/v horse blood. Bacteria were grown for 24 h and then counted as CFUs.
To assess the persistence of simultaneously injected wild-type vs. mgrA mutant strains, the kidney homogenate was plated on plain agar plates and cultures from joints were obtained using a cotton stick, and spread on blood agar plates. After incubation of the plates for 24 h, colonies were picked and screened for antibiotic resistance on agar plates containing chloramphenicol (5 μg/ml) to ascertain the frequency of respective strain.
2.7. Analyses of serum IL-6 levels
A bioassay method using the murine hybridoma cell line B9, which is dependent on interleukin-6 (IL-6) for growth was used to detect the serum levels of IL-6 [12,13]. B9 cells were seeded into microtiter plates (5000 cells/well), and dilutions of the serum samples were added to the wells. After 68 h incubation, 3H-thymidine (Radiochemical Centre, Amersham, UK) was added; 6 h later the cells were harvested and counted in a beta counter. The results were compared with a recombinant IL-6 standard. B9 cells were previously shown not to react with several recombinant cytokines, including IL-1α, IL-1β, IL-2, IL-3, IL-5, granulocyte-macrophage colony-stimulating factor, tumor necrosis factor alpha, and gamma-interferon. There was only a weak reactivity with IL-4 [13].
2.8. Phagocytosis and intracellular killing of staphylococci
The phagocytosis test was done using a modified method of Lissner as previously described [14,15]. In brief, peritoneal macrophages were collected from NMRI mice (n = 9) by injecting 3 ml of ice-cold Iscove’s complete medium containing 100 μg gentamycin per ml into the peritoneal cavity. After 1 min massage of the abdomen, the fluid was aspirated, cells were counted and adjusted to a concentration of 2 × 106 cells/ml. Two hundred μl of the cell suspension was seeded into each well in 24-well plates (Nunc, Roskilde, Denmark), and left at room temperature for 90 min allowing adherence of macrophages. Five hundred microliters of medium was added to each well and the cells were incubated at 37 °C for 4 h. Thereafter, the medium was replaced by 500 μl of new medium free of gentamycin. After incubation over night at 37 °C the cells were washed in PBS and 500 μl of a suspension with S. aureus Newman strain or its isogenic mgrA mutant were added in a concentration of 5 × 106 bacteria/ml. After 50 min of incubation the cells were washed three times with medium containing gentamycin (4 μg/ml) in order to remove and kill all the extra cellular bacteria. Thereafter the bacterial content in macrophages was analysed at three different time points starting directly after incubation with bacteria = time point 0, and then 4 and 24 h after extra cellular bacteria was removed, i.e. assessing intracellular killing of bacteria. To those cell cultures that were incubated for another 4 and 24 h, Iscove’s medium with 4 mg/ml gentamycin was added. The macrophages were lysed with distilled water for 20 min, and the lysate was serially diluted and cultured on blood agar plates, thereafter the CFU was determined.
2.9. Statistical analysis
Statistical evaluation was done by using the Mann–Whitney U-test, chi-square test or the Kaplan–Meier log-rank test. Wilcoxon signed rank sum test was used for evaluation of the phagocytosis and intracellular killing assay. Results are reported as median and interquartile range (IQR) or as mean ± standard error of the mean (SEM).
3. Results
3.1. mgrA expression is an important virulence factor in sepsis and septic arthritis
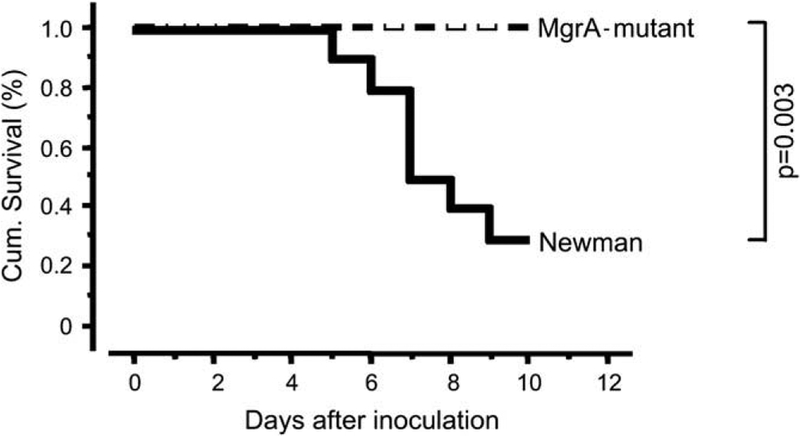
Mice inoculated with the mgrA mutant survived significantly better (p = 0.003) than mice inoculated with the wild-type strain expressing the mgrA gene, since seven of 10 mice inoculated with a septic dose of 6 × 106 wild-type staphylococci per mouse died during the course of experiment, while all 10 mice in the mgrA mutant inoculated group survived (Fig. 1). The mortality remained high in the wild-type inoculated mice also when a lower dose of bacteria of 4.5 × 106 was used. In this experiment three out of 10 wild-type strain inoculated mice died as compared to none out of 10 in the mutant inoculated group. Using an even lower bacterial dose of 3 × 106 per mouse, one mouse inoculated with the wild-type strain died, as compared to none of the mutant inoculated mice, during the course of experiment.
Fig. 1.
Cumulative survival of NMRI mice (10/group) inoculated with equal septic dose of Staphylococcus aureus Newman, or its isogenic mgrA mutant (CYL 1050). Mice inoculated with S. aureus Newman display significantly higher mortality (p = 0.003, Kaplan–Meier log-rank test) than mice inoculated with the MgrA mutant (CYL 1050).
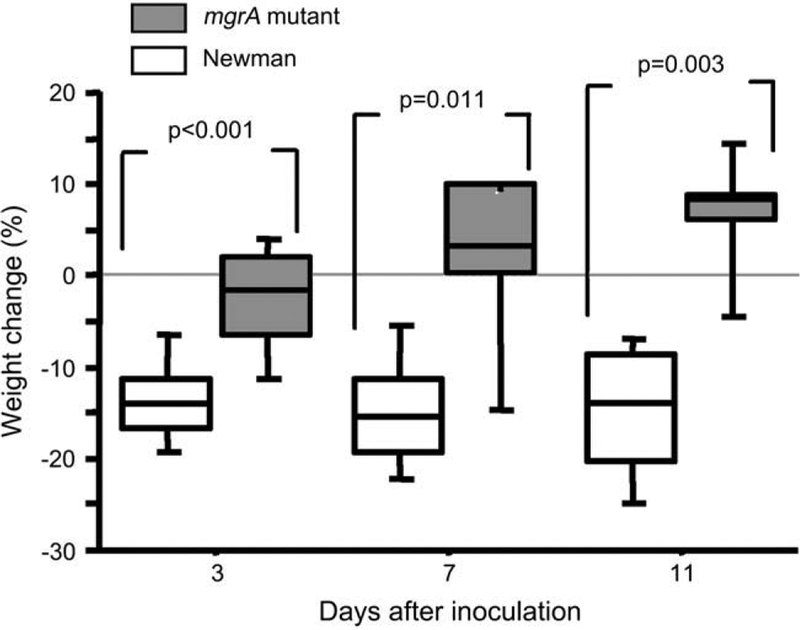
The mice inoculated with the wild-type bacteria lost significantly more weight in all experiments as compared to mutant inoculated mice. With an inoculum of 4.5 × 106 staphylococci per mouse the median weight loss, after 1 week was, 23% (IQR; 18–31%) for the wild-type inoculated mice, compared to a median weight loss of 4% (IQR; 1–5%; p = 0.0004, n = 9 and 10/group respectively) in the mutant inoculated mice. In the third experiment with an inoculum of 3 × 106 staphylococci per mouse, the same differences between the inoculation groups were found, i.e. the median weight loss for the wild-type inoculated mice was already after 3 days 14% (IQR; 11–17%) compared to the mutant inoculated mice which showed a median weight loss of only 2% (IQR; 2–6%; p < 0.001, n = 15/group). The weight loss for the wild-type inoculated mice persisted throughout the experiment while the mutant inoculated mice instead gained weight (Fig. 2).
Fig. 2.
Changes in body weight of NMRI mice inoculated with 3 × 106 CFU of S. aureus Newman or its isogenic mgrA mutant (CYL 1050). n = 15 mice/group on day 3, n = 7–8 mice/group on days 7 and 14 days following inoculation. Comparisons were made using the Mann–Whitney U-test.
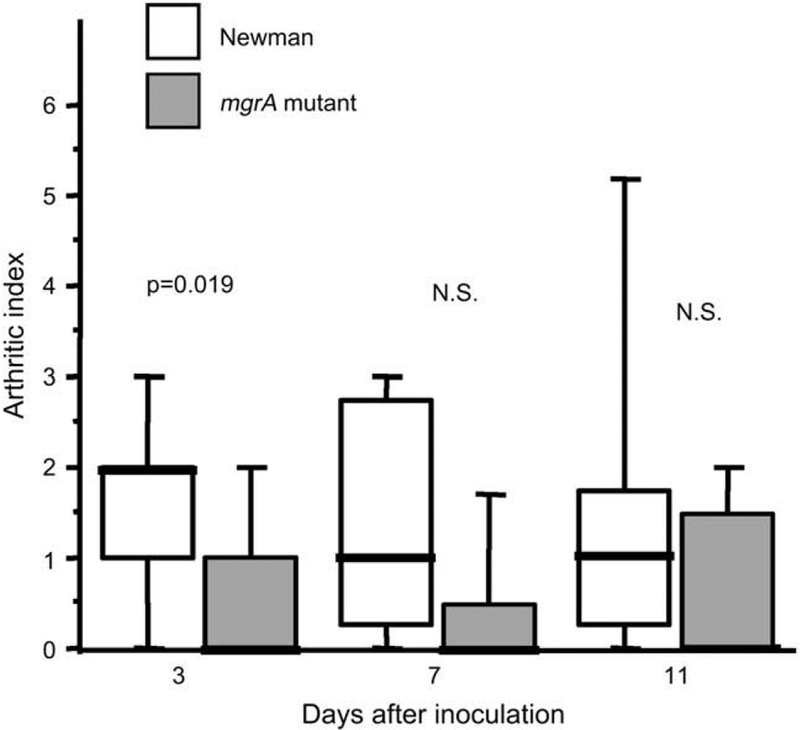
In all three in vivo experiments, the signs of clinical arthritis were analyzed. Mice inoculated with the wild-type staphylococci displayed both a higher frequency and severity of arthritis. With the highest dose of bacteria (6 × 106 per mouse) 8 of 9 (89%) of the wild-type inoculated mice developed arthritis within 5 days, as compared to only 3 of 10 (30%) of the mutant inoculated mice (p < 0.05). Also lower doses of bacteria; i.e. 3 × 106 per mouse gave arthritis in 13 of 15 (87%) of the wild-type inoculated mice as compared to 6 of 15 (40%) of the mutant inoculated mice (p < 0.05) within the first 4 days. Fig. 3 shows the severity of arthritis in those mice given a dose of 3 × 106 bacteria per mouse, demonstrating significantly less severe arthritis in the mgrA mutant inoculated group.
Fig. 3.
Severity of arthritis in NMRI mice inoculated with 3 × 106 CFU of S. aureus Newman or its isogenic mgrA mutant. n = 15 mice/group on day 3, n = 7–8 mice/group on days 7 and 14 days after the inoculation. Comparisons were made using the Mann–Whitney U-test, NS, not significant.
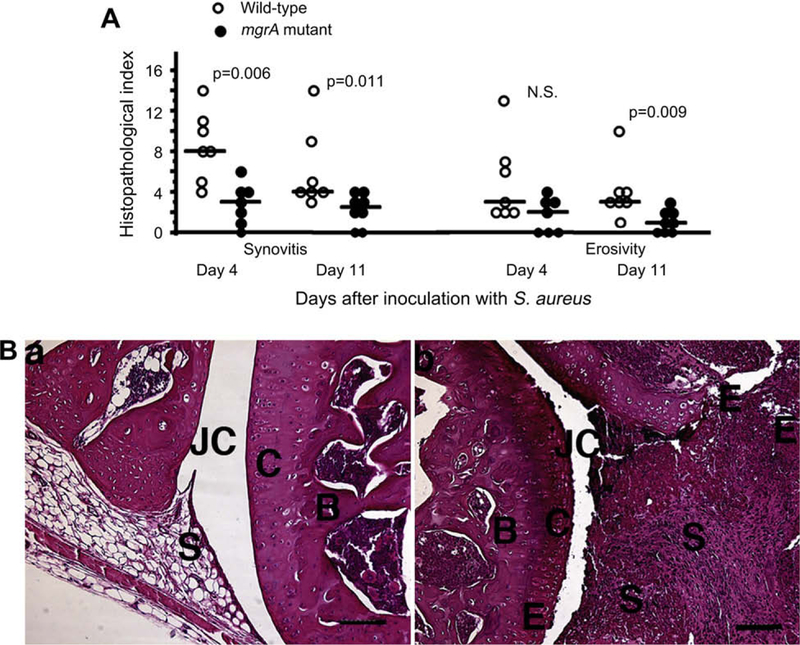
Next, all four paws from the mice inoculated with 3 × 106 staphylococci per mouse were examined for signs of synovitis and destruction of cartilage and bone following 4 and 11 days of infection. Mice inoculated with the wild-type mgrA expressing strain had significantly more severe synovitis at both time points investigated. Also destruction of cartilage and bone were more pronounced in joints from wild-type inoculated mice than in joints from the mgrA mutant inoculated mice (Fig. 4).
Fig. 4.
(A) Histologic evaluation of joints from all four limbs of NMRI mice, sacrificed 4 days (n = 7/group), and 11 days (n = 7 and 8 mice/group respectively) following i.v. inoculation with 3 × 106 CFU of S. aureus Newman or its isogenic mgrA mutant. Comparisons were made using the Mann–Whitney U-test, NS, not significant. (B) (a) Micrograph showing an apparently intact knee joint from a NMRI mouse, 11 days after inoculation with 3 × 106 CFU of the mgrA mutant. (b) Micrograph showing the knee joint from a mouse inoculated with the same dose of the wild-type strain Newman, displaying severe inflammation of synovial tissue and bone and cartilage erosion, JC, joint cavity; C, cartilage; B, bone; S, synovial tissue; E, erosion of bone and cartilage. Bar, 100 μm. Hematoxylin/eosin staining was used.
3.2. Bacterial persistence in host tissues
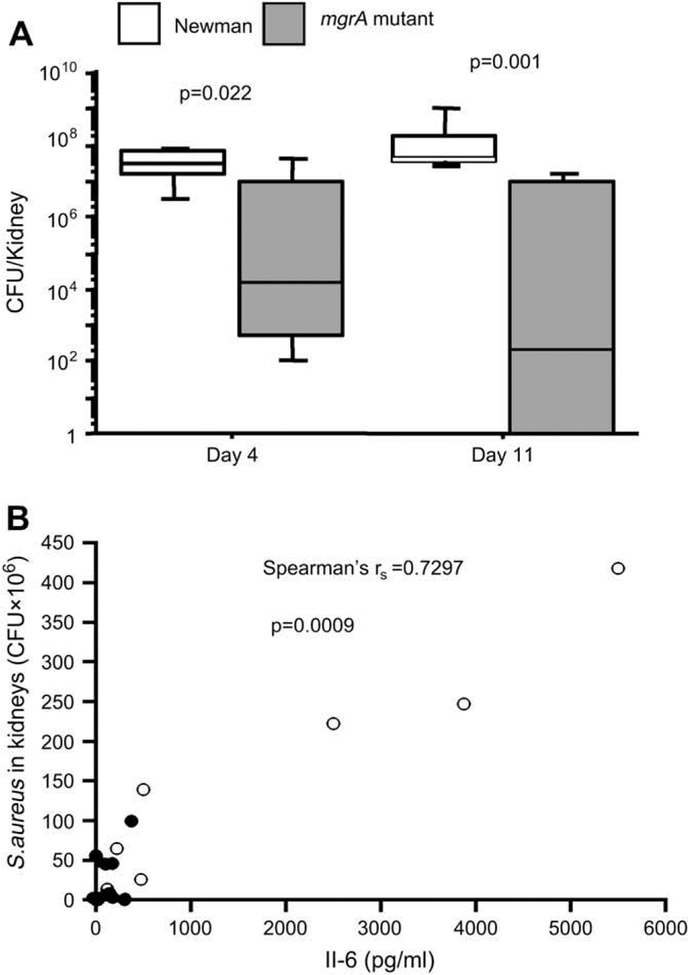
After sacrifice of the mice, the kidneys were removed for determination of bacterial load. To analyse the kinetics of bacterial kidney colonization, mice were sacrificed 4 and 11 days respectively after inoculation with 3 × 106 bacteria. Fig. 5A shows that the number of staphylococci was significantly higher in the wild-type inoculated mice than in the mutant inoculated mice at both time points. With an inoculum of 4.5 × 106 staphylococci per mouse, 14 days after the bacterial inoculation, the number of staphylococci was significantly higher in the wild-type inoculated mice than in mutant inoculated mice.
Fig. 5.
(A) Persistence of Staphylococcus aureus in kidneys after 4 days (n = 7 mice/group) and 11 days (n = 7 and 8 mice/group respectively) of inoculation with 3 × 106 CFU of Newman or its isogenic mgrA mutant. Statistical evaluation was made using Mann–Whitneys U-test. (B) Correlation between serum levels of IL-6 and numbers of Staphylococcus aureus in the kidneys of mice inoculated with 4.5 × 106 CFU/mouse 10 days earlier. Correlation was evaluated by using Spearman’s rank correlation test, filled circles represent values from the mgrA mutant inoculated group (n = 10), and opened circles represent values from wild-type Newman inoculated group (n = 7).
In an additional experiment the preferential persistence of the isogenic staphylococci in host tissue was assessed. Eleven days after the bacterial inoculation with a mixture containing both isogenic strains at equal amounts, we examined the bacterial growth in kidneys and joints as determined by viable counts. Ten mice were inoculated with the staphylococcal mixture. The mice harbored a median of 7 × 107 CFU (IQR; 1 × 107 to 1 × 109) of staphylococci in their kidneys. To compare the persistence of the mgrA mutant and the wild-type strain approximately 40% of the colonies were randomly picked and screened for antibiotic resistance. All of the screened colonies showed the phenotype of the wild-type Newman strain. Six out of the 10 mice harbored bacteria in their joints, and all isolated colonies were of the wild-type Newman phenotype.
3.3. Inflammatory responses and resistance to phagocytosis/intracellular killing as a function of mgrA expression
To assess the impact of mgrA gene expression on the inflammatory response during the infection, serum levels of IL-6 were measured 14 days after inoculation with 4.5 × 106 staphylococci per mouse. The levels of IL-6 was significantly higher for the wild-type inoculated mice then for the mgrA negative mutant inoculated mice (p = 0.006), and correlated well with the bacterial load in the animals as shown in Fig. 5B.
There were no significant differences between the groups with respect to degree of splenomegaly.
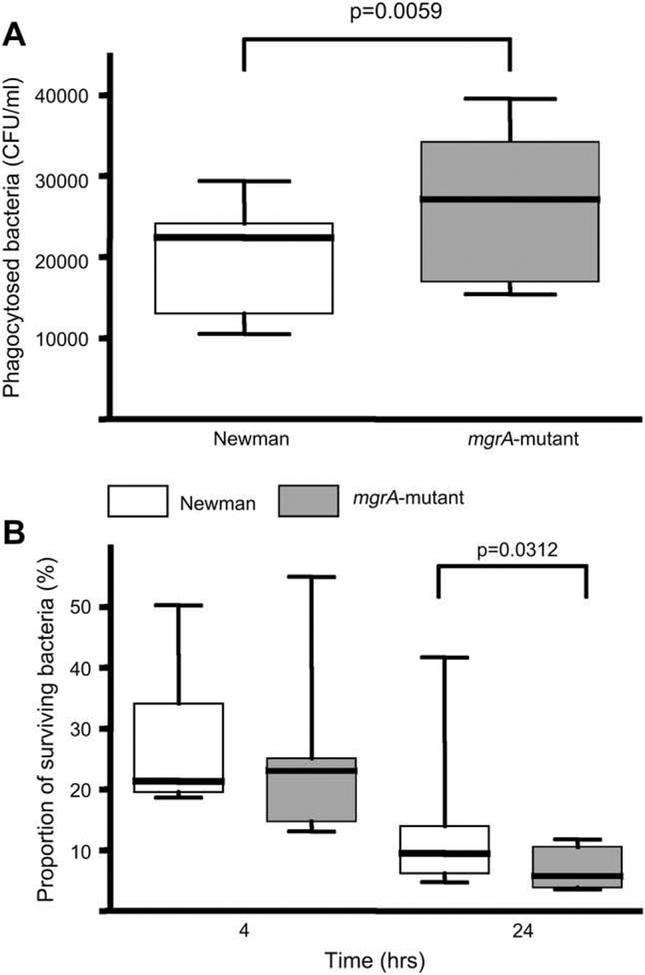
The importance of mgrA expression for bacterial resistance against phagocytosis and intracellular killing was tested in vitro using peritoneal macrophages. The mgrA mutant strain was more easily phagocytosed than the isogenic wild-type strain. In addition, the proportion of bacteria surviving intracellulary in the macrophages after 4 or 24 h of incubation, was larger for the wild-type strain than for the mgrA mutant strain. Thus, the mutant was more easily phagocytosed and killed by the peritoneal macrophages than the wild-type strain (Fig. 6A,B).
Fig. 6.
(A) Number of viable, in vitro phagocytized staphylococci by peritoneal macrophages. (B) Intracellular killing of staphylococci by peritoneal macrophages (n = 9). The proportions of surviving bacteria after 4 and 24 h are expressed as the percentage of the phagocytosed bacteria at time point 0. Statistical evaluation was made using Wilcoxon signed rank sum test.
4. Discussion
In this study we have used a murine model of hematogenously spread S. aureus arthritis to study the impact of mgrA gene expression on the disease onset and its progression. We show here that mice that were inoculated with the wild-type strain displayed significantly higher rate of mortality, showed a less favorable development of weight and displayed higher frequency and severity of arthritis than mice inoculated with the S. aureus mutant lacking mgrA gene expression. Thus, both clinically apparent arthritis as well as histological signs of synovitis and erosion was more severe in mice inoculated with the wild-type staphylococci.
Staphylococcal pathogenicity depends upon the host’s ability to deal with a huge variety of bacterial virulence factors, e.g. enzymes, toxins, cell wall components and capsular polysaccharide (CP). Any alteration in regulatory genes will affect the expression of these virulence factors. The different outcomes after inoculation with the wild-type MgrA expressing strain and its isogenic mgrA negative mutant is similar to findings in our previous experiments using strains isogenic for other regulatory genes, i.e. the agr or sarA loci [4,5]. In the latter cases (i.e. agr and sarA) just as in the case of mgrA, pore forming toxins such as leukotoxins and hemolysins are downregulated in the mgrA mutant strain [8]. We have earlier demonstrated that hemolysins are of importance for the development and progression of septic arthritis [16].
The first step in colonization is attachment of bacteria to host tissues using adhesins. Several bacterial adhesins are anchored to the surface by sortase A, being more expressed in the wild-type than in the mgrA mutant strain. Indeed, an upregulation of sortase A in vitro early in the culture [8] results in increased expression of proteins like protein A, clumping factor, and collagen adhesin [17,18] and thereby efficient adherence to host tissues by wild-type staphylococci. Mutants that lack the mgrA will thus have difficulties to colonize the host. We have earlier shown that not only sortase A, but also several of the individual adhesins that are anchored to the bacterial cell surface by sortase A, are important factors for S. aureus triggered arthritis [19–22]. In this study we also report significantly lower mgrA mutant bacteria load in kidneys both at early and late stages of infection, which at least partly is due to different colonization ability of this strain as compared to wild-type counterpart.
In vivo survival of S. aureus is also dependent on the ability to evade killing by host neutrophils [23]. Therefore S. aureus can avoid phagocytosis by producing capsular polysaccharides (CPs). The CPs enhance the bacterial virulence by allowing the bacterium to resist PMNC-mediated phagocytosis and intracellular killing [24,25]. We have earlier demonstrated that staphylococcal expression of CPs is of importance for its virulence by increasing the bacterial resistance to phagoytosis and intracellular killing by macrophages [15]. MgrA can upregulate capsular polysaccharide encoding genes, thus a lack of the mgrA gene can lead to diminished expression of CPs and thereby to decreased bacterial resistance to phagocytosis and intracellular killing. Indeed, we found that the mgrA mutant was less resistant to phagocytosis and intracellular killing by macrophages indicating that the mutant strain expressed less CPs. This diminished CP expression could be the reason for why mgrA mutant is less resistant. Moreover, the wild-type strain persisted significantly better in the host kidneys than the mgrA mutant. In addition, when both strains were co-inoculated into the mice in equal amounts the mutants was totally overgrown by the wild-type strain in kidneys, suggesting that MgrA also regulates virulence factors important for the persistence of infection.
Bacterial burden in mice is indeed reflected by the levels of the IL-6 in serum, as shown in Fig. 5B. IL-6 excerts pro-arthritogenic effects by recruitment of osteoclasts, thereby stimulating bone resorption [26]. IL-6 can also activate B-cells which in turn contribute to pathogenesis of S. aureus arthritis, by presenting autoantigens such as cartilage-derived molecules [27]. Indeed, the wild-type inoculated mice displayed significantly higher levels of this proinflammatory cytokine, along with a more severe and erosive arthritis, than the mice inoculated with the strain lacking functional mgrA gene.
This is the first in vivo study to demonstrate the role of mgrA locus in induction and progression of S. aureus triggered arthritis and sepsis. Our results suggest that virulence factors regulated by mgrA, will help wild-type S. aureus to evade host defence, invade the tissues and cause a more severe disease. We conclude that mgrA regulates virulence genes that are of importance for development and progression of septic arthritis and sepsis.
Acknowledgements
We thank Margareta Verdrengh and Berit Ertman-Ericsson for excellent technical assistance.
This work was supported by grants from The King Gustav V 80 Years Foundation, Göteborg Rheumatism Association, the Rune and Ulla Amlövs Foundation, the families Thölens and Kristlers Foundation, the Swedish Medical Association, The Swedish Association Against Rheumatism, The Foundation of Sigurd and Elsa Goljes Memory, and Göteborg University.
References
- [1].Lowy FD, Staphylococcus aureus infections, N. Engl. J. Med 339 (1998) 520–532. [DOI] [PubMed] [Google Scholar]
- [2].Cheung AL, Projan SJ, Gresham H, The genomic aspect of virulence, sepsis, and resistance to killing mechanisms in Staphylococcus aureus, Curr. Infect. Dis. Rep 4 (2002) 400–410. [DOI] [PubMed] [Google Scholar]
- [3].Arvidson S, Tegmark K, Regulation of virulence determinants in Staphylococcus aureus, Int. J. Med. Microbiol 291 (2001) 159–170. [DOI] [PubMed] [Google Scholar]
- [4].Abdelnour A, Arvidson S, Bremell T, Rydén C, Tarkowski A, The accessory gene regulator (agr) controls Staphylococcus aureus virulence in a murine arthritis model, Infect. Immun 61 (1993) 3879–3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Nilsson I-M, Bremell T, Rydén C, Cheung AL, Tarkowski A, Role of the staphylococcal accessory gene regulator (sar) in septic arthritis, Infect. Immun 64 (1996) 4438–4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ingavale SS, Van Wamel W, Cheung AL, Characterization of RAT, an autolysis regulator in Staphylococcus aureus, Mol. Microbiol 48 (2003) 1451–1466. [DOI] [PubMed] [Google Scholar]
- [7].Truong-Bolduc QC, Zhang X, Hooper DC, Characterization of NorR protein, a multifunctional regulator of norA expression in Staphylococcus aureus, J. Bacteriol 185 (2003) 3127–3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Luong TT, Dunman PM, Murphy E, Projan SJ, Lee CY, Transcription profiling of the mgrA regulon in Staphylococcus aureus, J. Bacteriol 188 (2006) 1899–1910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ingavale S, van Wamel W, Luong TT, Lee CY, Cheung AL, Rat/MgrA, a regulator of autolysis, is a regulator of virulence genes in Staphylococcus aureus, Infect. Immun 73 (2005) 1423–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tarkowski A, Collins LV, Gjertsson I, Hultgren OH, Jonsson I-M, Sakiniene E, Verdrengh M, Model systems: modeling human staphylococcal arthritis and sepsis in the mouse, Trends Microbiol. 9 (2001) 321–326. [DOI] [PubMed] [Google Scholar]
- [11].Duthie ES, Lorenz LL, Staphylococcal coagulase: mode of action and antigenicity, J. Gen. Microbiol 6 (1952) 95–107. [DOI] [PubMed] [Google Scholar]
- [12].Bremell T, Abdelnour A, Tarkowski A, Histopathological and serological progression of experimental Staphylococcus aureus arthritis, Infect. Immun 60 (1992) 2976–2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Helle M, Boeije L, Aarden L, Functional discrimination between interleukin 6 and interleukin 1, Eur. J. Immunol 18 (1988) 1525–1540. [DOI] [PubMed] [Google Scholar]
- [14].Lissner CR, Swanson R, O’Brien AD, Genetic control of the innate resistance of mice to Salmonella typhimurium: expression of the Ity gene in peritoneal and splenic macrophages isolated in vivo, J. Immunol 131 (1983) 3006–3013. [PubMed] [Google Scholar]
- [15].Nilsson I-M, Lee JC, Bremell T, Rydén C, Tarkowski A, The role of staphylococcal polysaccharide microcapsule expression in septicemia and septic arthritis, Infect. Immun 65 (1997) 4216–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Nilsson I-M, Hartford O, Foster T, Tarkowski A, Alpha-toxin and gamma-toxin jointly promote Staphylococcus aureus virulence in murine septic arthritis, Infect. Immun 67 (1999) 1045–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mazmanian SK, Liu G, Ton-That H, Schneewind O, Staphylococcus aureus sortase, an enzyme that anchors surface proteins to the cell wall, Science 285 (1999) 760–763. [DOI] [PubMed] [Google Scholar]
- [18].Mazmanian SK, Liu G, Jensen ER, Lenoy E, Schneewind O, Staphylococcus aureus sortase mutants defective in the display of surface proteins and in the pathogenesis of animal infections, Proc. Natl. Acad. Sci. U.S.A. 97 (2000) 5510–5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jonsson I-M, Mazmanian SK, Schneewind O, Verdrengh M, Bremell T, Tarkowski A, On the role of Staphylococcus aureus sortase and sortase-catalyzed surface protein anchoring in murine septic arthritis, J. Infect. Dis 185 (2002) 1417–1424. [DOI] [PubMed] [Google Scholar]
- [20].Palmqvist N, Foster T, Tarkowski A, Josefsson E, Protein A is a virulence factor in Staphylococcus aureus arthritis and septic death, Microb. Pathog 33 (2002) 239–249. [DOI] [PubMed] [Google Scholar]
- [21].Palmqvist N, Josefsson E, Tarkowski A, Clumping factor A-mediated virulence during Staphylococcus aureus infection is retained despite fibrinogen depletion, Microbes Infect. 6 (2004) 196–201. [DOI] [PubMed] [Google Scholar]
- [22].Patti JM, Bremell T, Krajewska-Pietrasik D, Abdelnour A, Tarkowski A, Rydén C, Höök M, The Staphylococcus aureus collagen adhesin is a virulence determinant in experimental septic arthritis, Infect. Immun 62 (1994) 152–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Voyich JM, Braughton KR, Sturdevant DE, Whitney AR, Said-Salim B, Porcella SF, Long RD, Dorward DW, Gardner DJ, Kreiswirth BN, Musser JM, DeLeo FR, Insights into mechanisms used by Staphylococcus aureus to avoid destruction by human neutrophils, J. Immunol 175 (2005) 3907–3919. [DOI] [PubMed] [Google Scholar]
- [24].Koening MG, Factors relating to the virulence of staphylococci. I Comparative studies on two colonial variants, Yale J. Biol. Med 34 (1962) 943–949. [PMC free article] [PubMed] [Google Scholar]
- [25].Peterson PK, Wilkinson BJ, Kim Y, Schmeling D, Quie PG, Influence of encapsulation on staphylococcal opzonization and phagocytosis by human polymorphonuclear leukocytes, Infect. Immun 19 (1978) 943–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Green J, Schotland S, Sella Z, Kleeman CR, Interleukin-6 attenuates agonist-mediated calcium mobilization in murine osteoblastic cells, J. Clin. Invest 93 (1994) 2340–2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao Y-X, Abdelnour A, Holmdahl R, Tarkowski A, Mice with the Xid B cell defect are less susceptible to developing Staphylococcus aureus-induced arthritis, J. Immunol 155 (1995) 2067–2076. [PubMed] [Google Scholar]