Abstract
Tumor progression to metastasis is not cancer cell autonomous, but rather involves the interplay of multiple cell types within the tumor microenvironment. Here we identify Asporin (ASPN) as a novel, secreted mesenchymal stromal cell (MSC) factor in the tumor microenvironment that regulates metastatic development. MSCs expressed high levels of ASPN, which decreased following lineage differentiation. ASPN loss impaired MSC self-renewal and promoted terminal cell differentiation. Mechanistically, secreted ASPN bound to BMP-4 and restricted BMP-4-induced MSC differentiation prior to lineage commitment. ASPN expression was distinctly conserved between MSC and cancer-associated fibroblasts (CAF). ASPN expression in the tumor microenvironment broadly impacted multiple cell types. Prostate tumor allografts in ASPN-null mice had a reduced number of tumor-associated MSCs, fewer cancer stem cells, decreased tumor vasculature, and an increased percentage of infiltrating CD8+ T cells. ASPN-null mice also demonstrated a significant reduction in lung metastases compared to wild type mice. These data establish a role for ASPN as a critical MSC factor that extensively effects the tumor microenvironment and induces metastatic progression.
Keywords: Asporin, Mesenchymal Stem Cell, Mesenchymal Stromal Cell, Activated Fibroblast, Reactive Stroma, Cancer-Associated Fibroblast, Tumor Microenvironment, Prostate Cancer, Bone Morphogenetic Protein-4, Differentiation, Self-renewal, Metastasis
Introduction
Tumors do not develop in isolation and components of the tumor microenvironment demonstrably influence many cancer hallmarks including metastasis (1, 2). A growing body of evidence now indicates that cells of mesenchymal origin including mesenchymal stem cells, mesenchymal stromal cells, and fibroblasts, collectively referred to herein as MSCs, reside in the tumor microenvironment and contribute to cancer progression (2-5). MSCs are a heterogeneous population of multipotent stromal cells that give rise to physiologic cell lineages including osteoblasts, adipocytes, and chondrocytes (6). MSCs also give rise in part to pathological cell lineages including cancer-associated fibroblasts (CAFs) and fibroblasts associated with injury or inflammation (2, 3, 7, 8). Similar to MSCs, CAFs are highly heterogeneous and dynamic, which may account for the reported pleiotropic roles of MSCs and CAFs in cancer progression as either potentiators of primary and metastatic cancer development (4, 5, 9-14) or restrainers of cancer growth (15-17). The seminal properties of MSCs in the tumor microenvironment have yet to be fully established. Due to the conservation between gene expression programs in development and cancer (18, 19), key factors may be similar between MSCs in development and MSCs in the tumor microenvironment.
Asporin (ASPN), a member of the small leucine-rich proteoglycan (SLRP) family of extracellular proteins, is markedly enriched in fetal prostate mesenchyme during development (18, 20). ASPN is similarly expressed in reactive stromal cells, including CAFs, in the tumor microenvironment of prostate (20-22), breast (23), scirrhous gastric (24), and pancreatic (25) cancers. The role of ASPN in prostate MSCs and in the tumor microenvironment is poorly understood. Intriguingly, ASPN has been reported to regulate mediators of MSC differentiation including BMP-2 (26) and TGF-β1 (27). Yet, the roles of ASPN in MSC self-renewal and multipotency have not been definitively determined.
ASPN has been shown to induce migration of scirrhous gastric (24, 28) and pancreatic (25) cancer cells; nevertheless, ASPN expression in the tumor microenvironment may have a dual role in regulating cancer progression (21-25, 29), possibly due to polymorphisms in the ASPN aspartic acid (D)-repeat domain length (21). ASPN expression in prostate reactive stromal cells is associated with adjacent local cancer aggressiveness and with worse oncologic outcomes (21, 22). However, two, of the over 10 reported, germline polymorphisms in the ASPN D-repeat domain length have been shown to be differentially associated with the development of metastatic prostate cancer following surgery (21). ASPN with 14 D-repeats (ASPN D14) was associated with an increased risk while ASPN D13 was associated with a reduced risk of metastatic progression (21).
Herein, we report that ASPN functions as a novel, secreted MSC factor and a key driver of metastatic development. We establish a role for ASPN in regulating fundamental properties of MSCs including self-renewal, differentiation, and migration. We demonstrate that ASPN expression is highly enriched in MSCs, and its expression decreases during differentiation to connective tissue lineages. Our data show that ASPN regulates MSC self-renewal and restricts MSC differentiation through regulation of BMP-4 signaling. ASPN null mice have fewer MSCs in the bone marrow and an enriched population of intermediate (or more differentiated) MSCs in the prostate. While most MSC-derived progeny have decreased ASPN expression, high ASPN expression is conserved between MSCs and CAFs in both primary and metastatic tumors. Prostate allograft tumors in ASPN null mice have an altered tumor microenvironment with fewer tumor-associated MSCs, decreased vasculature, and an increased percentage of infiltrating CD8+ T cells. Tumors in ASPN null mice also have a reduced number of cancer stem cells and a marked decrease in metastatic potential. These findings suggest that ASPN is an important regulator of MSC multipotency and metastatic development.
Materials and Methods
Immunohistochemistry (30), immunofluorescence (30), immunoblotting (21), RNA isolation and quantitative real-time PCR (30), colony forming unit assay (31), cell proliferation (32), migration (32), cytoskeletal remodeling (32), MSC isolation and differentiation (5, 30, 32-36), the PELICAN study (37), and the CP1 E. coli model of prostate inflammation (38) have been previously described and are detailed in the Supplementary Materials and Methods.
Cell lines and cell culture
PC-3, DU-145, WPMY-1, TRAMP-C2, and HEK293T cell lines were obtained from the American Type Culture Collection (ATCC). The B6MycCaP cancer cell line was a kind gift from Dr. Leigh Ellis (Roswell Park Cancer Institute). All cell lines were maintained in either DMEM (DU-145, WPMY-1, TRAMP-C2, B6MycCaP) or RPMI 1640 (PC-3) supplemented with 10% fetal bovine serum (Corning), and penicillin/streptomycin (Life Technologies). Following thaw from frozen stock, cell lines were used prior to passage 7. The WPMY-1-ASPN variant expressing cell lines were generated and cultured as previously described (21). Human MSCs were isolated from tissue and cultured in RoosterNourish™-MSC (RoosterBio) as previously described (5, 39). Mouse MSCs were cultured in DMEM supplemented with 10% fetal bovine serum (Corning), Glutamx (Life Technologies) and penicillin/streptomycin (Life Technologies). B6CaP organoids were generated from C57BL/6J Hi-Myc allografts and cultured using an adapted protocol from prior reports (40, 41). Briefly, B6CaP allograft tumors were finely minced with a scalpel, digested in DMEM/F12 + 10% FBS + 1:10 dilution of collagenase/hyaluronidase for one hour at 37°C, triturated in pre-warmed 1X PBS + DNAse I, and filtered through a 40μm cell strainer. Cells were embedded in growth factor reduced (GFR) Matrigel, plated on ultra-low attachment plates (Corning), and cultured in Advanced DMEM/F12 supplemented with 10% charcoal-stripped FBS, B-27, GlutaMAX, HEPES, and penicillin/streptomycin, recombinant mouse EGF (10ng/mL), TGF-β inhibitor A83-01 (200nM), ROCK inhibitor Y-27632 (10μM), and DHT (100nM). For harvest and passage, Matrigel-embedded organoids were incubated in pre-warmed Dispase (5U/mL) and subsequently trypsinized for single cell isolation. Cell lines were authenticated by STR analysis and confirmed mycoplasma free by PCR testing (JHU Genetic Resources Core Facility).
Prostate cancer and inflammation study
Tissue from radical prostatectomies performed at Johns Hopkins School of Medicine from 2009 to 2011 were examined for ASPN expression in cancer adjacent stroma and in inflammation adjacent stroma. Four-micrometer-cut radical prostatectomy sections were stained for ASPN (Sigma) by IHC. Cases were scored by a urologic pathologist for ASPN expression in stroma adjacent to cancer and in distinct areas of stroma adjacent to chronic inflammation. Chronic inflammation was defined by clusters of 20 or more lymphocytes. Of the 15 cases selected, 13 cases contained both cancer and distinct areas of chronic inflammation. Using established scoring schemes (21), ASPN intensity was evaluated and assigned an incremental score of 0 (negative), 1 (weak), 2 (moderate), or 3 (strong). The extent of staining was assigned a percentage from 0-100%. An ASPN score was calculated by multiplying the intensity score and the extent score (H-score).
Aspn−/− mice
All animal procedures were performed under a JHU approved Institutional Animal Care and Use Committee (IACUC) protocol. Mice were purchased from The Jackson Laboratory and maintained under standard pathogen free conditions. B6;129S5-Aspntm1Lex/Mmucd were generated through the Mutant Mouse Regional Resource Center (MMRRC) at UC Davis, a NIH funded strain repository. B6;129S5-Aspntm1Lex were donated to MMRRC by Genentech, Inc. Exon 2 was targeted (NCBI accession AF316825.1) by homologous recombination. The embryonic stem cell (ESC) line was derived from 129S5/SvEvBrd from Lexicon Genetics. B6;129S5-Aspntm1Lex mice were then fully backcrossed at least 9 generations to the C57BL/6J background. Mice were genotyped by PCR of genomic DNA with wild-type and mutation specific primers.
B6CaP model of metastasis
The B6CaP allograft model was derived from a C57BL/6J Hi-Myc mouse metastatic lesion. Cells from the metastatic tissue were isolated and serially passaged in C57BL/6J mice. When injected subcutaneously with B6CaP allograft cells, 60-70% of C57BL/6J mice develop lung metastases. For this study, we injected 5.5×106 B6CaP cells in 100μL PBS subcutaneously into Aspn+/+, Aspn+/−, and Aspn−/− mice (n=6 mice per group), resected tumors once they reached 1cm3, waited 11 weeks post-resection, euthanized the mice and inspected the lungs for metastases. Lungs were formalin-fixed, paraffin-embedded, sectioned, and stained as described previously (42).
Flow cytometry
To assess the in vivo MSC population in adult mouse prostates or B6CaP tumors by flow cytometry, the tissue of interest was isolated, mechanically dissociated, digested in RPMI + 10% FBS + 1:10 dilution of collagenase/hyaluronidase for one hour at 37°C, triturated in prewarmed PBS + DNAse I, and filtered through a 40μm cell strainer. If necessary, red blood cells were lysed by briefly incubating the cell pellet in ice-cold ACK lysis buffer (Quality Biological). Cells were Fc-blocked (Miltenyi) for 10 minutes in 4°C. Dead cells were discriminated using the LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) and samples were stained with the following antibodies from BioLegend: CD45, CD29, CD105, Sca-1, Ly6G, Ly6C, CD11b, CD11c, CCR2, F4/80, MHC II, CD3, CD4, CD8a, FoxP3, CD44, CD62L as well as CD3 from BD Pharmingen. Stained samples were analyzed on an LSR II flow cytometer (BD) and quantified using FlowJo.
Co-immunoprecipitation assays
HEK293T cells were transfected with a murine ASPN-Myc-FLAG tagged expression vector (Origene) or vehicle control using Lipofectamine 3000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Two days post-transfection, cells were washed with PBS and incubated in serum-free DMEM overnight. HEK293T cells were then treated with 0.3μg/mL recombinant murine BMP-4 protein (R&D Systems) or vehicle and incubated in 5% CO2 at 37°C for 30 minutes. Following BMP-4 incubation, conditioned media was filtered through a 0.45μm filtration unit (Millipore) and one third was set aside for the “input” sample. The remaining two thirds was treated with a protease inhibitor cocktail (Sigma), pre-cleared with mouse IgG agarose (Sigma) for one hour rotating at 4°C, and then incubated with anti-FLAG M2 magnetic beads (Sigma) overnight rotating at 4°C. Beads were washed three times with ice cold TBS, and proteins were eluted from the beads with 3XFLAG peptide (Sigma). Input conditioned media was concentrated using an Amicon Ultra-4 centrifugal filter unit with a 10kDa NMWL (Millipore) according to the manufacturer’s protocol.
Gene expression analysis
Gene expression profiling was performed for Aspn+/+ and Aspn−/− fetal MSCs expanded to passage 3 and cultured under normal growth conditions to 70-80% confluency prior to RNA isolation. Three biological replicates were analyzed per experimental group and Affymetrix mouse Clariom D arrays were run. Bioinformatics analyses were performed in R. Datasets were normalized at a transcript level for core transcripts with the R/Bioconductor package oligo and annotated with the NetAffx Biological Annotations. Artifacts from technical batches were removed with ComBat (43) implemented in the R/Bioconductor package SVA (44). Differential expression analyses were performed on ComBat adjusted data using the R/Bioconductor package LIMMA (45). Genes with FDR adjusted p-values below 0.05 and absolute log fold change greater than or equal to two were called statistically significant. Gene set analysis was performed on the KEGG cell cycle pathway and the GeneMAPP TGF-β family signaling pathway obtained from the NetAffx Biological Annotations to confirm hypothesized functional mechanisms with a one-sided Wilcoxon gene set test implemented in LIMMA.
Quantification and statistical analysis
Unless otherwise indicated, statistical comparisons between two groups were performed using a two-tailed unpaired Student’s t-test. Statistical comparisons between multiple groups were performed using one-way Analysis of Variance (ANOVA) with Tukey or Newman-Keuls multiple comparisons, as indicated in the figure legends. Statistical significance was defined as a P value <0.05, and P values were indicated with asterisks in the figures as follows: *P≤0.05; **P≤0.01; ***P≤0.001; ****P≤0.0001. All statistical comparisons were performed using GraphPad Prism software (v5.0).
Results
ASPN is Highly Expressed in MSCs
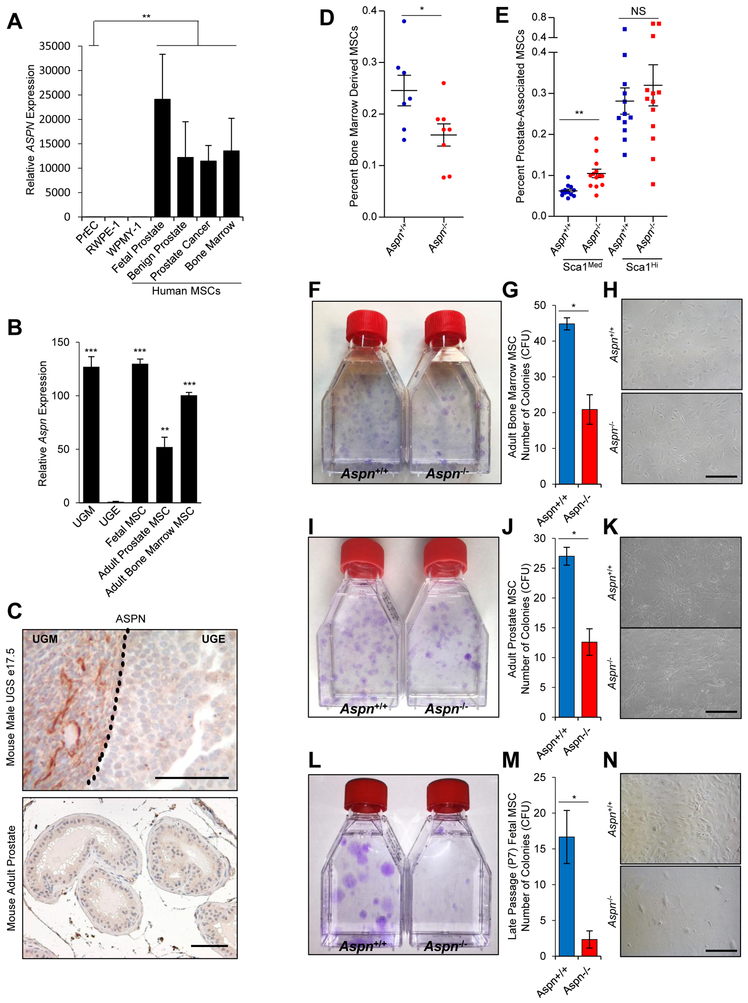
ASPN expression was examined in primary human MSCs isolated from fetal prostate, benign prostate, prostate cancer, and bone marrow. Primary heterogeneous cultures were expanded and shown to be enriched for MSCs by flow cytometry expression profile (CD73+, CD90+, CD105+, CD14−, CD20−, CD34−, CD45−, and HLA-DR−) and by multi-lineage (osteoblast, adipocyte, and chondrocyte) differentiation potential (5). Compared to human primary benign prostate epithelial cells (PreC) and a human benign prostate epithelial cell line (RWPE-1), MSCs from all sites had significantly elevated ASPN expression (Fig. 1A). Similar to human MSCs (20), Aspn mRNA and protein were distinctly expressed in urogenital sinus mesenchyme (UGM) compared to urogenital sinus epithelium (UGE) of the developing mouse prostate (Fig. 1B, C). MSCs were isolated from both fetal and adult mouse prostate and adult mouse bone-marrow, expanded in culture, and then examined for Aspn expression. Mouse MSCs showed increased Aspn expression compared to UGE (Fig. 1B). In contrast to MSCs and fetal prostate mesenchyme, ASPN expression was markedly reduced in adult mouse benign prostate stromal cells as determined by IHC (Fig. 1C). These data suggest that ASPN is highly expressed in both bone-marrow derived and tissue resident human and mouse MSCs. However, the role of ASPN in regulating key properties of MSCs such as self-renewal, differentiation, and migration has not been fully established.
Figure 1.
ASPN is expressed in MSCs and regulates MSC self-renewal. A, Relative ASPN expression in human primary prostate epithelial cells (PrEC), a benign human prostate epithelial cell line (RWPE-1), a human prostate stromal cell line (WPMY-1), and primary human MSCs as measured by qRT-PCR. Statistical analyses performed using Welch’s t-test (n≥3). B, Relative Aspn expression in mouse fetal prostate mesenchyme (UGM), mouse fetal prostate epithelium (UGE), and mouse MSCs as measured by qRT-PCR. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥3). C, ASPN expression as measured by IHC in the urogenital sinus (UGS) and in adult mouse prostate. D, E, Aspn+/+ and Aspn−/− (D) bone marrow and (E) prostates were analyzed by flow cytometry for MSCs (CD45−, CD105+, CD29+, and Sca-1+). Statistical analyses performed using Student’s t-test (n≥7). F-H, Aspn+/+ and Aspn−/− adult bone marrow-derived MSCs were isolated and then plated at equal densities for CFU assays. Displayed are the average number of colonies formed per 2.5×103 cells plated. Statistical analyses performed using Student’s t-test (n≥6). I-K, Aspn+/+ and Aspn−/− adult prostate MSCs were isolated and then plated at equal densities for CFU assays. Statistical analyses performed using Student’s t-test (n≥3). L-N, Aspn+/+ and Aspn−/− fetal MSCs were isolated and then plated at equal densities for CFU assays. Statistical analyses performed using Student’s t-test (n≥3). Graphs shown as mean±SEM, *P≤0.05, **P≤0.01, ***P≤0.001, NS=not significant, and black bars=100μM.
ASPN Regulates MSC Self-renewal
To determine the role of ASPN in MSCs, we generated Aspn−/− mice (B6J-Aspntm1Lex/Mmucd), which were then fully backcrossed at least 9 generations to the C57BL/6J background. The first coding exon of Aspn, exon 2, was targeted for deletion by homologous recombination (46) (Supplementary Fig. S1A, B), which resulted in the loss of full-length Aspn transcript and protein (Supplementary Fig. 1C, D). Aspn−/− mice were viable, fertile, had comparable prostate size and histology, and had similar expression of other SLRP family members including Decorin (Dcn), Biglycan (Bgn), and Extracellular matrix protein 2 (Ecm2) as Aspn+/+ mice (Supplementary Fig. S1E-K).
To determine how ASPN regulates MSCs in vivo, we compared bone marrow-derived MSCs from Aspn+/+ and Aspn−/− male mice at 24 weeks of age by flow cytometry for MSC markers (CD45−, CD105+, CD29+, and Sca-1+). By these markers, both Aspn+/+ and Aspn−/− mice had a single population of bone marrow-derived MSCs; however, Aspn−/− bone marrow had significantly fewer MSCs than bone marrow from Aspn+/+ mice (Fig. 1D and Supplementary Fig. S2A, B). We next compared prostate MSCs from Aspn+/+ and Aspn−/− male mice at 24 weeks of age. In contrast to the single MSC population detected in the bone marrow, both Aspn+/+ and Aspn−/− prostates had two distinct populations of MSCs which were delineated by the level of Sca-1 expression (Sca-1hi vs. Sca-1med) (Supplementary Fig. S2C, D). Prior studies have shown that MSC stem progenitors with the capacity for both self-renewal and differentiation are Sca-1hi, while more differentiated intermediate MSC progenitors with proliferative and differentiation capacity but with reduced self-renewal ability are Sca-1med (47). Interestingly, Aspn−/− prostates were slightly enriched for Sca-1med intermediate MSCs compared to Aspn+/+ prostates (Fig. 1E, Supplementary Fig. S2C, D) suggesting that in the absence of ASPN, prostate-derived MSCs are more likely to differentiate and may thereby have a decreased potential for self-renewal.
To examine MSC self-renewal in vitro, total MSCs were isolated from Aspn+/+ and Aspn−/− bone marrow, compact bone, and prostates at 24 weeks of age and then plated at equal numbers in vitro for colony forming unit (CFU) assays. Compared to Aspn+/+, Aspn−/− bone marrow-, compact bone-, and prostate-derived MSCs formed significantly fewer CFUs (Fig. 1F-K and Supplementary Fig. S3A, B). To determine further the role of ASPN in self-renewal, fetal MSCs were isolated from Aspn+/+ and Aspn−/− mice and examined at early (P2) and late (P7) passage for CFU ability. CFU number was comparable between early passage Aspn−/− and Aspn+/+ fetal MSCs; however, late passage Aspn−/− fetal MSCs formed significantly fewer colonies compared to late passage Aspn+/+ fetal MSCs (Fig. 1L-N and Supplementary Fig. S3C, D). When added to late passage Aspn−/− fetal MSCs, recombinant mouse ASPN did not increase CFU number. Collectively, these data suggest that ASPN is necessary to sustain a sub-population of MSCs with self-renewal capacity, but exogenous ASPN is not able to rescue self-renewal once MSCs have lost this capacity.
ASPN Restricts MSC Proliferation and Differentiation
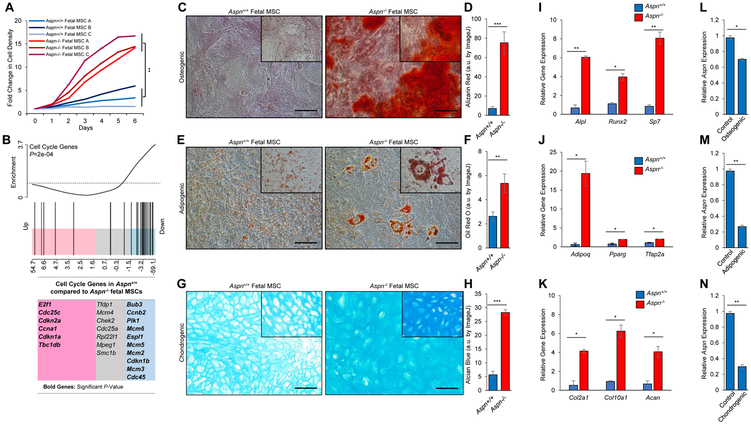
The loss of bone marrow MSCs in Aspn−/− mice suggests that ASPN may regulate MSC differentiation. Consistent with a more quiescent phenotype, Aspn+/+ fetal MSCs had a significantly decreased proliferation rate compared to Aspn−/− fetal MSCs (Fig. 2A). Microarray analyses of gene expression changes between Aspn+/+ and Aspn−/− fetal MSCs demonstrated that genes annotated to the cell cycle by KEGG were significantly downregulated in Aspn+/+ compared to Aspn−/− fetal MSCs (Fig. 2B). Consistent with a more proliferative phenotype, Aspn−/− fetal MSCs had elevated expression of several DNA synthesis regulatory genes including Cdc45, Mcm2, Mcm3, Mcm5, and Mcm6. In contrast, Aspn+/+ fetal MSCs showed increased expression of genes that restrict cell cycle progression including Cdkn2a. Interestingly, two genes with reported germline SNPs associated with aggressive prostate cancer, Cdkn1a and Cdkn1b (48), were differentially elevated between Aspn+/+ and Aspn−/− MSCs.
Figure 2.
ASPN restricts MSC proliferation and differentiation. A, Cell growth of Aspn+/+ and Aspn−/− fetal MSCs. B, Gene set enrichment analysis (GSEA) of cell cycle genes in Aspn+/+ and Aspn−/− fetal MSCs as determined from microarray data (n=3). C-H, Aspn+/+ and Aspn−/− fetal MSCs were cultured in (C, D) osteogenic, (E, F) adipogenic, and (G, H) chondrogenic differentiation-inducing media and then stained for Alizarin Red, Oil Red O, and Alcian Blue, respectively. Staining was quantified using ImageJ (n=3). I-K, Expression of (I) osteogenic, (J) adipogenic, and (K) chondrogenic differentiation-induced genes in Aspn+/+ and Aspn−/− fetal MSCs as determined by qRT-PCR (n≥3). L-N, Aspn expression in Aspn+/+ fetal MSCs cultured in (L) osteogenic, (M) adipogenic, and (N) chondrogenic differentiation-inducing media (n=3). Statistical analyses in A and C-N performed using Student’s t-test (mean±SEM; *P≤0.05, **P≤0.01, ***P≤0.001; n≥3). Black bars=100μM.
To more clearly define the role of ASPN in regulating MSC differentiation, early passage (P3) fetal MSCs from Aspn+/+ and Aspn−/− mice were cultured in osteogenic, adipogenic, and chondrogenic differentiation-inducing media and then analyzed for in vitro lineage differentiation. Differentiation to all three lineages (osteoblast, adipocyte, and chondrocyte) was significantly greater in Aspn−/− fetal MSCs compared to Aspn+/+ fetal MSCs as measured by Alizarin Red, Oil Red O, and Alcian Blue staining, respectively (Fig. 2C-H). In addition, lineage-specific differentiation markers such as Runx2, Adipoq, and Col10a1 were significantly higher in Aspn−/− fetal MSCs compared to Aspn+/+ fetal MSCs when cultured in osteogenic, adipogenic, and chondrogenic differentiation-inducing media, respectively (Fig. 2I-K). Compared to control media, Aspn expression in Aspn+/+ fetal MSCs significantly decreased in differentiation-inducing media, indicating that Aspn is downregulated during differentiation (Fig. 2L-N). Consistent with these findings, recombinant mouse ASPN decreased osteogenic differentiation of Aspn−/− fetal MSCs (Supplementary Fig. S3E, F). Interestingly, late passage (P8) Aspn−/− fetal MSCs showed increased differentiation in the absence of differentiation-inducing media (Supplementary Fig. S3G-K). Collectively, these data suggest that ASPN restricts MSC proliferation and inhibits early MSC differentiation prior to lineage specification.
ASPN Binds to BMP-4 and Restricts BMP-4-induced Signaling and Differentiation
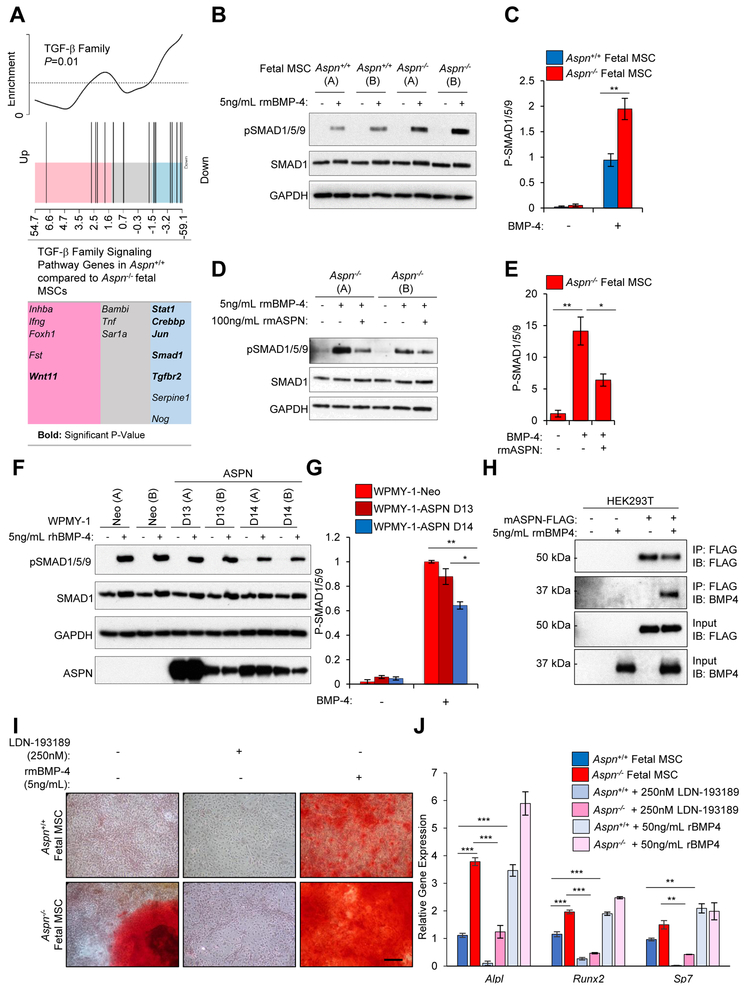
How ASPN regulates MSC differentiation is not fully known. ASPN has been reported to regulate many extracellular signaling molecules (27, 28, 49-52), including multiple TGF-β family members (26, 27, 49, 51-53). Most TGF-β family members have pleiotropic and lineage-specific roles during differentiation; however, BMP-4 distinctly induces progenitor differentiation to osteoblast, adipocyte, and chondrocyte lineages (54). Microarray analysis of gene expression changes between Aspn+/+ and Aspn−/− fetal mouse MSCs demonstrated that TGF-β family signaling pathway genes annotated in GeneMAPP, including genes restricted to BMP signaling, were significantly downregulated in Aspn+/+ compared to Aspn−/− fetal MSCs (Fig. 3A). Consistent with this, BMP-4-induced SMAD1/5/9 phosphorylation was significantly decreased in Aspn+/+ compared to Aspn−/− fetal mouse MSCs (Fig. 3B, C). Similarly, recombinant mouse ASPN also restricted BMP-4-induced signaling in Aspn−/− fetal mouse MSCs (Fig. 3D, E).
Figure 3.
ASPN binds to BMP-4 and restricts BMP-4-induced signaling. A, GSEA of TGF-β family pathway genes in Aspn+/+ and Aspn−/− fetal MSCs as determined from microarray (n=3). B, C, BMP-4-induced signaling in Aspn+/+ and Aspn−/− fetal MSCs (A and B represent independently derived MSCs) as measured by immunoblotting and quantified using ImageJ. Aspn+/+ and Aspn−/− fetal MSCs were serum-depleted and then incubated with recombinant mouse BMP-4 (5ng/mL) for 30 minutes. Statistical analyses performed using Student’s t-test (n≥3). D, E, Recombinant mouse ASPN restricts BMP-4-induced signaling in Aspn−/− fetal MSCs as measured by immunoblotting and quantified using ImageJ. Aspn+/+ and Aspn−/− fetal MSCs were serum-depleted and then incubated with recombinant mouse BMP-4 (5ng/mL) with vehicle or recombinant mouse ASPN (100ng/mL) for 30 minutes. Statistical analyses performed using Student’s t-test (n≥2). F, G, BMP-4-induced signaling in WPMY-1-Neo, WPMY-1-ASPN D13, and WPMY-1ASPN D14 (A and B represent independently derived clones) as measured by immunoblotting and quantified using ImageJ. WPMY-1-Neo, WPMY-1-ASPN D13, and WPMY-1ASPN D14 were serum-depleted and then incubated with recombinant human BMP-4 (5ng/mL) for 30 minutes. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥3). H, Co-immunoprecipitation of ASPN and BMP-4. FLAG tagged mouse ASPN was transfected in HEK293T cells and then incubated with recombinant mouse BMP-4. ASPN was immunoprecipitated with anti-FLAG beads and then examined by immunoblotting for ASPN and BMP-4 (n=2). I, Aspn+/+ and Aspn−/− fetal MSCs were cultured in osteogenic-inducing media with either vehicle, 250nM LDN-193189, or recombinant mouse BMP4 (5ng/mL) and then stained for Alizarin Red. J, Aspn+/+ and Aspn−/− fetal MSCs were cultured in osteogenic-inducing media with either vehicle, 250nM LDN-193189, or recombinant mouse BMP4 (5ng/mL) and then examined for osteogenic-induced genes by qRT-PCR. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥3). Graphs shown as mean±SEM, *P≤0.05, **P≤0.01, ***P≤0.001, and black bars=100μM.
Prior studies have shown that polymorphisms in the aspartic acid (D)-repeat length of human ASPN differentially regulate signaling (27) and that germline D-repeat variants are differentially associated with prostate oncologic outcomes (21). The germline variant of human ASPN containing 14-D repeats (ASPN D14) was shown to be significantly associated with metastatic prostate cancer recurrence after surgery while homozygosity for the ASPN D13 variant was significantly associated with a reduced risk of metastatic recurrence (21). To determine if ASPN D14 and ASPN D13 differentially regulate BMP-4 signaling in human prostate stromal cells, we generated independent clones (A and B) of the prostate fibroblast cell line, WPMY-1, overexpressing either ASPN D13 or ASPN D14. Independent clones of WPMY-1 expressing the neomycin empty vector alone (Neo) were also generated as control lines. WPMY-1, which are germline ASPN D13/13, did not express ASPN under standard conditions (Fig. 3F) (21). Similar to mouse ASPN, ASPN D14 significantly restricted BMP-4-induced signaling in WPMY-1 cells compared to WPMY-1-Neo and WPMY-1-ASPN D13 (Fig. 3F, G). BMP-4-induced signaling was slightly decreased in WPMY-1-ASPN D13 compared to WPMY-1-Neo, but was not significant (Fig. 3F, G).
To determine if ASPN directly binds to BMP-4, mouse ASPN was overexpressed in HEK293T cells and then incubated with recombinant mouse BMP-4. Immunoprecipitation of secreted ASPN from the media demonstrated binding to BMP-4 (Fig. 3H). To determine if BMP-induced signaling is necessary for differentiation in the absence of ASPN, we interrogated the ability of a BMP inhibitor to rescue differentiation in Aspn−/− MSCs. To do this, we compared osteogenic-induced differentiation in Aspn+/+ and Aspn−/− fetal MSCs in the absence of BMP-induced signaling by treating MSCs with the BMP inhibitor LDN-193189 or vehicle. Inhibition of BMP-induced signaling significantly restricted osteogenic-induced differentiation in Aspn−/− fetal MSCs as assayed by Alizarin Red staining and by expression of osteogenic-induced genes (Fig. 3I, J). To determine if BMP-4 could rescue differentiation in Aspn+/+ fetal MSCs, we compared osteogenic-induced differentiation in Aspn+/+ and Aspn−/− fetal MSCs in the presence of exogenous recombinant mouse BMP-4. BMP-4 strongly induced differentiation in Aspn+/+ fetal MSCs (Fig. 3I, J). Taken together, these findings support a model by which mouse ASPN and human ASPN D14 restrict BMP-4-induced signaling and differentiation of MSCs.
ASPN Expression Pattern is Distinctly Conserved between MSCs and Reactive Stromal Cells in the Tumor Microenvironment
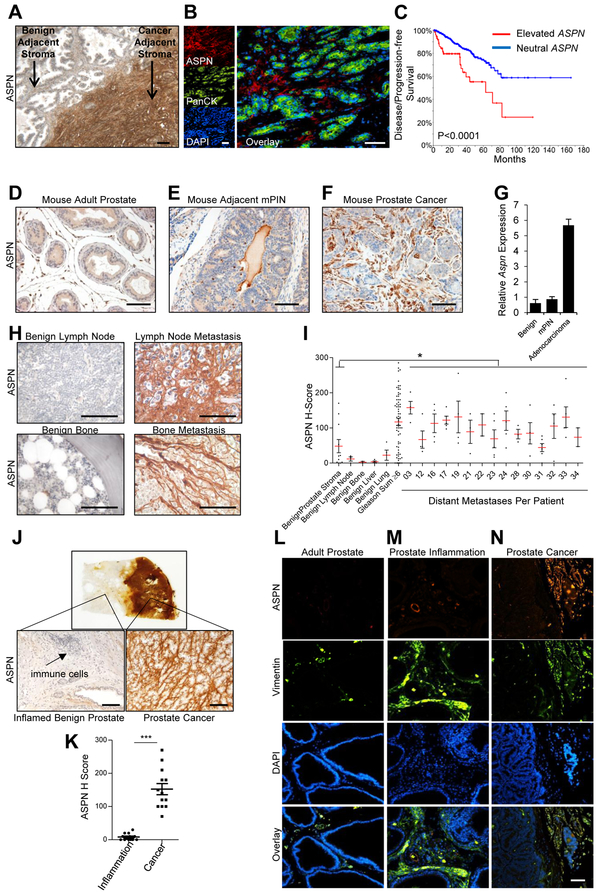
MSCs also give rise, in part, to pathological cell lineages including CAFs and injury or inflammation-associated fibroblasts (2, 3, 7, 8, 39, 47, 55, 56). We and others have previously reported that ASPN is highly expressed by reactive stromal cells in the microenvironment of primary tumors, with minimal to undetectable expression by primary adenocarcinoma cells or adenocarcinoma derived cell lines (21-25) (Fig. 4A, B, Supplementary Fig. S4A). In fact, ASPN expression in cancer cell lines negatively correlated with the epithelial marker EPCAM (Pearson r: −0.2478; P<0.0001) and positively correlated with MSC markers including THY1 (Pearson r: 0.4064; P<0.0001), ENG (Pearson r: 0.3607; P<0.0001), VCAM (Pearson r: 0.1237; P<0.0001), and ALCAM (Pearson r: 0.0952; P<0.0019) (Supplementary Fig. S4A-G) (57). Elevated ASPN in primary prostate cancer from the TCGA was significantly associated with a worse disease/progression-free survival (Fig. 4C) (58, 59). Similar to human prostate cancer, both Aspn mRNA and protein expression were elevated in the tumor microenvironment of mouse models of prostate adenocarcinoma compared to benign adjacent prostate stroma and stroma associated with mouse prostatic intraepithelial neoplasia (mPIN) (Fig. 4D-G and Supplementary Fig. S4H).
Figure 4.
ASPN is expressed in primary and metastatic prostate cancer tumor microenvironments, but not in the microenvironment of prostate inflammation. A, ASPN expression in human prostate cancer as measured by IHC. B, ASPN (red), pancytokeratin (green), and DAPI (blue) expression in human prostate cancer as measured by immunofluorescence (IF). C, Elevated ASPN (amplification, gain, expression>2) was associated with worse disease/progression-free survival in TCGA data as determined by Kaplan-Meier survival curves and visualized on cBioPortal (n=491; Logrank Test). D-F, ASPN expression in (D) mouse adult prostate, (E) murine prostatic intraepithelial neoplasia (mPIN) in the TRAMP model, and (F) mouse prostate adenocarcinoma in the TRAMP model as measured by IHC and (G) qRT-PCR. H, I, Mean ASPN H-score in prostate cancer metastases from the PELICAN rapid autopsy study of prostate cancer (n=15 patients with an average of 4 metastases per patient), benign adjacent prostate stroma (n=11), benign bone (n=2), benign lymph node (n=3), benign lung (n=4), and benign liver (n=4) as measured by IHC. Historical controls of stroma adjacent to benign prostate and stroma adjacent to Gleason grade ≥6 prostate cancer (G≥6) were measured and calculated using the same methodology and included for comparison. Statistical analyses performed using one-way ANOVA with Newman-Keuls multiple comparison. J, ASPN expression in human prostate inflammation and human prostate cancer as measured by IHC on radical prostatectomy sections that contained regions of both chronic inflammation and prostate cancer in non-overlapping locations. K, Quantification of ASPN expression by IHC (H-score) in stroma adjacent to inflammation and stroma adjacent to cancer on single sections from radical prostatectomy sections. Statistical analyses performed using Student’s t-test (n=13 patients). L-N, ASPN (red), Vimentin (green), and DAPI (blue) expression in mouse adult prostate (L), CP1 E. coli-induced mouse prostate inflammation (M), and mouse prostate adenocarcinoma in the TRAMP model (N) as measured by IF. Graphs shown as mean±SEM, *P≤0.05, **P≤0.01, ***P≤0.001, and black and white bars=100μM.
To determine if ASPN is also expressed in the metastatic tumor microenvironment, prostate cancer metastases from a Johns Hopkins School of Medicine rapid autopsy cohort (n=15 patients; 60 metastases) (37) were analyzed for ASPN expression by immunohistochemistry (IHC) and quantified by H-score. Elevated ASPN expression was detected in reactive stromal cells at various metastatic sites including lymph node, bone, and soft tissue such as lung and liver (Fig. 4H and Supplementary Fig. S4I). ASPN expression in metastatic prostate cancer adjacent stroma was elevated compared to benign prostate, lymph node, lung, and liver stroma, and it was comparable to ASPN expression in primary prostate cancer (Gleason Sum ≥6) adjacent stroma (Fig. 4I). Thus, we demonstrate that ASPN is highly expressed in MSCs isolated from multiple sites and in reactive stromal cells associated with both primary and metastatic prostate cancers.
To determine if ASPN expression is selective to reactive stroma in the tumor microenvironment or is generally conserved among reactive stroma, we next examined ASPN expression in human and mouse reactive stroma associated with prostate inflammation. Human prostate sections obtained after radical prostatectomy for prostate cancer that contained regions of both benign chronic inflammation and prostate cancer, but in distinct locations (≥2mm apart), were analyzed for ASPN expression by IHC. Compared to reactive stroma associated with human prostate cancer, stroma associated with distinct areas of chronic prostate inflammation expressed significantly lower levels of ASPN that were comparable to benign adjacent stroma (Fig. 4J, K). Similar to human prostate inflammation, CP1 E. coli-induced prostate inflammation, which is characterized by a marked vimentin-positive stromal response (38), was largely negative for ASPN expression (Fig. 4L-N). ASPN expression in inflammation adjacent stroma was comparable to benign prostate stroma and was only detected in perivascular regions. Collectively, these data suggest that ASPN expression is selectively conserved between MSCs and cancer-associated reactive stromal cells.
ASPN Induces MSC and Cancer Cell Migration
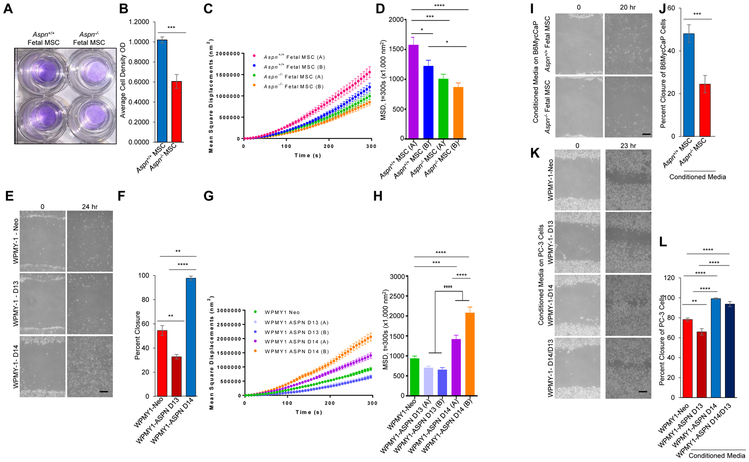
A fundamental property of MSCs is their ability to migrate to both local and distant areas of tissue injury or damage. Migration was significantly elevated in Aspn+/+ mouse fetal MSCs compared to Aspn−/− mouse fetal MSCs (Fig. 5A, B). Consistent with elevated motility, Aspn+/+ fetal MSCs cells had increased cytoskeletal remodeling compared to Aspn−/− MSCs as assayed by spontaneous motions of Arg-Gly-Asp (RGD) coated microbeads that bind to the cytoskeletal network through cell surface integrin receptors (Fig. 5C, D). Similar dynamics of increased migration and cytoskeletal remodeling were also observed in WPMY-1-ASPN D14 overexpressing cells compared to WPMY-1-ASPN D13 overexpressing cells and WPMY-1-Neo control cells (Fig. 5E-H).
Figure 5.
ASPN enhances MSC and cancer cell migration. A, B, Migration of Aspn+/+ and Aspn−/− fetal MSCs across a membrane. Statistical analyses performed using Student’s t-test (n=3). C, D, Cytoskeletal remodeling as measured by (C) mean square displacement and (D) analyzed at t=300s nm2 in Aspn+/+ and Aspn−/− fetal MSCs. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥3). E, F, Migration of WPMY-1-Neo, WPMY-1-ASPN D13, and WPMY-1-ASPN D14 as determined by scratch assay. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥9). G, H, Cytoskeletal remodeling as measured by (G) mean square displacement and (H) analyzed at t=300 nm2 in WPMY-1-Neo, WPMY-1-ASPN D13, and WPMY-1-ASPN D14 (n=2 independent clones per experimental group). Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥3). I, J, Migration of B6MycCaP cells in conditioned media from Aspn+/+ and Aspn−/− fetal MSCs as determined by scratch assay. Statistical analyses performed using Student’s t-test (n=3). K, L, Migration of PC-3 cells in conditioned media from WPMY-1-Neo, WPMY-1-ASPN D13, WPMY-1-ASPN D14, and a 1:1 mix from WPMY-1-ASPN D13:WPMY-1-ASPN D14 cells. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison (n≥9). Graphs shown as mean±SEM, *P≤0.05, **P≤0.01, ***P≤0.001, and black bars=100μM.
As a secreted protein in the tumor microenvironment, ASPN may also induce cancer cell migration. We examined cancer cell migration in response to conditioned media from Aspn+/+ and Aspn−/− fetal MSCs. Conditioned media from Aspn+/+ fetal MSCs increased B6MycCaP (60) and TRAMP-C2 mouse prostate cancer cell migration compared to Aspn−/− fetal MSC conditioned media (Fig. 5I, J and Supplementary Fig. S5A, B). Conditioned media from WPMY-1-ASPN D14 cells also increased PC-3 and DU-145 migration compared to conditioned media from WPMY-1-ASPN D13 cells and conditioned media from WPMY-1- Neo cells (Fig. 5K, L and Supplementary Fig. S5C, D). Interestingly, a 1:1 mix of conditioned media from WPMY-1-ASPN D14 and WPMY-1-ASPN D13 cells also increased PC-3 migration similar to conditioned media from WPMY-1-ASPN D14 alone, thereby suggesting a dominant function of ASPN D14 relative to ASPN D13 (Fig. 5K, L).
ASPN-Induced Migration is Calcium-Dependent
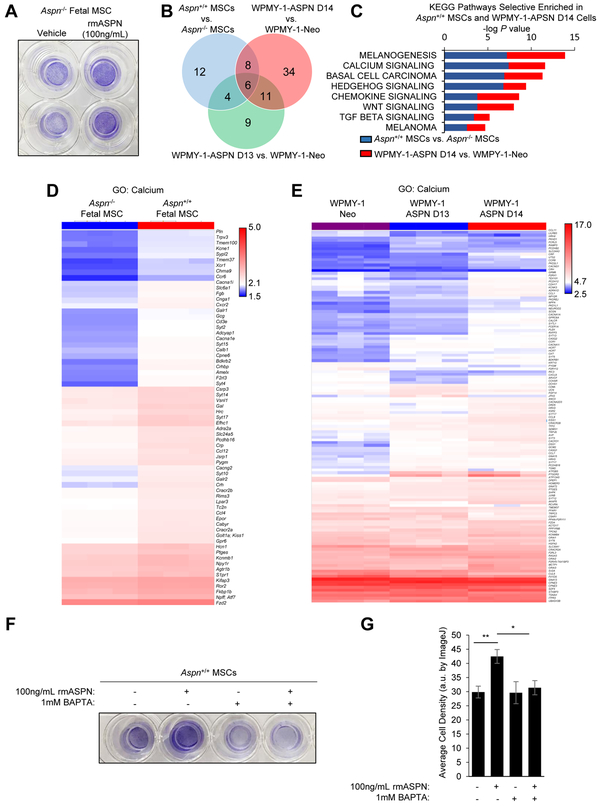
How ASPN regulates prostate stromal and cancer cell migration is not fully understood. Migration in Aspn−/− fetal MSCs was neither rescued by the BMP inhibitor LDN-193189 nor further restricted by BMP-4, suggesting that ASPN regulates migration by an alternative mechanism (Supplementary Fig. S5E). To determine if ASPN is able to directly enhance migration as opposed to functioning indirectly through the expression of other secreted factors, migration was examined in Aspn−/− cells in the presence of exogenous recombinant mouse ASPN. Elevated migration was observed in Aspn−/− cells when cultured with exogenous ASPN (Fig. 6A), suggesting that ASPN can, at least in part, directly enhance migration. To better determine how ASPN regulates migration, Aspn+/+ MSCs, Aspn−/− MSCs, WPMY-1-ASPN D14, WPMY-1-ASPN D13, and WPMY-1-ASPN Neo were compared by microarray for gene expression. Differentially expressed genes in Aspn+/+ MSCs compared to Aspn−/− MSCs as well as in WPMY-1-ASPN D14 compared to WPMY-1-ASPN Neo and in WPMY-1-ASPN D13 compared to WPMY-1-ASPN Neo were examined for KEGG Pathway enrichment. Genes annotated to cell adhesion, actin cytoskeleton, and cytokine-cytokine receptor interaction by KEGG were altered in cells expressing ASPN (Aspn+/+ MSCs, WPMY-1-ASPN D14, WPMY-1-ASPN D13) compared to cells deficient for ASPN (Aspn−/− MSCs and WPMY-1-ASPN Neo) (Supplementary Fig. S5F). Since mouse ASPN and human ASPN D14 enhanced migration compared to ASPN null and ASPN D13 cells, KEGG pathways selective to Aspn+/+ MSCs and WPMY-1-ASPN D14 were highlighted as a means to determine pathways potentially involved in ASPN-mediated migration. Interestingly, genes annotated to calcium signaling, Hedgehog signaling, chemokine signaling, WNT signaling, and TGFβ signaling by KEGG were altered in Aspn+/+ MSCs and WPMY-1-ASPN D14 when compared to cells deficient for ASPN (Fig. 6B, C). ASPN has been reported to bind calcium, likely through its poly-aspartate domain (61). Consistent with this, hierarchical clustering showed differential expression of several Gene Ontology (GO) calcium-related genes between Aspn+/+ and Aspn−/− fetal MSCs (Fig. 6D). Similarly, differential expression of calcium-related genes was also observed in WPMY-1-ASPN D14 compared to WPMY-1-ASPN D13 and WPMY-1-Neo (Fig. 6E). To determine if ASPN-mediated migration depends on calcium, Aspn−/− MSCs were examined for migration in the presence of exogenous recombinant mouse ASPN and an extracellular calcium chelator, BAPTA. BAPTA restricted ASPN-induced migration suggesting that extracellular calcium is necessary for ASPN’s pro-migratory function (Fig. 6F, G).
Figure 6.
ASPN-mediated migration is calcium-dependent. A, Migration of Aspn−/− cells in low calcium media with vehicle or 100ng/mL recombinant mouse ASPN as determined by transwell assay. B, Venn Diagram of altered KEGG Pathways between differentially expressed genes in Aspn+/+ MSCs compared to Aspn−/− MSCs as well as in WPMY-1-ASPN D14 compared to WPMY-1-ASPN Neo and in WPMY-1-ASPN D13 compared to WPMY-1-ASPN Neo. C, KEGG Pathway enrichment selective to Aspn+/+ MSCs and WPMY-1-ASPN D14 cells compared to cells deficient for ASPN. D, Hierarchical clustering of GO calcium-related genes in Aspn+/+ and Aspn−/− fetal MSCs. E, Hierarchical clustering of GO calcium-related genes in WPMY-1-ASPN D14, WPMY-1-ASPN Neo, and WPMY-1-ASPN D13 cells. F,G, Migration of Aspn−/− cells in low calcium media with vehicle, 100ng/mL recombinant mouse ASPN, 1mM BAPTA, or ASPN and BAPTA as determined by transwell assay. Quantification by ImageJ. Statistical analyses performed using one-way ANOVA with Newman-Keuls multiple comparison (mean±SEM; * P≤0.05, **P≤0.01; n≥3).
ASPN Alters the Primary Tumor Microenvironment
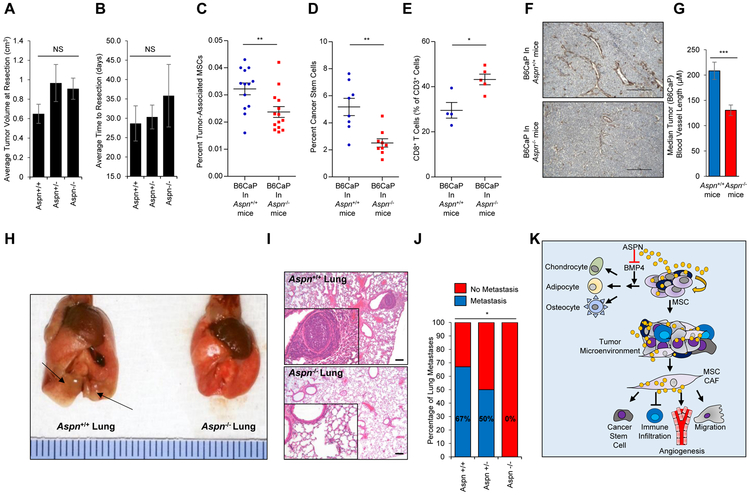
To determine the role of ASPN in the tumor microenvironment, we utilized a novel allograft generated from a C57BL/6J Hi-Myc mouse metastatic lesion (B6CaP). This allograft is not to be confused with the B6MycCaP cell line used for migration studies which was generated on a C57BL/6N background (60). B6CaP allografts consisted of both cancer and tumor infiltrating host cells including reactive stromal cells. Due to this stromal infiltration, B6CaP allografts highly expressed Aspn (Supplementary Fig. S6A). B6CaP organoids, which were enriched for cancer cells, had undetectable Aspn expression further establishing that Aspn expression was restricted to infiltrating stromal cells (Supplementary Fig. S6A). Subcutaneous growth of B6CaP allografts was similar in Aspn+/+, Aspn+/−, and Aspn−/− mice as average tumor volume at resection and average time to resection were not significantly different between mice (Fig. 7A, B). Despite comparable Aspn expression at tumor inoculation, B6CaP allografts in Aspn−/− mice had significantly reduced levels of Aspn at resection (Supplementary Fig. S6B).
Figure 7.
ASPN regulates the tumor microenvironment and promotes metastatic development. A, B, Tumor volume (A) and time to resection (B) of B6CaP subcutaneous allografts in Aspn+/+ (n=6), Aspn+/− (n=6), and Aspn−/− (n=6) mice. Statistical analyses performed using one-way ANOVA with Tukey multiple comparison. C, Percent tumor-associated MSCs (CD45−, CD105+, CD29+, and Sca-1+) in B6CaP subcutaneous allografts from Aspn+/+ and Aspn−/− mice as measured by flow cytometry. Statistical analyses performed using Student’s t-test (n≥13). D, Percent cancer stem cells (CD45−, CD105−, CD31−, CD29+, Sca-1+, CD44+) in B6CaP subcutaneous allografts from Aspn+/+ and Aspn−/− mice as measured by flow cytometry. Statistical analyses performed using Student’s t-test (n≥8). E, CD8+ T cells as a percent of CD3+ cells in B6CaP subcutaneous allografts from Aspn+/+ and Aspn−/− mice as measured by flow cytometry. Statistical analyses performed using Student’s t-test (n≥4). F, SMAα positive vasculature detected by IHC of B6CaP subcutaneous allografts from Aspn+/+ and Aspn−/− mice. G, Quantification of vasculature length in B6CaP subcutaneous allografts from Aspn+/+ and Aspn−/− mice. Statistical analyses performed using Student’s t-test (n≥4). H, I, Photograph (H) and H&E (I) of lungs from Aspn+/+ and Aspn−/− mice with B6CaP allograft. J, Percentage of Aspn+/+ (4/6), Aspn+/− (3/6), and Aspn−/− (0/6) mice with lung metastases from B6CaP subcutaneous allografts as determined by H&E. Statistical analyses performed using Chi-squared test. K, Schematic of the role of ASPN in MSCs and metastasis. Graphs shown as mean±SEM, *P≤0.05, **P≤0.01, ***P≤0.001, NS=not significant, and black bars=100μM.
B6CaP allografts were examined for MSCs and CAF-mediated properties including cancer stem cells, immune infiltration, and vascular development. Consistent with our in vivo and in vitro findings pertaining to the roles of ASPN in MSC self-renewal, differentiation, and migration, flow cytometry demonstrated that B6CaP tumors in Aspn−/− mice had significantly fewer MSCs (CD45−, CD105+, CD29+, and Sca-1+) compared to B6CaP tumors in Aspn+/+ mice (Fig. 7C). B6CaP tumors in Aspn−/− mice also had significantly fewer CD44+ cancer stem cells (CD45−, CD105−, CD31−, CD29+, Sca-1+, CD44+) compared to B6CaP tumors in Aspn+/+ mice (Fig. 7D). Flow cytometry for myeloid and lymphoid lineage cells demonstrated that Aspn−/− tumors had an increased percentage of tumor infiltrating CD8+ T cells compared to Aspn+/+ cells (Fig. 7E and Supplementary Fig. S6C, D). IHC for tumor associated vasculature showed that Aspn+/+ tumors were enriched for larger blood vessels compared to Aspn−/− tumors (Fig. 7F, G). These findings suggest that ASPN pleiotropically alters both the tumor and the tumor microenvironment.
ASPN Promotes Metastatic Progression
MSCs, cancer stem cells, angiogenesis, and immune infiltration have all been shown to regulate metastatic development. Prior overexpression studies indicate that ASPN (62) and specifically ASPN D14 (21) promotes invasion and metastasis; however, this has not been determined in a genetic, immune-competent mouse model. Thus, we examined metastatic progression in Aspn+/+ and Aspn−/− mice with B6CaP allografts. When grown subcutaneously and then resected, B6CaP allografts form lung metastases in 60-70% of C57BL/6J mice. While primary tumor size was similar between Aspn+/+ and Aspn−/− mice, the development of lung metastases was strikingly different. B6CaP subcutaneous allografts formed lung metastases in 67% (4/6) of Aspn+/+ mice and in 50% (3/6) of Aspn+/− mice, but not in any of the Aspn−/− (0/6) mice (Fig. 7H-J). Collectively, these findings indicate that ASPN in the tumor microenvironment broadly impacts multiple cell types, and ultimately influences metastatic progression (Fig. 7K).
Discussion
MSCs and CAFs reside in the tumor microenvironment and impact cancer growth and progression (4, 5, 9-13). These studies establish ASPN as a novel MSC factor expressed in the tumor microenvironment that regulates metastatic development. ASPN restricts early MSC differentiation, shown here by inhibiting BMP-4 signaling. While ASPN expression is reduced during differentiation to most MSC lineages, ASPN is highly expressed in CAFs and broadly impacts multiple cell types in the tumor microenvironment. Collectively, these findings underpin the role of ASPN as a key mediator of MSC multipotency and as a critical regulator of metastasis in the tumor microenvironment.
MSCs are a heterogeneous population of cells that include cells of mesenchymal origin including mesenchymal stem cells, mesenchymal stromal cells, and fibroblasts. We demonstrate that ASPN is expressed in MSCs isolated from distinct sites including bone-marrow, adult prostate, and fetal prostate in humans and mice. MSCs give rise to physiologic connective tissue lineages, and differentiation is regulated by both conserved and lineage-specific factors (6). ASPN expression is diminished during MSC differentiation to multiple lineages, and our findings indicate that ASPN functions prior to MSC lineage commitment to suppress the differentiation of a stem population of multipotent MSCs capable of self-renewal. In the absence of ASPN, fewer MSCs reside in the bone marrow and an intermediate population of multipotent MSCs with diminished self-renewal potential predominates in the prostate. This is evidenced by our flow cytometry data demonstrating that in contrast to Aspn+/+ MSCs in the prostate, MSCs from Aspn−/− prostates were enriched for intermediate MSCs (Sca-1med) as opposed to stem MSCs (Sca-1hi). Consistent with these in vivo findings, Aspn−/− bone marrow-derived, compact bone-derived, prostate-derived, and late passage fetal MSCs had a decreased capacity for self-renewal in vitro compared to Aspn+/+ MSCs. Studies using ASPN deficient cells demonstrated that ASPN restricts MSC differentiation in vitro along multiple lineages. Taken together, these data indicate that ASPN functions as a novel MSC factor that maintains MSC multipotency.
MSC lineage commitment and progression through differentiation is regulated by many TGF-β family members (54). ASPN is a secreted extracellular protein that has been shown to antagonize several members of the TGF-β family including BMP-2 (49), BMP-4 (51), and TGF-β1 (27). Most TGF-β family members have pleiotropic roles in regulating MSC differentiation to connective tissue lineages (54). In contrast to other TGF-β family members, BMP-4 distinctly induces early MSC differentiation along several lineages including osteoblasts, adipocytes, and chondrocytes (54). Our data demonstrate that ASPN binds to BMP-4 and restricts BMP-4-induced signaling in MSCs and support a model by which MSC-secreted ASPN locally restricts BMP-4-induced MSC differentiation.
Similar to MSCs, reactive stroma cells in multiple cancer types, including prostate (20-22), breast (23), scirrhous gastric (24), and pancreatic (25), also highly expresses ASPN while benign-associated stroma is largely negative for ASPN expression. In addition to localized cancer, we show that ASPN is widely expressed in prostate cancer metastases. In contrast to the tumor microenvironment, ASPN was not expressed in reactive stroma in the inflammatory microenvironment. This suggests that ASPN expression is not conserved across all types of reactive stroma, but has some specificity to reactive stroma in the tumor microenvironment. Tumor-associated reactive stroma is a heterogeneous population of cells that includes MSCs and CAFs. Studies support that CAFs are in part derived from MSC precursors (47, 55, 56). Accordingly, CAFs may be more similar to MSCs than inflammation-associated reactive stromal cells based on the data presented herein. Collectively, these findings indicate that ASPN expression is highly conserved between MSCs and the reactive stroma in both primary and metastatic tumor microenvironments.
These studies indicate that ASPN broadly impacts both the tumor and the tumor microenvironment. In the absence of ASPN, B6CaP allografts had fewer tumor-associated MSCs supporting that ASPN regulates the number of multipotent MSCs in the tumor microenvironment. In addition, the immune infiltration and vasculature was significantly altered in Aspn−/− mice. Effects on other cell types in the tumor microenvironment and on cancer stem cells may be directly mediated by ASPN or indirectly regulated through MSC deficiencies. In addition to impacting the tumor microenvironment, ASPN may promote tumor progression by directly enhancing migration of both stromal and cancer cells. ASPN has been implicated in the activation of multiple migratory pathways including FGF-2 (50), IGFR (51), RAC1 (24), CD44 (24, 25), and EGFR (28, 62). Our data support that exogenous ASPN is able to enhance migration, likely, by a mechanism independent of its ability to restrict BMP4-induced signaling. Our findings support that ASPN mediated migration is dependent on extracellular calcium and that genes annotated to the KEGG calcium signaling pathway are differentially expressed in ASPN expressing cells compared to ASPN null and ASPN D13 cells. Future studies will be needed to determine how ASPN mechanistically regulates MSC and prostate cancer cell migration.
Mouse and human ASPN are highly homologous, including the D-repeat domain. While C57BL/6 mice have 8 D-repeats interrupted by a single asparagine (N), the D-repeat length in humans is variable. Recent reports suggest that the D-repeat domain length may regulate cancer progression (21, 29). Patients harboring germline allele(s) of ASPN D14 were more likely to develop metastatic prostate cancer, while homozygous germline ASPN D13 was associated with a reduced risk of metastatic prostate cancer (21). Animal models have shown that ASPN D14 promotes metastatic prostate cancer progression (21), while ASPN D13 restricts breast (63) and prostate (21) cancer progression. Our data show that ASPN D14 increased stromal and cancer cell migration compared to ASPN D13. Intriguingly, prostate cancer patient-based data suggest that ASPN D14 may be dominant over ASPN D13. Consistent with this, mixed conditioned media from ASPN D13 and ASPN D14 expressing cells increased cancer cell migration similar to conditioned media from ASPN D14 expressing cells alone while conditioned media from ASPN D13 did not increase cancer cell migration. Collectively, our findings reveal that ASPN D14 and ASPN D13 may differentially regulate molecular and cellular pathways that are fundamental to metastasis.
In conclusion, our data identifies ASPN as a novel MSC factor that is conserved in the tumor microenvironment and promotes metastatic progression. Further studies are warranted to determine the efficacy and feasibility of therapeutically targeting ASPN in prostate cancer, and potentially other solid tumor types.
Supplementary Material
Key Resources
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Rabbit Anti-ASPN antibody | Sigma Prestige | Cat# HPA008435 RRID:AB_1845112 |
| Mouse Anti-Vimentin antibody | Sigma | Cat# V2258 RRID:AB_261856 |
| Rabbit anti-phospho SMAD1/5/9 antibody | Cell Signaling Technology | Cat# 13820 RRID:AB_2493181 |
| Rabbit anti-SMAD1 antibody | Cell Signaling Technology | Cat# 9743 RRID:AB_2107780 |
| Mouse anti-GAPDH antibody | Santa Cruz Biotechnology | Cat# sc-32233 RRID:AB_627679 |
| Mouse anti-CK8 antibody | Covance Research Products Inc | Cat# MMS-162P-250 RRID:AB_291334 |
| Rabbit anti-CK14 antibody | Covance Research Products Inc | Cat# PRB-155P RRID:AB_292096 |
| Rabbit anti-Ki67 antibody | Abcam | Cat# ab15580 RRID:AB_443209 |
| Mouse anti-FLAG | Sigma | Cat# F1804 |
| Rabbit anti-BMP4 | R&D | Cat# MAB5020 |
| Mouse anti-SMA | Dako | Cat# M085129 |
| APC/Cy7 Rat anti-mouse CD45 antibody | BioLegend | Cat# 103115 RRID:AB_312980 |
| APC Rat anti-mouse CD105 antibody | BioLegend | Cat# 120413 RRID:AB_2277915 |
| PE/Cy7 Armenian Hamster anti-mouse/rat CD29 antibody | BioLegend | Cat# 102221 RRID:AB_528789 |
| FITC Rat anti-mouse Ly-6A/E (Sca-1) antibody | BioLegend | Cat# 108105 RRID:AB_313342 |
| Ly6G | BioLegend | Clone 1A8 |
| Ly6C | BioLegend | Clone HK1.4 |
| CD11b | BioLegend | Clone M1/70 |
| CD11c | BioLegend | Clone N418 |
| CCR2 | BioLegend | Clone SA203G11 |
| F4/80 | BioLegend | Clone BM8 |
| CD3 | BD Pharmingen | Clone 145-2C11 |
| CD4 | BioLegend | Clone GK1.5 |
| CD8a | BioLegend | Clone 53-6.7 |
| FoxP3 | BioLegend | Clone 150D |
| CD44 | BioLegend | Clone IM7 |
| ImmPRESS HRP anti-mouse IgG antibody, made in Horse | Vector Laboratories | Cat# MP-7402 RRID:AB_2336528 |
| ImmPRESS HRP anti-rabbit IgG antibody, made in Horse | Vector Laboratories | Cat# MP-7401 RRID:AB_2336529 |
| F(ab)2-Goat anti-Mouse IgG (H+L) Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | Thermo Fisher Scientific | Cat# A-11017 RRID:AB_2534084 |
| Donkey anti-Rabbit IgG (H+L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 594 | Thermo Fisher Scientific | Cat# A-21207 RRID:AB_141637 |
| IgG agarose | Sigma | Cat# A0919 |
| anti-FLAG M2 magnetic beads | Sigma | Cat# M8823 |
| 3x FLAG peptide | Sigma | Cat# F4799 |
| Bacterial and Virus Strains | ||
| CP1 E. coli strain | Brian W. Simons (Simons et al., 2015) | |
| Biological Samples | ||
| Human Primary Prostate Cancer | Mayo Clinic Progression cohort (Hurley et al., 2016) | |
| Metastatic Prostate Cancer TMA | PELICAN rapid autopsy study of prostate cancer | |
| Matched prostate inflammation & cancer tissue | Johns Hopkins School of Medicine | |
| Chemicals, Peptides, and Recombinant Proteins | ||
| RGD-coated microbeads | Steven S. An (An et al., 2004) | |
| FuGENE HD Transfection Reagent | Promega | Cat# E2311 |
| LIVE/DEAD Fixable Aqua Dead Cell Stain kit (405nm excitation) | Thermo Fisher Scientific | Cat# L34957 |
| MesenCult Osteogenic Stimulatory Kit (Mouse) | STEMCELL Technologies | Cat# 05504 |
| MesenCult Adipogenic Stimulatory Supplement (Mouse) | STEMCELL Technologies | Cat# 05503 |
| MesenCult ACF Chondrogenic Differentiation Medium | STEMCELL Technologies | Cat# 05455 |
| Alizarin Red Staining solution | Millipore | Cat# TMS-008-C |
| Oil Red O solution | Sigma | Cat# O1391 |
| Alcian-Blue Staining solution | Millipore | Cat# TMS-010-C |
| Recombinant Human BMP4 | Peprotech | Cat# 120-05 |
| Recombinant Mouse BPM4 | R & D | Cat#5020-BP |
| Recombinant Mouse ASPN generated in 293T cells | Origene | Custom |
| Matrigel, Growth Factor Reduced (GFR) | Corning | Cat# 356230 |
| Recombinant Mouse EGF | BioLegend | Cat# 713108 |
| Y-27632 (ROCK inhibitor) | STEMCELL Technologies | Cat# 72302 |
| 5α-Androstan-17β-ol-3-one (DHT) | Sigma | Cat# A8380 |
| A83-01 (TGFb inhibitor) | Tocris | Cat# 2939 |
| Advanced DMEM/F12 | Thermo Fisher Scientific | Cat# 12634-010 |
| 50X B-27 Supplement | Life Technologies | Cat# 17504-044 |
| GlutaMAX | Life Technologies | Cat# 35050-079 |
| HEPES | Life Technologies | Cat# 15630-080 |
| Penicillin/Streptomycin (10,000 U/mL) | Life Technologies | Cat# 15140-122 |
| 10X Collagenase/Hyaluronidase | STEMCELL Technologies | Cat# 07912 |
| Dispase (5 U/mL) | STEMCELL Technologies | Cat# 07913 |
| 10X DNAse I | STEMCELL Technologies | Cat# 07900 |
| Thiazolyl Blue Tetrazolium Bromide | Sigma | Cat# M2128 |
| Target Retrieval Solution, Ready to Use | Dako | Cat# S1700 |
| Protein Block Serum-Free | Dako | Cat# X0909 |
| Antibody Diluent Reagent Solution, Ready-to-use | Invitrogen | Cat# 003218 |
| ImmPACT DAB (3,3'-Diaminobenzidine) Peroxidase Substrate | Vector Laboratories | Cat# SK-4105 |
| VECTASHIELD HardSet Antifade Mounting Medium with DAPI counterstain | Vector Laboratories | Cat# H-1500 |
| FcR Blocking Reagent, mouse | Miltenyi | Cat# 130-092-575 |
| TaqMan Fast Advanced Master Mix | Thermo Fisher Scientific | Cat# 4444557 |
| SimplyBlue SafeStain | Invitrogen | Cat# LC6060 |
| Critical Commercial Assays | ||
| Pierce BCA Protein Assay Kit, Reducing Agent Compatible | Thermo Fisher Scientific | Cat# 23250 |
| SuperSignal West Pico PLUS Chemiluminescent Substrate | Thermo Fisher Scientific | Cat# 34580 |
| QCM 24-well Colorimetric Cell Migration Assay | Millipore | Cat# ECM508 |
| RNeasy Plus Mini Kit | Qiagen | Cat# 74134 |
| Ready-To-Go You-Prime-First-Strand Beads | GE Healthcare | Cat# 27-9264-01 |
| Amicon Ultra-4 10K Centrifugal Filter Device | Millipore | Cat# UFC801008 |
| EasySep Mouse Mesenchymal Stem/Progenitor Cell Enrichment Kit | STEMCELL Technologies | Cat# 19771 |
| MesenCult Expansion Kit (Mouse) | STEMCELL Technologies | Cat# 05513 |
| Deposited Data | ||
| SuperSeries (GSE109646) | This study | GEO database |
| Experimental Models: Cell Lines | ||
| Human: WPMY-1 | ATCC | |
| Human: WPMY-1-ASPN variants | Paula J. Hurley (Hurley et al., 2016) | |
| Human: MSCs (various sources) | W. Nathaniel Brennen (Brennen et al., 2017) | |
| Human: PC-3 | ATCC | |
| Human: DU-145 | ATCC | |
| Mouse: MSCs (various sources) | this study | |
| Mouse: B6MycCaP | Leigh Ellis (Ellis et al., 2016) | |
| Mouse: TRAMP-C2 | ATCC | |
| Mouse: B6CaP organoids | this study | |
| Experimental Models: Organisms/Strains | ||
| C57BL/6J mice | Jackson Labs | |
| B6;129S5-Aspn tm1Lex/Mmucd mice | Mutant Mouse Regional Resource Center (UC Davis) | |
| C57BL/6-Tg(TRAMP)8247Ng/J mice | Jackson Labs | |
| Oligonucleotides | ||
| murine Aspn wild-type specific forward primer | 5’ AGTCTACTTTGCCACATTCACC 3’ | |
| murine Aspn wild-type specific reverse primer | 5’ GCTTTTGGCTGTGTGCTCTGC 3’ | |
| murine Aspn mutation specific forward primer | 5’CGAGAGTAACATTGGCACCCAAATG 3 | |
| murine Aspn mutation specific reverse primer | 5’ GCAGCGCATCGCCTTCTATC 3’ | |
| Random hexamer primers | Promega | Cat# C118A |
| Human ASPN TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Hs01550903_m1 |
| Human HPRT1 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Hs02800695_m1 |
| Human GAPDH TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Hs02758991_g1 |
| Mouse Aspn TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00445939_m1 |
| Mouse Alpl TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00475834_m1 |
| Mouse Runx2 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00501584_m1 |
| Mouse Sp7 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm04209856_m1 |
| Mouse Adipoq TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00456425_m1 |
| Mouse Pparg TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00440940_m1 |
| Mouse Tfap2a TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00495574_m1 |
| Mouse Col2a1 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm01309565_m1 |
| Mouse Col10a1 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00487041_m1 |
| Mouse Acan TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00545794_m1 |
| Mouse Dcn TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm00514535_m1 |
| mouse Bgn TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm01191753_m1 |
| mouse Ecm2 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm01349471_m1 |
| mouse Hprt1 TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm03024075_m1 |
| mouse Gapdh TaqMan qRT-PCR Gene Expression assay | Thermo Fisher Scientific | ID# Mm99999915_g1 |
| Recombinant DNA | ||
| pIRESneo3 vector | Clontech | Cat# 631621 |
| ASPN (NM_017680) Human cDNA clone | OriGene | Cat# SC321136 |
| Software and Algorithms | ||
| ImageJ | NIH | https://fiji.sc |
| PRISM | Graphpad | https://ww.graphpad.com |
| RStudio | RStudio Team | http://www.rstudio.com |
| FlowJo | FLOWJO LLC | FlowJo |
| NIS Elements (vAR 3.0) | Nikon | https://www.nikoninstruments.com |
| StepOne Software (v2.3) | Applied Biosystems (Thermo Fisher Scientific) | https://www.thermofisher.com |
| Transcriptome Analysis Console (TAC, v4.0) | Thermo Fisher Scientific | https://www.thermofisher.com |
Significance.
Findings show that asporin regulates key properties of mesenchymal stromal cells including self-renewal and multipotency, and asporin expression by reactive stromal cells alters the tumor microenvironment and promotes metastatic progression.
Acknowledgments
We thank Edward M. Schaeffer, Ashley E. Ross, Daniel Ardeljan, Leigh Ellis, Daniele M. Gilkes, Inês Godet, Timothy E. Krueger, and Karen Cravero for thoughtful discussions and for sharing resources and experimental protocols. We thank the Johns Hopkins Sidney Kimmel Comprehensive Cancer Center Flow Cytometry and Cell Imaging Core Facilities supported by the Cancer Center Core Grant P30 CA006973. We also thank the Prostate Cancer Biorepository Network (PCBN), supported by the Department of Defense Prostate Cancer Research Program, DOD Award No W81XWH-10-2-0056 and W81XWH-10-2-0046. We acknowledge the use of tissues procured by the National Disease Research Interchange (NDRI) with support from NIH grant 2 U42 OD011158.
Financial Support
This work was supported by Department of Defense Prostate Cancer Research Program W81XWH-11-PCRP-IDA (PJH), The Patrick C. Walsh Prostate Cancer Fund (PJH), The Willowcroft Foundation (PJH), The Johns Hopkins Catalyst Award (PJH), The American Cancer Society 131356-RSG-17-160-01-CSM (PJH), The National Cancer Institute/National Institute of Health RO1CA211695-01A1 (PJH; BWS; TL; WNB; RMH), R01CA194024 (BHP), and R01CA201035 (DLJT), and the Commonwealth Foundation (BHP). WNB acknowledges support from the Prostate Cancer Foundation as the Clay and Lynn Hamlin Young Investigator. BHP acknowledges support from Susan G. Komen as a Komen Scholar.
Footnotes
Disclosure of Potential Conflicts of Interest
Elai Davicioni is the President and CSO, Director of GenomeDx. Ben H. Park is a consultant for Foundation Medicine, Roche, H3 Biomedicine, Casdin Capital, Loxo Oncology, and Lilly, all outside the submitted work. No potential conflicts of interest were disclosed by the other authors.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–74. [DOI] [PubMed] [Google Scholar]
- 2.Kalluri R The biology and function of fibroblasts in cancer. Nature reviews Cancer. 2016;16:582–98. [DOI] [PubMed] [Google Scholar]
- 3.LeBleu VS, Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018;11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ridge SM, Sullivan FJ, Glynn SA. Mesenchymal stem cells: key players in cancer progression. Mol Cancer. 2017;16:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennen WN, Zhang B, Kulac I, Kisteman LN, Antony L, Wang H, et al. Mesenchymal stem cell infiltration during neoplastic transformation of the human prostate. Oncotarget. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–7. [DOI] [PubMed] [Google Scholar]
- 7.Paunescu V, Bojin FM, Tatu CA, Gavriliuc OI, Rosca A, Gruia AT, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mishra PJ, Mishra PJ, Humeniuk R, Medina DJ, Alexe G, Mesirov JP, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68:4331–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kansy BA, Dissmann PA, Hemeda H, Bruderek K, Westerkamp AM, Jagalski V, et al. The bidirectional tumor--mesenchymal stromal cell interaction promotes the progression of head and neck cancer. Stem Cell Res Ther. 2014;5:95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–63. [DOI] [PubMed] [Google Scholar]
- 11.Prantl L, Muehlberg F, Navone NM, Song YH, Vykoukal J, Logothetis CJ, et al. Adipose tissue-derived stem cells promote prostate tumor growth. Prostate. 2010;70:1709–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suzuki K, Sun R, Origuchi M, Kanehira M, Takahata T, Itoh J, et al. Mesenchymal stromal cells promote tumor growth through the enhancement of neovascularization. Molecular medicine. 2011;17:579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Connell JT, Sugimoto H, Cooke VG, MacDonald BA, Mehta AI, LeBleu VS, et al. VEGF-A and Tenascin-C produced by S100A4+ stromal cells are important for metastatic colonization. Proc Natl Acad Sci U S A. 2011;108:16002–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khakoo AY, Pati S, Anderson SA, Reid W, Elshal MF, Rovira II, et al. Human mesenchymal stem cells exert potent antitumorigenic effects in a model of Kaposi’s sarcoma. J Exp Med. 2006;203:1235–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qiao L, Xu Z, Zhao T, Zhao Z, Shi M, Zhao RC, et al. Suppression of tumorigenesis by human mesenchymal stem cells in a hepatoma model. Cell Res. 2008;18:500–7. [DOI] [PubMed] [Google Scholar]
- 17.Ozdemir BC, Pentcheva-Hoang T, Carstens JL, Zheng X, Wu CC, Simpson TR, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer cell. 2014;25:719–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaeffer EM, Marchionni L, Huang Z, Simons B, Blackman A, Yu W, et al. Androgen-induced programs for prostate epithelial growth and invasion arise in embryogenesis and are reactivated in cancer. Oncogene. 2008;27:7180–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pritchard C, Mecham B, Dumpit R, Coleman I, Bhattacharjee M, Chen Q, et al. Conserved gene expression programs integrate mammalian prostate development and tumorigenesis. Cancer Res. 2009;69:1739–47. [DOI] [PubMed] [Google Scholar]
- 20.Orr B, Riddick AC, Stewart GD, Anderson RA, Franco OE, Hayward SW, et al. Identification of stromally expressed molecules in the prostate by tag-profiling of cancer-associated fibroblasts, normal fibroblasts and fetal prostate. Oncogene.31:1130–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hurley PJ, Sundi D, Shinder B, Simons B, Hughes RM, Miller RM, et al. Germline Variants in Asporin Vary by Race, Modulate the Tumor Microenvironment and are Differentially Associated with Metastatic Prostate Cancer. Clin Cancer Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rochette A, Boufaied N, Scarlata E, Hamel L, Brimo F, Whitaker HC, et al. Asporin is a stromally expressed marker associated with prostate cancer progression. Br J Cancer. 2017;116:775–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Castellana B, Escuin D, Peiro G, Garcia-Valdecasas B, Vazquez T, Pons C, et al. ASPN and GJB2 Are Implicated in the Mechanisms of Invasion of Ductal Breast Carcinomas. J Cancer.3:175–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Satoyoshi R, Kuriyama S, Aiba N, Yashiro M, Tanaka M. Asporin activates coordinated invasion of scirrhous gastric cancer and cancer-associated fibroblasts. Oncogene. 2014. [DOI] [PubMed] [Google Scholar]
- 25.Wang L, Wu H, Wang L, Zhang H, Lu J, Liang Z, et al. Asporin promotes pancreatic cancer cell invasion and migration by regulating the epithelial-to-mesenchymal transition (EMT) through both autocrine and paracrine mechanisms. Cancer Lett. 2017;398:24–36. [DOI] [PubMed] [Google Scholar]
- 26.Kajikawa T, Yamada S, Tauchi T, Awata T, Yamaba S, Fujihara C, et al. Inhibitory effects of PLAP-1/asporin on periodontal ligament cells. Journal of dental research. 2014;93:400–5. [DOI] [PubMed] [Google Scholar]
- 27.Kizawa H, Kou I, Iida A, Sudo A, Miyamoto Y, Fukuda A, et al. An aspartic acid repeat polymorphism in asporin inhibits chondrogenesis and increases susceptibility to osteoarthritis. Nat Genet. 2005;37:138–44. [DOI] [PubMed] [Google Scholar]
- 28.Ding Q, Zhang M, Liu C. Asporin participates in gastric cancer cell growth and migration by influencing EGF receptor signaling. Oncology reports. 2015;33:1783–90. [DOI] [PubMed] [Google Scholar]
- 29.Simkova D, Kharaishvili G, Korinkova G, Ozdian T, Suchankova-Kleplova T, Soukup T, et al. The dual role of asporin in breast cancer progression. Oncotarget. 2016;7:52045–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hurley PJ, Marchionni L, Simons BW, Ross AE, Peskoe SB, Miller RM, et al. Secreted protein, acidic and rich in cysteine-like 1 (SPARCL1) is down regulated in aggressive prostate cancers and is prognostic for poor clinical outcome. Proc Natl Acad Sci U S A.109:14977–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hurley PJ, Wilsker D, Bunz F. Human cancer cells require ATR for cell cycle progression following exposure to ionizing radiation. Oncogene. 2007;26:2535–42. [DOI] [PubMed] [Google Scholar]
- 32.Hurley PJ, Hughes RM, Simons B, Huang J, Miller RM, Shinder B, et al. Androgen Regulated SPARCL1 in the Tumor Microenvironment Inhibits Metastatic Progression. Cancer Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guo C, Liu H, Zhang BH, Cadaneanu RM, Mayle AM, Garraway IP. Epcam, CD44, and CD49f distinguish sphere-forming human prostate basal cells from a subpopulation with predominant tubule initiation capability. PLoS One. 2012;7:e34219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phinney DG. Isolation of mesenchymal stem cells from murine bone marrow by immunodepletion. Methods Mol Biol. 2008;449:171–86. [DOI] [PubMed] [Google Scholar]
- 35.Boregowda SV, Krishnappa V, Chambers JW, Lograsso PV, Lai WT, Ortiz LA, et al. Atmospheric oxygen inhibits growth and differentiation of marrow-derived mouse mesenchymal stem cells via a p53-dependent mechanism: implications for long-term culture expansion. Stem cells. 2012;30:975–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhu H, Guo ZK, Jiang XX, Li H, Wang XY, Yao HY, et al. A protocol for isolation and culture of mesenchymal stem cells from mouse compact bone. Nat Protoc. 2010;5:550–60. [DOI] [PubMed] [Google Scholar]
- 37.Gundem G, Van Loo P, Kremeyer B, Alexandrov LB, Tubio JM, Papaemmanuil E, et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520:353–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simons BW, Durham NM, Bruno TC, Grosso JF, Schaeffer AJ, Ross AE, et al. A human prostatic bacterial isolate alters the prostatic microenvironment and accelerates prostate cancer progression. J Pathol. 2015;235:478–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brennen WN, Kisteman LN, Isaacs JT. Rapid selection of mesenchymal stem and progenitor cells in primary prostate stromal cultures. Prostate. 2016;76:552–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Karthaus WR, Iaquinta PJ, Drost J, Gracanin A, van Boxtel R, Wongvipat J, et al. Identification of multipotent luminal progenitor cells in human prostate organoid cultures. Cell. 2014;159:163–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chua CW, Shibata M, Lei M, Toivanen R, Barlow LJ, Bergren SK, et al. Single luminal epithelial progenitors can generate prostate organoids in culture. Nature cell biology. 2014;16:951–61, 1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hughes RM, Simons BW, Hurley PJ. A Murine Orthotopic Allograft to Model Prostate Cancer Growth and Metastasis. Bio Protoc. 2017;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson WE, Li C, Rabinovic A. Adjusting batch effects in microarray expression data using empirical Bayes methods. Biostatistics. 2007;8:118–27. [DOI] [PubMed] [Google Scholar]
- 44.Leek JT, Johnson WE, Parker HS, Jaffe AE, Storey JD. The sva package for removing batch effects and other unwanted variation in high-throughput experiments. Bioinformatics. 2012;28:882–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ritchie ME, Phipson B, Wu D, Hu Y, Law CW, Shi W, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic acids research. 2015;43:e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tang T, Li L, Tang J, Li Y, Lin WY, Martin F, et al. A mouse knockout library for secreted and transmembrane proteins. Nat Biotechnol. 2010;28:749–55. [DOI] [PubMed] [Google Scholar]
- 47.Quante M, Tu SP, Tomita H, Gonda T, Wang SS, Takashi S, et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer cell. 2011;19:257–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kibel AS, Suarez BK, Belani J, Oh J, Webster R, Brophy-Ebbers M, et al. CDKN1A and CDKN1B polymorphisms and risk of advanced prostate carcinoma. Cancer research. 2003;63:2033–6. [PubMed] [Google Scholar]
- 49.Tomoeda M, Yamada S, Shirai H, Ozawa Y, Yanagita M, Murakami S. PLAP-1/asporin inhibits activation of BMP receptor via its leucine-rich repeat motif. Biochem Biophys Res Commun. 2008;371:191–6. [DOI] [PubMed] [Google Scholar]
- 50.Awata T, Yamada S, Tsushima K, Sakashita H, Yamaba S, Kajikawa T, et al. PLAP-1/Asporin Positively Regulates FGF-2 Activity. Journal of dental research. 2015. [DOI] [PubMed] [Google Scholar]
- 51.Luehders K, Sasai N, Davaapil H, Kurosawa-Yoshida M, Hiura H, Brah T, et al. The small leucine-rich repeat secreted protein Asporin induces eyes in Xenopus embryos through the IGF signalling pathway. Development. 2015;142:3351–61. [DOI] [PubMed] [Google Scholar]
- 52.Nakajima M, Kizawa H, Saitoh M, Kou I, Miyazono K, Ikegawa S. Mechanisms for asporin function and regulation in articular cartilage. J Biol Chem. 2007;282:32185–92. [DOI] [PubMed] [Google Scholar]
- 53.Yamada S, Tomoeda M, Ozawa Y, Yoneda S, Terashima Y, Ikezawa K, et al. PLAP-1/asporin, a novel negative regulator of periodontal ligament mineralization. J Biol Chem. 2007;282:23070–80. [DOI] [PubMed] [Google Scholar]
- 54.Grafe I, Alexander S, Peterson JR, Snider TN, Levi B, Lee B, et al. TGF-beta Family Signaling in Mesenchymal Differentiation. Cold Spring Harbor perspectives in biology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Spaeth EL, Dembinski JL, Sasser AK, Watson K, Klopp A, Hall B, et al. Mesenchymal stem cell transition to tumor-associated fibroblasts contributes to fibrovascular network expansion and tumor progression. PLoS One. 2009;4:e4992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zeisberg EM, Potenta S, Xie L, Zeisberg M, Kalluri R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007;67:10123–8. [DOI] [PubMed] [Google Scholar]
- 57.Barretina J, Caponigro G, Stransky N, Venkatesan K, Margolin AA, Kim S, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer discovery. 2012;2:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ellis L, Ku S, Li Q, Azabdaftari G, Seliski J, Olson B, et al. Generation of a C57BL/6 MYC-Driven Mouse Model and Cell Line of Prostate Cancer. Prostate. 2016;76:1192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kalamajski S, Aspberg A, Lindblom K, Heinegard D, Oldberg A. Asporin competes with decorin for collagen binding, binds calcium and promotes osteoblast collagen mineralization. Biochem J. 2009;423:53–9. [DOI] [PubMed] [Google Scholar]
- 62.Wu H, Jing X, Cheng X, He Y, Hu L, Wu H, et al. Asporin enhances colorectal cancer metastasis through activating the EGFR/src/cortactin signaling pathway. Oncotarget. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maris P, Blomme A, Palacios AP, Costanza B, Bellahcene A, Bianchi E, et al. Asporin Is a Fibroblast-Derived TGF-beta1 Inhibitor and a Tumor Suppressor Associated with Good Prognosis in Breast Cancer. PLoS medicine. 2015;12:e1001871. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.