Abstract
Introduction:
The relationships between morbid obesity, changes in body mass index (BMI) before cancer diagnosis, and lung cancer outcomes by histology (SCLC and NSCLC) have not been well studied.
Methods:
Individual level data analysis was performed on 25,430 patients with NSCLC and 2787 patients with SCLC from 16 studies of the International Lung Cancer Consortium evaluating the association between various BMI variables and lung cancer overall survival, reported as adjusted hazard ratios (aHRs) from Cox proportional hazards models and adjusted penalized smoothing spline plots.
Results:
Overall survival of NSCLC had putative U-shaped hazard ratio relationships with BMI based on spline plots: being underweight (BMI < 18.5 kg/m2; aHR = 1.56; 95% confidence interval [CI]:1.43–1.70) or morbidly overweight (BMI > 40 kg/m2; aHR = 1.09; 95% CI: 0.95–1.26) at the time of diagnosis was associated with worse stage-specific prognosis, whereas being overweight (25 kg/m2 ≤ BMI < 30 kg/m2; aHR= 0.89; 95% CI: 0.85–0.95) or obese (30 kg/m2 ≤ BMI ≤ 40 kg/m2; aHR = 0.86; 95% CI: 0.82–0.91) was associated with improved survival. Although not significant, a similar pattern was seen with SCLC. Compared with an increased or stable BMI from the period between young adulthood until date of diagnosis, a decreased BMI was associated with worse outcomes in NSCLC (aHR = 1.24; 95% CI: 1.2–1.3) and SCLC patients (aHR=1.26 (95% CI: 1.0–1.6). Decreased BMI was consistently associated with worse outcome, across clinicodemographic subsets.
Conclusions:
Both being underweight or morbidly obese at time of diagnosis is associated with lower stage-specific survival in independent assessments of NSCLC and SCLC patients. In addition, a decrease in BMI at lung cancer diagnosis relative to early adulthood is a consistent marker of poor survival.
Keywords: Body mass index, Lung cancer, Survival
Introduction
The relationship between weight and cancer survival is complex. Being significantly obese or underweight may impair the efficacy of and tolerance to treatment. Examples include the impact of such extreme weight on surgical comorbidities and when dosing chemotherapeutic agents.1–5
Obesity has long been associated with worse cancer outcomes. In the United States, being overweight was estimated to account for 14% of all cancer deaths in men and 20% in women, but this was studied in a cohort that was initially cancer-free, as opposed to a cohort of incident cancer patients; therefore, the reported mortality rates combined the effect of obesity on both cancer incidence and cancer outcomes.6 Obesity can cause systemic physiologic alterations, such as higher insulin resistance, which has been linked to poor cancer outcomes, chronic inflammation, and abnormal nutrient homeostasis, which may lower the barrier for oncogenic transformation by driving cellular proliferation and resisting apoptosis.7–9 The American Society of Clinical Oncology has investigated the association of obesity with cancer in one of its core initiatives in 2014, aiming to raise awareness of this relationship.10,11 Lung cancer stands apart from other solid tumors. In previous studies, an excess mortality due to obesity was not described for lung cancer; instead, overweight and obese patients had improved outcomes.6,12–19
In studies covering both resectable and metastatic lung cancers, the worst outcomes were observed in underweight patients as defined by having a body mass index (BMI) less than 18.5 kg/m2.6,12–15,20–29 Being severely underweight may be an indicator of cancer cachexia, which is a well-described marker of poor outcome on cancer mortality.30–35 Weight in the years before lung cancer diagnosis has also been assessed. For example, a prior case control study of 2285 patients reported no significant association between BMI at 2 years before lung cancer diagnosis and mortality, whereas a strong association was reported between BMI less than 18.5 kg/m2 at diagnosis and death.16 Associations with temporal changes in BMI before diagnosis were not reported.
There remain multiple key knowledge gaps in this research field, most commonly due to limited sample size and the single-site nature of many published series. Firstly, as most published reports focused on NSCLC, separate analyses of SCLC are scarce, whereas none have evaluated NSCLC and SCLC in parallel.36–39 Secondly, past studies have not assessed the role of morbid obesity (defined as BMI > 40 kg/m2) on survival, but have focused on complication rates in both obese and morbidly obese patients.17,40,41 This is an important knowledge gap, as the only available data suggest that all overweight and obese patients have improved survival regardless of the magnitude of the BMI value. Thirdly, prior analyses have mostly assessed the prognostic role of BMI captured at the time of diagnosis, but have not evaluated BMI in a patients’ prior healthy state. Although recent weight loss around the time of diagnosis has been associated with poor prognosis, longer-term changes in BMI (i.e., from the time of young adulthood until diagnosis) have not been studied previously.21,32,35,42, Evaluation of BMI changes over a longer time may reflect metabolic or biologic effects that can both impact cancer risk and prognosis.7–9 In a large, multicenter, multinational cohort with special consideration of morbid obesity and SCLC patient subsets, we describe the prognostic association of three main BMI measurements: BMI at diagnosis, BMI at young adulthood (a surrogate for BMI when healthy), and change in BMI (ΔBMI) from a young adulthood to the time of diagnosis.
Methods
Study Population
The International Lung Cancer Consortium was established in 2004 with the aims to share compatible data and maximize resource sharing for lung cancer epidemiology research. Full details have been provided previously and are available at http://ilcco.iarc.fr.43–48 To be included in the present pooled analysis, studies had to have data on BMI at lung cancer diagnosis, lung cancer type (SCLC versus NSCLC), date of diagnosis, stage at diagnosis, vital status at last follow-up, and date of death. Optional variables included BMI at periods other than at diagnosis. The individual-level data across studies were then pooled and checked for inconsistency, inadmissible values, aberrant distributions, and outliers before being harmonized into a common data set. Written informed consents were obtained from all study participants, and each study was approved by its respective local institutional human subject review board.
Statistical Analysis
Harmonization of epidemiologic data elements has been previously described.44 Harmonization of outcomes-related variables is described in the Supplemental Data. Separate analyses were performed for NSCLC and SCLC. Overall survival (OS) was assessed using Kaplan-Meier curves and log-rank tests in univariable analyses. OS was assessed using penalized smoothing spline (PSS) curves (continuous BMI variable) and Cox proportional hazards models (continuous and categorical variables) in multivariable analyses, adjusting for clinically relevant factors identified in the univariable analyses.49,50 A detailed description of the PSS models is provided in the Supplemental Data. Spline curves are functions that are defined piecewise by a polynomial, allowing complex shapes of relationships with continuous variables to be modeled. In addition to treating each BMI variable as a continuous variable, BMI at diagnosis and BMI during young adulthood (defined as ages 18 to 25 years) were also categorized into standard clinical groupings of less than 18 kg/m2 (underweight), 18 to less than 25 kg/m2 (normal weight), 25 to less than 30 kg/m2 (overweight), and 30 to less than 40 kg/m2 (obese) with the morbidly obese defined as greater than 40 kg/m2. Analyses were performed based on the pooled data, but subset analyses within individual studies were performed to evaluate consistency across studies. The clinical multivariable survival analysis that generated the base models included all variables with p values less than 0.05 on univariable analysis. To this base model, various definitions of BMI (BMI at diagnosis, BMI at young adulthood, ΔBMI), were added to the clinical multivariable model individually, as these variables were partially correlated. The association between BMI variables was tested using Pearson’s correlation test. Change in BMI (ΔBMI) from young adulthood to the time of diagnosis was used to correct partially for heterogeneity of baseline (pre-illness) BMI across the population because it uses the same person’s BMI at a prior, presumed healthy state (young adulthood) as a self-control. This study focuses on the primary relationships between BMI and survival; interaction analyses between BMI and other variables on survival will be reported in separate articles.
Sensitivity analyses were pre-planned to deal with potential issues related to study heterogeneity, including performing analyses that omitted participants/studies that had the following conditions, one at a time: the two Surveillance, Epidemiology, and End Results Program-staged studies; one study that used grade as a surrogate for stage, any single large studies that had more than 15% of the total population, and individual participants who were originally staged before the A/B substages were incorporated into the staging system (conservatively estimated to be before the year 2000, as the sixth edition of the American Joint Committee on Cancer staging manual was released in 1998). The fixed-effect model was used when evaluating the impact of different study groups. Given that BMI norms may be different by race, sensitivity analyses by ethnicity were performed that omitted any minority ethnicities that contributed more than 15% of the total sample.
Results
Patient and Characteristics
A total of 29,217 patients met the inclusion criteria from the 16 studies and were included in the base (clinical) model analysis (Supplementary Table 1). Patient characteristics of the pooled population according to lung cancer type are shown in Table 1. Studies were from North America, Europe, and Asia; median age was 65 years; 54% were males; the majority was ever-smokers; 10% had SCLC and the most common NSCLC subtype was adenocarcinoma; overall median follow-up time was 3.9 years; and 71% patients had died during follow-up.
Table 1.
Patients Characteristics According to Lung Cancer Type
| NSCLC |
SCLC |
||||
|---|---|---|---|---|---|
| Variable | Categories | Summary Statistics |
No. of Studies Providing Data |
Summary Statistics |
No. of Studies Providing Data |
| Total counts | N (%) | 26,430 (100%) | 16 | 2787 (100%) | 16 |
| Age, years | Median (range) | 65 (17–97) | 16 | 65 (22–92) | 16 |
| Year of diagnosis | Median (range) | 2006 (1974–2015) | 16 | 2005 (1987–2015) | 16 |
| Sex, n (%) | Males | 14150 (54%) | 16 | 1561 (56%) | 16 |
| High school graduate | No | 1927 (11%) | 14 | Low: 220 (12%) | 14 |
| Yes | 15,373 (89%) | High: 1643 (88%) | |||
| Missing | 7558 | 897 | |||
| Ethnicity | Caucasian | 18,141 (76%) | 16 | 2484 (93%) | 16 |
| Asian | 3938 (17%) | 59 (2%) | |||
| Black | 1020 (4%) | 33 (1%) | |||
| Other | 686 (3%) | 98 (4%) | |||
| Missing | 2645 | 113 | |||
| Stage | 1A | 5478 (21%) | 16 | – | 16 |
| 1B | 2448 (9%) | – | |||
| 2A | 1131 (4%) | – | |||
| 2B | 1884 (7%) | – | |||
| 3A | 3905 (15%) | – | |||
| 3B | 2434 (9%) | – | |||
| 4 | 9150 (35%) | – | |||
| Limited stage | – | 1135 (41%) | |||
| Extensive stage | 1652 (59%) | ||||
| Histology | Squamous cell | 6024 (23%) | 16 | – | 16 |
| Adenocarcinoma | 15,812 (60%) | – | |||
| Other | 4527 (17%) | – | |||
| Small cell | – | 2787 (100%) | |||
| Missing | 67 | – | |||
| Smoking Status | Ever-smoker | 17,118 (84%) | 14 | 2389 (98%) | 14 |
| Never-smoker | 3201 (17%) | 54 (2%) | |||
| Missing | 3847 | 57 | |||
| Pack years among ever-smokers | Median (range) | 43 (0–275) | 13 | 50 (0.5–200) | 13 |
| Missing | 6304 | 748 | |||
| BMI at diagnosis, kg/mg2 | Median (range) | 25.2 (11-87) | 16 | 26.3 (12-70) | 16 |
| BMI < 18.5 (underweight) | 906 (4%) | 65 (3%) | |||
| 18.5 ≥ BMI < 25 (normal BMI) | 9189 (44%) | 765 (36%) | |||
| 25 ≥ BMI < 30 (overweight) | 7086 (34%) | 815 (38%) | |||
| 40 ≥ BMI ≥ 30 (obese) | 3435 (16%) | 487 (23%)a | |||
| BMI ≥ 40 (morbidly obese) | 321 (2%) | 655 | |||
| Missing | 5493 | ||||
| BMI at young adult age, kg/mg2 | Median (range) | 22.7 (10-71) | 7 | 22.7 (14-43) | 7 |
| BMI < 18.5 (underweight) | 397 (7%) | 31 (6%) | |||
| 18.5 ≤ BMI <25 (normal BMI) | 3518 (65%) | 398 (71%) | |||
| 25 ≤ BMI <30 (overweight) | 1121 (21%) | 95(17%) | |||
| 30 ≤ BMI ≤ 40 (obese) | 378 (7%) | 35 (6%)a | |||
| BMI ≥ 40 (morbidly obese) | 40 (1%) | 134 | |||
| Missing | 943 | ||||
| BMI change from young adult age to diagnosis, kg/mg2 | Decreased BMI | 1639 (30%) | 7 | 112 (20%) | 7 |
| No change/ Increased BMI | 3790 (70%) | 442 (80%) | |||
| Missing | 968 | 139 | |||
There were too few morbidly obese individuals to form its own category in SCLC; instead, obese and morbidly obese were grouped together. BMI, body mass index.
BMI at diagnosis was available for 79% of patients, whereas BMI before diagnosis was available for 22% of patients. Median BMI at diagnosis and young adulthood was 25 kg/mg2 and 23 kg/mg2, respectively; the correlation between these two values was 0.46 (p < 0.001). Supplementary Table 2 describes the median OS and median follow-up times by stage, showing consistency with stage-specific expected median OS.
Patient Characteristics and OS
The results of the univariable analysis for OS are summarized in Table 2. Higher cancer stage, being older, being male, and not graduating from high school were each associated with lower survival rates for both NSCLC and SCLC. Cumulative smoking exposure, squamous cell histology, recent year of diagnosis, and being of African (black) ancestry were associated with lower survival rates for NSCLC. Multivariable analysis confirmed these variables as independently associated with survival (Table 2). Cumulative smoking was not included in the final multivariable model due to missing data for a large number of patients (Table 2). However, results remained unchanged in the subgroup of patients with available cumulative smoking data (Supplementary Table 3).
Table 2.
Association Between Patient Characteristics and Overall Survival: Univariable and Multivariable Analysis
| NSCLC, HR (95%CI), p value |
SCLC, HR (95% CI), p value |
||||
|---|---|---|---|---|---|
| Variable | Comparisons | Univariable Analysis | Multivariable Analysisa | Univariable Analysis | Multivariable Analysis |
| Base (clinical) model variables | |||||
| Stage | 1B vs. 1A | 1.52 (1.4–1.6), <0.001 | 1.46 (1.35,1.58), <0.001 | – | – |
| 2A vs. 1A | 1.72 (1.6–1.9), <0.001 | 1.60 (1.45,1.76), <0.001 | – | – | |
| 2B vs. 1A | 2.36(2.2–2.5), <0.001 | 2.20 (2.04,2.38), <0.001 | – | – | |
| 3A vs. 1A | 3.40 (3.2–3.6), <0.001 | 3.15 (2.96,3.35), <0.001 | – | – | |
| 3B vs. 1A | 4.60 (4.3–4.9), <0.001 | 4.29 (4,4.59), <0.001 | – | – | |
| 4 vs. 1A | 7.79 (7.4–8.2), <0.001 | 7.55 (7.14,7.99), <0.001 | – | – | |
| Extensive vs. limited | – | – | 2.50 (2.3–2.7), <0.001 | 2.50 (2.3–27), <0.001 | |
| Age, years | Per increase in 10 | 1.21 (1.19–1.22), <0.001 | 1.20 (1.18–1.21), <0.001 | 1.30 (1.24–1.35) <0.001 | 1.28 (1.22–1.34), <0.001 |
| Sex | Female vs. male | 0.75 (0.73–0.77), <0.001 | 0.78 (0.75,0.8), <0.001 | 0.82 (0.76–0.89) <0.001 | 0.86 (0.79–0.93), <0.001 |
| Secondary school | Graduate vs. not | 0.77 (0.73–0.82), <0.001 | 0.84 (0.80–0.90), <0.001 | 0.72 (0.62–0.85) <0.001 | 0.82 (0.70–0.97), 0.02 |
| Ethnicity | Asian vs. Caucasian | 0.86 (0.78–0.96), 0.005 | 0.93 (0.84,1.03), 0.17 | 1.12 (0.79–1.60) 0.53 | – |
| Black vs. Caucasian | 1.06 (0.97–1.20), 0.18 | 1.11 (1.02–1.20), 0.02 | 0.97 (0.63–1.50) 0.89 | ||
| Other vs. Caucasian | 0.83 (0.76–.91), <0.001 | 0.87 (0.80–0.96), 0.004 | 0.92 (0.74–1.15) 0.48 | ||
| Pack yearsb | Per increase in 10 | 1.04 (1.03–1.04), <0.001 | – | 1.01 (1.00–1.03) 0.06 | – |
| Year of diagnosisc | 2000 onward vs. before 2000 | 1.03 (0.98–1.07), 0.22 | – | 1.06 (0.95–1.18) 0.28 | – |
| Histology | Adeno vs. squam | 0.73 (0.70–0.76), <0.001 | 0.80 (0.77–0.83), <0.001 | Not applicable | Not applicable |
| Other vs. squam | 0.95 (0.91–1.0), 0.04 | 1.03 (0.98–1.1), 0.24 | |||
| BMI variables, kg/mg2 BMI at diagnosisd | Per increase of 5 | 0.95 (0.93–0.96), <0.001 | 0.92 (0.91–0.94), <0.001 | 1.00 (0.96–1.04) 0.98 | 1.01 (0.97–1.06), 0.53 |
| Underweight vs. normal | 1.43 (1.32–1.55), <0.001 | 1.56 (1.43–1.70), <0.001 | 1.16 (0.89–1.51) 0.28 | 1.20 (0.92–1.6), 0.18 | |
| Overweight vs. normal | 0.94 (0.90–0.97), <0.001 | 0.89 (0.85–0.93), <0.001 | 0.97 (0.87–1.07) 0.51 | 0.93 (0.84–1.0), 0.20 | |
| Obese vs. normal | 0.92 (0.88–0.97), <0.001 | 0.86 (0.82–0.91), <0.001 | 1.05 (0.94–1.19) 0.39e | 1.07 (0.95–1.2), 0.24e | |
| Morbidly obese vs. normal | 1.04 (0.91–1.19), 0.56 | 1.09 (0.95–1.26), 0.22 | |||
| BMI at young adult aged | Per increase of 5 | 1.05 (1.01–1.09), 0.02 | 1 (0.96,1.05), 0.83 | 1.01 (0.89,1.14) 0.89 | 1.03 (0.9–1.2), 0.68 |
| Underweight vs. normal | 1.06 (0.93–1.20), 0.38 | 1.15 (1–1.31), 0.04 | 1.70 (1.1–2.5), 0.009 | 1.93 (1.3–2.9), 0.001 | |
| Overweight vs. normal | 1.06 (0.97–1.15), 0.22 | 0.98 (0.9–1.07), 0.69 | 1.22 (0.95–1.6), 0.12 | 1.26 (0.98–1.6), 0.07 | |
| Obese vs. normal | 1.16 (1.02–1.32), 0.03 | 1.07 (0.93–1.23), 0.33 | 1.22 (0.83–1.8), 0.30e | 1.39 (0.94–2.0), 0.10e | |
| Morbidly obese vs. normal | 1.28 (0.88–1.85), 0.19 | 1.27 (0.88–1.84), 0.20 | |||
| Change in BMI from young adult age to diagnosis | Per increase of 5 | 0.87 (0.8–0.9), <0.001 | 0.89 (0.86–0.92), <0.001 | 1.03 (0.93–1.2), 0.55 | 1.03 (0.93–1.2), 0.57 |
| Decrease vs. increase/stable | 1.31 (1.2–1.4), <0.001 | 1.24 (1.2–1.3), <0.001 | 1.25 (1.0–1.6), 0.06 | 1.26 (1.0–1.6), 0.06 | |
The multivariable base models included either 26,430 NSCLC or 2787 SCLC patients with data on all assessed variables in the table; for multivariable analysis of BMI at diagnosis, BMI at young adult age, and change in BMI from young adult age to time of diagnosis, each of these BMI variables was added individually to the multivariable base model. BMI at diagnosis was available for 20,937 NSCLC and 2132 SCLC patients. BMI at young adult age was available for 5454 NSCLC and 559 SCLC patients. Change in BMI was available for 5429 NSCLC and 554 SCLC patients.
For every 10 pack years smoked. Not included in the base multivariable model for NSCLC due missing data for 14,359 patients.
The year 2000 was chosen because it was the first full implementation year of AJCC sixth edition staging (published in 1998), which was significantly different than the fifth edition; the fourth and fifth edition are similar and the sixth and seventh edition are similar.
Underweight, BMI < 18.5 kg/mg2; normal weight 18.5 kg/mg2 ≤ BMI < 25 kg/mg2; overweight 25 kg/mg2 ≤ BMI < 30 kg/mg2; obese, 30 kg/mg2 ≤ BMI ≤ 40 kg/mg2; morbidly obese, BMI > 40 kg/mg2.
For SCLC cases, there were not enough morbidly obese individuals to study separately, and the obese and morbidly obese categories were combined together. HR, hazard ratio; CI, confidence interval; Adeno, adenocarcinoma; Squam, squamous cell carcinoma; BMI, body mass index; AJCC, American Joint Committee on Cancer.
OS and BMI at Diagnosis, BMI in Young Adulthood, and Change in BMI Between These Two Timepoints
Univariable and multivariable analyses of the association of BMI at a young adult age, BMI at diagnosis, and change in BMI with OS are shown in Table 2.
The association of BMI at diagnosis and OS is depicted in PSS curves adjusted for the clinical base model (Figs. 1A and B, Table 2) and the unadjusted KaplanMeier survival curves (Figs. 1C and D, Table 2). For patients with NSCLC (Figs. 1A and C), there was a strong association with higher risk of death in underweight patients when compared to “normal” weight individuals; risk of death was lowest in “normal,” overweight, and obese patients, but when the BMI was greater than 40 kg/mg2 (morbid obesity), the risk of death increased again (Figs. 1A and C, Table 2). For SCLC (Figs. 1B and D), although there was no statistically significant association and the magnitude of hazard ratios (HRs) were smaller, the overall shape of HRs across different BMIs was similar to that of NSCLC with greater risks in the lowest and highest BMI groups (Fig. 1B versus A, Table 2). Analysis of the association between BMI at diagnosis and lung cancer-free survival showed similar findings (Supplementary Fig. 1), except for an attenuation of the increased risk of lung cancer-specific death in morbidly obese individuals.
Figure 1.
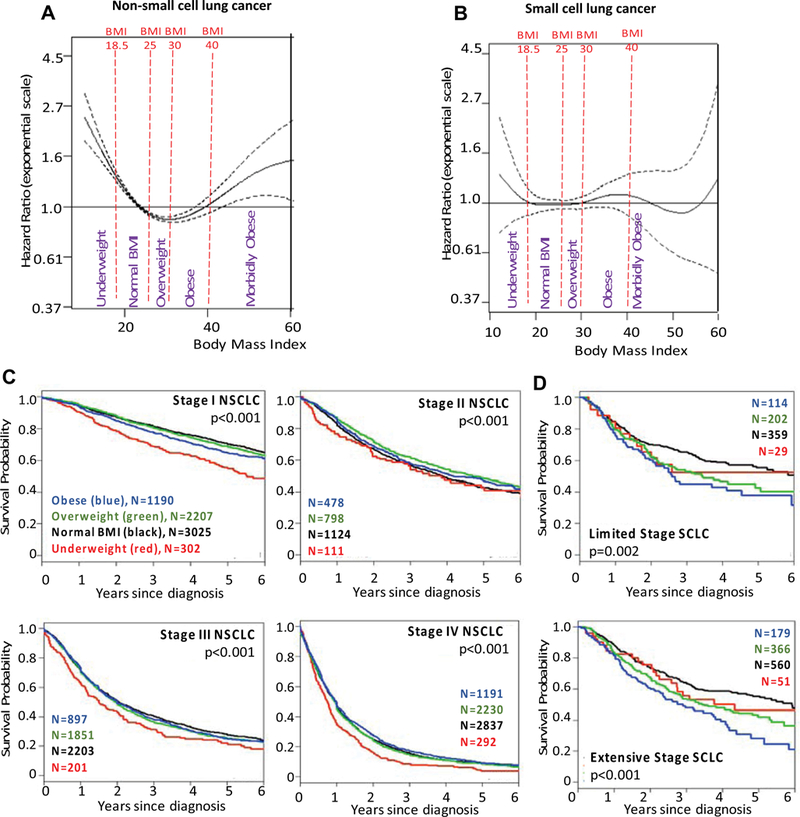
The hazard ratio of overall survival based on penalized smoothing spline by body mass index (BMI [kg/m2]) at diagnosis for (A) NSCLC and (B) SCLC, and Kaplan Meier survival curves for (C) NSCLC and (D) SCLC patients. BMI data points above 60 kg/m2 are sparse, explaining the wide confidence intervals in A and B. Data are sparse when BMI is greater than 60 kg/m2, and interpretation should be made with caution.
The corresponding associations between BMI in young adulthood and OS are shown in the PSS curves (Figs. 2A and B), Kaplan-Meier survival curves (Figs. 2C and D), and summarized in Table 2. There was no strong association between BMI in young adulthood and OS in NSCLC (Figs. 2A and C, Table 2). However, there was a statistically significant relationship between being underweight during young adulthood and having poorer survival after diagnosis with SCLC; this relationship is revealed in the multivariable analysis (Fig. 2B, Table 2) that corrected for confounding prognostic variables than in the univariable analysis (Fig. 2D, Table 2).
Figure 2.
The hazard ratio of overall survival based on penalized smoothing spline by body mass index at young adulthood (BMI, kg/m2) for (A) NSCLC and (B) SCLC, and Kaplan Meier survival curves for (C) NSCLC patients and (D) SCLC patients. Young adulthood is defined as an age between 18 and 25 years, or approximately 20 years.
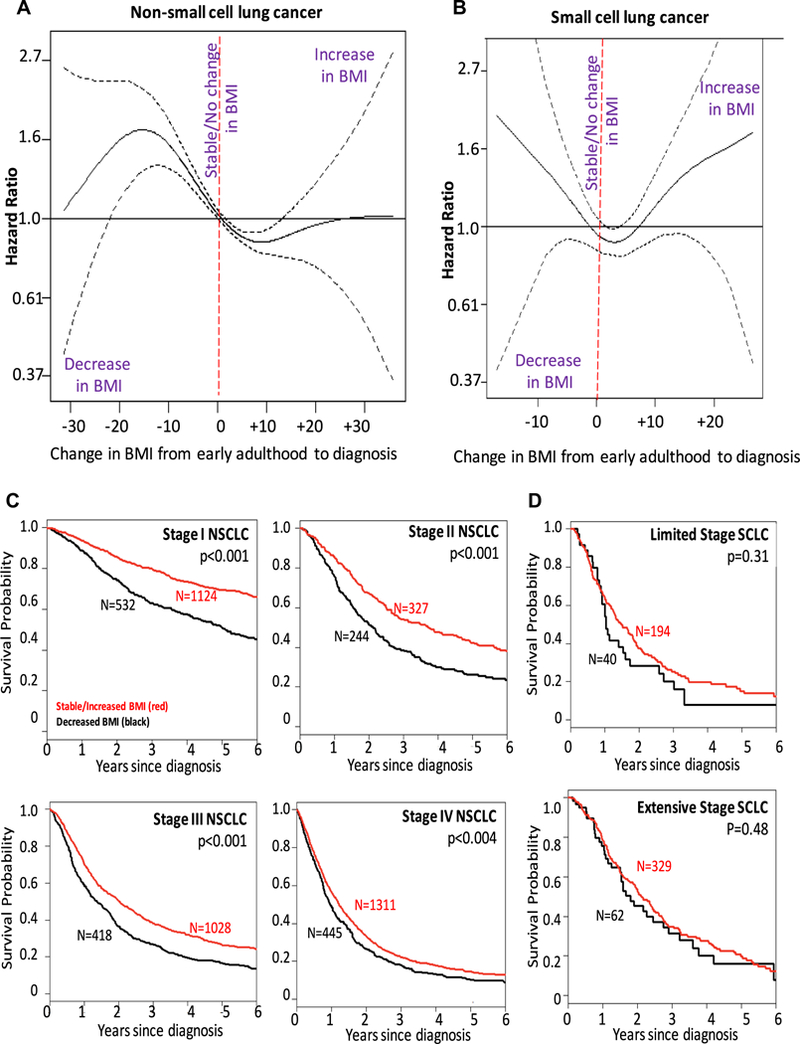
The association between the change in BMI (ΔBMI) from early adulthood to the time of lung cancer diagnosis and OS is depicted in the PSS curves (Figs. 3A and B), the Kaplan-Meier survival curves (Figs. 3C and D), and summarized in Table 2. Relative to the BMI during early adulthood, a decrease in BMI at diagnosis was associated with worse OS when compared to patients who had similar or increased BMI at the time of diagnosis for patients with NSCLC. The benefit of an increase in BMI was present significantly for increases as large as ΔBMI of +12. There was a similar association in SCLC (Table 2, Figs. 3B and D), except that the benefit of a stable/increased BMI only occurred up to DBMI of +6 (an increase of 6 kg/m2 of BMI). Fewer than 10% of patients had a ΔBMI greater than +6, suggesting that the estimates more than ΔBMI greater than +6 may be hard to interpret.
Figure 3.
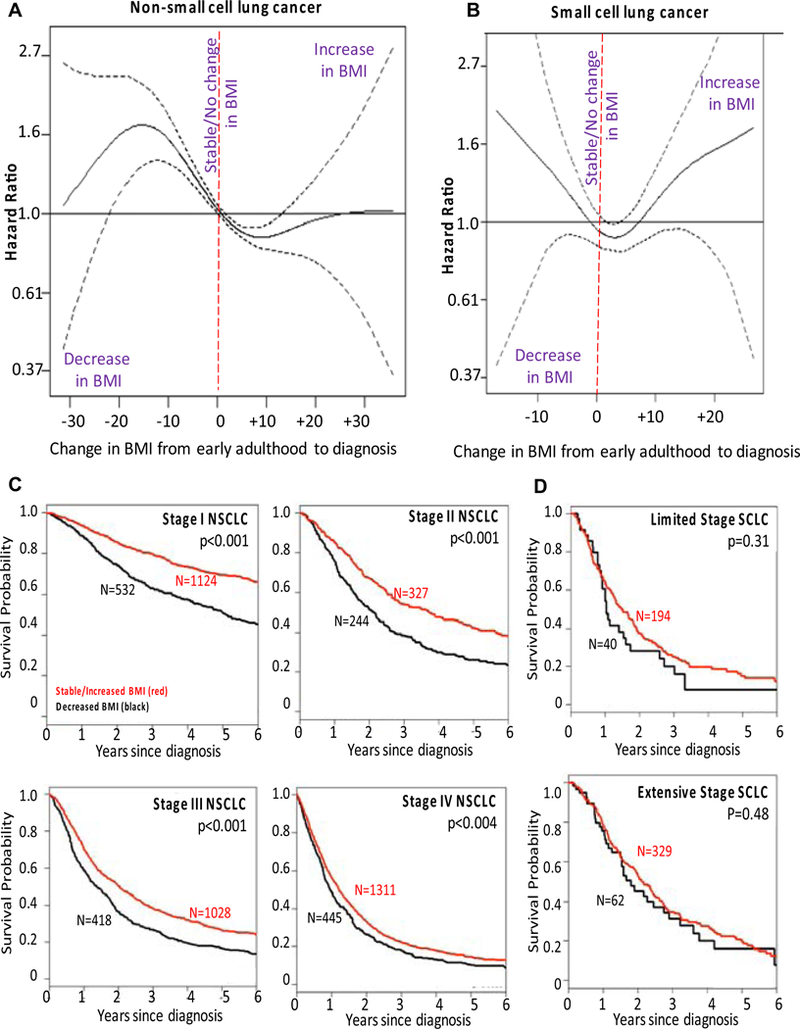
The hazard ratio of overall survival based on penalized smoothing spline by change in body mass index at diagnosis (ΔBMI, kg/m2) for (A) NSCLC and (B) SCLC, and Kaplan Meier survival curves for (C) NSCLC patients and (D) SCLC patients. The change compares the relationship between BMI at young adulthood (around 20 years of age) to the BMI at the time of the diagnosis as a means of correcting for heterogeneity of BMI in a healthy population.
Subset Analyses and Sensitivity Analyses
Subset analyses of the individual studies confirmed that 15 of 16 individual studies reported that underweight patients had numerical HRs above unity, consistent with the pooled analysis.
When evaluating subset relationships between BMI at diagnosis and OS, BMI at young adulthood, and ΔBMI (Supplementary Figs. 2–4) by age, sex, education, smoking status, ethnicity, histology, and stage, the most consistent relationship seen across all subsets was observed with ΔBMI. A decrease in BMI was associated with an increase in risk of death in all subsets of NSCLC and in most subsets of SCLC (where none of the subsets were associated with a decrease in risk). In contrast, both BMI at diagnosis and BMI in young adulthood showed much more heterogeneous associations.
The association between OS and BMI at diagnosis, in young adulthood, or changes in BMI before diagnosis remained similar across multiple pre-planned sensitivity analyses (Supplementary Table 3). These sensitivity analyses removed patients with data variables one-by-one and assessed whether the subsequent primary association remained similar after removal. Sensitivity studies confirmed consistency of the primary associations reported, despite minor variation in the magnitude of associations.
Discussion
This large pooled analysis identified a number of novel findings of the relationship between BMI variables measured in young adulthood, changes before diagnosis and at the time of diagnosis, and lung cancer survival outcomes. We describe that DBMI, that is, a change in BMI between early adulthood and the diagnosis date, was associated with OS in lung cancer. Specifically, a decrease in BMI when compared to a remote period at young adulthood is consistently associated with poorer lung cancer survival across age groups, sex, smoking status, stage, and histology with adjusted HRs of approximately 1.25. Its consistency in association across many subgroups suggests its potential utility as a clinically useful global marker of lung cancer prognosis.
We also report a potential U-shaped association between BMI at diagnosis and OS with greater mortality in the extreme groups of underweight and morbidly obese patients, relative to patients who are normal weight, with the best outcomes in those who are overweight or obese (but not morbidly obese). These relationships appear to be similar between NSCLC and SCLC patients, but more pronounced in the NSCLC patients. Whereas the increase in mortality in the underweight lung cancer patients is consistent across all analyses, the increase in mortality in the morbidly obese lung cancer patients is not as clear. The number of morbidly obese patients is modest, and the relationship is attenuated when evaluating lung cancer-specific mortality. Thus, the increase in mortality in the morbidly obese patients may be due to non-lung cancer-related causes, especially given the known increase in risk of death from all causes associated with morbid obesity. Our results also confirm findings in other patient cohorts that being overweight or obese at lung cancer diagnosis was associated with improved OS when compared to patients with normal BMI.6,12–19 The association between low BMI and lower OS rates have been described for several malignancies, including lung cancer, with similar effect size.6,12–15,20–29 However, the positive association between high BMIs between 25 and 40 kg/mg2 and OS for NSCLC patients is contrary to the inverse association described for most other malignancies.6,10,51–53 The reasons for such findings in lung cancer remain unclear, but several biologic explanations have been postulated.
In a meta-analysis of more than 10,000 patients, Zhu et al.54 reported that increasing BMI is associated with lower lung cancer risk in never-smokers, especially in women, raising questions whether estrogens play a protective role in lung cancer carcinogenesis. Effects on prognosis were not studied. A sex difference in outcomes is suggested by our results. Both low BMI at diagnosis and a decrease in ΔBMI appear to adversely affect OS to a greater extent in women than in men (Supplementary Figs. 2 and 4), indirectly suggesting a potential hormonal influence on survival. In exploratory analyses, these sex differences were not found to be ethnically driven (data not reported), and are thus unlikely to be driven solely by molecular profiles (as Asian women have a much higher chance of carrying an EGFR-activating mutation).
Biologically, the finding of similar prognostic relationships between BMI at diagnosis and ΔBMI in Asians is important (Supplementary Figs. 2–4), as Asians diagnosed with NSCLC have different molecular profiles and outcomes compared to other ethnicities.55 Thus, our results suggest that these BMI-survival relationships transcend histomolecular subtype differences, although conclusive evidence would need to be based on molecular profiling data, which we do not have access to for this project.
Dahlberg et al.13 found a time-dependent relationship whereby obesity initially led to improved outcomes in stage IV patients treated with chemotherapy early in follow-up, but that the risk of death increased in obese patients after 16 months. A time-dependent analysis of our stage IV patients did not confirm such an association in our sample (data not reported).
In our pooled analysis, the relationships in both BMI at diagnosis and ΔBMI were consistent across different disease stages, including stage IA patients who typically undergo only surgical resection, and stage IV patients, who typically undergo only systemic therapy. Such consistency suggests that either the effects of BMI on survival are treatment-independent, or that multiple treatments interact with BMI in a similar manner on survival outcomes.
Compared to normal BMI during early adulthood, a significantly worse prognosis in patients with SCLC who were underweight during early adulthood was an unexpected finding, but must be interpreted with caution given the small numbers of patients. Further, because of missing data, we were not able to account for cumulative smoking exposure or comorbidities in this specific analysis. Where data were available, adjustment for smoking did not influence most results; the exception was a larger HR when comparing the underweight versus normal BMI patients at both diagnosis and in young adulthood, which was observed in both NSCLC and SCLC. These data suggest that it is possible that being underweight during early adulthood was also associated with heavier tobacco consumptions, which led to greater comorbidities at the time of diagnosis, and thus a worse prognosis. Future analyses could attempt to quantify directly cumulative smoking exposure, and particularly intensity of smoking in early adulthood, and compare it OS after lung cancer diagnosis.
The relatively better OS in patients with BMI from 18.5 kg/mg2 to 40 kg/mg2, specifically in stage II-IV patients, is reassuring from a chemotherapy dosing perspective, as the vast majority of patients will fall in this range of BMIs. Although there are data regarding the importance and safety of full dosing based on true body weight, some overweight/obese patients are still under-dosed based on an assumed ideal body weight, or a capped body surface area of 2 m2.56 Although we had no dosing data for the patients included in this analysis, it is reassuring that OS for overweight patients is actually better than for those with BMI values within normal limits in patients with disease stages that are generally treated with chemotherapy. OS for patients with BMI greater than or equal to 40 kg/mg2 were found to be worse comparable to patients with normal BMI. Whether this loss of the protective effect of high BMI represents the OS effect of comorbidities associated with higher BMI, suboptimal dosing, or other factors is unknown.
Our study has several limitations. First, the harmonization of different datasets collected in different countries and periods, with lack of treatment data, might have introduced external bias, although multiple sensitivity analyses showed similar results. Secondly, BMI data was derived from self-reported data, a method known to be highly correlated with measured height and weight, with slight overestimation of height and underestimation of weight.57–59 Thus, reported BMI probably slightly underestimates true BMI values. Thirdly, BMI during early adulthood is also prone to recall bias and the reported changes may well have occurred recently, rendering ΔBMI a surrogate for recent weight loss. However, BMI at additional timepoints between young adulthood and at diagnosis was unavailable for this analysis. That the association between DBMI and OS was observed consistently across stages, including stages I and II NSCLCs where patients are least likely to be symptomatic from their cancer, suggests that the ΔBMI relationship is not completely attributable to recent weight loss as a symptom of the lung cancer. Fourthly, the strength of the association between BMI and OS in the morbidly obese group is not as strong as the associations with underweight patients. Thus, the finding of adverse outcomes associated with morbid obesity is more preliminary in nature. Fifthly, the analysis did not include data on different lung cancer treatments, a potential confounding factor. Some individual studies did provide treatment data, but when treatment and stage were included in the same model, there was significant collinearity such that either stage or treatment needed to be removed. Because data for stage was complete whereas treatment data was limited, stage was ultimately left in the final models. Finally, some patients were excluded from the analysis due to missing data, potentially introducing additional selection bias.
Recent data indicate that measures of body composition, capable of distinguishing muscle and fat, and a diagnosis of sarcopenia may be a better predictor for mortality in cancer.60–64 However, in the absence of data from these markers, as our results suggest, changes in BMI from a healthy pre-morbid state may be a better prognosis surrogate than BMI at diagnosis.
In summary, we identified a U-shaped relationship between BMI at diagnosis and OS in patients with NSCLC, with the worst prognosis in underweight and morbidly obese patients. However, we also reported sex, ethnicity, and smoking heterogeneity in the prognostic relationship with BMI at diagnosis in our study. Thus, there should be caution regarding generalizing this relationship, given that each of these demographic variables can also influence baseline pre-morbid BMI. Instead, ΔBMI generated a more consistent prognostic relationship with OS across clinicodemographic groups. A decrease in ΔBMI from early adulthood to the time of diagnosis was associated with a modest, but significant 20% to 30% increase in risk of dying.
Supplementary Material
Acknowledgments
Drs. Shepshelovich, Fares, Brown, and G. Liu were supported by funding from the Alan Brown Chair in Molecular Genomics, the Posluns Family Fund, and the Lusi Wong Fund. Drs. Schwartz and Wenzlaff and data collection at Karmanos were supported by National Institutes of Health (NIH) grants and contracts R01CA60691, R01CA87895, P30CA022453, and HHSN261201300011I. CARET is funded by the National Cancer Institute (NCI), NIH through grants U01-CA063673, UM1-CA167462, and U01-CA167462. Drs. Z. Zhang and Morgenstern were supported by DA/CA11386 and CA90833. Drs. Shirashi and Kohno were supported by the Health and Labour Sciences Research Grant. Drs. Ryan and Harris were supported by the Intramural Research Program of the Center for Cancer Research, NCI.
Footnotes
Supplementary Data
Note: To access the supplementary material accompanying this article, visit the online version of the Journal of Thoracic Oncology at www.jto.org and at https://doi.org/10.1016/j.jtho.2019.05.031.
Disclosures: The authors declare no conflict of interest.
References
- 1.Renehan AG, Harvie M, Cutress RI, et al. How to manage the obese patient with cancer. J Clin Oncol. 2016;34:4284–4294. [DOI] [PubMed] [Google Scholar]
- 2.Kizer NT, Thaker PH, Gao F, et al. The effects of body mass index on complications and survival outcomes in patients with cervical carcinoma undergoing curative chemoradiation therapy. Cancer. 2011;117:948–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horowitz NS, Wright AA. impact of obesity on chemotherapy management and outcomes in women with gynecologic malignancies. Gynecol Oncol. 2015;138: 201–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kashiwabara K, Yamane H, Tanaka H. Toxicity and prognosis in overweight and obese women with lung cancer receiving carboplatin-paclitaxel doublet chemotherapy. Cancer Invest. 2013;31:251–257. [DOI] [PubMed] [Google Scholar]
- 5.Furlanetto J, Eiermann W, Marme F, et al. Higher rate of severe toxicities in obese patients receiving dose-dense (DD) chemotherapy according to unadjusted body surface area: results of the prospectively randomized gain study. Ann Oncol. 2016;27:2053–2059. [DOI] [PubMed] [Google Scholar]
- 6.Calle EE, Rodriquez C, Walker Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. [DOI] [PubMed] [Google Scholar]
- 7.Lohmann AE, Goodwin PJ, Chlebowski RT, Pan K, Stambolic V, Dowling RJO. Association of obesity-related metabolic disruptions with cancer risk and outcome. J Clin Oncol. 2016;34:4249–4255. [DOI] [PubMed] [Google Scholar]
- 8.Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34:4270–4276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. 2016;34:4277–4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32:3568–3574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ligibel JA, Wollins D. American Society of Clinical Oncology obesity initiative: rationale, progress, and future directions. J Clin Oncol. 2016;34:4256–4260. [DOI] [PubMed] [Google Scholar]
- 12.Attaran S, Mcshane J, Whittle I, Poullis M, Shackcloth M. A propensity-matched comparison of survival after lung resection in patients with a high versus low body mass index. Eur J Cardiothorac Surg. 2012;42:653–658. [DOI] [PubMed] [Google Scholar]
- 13.Dahlberg SE, Schiller JH, Bonomi PB, et al. Body mass index and its association with clinical outcomes for advanced non-small-cell lung cancer patients enrolled on Eastern Cooperative Oncology Group clinical trials. J Thorac Oncol. 2013;8:1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie H-J, Zhang X, Wei Z-Q, Long H, Rong T-H, Su X-D. Effect of body mass index on survival of patients with stage i non-small cell lung cancer. Chin J Cancer [internet]. 2017. December;36(1). https://cancercommun.biomedcentral.com/articles/10.1186/s40880-016-0170-7. Accessed August 20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tomita M, Ayabe T, Maeda R, Nakamura K. Combination of advanced lung cancer inflammation index and C-reactive protein is a prognostic factor in patients with operable non-small cell lung cancer. World J Oncol. 2017;8:175–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanikini H, Clain G, Sanchez M, et al. Body mass index and lung cancer survival: results from the icare study. Ann Oncol [internet]. 2015. April 1;26(suppl). https://academic.oup.com/annonc/article-lookup/doi/10.1093/annonc/mdv044.02. Accessed August 21, 2018. [Google Scholar]
- 17.Gupta A, Majumder K, Arora N, et al. Premorbid body mass index and mortality in patients with lung cancer: a systematic review and meta-analysis. Lung Cancer Amst Neth. 2016;102:49–59. [DOI] [PubMed] [Google Scholar]
- 18.Lam VK, Bentzen SM, Mohindra P, et al. Obesity is associated with long-term improved survival in definitively treated locally advanced non-small cell lung cancer (NSCLC). Lung Cancer. 2017;104:52–57. [DOI] [PubMed] [Google Scholar]
- 19.Greenlee H, Unger JM, Leblanc M, Ramsey S, Hershman DL. Association between body mass index and cancer survival in a pooled analysis of 22 clinical trials. Cancer Epidemiol Biomarkers Prev. 2017;26: 21–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoang T, Dahlberg SE, Sandler AB, Brahmer JR, Schiller JH, Johnson DH. Prognostic models to predict survival in non-small-cell lung cancer patients treated with first-line paclitaxel and carboplatin with or without bevacizumab. J Thorac Oncol. 2012;7: 1361–1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakagawa T, Toyazaki T, Chiba N, Ueda Y, Gotoh M. Prognostic value of body mass index and change in body weight in postoperative outcomes of lung cancer surgery. Interact Cardiovasc Thorac Surg. 2016;23: 560–566. [DOI] [PubMed] [Google Scholar]
- 22.Mandrekar SJ, Schild SE, Hillman SL, et al. A prognostic model for advanced stage nonsmall cell lung cancer: pooled analysis of north central cancer treatment group trials. Cancer. 2006;107:781–792. [DOI] [PubMed] [Google Scholar]
- 23.Luo J, Chen Y-J, Narsavage Gl, Ducatman A. Predictors of survival in patients with non-small cell lung cancer. Oncol Nurs Forum. 2012;39:609–616. [DOI] [PubMed] [Google Scholar]
- 24.Masel EK, Berghoff AS, Füreder LM, et al. Decreased body mass index is associated with impaired survival in lung cancer patients with brain metastases: a retrospective analysis of 624 patients. Eur J Cancer Care (Engl). 2017;26:e12707. [DOI] [PubMed] [Google Scholar]
- 25.Bowden JCS, Williams LJ, Simms A, et al. Prediction of 90 day and overall survival after chemo-radiotherapy for lung cancer: role of performance status and body composition. Clin Oncol. 2017;29: 576–584. [DOI] [PubMed] [Google Scholar]
- 26.Matsuoka K, Yamada T, Matsuoka T, Nagai S, Ueda M, Miyamoto Y. Significance of body mass index for postoperative outcomes after lung cancer surgery in elderly patients. World J Surg. 2018;42:153–160. [DOI] [PubMed] [Google Scholar]
- 27.Mitsuyoshi T, Matsuo Y, Itou H, et al. Evaluation of a prognostic scoring system based on the systemic inflammatory and nutritional status of patients with locally advanced non-small-cell lung cancer treated with chemoradiotherapy. J Radiat Res (Tokyo). 2018;59: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hsu C-L, Chen K-Y, Shih J-Y, et al. Advanced non-small cell lung cancer in patients aged 45 years or younger: outcomes and prognostic factors. BMC Cancer [internet]. 2012. December;12(1). http://bmccancer.biomedcentral.com/articles/10.1186/1471-2407-12-241. Accessed August 20, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong KM, Cuffe S, Coate LE, et al. The impact of body mass index on survival in stage 3 and 4 lung cancer. 2013. June 3 https://meetinglibrary.asco.org/record/81629/abstract. Accessed August 21,2018. [Google Scholar]
- 30.Martin L, Birdsell L, Macdonald N, et al. Cancer cachexia in the age of obesity: skeletal muscle depletion is a powerful prognostic factor, independent of body mass index. J Clin Oncol. 2013;31:1539–1547. [DOI] [PubMed] [Google Scholar]
- 31.Kimura M, Naito T, Kenmotsu H, et al. Prognostic impact of cancer cachexia in patients with advanced non-small cell lung cancer. Support Care Cancer. 2015;23: 1699–1708. [DOI] [PubMed] [Google Scholar]
- 32.Mytelka DS, Li L, Benoit K. Post-diagnosis weight loss as a prognostic factor in non-small cell lung cancer: post-diagnosis weight loss in non-small cell lung cancer. J Cachexia Sarcopenia Muscle. 2018;9:86–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Strulov Shachar S, Williams GR. The obesity paradox in cancer—moving beyond BMI. Cancer Epidemiol Bio-markers Prev. 2017;26:13–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anandavadivelan P, Lagergren P. Cachexia in patients with oesophageal cancer. Nat Rev Clin Oncol. 2016;13:185–198. [DOI] [PubMed] [Google Scholar]
- 35.Martin L, Senesse P, Gioulbasanis I, et al. Diagnostic criteria for the classification of cancer-associated weight loss. J Clin Oncol. 2015;33:90–99. [DOI] [PubMed] [Google Scholar]
- 36.Abdel-Rahman O Impact of baseline characteristics on extensive-stage SCLC patients treated with etoposide/carboplatin; a secondary analysis of a phase III study. Clin RespirJ. 2018;12:2519–2524. [DOI] [PubMed] [Google Scholar]
- 37.Georgiadis MS, Steinberg SM, Hankins LA, Ihde DC, Johnson BE. Obesity and therapy-related toxicity in patients treated for small-cell lung cancer. J Natl Cancer Inst. 1995;87:361–366. [DOI] [PubMed] [Google Scholar]
- 38.Kim EY, Kim N, Kim YS, et al. Prognostic significance of modified advanced lung cancer inflammation index (ALI) in patients with small cell lung cancer: comparison with original ALI. Plos One. 2016;11:e0164056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee C-H, Lin C, Wang C-Y, et al. Premorbid bmi as a prognostic factor in small-cell lung cancer—a single institute experience. Oncotarget. 2018;9:24642–24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams T, Gulack BC, Kim S, Fernandez FG, Ferguson MK. Operative risk for major lung resection increases at extremes of body mass index. Ann Thorac Surg. 2017;103:296–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Petrella F, Radice D, Borri A, et al. The impact of pre-operative body mass index on respiratory complications after pneumonectomy for non-small-cell lung cancer. Results from a series of 154 consecutive standard pneumonectomies. Eur J Cardiothorac Surg. 2011;39: 738–744. [DOI] [PubMed] [Google Scholar]
- 42.Fearon K, Strasser F, Anker SD, et al. Definition and classification of cancer cachexia: an international consensus. Lancet Oncol. 2011;12:489–495. [DOI] [PubMed] [Google Scholar]
- 43.Hung RJ, Christiani DC, Risch A, et al. International Lung Cancer Consortium: pooled analysis of sequence variants in DNA repair and cell cycle pathways. Cancer Epidemiol Biomarkers Prev. 2008;17:3081–3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brenner DR, Boffetta P, Duell EJ, et al. Previous lung diseases and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium. Am J Epidemiol. 2012;176:573–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coté ML, Liu M, Bonassi S, et al. Increased risk of lung cancer in individuals with a family history of the disease: a pooled analysis from the International Lung Cancer Consortium. Eur J Cancer. 2012;48: 1957–1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ben Khedher S, Neri M, Papadopoulos A, et al. Menstrual and reproductive factors and lung cancer risk: a pooled analysis from the International Lung Cancer Consortium: menstrual and reproductive factors and lung cancer risk. Int J Cancer. 2017;141:309–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fehringer G, Brenner DR, Zhang Z-F, et al. Alcohol and lung cancer risk among never smokers: a pooled analysis from the International Lung Cancer Consortium and the synergy study: alcohol and lung cancer risk among never smokers. Int J Cancer. 2017;140: 1976–1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pesatori AC, Carugno M, Consonni D, et al. Hormone use and risk for lung cancer: a pooled analysis from the International Lung Cancer Consortium (ILCCO). Br J Cancer. 2013;109:1954–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York, New York: Springer; 2000. [Google Scholar]
- 50.Eilers PHC, Marx BD. Flexible smoothing with B-splines and penalties. Statist Sci. 1996;11:89–121. [Google Scholar]
- 51.Sun L, Zhu Y, Qian Q, Tang L. Body mass index and prognosis of breast cancer: an analysis by menstruation status when breast cancer diagnosis. Medicine (Baltimore). 2018;97:e11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chan DSM, Vieira AR, Aune D, et al. Body mass index and survival in women with breast cancer—systematic literature review and meta-analysis of 82 follow-up studies. Ann Oncol. 2014;25:1901–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haque R, Van Den Eeden SK, Wallner LP, et al. Association of body mass index and prostate cancer mortality. Obes Res Clin Pract. 2014;8:e374–e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu H, Zhang S. Body mass index and lung cancer risk in never smokers: a meta-analysis. BMC Cancer [internet]. 2018. December;18(1). http://https://bmccancer.biomedcentral.com/articles/10.1186/s12885-018-4543-y. Accessed August 23, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Soo RA, Kawaguchi T, Loh M, et al. Differences in outcome and toxicity between Asian and Caucasian patients with lung cancer treated with systemic therapy. Future Oncol. 2012;8:451–462. [DOI] [PubMed] [Google Scholar]
- 56.Thigpen JT. Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. Yearb Med. 2012;2012:121–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115:223–230. [DOI] [PubMed] [Google Scholar]
- 58.Stewart AW, Jackson RT, Ford MA, Beaglehole R. Underestimation of relative weight by use of self-reported height and weight. Am J Epidemiol. 1987;125:122–126. [DOI] [PubMed] [Google Scholar]
- 59.Stevens J, Keil JE, Waid LR, Gazes PC. Accuracy of current, 4-year, and 28-year self-reported body weight in an elderly population. Am J Epidemiol. 1990;132:1156–1163. [DOI] [PubMed] [Google Scholar]
- 60.Suzuki Y, Okamoto T, Fujishita T, et al. Clinical implications of sarcopenia in patients undergoing complete resection for early non-small cell lung cancer. Lung Cancer. 2016;101:92–97. [DOI] [PubMed] [Google Scholar]
- 61.Nattenmüller J, Wochner R, Muley T, et al. Prognostic impact of CT-quantified muscle and fat distribution before and after first-line-chemotherapy in lung cancer patients. Plos One. 2017;12, e0169136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kinsey E, Ajazi E, Wang X, Johnston MAM, Crawford J. Predictors of physical and functional loss in advanced stage lung cancer patients receiving platinum chemo-therapy. J Thorac Oncol. 2018;13:1294–1301. [DOI] [PubMed] [Google Scholar]
- 64.Hervochon R, Bobbio A, Guinet C, et al. Body mass index and total psoas area affect outcomes in patients undergoing pneumonectomy for cancer. Ann Thorac Surg. 2017;103:287–295. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


