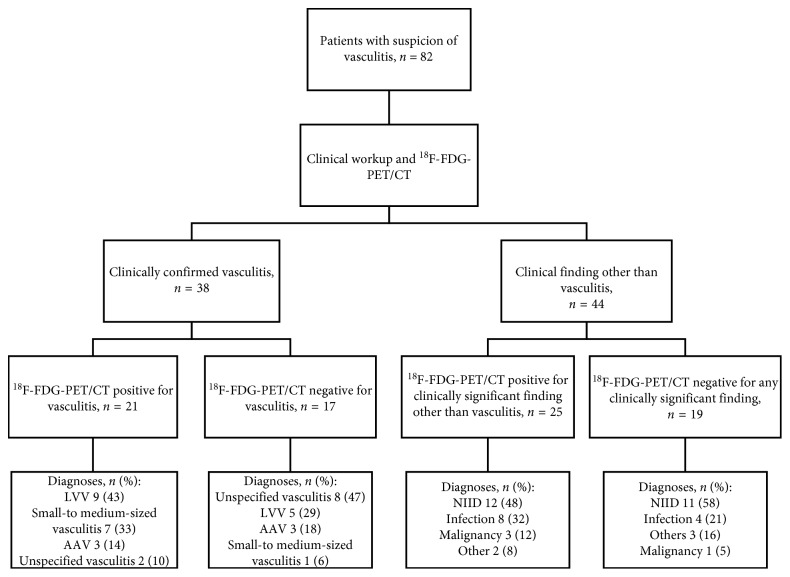
Abstract
18F-Fluorodeoxyglucose positron-emission tomography (18F-FDG-PET) with computed tomography (CT) is effective for diagnosing large vessel vasculitis, but its usefulness in accurately diagnosing suspected, unselected vasculitis remains unknown. We evaluated the feasibility of 18F-FDG-PET/CT in real-life cohort of patients with suspicion of vasculitis. The effect of the dose and the timing of glucocorticoid (GC) medication on imaging findings were in special interest. 82 patients with suspected vasculitis were evaluated by whole-body 18F-FDG-PET/CT. GC treatment as prednisolone equivalent doses at the scanning moment and before imaging was evaluated. 38/82 patients were diagnosed with vasculitis. Twenty-one out of 38 patients had increased 18F-FDG accumulation in blood vessel walls indicating vasculitis in various sized vessels. Vasculitis patients with a positive vasculitis finding in 18F-FDG-PET/CT had a significantly shorter duration of GC use (median = 4.0 vs 7.0 days, P=0.034), and they used lower GC dose during the PET scan (median dose = 15.0 mg/day vs 40.0 mg/day, p=0.004) compared to 18F-FDG-PET/CT-negative patients. Vasculitis patients with a positive 18F-FDG-PET/CT result had significantly higher C-reactive protein (CRP) than patients with a negative 18F-FDG-PET/CT finding (mean value = 154.5 vs 90.4 mg/L, p=0.018). We found that 18F-FDG-PET/CT positivity was significantly associated with a lower dose and shorter duration of GC medication and higher CRP level in vasculitis patients. 18F-FDG-PET/CT revealed clinically significant information in over half of the patients and was effective in confirming the final diagnosis.
1. Introduction
The diagnosis of vasculitis is a challenge, especially when vasculitis affects vital organs, and the patient presents nonspecific symptoms [1]. Vasculitis requires prompt recognition and initiation of treatment even if the diagnosis is uncertain.
The diagnostic process is often laborious. A biopsy is considered as a gold standard for diagnosing vasculitis, but in many cases, the optimal biopsy location is unavailable. The combination of 18F-fluorodeoxyglucose-positron emission tomography (18F-FDG-PET) with computed tomography (CT) is a promising diagnostic tool in the workup for vasculitis [2–4].
The accuracy and usefulness of 18F-FDG-PET/CT in the diagnostic procedure of vasculitis are still under debate. 18F-FDG-PET/CT has showed good performance in detecting large-vessel vasculitis (LVV) [4–7]. European League Against Rheumatism (EULAR) recommendation for the use of imaging in LVV in clinical practice recommends an early imaging test with ultrasound or MRI as first choices. PET may be used alternatively especially considering its ability to identify other serious differential diagnostic conditions [8]. In 2018, nuclear medicine interest committees gave a joint procedural recommendation on 18F-FDG-PET/CTA (angiography) imaging advising in data acquisition and interpretation in LVV and polymyalgia rheumatica [9]. Less is known about how 18F-FDG-PET/CT performs in other types of vasculitis than LVV. There is some evidence that PET may be useful in detecting small-vessel vasculitis [10, 11]. The ongoing multinational Diagnostic and Classification Criteria for Vasculitis (DCVAS) study aims to validate diagnostic criteria and to improve classification criteria for primary systemic vasculitis [12].
With a strong suspicion of vasculitis, rapid initiation of treatment is necessary. Glucocorticoids (GCs) are the most important first-line immunosuppressive treatment of noninfectious vasculitidies [13, 14]. Unfortunately, the use of immunosuppressive medication probably deteriorates the diagnostic accuracy of 18F-FDG-PET [7]. GC may attenuate 18F-FDG-uptake as early as after three days, but more confirmation is needed, since this is clinically a crucial question [15–18].
Here, we evaluated the impact of using 18F-FDG-PET/CT for accurately diagnosing vasculitis in real-life cohort of patients. We had a special interest in observing the effect of GC treatment prior to the 18F-FDG-PET/CT scan. We evaluated also differential diagnostic findings in patients with vasculitis suspicion.
2. Materials and Methods
2.1. Patients and Study Design
Eighty-two patients with suspected vasculitis were evaluated by whole-body 18F-FDG-PET/CT. The enrolment was done prospectively among inpatients. All diagnostic procedures were done at Turku University Hospital, Turku, Finland, between May 2011 and June 2015. The hospital is a tertiary-care centre for a population of 470 000. The institutional ethical committee approved the study protocol. All patients gave a written informed consent, according to the Declaration of Helsinki. This study is part of the Positron Emission Tomography of Infection and Vasculitis (PETU) study, which is registered as a clinical trial (NCT01878721). The PETU study researched different branches of infectious and inflammatory diseases which are reported separately [19–22]. Our series of vasculitis patients are previously unpublished.
The inclusion criterion of this study was vasculitis suspicion. Vasculitis suspicion was raised by an experienced specialist based on the clinical symptoms and signs of the patient. Vasculitis was confirmed or excluded by a consensus-based decision made by the specialists, while taking notice of the medical history, results of clinical examination, extensive laboratory work, 18F-FDG-PET/CT result, other imaging modalities, response to GC therapy, and follow-up. A minimum of 6 months clinical follow-up was considered sufficient to establish the diagnosis.
Special attention was paid to examine features and GC use in patients with diagnosed vasculitis in relation to 18F-FDG-PET/CT results. The cumulative GC dose was calculated from patients with a history of continuous GC use. GC use was evaluated as prednisolone equivalent doses.
2.2. Evaluation of the Diagnoses
The final diagnosis was based on the clinical picture as well as on the imaging findings of different sizes of affected vessels and histology. Based on the diagnosis, we divided the vasculitis patients into the following groups: LVV, medium- and small-vessel vasculitis, and unspecified vasculitis or antineutrophilic cytoplasmic antibodies- (ANCA-) associated vasculitis (AAV). Due to a low number of patients, vasculitis patients with granulomatous polyangiitis (GPA), eosinophilic granulomatous polyangiitis (EGPA), and microscopic polyangiitis (MPA) were combined into a group called AAV. In this group, five out of six patients were ANCA-positive, and an ANCA-negative patient had a histological finding of vasculitis.
All patients were evaluated by using the clinical criteria for vasculitis by American College of Rheumatology (ACR) 1990 [23, 24]. We evaluated the ACR criteria for GCA, GPA, EGPA, MPA, and polyarteritis nodosa (PAN). Due to a limited number of patients, cases from GPA, EGPA, and MPA formed a single group.
2.3. 18F-FDG-PET/CT Imaging Protocol
A whole-body 18F-FDG-PET/CT scan (64-slice Discovery VCT, General Electric Medical Systems, Milwaukee, WI, USA) was performed in all patients. Patients fasted at least 10 hours before the study. The mean injected radioactive dose of 18F-FDG was 273 MBq (range = 197–390 MBq). After an average of 57 minutes (range = 44–79 minutes), a whole-body PET acquisition (3 min/bed position) was performed following low-dose CT (kV 120, Smart mA range 10–80). In some patients, this was followed by a diagnostic contrast-enhanced CT scan (kV 120, Smart mA range 100–440) during the arterial phase after an automated i.v. injection of contrast agent.
Blood glucose levels were <10 mmol·L−1 prior to injection of the tracer in all patients. PET images were reconstructed in 128 × 128 matrix size in full 3D mode using maximum-likelihood reconstruction with an ordered-subset expectation maximization algorithm (VUE Point, GE Healthcare).
Visual analysis of the images was performed by an experienced nuclear medicine specialist, and the results were re-evaluated by the research team for a consensus-based diagnosis. All image analyses were done blinded with respect to patient's clinical details. 18F-FDG-PET/CT scans were considered positive, when a linear uptake pattern was found in the large arterial walls and/or its branches with an intensity similar or higher than the liver [25]. A positive finding for small- to medium-sized vasculitis was considered, when activity was higher than the vascular background activity and showed a tree-root-like uptake pattern [21] (Figure 1).
Figure 1.
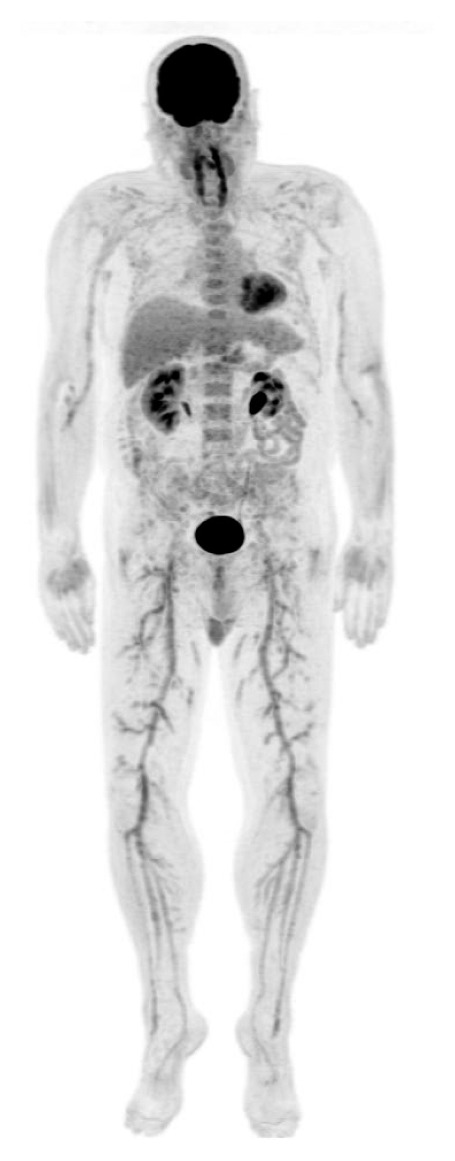
PET scan showing 18F-FDG-uptake in large- and medium- sized vessels. Maximum intensity projection (MIP) image of a whole-body PET-image of a 67-year-old male with high fever, mild headache, and a CRP value of 300 mg/l. After an extensive clinical workup, suspicion of vasculitis occurred, when there was no response to antibiotics. Whole-body CT showed no infection or malignant focus. Temporal arterial biopsy was equivocal. A PET/CT scan confirmed the vasculitis diagnosis by showing a tree-root-like 18F-FDG uptake pattern in large- and medium-sized arteries in the lower limbs. Physiological tracer uptake is noted in the brain, the neck muscles, the myocardium, the kidneys, and the bladder.
2.4. Statistical Analysis
Normally distributed continuous data were expressed as mean (standard deviation, SD), and for skewed distributions, data were expressed as median (interquartile range, IQR), unless stated otherwise. Categorical variables were described with absolute and relative (percentage) frequencies. An independent sample t test or Mann–Whitney U test was applied to determine the significance of differences for continuous variables as appropriate and a chi-squared or Fischer's exact test for categorical variables. All statistical analyses were calculated using SPSS Software Package (IBM SPSS Statistics Version 24). P values ≤0.05 were considered significant.
3. Results
3.1. Patients' Characteristics, Diagnosis, and 18F-FDG-PET/CT Findings
A total of 82 patients with a clinical suspicion of vasculitis were referred for 18F-FDG-PET/CT and prospectively screened for this study (38 males and 44 females) (Figure 2). The mean age for patients was 62.7 years (age range = 19–89 years, SD = 16.0 years). An abnormal or clinically significant 18F-FDG-PET/CT finding was encountered in 46/82 patients (56%) (Table 1). A clinically significant 18F-FDG-PET/CT finding in different diagnostic subgroups is depicted in Table 1.
Figure 2.
Diagram of the study design. 82 patients with a clinical suspicion of vasculitis referred for 18F-FDG-PET/CT were included. Diagnoses were confirmed by consensus-based decisions made by specialists after evaluation of a standard extensive workup, 18F-FDG-PET/CT scan, and a minimum of 6 months follow-up. Vasculitis patients with a negative 18F-FDG-PET/CT for vasculitis had other minor findings in PET/CT: mild infection (n = 2, 12%), pericarditis (n = 1, 6%), and pleuritis (n = 1, 6%). Among nonvasculitis patients, clinically significant 18F-FDG-PET/CT findings were as follows: NIID (n = 12), infection (n = 8), malignancy (n = 3), and miscellaneous (n = 2). LVV = large-vessel vasculitis. AAV = antineutrophil cytoplasmic antibody- (ANCA-) associated vasculitis. NIID = noninfectious inflammatory disease other than vasculitis.
Table 1.
Final clinical diagnosis and significance of PET/CT by each diagnosis.
| Category | Number of cases | Clinically significant PET/CT finding |
|---|---|---|
| Other autoimmune diseases | 18 | 10/18 |
| Adult-onset Still's disease | 3 | 0/3 |
| Sarcoidosis | 2 | 1/2 |
| Collagenosis | 2 | 2/2 |
| Pericarditis | 2 | 1/2 |
| Morbus Crohn/IBD | 2 | 1/2 |
| Myositis | 2 | 2/2 |
| SLE | 2 | 1/2 |
| Unspecified | 2 | 1/2 |
| Rheumatoid arthritis | 1 | 1/1 |
|
| ||
| Large vessel vasculitis | 14 | 9/14 |
| Giant cell arteritis | 13 | 9 |
| Takayasu arteritis | 1 | 0 |
|
| ||
| Infection | 12 | 8/12 |
| Infection NAS/FUO | 3 | 2/3 |
| Deep abscess | 3 | 2/3 |
| Septic arthritis | 1 | 1/1 |
| Septic spondylodiscitis | 1 | 1/1 |
| Pneumonia | 1 | 1/1 |
| Urinary tract infection | 1 | 0/1 |
| Cholecystitis | 1 | 1/1 |
| Tuberculosis | 1 | 0/1 |
|
| ||
| Unspecified vasculitis∗ | 10 | 2/10 |
| Vasculitis NAS | 8 | 2 |
| Secondary vasculitis | 2 | 0 |
|
| ||
| Small- and medium-sized vasculitis (other than ANCA-associated vasculitis) | 8 | 7/8 |
|
| ||
| ANCA-associated vasculitis∗∗ | 6 | 3/6 |
| EGPA | 3 | 1/3 |
| GPA | 2 | 2/2 |
| MPA | 1 | 0/1 |
|
| ||
| Polymyalgia rheumatica | 5 | 2/5 |
|
| ||
| Malignancy | 4 | 3/4 |
| Lymphoma | 3 | 2/3 |
| Lung cancer | 1 | 1/1 |
|
| ||
| Miscellaneous | 4 | 1/4 |
| Cardiac disease | 2 | 0/2 |
| Calciphylaxis | 1 | 0/1 |
| Leg ulcers | 1 | 1/1 |
|
| ||
| Unknown diagnosis | 1 | 1/1 |
ANCA, antineutrophil cytoplasmic antibody; EGPA, eosinophilic granulomatosis with polyangiitis; GPA, granulomatosis with polyangiitis; MPA, microscopic polyangiitis; FUO, fever of unknown origin. ∗Vasculitis diagnosis confirmed by either imaging or biopsy. ∗∗5/6 patients were ANCA-positive. The ANCA-negative patient had biopsy confirmed diagnosis.
The vasculitis diagnosis was confirmed in 38 (46%) of the patients (Table 2). Most common cases of vasculitis were LVV (n = 14, 37%) and unspecified vasculitis (n = 10, 26%). Increased 18F-FDG accumulation in blood vessels suitable for vasculitis was detected in 21 of these 38 (55%) patients (Tables 2 and 3). 18F-FDG-PET/CT-positive patients fulfilled the ACR criteria for GCA significantly more often than 18F-FDG-PET/CT-negative patients (38% vs 8%, p=0.015). No accumulation of 18F-FDG in blood vessels was detected in 44 patients who did not fulfil the vasculitis diagnosis. Among patients without vasculitis diagnosis, the most common diagnostic groups were autoimmune diseases other than vasculitis (not including polymyalgia rheumatica, PM) (n = 18, 41%), infection (n = 12, 27%), PM (n = 5, 11%), and malignancy (n = 4, 9%) (Figure 2). In the PM group, one patient had 18F-FDG accumulation in the shoulder area relating to PM. One patient had a biopsy proven panniculitis which was clinically significant but not related to PM. Rest of the three patients did not have significant 18F-FDG-PET/CT findings.
Table 2.
Patients' characteristics based on vasculitis diagnosis.
| Vasculitis (n = 38) | No vasculitis (n = 44) | P value | |
|---|---|---|---|
| Female sex, n (%) | 23 (60.5) | 21 (47.7) | 0.246 |
| Age, years, mean (SD) | 66.3 (13.4) | 59.5 (17.5) | 0.056 |
| CRP max, mg/l, mean (SD) | 125.8 (88.3) | 131.8 (91.4) | 0.765 |
| PCT max, µg/l, mean (SD) | 0.16 (0.16), n = 29 | 0.16 (0.18), n = 33 | 0.872 |
| Prednisolone at scanning moment, mg, median (IQR) | 30.0 [33] | 1.0 [20] | 0.001∗ |
| Patients using prednisolone | 29/38 | 20/44 | |
| Prednisolone prior scanning, d, median (IQR) | 6.0 [11] | 0.0 [52] | 0.135 |
| Prednisolone cumulative dose, mg, median (IQR) | 260.0 [1500] | 1.00 [1706] | 0.075 |
| Fulfills ACR criteria for GCA, n (%) | 10 (26.3) | 3 (6.8) | 0.016∗ |
| Fulfills ACR criteria for EGPA, GPA, or MPA, n (%) | 12 (31.6) | 8 (18.2) | 0.159 |
| Fulfills ACR criteria for PAN, n (%) | 5 (13.2) | 2 (4.5) | 0.164 |
| Fever over 38°C, n = 79, n (%) | 22 (57.9) | 26 (63.4) | 0.616 |
SD, standard deviation; CRP, C-reactive protein; PCT, procalcitonin; IQR, interquartile range; ACR, American College of Rheumatology; GCA, giant cell arteritis; EGPA, eosinophilic granulomatous polyangiitis; GPA, granulomatous polyangiitis; MPA, microscopic polyangiitis; PAN, polyarteritis nodosa. ∗Significant at P value <0.05.
Table 3.
Characteristics of vasculitis patients.
| 18F-FDG-PET/CT positive (n = 21) | 18F-FDG-PET/CT negative (n = 17) | P value | |
|---|---|---|---|
| Female sex, n (%) | 14 (66.7) | 9 (52.9) | 0.389 |
| Age, years, mean (SD) | 68.0 (12.1) | 64.2 (15.0) | 0.390 |
| CRP max, mg/l, mean (SD) | 154.5 (100.2) | 90.4 (55.6) | 0.018∗ |
| PCT max, μg/l, mean (SD) | 0.12 (0.09), n = 17 | 0.22 (0.02), n = 12 | 0.137 |
| ANCA positive, n (%) | 3 (14.3) | 4 (23.5) | 0.478 |
| Prednisolone at scanning moment, mg, median [IQR] | 15.0 [40.0] | 40.0 [30.0] | 0.004∗ |
| Prednisolone prior scanning, d, median [IQR] | 4.0 [9] | 7.0 [154] | 0.034∗ |
| Prednisolone cumulative dose, mg, median [IQR] | 120 [1120] | 360 [1965] | 0.096 |
| Fever over 38°C | 14 (66.7) | 8 (47.1) | 0.224 |
SD, standard deviation; CRP, C-reactive protein; PCT, procalcitonin; IQR, interquartile range; ANCA, antineutrophil cytoplasmic antibody. ∗Significant at P value <0.05.
3.2. Effect of Glucocorticoid Treatment on 18F-FDG-PET/CT Findings among the Vasculitis Patients
The duration and dose of GC treatment had a significant effect on the outcomes of the 18F-FDG-PET/CT scans. Out of 38 vasculitis patients, 9 patients (24%) had no GC treatment previously and 8 (21%) had used GC over 31 days.
Vasculitis patients with positive 18F-FDG-PET/CT had significantly fewer days of GC use before imaging than patients with negative 18F-FDG-PET/CT (median = 4.0 (IQR 9) vs 7.0 (IQR 154) days, p=0.034) (Table 3). In patients scanned within 3 days of GC treatment, 77% had vascular 18F-FDG uptake suitable for vasculitis in comparison to 42% after one week of treatment (Figure 3). Among these 38 vasculitis patients, there was a significant association of 18F-FDG-PET/CT positivity with a lower GC dose on the scanning day with a median dose 15.0 (IQR 40.0) mg/day vs 40.0 (IQR 30.0) mg/day (p=0.004) (Table 3).
Figure 3.
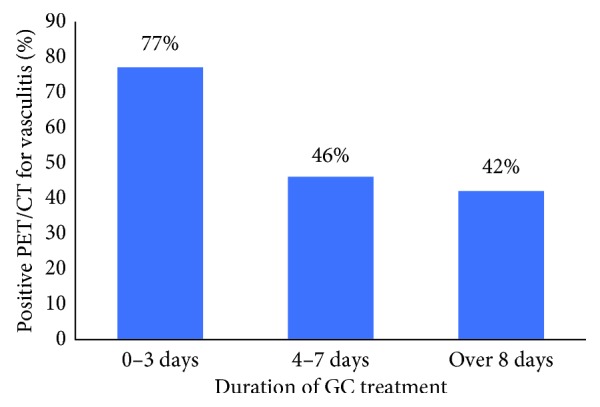
Positive 18F-FDG-PET/CT scans (%) for vasculitis and the duration of glucocorticoid (GC) treatment (days). In our study population, 21 of 38 vasculitis patients had positive 18F-FDG-PET/CT finding. In patients scanned within 3 days of GC treatment, 77% had vascular 18F-FDG uptake suitable for vasculitis in comparison to 42% after 8 days of treatment.
Patients with vasculitis used a higher GC dose during 18F-FDG-PET/CT scan than patients without vasculitis having a median prednisolone use of 30.0 (IQR 33.0) mg/day vs 0 (IQR 20.0) mg/day (p=0.001). Among vasculitis patients, 9 patients (24%) used no GC on the scanning day in comparison to the nonvasculitis group, where 24 patients (55%) used no GC on the scanning day.
3.3. Laboratory and Clinical Findings of the Patients
Among all 82 patients with suspicion of vasculitis, C-reactive protein (CRP) was elevated in 75 patients (91.5%), with a mean CRP value of 129.0 mg/L (SD = 89.5 mg/L). Vasculitis patients with a positive 18F-FDG-PET/CT scan had significantly higher CRP values than vasculitis patients with a negative 18F-FDG-PET/CT scan (mean CRP = 154.5 mg/L; SD 100.2 mg/L vs 90.4 mg/L; SD 55.6 mg/L, respectively; p=0.018) (Table 3). No difference was found in procalcitonin (PCT) values (data available from 62 patients) between vasculitis patients with positive or negative 18F-FDG-PET/CT findings (Table 3). There was no difference in CRP or PCT values between vasculitis and nonvasculitis patients (Table 2).
Forty-eight (out of 79) patients (60.8%) had a fever over 38°C. Other common clinical symptoms were haematuria (n = 38/75, 46.3%), myalgia (n = 36/79, 43.9%), hip pain (n = 24/76, 29.3%), bilateral shoulder pain (n = 18/78, 22%), a new headache (n = 14/78, 17.1%), and new neuropathy (n = 11/79, 13.3%). When comparing vasculitis with nonvasculitis patients, vasculitis patients had significantly more often a new headache (29% vs 7%, p=0.008).
4. Discussion
The spectrum of conditions causing vasculitis-like symptoms is wide. We found that in real-life cohort of patients, 18F-FDG-PET/CT was effective in confirming the final diagnosis among inpatients with vasculitis suspicion. 18F-FDG-PET/CT showed vasculitis in 26% of all patients and revealed clinically significant information in over half of the patients.
We found that among vasculitis patients, a shorter duration of prednisolone use is significantly associated with positive 18F-FDG-PET/CT vasculitis findings (Table 3). Vasculitis patients with positive 18F-FDG-PET/CT imaging had a median of 4 days of prednisolone treatment versus 7 days in the negative 18F-FDG-PET/CT group. This implicates that withholding diagnostic imaging for over one week during GC treatment increases the risk of a false-negative diagnosis. In the vasculitis group, a lower GC dose at the scanning moment was significantly associated with an 18F-FDG-PET/CT-based vasculitis diagnosis (Table 3).
In a previous study, good sensitivity, at 80%, and specificity, at 79%, have been reported for 18F-FDG-PET/CT in patients with GCA receiving GC less than 3 days [6]. In another study, Fuchs et al. reported that the sensitivity of 18F-FDG-PET/CT lowers from 99% to 53% in patients with GCA receiving an immunosuppressant [7]. A reduction of 18F-FDG accumulation under treatment has been reported in follow-up studies [16, 26]. A study by Imfeld et al. shows that prednisone treatment ≥10 days significantly reduced 18F-FDG-PET/CT sensitivity. The first effect of lowered sensitivity was seen as early as 3 days after treatment initiation in the abdominal aorta [17] and in supra-aortic vessels [15]. Surprisingly, a study by Clifford et al. [18] did not find a correlation. Clifford et al. explained that their study subject number was low (n = 28), patients had received treatment over long time (on an average of 11.9 days), and doses were similarly high among all patients.
In a real clinical setting, withholding the treatment initiation until imaging is often impossible, so the knowledge of the GC effect on 18F-FDG-PET diagnostic performance is important. Our study supports the data that GC treatment reduces the diagnostic power of 18F-FDG-PET/CT after one week. Thus, there is a need for fast 18F-FDG-PET/CT availability for suspected vasculitis patients. These patients represent often a diffuse clinical picture. Ultrasound, which nowadays is the recommended standard protocol in LVV, performs poorly in thoracic aorta area or in small, deep vessels without focal symptoms. In our material, 18F-FDG-PET/CT was useful also in other vasculitis than LVV and performs well in thoracic vessels. A lower GC dose during PET/CT scanning was associated with vasculitis findings in 18F-FDG-PET/CT, but our study cannot answer the question that, if lowering temporarily the GC dose helps avoid false-negative results. In few patients, 18F-FDG-PET/CT showed vascular uptake suitable for vasculitis even after long GC treatment. In our cohort, the duration of use and dosage of GC treatment varied in patients at the 18F-FDG-PET/CT imaging due to the study design.
We found a significant correlation between higher CRP value and 18F-FDG-PET/CT positivity in patients diagnosed with vasculitis. A high CRP value might reflect more active inflammation and less use of GC at the scanning moment. There are several studies testing the correlation of laboratory parameters and diagnostic performance of 18F-FDG-PET/CT in GCA, in fever of unknown origin (FUO), or in inflammation of unknown origin (IUO) [17, 27–29]. FUO and IUO are essential differential diagnostic challenges for vasculitis. Schönau et al. reported that an age over 50 years, a CRP level over 30 mg/L, and the absence of fever predicted the helpfulness of 18F-FDG-PET/CT [27] in FUO and IUO. Papathanasiou et al. noticed a significant positive association between maximal aortic 18F-FDG uptake and inflammatory markers [29].
Our study had limitations that should be considered. The study was done in a real clinical setting, and the inclusion criterion was vasculitis suspicion; therefore, the vasculitis patient group was heterogeneous. The vasculitis diagnosis was confirmed later, and the spectrum of different vasculitis was detected. Our study did not exclude patients who did not fulfil the ACR inclusion criteria. This might be a limitation when comparing the results to previous studies with more restricted inclusion criteria.
5. Conclusions
We found that in patients with confirmed vasculitis diagnosis, 18F-FDG-PET/CT positivity was significantly related to a lower dose and shorter duration of GC medication and a higher CRP level. In real-life circumstances, 18F-FDG-PET/CT revealed different types of vasculitidies as well as other clinically significant information in over half of the patients and had an impact in confirming the final diagnosis.
Acknowledgments
The authors wish to thank Mia Koutu and Laura Kontto for helping with 18F-FDG-PET/CT imaging and the staff of Department of Rheumatology for recruiting study subjects. Robert M. Badeau, M.Sc., Ph.D., of Aura Professional English Language Consulting, Ltd. (http://auraenglish.com) performed this manuscript's English language checking and proofreading service. This study was supported by the State Research Funds of Turku University Hospital and the Finnish Society for Rheumatology.
Data Availability
The data included in this study are available upon request from the corresponding author.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Weyand C. M., Goronzy J. J. Giant-cell arteritis and polymyalgia rheumatica. New England Journal of Medicine. 2014;371(17):1652–1653. doi: 10.1056/NEJMc1409206. [DOI] [PubMed] [Google Scholar]
- 2.Prieto-González S., Arguis P., Cid M. C. Imaging in systemic vasculitis. Current Opinion in Rheumatology. 2015;27(1):53–62. doi: 10.1097/bor.0000000000000130. [DOI] [PubMed] [Google Scholar]
- 3.Puppo C., Massollo M., Paparo F., et al. Giant cell arteritis: a systematic review of the qualitative and semiquantitative methods to assess vasculitis with 18F-fluorodeoxyglucose positron emission tomography. BioMed Research International. 2014;2014:11. doi: 10.1155/2014/574248.574248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besson F. L., Parienti J.-J., Bienvenu B., et al. Diagnostic performance of 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a systematic review and meta-analysis. European Journal of Nuclear Medicine and Molecular Imaging. 2011;38(9):1764–1772. doi: 10.1007/s00259-011-1830-0. [DOI] [PubMed] [Google Scholar]
- 5.Soussan M., Nicolas P., Schramm C., et al. Management of large-vessel vasculitis with FDG-PET. Medicine. 2015;94(14):p. e622. doi: 10.1097/md.0000000000000622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Prieto-González S., Depetris M., García-Martínez A., et al. Positron emission tomography assessment of large vessel inflammation in patients with newly diagnosed, biopsy-proven giant cell arteritis: a prospective, case-control study. Annals of the Rheumatic Diseases. 2014;73(7):1388–1392. doi: 10.1136/annrheumdis-2013-204572. [DOI] [PubMed] [Google Scholar]
- 7.Fuchs M., Briel M., Daikeler T., et al. The impact of 18F-FDG PET on the management of patients with suspected large vessel vasculitis. European Journal of Nuclear Medicine and Molecular Imaging. 2012;39(2):344–353. doi: 10.1007/s00259-011-1967-x. [DOI] [PubMed] [Google Scholar]
- 8.Dejaco C., Ramiro S., Duftner C., et al. EULAR recommendations for the use of imaging in large vessel vasculitis in clinical practice. Annals of the Rheumatic Diseases. 2018;77(5):636–643. doi: 10.1136/annrheumdis-2017-212649. [DOI] [PubMed] [Google Scholar]
- 9.Slart R. H. J. A., Writing Group, Reviewer Group, et al. FDG-PET/CT(A) imaging in large vessel vasculitis and polymyalgia rheumatica: joint procedural recommendation of the EANM, SNMMI, and the PET interest group (PIG), and endorsed by the ASNC. European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(7):1250–1269. doi: 10.1007/s00259-018-3973-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Soussan M., Abisror N., Abad S., et al. FDG-PET/CT in patients with ANCA-associated vasculitis: case-series and literature review. Autoimmunity Reviews. 2014;13(2):125–131. doi: 10.1016/j.autrev.2013.09.009. [DOI] [PubMed] [Google Scholar]
- 11.Kemna M. J., Vandergheynst F., Vöö S., et al. Positron emission tomography scanning in anti-neutrophil cytoplasmic antibodies-associated vasculitis. Medicine. 2015;94(20):p. e747. doi: 10.1097/md.0000000000000747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luqmani R. A., Suppiah R., Grayson P. C., Merkel P. A., Watts R. Nomenclature and classification of vasculitis—update on the ACR/EULAR diagnosis and classification of vasculitis study (DCVAS) Clinical and Experimental Immunology. 2011;164(1):11–13. doi: 10.1111/j.1365-2249.2011.04358.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buttgereit F., Dejaco C., Matteson E. L., Dasgupta B. Polymyalgia rheumatica and giant cell arteritis. JAMA. 2016;315(22):p. 2442. doi: 10.1001/jama.2016.5444. [DOI] [PubMed] [Google Scholar]
- 14.Muratore F., Pazzola G., Soriano A., et al. Unmet needs in the pathogenesis and treatment of vasculitides. Clinical Reviews in Allergy and Immunology. 2018;54(2):244–260. doi: 10.1007/s12016-017-8643-2. [DOI] [PubMed] [Google Scholar]
- 15.Nielsen B. D., Gormsen L. C., Hansen I. T., Keller K. K., Therkildsen P., Hauge E.-M. Three days of high-dose glucocorticoid treatment attenuates large-vessel 18F-FDG uptake in large-vessel giant cell arteritis but with a limited impact on diagnostic accuracy. European Journal of Nuclear Medicine and Molecular Imaging. 2018;45(7):1119–1128. doi: 10.1007/s00259-018-4021-4. [DOI] [PubMed] [Google Scholar]
- 16.Blockmans D., De Ceuninck L., Vanderschueren S., Knockaert D., Mortelmans L., Bobbaers H. Repetitive 18F-fluorodeoxyglucose positron emission tomography in giant cell arteritis: a prospective study of 35 patients. Arthritis and Rheumatism. 2006;55(1):131–137. doi: 10.1002/art.21699. [DOI] [PubMed] [Google Scholar]
- 17.Imfeld S., Rottenburger C., Schegk E., et al. [18F]FDG positron emission tomography in patients presenting with suspicion of giant cell arteritis—lessons from a vasculitis clinic. European Heart Journal-Cardiovascular Imaging. 2017;19(8):933–940. doi: 10.1093/ehjci/jex259. [DOI] [PubMed] [Google Scholar]
- 18.Clifford A. H., Murphy E. M., Burrell S. C., et al. Positron emission tomography/computerized tomography in newly diagnosed patients with giant cell arteritis who are taking glucocorticoids. The Journal of Rheumatology. 2017;44(12):1859–1866. doi: 10.3899/jrheum.170138. [DOI] [PubMed] [Google Scholar]
- 19.Salomäki S. P., Saraste A., Kemppainen J., et al. 18F-FDG positron emission tomography/computed tomography in infective endocarditis. Journal of Nuclear Cardiology. 2017;24(1):195–206. doi: 10.1007/s12350-015-0325-y. [DOI] [PubMed] [Google Scholar]
- 20.Salomaki S. P., Hohenthal U., Kemppainen J., Pirila L., Saraste A. Visualization of pericarditis by fluorodeoxyglucose PET. European Heart Journal-Cardiovascular Imaging. 2014;15(3):p. 291. doi: 10.1093/ehjci/jet179. [DOI] [PubMed] [Google Scholar]
- 21.Salomäki S. P., Kemppainen J., Aho H., et al. Widespread vascular inflammation in a patient with antineutrophil cytoplasmic antibody-associated vasculitis as detected by positron emission tomography. European Journal of Nuclear Medicine and Molecular Imaging. 2014;41(11):2167–2168. doi: 10.1007/s00259-014-2847-y. [DOI] [PubMed] [Google Scholar]
- 22.Salomäki S. P., Kemppainen J., Hohenthal U., et al. Head-to-head comparison of 68Ga-citrate and 18F-FDG PET/CT for detection of infectious foci in patients with Staphylococcus aureus bacteraemia. Contrast Media & Molecular Imaging. 2017;2017:8. doi: 10.1155/2017/3179607.3179607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hunder G. G., Bloch D. A., Michel B. A., et al. The American college of rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis and Rheumatism. 1990;33(8):1122–1128. doi: 10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 24.Fries J. F., Hunder G. G., Bloch D. A., et al. The American college of rheumatology 1990 criteria for the classification of vasculitis: summary. Arthritis and Rheumatism. 1990;33(8):1135–1136. doi: 10.1002/art.1780330812. [DOI] [PubMed] [Google Scholar]
- 25.Jamar F., Buscombe J., Chiti A., et al. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. Journal of Nuclear Medicine. 2013;54(4):647–658. doi: 10.2967/jnumed.112.112524. [DOI] [PubMed] [Google Scholar]
- 26.Martínez-Rodríguez I., Jiménez-Alonso M., Quirce R., et al. 18F-FDG PET/CT in the follow-up of large-vessel vasculitis: a study of 37 consecutive patients. Seminars in Arthritis and Rheumatism. 2018;47(4):530–537. doi: 10.1016/j.semarthrit.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Schönau V., Vogel K., Englbrecht M., et al. The value of 18F-FDG-PET/CT in identifying the cause of fever of unknown origin (FUO) and inflammation of unknown origin (IUO): data from a prospective study. Annals of the Rheumatic Diseases. 2018;77(1):70–77. doi: 10.1136/annrheumdis-2017-211687. [DOI] [PubMed] [Google Scholar]
- 28.Balink H., Veeger N. J. G. M., Bennink R. J., et al. The predictive value of C-reactive protein and erythrocyte sedimentation rate for 18F-FDG PET/CT outcome in patients with fever and inflammation of unknown origin. Nuclear Medicine Communications. 2015;36(6):604–609. doi: 10.1097/mnm.0000000000000300. [DOI] [PubMed] [Google Scholar]
- 29.Papathanasiou N. D., Du Y., Menezes L. J., et al. 18F-fludeoxyglucose PET/CT in the evaluation of large-vessel vasculitis: diagnostic performance and correlation with clinical and laboratory parameters. The British Journal of Radiology. 2012;85(1014):e188–e194. doi: 10.1259/bjr/16422950. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data included in this study are available upon request from the corresponding author.