Abstract
There is increasing evidence that several metals are endocrine disrupting chemicals (EDCs). In utero development and adolescence are critical windows of susceptibility to EDC exposure. With the exception of a few heavy metals, few human studies have evaluated the impact of metal exposure on pubertal development. Our aim was to investigate measures of in utero and peripubertal metal exposure in relation to reproductive hormone levels and sexual maturation and progression among girls from the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) cohorts. We measured urinary concentrations of aluminum (Al), arsenic (As), barium (Ba), cadmium (Cd), cobalt (Co), copper (Cu), iron (Fe), manganese (Mn), molybdenum (Mo), nickel (Ni), antimony (Sb), selenium (Se), and zinc (Zn) in samples collected from women during their third trimester of pregnancy and from their female children at 8-13 years (n=132). We measured serum testosterone, estradiol, dehydroepiandrosterone sulfate (DHEA-S), inhibin B, and sex hormone-binding globulin (SHBG) at age 8–13, and assessed Tanner stages for sexual maturation (breast, pubic hair development, and menarche status), at two time points (8–13, 14-18 years). We used linear regression to independently examine in utero and peripubertal metal concentrations as predictors of peripubertal hormones. In a longitudinal analysis using generalized estimation equations, we evaluated Tanner stage and menarche progression in relation to individual in utero and peripubertal metal concentrations. We found that higher in utero Zn was associated with increased inhibin B. Several metals at 8-13 years were associated with higher DHEA-S and estradiol, while Ni was positively but Cu was negatively associated with testosterone. In utero Ni, Al, and Cd were associated with slower progression of breast development after adjustment for child age and BMI z-score. For example, an IQR increase in in utero Al exposure was associated with 0.82 times lower odds of progressing to a higher Tanner stage for breast development per year (95% CI: 0.68, 0.99). Peripubertal concentrations of Ba and Al were also associated with being at a higher pubic hair Tanner stage and menarche at 8-13, but lower odds of progressing to the next stage at 14-18 years. We used Bayesian kernel machine regression (BKMR) to model the joint effect of multiple metals while accounting for correlated exposures, as well as potential non-linear relationships between metals and outcomes of interest, which yielded results similar to individual analyses. These findings suggest that female reproductive development may be vulnerable to the effects of metal exposure, and using both Tanner stages and hormone levels may provide clues about underlying mechanisms in two sensitive periods of development.
Keywords: Metal, Hormone, In utero exposure, Pregnancy, Puberty
INTRODUCTION
Puberty, the process by which adolescents reach sexual maturity and become capable of reproduction, is a period of rapid physical and psychological development, including increased weight and height velocity, sexual maturation, and cognitive and social maturation (Blakemore et al. 2010; Sisk and Foster 2004). Several studies have reported a temporal trend of earlier pubertal onset among girls (Anderson et al. 2003; Herman-Giddens et al. 1997; Wyshak and Frisch 1982). This trend is partly attributable to changes in diet (Cheng et al. 2012; Villamor and Jansen 2016; Wyshak and Frisch 1982; Zacharias and Wurtman 1969), obesity (Anderson et al. 2003; Kaplowitz 2008; Lee et al. 2007), and changes in various types of stress (Lee and Styne 2013; Parent et al. 2003). However, a growing body of evidence suggests that environmental factors may also be contributing to earlier pubertal onset and sexual maturation, with potential adverse effects (Buck Louis et al. 2008; Lee and Styne 2013).
Girls who begin puberty early may be at greater risk for alcohol and substance abuse (Castellanos-Ryan et al. 2013; Collado-Rodriguez et al. 2014; Patton et al. 2004), and psychological disorders (Klump 2013; Mendle et al. 2012; Tremblay and Lariviere 2009) during adolescence. Earlier pubertal onset is also associated with increased risk for certain types of cancer (Ali 2014; Collaborative Group on Hormonal Factors in Breast 2012; Jordan et al. 2005; Lacey et al. 2009; Stockl et al. 2011), metabolic syndrome and type 2-diabetes (Elks et al. 2013; Frontini et al. 2003; Janghorbani et al. 2014; Stockl et al. 2011; Widen et al. 2012), cardiovascular disease (Jacobsen et al. 2009; Lakshman et al. 2009; Prentice and Viner 2013), and other illnesses later in life (Mueller et al. 2014; Widen et al. 2012).
Early life exposure to endocrine disrupting chemicals (EDCs), including metals, are thought to play a role in altered pubertal timing. In utero exposure can pose estrogenic or anti-androgenic effects potentially resulting in long term deviation from normal homeostatic control of the hypothalamic-pituitary-gonadal (HPG) axis or hypothalamic-pituitary-adrenal (HPA) axis (Bellingham et al. 2009; Buck Louis et al. 2008; Den Hond and Schoeters 2006; Diamanti-Kandarakis et al. 2009; Jacobson-Dickman and Lee 2009; Massart et al. 2006; McGivern et al. 1991; Pescovitz and Walvoord 2007; Roy et al. 2009). Because in utero development is a period of organogenesis and increased hormonal activity, effects of exposure might persist long after birth and result in effects not observed with exposure at other life stages (Doherty et al. 2010; Su et al. 2010). Adolescence is also a susceptible window to the impact of endocrine disruptors, as dramatic hormonal changes during adolescence have profound effects on brain maturation, behavior, and reproductive development (Cahill 2006; Parent et al. 2015; Sisk and Foster 2004; Spear 2000).
Metals enter the human body through ingestion of food, water, and dietary supplements, and the use of metal-containing products, through inhalation and skin contact (Martin and Griswold 2009; Singh et al. 2011). In the United States, reports from the National Health and Nutrition Examination Survey (NHANES) show that children and adults, including pregnant women, have detectable concentrations of a range of metals in their bodies (Centers for Disease Control and Prevention (CDC) 2019). Because metals readily transfer cross human placenta and through breast milk, fetuses/infants are also exposed to multiple metals (Caserta et al. 2013; Chen et al. 2014; Ettinger et al. 2014; Punshon et al. 2016). Some metals are essential for human health such as Co, Cu, Fe, Mg, Mn, Mo, Ni, Se, and Zn, however, they can be toxic depending on their concentration; while other non-essential metals like Cd, Pb, Hg and As can be toxic if present even at low concentrations (Singh et al. 2011). A number of metals are reproductive toxicants and suspected endocrine disruptors (Bloom et al. 2010; De Coster and van Larebeke 2012; Diamanti-Kandarakis et al. 2009; Mendiola et al. 2011). Although exposures are highly prevalent (Centers for Disease Control and Prevention 2018), metals have received limited attention with respect to their endocrine disrupting potential. There has been some consistency with regard to the positive relationship between Cd exposure and testosterone levels among both males and females (Garcia-Morales et al. 1994; Jurasovic et al. 2004; Meeker et al. 2010; Menke et al. 2008; Nagata et al. 2005; Telisman et al. 2007; Zeng et al. 2002; Zeng et al. 2004). Urinary Cd was associated with increased serum testosterone in postmenopausal Japanese women when examined cross-sectionally (Nagata et al. 2005). In another study, Cd-treated human breast cancer cells showed a significant decrease in transcription of the estrogen receptor gene (Garcia-Morales et al. 1994).
A number of studies indicate that Pb exposure leads to delay in pubertal development in girls (De Craemer et al. 2017; Denham et al. 2005; Jansen et al. 2018; Liu et al. 2019; Selevan et al. 2003; Wu et al. 2003). Results from Denham et al. 2005 suggested that Hg concentrations were associated with earlier menarche, while other studies found that Hg was associated with increased estradiol levels in adults in Cambodia and among women with repeated miscarriages (Agusa et al. 2007; Gerhard et al. 1998). Human research regarding pubertal timing in relation to As exposure has been limited, but animal studies have found exposure to As resulted in delayed sexual maturation in female rats (Davila-Esqueda et al. 2012; Reilly et al. 2014).
In summary, most studies performed to assess the reproductive effect of metals focused on non-essential metals (Cd, Hg, As, and Pb) and adults, whereas information on a number of other metals that may act as endocrine disruptors is still scant and incomplete. A few recent studies found associations between certain essential or trace metals such as Cu, Mn, and Mo and adverse effects on male reproduction (Jeng et al. 2015; Meeker et al. 2008; Telisman et al. 2000). However, limited studies have measured associations in adolescents, and none have examined the impact of in utero exposures on sex hormone levels and timing of puberty. Therefore, our objective was to examine the association between in utero exposure to both essential and nonessential metals measured in maternal urine collected during the 3rd trimester of pregnancy, and sex hormone levels in girls aged 8 to 13 years, and measures of sexual maturation at age 8 to 13 and again at 14-18 years. Additionally, we examined cross-sectional associations between metal exposures, hormone levels, and sexual maturation assessed in adolescence.
METHOD
2.1. Study Population
This study used data collected from participants in the Early Life Exposure in Mexico to Environmental Toxicants (ELEMENT) project. ELEMENT is a longitudinal cohort study of women in Mexico City and their children. Mothers were recruited at maternity hospitals during their first trimester between 1997 and 2004 as previously described (Lewis et al. 2013; Liu et al. 2019; Wu et al. 2018). Spot urine samples were collected from pregnant women at third trimester visits [mean gestational age at visit: 34.4 (range: 28–43) weeks].
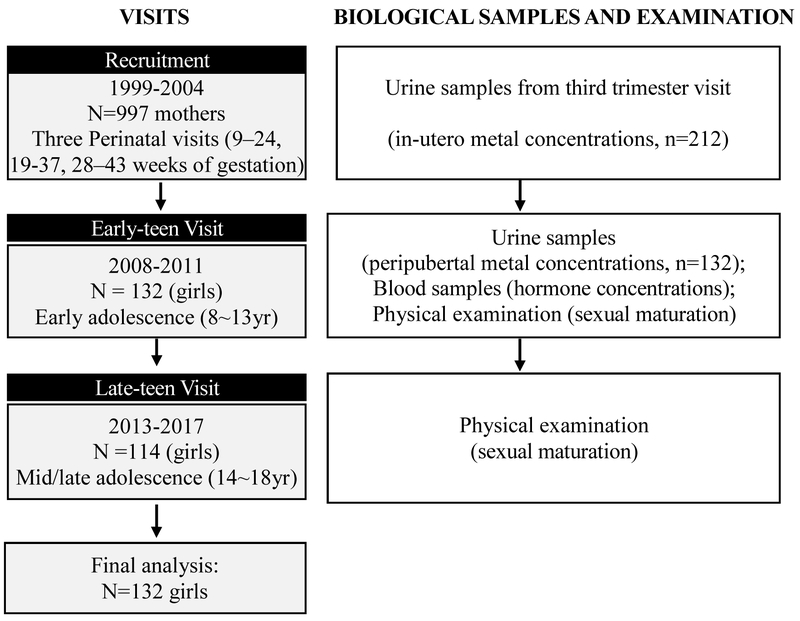
Between 2008 and 2011, 250 child participants (132 girls and 118 boys), then aged 8-13 years (childhood/early adolescence), were selected based on the availability of archived maternal biological specimens and invited to participate in a follow-up study (i.e. early-teen visit). Of those 250 children, 223 (89%) were enrolled again at age 14–18 years (late adolescence/adulthood) in a second follow-up study (i.e. late-teen visit), in 2013-2017. Among those 223 children, 114 were girls (86% retention rate from early to late-teen visit). Figure 1 shows the study design and the timing of biological sample collection and physical examination. Participants provided spot urine and fasting blood samples, anthropometry, and reported socio-demographic information via an interviewer-administered questionnaire. In the current analyses, we included female children who finished the early-teen visit (majority also completed late-teen visit) and had maternal urinary metal concentration measurements and/or their early-teen visit urinary metal measurements available (n=132). Research protocols were approved by the ethics and research committees of the Mexico National Institute of Public Health and the University of Michigan, and all participants provided informed consent prior to enrollment.
Figure 1.
Recruitment, sample collection and examination timeline for the ELEMENT study, a prospective birth cohort in Mexico City, Mexico.
2.2. Metal Concentrations
Urinary metal concentrations (Al, As, Ba, Cd, Co, Cu, Fe, Mn, Mo, Ni, Pb, Sb, Se, Zn) were measured in maternal third trimester urine and peripubertal urine samples collected during the early-teen visit at age 8–13 years. Prenatal and peripubertal urine samples were collected in sterile cups, aliquoted within one hour after collection, frozen and stored at −80°C, and shipped on dry ice to McGill University (Montreal, Canada) for analysis. Urinary metals were measured using inductively coupled plasma mass spectrometry (ICPMS; Varian 820-MS, Inc., Palo Alto, California) as described previously (Basu et al. 2010; Srigboh et al. 2016). Accuracy and precision were measured using certified reference standards (Institut National de Santé Publique du Québec, or INSPQ) with coefficients of variation (CVs) ranging from 3 to 14%, and each batch run contained procedural blanks and replicate runs (Lewis et al. 2018; Srigboh et al. 2016). More details regarding quality control (QC) were previously described (Lewis et al. 2018).
Values below the limit of detection (LOD) were replaced with the LOD/√2. Urinary specific gravity (SG) was measured using a handheld digital refractometer. Metal concentration values were corrected for urinary SG using the following equation: Pc = P[(SGp – 1)/(SGi – 1)] where Pc is the SG corrected metal concentration (μg/L), P is the measured metal concentration, SGp is the median urinary specific gravity, and SGi is the individual’s urinary specific gravity. Pb exposure was also measured in maternal patella/blood and early childhood blood, and results of these biomarkers in relation to pubertal development within this population have already been published (Jansen et al. 2018; Liu et al. 2019). As patella and blood are better biomarkers of Pb exposure, we excluded urinary Pb from the current analyses.
2.3. Hormones
Children provided fasting blood samples during the early-teen visit at age 8–13 years. Serum aliquots were separated and frozen at−80 °C, and then sent to the Clinical Ligand Assay Service Satellite (CLASS) Laboratory at the University of Michigan (Ann Arbor, MI) for hormone analysis. Estradiol, testosterone, inhibin B, and sex hormone-binding globulin (SHBG) were measured in serum samples as biomarkers of puberty, and dehydroepiandrosterone sulfate (DHEA-S) was measured as a biomarker of adrenarche. Estradiol, total testosterone, SHBG, and DHEA-S were measured using an automated chemiluminescent immunoassay (Bayer Diagnostics ACS:180). Active inhibin B was assayed using Gen II ELISA (Beckman Coulter, Webster, TX). Values below the LOD were replaced with the LOD/√2.
2.4. Sexual Maturation
Each participant underwent a physical examination during both follow-up visits at age 8–13 and 14-18 years. Exams were completed by two pediatricians at the early-teen visit, and by one of the same pediatricians at the late-teen visit. To ensure consistency, pediatricians were trained prior to the start of each follow-up as previously described (Chavarro et al. 2017) to evaluate Tanner staging in female participants using standardized protocols. Breast developmental stage and pubic hair stage were assessed as indicators of puberty and adrenarche, respectively. Tanner Stage 1 corresponds to the no development with progression to Tanner Stage 5, indicating full development (Marshall and Tanner 1969). Girls were also asked if they had had their first period (menarche) at both visits as an additional marker of sexual maturation.
2.5. Covariates
Age-specific BMI z-scores were calculated based on the World Health Organization child reference curves for age and sex (WHO, 2007) for each study visit. Socioeconomic status (SES) was estimated using a validated scale consisting of thirteen questions on housing quality, services, material goods and head of household education (Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública, AMAI version 13×6), which classifies households into six SES categories (AMAI 2000; López 2008).
2.6. Statistical Methods
We calculated geometric mean concentrations of each metal in prenatal and peripubertal urine samples. The percent of urine samples with concentrations below the LOD were reported for each metal, and metals that were detected in less than 50% of samples were excluded from further analysis.
Serum hormones were natural log-transformed prior to analysis to achieve normal distribution. Associations of prenatal and peripubertal urinary metal concentrations with peripubertal serum hormone concentrations were assessed using separate linear regression models for each metal, adjusting for potential confounders including child age and BMI z-score. SG was included as a covariate in all models as a measure of urinary dilution. The percent difference in hormones (95% confidence interval) per interquartile range (IQR) increase in urinary metal concentrations were calculated from model estimates. As a sensitivity analysis, we restricted analyses to girls that had not yet undergone menarche at the early-teen visit.
Longitudinal analyses were conducted to explore the association between repeated measures of metal exposure and sexual maturation using repeated generalized estimating equation models (GEE). With separate models for each metal, the GEE approach was used to fit ordinal regression models for pubertal stages at each visit:
where Y represents the repeated measures of Tanner stage and menarche at two visits, g () is a link function (cumulative logit), i denotes subject number (1,…,n), and j denotes visit number (1, 2). M represents the ln-transformed, sg-corrected metal concentration, Age is the age at the early-teen visit, and Time is the change in time between the early-teen and late-teen visit. We included age at early-teen visit and change in time between two visits to account for 1) the effect of baseline age on attained Tanner stage or menarche status, 2) the natural pubertal progression across time, and 3) the effect of baseline age on the natural pubertal progression. Hence, the coefficients of interest are the cross-sectional effects of prenatal or peripubertal metal concentrations on Tanner stage or menarche status at baseline (β1) and the effect of metal concentrations on the progression of Tanner stage or menarche status (β4). These were estimated using GEE with working independence correlation to ensure the validity of parameter estimates (Sullivan Pepe and Anderson 1994). We included SG-corrected metal concentrations rather than entering SG as a separate covariate to minimize the number of covariates in the models. In our final model we adjusted for BMI, specifically adding terms β6BMIbase+ β7BMIvar to the model described above, where BMIbase is BMI z-score at the early-teen visit and BMIvar is the change in the BMI z-score measure that occurred between the two visits. Results are presented as odds ratios (OR) and 95% confidence intervals (95% CI) per IQR increase in exposure.
To evaluate the joint effect of multiple metals, interactions between metals, and potential non-linear relationships between metals and outcomes of interest, we conducted mixture analyses using Bayesian kernel machine regression (BKMR) (Bobb et al. 2015). BKMR is designed for cross-sectional studies and can analyze continuous and binary outcomes in relation to exposure mixtures (Bobb et al. 2018). Therefore, for this analysis, only reproductive hormone levels and measures of sexual maturation from the early-teen visit were included and Tanner stages were converted to dichotomous variables (stage=1, stage >1). Because metal exposures in our study are correlated, we implemented BKMR with hierarchical variable selection (10,000 iterations by a Markov Chain Monte Carlo (MCMC) algorithm). This approach requires grouping of exposures based on similar potential mechanisms of action (e.g. toxic metals vs essential metals) and correlations between exposures. Therefore, we grouped Ni, Cu, Ba, Se, Al, and Zn into group 1, Mo, Mn, and Co into group 2, and As and Pb into group 3. The BKMR model for a continuous outcome (hormones) can be expressed as:
Where h denotes the unknown exposure-response function of the predictor variables, β represents the effect of the covariates (we controlled for the same covariates as in the main analysis). The same model can be extended to binary outcomes (sexual maturation measurements) via generalized linear modeling using the probit regression function. Posterior inclusion probabilities (PIP) were extracted from each BKMR model, which provides a measure of variable importance for each exposure group (groupPIP) and how each exposure in that group is driving that group-outcome association (condPIP). To determine the importance of each group/exposure for each study outcome a threshold of PIP>0.5 was used (Coker et al. 2018; Zhang et al. 2019).
All analyses were performed using R version 3.5.2 and SAS 9.4. R package bkmr was used to implement BKMR.
RESULTS
3.1. Exposure and Outcome Distributions
Distributions of metal concentrations (geometric means, standard deviations, and selected percentiles) from prenatal and early-teen visits, as well as the percent of samples with concentrations below the limit of detection are shown in Table 1. Spearman correlations between the prenatal and early-teen visit metal concentrations adjusted for SG are also presented in the table. With the exception of Sb and Fe, the majority of metals were detected in >50% of the urine samples, therefore, Sb and Fe were excluded from further analysis. Distributions and Spearman correlations of metals among all children (including male children) and their mothers in this cohort were previously reported elsewhere (Lewis et al. 2018); the analysis found weak correlations between maternal and childhood metal levels, weak to moderate correlations between different urinary metal concentrations within maternal and child samples were also reported. The associations between metal concentration and the covariates child age and child BMI-z-score are presented in SI Table S1.
Table1.
Distribution of uncorrected metal concentration during pregnancy (μg/L) among ELEMENT mothers who gave birth to girls and in their female children.
| In utero | Peripubertal | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LOD | %< LOD |
GM | GSD | 25% | 50% | 75% | MAX | %< LOD |
GM | GSD | 25% | 50% | 75% | MAX | P value a | |
| SG | <0.01 | |||||||||||||||
| Ni | 3.0 | 0.9 | 9.3 | 2.2 | 5.9 | 8.1 | 12.2 | 950.5 | 0.8 | 7.9 | 1.7 | 5.7 | 7.6 | 10.5 | 48.0 | 0.56 |
| Cu | 48.2 | 43.6 | 113.3 | 5.4 | 34.1 | 52.9 | 111.5 | 2610.4 | 47.0 | 46.9 | 1.4 | 34.1 | 49.3 | 62.1 | 119.8 | 0.71 |
| Mo | 2.9 | 23.1 | 14.5 | 3.4 | 7.1 | 20.9 | 37.8 | 85.9 | 0.0 | 41.6 | 1.9 | 28.4 | 44.6 | 64.8 | 186.4 | 0.93 |
| Ba | 1.1 | 6.8 | 3.7 | 2.2 | 2.1 | 4.3 | 6.4 | 23.3 | 7.6 | 2.8 | 2.0 | 1.7 | 2.8 | 4.2 | 24.4 | 0.55 |
| As | 0.3 | 0.0 | 12.4 | 2.3 | 8.7 | 12.4 | 18.2 | 546.1 | 0.0 | 12.9 | 1.9 | 8.4 | 12.9 | 19.3 | 126.8 | 0.29 |
| Se | 10.5 | 6.0 | 30.4 | 1.8 | 20.9 | 35.8 | 45.5 | 95.2 | 2.3 | 44.6 | 1.8 | 32.2 | 45.4 | 67.6 | 130.0 | 0.23 |
| Al | 8.6 | 12.1 | 23.7 | 2.3 | 12.8 | 28.3 | 39.5 | 486.1 | 13.6 | 16.1 | 1.9 | 10.4 | 15.1 | 23.1 | 114.4 | 0.19 |
| Mn | 0.4 | 6.8 | 0.8 | 1.9 | 0.6 | 0.7 | 1.0 | 9.1 | 5.3 | 1.0 | 1.8 | 0.7 | 1.0 | 1.6 | 5.9 | 0.09 |
| Co | 0.4 | 0.0 | 1.1 | 2.2 | 0.7 | 1.1 | 1.8 | 26.5 | 0.0 | 0.7 | 1.8 | 0.5 | 0.7 | 1.0 | 4.3 | 0.71 |
| Zn | 0.1 | 3.7 | 268.3 | 2.1 | 182.7 | 305.9 | 449.4 | 1545.4 | 0.8 | 336.2 | 1.9 | 218.9 | 357.4 | 543.4 | 1120.6 | 0.51 |
| Cd | 54.0 | 1.7 | 0.2 | 2.3 | 0.1 | 0.2 | 0.3 | 7.9 | 6.1 | 0.1 | 1.9 | 0.1 | 0.1 | 0.2 | 2.0 | 0.43 |
P value from Spearman correlation test between in utero and peripubertal specific urinary specific gravity and metal concentration measurements.
The distribution of measures of Tanner stages of sexual maturation at the two visits among this population is reported (SI Table S2). Among 132 female participants at the early-teen visit, 98 girls (74.2%) were at stage 1 for pubic hair development, 87 girls (65.9%) were at stage 1 for breast development, and only one girl had reached stage 5 (full maturity) for pubic hair development. At the late-teen visit, only 9 girls (8%) were still at stage 1 for pubic hair development and only 5 (4.4%) were at stage 1 for breast development, while 14 (12.4%) and 18 girls (15.9%) had reached full maturity (stage 5) for pubic hair and breast development, respectively. There were 22 girls (19.3%) at the early-teen visit and 90 girls (78.9%) at the late-teen visit who had attained menarche.
3.2. In utero and peripubertal metal exposure and peripubertal hormone levels
Associations of in utero and peripubertal metal concentrations with sex hormone levels are presented in Figure 2 and SI Table S4. In utero concentrations of metals were not associated with peripubertal serum hormone levels in girls, with the exception of Zn, for which an IQR increase was associated with 17.4% (95%CI: −31.5, −0.6) lower serum inhibin B after adjustment for child age, BMI z-score, and urinary SG. Although only marginally significant, girls exposed to higher Mo concentrations in utero had higher estradiol levels ((%Δ/IQR: 14.7 %, 95%CI: −0.6, −32.2). In models where sex hormones were regressed on concurrent peripubertal urinary metal concentrations, an IQR increase in Mn was associated with 18.4% higher (95%CI: 1.2, 38.5) DHEA_S and an IQR increase in Co was associated with 12.6% higher estradiol (95%CI: 0.04, 26.8). Peripubertal Ni concentrations were associated with higher testosterone levels (%Δ/IQR: 40.8, 95%CI: 18.0, 68.0). In contrast, peripubertal exposure to Cu was associated with lower testosterone levels (%Δ/IQR: −28.0, 95%CI: −45.5, −4.8). No significant associations were detected between peripubertal metal levels and inhibin B or SHBG.
Figure 2.
Percent difference in peripubertal hormone levels associated with an interquartile range (IQR) increase in in utero and peripubertal metal concentration among ELEMENT girlsa.
a Linear regression models were adjusted for child age, BMI z-score and specific gravity
* Significant associations detected (p value<0.05)
3.3. In utero and peripubertal metal exposure and sexual maturation
Results from multiple ordinal regression models of in utero metal concentrations and Tanner stage and menarche are shown in Figure 3 and SI Table S5. An IQR increase in in utero Mo concentrations was associated with 2.9 times greater odds (95% CI: 1.14, 7.55) of being at a higher stage of pubic hair maturation versus any lower stages. In utero concentrations of other metals were not significantly associated with Tanner stages. The odds of having undergone menarche at the early-teen visit was reduced with higher in utero Mn concentrations (OR/IQR: 0.29, 95% CI: 0.08, 0.98).
Figure 3.
Odds Ratios and 95% Confidence Intervals for the Ordinal Generalized Linear Regression of in utero Metal Exposure and Tanner Stagea
a GEE models were adjusted for child age and BMI z-score (baseline and change)
* Significant associations detected (p value<0.05)
As shown in Figure 4 and SI Table S5, peripubertal concentrations of some urinary biomarkers of exposure to metals were associated with higher odds of being at higher Tanner stage at age 8–13 years, adjusting for child age and BMI z-score, most notably with Al exposure. For an IQR increase in peripubertal Al, the odds of being at higher versus any lower stages for pubic hair development at the early-teen visit was 1.9 times greater (95% CI: 1.11, 3.36); similar associations were observed between odds of being at higher pubic hair stage and peripubertal exposure to Ba (OR/IQR: 1.69, 95% CI: 1.04, 2.75). Menarche was also cross-sectionally associated with peripubertal Co concentrations, for which an IQR increase was associated 3.8 times higher odds of having had menarche at the early-teen visit (95%CI: 1.14, 12.58).
Figure 4.
Odds Ratios and 95% Confidence Intervals for the Ordinal Generalized Linear Regression of peripubertal Metal Exposure and Tanner Stagea
a GEE models are adjusted for child age and BMI z-score (baseline and change)
* Significant associations detected (p value<0.05)
We also explored associations between in utero and peripubertal metal concentrations and pubertal development over time in girls. Results are also shown in Figure 3 and Figure 4 (SI Table S5). After controlling for age, BMI, and Tanner stage at the early-teen visit, in utero concentrations of Ni, Al, and Cd were associated with decreased odds of breast development progression during follow-up. The change in odds of being at higher Tanner stage for breast development per year after the early-teen visit was decreased by 27% (OR/IQR: 0.73 95% CI: 0.59, 0.89), 18% (OR/IOR: 0.82, 95% CI: 0.68, 0.99), and 17% (OR/IQR:0.83, 95% CI: 0.71, 0.97) for an IQR increase in in utero concentrations of Ni, Al, and Cd respectively. No significant associations were found between in utero urinary metal concentrations with the progression of pubic hair development or menarche, or between peripubertal urinary metal concentrations and the progression of puberty estimated by Tanner stage of breast development. However, higher peripubertal urinary Ni and Ba were associated with decreased odds of pubic hair development progression, for which IQR increases were associated with 25% (OR/IQR: 0.75, 95% CI: 0.59, 0.95) and 18% (OR/IQR:0.82, 95%CI: 0.67, 0.1) lower odds of being at a higher pubic hair Tanner stage, respectively. Similarly, we found associations between peripubertal Cd concentrations with decreased odds of menarche status progression (OR/IQR: 0.69, 95% CI: 0.48,1).
3.4. Sensitivity Analyses
When we restricted the hormone analysis to girls that had not yet undergone menarche at the early-teen visit (102 out of 132), the direction and magnitude of observed associations from the main analysis did not change (SI Table S6), and we observed one additional association: An IQR increase in in utero Mo was associated with 25% higher estradiol level (95% CI: 1.36, 53.98). Because BMI may be on the causal pathway between exposure and puberty, we ran the GEE models both with and without BMI z-score. The magnitude of estimates from both models were similar when BMI z-score was not included (SI Table S7), with slightly attenuated associations between in utero Mo concentration and pubic hair development, and peripubertal Cd and menarche progression, as well as a slightly stronger association between peripubertal As concentrations and pubic hair development. In another sensitivity analysis, SES was included as a covariate in models of hormones and maturation stages because it could be a potential confounder, associated with metal exposure and puberty. Findings were generally consistent with metal and hormone associations observed in our main analyses; in SES-adjusted models, association between in utero Cd concentrations and lower inhibin B (%Δ/IQR=−15.27, 95%CI=−26.72,−2.04), was stronger and statistically significant, while the association between peripubertal Cu and testosterone was slightly attenuated and no longer significant. In GEE models for Tanner stage or menarche status including SES, cross-sectional associations between pubic hair Tanner stage and Al (OR/IQR: 2.5, 95% CI :1.04, 5.99) and Zn (OR/IQR: 2.8, 95% CI: 1.01, 7.77) were significant while other findings remain consistent with the main models. As Cu was below the detection limit in 44% of samples, we categorized urinary Cu concentration into three groups in a secondary analysis. The low group consisted of values below the LOD, while the medium and high groups were made up of equalized bins among the detected values. We estimated model parameters using this categorical variable, and the parameter results were similar to those from the main analysis.
3.5. Mixture Analysis
Finally, the estimates of PIPs from sexual maturation and reproductive hormone BKMR models are presented in Table 2 and Table S8, respectively. Mixture analysis results were comparable to the findings from individual analyses; 1) Among both models where breast development regressed on in utero and prepubertal metal exposure mixtures, all three group PIPs were <0.5. (i.e. no group was identified as “important”) 2) In the model for menarche and in utero metal mixtures, PIP for group 2 (Mo, Mn, and Co) was > 0.5 and condPIP of Mn was the highest (0.71) within this group; 3) Similarly, peripubertal Co was identified as the most important exposure for menarche in relation to peripubertal metal mixture; 4) In reproductive hormone BKMR models, few metals with both group PIPs and CondPIPs > 0.5 were in agreement with the significant or borderline significant in the individual analyses. However, for pubic hair development models regressed on exposure mixtures, all three groupPIPs were <0.5, whereas individual analysis found associations between in utero Mo, peripubertal Ba and Al and pubic hair stages at the early-teen visit. BKMR also generated figure outputs corresponding to exposure-response relationship for each metal when fixing a second metal at various quantiles (10th, 50th, 90th percentiles). SI Figure S1 shows an example of the exposure-response relationship where menarche status was regressed on the peripubertal metal mixture which indicated that there is no interaction between different metals. Similarly, no interaction between any of the metals with any other outcome is detected.
Table 2.
Posterior inclusion probabilities (PIPs) for group inclusion and conditional inclusion into sexual maturation measurements models, using Bayesian kernel machine regression (BKMR) model (N=132)a
|
In utero exposure |
Peripubertal exposure |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Metals | Group | Breast Development | Pubic Hair Development | Menarche | Breast Development | Pubic Hair Development | Menarche | ||||||
| groupPIP | condPIP | groupPIP | condPIP | groupPIP | condPIP | groupPIP | condPIP | groupPIP | condPIP | groupPIP | condPIP | ||
| Ni | 1 | 0.38 | 0.48 | 0.34 | 0.03 | 0.27 | 0.11 | 0.33 | 0.13 | 0.34 | 0.11 | 0.28 | 0.27 |
| Cu | 1 | 0.38 | 0.05 | 0.34 | 0.17 | 0.27 | 0.06 | 0.33 | 0.23 | 0.34 | 0.23 | 0.28 | 0.19 |
| Mo | 2 | 0.22 | 0.28 | 0.32 | 0.52 | 0.72 | 0.19 | 0.40 | 0.28 | 0.42 | 0.29 | 0.59 | 0.10 |
| Ba | 1 | 0.38 | 0.09 | 0.34 | 0.09 | 0.27 | 0.26 | 0.33 | 0.11 | 0.34 | 0.11 | 0.28 | 0.04 |
| As | 3 | 0.20 | 0.76 | 0.20 | 0.58 | 0.29 | 0.30 | 0.37 | 0.66 | 0.36 | 0.65 | 0.23 | 0.43 |
| Se | 1 | 0.38 | 0.14 | 0.34 | 0.29 | 0.27 | 0.34 | 0.33 | 0.34 | 0.34 | 0.31 | 0.28 | 0.16 |
| Al | 1 | 0.38 | 0.16 | 0.34 | 0.24 | 0.27 | 0.03 | 0.33 | 0.03 | 0.34 | 0.10 | 0.28 | 0.05 |
| Mn | 2 | 0.22 | 0.26 | 0.32 | 0.17 | 0.72 | 0.71 | 0.40 | 0.31 | 0.42 | 0.32 | 0.59 | 0.08 |
| Co | 2 | 0.22 | 0.46 | 0.32 | 0.32 | 0.72 | 0.10 | 0.40 | 0.41 | 0.42 | 0.39 | 0.59 | 0.82 |
| Zn | 1 | 0.38 | 0.08 | 0.34 | 0.19 | 0.27 | 0.19 | 0.33 | 0.17 | 0.34 | 0.14 | 0.28 | 0.28 |
| Cd | 3 | 0.20 | 0.24 | 0.20 | 0.42 | 0.29 | 0.70 | 0.37 | 0.34 | 0.36 | 0.35 | 0.23 | 0.57 |
Models were adjusted for age and BMI z-score at early teen visit
DISCUSSION
In this study, we investigated the effects of in utero and peripubertal metal exposure on subsequent peripubertal steroid hormone levels and progression of puberty in Mexican girls. Average urinary concentrations of metals measured in the present study were generally lower than levels reported in studies of pregnant women and their children living in regions of Mexico where metals-related industries are prevalent (Garcia-Vargas et al. 2014; Moreno et al. 2010; Roy et al. 2011). However, most of the metal concentrations among women and their children in this study were largely higher than the US women and children within 12-19 years old age group, participants of NHANES, including various cycles from 1999 to 2014 (Centers for Disease Control and Prevention 2018) (SI Table S3). We found associations between in utero and peripubertal exposure to a number of metals, reproductive hormones, and progression of pubertal development.
In Utero Exposure:
Higher in utero Zn exposure was negatively associated with inhibin B levels among female children, and marginally associated with decreased odds of pubic hair development progression. This finding is consistent with one animal study where zinc-supplemented testicular tissue from adult male rats showed reduced levels of inhibin-B and spermatogenetic activity (Semercioz et al. 2017). The “inhibin B pubertal surge” signals gonadal maturation among girls as it is a prominent marker of follicular development through the peripubertal stages (Burger et al. 2000; Lahlou and Roger 2004; Welt et al. 1999); therefore, the observed inverse association between in utero Zn exposure, inhibin B, and progression of pubic hair development adds further evidence that in utero Zn exposure may be associated with the timing of pubertal progression.
We found that one IQR increase in utero Mo concentrations was associated with 2.9 times greater odds of being at a higher stage of pubic hair development at early-teen visit among girls in our study; and among those who had not undergone menarche, Mo was also associated with 25% higher estradiol levels. While no prior studies have investigated these associations in girls, inverse associations between urinary concentrations of Mo and testosterone and sperm concentration were reported among men of reproductive age in the general US population (Lewis and Meeker 2015; Meeker et al. 2008; Meeker et al. 2010). Although no association with higher Mo levels were evaluated in animal studies, Mo deficiency was reported to be associated with delayed puberty among female cattle; with our findings on high Mo concentrations associated with advanced sexual maturation, these may reflect the lower and higher end of the dose-response relationship between Mo and puberty (Deb et al. 2014).
We observed 17–27% decreased odds of progression to a higher stage of breast development over the study period associated with an IQR increase in Ni, Al, and Cd concentration. These findings are consistent with results of a recent cross-sectional study that showed blood Cd was associated with delayed breast and pubic hair development among Flemish adolescent females (De Craemer et al. 2017). Previously, another study suggested that higher urinary Cd was associated with decreased inhibin B levels and might be linked to pubertal delays in 705 girls 6-11years of age who participated in NHANES III (1988-1994) (Gollenberg et al. 2010). However, both studies were cross-sectional and therefore unable to clearly establish temporal relationships between metal exposures and pubertal development. Animal studies have also found that rats exposed to Cd during gestation had delayed sexual maturation together with increased oxidative stress and impaired steroid hormone levels (Samuel et al. 2011). There have been no human studies of in utero Al and Ni exposure and sex hormone levels during the peripubertal period, although our findings are consistent with the previous animal and in vitro studies that have reported associations between Ni exposure and disruption of mammalian reproductive functions (Iscan et al. 2002).
There are no previous studies reporting relationships between Mn exposure and sexual maturation in humans, although experimental studies assessing the ability of Mn to stimulate critical hypothalamic actions among female rats reported that Mn is capable of enhancing puberty-related hormone secretions, and thus, may facilitate the normal onset and progression of puberty (Dees et al. 2017; Pine et al. 2005). This is in contrast to our finding that in utero Mn concentrations were negatively associated with having experienced menarche prior to the early-teen visit.
Peripubertal Exposure:
Higher peripubertal Co concentrations were positively associated with both estradiol levels and odds of having had menarche at the early-teen visit. This is in contrast to two experimental studies that explored the reproductive effects of Co exposure in mice, of which one showed that Co exposure was associated with significantly reduced reproductive organ development (Pedigo et al. 1988), while the other observed a null association (Madzharova et al. 2010). However, it has been shown that circulating estradiol drives the activation of kisspeptin, which in turn activates gonadotropin-releasing hormone (GnRH) neurons, which is essential for the onset of puberty (Clarkson et al. 2009; Karapanou and Papadimitriou 2010). Therefore, the observed positive association of peripubertal Co exposure with estradiol and odds of menarche adds further evidence that Co exposure may be associated with the timing of puberty.
We found a negative association between peripubertal Cu concentration and testosterone levels among female children, which was consistent with findings from the Flemish study (De Craemer et al. 2017). We also observed that being at higher pubic hair development stage at the early-teen visit was suggestively associated with peripubertal Cu concentration, while the Flemish study found that blood Cu was associated with a delay in maturation among 14–15 year-old adolescents. However, caution has to be taken when comparing the two studies because we measured urinary Cu while the Flemish study assessed Cu in blood. Cu is a trace element which is subject to a complex system to maintain homeostasis in the body (Blazewicz et al. 2013). Nonessential metals such as Cd can affect the metabolism and urinary excretion of essential metals like Cu (Ashby et al. 1980; Chmielnicka et al. 1989). As such, differences in Cu concentrations in urine might not only be caused by a higher exposure, but also by the individual differences in non-essential metal concentrations and their impacts on homeostasis.
We found that an IQR increase in Ba concentrations was associated with a 1.7 fold increase in odds of being at higher pubic hair stage at the early-teen visit and 18% decreased odds of pubic hair development progression. No previous studies were located reporting effects of Ba on maturation. Decreased progression of maturation for those female children at higher Tanner stages at the early-teen visit (i.e. increased progression for those at a lower stage) may be explained by a concept similar to “catch-up growth” (Wu et al. 2018). Catch up growth is defined as an increased growth following a period of growth inhibition (Ashworth and Millward 1986; Wit and Boersma 2002). Since some girls had experienced an advanced pubertal development in relation to exposure to metals at early-teen, their body systems may have responded by slowing down the tempo of pubertal progression, attenuating the accelerating effect exerted by metal; while others who had experienced a delayed puberty may respond by accelerating the change from lower stages of puberty to higher stages. How catch-up growth or “catch-up puberty” may be achieved is not understood and future research is needed to confirm these findings.
We also found a negative association between peripubertal Cd concentrations and timing of menarche, which is consistent with previous cross-sectional analyses of menarche onset (De Craemer et al. 2017; Gollenberg et al. 2010). However, caution has to be taken while comparing these studies as we considered a longitudinal association between metal exposure and pubertal development by introducing an interaction term with time and exposure in the model.
Results from mixture analyses using BKMR identified the same critical metals as reported in the individual analysis, except for pubic hair development models. This may be due to different distribution assumptions for the outcome in BKMR and GEE models, as the probit regression in BKMR required dichotomous Tanner stage variables, whereas ordinal generalized regression models utilized each category of the outcome.
Potential Mechanisms:
The onset and progression of puberty depend on a complex interplay between the central neuroendocrine system, the gonads, and the adrenal cortex modulated by a sex steroid-mediated negative feedback mechanism (Bordini and Rosenfield 2011), and metals exposure may affect this process through a few different mechanisms. Puberty changes occur as a consequence of the activation of the HPG axis: the secretion of neuroendocrine factors stimulates the pulsatile secretion of GnRH, which eventually result in the secretion of the sex hormones estradiol and progesterone from the ovaries. Estrogen stimulates breast development and genital growth (Bordini and Rosenfield 2011; Stattin and Magnusson 1990). Pubertal changes are also a result of HPA axis activation: the adrenal zona reticularis increases adrenal androgen production. Pubic and axillary hair growth can be stimulated by the adrenal androgens dehydroepiandrosterone and DHEA-S (Bordini and Rosenfield 2011).
In vitro and animal studies suggest that non-essential metals can disrupt the onset and progression of puberty via interrupting the critical neuroendocrine and hormonal pathways (Dees et al. 2017; Dyer 2007; Garcia-Morales et al. 1994; Iavicoli et al. 2009; Nagata et al. 2005; Pine et al. 2005; Shen et al. 2016). Moreover, essential metals necessary for pubertal development can also be harmful when at deficient or excessive concentrations as they can accelerate or delay timing and progression of normal puberty (Dees et al. 2017; Kozielec et al. 1996).
Considering the positive associations between certain reproductive hormone levels and sexual maturation in normal pubertal development, the observed associations of Co and Zn with sexual maturation (increased odds of menarche; decreased odds of pubic hair development) are in line with their associations with hormone levels (increased estradiol; decreased inhibin B). Several animal studies on Pb and Cd have suggested that metals may impact sexual maturation through changes in reproductive hormones; by suppressing the secretion of sex steroid hormones involved in the initiation of puberty, sexual maturation is delayed (Iavicoli et al. 2009; Lafuente et al. 2003; Sokol and Berman 1991). It is possible that Co and Zn also exert their influence on the endocrine system through the same mechanism of action, but different mechanisms are also possible.
Human studies also suggest that change in body fat (primarily measured by BMI) is another potential pathway for metals to affect puberty. When we did not include BMI z-score in models evaluating the relationship between metals and sexual maturation measurements, effect estimates for a few associations were attenuated whereas others became stronger compared to models with BMI z-score included (Supplemental SI Table S7). These findings suggest that in utero and peripubertal metal exposure may be related to pubertal development both independent of BMI as well as via this pathway. However, the relationship between in utero or peripubertal metal exposure and peripubertal BMI is not well understood. Some studies have shown a relationship with early growth, but these findings have also been inconsistent between studies (Fan et al. 2017; Padilla et al. 2010; Park et al. 2017), therefore, additional research is needed.
This longitudinal analysis allowed us to capture associations of both in utero and peripubertal metal exposure on hormone levels and measures of sexual maturation, as well as Tanner stage progression during the peripubertal period. However, this analysis also had a number of limitations, including a somewhat small sample size and few observations for certain Tanner stages, resulting in imprecise effect estimates. In addition, because we measured several metals, a large number of comparisons were made which likely increased the probability of chance findings, although the considered outcome measures are not independent of one another. As some urinary metal concentrations have been shown to vary over relatively short periods of time, a single sample collected during third trimester visit and at the early-teen visit may not fully characterize exposure during each specific window of development or reflect trends in exposure. We were not able to assess the combined effect of metals and other EDCs (e.g. phthalates) due to sample size constraints, however, metal concentrations are not likely to be correlated with other chemicals or share sources of exposure. Nevertheless, a larger cohort mixture analysis considering the multitude of environmental toxicants humans are exposed to daily is needed. Lastly, the diurnal variation of hormones may result in non-differential misclassification of the concentrations as they were measured at only one time point.
CONCLUSION
Our study found a variety of associations among several in utero and peripubertal metal concentrations with reproductive hormone levels and sexual maturation among girls, several of which are comparable to previous reports from human and animal research and supported by the current understanding of female pubertal development. These findings add to a growing body of literature on the reproductive effects of environmental metal exposure. Our work also suggests that aspects of reproductive development may be more vulnerable to the effects of metal exposure during critical periods of in utero and/or peripubertal development. The potential impact of metals exposure on pubertal timing is an important public health concern as an advance or delay in normal pubertal development is associated with adverse health outcomes during adolescence and adult reproductive life. Therefore, more epidemiological studies are needed to confirm these findings and to better understand the underlying mechanism for the effect of metals on sexual maturation and progression.
Supplementary Material
HIGHLIGHTS.
In utero and prepubertal reproductive development are vulnerable to metal exposure
Essential and non-essential metals are associated with reproductive hormone levels
In utero Ni, Al, and Cd were associated with slower progression of breast development
Mixture analyses of eleven metals revealed similar results as individual analyses.
Acknowledgments
We would like to thank American British Cowdray (ABC) Hospital for providing facilities for this research.
Funding
This work was supported by U.S. Environmental Protection Agency (US EPA) grants RD834800 and RD83543601 and National Institute for Environmental Health Sciences (NIEHS) grants P20 ES018171, P01 ES02284401, R01 ES007821, and P30 ES017885. Its contents are solely the responsibility of the grantee and do not necessarily represent the official views of the US EPA. Further, the US EPA does not endorse the purchase of any commercial products or services mentioned in the publication. This work was also supported and partially funded by the National Institute of Public Health, Ministry of Health of Mexico.
Abbreviations
- DHEA-S
dehydroepiandrosterone sulfate
- ELEMENT
Early Life Exposure in Mexico to Environmental Toxicants
- GM
geometric mean
- HPG
hypothalamus-pituitary-gonadal
- HPA
hypothalamic-pituitary-adrenal
- IQR
interquartile range
- LOD
limit of detection
- SHBG
sex hormone-binding globulin
- SG
specific gravity
Footnotes
Competing interests
The authors declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Agusa T, Kunito T, Iwata H, Monirith I, Chamnan C, Tana TS, et al. 2007. Mercury in hair and blood from residents of phnom penh (cambodia) and possible effect on serum hormone levels. Chemosphere 68:590–596. [DOI] [PubMed] [Google Scholar]
- Ali AT. 2014. Reproductive factors and the risk of endometrial cancer. Int J Gynecol Cancer 24:384–393. [DOI] [PubMed] [Google Scholar]
- AMAI. 2000. Avances del comité de niveles socioeconómicos. Vol. 2018:Comité de Niveles Socioeconómicos. Asociación Mexicana de Agencias de Investigación de Mercados y Opinión Pública, A.C.
- Anderson SE, Dallal GE, Must A. 2003. Relative weight and race influence average age at menarche: Results from two nationally representative surveys of us girls studied 25 years apart. Pediatrics 111:844–850. [DOI] [PubMed] [Google Scholar]
- Ashby SL, King LJ, Parke DV. 1980. Effect of acute administration of cadmium on the disposition of copper, zinc, and iron in the rat. Environ Res 21:177–185. [DOI] [PubMed] [Google Scholar]
- Ashworth A, Millward DJ. 1986. Catch-up growth in children. Nutr Rev 44:157–163. [DOI] [PubMed] [Google Scholar]
- Basu N, Abare M, Buchanan S, Cryderman D, Nam DH, Sirkin S, et al. 2010. A combined ecological and epidemiologic investigation of metal exposures amongst indigenous peoples near the marlin mine in western guatemala. Sci Total Environ 409:70–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham M, Fowler PA, Amezaga MR, Rhind SM, Cotinot C, Mandon-Pepin B, et al. 2009. Exposure to a complex cocktail of environmental endocrine-disrupting compounds disturbs the kisspeptin/gpr54 system in ovine hypothalamus and pituitary gland. Environ Health Perspect 117:1556–1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ, Burnett S, Dahl RE. 2010. The role of puberty in the developing adolescent brain. Hum Brain Mapp 31:926–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazewicz A, Klatka M, Astel A, Partyka M, Kocjan R. 2013. Differences in trace metal concentrations (co, cu, fe, mn, zn, cd, and ni) in whole blood, plasma, and urine of obese and nonobese children. Biol Trace Elem Res 155:190–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Parsons PJ, Steuerwald AJ, Schisterman EF, Browne RW, Kim K, et al. 2010. Toxic trace metals and human oocytes during in vitro fertilization (ivf). Reprod Toxicol 29:298–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Valeri L, Claus Henn B, Christiani DC, Wright RO, Mazumdar M, et al. 2015. Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures. Biostatistics 16:493–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobb JF, Henn BC, Valeri L, Coull BA. 2018. Statistical software for analyzing the health effects of multiple concurrent exposures via bayesian kernel machine regression. Environmental Health 17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordini B, Rosenfield RL. 2011. Normal pubertal development: Part i: The endocrine basis of puberty. Pediatr Rev 32:223–229. [DOI] [PubMed] [Google Scholar]
- Buck Louis GM, Gray LE Jr., Marcus M, Ojeda SR, Pescovitz OH, Witchel SF, et al. 2008. Environmental factors and puberty timing: Expert panel research needs. Pediatrics 121 Suppl 3:S192–207. [DOI] [PubMed] [Google Scholar]
- Burger HG, Dudley E, Mamers P, Groome N, Robertson DM. 2000. Early follicular phase serum fsh as a function of age: The roles of inhibin b, inhibin a and estradiol. Climacteric 3:17–24. [DOI] [PubMed] [Google Scholar]
- Cahill L 2006. Why sex matters for neuroscience. Nat Rev Neurosci 7:477–484. [DOI] [PubMed] [Google Scholar]
- Caserta D, Graziano A, Lo Monte G, Bordi G, Moscarini M. 2013. Heavy metals and placental fetal-maternal barrier: A mini-review on the major concerns. Eur Rev Med Pharmacol Sci 17:2198–2206. [PubMed] [Google Scholar]
- Castellanos-Ryan N, Parent S, Vitaro F, Tremblay RE, Seguin JR. 2013. Pubertal development, personality, and substance use: A 10-year longitudinal study from childhood to adolescence. J Abnorm Psychol 122:782–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2018. Fourth report on human exposure to environmental chemicals. Available: https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Mar2018.pdf [accessed 04/04/2018.
- Centers for Disease Control and Prevention (CDC). 2019. Fourth national report on human exposure to environmental chemicals updated tables.
- Chavarro JE, Watkins DJ, Afeiche MC, Zhang Z, Sanchez BN, Cantonwine D, et al. 2017. Validity of self-assessed sexual maturation against physician assessments and hormone levels. J Pediatr 186:172–178 e173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Myers R, Wei T, Bind E, Kassim P, Wang G, et al. 2014. Placental transfer and concentrations of cadmium, mercury, lead, and selenium in mothers, newborns, and young children. J Expo Sci Environ Epidemiol 24:537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Buyken AE, Shi L, Karaolis-Danckert N, Kroke A, Wudy SA, et al. 2012. Beyond overweight: Nutrition as an important lifestyle factor influencing timing of puberty. Nutr Rev 70:133–152. [DOI] [PubMed] [Google Scholar]
- Chmielnicka J, Halatek T, Jedlinska U. 1989. Correlation of cadmium-induced nephropathy and the metabolism of endogenous copper and zinc in rats. Ecotoxicol Environ Saf 18:268–276. [DOI] [PubMed] [Google Scholar]
- Clarkson J, Boon WC, Simpson ER, Herbison AE. 2009. Postnatal development of an estradiol-kisspeptin positive feedback mechanism implicated in puberty onset. Endocrinology 150:3214–3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coker E, Chevrier J, Rauch S, Bradman A, Obida M, Crause M, et al. 2018. Association between prenatal exposure to multiple insecticides and child body weight and body composition in the vhembe south african birth cohort. Environ Int 113:122–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collaborative Group on Hormonal Factors in Breast C. 2012. Menarche, menopause, and breast cancer risk: Individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol 13:1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collado-Rodriguez A, MacPherson L, Kurdziel G, Rosenberg LA, Lejuez CW. 2014. The relationship between puberty and risk taking in the real world and in the laboratory. Pers Individ Dif 68:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davila-Esqueda ME, Jimenez-Capdeville ME, Delgado JM, De la Cruz E, Aradillas-Garcia C, Jimenez-Suarez V, et al. 2012. Effects of arsenic exposure during the pre- and postnatal development on the puberty of female offspring. Exp Toxicol Pathol 64:25–30. [DOI] [PubMed] [Google Scholar]
- De Coster S, van Larebeke N. 2012. Endocrine-disrupting chemicals: Associated disorders and mechanisms of action. J Environ Public Health 2012:713696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Craemer S, Croes K, van Larebeke N, De Henauw S, Schoeters G, Govarts E, et al. 2017. Metals, hormones and sexual maturation in flemish adolescents in three cross-sectional studies (2002–2015). Environ Int 102:190–199. [DOI] [PubMed] [Google Scholar]
- Deb R, Chakraborty S, Mahima, Verma AK, Tiwari R, Dhama K 2014. Nutrigenomics and its role in male puberty of cattle: A mini review. Pak J Biol Sci 17:329–334. [DOI] [PubMed] [Google Scholar]
- Dees WL, Hiney JK, Srivastava VK. 2017. Influences of manganese on pubertal development. J Endocrinol 235:R33–R42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Den Hond E, Schoeters G. 2006. Endocrine disrupters and human puberty. Int J Androl 29:264–271; discussion 286–290. [DOI] [PubMed] [Google Scholar]
- Denham M, Schell LM, Deane G, Gallo MV, Ravenscroft J, DeCaprio AP, et al. 2005. Relationship of lead, mercury, mirex, dichlorodiphenyldichloroethylene, hexachlorobenzene, and polychlorinated biphenyls to timing of menarche among akwesasne mohawk girls. Pediatrics 115:e127–134. [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, et al. 2009. Endocrine-disrupting chemicals: An endocrine society scientific statement. Endocr Rev 30:293–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty LF, Bromer JG, Zhou Y, Aldad TS, Taylor HS. 2010. In utero exposure to diethylstilbestrol (des) or bisphenol-a (bpa) increases ezh2 expression in the mammary gland: An epigenetic mechanism linking endocrine disruptors to breast cancer. Horm Cancer 1:146–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer CA. 2007. Heavy metals as endocrine-disrupting chemicals In: Endocrine-disrupting chemicals:Springer, 111–133. [Google Scholar]
- Elks CE, Ong KK, Scott RA, van der Schouw YT, Brand JS, Wark PA, et al. 2013. Age at menarche and type 2 diabetes risk: The epic-interact study. Diabetes Care 36:3526–3534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ettinger AS, Roy A, Amarasiriwardena CJ, Smith D, Lupoli N, Mercado-Garcia A, et al. 2014. Maternal blood, plasma, and breast milk lead: Lactational transfer and contribution to infant exposure. Environ Health Perspect 122:87–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Zhang C, Bu J. 2017. Relationship between selected serum metallic elements and obesity in children and adolescent in the u.S. Nutrients 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frontini MG, Srinivasan SR, Berenson GS. 2003. Longitudinal changes in risk variables underlying metabolic syndrome x from childhood to young adulthood in female subjects with a history of early menarche: The bogalusa heart study. Int J Obes Relat Metab Disord 27:1398–1404. [DOI] [PubMed] [Google Scholar]
- Garcia-Morales P, Saceda M, Kenney N, Kim N, Salomon DS, Gottardis MM, et al. 1994. Effect of cadmium on estrogen receptor levels and estrogen-induced responses in human breast cancer cells. J Biol Chem 269:16896–16901. [PubMed] [Google Scholar]
- Garcia-Vargas GG, Rothenberg SJ, Silbergeld EK, Weaver V, Zamoiski R, Resnick C, et al. 2014. Spatial clustering of toxic trace elements in adolescents around the torreon, mexico lead-zinc smelter. J Expo Sci Environ Epidemiol 24:634–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerhard I, Waibel S, Daniel V, Runnebaum B. 1998. Impact of heavy metals on hormonal and immunological factors in women with repeated miscarriages. Hum Reprod Update 4:301–309. [DOI] [PubMed] [Google Scholar]
- Gollenberg AL, Hediger ML, Lee PA, Himes JH, Louis GM. 2010. Association between lead and cadmium and reproductive hormones in peripubertal u.S. Girls. Environ Health Perspect 118:1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman-Giddens ME, Slora EJ, Wasserman RC, Bourdony CJ, Bhapkar MV, Koch GG, et al. 1997. Secondary sexual characteristics and menses in young girls seen in office practice: A study from the pediatric research in office settings network. Pediatrics 99:505–512. [DOI] [PubMed] [Google Scholar]
- Iavicoli I, Fontana L, Bergamaschi A. 2009. The effects of metals as endocrine disruptors. Journal of Toxicology and Environmental Health, Part B 12:206–223. [DOI] [PubMed] [Google Scholar]
- Iscan M, Ada AO, Coban T, Kapucuoglu N, Aydin A, Isimer A. 2002. Combined effects of cadmium and nickel on testicular xenobiotic metabolizing enzymes in rats. Biol Trace Elem Res 89:177–190. [DOI] [PubMed] [Google Scholar]
- Jacobsen BK, Oda K, Knutsen SF, Fraser GE. 2009. Age at menarche, total mortality and mortality from ischaemic heart disease and stroke: The adventist health study, 1976–88. Int J Epidemiol 38:245–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson-Dickman E, Lee MM. 2009. The influence of endocrine disruptors on pubertal timing. Curr Opin Endocrinol Diabetes Obes 16:25–30. [DOI] [PubMed] [Google Scholar]
- Janghorbani M, Mansourian M, Hosseini E. 2014. Systematic review and meta-analysis of age at menarche and risk of type 2 diabetes. Acta Diabetol 51:519–528. [DOI] [PubMed] [Google Scholar]
- Jansen EC, Zhou L, Song PXK, Sanchez BN, Mercado A, Hu H, et al. 2018. Prenatal lead exposure in relation to age at menarche: Results from a longitudinal study in mexico city. J Dev Orig Health Dis 9:467–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeng HA, Huang YL, Pan CH, Diawara N. 2015. Role of low exposure to metals as male reproductive toxicants. Int J Environ Health Res 25:405–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SJ, Webb PM, Green AC. 2005. Height, age at menarche, and risk of epithelial ovarian cancer. Cancer Epidemiol Biomarkers Prev 14:2045–2048. [DOI] [PubMed] [Google Scholar]
- Jurasovic J, Cvitkovic P, Pizent A, Colak B, Telisman S. 2004. Semen quality and reproductive endocrine function with regard to blood cadmium in croatian male subjects. Biometals 17:735–743. [DOI] [PubMed] [Google Scholar]
- Kaplowitz PB. 2008. Link between body fat and the timing of puberty. Pediatrics 121 Suppl 3:S208–217. [DOI] [PubMed] [Google Scholar]
- Karapanou O, Papadimitriou A. 2010. Determinants of menarche. Reprod Biol Endocrinol 8:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klump KL. 2013. Puberty as a critical risk period for eating disorders: A review of human and animal studies. Horm Behav 64:399–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozielec T, Drybanska-Kalita A, Hornowska I, Salacka A. 1996. [levels of calcium, magnesium, zinc, copper and iron in hair of children and adolescents]. Pol Merkur Lekarski 1:150–154. [PubMed] [Google Scholar]
- Lacey JV Jr., Kreimer AR, Buys SS, Marcus PM, Chang SC, Leitzmann MF, et al. 2009. Breast cancer epidemiology according to recognized breast cancer risk factors in the prostate, lung, colorectal and ovarian (plco) cancer screening trial cohort. BMC Cancer 9:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafuente A, Cano P, Esquifino AI. 2003. Are cadmium effects on plasma gonadotropins, prolactin, acth, gh and tsh levels, dose-dependent? Biometals 16:243–250. [DOI] [PubMed] [Google Scholar]
- Lahlou N, Roger M. 2004. Inhibin b in pubertal development and pubertal disorders. Semin Reprod Med 22:165–175. [DOI] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, et al. 2009. Early age at menarche associated with cardiovascular disease and mortality. J Clin Endocrinol Metab 94:4953–4960. [DOI] [PubMed] [Google Scholar]
- Lee JM, Appugliese D, Kaciroti N, Corwyn RF, Bradley RH, Lumeng JC. 2007. Weight status in young girls and the onset of puberty. Pediatrics 119:e624–630. [DOI] [PubMed] [Google Scholar]
- Lee Y, Styne D. 2013. Influences on the onset and tempo of puberty in human beings and implications for adolescent psychological development. Horm Behav 64:250–261. [DOI] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Peterson KE, Lee JM, Pace GG, Cantoral A, et al. 2013. Predictors of urinary bisphenol a and phthalate metabolite concentrations in mexican children. Chemosphere 93:2390–2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD. 2015. Biomarkers of exposure to molybdenum and other metals in relation to testosterone among men from the united states national health and nutrition examination survey 2011–2012. Fertil Steril 103:172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis RC, Meeker JD, Basu N, Gauthier AM, Cantoral A, Mercado-Garcia A, et al. 2018. Urinary metal concentrations among mothers and children in a mexico city birth cohort study. Int J Hyg Environ Health 221:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Tellez-Rojo MM, Sanchez BN, Zhang Z, Afeiche MC, Mercado-Garcia A, et al. 2019. Early lead exposure and pubertal development in a mexico city population. Environ Int 125:445–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López H 2008. Nivel socieconómico amai. (AMAI, ed):INEGI. [Google Scholar]
- Madzharova M, Gluhcheva Y, Pavlova E, Atanassova N. 2010. Effect of cobalt on male reproductive organs during puberty. Biotechnology & Biotechnological Equipment 24:321–324. [Google Scholar]
- Marshall WA, Tanner JM. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Griswold W. 2009. Human health effects of heavy metals. Environmental Science and Technology briefs for citizens 15:1–6. [Google Scholar]
- Massart F, Parrino R, Seppia P, Federico G, Saggese G. 2006. How do environmental estrogen disruptors induce precocious puberty? Minerva Pediatr 58:247–254. [PubMed] [Google Scholar]
- McGivern RF, Sokol RZ, Berman NG. 1991. Prenatal lead exposure in the rat during the third week of gestation: Long-term behavioral, physiological, and anatomical effects associated with reproduction. Toxicol Appl Pharmacol 110:206–215. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Diamond MP, Puscheck E, Daly D, et al. 2008. Cadmium, lead, and other metals in relation to semen quality: Human evidence for molybdenum as a male reproductive toxicant. Environ Health Perspect 116:1473–1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Rossano MG, Protas B, Padmanahban V, Diamond MP, Puscheck E, et al. 2010. Environmental exposure to metals and male reproductive hormones: Circulating testosterone is inversely associated with blood molybdenum. Fertil Steril 93:130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Moreno JM, Roca M, Vergara-Juarez N, Martinez-Garcia MJ, Garcia-Sanchez A, et al. 2011. Relationships between heavy metal concentrations in three different body fluids and male reproductive parameters: A pilot study. Environ Health 10:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendle J, Harden KP, Brooks-Gunn J, Graber JA. 2012. Peer relationships and depressive symptomatology in boys at puberty. Dev Psychol 48:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke A, Guallar E, Shiels MS, Rohrmann S, Basaria S, Rifai N, et al. 2008. The association of urinary cadmium with sex steroid hormone concentrations in a general population sample of us adult men. Bmc Public Health 8:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno ME, Acosta-Saavedra LC, Meza-Figueroa D, Vera E, Cebrian ME, Ostrosky-Wegman P, et al. 2010. Biomonitoring of metal in children living in a mine tailings zone in southern mexico: A pilot study. Int J Hyg Environ Health 213:252–258. [DOI] [PubMed] [Google Scholar]
- Mueller NT, Duncan BB, Barreto SM, Chor D, Bessel M, Aquino EM, et al. 2014. Earlier age at menarche is associated with higher diabetes risk and cardiometabolic disease risk factors in brazilian adults: Brazilian longitudinal study of adult health (elsa-brasil). Cardiovasc Diabetol 13:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata C, Nagao Y, Shibuya C, Kashiki Y, Shimizu H. 2005. Urinary cadmium and serum levels of estrogens and androgens in postmenopausal japanese women. Cancer Epidemiol Biomarkers Prev 14:705–708. [DOI] [PubMed] [Google Scholar]
- Padilla MA, Elobeid M, Ruden DM, Allison DB. 2010. An examination of the association of selected toxic metals with total and central obesity indices: Nhanes 99–02. Int J Environ Res Public Health 7:3332–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. 2003. The timing of normal puberty and the age limits of sexual precocity: Variations around the world, secular trends, and changes after migration. Endocr Rev 24:668–693. [DOI] [PubMed] [Google Scholar]
- Parent AS, Franssen D, Fudvoye J, Gerard A, Bourguignon JP. 2015. Developmental variations in environmental influences including endocrine disruptors on pubertal timing and neuroendocrine control: Revision of human observations and mechanistic insight from rodents. Front Neuroendocrinol 38:12–36. [DOI] [PubMed] [Google Scholar]
- Park SS, Skaar DA, Jirtle RL, Hoyo C. 2017. Epigenetics, obesity and early-life cadmium or lead exposure. Epigenomics 9:57–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton GC, McMorris BJ, Toumbourou JW, Hemphill SA, Donath S, Catalano RF. 2004. Puberty and the onset of substance use and abuse. Pediatrics 114:e300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedigo NG, George WJ, Anderson MB. 1988. Effects of acute and chronic exposure to cobalt on male reproduction in mice. Reprod Toxicol 2:45–53. [DOI] [PubMed] [Google Scholar]
- Pescovitz OH, Walvoord EC. 2007. When puberty is precocious: Scientific and clinical aspects:Springer Science & Business Media. [Google Scholar]
- Pine M, Lee B, Dearth R, Hiney JK, Dees WL. 2005. Manganese acts centrally to stimulate luteinizing hormone secretion: A potential influence on female pubertal development. Toxicol Sci 85:880–885. [DOI] [PubMed] [Google Scholar]
- Prentice P, Viner RM. 2013. Pubertal timing and adult obesity and cardiometabolic risk in women and men: A systematic review and meta-analysis. Int J Obes (Lond) 37:1036–1043. [DOI] [PubMed] [Google Scholar]
- Punshon T, Li Z, Marsit CJ, Jackson BP, Baker ER, Karagas MR. 2016. Placental metal concentrations in relation to maternal and infant toenails in a u.S. Cohort. Environ Sci Technol 50:1587–1594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly MP, Saca JC, Hamilton A, Solano RF, Rivera JR, Whitehouse-Innis W, et al. 2014. Prepubertal exposure to arsenic(iii) suppresses circulating insulin-like growth factor-1 (igf-1) delaying sexual maturation in female rats. Reprod Toxicol 44:41–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy A, Kordas K, Lopez P, Rosado JL, Cebrian ME, Vargas GG, et al. 2011. Association between arsenic exposure and behavior among first-graders from torreon, mexico. Environ Res 111:670–676. [DOI] [PubMed] [Google Scholar]
- Roy JR, Chakraborty S, Chakraborty TR. 2009. Estrogen-like endocrine disrupting chemicals affecting puberty in humans--a review. Med Sci Monit 15:RA137–145. [PubMed] [Google Scholar]
- Samuel JB, Stanley JA, Princess RA, Shanthi P, Sebastian MS. 2011. Gestational cadmium exposure-induced ovotoxicity delays puberty through oxidative stress and impaired steroid hormone levels. J Med Toxicol 7:195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selevan SG, Rice DC, Hogan KA, Euling SY, Pfahles-Hutchens A, Bethel J. 2003. Blood lead concentration and delayed puberty in girls. N Engl J Med 348:1527–1536. [DOI] [PubMed] [Google Scholar]
- Semercioz A, Baltaci AK, Mogulkoc R, Avunduk MC. 2017. Effect of zinc and melatonin on oxidative stress and serum inhibin-b levels in a rat testicular torsion-detorsion model. Biochem Genet 55:395–409. [DOI] [PubMed] [Google Scholar]
- Shen W, Chen J, Yin J, Wang SL. 2016. Selenium protects reproductive system and foetus development in a rat model of gestational lead exposure. Eur Rev Med Pharmacol Sci 20:773–780. [PubMed] [Google Scholar]
- Singh R, Gautam N, Mishra A, Gupta R. 2011. Heavy metals and living systems: An overview. Indian J Pharmacol 43:246–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Foster DL. 2004. The neural basis of puberty and adolescence. Nat Neurosci 7:1040–1047. [DOI] [PubMed] [Google Scholar]
- Sokol R, Berman N. 1991. The effect of age of exposure on lead-induced testicular toxicity. Toxicology 69:269–278. [DOI] [PubMed] [Google Scholar]
- Spear LP. 2000. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev 24:417–463. [DOI] [PubMed] [Google Scholar]
- Srigboh RK, Basu N, Stephens J, Asampong E, Perkins M, Neitzel RL, et al. 2016. Multiple elemental exposures amongst workers at the agbogbloshie electronic waste (e-waste) site in ghana. Chemosphere 164:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stattin H, Magnusson D. 1990. Pubertal maturation in female development:Lawrence Erlbaum Associates, Inc. [Google Scholar]
- Stockl D, Meisinger C, Peters A, Thorand B, Huth C, Heier M, et al. 2011. Age at menarche and its association with the metabolic syndrome and its components: Results from the kora f4 study. PLoS One 6:e26076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su PH, Chen JY, Chen JW, Wang SL. 2010. Growth and thyroid function in children with in utero exposure to dioxin: A 5-year follow-up study. Pediatr Res 67:205–210. [DOI] [PubMed] [Google Scholar]
- Sullivan Pepe M, Anderson GL. 1994. A cautionary note on inference for marginal regression models with longitudinal data and general correlated response data. Communications in Statistics-Simulation and Computation 23:939–951. [Google Scholar]
- Telisman S, Cvitkovic P, Jurasovic J, Pizent A, Gavella M, Rocic B. 2000. Semen quality and reproductive endocrine function in relation to biomarkers of lead, cadmium, zinc, and copper in men. Environ Health Perspect 108:45–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telisman S, Colak B, Pizent A, Jurasovic J, Cvitkovic P. 2007. Reproductive toxicity of low-level lead exposure in men. Environ Res 105:256–266. [DOI] [PubMed] [Google Scholar]
- Tremblay L, Lariviere M. 2009. The influence of puberty onset, body mass index, and pressure to be thin on disordered eating behaviors in children and adolescents. Eat Behav 10:75–83. [DOI] [PubMed] [Google Scholar]
- Villamor E, Jansen EC. 2016. Nutritional determinants of the timing of puberty. Annu Rev Public Health 37:33–46. [DOI] [PubMed] [Google Scholar]
- Welt CK, Adams JM, Sluss PM, Hall JE. 1999. Inhibin a and inhibin b responses to gonadotropin withdrawal depends on stage of follicle development. J Clin Endocrinol Metab 84:2163–2169. [DOI] [PubMed] [Google Scholar]
- Widen E, Silventoinen K, Sovio U, Ripatti S, Cousminer DL, Hartikainen AL, et al. 2012. Pubertal timing and growth influences cardiometabolic risk factors in adult males and females. Diabetes Care 35:850–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wit JM, Boersma B. 2002. Catch-up growth: Definition, mechanisms, and models. J Pediatr Endocrinol Metab 15 Suppl 5:1229–1241. [PubMed] [Google Scholar]
- Wu T, Buck GM, Mendola P. 2003. Blood lead levels and sexual maturation in u.S. Girls: The third national health and nutrition examination survey, 1988–1994. Environ Health Perspect 111:737–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Peterson KE, Sanchez BN, Dolinoy DC, Mercado-Garcia A, Tellez-Rojo MM, et al. 2018. Association of blood leukocyte DNA methylation at line-1 and growth-related candidate genes with pubertal onset and progression. Epigenetics 13:1222–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyshak G, Frisch RE. 1982. Evidence for a secular trend in age of menarche. N Engl J Med 306:1033–1035. [DOI] [PubMed] [Google Scholar]
- Zacharias L, Wurtman RJ. 1969. Age at menarche. Genetic and environmentalinfluences. N Engl J Med 280:868–875. [DOI] [PubMed] [Google Scholar]
- Zeng X, Lin T, Zhou Y, Kong Q. 2002. Alterations of serum hormone levels in male workers occupationally exposed to cadmium. J Toxicol Environ Health A 65:513–521. [DOI] [PubMed] [Google Scholar]
- Zeng X, Jin T, Buchet JP, Jiang X, Kong Q, Ye T, et al. 2004. Impact of cadmium exposure on male sex hormones: A population-based study in china. Environ Res 96:338–344. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Dong T, Hu W, Wang X, Xu B, Lin Z, et al. 2019. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ Int 123:325–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.