Abstract
Chemical mitochondrial uncouplers are lipophilic weak acids that transport protons into the mitochondrial matrix via a pathway that is independent of ATP synthase, thereby uncoupling nutrient oxidation from ATP production. These uncouplers have potential for the treatment of diseases such as obesity, Parkinson’s disease, and aging. We have previously identified a novel mitochondrial protonophore, named BAM15, which stimulates mitochondrial respiration across a broad dosing range compared to carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP). Herein, we report our investigations on the structure–activity relationship profile of BAM15. Our studies demonstrate the importance of the furazan, pyrazine, and aniline rings as well as pKa in maintaining its effective protonophore activity.
Keywords: Mitochondrial uncoupler, Mitochondrial bioenergetics, Protonophore, Structure-activity relationships, Pyrazines
Mitochondrial uncouplers or protonophores are small molecule organic compounds, typically lipophilic weak acids, that utilize the pH gradient in the mitochondria to shuttle protons from the inner membrane space to the mitochondrial matrix.1 Their uncoupling activity is dependent on the efficiency in which they shuttle protons across the lipid bilayer of the mitochondrial inner membrane,2 which is determined by the diffusion of the uncoupler into and out of a phospholipid bilayer and the efficiency by which the protonophore transports protons in the intermembrane space and releases them into the mitochondrial matrix. The net result is the dissipation of a proton gradient across the mitochondrial inner membrane that is associated with an increase in energy expenditure, a phenomenon that has implications in several disease states. For example, mitochondrial uncouplers have therapeutic potential as an anti-obesity drugs1,2 or as treatments of disorders linked to mitochondrial oxidative stress such Parkinson’s disease,3 insulin resistance,4,5 aging,6 heart failure,7 and ischemia-reperfusion injury.8
A number of mitochondrial uncouplers have been reported (Fig. 1). Among these, 2,4-dinitrophenol (DNP) was temporarily used for weight loss, but was removed from the market by the FDA because of its narrow therapeutic window.9 Carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP) is a widely used potent protonophore;10,11 however, it has off-target effects such as the depolarization of the plasma membrane.12–14 Other mitochondrial uncouplers such as bupivacaine and ellipticine have been disclosed.15,16 The properties of an ideal mitochondrial uncoupler includes exclusive selectivity for the mitochondria and wide therapeutic window. To date, there is a paucity of such compounds.
Figure 1.
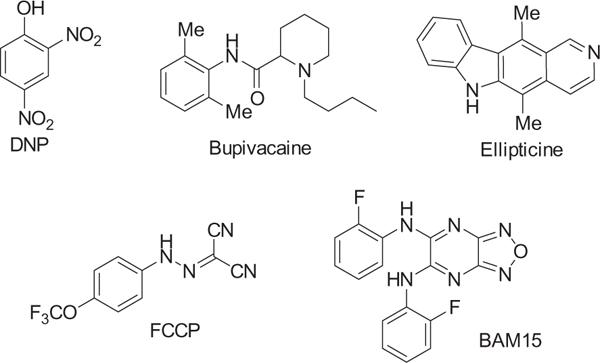
Small molecule protonophores
Due to our interest in this area, we developed a phenotypic assay to identify compounds that meet these criteria, and reported the discovery of BAM15, which stimulates mitochondrial respiration across a broad dosing range compared to FCCP.17 Herein, we disclose the structure–activity relationship studies of BAM15. Our investigations suggest the pivotal role of the furazan, pyrazine, and aniline in the scaffold as well as the substituents in the aniline ring. Further, our studies indicate the pKa of BAM15 is important in the ability to transport protons from the mitochondrial inner membrane to the matrix.
The diffusion of uncouplers in and out of the mitochondrial inner membrane is determined by the energy barrier required to permeate the hydrophobic membrane core.18 Hence, lipophilicity of the compound plays a significant role in its ability to accumulate in the membrane and also to penetrate through the membrane.19,20 To be effective as a mitochondrial protonophore, the uncoupler must transport hydrogen ions (H+) across the inner mitochondrial membrane. Two characteristics are necessary to facilitate this transport: (1) an ionizable proton that is donated in the matrix19 and (2) a large surface area that functions to delocalize the resulting charge.21,22 These are properties that are shared among uncouplers shown in Figure 1.
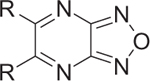
We started our investigation by determining the importance of the aniline, pyrazine, and furazan rings of BAM15 (Fig. 2). The synthesis of these compounds is shown in Scheme 1. Following standard Buchwald–Hartwig coupling conditions, 2,3-dichloropyrazine and 2-fluoroaniline were coupled in the presence of Pd(dba)2 under reflux to afford compound 1.23 Compound 2 was achieved in a similar manner while 3 was commercially available. Once the importance of each ring system was established, a series of substitutions were placed on the pyrazine ring via nucleophilic aromatic substitution of 5,6-dichlorofurazano[3,4-b]pyrazine with a variety of anilines or with alcohol (Scheme 2).24,25 It should be noted that N,N-diethylaniline was crucial for the reactions with anilines.
Figure 2.
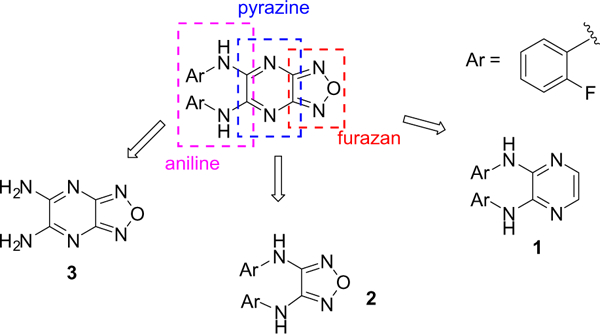
Three regions of BAM15.
Scheme 1.
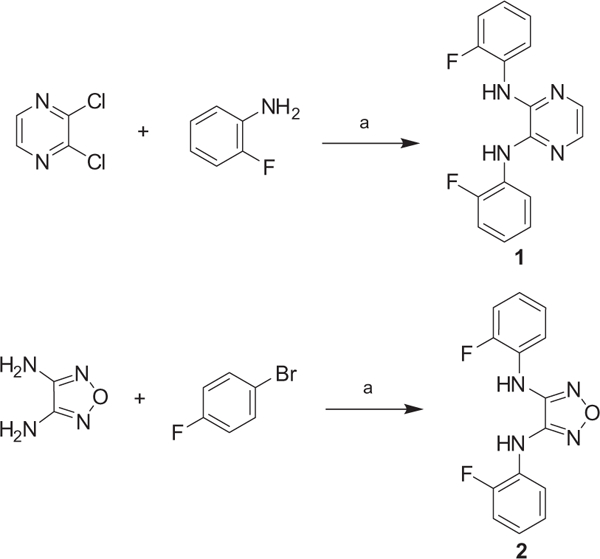
Synthesis of compounds 1 and 2. Reagents and conditions: (a) 10 mol % Pd(dba)2, 20 mol % BINAP, 4 equiv NaOtBu, toluene, reflux, 13–25%.
Scheme 2.
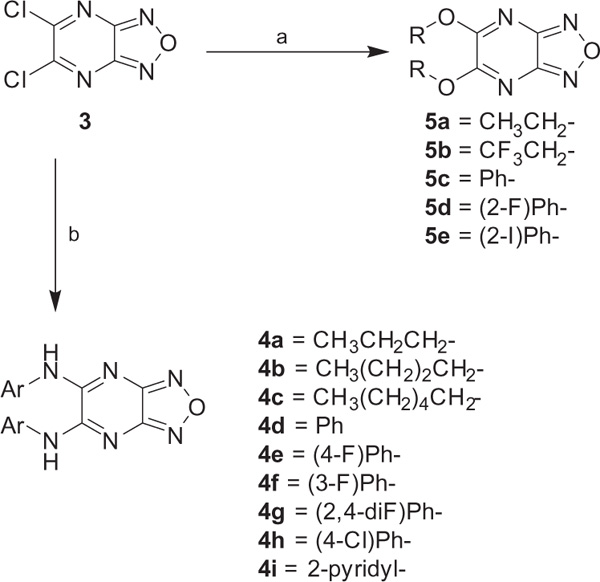
Synthesis of compounds 4a–i and 5a–e. Reagents and conditions: (a) alcohol, K2CO3, acetonitrile, 53–96%; (b) alkylamine or substituted aniline, (2 equiv) N,N-diethylaniline, acetonitrile, reflux, 6–60%.
With the key compounds available (obtained either by chemical synthesis or bought commercially), we determined their protonophoric effect by stimulating respiration in cells. Changes in cellular respiration following uncoupler treatment were measured in intact L6 myoblasts using a Seahorse XF-24 Flux Analyzer as previously described.17 In this assay, mitochondrial uncoupling activity was determined as a function of oxygen consumption rate (OCR). As shown in Figure 3, OCR increased as a function of BAM15 concentration. At the outset, we investigated the importance of the furazan (1), pyrazine (2), and aniline (3) rings. Our studies suggest that removal of each of these moieties individually resulted in complete loss in the ability to stimulate respiration in concentrations up to 10 μM (data not shown). Hence, we focused our attention with 5,6-disubstituted furazano[3,4-b]pyrazines and performed a structure–activity profile. As shown in Table 1, substitution of the aniline ring with short propyl to much larger butyl or hexyl amines or various alkyl ethers resulted in molecules with a poor a poor capacity to increase cellular respiration (entries 1–5). For reference, in L6 myoblasts BAM15 has an EC50 value of 270 nM that reaches a maximum respiration of 215% above basal levels (Fig. 3). Removal of the fluorine atom of BAM15 to generate compound 4d significantly decreased its uncoupling ability (entry 7). On the one hand, moving the position of the fluorine atom to the para (4e) position decreased EC50 value (entry 8). On the other hand, placement of the fluorine atom to the meta position (4f) improved the activity to 180 nM (entry 9). These results indicate the importance of the fluorine atom around the ring. Remarkably, fluorination on the ortho and para positions in 4g improved the EC50 value to 40 nM (entry 10). Introduction of a chlorine moiety on the 4-position (4h) afforded similar activity as BAM15 although the maximum oxygen consumption level decreased slightly (compare entry 6 vs 11). To determine the role of aniline nitrogen linker to the pyrazine ring, we synthesized various ether linkages. Phenyl (5c), 2-fluorophenyl (5d), and 2-iodophenyl (5e) ethers were found to be inactive (entries 12–14). Our results indicate the possibility of the key role of nitrogen for its protonophoric activity to either affect the protonation state of the scaffold and its ability to transport a proton across the membrane.
Figure 3.
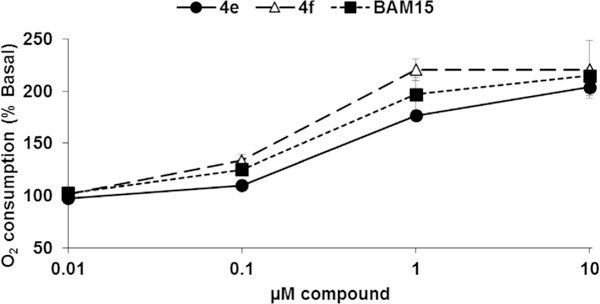
Changes in cellular oxygen consumption rate relative to DMSO control for BAM15 and compounds 4e and 4f. Each data point is from 5 to 6 replicates.
Table 1.
Activity of furazano[3,4-b]pyrazines
 | ||||
|---|---|---|---|---|
| Entry | Cmpd | R | EC50a (μM) | Max% basalb |
| l | 4a | 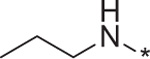 |
– | 98.0 ± 2.8c |
| 2 | 4b | – | 116.0 ± 1.9c | |
| 3 | 4c | – | 160.2 ± 8.2c | |
| 4 | 5a | – | 100.0 ± 0.6c | |
| 5 | 5b | – | 96.3 ± 2.2c | |
| 6 | BAM15 | 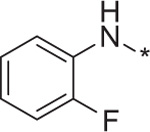 |
0.27 | 214.8 ± 3.0 |
| 7 | 4d | 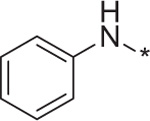 |
– | 172.8 ± 3.0c |
| 8 | 4e | 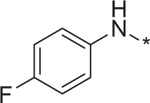 |
0.46 | 204.0 ± 3.5 |
| 9 | 4f | 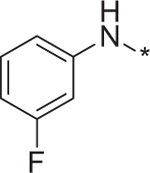 |
0.18 | 220.8 ± 4.6 |
| 10 | 4g | 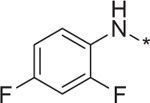 |
0.04 | 222.6 ± 6.4 |
| 11 | 4h | 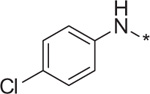 |
0.27 | 190.4 ± 4.3 |
| 12 | 4i | 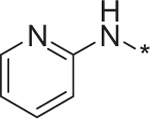 |
–d | – |
| 12 | 5c | 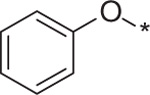 |
– | 98.3 ± 0.5c |
| 13 | 5d | 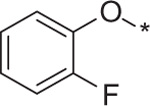 |
– | 93.0 ± 2.6c |
| 14 | 5e | 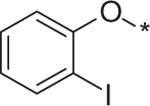 |
– | 98.8 ± 0.5c |
Cellular oxygen consumption rates following a dose response of 0.01–10 μM were normalized from 0% to 100% and subjected to nonlinear regression analysis to obtain EC50 and 95% confidence intervals.
The maximum percentage of basal (100%) respiration that could be stimulated using uncoupler concentrations from 0.01 to 10 μM.
The maximum oxygen consumption rate was not obtained using uncoupler concentrations of 0.01–10 μM. The highest oxygen consumption rate using uncoupler concentrations from 0.01 to 10 μM are reported.
Compound is insoluble.
To understand the potential mechanism by which BAM15 transports protons from the inner membrane space to the mitochondrial matrix, we determined the pKa of BAM15. Because BAM15 was insoluble in water at required concentrations of the assay, a water-methanol mixture was used (see Supporting information). Titrations using UV spectroscopic methods and Yasuda-Shedlovsky extrapolation, the pKa of BAM15 was determined to be 7.56 ± 0.08. As the pH in the inner membrane space is 6.8,1 BAM15 is expected to be protonated and allow it to shuttle a proton to the mitochondrial matrix where the pH is significantly increased to 8.0. We suspect that this pH differential as well as the lipophilic nature of BAM15 (cLogP = 6.52) facilitates the efficient transfer of protons across the membrane.
In summary, our investigations demonstrate the importance of the furazan, pyrazine, and aniline rings in the BAM15 pharmacophore as deletion of these rings led to completely inactive compounds. Further, both the nitrogen and phenyl ring of the ‘aniline’ moiety are essential to the uncoupling activity, suggesting an essential structural role in the protonation of BAM15. Finally, our studies indicate the importance of pKa in the ability to transport protons across the membrane, which is affected by halogens such as fluorine atoms. Efforts to fully elucidate the mechanism by which BAM15 transports protons as well as further development of molecules towards improved uncoupling capacity is currently underway.
Supplementary Material
Acknowledgments
We acknowledge financial support by the Department of Chemistry at Virginia Tech and the Department of Pharmacology at the University of Virginia.
Footnotes
Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bmcl.2015.06.040.
References and notes
- 1.Harper JA; Dickinson K; Brand MD Obes. Rev. 2001, 2, 255. [DOI] [PubMed] [Google Scholar]
- 2.Tseng YH; Cypess AM; Kahn CR Nat. Rev. Drug Disc. 2010, 9, 465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu Y-N; Munhall AC; Johnson SW Brain Res. 2011, 1395, 86. [DOI] [PubMed] [Google Scholar]
- 4.Anderson EJ; Lustig ME; Boyle KE; Woodlief TL; Kane DA; Lin CT; Price JW 3rd; Kang L; Rabinovitch PS; Szeto HH; Houmard JA; Cortright RN; Wasserman DH; Neufer PD J. Clin. Invest. 2009, 119, 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoehn KL; Salmon AB; Hohnen-Behrens C; Turner N; Hoy AJ; Maghzal GJ; Stocker R; Van Remmen H; Kraegen EW; Cooney GJ; Richardson AR; James DE Proc. Natl. Acad. Sci U.S.A. 2009, 106, 17787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Caldeira da Silva CC; Cerqueira FM; Barbosa LF; Medeiros MH; Kowaltowski AJ Aging Celt 2008, 7, 552. [DOI] [PubMed] [Google Scholar]
- 7.Brennan JP; Southworth R; Medina RA; Davidson SM; Duchen MR; Shattock MJ Cardiovasc. Res. 2006, 72, 313. [DOI] [PubMed] [Google Scholar]
- 8.Korde AS; Pettigrew LC; Craddock SD; Maragos WF J. Neurochem. 2005, 94, 1676. [DOI] [PubMed] [Google Scholar]
- 9.Grundlingh J; Dargan PI; El-Zanfaly M; Wood DM J. Med. Toxicol. 2011, 7, 205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benz R; McLaughlin S Biophys. J. 1983, 41, 381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasianowicz J; Benz R; McLaughlin SJ Membr. Biol. 1984, 82, 179. [DOI] [PubMed] [Google Scholar]
- 12.Park KS; Jo I; Pak K; Bae SW; Rhim H; Suh SH; Park J; Zhu H; So I; Kim KW Pflugers Arch. 2002, 443, 344. [DOI] [PubMed] [Google Scholar]
- 13.Juthberg SK; Brismar T Cell. Mol. Neurobiol. 1997, 17, 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buckler KJ; Vaughan-Jones RD J. Physiol. 1998, 513, 819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun X; Garlid KD J. Biol. Chem. 1992, 267, 19147. [PubMed] [Google Scholar]
- 16.Schwaller M-A; Allard B; Lescot E; Moreau FJ Biol. Chem. 1995, 270, 22709. [DOI] [PubMed] [Google Scholar]
- 17.Kenwood BM; Weaver JL; Bajwa A; Poon IK; Byrne FL; Murrow BA; Calderone JA; Huang L; Divakaruni AS; Tomsig JL; Okabe K; Lo RH; Cameron Coleman G; Columbus L; Yan Z; Saucerman JJ; Smith JS; Holmes JW; Lynch KR; Ravichandran KS; Uchiyama S; Santos WL; Rogers GW; Okusa MD; Bayliss DA; Hoehn KL Mol. Met. 2014, 3, 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spycher S; Smejtek P; Netzeva TI; Escher BI Chem. Res. Toxicol. 2008,21, 911. [DOI] [PubMed] [Google Scholar]
- 19.Miyoshi H; Tsujishita H; Tokutake N; Fujita T Biochim. Biophys. Acta 1990, 1016, 99. [DOI] [PubMed] [Google Scholar]
- 20.Martineau LC J. Theor. Biol. 2012, 303, 33. [DOI] [PubMed] [Google Scholar]
- 21.Skulachev VP; Sharaf AA; Liberman EA Nature 1967, 216, 718. [DOI] [PubMed] [Google Scholar]
- 22.Escher BI; Hunziker R; Schwarzenbach RP; Westall JC Environ. Sci. Technol. 1999, 33, 560. [Google Scholar]
- 23.Xie X; Zhang TY; Zhang ZJ Org. Chem. 2006, 71, 6522. [DOI] [PubMed] [Google Scholar]
- 24.Starchenkov IB; Andrianov VG Chem. Heterocycl. Compd. 1997, 33,1219. [Google Scholar]
- 25.Fernández E; García-Ochoa S; Huss S; Mallo A; Bueno JM; Micheli F; Paio A; Piga E; Zarantonello P Tetrahedron Lett. 2002, 43, 4741. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


