Abstract
Sheep and goats are valuable livestock species that have been raised for their production of meat, milk, fiber, and other by-products. Due to their suitable size, short gestation period, and abundant secretion of milk, sheep and goats have become important model animals in agricultural, pharmaceutical, and biomedical research. Genome engineering has been widely applied to sheep and goat research. Pronuclear injection and somatic cell nuclear transfer represent the two primary procedures for the generation of genetically modified sheep and goats. Further assisted tools have emerged to enhance the efficiency of genetic modification and to simplify the generation of genetically modified founders. These tools include sperm-mediated gene transfer, viral vectors, RNA interference, recombinases, transposons, and endonucleases. Of these tools, the four classes of site-specific endonucleases (meganucleases, ZFNs, TALENs, and CRISPRs) have attracted wide attention due to their DNA double-strand break-inducing role, which enable desired DNA modifications based on the stimulation of native cellular DNA repair mechanisms. Currently, CRISPR systems dominate the field of genome editing. Gene-edited sheep and goats, generated using these tools, provide valuable models for investigations on gene functions, improving animal breeding, producing pharmaceuticals in milk, improving animal disease resistance, recapitulating human diseases, and providing hosts for the growth of human organs. In addition, more promising derivative tools of CRISPR systems have emerged such as base editors which enable the induction of single-base alterations without any requirements for homology-directed repair or DNA donor. These precise editors are helpful for revealing desirable phenotypes and correcting genetic diseases controlled by single bases. This review highlights the advances of genome engineering in sheep and goats over the past four decades with particular emphasis on the application of CRISPR/Cas9 systems.
Keywords: sheep, goats, farm animals, genetic modification, genome engineering, gene editing, CRISPR/Cas9
Introduction
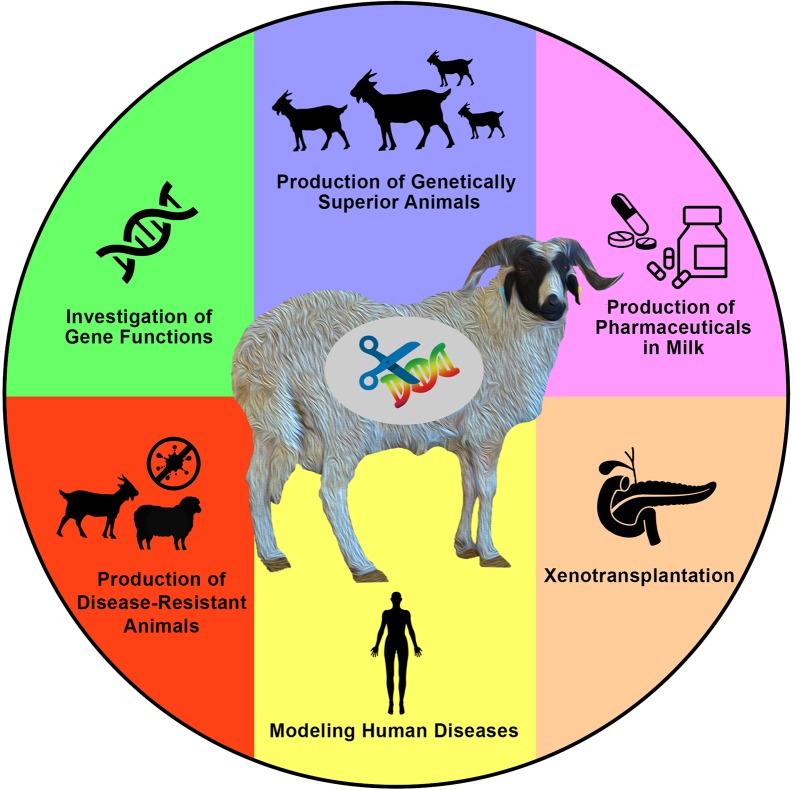
Generating new and variable phenotypes via direct alteration of DNA sequences is an interesting idea that has sparked the curiosity of a wide spectrum of researchers over the past few decades. Based on significant efforts, tremendous advances have been achieved in animal genetics and reproductive physiology. These have enabled what is now known as the genome-editing revolution that can be applied to generate gene-edited animals including sheep and goats for various purposes (Figure 1).
Figure 1.
Applications and aims of genome engineering in sheep and goats. Genome engineering has been applied in both sheep and goats (or generally in farm animals) for various purposes such as to investigate the biological and functional roles of genes, to introduce novel economically important traits for agricultural purposes, to produce valuable proteins in milk, to produce animals that are resistant to epidemic diseases, to model human diseases, and to produce hosts for the growth of human organs for xenotransplantation research, among other valuable purposes that mainly aim to increase human knowledge, as well as human and animal health and welfare.
About 40 years ago, a set of basic techniques were applied to sheep embryos with the desire to generate identical twins, multiplets, and chimeras. The further development of these tools has led to the generation of identical individuals by embryo splitting (Willadsen, 1979), chimeras by aggregating embryonic cells (Fehilly et al., 1984b), and even the first cloned sheep prior to the famous Dolly from undifferentiated embryonic cells (Willadsen, 1986). During that time, in 1985, the first report about the generation of transgenic farm animals (including sheep) via pronuclear injection (PNI) was published, announcing the first procedure for the production of transgenic farm animals (Hammer et al., 1985). About 10 years later, in 1996, success of cloning sheep from more differentiated embryonic cells has been reported (Campbell et al., 1996). One year later, the same group announced unprecedented success by cloning the sheep Dolly from adult somatic cells (Wilmut et al., 1997). In the same year, another remarkable advance had been achieved by using transfected fetal fibroblast cells for the generation of the first transgenic cloned sheep (Schnieke et al., 1997). Based on these advances, somatic cell nuclear transfer (SCNT) has been established as an essential tool for the creation of transgenic animals. Using these two approaches (PNI and SCNT), a large number of transgenic sheep and goats have been made for various purposes (Tables 1 and 2). From that time, various strategies have been applied to facilitate the generation of gene-modified animals that express specific and desired traits, employing spermatozoa, viral vectors, transposons, recombinases, RNA interference (RNAi) molecules, and endonucleases (Figure 2). Of these gene manipulation tools, the clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9 (CRISPR/Cas9) is currently revolutionizing the field of genome editing throughout virtually all biological kingdoms (Doudna and Charpentier, 2014; Hsu et al., 2014).
Table 1.
Examples of transgenic sheep and goats produced using a pronuclear microinjection (PNI) approach.
| Species | Construct abbreviation | Construct name | Main trait | References |
|---|---|---|---|---|
| Sheep | mMT/hGH | Mouse metallothionein-I/human growth hormone | Growth | Hammer et al., 1985 |
| oMT/oGH | Ovine metallothionein-I/ovine growth hormone | Growth | Murray et al., 1989 | |
| mMT/bGH | Mouse metallothionein-I/bovine growth hormone | Growth | Rexroad et al., 1989 | |
| mMT/hGRF | Mouse metallothionein-I/human growth hormone–releasing factor | Growth | Rexroad et al., 1989 | |
| mTRF/bGH | Mouse transferrin/bovine growth hormone | Growth | Rexroad Jr. et al., 1991 | |
| mALB/hGRF | Mouse albumin/human growth hormone–releasing factor | Growth | Rexroad Jr. et al., 1991 | |
| oBLG/hFIX | Ovine β-lactoglobulin/human factor IX | Therapeutic proteins in milk | Clark et al., 1989 | |
| oBLG/hα1AT | Ovine β-lactoglobulin/human α1-antitrypsin | Therapeutic proteins in milk | Wright et al., 1991 | |
| oBLG/hFVIII | Ovine β-lactoglobulin/human factor VIII | Therapeutic proteins in milk | Niemann et al., 1996 | |
| vLRT/vvENV | Virus long terminal repeat/visna virus envelope | Disease model | Clements et al., 1994 | |
| hHTT/hHTT | Human huntingtin/human huntingtin | Disease model | Jacobsen et al., 2010 | |
| CMVp/oTLR4 | Cytomegalovirus promoter/ovine toll-like receptor 4 | Disease resistance | Deng et al., 2012a | |
| mKER/oIGF1 | Mouse ultrahigh-sulfur keratin/ovine insulin-like growth factor 1 | Wool | Damak et al., 1996b | |
| mKER/baCAT | Mouse ultrahigh-sulfur keratin/bacterial chloramphenicol acetyl transferase | Wool | Damak et al., 1996a | |
| Goat | mWAP/hLAtPA | Mouse whey acidic protein/human longer-acting tissue plasminogen activator | Therapeutic proteins in milk | Ebert et al., 1991 |
| bβCas/hFIX | Bovine β-casein/human factor IX | Therapeutic proteins in milk | Huang et al., 1998 | |
| cβCas/hAT | Caprine β-casein/human antithrombin | Therapeutic proteins in milk | Edmunds et al., 1998 | |
| cβCas/hG-CSF | Caprine β-casein/human granulocyte colony-stimulating factor | Therapeutic proteins in milk | Ko et al., 2000 | |
| cβCas/hLF | Caprine β-casein/human lactoferrin | Therapeutic proteins in milk | Zhang et al., 2008a | |
| bαS1Cas/hLZ | Bovine αs1-casein/human lysozyme | Therapeutic proteins in milk | Maga et al., 2003 | |
| cβCas/hBChE | Caprine β-casein/human butyrylcholinesterase | Therapeutic proteins in milk | Huang et al., 2007 | |
| bBLG/rSCD | Bovine β-lactoglobulin/rat stearoyl-coa desaturase | Alteration of milk composition | Reh et al., 2004 | |
| CMVp/cTLR2 | Cytomegalovirus promoter/caprine toll-like receptor 2 | Disease resistance | Deng et al., 2012b |
Table 2.
Examples of transgenic and gene-targeted sheep and goats produced using a somatic cell nuclear transfer (SCNT) approach.
| Species | Gene or construct abbreviation | Gene or construct name | Main trait | References |
|---|---|---|---|---|
| Sheep | oBLG/hFIX | Ovine β-lactoglobulin/human factor IX | Therapeutic proteins in milk | Schnieke et al., 1997 |
| oBLG/hα1AT | Ovine β-lactoglobulin/human α1-antitrypsin | Therapeutic proteins in milk | McCreath et al., 2000 | |
| CAGp/ceFat1 | Chicken β-actin promoter/Caenorhabditis elegans fat-1 | Enrich n-3 fatty acids | Duan et al., 2012 | |
| CMVp/oTLR4 | Cytomegalovirus promoter/ovine toll-Like receptor 4 | Disease resistance | Deng et al., 2013 | |
| oPrP* | Ovine prion protein | Disease resistance | Denning et al., 2001 | |
| Goat | cβCas/hAT | Caprine β-casein/human antithrombin | Therapeutic proteins in milk | Baguisi et al., 1999 |
| bαS1Cas/hLF | Bovine αS1-casein/human lactoferrin | Therapeutic proteins in milk | An et al., 2012 | |
| cβCas/hLF | Caprine β-casein/human lactoferrin | Therapeutic proteins in milk | Wan et al., 2012 | |
| bβCas/hLZ | Bovine β-casein/human lysozyme | Therapeutic proteins in milk | Liu et al., 2013c | |
| cβCas/hLZ | Caprine β-casein/human lysozyme | Therapeutic proteins in milk | Yu et al., 2013a | |
| bBLG/hLZ | Bovine β-lactoglobulin/human lysozyme | Therapeutic proteins in milk | Yu et al., 2013a | |
| cβCas/hAFP | Caprine β-casein/human α-fetoprotein | Therapeutic proteins in milk | Parker et al., 2004 | |
| cBLG/hPA | Caprine β-lactoglobulin/human plasminogen activator | Therapeutic proteins in milk | He et al., 2018a | |
| cβCas/hCuZn-SOD | Caprine β-casein/human copper-zinc superoxide dismutase | Therapeutic proteins in milk | Lu et al., 2018 | |
| cβCas/hEC-SOD | Caprine β-casein/human extracellular superoxide dismutase | Therapeutic proteins in milk | Lu et al., 2018 | |
| cBLG/hLA | Caprine β-lactoglobulin/human α-lactalbumin | Valuable molecule in milk | Yuan et al., 2014 | |
| cβCas/pfMSP142 | Caprine β-casein/Plasmodium falciparum merozoite surface protein 1 | Candidate malaria vaccine | Behboodi et al., 2005 | |
| cBLG/cGH | Caprine β-lactoglobulin/caprine growth hormone | Improve milk production | Zhang et al., 2014b | |
| cβCas/cIGF-1 | Caprine β-casein/caprine insulin-like growth factor I | Improve milk production | Lin et al., 2014 | |
| hEF1a1/eGFP | Human elongation factor-1α/enhanced green fluorescent protein | Marker gene | Keefer et al., 2001 | |
| CMVp/DsRed | Cytomegalovirus promoter/Discosoma sp. red fluorescent protein | Marker gene | Nuo et al., 2016 | |
| hsvTK/Neor | Herpes simplex virus thymidine kinase/neomycin | Marker gene | Zou et al., 2002 | |
| mαMHC/hTGF-β1 | Mouse alpha myosin heavy chain/human transforming growth factor-β1 | Disease model | Polejaeva et al., 2016 | |
| bβCas/hBD3 | Bovine β-casein/human β-defensin-3 | Disease resistance | Liu et al., 2013b | |
| CMVp/oAANAT | Cytomegalovirus promoter/ovine aralkylamine N-acetyltransferase | Disease resistance | Tao et al., 2018 | |
| cPRNP* | Caprine prion protein | Disease resistance | Yu et al., 2006 | |
| cBLG* | Caprine β-lactoglobulin | Alteration of milk composition | Zhu et al., 2016a |
*Gene targeting by homologous recombination.
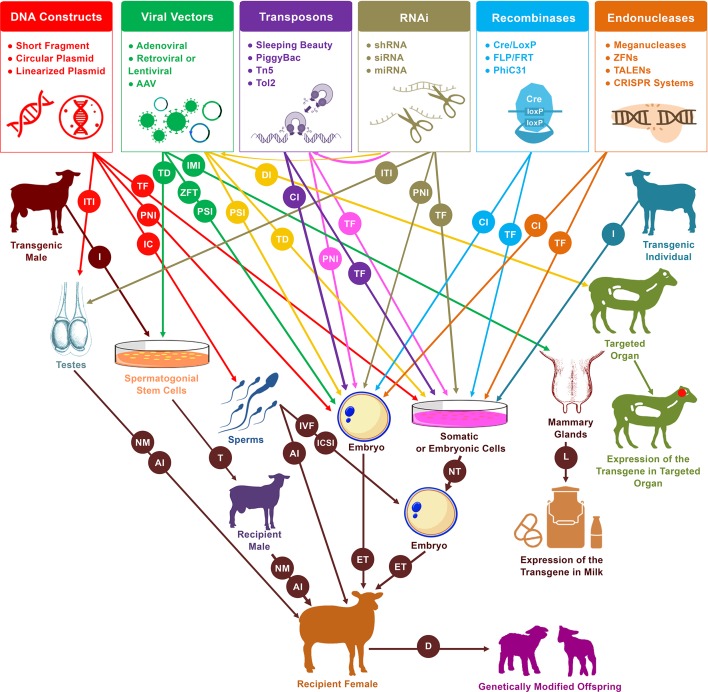
Figure 2.
Schematic representation of practical and likely pathways of genetic modification in sheep and goats. Pronuclear injection (PNI) and nuclear transfer (NT) are the two primary procedures for the generation of live founders with desired genetic modifications. In addition to these two approaches, several new tools have emerged that increase the efficiency and simplify the process of mediating genetic modification. These tools include sperm-mediated gene transfer (SMGT), viral vectors, recombinases, transposons, RNA interference (RNAi), and endonucleases. These have served to mediate manipulations in a variety of cells and organs, including somatic cells, embryonic cells, embryos, spermatozoa, spermatogonial stem cells (SSCs), testes, mammary glands, and other targeted organs. Different procedures are involved in the delivery of DNA constructs as well as the various enzymes and systems that induce genetic modification events within genomes. PNI, cytoplasmic injection (CI), perivitelline space injection (PSI), and zona-free transduction (ZFT) have been used for the delivery to embryos, transfection (TF), and transduction (TD) for the delivery to cells, incubation (IC) for the delivery to spermatozoa, intratesticular injection (ITI) for the delivery to testes, intramammary injection (IMI) for the delivery to mammary glands, and direct injection (DI) for the delivery to targeted organs (mainly for medical purposes). In vitro fertilization (IVF), intracytoplasmic sperm injection (ICSI), artificial insemination (AI) or even natural mating (NM) have been used for the delivery of transgenic sperms that resulted from incubation treatment, male germ cell transplantation, or intratesticular injection. In the diagram, from left to right, red arrows indicate the uses of DNA constructs for mediating DNA modification, green arrows indicate the uses of viral vectors, yellow arrows indicate the uses of RNAi molecules via delivery by viral vectors, purple arrows indicate the uses of transposons, pink arrows indicate the uses of RNAi molecules via integration by transposons, tan arrows indicate the independent uses of RNAi molecules, light blue arrows indicate the uses of recombinases, and orange arrows indicate the uses of endonucleases. Isolation (I) of spermatogonial stem cells from transgenic males can be used via transplantation (T) into infertile males to generate donor-derived spermatogenesis, which can then be used to generate transgenic founders. Furthermore, isolation of cells from transgenic individuals can also be used by nuclear transfer (NT) to generate transgenic progeny. Other abbreviations used in the diagram include embryo transfer (ET), lactation (L), and delivery (D).
CRISPR/Cas9 is a particularly simple, precise, and efficient gene-editing tool, which enabled its rapid and widespread application to different research fields. In our laboratory, CRISPR/Cas9 has been utilized to generate gene-edited sheep and goats both to investigate gene functions and to enhance economically important traits, such as muscle mass, fiber length, coat color, fat color, and litter size, among other traits (Table 3). In addition, a growing list of publications report applications of CRISPR/Cas9 in sheep and goats for other purposes such as manipulating the milk components, modeling human diseases, generating disease-resistant individuals, and developing hosts for growing human organs (Figure 1 and Table 3). In general, CRISPR/Cas9 has been used in sheep and goats to introduce different forms of modifications including gene knockout, multiplex gene knockout, gene knockin, point mutation using DNA oligo templates, single-nucleotide alteration using base editors, simultaneous gene knockout and gene knockin, and deletion via dual single-guide RNAs (sgRNAs) (Table 3). In addition to the other toolkits of genome engineering, CRISPR systems have shown unprecedented potential for the generation of gene-edited animals with defined genetic alterations.
Table 3.
Examples of gene-edited sheep and goats produced using the CRISPR/Cas9 system
| Species | Gene(s)* | Editing type | Delivery method | Transferred embryos/recipients/pregnancies | Pregnancy rate** | Obtained founders+ (birth rate***) | Mutated founders+ (targeting efficiency****) | References |
|---|---|---|---|---|---|---|---|---|
| Sheep | MSTN | KO | MI | 213/55/31 | 56.3% | 35 (16.4%) | 2 (5.7%) | Han et al., 2014 |
| MSTN | KO | MI | 53/29/19 | 65.5% | 22 (41.5%) | 10 (45.4%) | Crispo et al., 2015a | |
| MSTN, ASIP, BCO2 | M-KO | MI | 578/82/34 | 41.4% | 49 (8.4%) | 36++ (73.4%) | Wang et al., 2016b# | |
| MSTN | KO | MI | 130/N.A./N.A. | N.A. | 32 (24.6%) | 5 (15.6%) | Wu et al., 2018 | |
| MSTN | KO | SCNT | 415/20/8 | 40.0% | 6 (1.4%) | 3 (50.0%) | Zhang et al., 2018c | |
| FGF5 | KO | MI | 100/53/14 | 26.4% | 18 (18.0%) | 3 (16.6%) | Hu et al., 2017 | |
| FGF5 | KO | MI | 170/101/20 | 19.8% | 20 (11.7%) | 16 (80.0%) | Li et al., 2017c | |
| ASIP | KO | MI | 92/60/6 | 10.0% | 6 (6.5%) | 5 (83.3%) | Zhang et al., 2017a | |
| BMPR1B (FecBB) | PM | MI | 279/39/16 | 41.0% | 21 (7.5%) | 7+++ (33.3%) | Zhou et al., 2018# | |
| AANAT, ASMT | KI | MI | 593/150/77 | 51.3% | 98 (16.5%) | 34 (34.6%) | Ma et al., 2017 | |
| AANAT | KI | MI | 977/181/59 | 31.5% | 79 (8.0%) | 50 (63.2%) | Tian et al., 2018b | |
| tGFP | KI | MI | 30/N.A./N.A. | N.A. | 8 (26.0%) | 1 (12.5%) | Wu et al., 2016 | |
| CFTR | KO | SCNT | 1029/73/34 | 46.5% | 33 (3.2%) | 33 (100.0%) | Fan et al., 2018 | |
| ALPL | PM | MI | 41/17/9 | 52.9% | 15 (36.5%) | 6+++ (40.0%) | Williams et al., 2018 | |
| SOCS2 | BE | MI | 20/8/3 | 37.5% | 4 (20.0%) | 3+++ (75.0%) | Zhou et al., 2019# | |
| Goat | MSTN | KO | SCNT | 269/21/7 | 33.3% | 3 (1.1%) | 3 (100.0%) | Ni et al., 2014 |
| MSTN, FGF5 | M-KO | MI | 416/137/64 | 46.7% | 93 (22.3%) | 26++ (27.9%) | Wang et al., 2015# | |
| MSTN | KO | MI | 18/5/3 | 60.0% | 4 (22.2%) | 1 (25.0%) | Guo et al., 2016 | |
| MSTN | KO | MI | N.A./7/6 | 85.7% | 8 (N.A). | 6 (75.0%) | He et al., 2018b | |
| fat-1, MSTN | KI, KO | SCNT | 134/56/8 | 14.2% | 1 (0.74%) | 1 (100.0%) | Zhang et al., 2018a | |
| BLG | KO | MI | 103/67/18 | 26.8% | 26 (25.2%) | 4 (15.3%) | Zhou et al., 2017 | |
| GDF9 | PM | MI | 56/17/13 | 76.4% | 18 (32.1%) | 6+++ (33.3%) | Niu et al., 2018# | |
| EDAR | KO | SCNT | 257/79/5 | 6.3% | 6 (2.3%) | 6 (100.0%) | Hao et al., 2018 | |
| FGF5 | BE | MI | 22/7/3 | 42.8% | 5 (22.7%) | 5+++ (100.0%) | Li et al., 2018b# |
N.A., not available; KO, knockout; M-KO, multiplex knockout; KI, knockin; PM, point mutation (using HDR); BE, base editing; MI, microinjection; SCNT, somatic cell nuclear transfer; *(MSTN, myostatin also known as GDF8 growth differentiation factor 8; ASIP, agouti-signaling protein; BCO2, β-carotene oxygenase 2; FGF5, fibroblast growth factor 5; BMPR-1B “FecB”, bone morphogenetic protein receptor type 1B “Booroola fecundity gene FecBB mutation”; AANAT, arylalkylamine N-acetyltransferase or SANT serotonin N-acetyltransferase; ASMT, acetylserotonin methyltransferase; tGFP, turbo green fluorescent protein; CFTR, cystic fibrosis transmembrane conductance regulator; ALPL, alkaline phosphatase biomineralization associated; SOCS2, suppressor of cytokine signaling 2; BLG, β-lactoglobulin; GDF9, growth differentiation factor 9; EDAR, ectodysplasin receptor); *indicates to the full names (in the table legend) of the used gene abbreviations in this column. **pregnancy rate (%) = no. of pregnancies/no. of recipients; ***birth rate (%) = no. of obtained founders/no. of transferred embryos; ****targeting efficiency (%) = no. of mutated founders/no. of obtained founders; +alive and dead; ++total number of the obtained mutated founders whether they carry mutations in a single gene or in more than one gene; +++the founders might be mutated but not all of them only show the defined point mutation. #Models generated by our research team.
Menchaca et al. (2016) previously reported the tools used for the genetic modification of small ruminants. Here, we further extend this effort by presenting key examples of generated sheep and goat models and by providing an update on the evolution and potential of CRISPR/Cas9 applications in sheep and goats.
Overview of Genetic Modification and Relevant Biotechnological Advances in Sheep and Goats
Tremendous advances in the field of genetic engineering in animals have been achieved over the past few decades. Various strategies have been used to generate genetically modified animals with desired traits (Figures 1 and 2). Increasing the efficiencies of mediating specific genetic modifications and simplifying the procedures for generating genetically modified organisms were the main aims that challenged specialists in this field. Enormous and collective efforts have been made in science and technology to facilitate the ability to induce specific genomic manipulations using procedures that have become more efficient and simpler to use. In the following paragraphs, the various tools for mediating genetic manipulations in sheep and goats (Figure 2) as well as some other relevant biotechnological advances are presented.
The First Waves
Cloning by Embryo Splitting
Cloning by embryo splitting, also known as twinning by separation of blastomeres, is a set of primary reproductive techniques that have been applied to fulfill purposes related to the production of identical twins, multiplets, and chimeras. Embryo-splitting techniques were among the first strategies that led to the emergence of cloning. Based on the concept of cloning, researchers could produce genetically modified sheep and goats by using SCNT. Therefore, these multiplication strategies are discussed in this review. Previous work on sea urchins, salamanders, rats, rabbits, and mice have led to the application of these techniques in sheep and goats (Seidel, 1983; McLaren, 2000; Vajta and Gjerris, 2006). Sheep were among the first domestic animals that are subjected to embryo-splitting techniques. In 1979, Willadsen introduced a simple and successful procedure for blastomere separation in sheep (Willadsen, 1979). Willadsen aimed to develop new and highly selective methods for the breeding of farm animals, in addition to utilizing cells of cleaved embryos to increase the number of available embryos from superior mothers for the production of valuable offspring. Basically, the established procedure included the collection of cleaved embryos from super-ovulated donor females, blastomere separation, placement of each half into the zona pellucida, embedding in agar, and finally, transfer to recipient mothers. Willadsen summarized the results of ovine blastomere separation as follows: half embryos [single cells from two-cell embryos (1/2), pairs of cells from four-cell embryos (2/4), and a group of four cells from eight-cell embryos (4/8)] obtained ∼66% pregnancy rates. Quarter embryos [single cells from four-cell embryos (1/4), and pairs of cells from eight-cell embryos (2/8)] obtained ∼50% pregnancy rates. Eighth embryos (single cells from eight-cell embryos 1/8) obtained ≳5% pregnancy rates (Willadsen, 1989).
Embryo splitting was also applied to other livestock species including goats for both experimental and commercial purposes (Tsunoda et al., 1985; Udy, 1987). Different strategies have been adapted to improve the efficiency to produce an increased number of monozygotic animals. These strategies include the evaluation of isolated cells from different stages of development (Willadsen, 1980; Willadsen, 1981), presence or absence of intermediate hosts (Gatica et al., 1984), and presence or absence of zona pellucida (Shelton and Szell, 1988). The production of monozygotic animals via embryo splitting was a useful procedure for research and study of embryo development; however, the application of this procedure remained limited. Moreover, technical difficulties and suboptimal pregnancy rates lead to the production of only a relatively small number of individuals using this procedure. This is due to the limited divisibility of embryos to obtain two or occasionally up to four genetically identical animals. Although blastomere separation is considered as one of the basic cloning approaches, more promising approaches (such as nuclear transplantation) have opened the way for the large-scale production of genetically identical individuals.
Sheep–Goat Interspecific Chimerism
Despite the family relationship between sheep and goats, hybridization between both genera is an extremely rare event. Natural hybridization between sheep and goats is in most cases accompanied by high mortality rates of hybrid fetuses during the second month of pregnancy, likely as a result of fetal/maternal immunological incompatibility (Dent et al., 1971). By using an embryo aggregation strategy, researchers were able to produce sheep–sheep intraspecific chimeras (Tucker et al., 1974; Fehilly et al., 1984a). These fundamental studies indicated the aggregation ability of isolated blastomeres from early cleavage stage embryos to produce chimeric blastocysts that can be transferred to foster mothers (recipients) for the production of intraspecific chimeras. Based on these experiences and with the aim to increase the understanding of reproductive incompatibilities between species, in addition to providing a successful approach for interspecific hybridization, researchers were able to generate sheep–goat chimeras by using two basic techniques: embryo aggregation (Meinecke-Tillmann and Meinecke, 1984; Fehilly et al., 1984b) and embryonic cell injection into host blastocysts (Polzin et al., 1987). These procedures were based on the combination of blastomeres of two species, surrounding blastomeres of two species with each other, or injecting cells of the inner cell mass of one species into the blastocyst cavity of different species.
Sheep–goat chimeras differ from sheep–goat hybrids, which can be obtained when a goat naturally mates with a sheep. The phenotypic characteristics of sheep–goat chimeras include regions of both sheep-like wool and goat-like hair. Due to the mosaic nature of goat and sheep tissues in the produced chimeras, chimeric characteristics cannot be transferred to the next generation. Fertile sheep–goat chimeras can either pass on sheep or goat characteristics to their progeny depending on whether the reproductive organs of the chimera formed from caprine or ovine origins (Amoah and Gelaye, 1997). Interspecific chimerism may offer experimental approaches for developmental biology to investigate cell linkages, embryonic development interactions, reproductive incompatibilities, and embryo transfer opportunities. Although these approaches have been used to remove the reproductive barriers between species, the expanded use of these techniques to create new hybrids remained limited.
PNI
PNI was among the first techniques that have been applied to generate transgenic animals. It was the dominant methodology for the generation of transgenic animals during the first decade of animal transgenesis studies. By introducing DNA constructs into the pronuclei of fertilized eggs and transferring the injected eggs to foster mothers, researchers were able to generate transgenic animals (Figure 2). After reporting the generation of transgenic mice using this technique (Gordon et al., 1980; Gordon and Ruddle, 1981), in 1985, Hammer et al. (1985) were the first to report the generation of transgenic livestock. Sheep were among the first reported transgenic domestic animals; however, although Hammer et al. were able to generate a transgenic lamb, carrying the mouse metallothionein-I/human growth hormone (mMT/hGH) transgene, it did not express the integrated gene. The authors speculated that the reasons behind the low efficiencies might be due to the concentration of injected DNA, the composition of the used buffer, the stage of the collected embryos, and other structural aspects of the chromosomes. Further attempts have been made to overcome these obstacles and to facilitate the generation of transgenic animals using this technique. A few years later, in 1991, Ebert et al. (1991) reported the first generation of transgenic goats carrying the mouse whey acidic protein/human longer-acting tissue plasminogen activator (mWAP/hLAtPA) transgene using the same technique.
In both sheep and goats, the reported efficiencies of conventional PNI of DNA constructs were ∼1% of the injected zygotes (Clark, 2002). Several challenges caused these low efficiencies such as the random integration and the variable copy number of integrated DNA constructs. Because of these factors, the expression of the transgene can be unpredictable. A further technical challenge related to the application of PNI in livestock is the visualization of pronuclei. This is obstructed by the presence of a large amount of lipid granules in livestock eggs, which results in a nontransparent cytoplasm, thus hampering the localization of pronuclei. The pronuclei of ovine eggs can be visualized using differential interference contrast (DIC) microscopy (Hammer et al., 1985). In other species such as goats, a centrifugation step (12,000 × g for 5 min) improves the visualization of pronuclei (Freitas et al., 2016). Despite the suboptimal efficiencies of the conventional PNI, a large number of transgenic sheep and goats have been generated. Prominent examples of generated transgenic sheep and goats using PNI are shown in Table 1. The contributions of classical PNI of DNA constructs equipped the global transgenic sheep and goat sector with novel and useful genetically modified models. Furthermore, new forms of oocyte/zygote microinjection have emerged that provide simpler strategies for the introduction of desirable manipulations to the genomes of sheep and goats.
Embryonic Cell Cloning
Cloning can happen naturally in a number of living organisms via asexual reproduction and can also be artificially introduced in mammals by using primary techniques such as embryo splitting (Vajta and Gjerris, 2006). Efforts in embryo manipulation research led to the development of technical tools that enabled the production of identical individuals as well as intra- and inter-specific chimeric individuals (Willadsen, 1979; Fehilly et al., 1984a; Fehilly et al., 1984b). During the second wave of the development of these enabling technical tools, new and more advanced techniques emerged. Nuclear transfer, or nuclear transplantation, as it was first called, was developed to overcome the limitations of embryo-splitting techniques such as the limitation of the number of individuals that can be produced from a single split embryo. Despite the first attempts of nuclear transplantation in non-mammalian animal species and laboratory mice (Meissner and Jaenisch, 2006), the first cloned mammal (sheep) from undifferentiated embryonic blastomeres was reported in 1986 by Willadsen (Willadsen, 1986). Willadsen used ovine 8 to 16-cell stage embryos as nuclear donors in combination with ovine enucleated metaphase II oocytes as recipient cytoplasts to produce live lambs. Willadsen aimed to define suitable conditions required for the large-scale cloning of domestic animals. Despite the scientific significance of Willadsen’s work, academic and public attention was attracted later when more technically challenging cells have been used to produce viable cloned offspring (Vajta and Gjerris, 2006).
About 10 years later, in 1996, Campbell et al. reported the production of cloned lambs using long-term cultured and more differentiated embryonic cells rather than early embryonic or primary cultured embryo-derived cells (Campbell et al., 1996). Campbell et al. offered more choices for nuclei sources and attempted to overcome the limitations of the use of embryonic blastomeres (e.g., the limited number of this type of cells and their uncertain ability of long-term culture). This was in response to the unsuitability of the utilization of this type of cells in genetic modification programs (Colman, 1999). 1 year later, the same group reported pioneering and unprecedented work of utilizing fetal and adult mammalian cells to produce viable offspring, which resulted in the generation of the first and most famous somatic cloned animal in the world, the sheep Dolly (Wilmut et al., 1997). In the same year, a further advance has been accomplished by the generation of the first transgenic cloned sheep carrying human coagulation factor IX (hFIX) gene from transfected fetal fibroblasts (Schnieke et al., 1997). This success of cloning approaches in sheep was followed by several attempts to clone various species, including goats. Cloned goats were first produced by early embryonic blastomeres (Yong et al., 1991; Yong and Yuqiang, 1998). Subsequently, fetal somatic cells were used for the generation of transgenic cloned goats carrying human antithrombin III (hAT) gene (Baguisi et al., 1999). The aims of the cloning of domestic animals have been altered due to rapid and significant advances in the field. In addition to utilizing this approach as a valuable tool in embryological studies and to achieve the multiplication of desired genetics, nuclear transfer has become one of the basic methods to generate genetically modified animals with useful and desired traits.
Somatic Cell Cloning
Somatic cell cloning or SCNT has emerged with the creation of Dolly, the sheep from a mammary gland cell of a 6-year-old ewe, taken by Wilmut and his colleagues in 1997 (Wilmut et al., 1997). In addition to the putative aims of animal cloning such as multiplying superior animals for the construction of highly productive flocks for agricultural purposes and the restoration of endangered or even extinct species, the application of SCNT in genetic modification programs of farm animals has attracted wide attention. The emergence of SCNT has removed the barriers that inhibited the implementation of gene targeting by homologous recombination (HR) in species that lack embryonic stem cells (ESCs) to generate authentic genetically modified individuals. Moreover, implementation of SCNT for the manipulation of animal genomes has overcome several of the drawbacks of previously emerged PNI such as the low level of transgene integration, the variability of transgene expression, the unpredictable transmission of the transgene to the next generation, and founder mosaicism.
Transgenic farm animals can be produced using SCNT via transfection of donor cell nuclei with DNA expression constructs or vectors or by cloning transgenic founder animals (Figure 2). Various cell types have been utilized as nucleic donors to generate cloned sheep and goats. These include adult mammary gland cells (Wilmut et al., 1997), adult granulosa cells (Keefer et al., 2002; Loi et al., 2002), adult cumulus cells (Zou et al., 2001), fetal fibroblast cells (Wilmut et al., 1997; Baguisi et al., 1999), and other potentially utilizable cells such as fetal skeletal muscle–derived satellite cells (Ren et al., 2014). Fetal fibroblast cells have been used dominantly for the generation of transgenic cloned sheep and goats among other reported cell types. After the first generation of transgenic cloned sheep reported in 1997 (Schnieke et al., 1997), and the first transgenic cloned goats in 1999 (Baguisi et al., 1999), a large number of transgenic and gene-targeted cloned sheep and goats have been generated. Examples of generated transgenic sheep and goats using SCNT are listed in Table 2. Despite the relatively low efficiency of SCNT and the potential for developmental anomalies, in parallel with the PNI technique, SCNT has become a basic and dominant methodology to generate transgenic and gene-targeted sheep and goats.
Interspecific Cloning
In addition to the promising advantages of SCNT for the multiplication of genetically valuable or superior livestock and the manipulation of the genomes of experimentally, biomedically, and agriculturally important animals, SCNT offers promising potential for the conservation of genomes of endangered species and for restoring or reviving the genomes of extinct species. Finding effective tools to conserve and restore threatened genomes is equally important to finding new tools for the manipulation of existing genomes to generate novel and desirable phenotypes. Interspecies cloning or interspecies somatic cell nuclear transfer (iSCNT) is one of the emerging strategies to conserve genetic diversity and prevent the rapid loss of animal genetic resources. Genetic rescue programs based on iSCNT use nuclei from endangered species in the wild, whose oocytes are difficult to obtain, with oocytes from closely related domesticated species to reconstruct embryos that can then be transferred to foster mothers. The resultant offspring of this process resembles nucleic donors.
Sheep and goats were among the closely related domesticated species that were utilized in the conservation cloning programs of threatened species that belong to the genera Ovis and Capra. Loi et al. (2001) reported the successful generation of a cloned mouflon from reconstructed embryos, combining mouflon post mortem somatic cells, and domestic sheep oocytes. This provided an encouraging example of the application of iSCNT for the generation of live founders. Further examples of the implementation of iSCNT using sheep and goats to reconstruct embryos between species within the same genera are listed in Table 4. Despite the successful attempts of reconstructing embryos between two species from closely related genera, the number of viable offspring produced using this strategy was very low. Such low efficiency might be a result of implantation failure or of immunological rejection (Wang et al., 2001). Other embryonic combinations have also been reported for the study of developmental ability, mitochondrial heteroplasmy, and nuclear-cytoplasmic interactions between different species (Table 4). Despite the low numbers of publications reporting the successful generation of viable offspring using iSCNT, research in this field still offers great potential in interspecies embryological studies and genetic resource conservation programs.
Table 4.
Examples of interspecies somatic cell nuclear transfer (iSCNT) applications in sheep and goats for the reconstruction of embryos between different species.
| Intra-/Inter-genera | Nucleus donor | × | Oocyte donor | References |
|---|---|---|---|---|
| Within the same genus | Argali (Ovis ammon) | × | Sheep (Ovis aries) | White et al., 1999 |
| European mouflon (Ovis orientalis musimon) | × | Sheep (Ovis aries) | Loi et al., 2001 | |
| Esfahan mouflon (Ovis orientalis isphahanica) | × | Sheep (Ovis aries) | Hajian et al., 2011 | |
| Ibex (Capra ibex) | × | Goat (Capra hircus) | Wang et al., 2007 | |
| Between different genera | Tibetan antelope (Pantholops hodgsonii) | × | Goat (Capra hircus) | Zhao et al., 2007 |
| Goat (Capra hircus) | × | Sheep (Ovis aries) | Ma et al., 2008 | |
| Goat (Capra hircus) | × | Bovine (Bos taurus) | Tao et al., 2008 | |
| Goat (Capra hircus) | × | Buffalo (Bubalus bubalis) | Selokar et al., 2011 | |
| Sheep (Ovis aries) | × | Bovine (Bos taurus) | Hua et al., 2008 | |
| Human (Homo sapiens) | × | Goat (Capra hircus) | Sha et al., 2009 | |
| Human (Homo sapiens) | × | Sheep (Ovis aries) | Hosseini et al., 2012 |
Handmade Cloning
Handmade cloning (HMC) is a simplified version of the SCNT technique. This modified technique has emerged to overcome the technical difficulties that obstruct the improvement and widespread application of SCNT. In traditional SCNT, oocyte enucleation is one of the technical steps which encounter major obstacles due to the presence of the zona pellucida (the outer thick membrane of mammalian oocytes) (Lagutina et al., 2007). Thus, introducing careful manipulations inside the zona pellucida to replace the nuclei (enucleating the oocyte nucleus and transferring the somatic cell nucleus) requires expensive instruments such as micromanipulators as well as both skill and time (Vajta and Gjerris, 2006). The simplicity of HMC is mainly based on the removal of the zona pellucida after maturation and before enucleation. In this case, sophisticated micromanipulators are not necessary because the manipulations required for both enucleation and nucleus transfer are performed by hand as indicated by the name (Vajta, 2007). Basically, the procedure of HMC includes handmade bisection of zona-free oocytes, staining and selection of cytoplasts, and fusion of the somatic cell with two cytoplasts to generate an equally sized reconstructed embryo (Vajta et al., 2006). Thus, the implementation of SCNT using this approach requires less expertise, time, and cost.
Initial attempts to use zone-free procedures, especially in embryonic cell nuclear transfer, have led to the first successful report to produce cloned cattle, using a somatic cell as nucleus donor (Vajta et al., 2001). In sheep and goats, initial publications have reported the application of this technique for successful embryo development (Peura and Vajta, 2003; Akshey et al., 2008), followed by a number of publications reporting the application of this technique to produce cloned (Malik et al., 2014; Khan et al., 2018), transgenically cloned (Lagutina et al., 2007; Pereira et al., 2013), or interspecies cloned embryos (Selokar et al., 2011; Yu et al., 2011). An interesting example of the application of this technique in sheep is the generation of transgenic cloned lambs carrying a modified nematode mfat-1 gene to enrich muscles and other organs and tissues with omega-3 fatty acids (Zhang et al., 2013). In this study, one of the three generated founders showed a lower omega-6/omega-3 ratio, indicating the converting role of the integrated mfat1 gene. Despite the relatively equivalent efficiency of HMC compared to traditional SCNT, as well as the further advantages of HMC (simpler to use, cheaper, and more time-saving), the applications of HMC in sheep and goats to produce viable founders have not been studied in detail. In general, despite the simplification provided by HMC and other emerging strategies, SCNT remains technically challenging, and few research groups around the world are able to perform it efficiently (Tan et al., 2016). Further advances in this field are required to enable the widespread application of these techniques to facilitate multiplication, transgenesis, and genetic rescue of threatened genomes.
Additional Tools for Transfer and Manipulation
Sperm-Based Transgenesis
Spermatozoa have the natural ability to obtain exogenous DNA by a simple incubation procedure (Brackett et al., 1971; Lavitrano et al., 1989). This significant observation opened the way for further alternative strategies that can be utilized in transgenesis programs. Basically, three main strategies have been used to mediate transgenesis that utilize the male side (spermatozoon), namely, male germ cell transplantation–mediated transgenesis, sperm-mediated gene transfer (SMGT), and testis-mediated gene transfer (TMGT) (Figure 2).
In addition to its importance for spermatogenesis and fertility studies, male germ cell transplantation has been suggested to be an alternative tool to mediate transgenesis. Spermatogonial transplantation uses isolated spermatogonial stem cells (SSCs) from desirable male donors and injects and transplants these into the seminiferous tubules of an infertile recipient males, which results in donor-derived spermatogenesis. In this case, transgenesis can be mediated by manipulating SSCs prior to transplantation into the recipient males or by transferring transgenic donor germ cells from transgenic individuals (Hill and Dobrinski, 2006). This technique has been initially established in rodents (Brinster and Avarbock, 1994; Brinster and Zimmermann, 1994). Although this technique has basically been extended to farm animals including goats (Honaramooz et al., 2003a; Honaramooz et al., 2003b; Zeng et al., 2012) and sheep (Rodriguez-Sosa et al., 2006), the application of this technique to produce transgenic founders is limited. A recent study in a pig model used genetic manipulation via CRISPR/Cas9 for the generation of male recipient models for SSC transplantation by targeting the nanos C2HC-type zinc finger 2 (NANOS2) gene (Park et al., 2017). Homozygous knockout males showed an ablation of the male specific germline with intact testicular development, while heterologous knockout males and females were fertile. This offers an advantage in agriculture where these models serve as recipients for donor spermatogonial stem cells from genetically valuable males, thus expanding the availability of desirable genetics.
SMGT is directly based on the intrinsic ability of sperm cells to capture and internalize exogenous DNA (Lavitrano et al., 2006). After a simple step of incubating sperm cells with exogenous DNA, transfected sperm cells can then be transferred to female (eggs) using various strategies, such as artificial insemination (AI) (Zhao et al., 2010), intracytoplasmic sperm injection (ICSI), in vitro fertilization (IVF), or laparoscopic insemination (LI) (Pereyra-Bonnet et al., 2011), with varied efficiencies. SMGT application in sheep and goats includes the production of both transgenic embryos (Pereyra-Bonnet et al., 2008; Shadanloo et al., 2010; Pereyra-Bonnet et al., 2011; Pramod et al., 2016) and transgenic founders (Zhao et al., 2010) using marker transgenes. Despite the simplicity this approach offers, its application for the generation of transgenic sheep and goats remained limited. This might be a result of a number of drawbacks of this approach such as the low incorporation of the exogenous genes. In general, several attempts have been reported to enhance the ability of sperm to obtain exogenous DNA. These include electroporation-, liker-, retroviral-, liposome (lipofection)-based SMGT, restriction enzyme–mediated integration, and further techniques (Smith, 2012). The optimization of this approach might increase its efficiency.
TMGT or intratesticular injection are further alternative tools based on the direct injection of testes with exogenous DNA. After a specific interval, injected males can then be used to naturally mate with females to produce transgenic founders. TMGT was initially applied in sheep and goats to produce transgenic founders with inserted genes including lipoprotein lipase (LPL) (Qin et al., 2012), solute carrier family 7 member 11 (SLC7A11) (He et al., 2012), peroxisome proliferator–activated receptor gamma (PPARγ) (Qin et al., 2013), myogenin (MyoG) (Zhang et al., 2014c), and enhanced green fluorescent protein (eGFP) (Raina et al., 2015; Pramod and Mitra, 2018). In general, despite the potential possibilities of sperm-based strategies to mediate transgenesis, these strategies still require optimization. Integration between these strategies and newly emerging targeting tools might be of great importance for the generation of desirable genetically modified sheep and goats.
Virus-Based Transgenesis
Viral vectors have been used to mediate transgenesis by delivering and integrating transgenes into the host genome. Viral vectors can be divided into non-integrating viral vectors (e.g., adenoviral vectors), and integrating viral vectors that are mostly derived from a retrovirus, lentivirus, and adeno-associated virus (AAV) (Pfeifer and Hofmann, 2009). Viral vectors have been used after being made replication-deficient by deleting genes that are essential for viral pathogenesis and/or replication (Nakagawa and Hoogenraad, 2011).
Serval strategies have been utilized that use of viral vectors as vehicles or carriers to deliver chosen exogenous DNA constructs into targeted expression positions (Figure 2). One of the basic strategies that uses viral vectors is the direct intramammary injection via the teat canal for the transient production of valuable proteins in milk. Goats are an ideal model for the production of pharmaceutical molecules in milk and have been subjected to this protocol. Human growth hormone (hGH) was among the first published genes to be infused into the goat mammary gland using retroviral vectors (Archer et al., 1994). The same strategy has also been applied using adenoviral vectors to direct the expression of functional proteins into mammary secretory epithelial cells. Examples of the adenoviral vector–mediated transfer of genes infused via the teat canal of goat mammary glands are listed in Table 5 .
Table 5.
Examples of adenoviral-mediated gene transfer into the teat canal of caprine mammary glands.
| Gene abbreviation | Gene full name | References |
|---|---|---|
| Lys | Lysostaphin | Fan et al., 2002 |
| hGH | Human growth hormone | Sánchez et al., 2004; Han et al., 2009 |
| hEPO | Human erythropoietin | Toledo et al., 2006; Liu et al., 2010 |
| hLTF | Human lactoferrin | Han et al., 2007 |
| CSFV-E2 | Classical swine fever virus E2 | Toledo et al., 2008; Sánchez et al., 2014 |
| hNGF-β | Human nerve growth factor beta | Xiao et al., 2009 |
| hAT | Human antithrombin | Yang et al., 2009 |
| hGCase | Human glucocerebrosidase | Tavares et al., 2016 |
A further strategy that involves the transduction of goat male germline stem cells with an adeno-associated viral vector carrying eGFP marker gene, resulted in transgene transmission after germ cell transplantation (Honaramooz et al., 2008). Lentiviral vectors carrying the eGFP marker gene have also been applied to transduce sheep (Rodriguez-Sosa et al., 2009) and goat (Abbasi et al., 2015) spermatogonia prior to transplantation and colonization into male recipients. This approach might be a useful tool for the generation of transgenic founders, in particular, since it requires minimal embryo handling.
Viral vectors coupled with an RNAi mechanism have been used to mediate the loss of gene expression for the investigation of potential biological functions of genes, to suppress the expression of disease-related genes, and to inhibit the expression of genes that negatively regulate economically important traits. Recombinant adenoviruses that carry short hairpin RNA (shRNA) and that target goat parathyroid hormone–related protein (PTHrP) in mammary epithelial cells successfully inhibited PTHrP gene expression (Zheng et al., 2013). Lentiviral vector–based delivery of shRNA has also been used in both ovine and caprine cells to suppress the expression of target genes ( Table 6 ). Additionally, injecting AAV9-miRNA targeting human huntingtin (HTT) in the striatum of transgenic Huntington’s disease (HD) sheep so that these express the full-length HTT gene reduced the mRNA and protein of human HTT by 50–80% in the striatum at 1 and 6 months postinjection (Pfister et al., 2018).
Table 6.
Examples of ovine and caprine targeted gene expressions using RNA interference (RNAi).
| Species | Gene abbreviation | RNAi molecule | Delivery tool | Cell type | References |
|---|---|---|---|---|---|
| Sheep | MSTN | shRNA | Lentiviral vector | Myoblasts | Liu et al., 2012; Liu et al., 2014 |
| MSTN | shRNA | Lentiviral vector | Fibroblasts | Tang et al., 2012 | |
| MSTN | siRNA | Synthesized construct | Fibroblasts | Lu et al., 2012 | |
| TRIM28 | siRNA | Synthesized construct | Fibroblasts | Luo et al., 2017 | |
| INHα | siRNA | Synthesized construct | Granulosa cells | Li et al., 2017a | |
| Goat | MSTN | shRNA | Lentiviral vector | Fibroblasts | Lu et al., 2013; Patel et al., 2015 |
| MSTN | shRNA | Lentiviral vector | Myoblasts | Patel et al., 2014; Kumar et al., 2018 | |
| MSTN | shRNA | Expression construct | Fibroblasts | Jain et al., 2010; Singh et al., 2012; Jain et al., 2015; Hati Boruah et al., 2016 | |
| MSTN | shRNA | Expression construct | Myoblasts | Tripathi et al., 2013 | |
| MSTN | siRNA | Synthesized construct | Myoblasts | Kumar et al., 2014 | |
| MSTN | miRNA | Expression construct | Fibroblasts | Zhong et al., 2014 | |
| PrP | shRNA | Lentiviral vector | Fibroblasts | Golding et al., 2006 | |
| BLG | shRNA | Lentiviral vector | Fibroblasts | Zhang et al., 2012 | |
| PPARγ | shRNA | Lentiviral vector | Fat cells | Du et al., 2018 | |
| PGC-1α | shRNA | Lentiviral vector | Granulosa cells | Zhang et al., 2016a | |
| DNMT1 | shRNA | Expression construct | Fibroblasts | Lan et al., 2010 | |
| FKBP38 | shRNA | Expression construct | Fibroblasts | Fu et al., 2015 | |
| BZW2 | shRNA | Expression construct | Mammary epithelial cells | Sun et al., 2012 |
shRNA, short hairpin RNA; siRNA, small interfering RNA; miRNA, microRNA; MSTN, myostatin; TRIM28, tripartite motif containing 28; INHα, inhibin α-subunit; PrP, prion protein; BLG, β-lactoglobulin; PPARγ, peroxisome proliferator-activated receptor γ; PGC-1α, peroxisome proliferator-activated receptor γ coactivator-1 α; DNMT1, DNA methyltransferase 1; FKBP38, FK506-binding protein 38; BZW2, basic leucine zipper and W2 domains 2.
Viral vectors can also be used to mediate transgenesis into zygotes by using two main ways: viral transduction of zona-free embryos and perivitelline space (subzonal) injection (Pfeifer and Hofmann, 2009). Both strategies have been applied using lentiviral vectors in sheep. Ritchie et al. have applied a lentiviral transduction protocol with eGFP marker gene for both zona-free and split embryos and reported potential possibilities for the application of this approach in small ruminants (Ritchie et al., 2005; Ritchie et al., 2009). Perivitelline space injection of lentiviral vectors that carry eGFP marker genes has shown efficient expression of green fluorescence in transgenic lambs (Liu et al., 2013a; Crispo et al., 2015b). Despite these results, using a 2A peptide–based tricistronic lentiviral vector for the expression of three fluorescent protein genes subjected to hypermethylation and silenced the expression of transgenes in transgenic sheep (Tian et al., 2013). Furthermore, cells derived from these transgenic sheep could achieve expression of transgenes when the epigenetic status has been regulated via methyltransferase and deacetylase inhibitors (Tian et al., 2013).
Other interesting examples include the production of transgenic sheep via perivitelline space injection of lentiviral vectors encoding shRNAs that have been specifically designed to inhibit the replication of foot-and-mouth disease virus (FMDV), or to silence the expression of myostatin (MSTN) to promote muscle growth (Cornetta et al., 2013). This report also provides additional safety information in support of the application of this technology. Additionally, recombinant lentivirus carrying the fibroblast growth factor 5–short alternative transcript (FGF5s) was used to produce transgenic sheep with enhanced fiber growth (Li et al., 2017b). Viral vector–based transgenic strategies have shown moderate efficiency compared to other conventional methodologies that have been used to mediate transgenesis. Despite the moderate efficiency of these strategies, newly emerging gene modifying tools are likely a better choice or are at least simpler and safer to use.
Recombinases
Recombinases are enzymes that promote site-specific genetic recombination. Recombinases are derived from nature and possess the ability to perform deletions, insertions, and inversions into DNA sequences via interaction between the recombinases and their own recognition sites (Olorunniji et al., 2016). Site-specific recombinases have been integrated into genome engineering programs for a variety of purposes. Manipulations using recombinases have been applied to sheep and goat genomes via Cre recombinase, Flp recombinase, and PhiC31 integrase (Figure 2).
In general, the Cre/loxP system uses bacteriophage P1-derived Cre recombinase, which acts on a 34-bp sequence called loxP. This 34-bp sequence consists of two 13-bp inverted or palindromic repeats separated by an 8-bp spacer region (Hoess and Abremski, 1984). The Cre/loxP site–specific recombination system was used to excise the selectable genes from goat transgenic cells (Xu et al., 2008). Xu et al. have combined both the Cre/loxP recombination system and protein transduction technology and produced TAT-Cre recombinase, which is a recombinant cell-permeable fusion protein. This recombinant protein was used to optimize the efficiency of delivery and to eliminate cytotoxic and genotoxic effects of both the integration and continuous expression of Cre recombinase-expressing vectors (Xu et al., 2008). Briefly, the authors used primary skin fibroblasts from β-lactoglobulin (BLG) transgenic goats that carried both the human lysozyme (LYZ) vector and selectable gene-expressing vector, which contained a left loxP site, neomycin resistance gene Neo, a thymidine kinase gene TK, and a right loxP site. After TAT-Cre protein transduction, one of the TAT-Cre treated cell colonies was used as a nuclear donor to perform nuclear transfer. Two selectable gene-free cloned goats were produced with removed Neo/TK cassette (Xu et al., 2008).
The yeast Saccharomyces cerevisiae derived Flp/FRT system functions in an analogous fashion to the Cre/loxP system, in which flippase (Flp) recognizes and cleaves two FRT recognition sites (Rafferty and Quinn, 2018). The Flp/FRT site–specific recombination system has been applied in goat somatic cells to mediate the site-specific integration and to eliminate the problematic random integration of transgenes (Yu et al., 2013b). Briefly, Yu et al. first performed gene targeting by HR thus introducing an FRT-docking site into the α1 (I) procollagen (ColA1) locus (HR efficiency of 5.9% “11/185”). Cell clones with successful targeting have been subjected to embryo cloning to achieve rejuvenation or regeneration. Cells with the FRT-homing site were isolated from cloned fetuses and co-transfected with two vectors, an eGFP replacement vector, and an Flp recombinase-expressing vector. After the second round of recombination, transgenic cells exhibited functional expression of eGFP with a gene replacement efficiency of 38.4% (15/39) (Yu et al., 2013b).
Streptomyces bacteriophage–derived ΦC31 (PhiC31) integrase has been applied to mediate HR between attB and corresponding pseudo attP sites (Andreas et al., 2002). Three pseudo-attP sites were identified in the sheep genome (Ni et al., 2012), while eight pseudo-attP sites were identified in the goat genome (Ma et al., 2014). These pseudo-attP sites were located in intron or intergenic regions. PhiC31 integrase has been applied in sheep (Ni et al., 2012) and goat (Ma et al., 2014) primary fibroblasts to integrate the eGFP marker cassette, showing an increase in gene integration efficiency. Recombinases have been used to perform modifications such as selectable cassette excision or the integration of marker or overexpression cassettes. Further strategies can also be used to achieve desirable modifications in a relatively simple and efficient manner.
Transposons
Transposable elements or transposons have been used for genetic manipulation after highlighting their role as active non-viral DNA delivery systems (Largaespada, 2003). These systems have been applied to sheep and goat genomes such as the Sleeping Beauty transposon and the PiggyBac transposon (Figure 2). The Sleeping Beauty transposon system works through the mediation of transposase to directly integrate Sleeping Beauty transposon into, mainly, thymine-adenine TA-dinucleotide sites of the target genome (Ivics et al., 1997). In sheep, the Sleeping Beauty transposon system was used in combination with RNAi to knockdown MSTN gene expression (Hu et al., 2011). Sleeping Beauty–mediated shRNA expression in transfected sheep fetal fibroblasts showed a significant decrease of MSTN expression, exceeding that of the random integration of anti-MSTN shRNA (Hu et al., 2011). This highlights the potential for the combination of the Sleeping Beauty transposon with RNAi to generate knockdown donor cells for animal cloning. This combined system was also used to generate transgenic sheep with resistance to FMDV by knocking down the FMDV-VP1 gene (Deng et al., 2017). After pronuclear microinjection, eight out of 92 generated lambs showed positive integration of VP1-shRNA. This study also reported that shRNA mediated by the Sleeping Beauty transposon achieved an increased integration rate over random integration of anti-VP1 shRNA. Furthermore, Sleeping Beauty transposase and Tn5 transposase were cytoplasmically injected into sheep zygotes to integrate transposon containing recombinant human factor IX (rhFIX) driven by the BLG promoter (Bevacqua et al., 2017). No transgenic lambs have been obtained from Tn5 transposase injection, while injection of Sleeping Beauty transposase resulted in two lambs that carried the transgene 2/7 (29%) (Bevacqua et al., 2017). More animals have to be produced for the accurate detection of Tn5 efficiency.
Another interesting transposon system, called the PiggyBac, has been applied by our team in cashmere goats. The PiggyBac transposon system was tested to mediate eGFP gene expression and to generate stably transfected cell lines with the use of goat fetal fibroblasts (Bai et al., 2012). The generated cell lines have shown a high level of eGFP mRNA. Furthermore, transfected cells with the same set of vectors (eGFP/Neo gene replacement vector and PiggyBac transposase–expressing vector) were used as donor cells to produce transgenic goats via SCNT (Bai et al., 2017). 14 cloned embryos were implanted into 20 recipient females; however, only one live transgenic kid was produced with a strong expression of the eGFP gene in the horns, hooves, nose, and hair. The PiggyBac transposon system has also been used to mediate overexpression of the thymosin β-4 (Tβ4) gene to improve the production of fine hair in cashmere goats (Shi et al., 2017). The hair follicle-specific keratin-associated protein 6.1 (KAP6.1) promoter was used to construct the Tβ4-overexpressing cassette. Using the same strategy, transfected goat fetal fibroblasts with the integration of Tβ4 gene–expressing cassette have been used as donor cells to generate transgenic goats via SCNT (Shi et al., 2017). Five transgenic cloned founders were produced and showed an increased number of secondary hair follicles that produce the commercially desirable fine cashmere hair. Transposon systems have shown potential for the mediation of integrations that can result in gene knockdown or gene overexpression. In addition to the newly emerging toolkits of genetic engineering, transposons can contribute to the generation of desirable genetically modified cell lines and organisms.
RNAi
RNAi is an interesting natural mechanism that has been applied in genetic manipulation programs due to its ability to simply suppress (knockdown) gene expression by silencing the mRNA of the targeted gene. RNAi has mainly been used to understand the biological and functional roles of specific genes, investigate the inhibitory effects of gene expression, suppress the expression of pathogenic genes, and inhibit the expression of genes that negatively regulate desirable phenotypes. shRNA and small interfering RNA (siRNA) are the main forms of RNAi molecules that have been used to mediate gene knockdown in sheep and goat genomes. In sheep and goats, a set of strategies has been used to deliver RNAi-expressing constructs, to mediate the generation of knockdown cell lines, and to obtain transgenic individuals. These methods include the transfection of RNAi-expressing vectors, viral vectors (mainly lentiviral vectors), transposons (e.g., the Sleeping Beauty transposon system), SCNT, PNI, intratesticular injection, and direct injection into the targeted organ (Figure 2).
To equip sheep and goat models with potential disease resistance, RNAi has been used to suppress the expression of goat prion protein (PrP). The examined brain tissues of a transgenic cloned fetus from lentiviral-shRNA transfected fibroblasts showed a significant decrease in PrP expression (Golding et al., 2006). Tongue epithelium cells isolated from transgenic goats carrying shRNA against the FMDV 3Dpol gene showed effective resistance after FMDV challenge (Li et al., 2015). Moreover, ear fibroblasts isolated from transgenic lambs that carried shRNA against the FMDV-VP1 gene showed a significant inhibitory effect on the VP1 gene (Deng et al., 2017). These studies highlight the possibilities for the use of RNAi strategies to confer potential disease resistance in farm animals.
In addition to providing models with resistance to epidemic diseases, the provision of models with enhanced economical traits is of great importance. Disrupting the normal function of the negative muscle-mass regulator MSTN offers promising potential for the promotion of meat production. Increasing animal muscle growth and body weight is one of the ultimate goals in agriculture. The MSTN gene is an attractive target to fulfill this purpose. Many studies have been published about the disruption of ovine and caprine MSTN using RNAi and a significant decrease of MSTN expression has been reported ( Table 6). Moreover, MSTN-shRNA-expressing transgenic lambs have been produced via SCNT and showed a faster increase in body weight than control individuals (Hu et al., 2013).
In addition, a set of genes have also been investigated using RNAi in a variety of ovine and caprine cells. Examples of these genes are shown in Table 6. A further strategy is based on the injection of testis with shRNA vectors, which was performed to target the zinc finger protein Y-linked (ZFY) gene. This strategy was applied as a genetic method of sex control and to bias the sex ratio toward females in sheep (Zhang et al., 2018b). In general, RNAi-based approaches are an interesting tool for mediating loss of gene expression by targeting the products of gene transcription (mRNA). Currently, more advanced strategies can be easily and directly applied to knockout the gene sequence instead of its mRNA, thus ensuring complete disruption of the associated gene function (Figure 3).
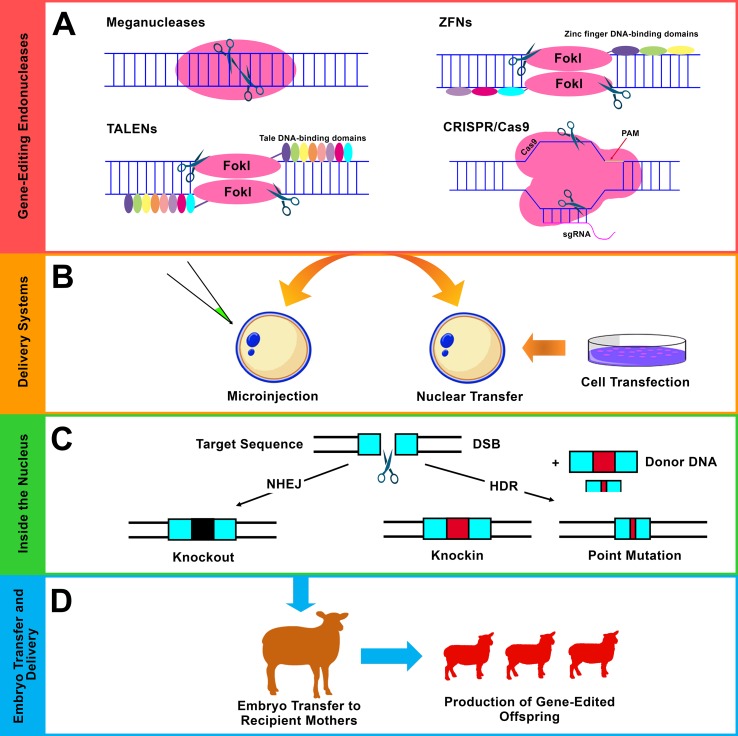
Figure 3.
Gene editing using site-specific endonucleases. (A) The four major classes of endonucleases: meganucleases, ZFNs, TALENs, and CRISPR/Cas9. Flavobacterium okeanokoites, type IIS restriction enzyme (FokI), protospacer adjacent motif (PAM), and single-guide RNA (sgRNA). (B) The commonly used delivery approaches of the endonucleases are the direct microinjection into embryos (mainly, cytoplasmic injection in sheep and goats) and somatic cell nuclear transfer (SCNT) (mainly, using fibroblast cells). (C) Different forms of modifications that result from the two main DNA repair pathways after induction of double-strand break (DSB) using endonucleases. i) Non-homologous end joining (NHEJ), which results in small insertions and/or deletions (indels), leads to gene knockout (disruption). ii) Homology-directed repair (HDR), which acts in the presence of exogenous donor DNA and mediates precise genetic modification including knockin (site-specific integration) and point mutation (single-nucleotide alteration). (D) Embryo transfer, gestation, and the generation of genetically edited offspring.
The Recent Revolution
Meganucleases
Site-specific endonucleases are enzymes that can break down polynucleotide chains and make cleavages in DNA sequences. Generally, there are four main classes of endonucleases that have been utilized in gene-editing programs: i) meganucleases or homing endonucleases, ii) zinc finger nucleases (ZFNs), iii) transcription activator-like effector nucleases (TALENs), and (iv) CRISPR systems (Figures 2 and 3A ) (Hsu et al., 2014). Of these four different classes, three are commonly used: ZFNs, TALENs, and CRISPR systems. The use of endonucleases has attracted significant attention among scientists after the role of double-strand breaks (DSBs) in increasing the efficiency of HR event has been highlighted (Rouet et al., 1994; Choulika et al., 1995). Gene editing using endonucleases is based on their ability to promote DSBs. After such induction of DSBs, the DNA repair mechanisms function in different pathways depending on the situation and generates different forms of manipulations. In the absence of donor DNA, non-homologous end joining (NHEJ) functions via random formation of small insertions and/or deletions (indels) that result in gene disruption (knockout). In the presence of donor DNA, homology-directed repair (HDR) functions to mediate more precise modifications such as site-specific integration (knockin) and single-base alteration (point mutation) (Figure 3C) (Gaj et al., 2013). Other forms of modifications, such as large deletions and inversions, can also be achieved using optimized endonucleases. In addition to NHEJ and HDR, a single-strand annealing (SSA) repair mechanism can also be utilized to mediate modifications by inducing the deletion of an intervening fragment between two homogenous repeat sequences (Li et al., 2018c).
Meganucleases are rare-cutting enzymes that can be classified as the first class of sequence-specific nucleases. These have been employed to create targeted DSBs in eukaryotic genomes (Daboussi et al., 2015). Meganucleases include five families, and the family LAGLIDADG is the largest and best characterized. This family also contains the most specific cutters, such as I-SceI of yeast S. cerevisiae (Galetto et al., 2009). Strategies have been developed to engineer meganucleases with new properties and DNA-binding specificities to widen their applications (Galetto et al., 2009). Meganucleases have mostly been applied to experimental model organisms. However, the number of publications regarding the applications of meganucleases in farm animals is limited (Bevacqua et al., 2013; Wang et al., 2014), and no publications can be found regarding their application in sheep and goats. Presumably, challenges related to both the engineering of meganucleases and protein redesign to direct meganucleases to novel DNA sequences result in their limited application in farm animals including sheep and goats (Petersen, 2017). In addition to the initial role of meganucleases for understanding the significance of DSB-based gene-editing events, simpler and more customizable gene-editing tools such as ZFNs, TALENs, and CRISPR systems have emerged, providing further options and opportunities to apply gene editing to a wide range of organisms.
ZFNs
ZFNs are site-specific custom-designed endonucleases that act through a combination between zinc finger proteins, which direct the gene-editing event to predetermined DNA sequences, and FokI DNA restriction enzymes, which introduce cleavages into the intended DNA sequences (Urnov et al., 2010). ZFNs apply the same principle of mediating site-specific modifications via induction of DSB repair pathways ( Figures 2 and 3). ZFNs are considered as the first “practical” DSB-assisted gene-editing tool that has been applied for the introduction of desired manipulations to cell lines as well as to organisms in a relatively easier way than the previously reported meganucleases. Cleaving the target DNA can be achieved by binding and aligning two ZFN monomers to their corresponding DNA target sequences in a tail-to-tail orientation (Weinthal et al., 2010). Each ZFN monomer is composed of a ZF domain (DNA-binding domain) and a non-specific FokI domain (DNA-cleavage domain). Typically, the ZF domain is composed of three to four individual fingers, each being capable to recognize and bind to an approximately 3-bp-long sequence (triplet bp within the DNA substrate) (Wu et al., 2007; Weinthal et al., 2010). This means that the ZF domain of three or four individual fingers can recognize and bind to DNA sequences with lengths of 9 or 12 bp.
ZFNs have been applied to farm animals including sheep and goats. In sheep, ZFNs have been applied to target the MSTN gene in fetal fibroblasts (Zhang et al., 2014a; Zhang et al., 2016b), primary satellite cells (Salabi et al., 2014), and embryos (Zhang et al., 2016b). The results of these reports indicated the potential of ZFNs to introduce gene disruption in both MSTN exon 1 (Zhang et al., 2014a), and exon 3 (Salabi et al., 2014; Zhang et al., 2016b) with both mono- and bi-allelic knockouts. Cytoplasmic injection of MSTN-ZFN mRNA into ovine embryos at the one-cell stage achieved 35% (13/37) efficiency (Zhang et al., 2016b). These studies highlight the potential of ZFNs to generate MSTN gene knockout founders using somatic cloning or ZFN microinjection into embryos (Figure 3B).
In addition to targeting MSTN to promote the growth and muscle mass in meat-producing lambs, BLG, a dominant allergen in milk, has also been targeted with desirable outcomes in dairy goats. ZFNs have been designed to mediate disruption in the goat BLG gene. Different methods have been used to deliver ZFNs that specifically target BLG in goat fibroblasts, including transfection of ZFN-expressing plasmid (Xiong et al., 2013), direct delivery of ZFNs as purified proteins (Song et al., 2015), and electroporation of ZFN-expressing plasmid (Yuan et al., 2016). Using purified ZFN proteins for targeting has potential to reduce insertional mutagenesis, toxicity, and off-target events (Gaj et al., 2012; Song et al., 2015). In general, the results of these initial studies have corroborated the ability of ZFNs to mediate BLG disruption in goats. Another interesting example for the application of ZFNs in goats includes the generation of gene knockout fetuses by ZFN-mRNA cytoplasmic injection into fertilized oocytes. For this, ZFNs have been designed to introduce biallelic disruption in forkhead box L2 (FOXL2) to investigate the function of this gene in female sex determination (Boulanger et al., 2014).
Although, no reports indicate the production of live gene-edited sheep and goats using ZFNs, the published data on the use of ZFNs to target cell lines, embryos, and fetuses indicate their potential ability for the generation of live genetically modified sheep and goats. However, the further emergence of simpler tools such as TALENs and CRISPRs has facilitated the application of gene editing for the generation of live founders with intended genomic modifications.
TALENs
TALENs are customizable DNA nucleases that have rapidly emerged as a desirable alternative for ZFNs with the ability to mediate site-specific modifications based on the principle of introducing DSBs (Bogdanove and Voytas, 2011; Joung and Sander, 2013) (Figures 2 and 3). TALENs resemble ZFNs in which a nonspecific FokI DNA-cleaving domain is fused to a customizable DNA-binding domain to generate functional DSB-introducing nucleases. TALEs are naturally occurring proteins that are secreted by plant pathogenic bacteria Xanthomonas species. These can cause disease in plants after injection into host cells and via interference with cellular activities by activating the transcription of specific target genes (Chen and Gao, 2013). TALENs function as dimers, and each DNA-binding domain is composed of a series of tandem repeats, each of which comprises 33–35 amino acids that can recognize and specifically bind to a single DNA nucleotide (Sun and Zhao, 2013). Each TALEN is designed to bind to ∼20 nucleotides with a DNA spacer consisting of ∼14–20 nucleotides between both TALEN dimers. This forms a range of ∼54–60 nucleotides for recognition and targeting (Sanjana et al., 2012). ZFNs and TALENs cleave DNA with relatively similar efficiency; however, the main advantage of TALENs over ZFNs is that TALENs are easier for design and construction (Joung and Sander, 2013). The simplicity of TALENs over ZFNs has extended the application of gene editing and facilitated the generation of organisms as well as cell lines with specific genetic alterations.
TALENs have been applied to sheep and goats and live founders with desired genetic alterations have been generated. In sheep, TALENs have been used to generate MSTN-knockout lambs. One out of nine live births produced using TALEN-mRNA cytoplasmic injection was MSTN-edited (Proudfoot et al., 2015). TALENs combined with single-stranded oligodeoxynucleotides (ssODNs) carrying a stop codon to target exon 2 of the MSTN gene have been transfected to sheep primary fibroblasts; 11.4% (4/35) of the sequenced colonies contained the desired insertion (Zhao et al., 2016). Fibroblasts with modified MSNT were used as nuclear donor for SCNT. After full-term gestation, one lamb was born and died soon after birth. DNA sequencing of tissues from the cloned lamb showed identical insertion of a stop codon site in the MSTN gene with donor cells (Zhao et al., 2016). In a further report, TALEN-mediated MSTN biallelic-knockout somatic cells were used as nuclear donor cells for SCNT (Li et al., 2016). 16 out of 23 lambs were obtained (12 live and 11 dead) that showed expected biallelic mutations of the MSTN gene. The live founders showed a remarkable increase in body weight compared to their wild-type counterparts (Li et al., 2016).
In goats, TALENs were first reported in fibroblasts to introgress SNP alleles that are responsible for fecundity in sheep (bone morphogenetic protein receptor type 1B, BMPR-IB; also known as Booroola fecundity, FecB) and muscle hypertrophy (callipyge, CLPG) into the goat genome using TALEN mRNA and oligonucleotide transfection (Tan et al., 2013). This initial report presented the potential of the TALEN endonucleases for the introduction of desirable allelic introgressions into the genomes of farm animals. TALENs have also been applied in goats to target the BLG gene (Cui et al., 2015; Ge et al., 2016; Zhu et al., 2016b; Yuan et al., 2017). Furthermore, gene-edited goats that carry both BLG knockout and enriched expression of human lactoferrin (hLF) (Cui et al., 2015) or human α-lactalbumin (hLA) (Zhu et al., 2016b) have been generated using SCNT. Cui et al. performed two rounds of cloning to generate BLG biallelic knockout goats with an enriched expression of hLF in milk. 10 kids were generated during the first round of cloning (seven BLG+/- and three BLG+/hLF), and after the second targeting and cloning, five cloned kids were generated (three BLG-/- and two BLG-/hLF) (Cui et al., 2015). Furthermore, Zhu et al. reported the generation of BLG knockout goats with enriched expression of hLA in milk, six live births were obtained, one was a biallelic targeted goat (which died after 1 month), and the other five goats were BLGhLA/+. BLG expression in the milk of transgenic goats was reduced, while hLA was highly expressed compared to normal goats (Zhu et al., 2016b). Caprine MSTN knockout using TALENs has also been reported. Three cloned kids have been produced, two have died after birth (MSTN-/- and MSTN+/+), and one was alive and healthy (MSTN+/-) (Yu et al., 2016). TALENs have contributed to the generation of live and genetically altered sheep and goats with desired phenotypes; however, the procedure of gene editing has to become simpler and quicker with more advanced modification systems.
CRISPR/Cas9
Rapid and promising advances have been reported in the field of genetic modification during the past decade. The revolution of genetic engineering has culminated in the emergence of CRISPR systems. These are simple but sophisticated mechanisms derived from nature that act in prokaryotes as an adaptive immune system against phage and foreign DNA infection by the cooperation of CRISPR sequences with Cas proteins (Mojica and Montoliu, 2016). Based on many years of research, CRISPR systems were developed from prokaryotic adaptive defense systems to robust gene-editing tools applicable throughout the entire biological kingdom (Doudna and Charpentier, 2014).
CRISPR systems have attracted scientific attention after their role as simple, precise, and efficient nucleases that can introduce DSBs within DNA sequences in a site-specific manner has been highlighted. Of the three types of CRISPR/Cas systems, type II Streptococcus pyogenes CRISPR-Cas9 is the most widely used CRISPR system. The CRISPR/Cas9 system uses two main components: an RNA-directed Cas9 protein and ∼20-nucleotide sgRNA, which leads the Cas9 protein to a user-defined DNA target site as long as it is next to a protospacer adjacent motif (PAM) sequence (Doudna and Charpentier, 2014). PAM is a short sequence within the targeted DNA that acts as a recognition site. Introducing DSBs into the targeted genomes stimulates various forms of gene-editing events based on the natural DNA repair ability (Gaj et al., 2013) (Figure 3C). The CRISPR/Cas9 system differs from ZFNs and TALENs since it is an RNA-directed system based on the Cas9 protein that introduces DSBs instead of protein-directed FokI restriction endonuclease in both ZFNs and TALENs. One of the main advantages of directing the nuclease via RNA is the simple construction of RNA-expressing constructs and thus the expansion of the ability to direct the nuclease to any desired target sequence.
CRISPR systems were first applied to mammalian genomes in 2013 (Cong et al., 2013; Mali et al., 2013); during the same year, CRISPR systems have been applied to generate mutant mice with a number of modifications (Shen et al., 2013; Wang et al., 2013; Yang et al., 2013). Consequently, CRISPR systems have been applied to a wide range of cell lines as well as living organisms. Recently, new CRISPR systems have been discovered, thus extending the toolbox of gene editing with further options for efficient and precise targeting and/or manipulation (Komor et al., 2017). These systems include catalytically inactive/dead Cas9 (dCas9), which has been employed in gene regulation, epigenetic modification, chromatin engineering, and base editing (reviewed by Adli, 2018). Single-nucleotide alterations have been applied using base editor systems composed of dCas9, and further versions have been developed using Cas9 nickase (nCas9) (Komor et al., 2016; Eid et al., 2018). These enable new and more precise forms of genomic modifications. Due to their simplicity, affordability, and customizability, CRISPR systems (especially Cas9-based systems), have initiated a great biotechnological revolution in different fields including basic research, biomedicine, and agriculture.
In general, despite the advantages and disadvantages of the above-mentioned genetic modification techniques (see Table 7), all of these have significantly increased our knowledge of the nature of ovine and caprine genomes. Furthermore, they enabled the generation of a large variety of useful, genetically manipulated sheep and goat models.
Table 7.
The advantages and disadvantages of genetic manipulation tools applicable to ovine and caprine genomes
| Tool | Uses | Advantages | Disadvantages | References |
|---|---|---|---|---|
| PNI | Insertional transgenesis | The first tool to be applied for the generation of transgenic animals | Random integration, variable transgene copy number, low efficiency | Hammer et al., 1985; Clark, 2002 |
| SCNT | Gene targeting, editing | An alternative that facilitated the implementation of HR gene targeting in species that lack ESCs, a low-level mosaicism | Development of a small proportion of reconstructed embryos that become live offspring, potential complications at birth of offspring as a result of developmental abnormalities | Schnieke et al., 1997; Wilmut et al., 1997; Wilmut et al., 1999 |
| SMGT | Gene transfer, integration | Simple, cost-effective, minimal embryo handling required | Initial doubt with regard to its repeatability, variable results, low incorporation of the exogenous gene | Lavitrano et al., 1989; Wall, 2002; Lavitrano et al., 2006 |
| VMGT | Gene transfer, integration | Able to infect germline cells and dividing or non-dividing somatic cells, delivery of the system to the egg/zygote is less damaging compared to pronuclear injection, high integration | Variability of transgenic expression, potential health risks, limited DNA capacity | Whitelaw et al., 2008; Modric and Mergia, 2009 |
| Recombinases | Integration, selectable cassette excision | Increased gene integration efficiency, offer different forms of modifications including the removal of unwanted DNA | Conservative specificity, in specific cases, pre-introduction of specific target sites within the host genome is required which is an inefficient and time-consuming process, potential toxicity | Xu et al., 2008; Gaj et al., 2014; Olorunniji et al., 2016 |
| Transposons | Integration | Able to integrate transgenes and RNAi-expressing constructs for the mediation of knockdown expression, lower immunogenicity and larger DNA capacity compared to viral systems | Classical transposons are less efficient for gene transfer compared to viral systems, potential cytotoxicity | Muñoz-López and García-Pérez, 2010; Meir and Wu, 2011; Hudecek et al., 2017 |
| RNAi | Gene knock down | Targeting gene expression at mRNA level, useful tool to elucidate gene functions | Variability and incompleteness of knockdowns, potential off-target | Boutros and Ahringer, 2008; Boettcher and McManus, 2015; Bradford et al., 2017 |
| ZFNs | Gene editing | First “practical” endonuclease that has been applied to mediated gene-editing events | Difficult to design, potential off-target, mosaicism in offspring generated from microinjected embryos | Gaj et al., 2013; Gupta and Musunuru, 2014; Oliver et al., 2015; Zhang et al., 2019a |
| TALENs | Gene editing | A simplified alternative of the previously emerged ZFNs | Moderate difficulty in design, potential off-target, mosaicism in offspring generated from microinjected embryos | Bedell et al., 2012; Gaj et al., 2013; Gupta and Musunuru, 2014; Zhang et al., 2019a |
| CRISPR/Cas9 | Gene editing | Simple, cost-effective, customizable, precise compared to other endonucleases, able to mediate multiplex editing | Potential off-target, mosaicism in offspring generated from microinjected embryos | Gaj et al., 2013; and Gupta and Musunuru, 2014; Mehravar et al., 2019; Zhang et al., 2019a |
PNI, pronuclear injection; SCNT, somatic cell nuclear transfer; SMGT, sperm-mediated gene transfer; VMGT, virus-mediated gene transfer; RNAi, RNA interference; ZFNs, zinc finger nucleases; TALENs, transcription activator-like effector nucleases; CRISPR/Cas9, clustered regularly interspaced short palindromic repeat/CRISPR-associated protein 9.
Applications of CRISPR/Cas9 in Sheep and Goats
Gene editing has been revolutionized as a result of the rapid emergence of novel varieties of tools that can simply, precisely, and more efficiently mediate different forms of DNA modifications than previously reported tools. CRISPR/Cas9 systems have been applied to sheep and goats to fulfill various promising purposes (Figure 1). To date, a number of sheep and goat models have been generated via CRISPR/Cas9 systems (Table 3). Moreover, further studies are ongoing for the provision of useful sheep and goat models for agriculture and biomedicine. The following paragraphs outline the applications of CRISPR/Cas9 in sheep and goats.
Promotion of Muscle Growth and Development
Increasing the body weight and accelerating the growth rates of farm animals are important aims in agriculture. Genes that affect these traits are attractive targets for emerging disruptive gene-editing techniques. MSTN was among the first genes that have been subjected to CRISPR/Cas9 targeting, as a strategy to achieve an economically important trait by applying gene-editing tools in sheep and goats. CRISPR/Cas9 targeting of MSTN was first applied in sheep, where 35 founders were obtained, two of which contained the mutation (5.7%) (Han et al., 2014). Later, Crispo et al. reported the production of MSTN-disrupted sheep using CRISPR/Cas9, where 10 lambs out of 22 obtained founders (45.4%) showed the mutation with heavier body weight compared to their wild-type counterparts (Crispo et al., 2015a). These initial reports encouraged the further application of CRISPR/Cas9 systems in small ruminants.
In our team, CRISPR/Cas9 has been applied to achieve multiplex gene editing of MSTN with two other economically important genes, including the agouti-signaling protein (ASIP) and β-carotene oxygenase 2 (BCO2) in sheep (Wang et al., 2016b). 49 founders have been obtained, 36 of which were alive. Among these 36 live lambs, the targeting efficiencies were 27.7% (10/36) for MSTN, 33.3% (12/36) for ASIP, and 27.7% (10/36) for BCO2; 5.6% (2/36) showed the simultaneous targeting of all three genes. No off-target has been detected and founders with MSTN mutations showed enlarged myofibers and enhanced body weight compared to wild-type individuals (Wang et al., 2016b). In addition, sheep with biallelic modification in the BCO2 gene showed yellow fat compared to the white fat color of monoallelic and wild-type individuals, highlighting the role of BCO2 in the fat color determination in sheep (Niu et al., 2017). To ensuring the biosafety of CRISPR/Cas9 in large animals, further steps have been taken by performing trio-based whole genome sequencing (for the edits and their parents) to investigate the origins of the variations in the generated edits, which might be parentally inherited, naturally obtained, or induced by a specific targeting event (Wang et al., 2018a). The results that were obtained from the multiplex edited sheep showed negligible off-target modifications that did not affect the application of CRISPR/Cas9 in large animals. In summary, these results highlight the potential of the CRISPR/Cas9 system to introduce multiplex editing in farm animals.
Moreover, disrupting the normal function of MSTN in sheep skeletal muscle satellite cells (sSMSCs) has been shown to promote sSMSC differentiation in both number and length. This study has also reported the generation of MSTN-disputed sheep using SCNT from CRISPR/Cas9 transfected ear fibroblasts (Zhang et al., 2018c). A further and more recent report, published by our team, described the application of CRISPR/Cas9-based base editors for the introduction of a point mutation within the suppressor of cytokine signaling 2 (SOCS2) gene in sheep (Zhou et al., 2019). This single-nucleotide variant exerts profound effects on both body weight and size as well as milk production. This study highlights the potential role of base editors in sheep and goats, which can be utilized to introduce single alterations of bases that harbor desirable economical traits.
In goats, CRISPR/Cas9 was used in a study that targeted the four important genes, MSTN, BLG, PrP, and nucleoporin 155 (NUP155) in goat fibroblasts and generated three MSTN knockout goats using SCNT (Ni et al., 2014). The reported efficiencies of CRISPR/Cas9 in goat fibroblasts ranged from 9 and 70%, indicating the ability of CRISPR/Cas9 to efficiently work in the caprine system. Later, gene-modified goats that carry knockouts either in MSTN or fibroblast growth factor 5 (FGF5) or both genes have been reported by our team (Wang et al., 2015). Of 98 obtained individuals (including 79 delivered alive, 14 delivered but died shortly after birth, and five aborted), 15/98 (15.3%) carried a disruption in MSTN, 21/98 (21.4%) carried a disruption in FGF5, and 10/98 (10.2%) showed simultaneous disruption of both genes. These results confirm the efficient induction of multiplex targeting via CRISPR/Cas9, which is of great importance in farm animals. Especially, since most of the economically important traits are controlled by multiple loci. Further studies have been conducted that used the MSTN mutated founders generated from this experiment to confirm the occurrence of gene disruption and the transmission of the knockout alleles (Wang et al., 2018b), as well as to analyze the transcriptomic changes of MSTN knockout goats (Wang et al., 2017). The occurrence and transmission of editing events have been confirmed, and substantial changes in gene expressions have been determined at the transcriptome level. These expressional changes were found in genes that are involved in fatty acid metabolism and unsaturated fatty acid biosynthesis, suggesting a regulatory role of MSTN in the expression of these genes. Furthermore, family trio-based deep sequencing for gene-edited goats and their progenies was performed to investigate the occurrence of de novo mutations, indels, and other structural variants (Li et al., 2018a). The obtained results of this report support the reliability of CRISPR/Cas9 application in large animals.
Further publications have also reported the generation of MSTN knockout sheep and goats via the CRISPR/Cas9 system (see Table 3). Among these, an interesting report described the generation of a goat kid carrying simultaneous MSTN knockout and fat1 knockin using CRISPR/Cas9 combined with SCNT (Zhang et al., 2018a). The efficiency of simultaneous targeting was 25.6% (40/156) in goat fibroblasts. Despite this moderate efficiency at the cellular level, one edited founder out of 134 transferred cloned embryos was generated. This ratio might be increased by improving SCNT conditions. The efficiencies of TALEN and CRISPR/Cas9 for the targeting of caprine MSTN have also been compared. Despite several advantages of the former endonuclease, the latter has shown a higher generation frequency of biallelic disruptions and longer deletions (Zhang et al., 2019b). CRISPR/Cas9 has significantly highlighted the functional role of genes related to muscle growth and body weight both in sheep and goats, further emphasizing the potential role of gene editing to provide the farm animal sector with novel breeds that carry desirable and valuable traits.
Promotion of Fiber Length and Growth
Sheep and goats form a valuable source for the production of fibers. Genes associated with fiber quality and quantity are a source of attraction, and many researchers hope to alter these genes in attempts to obtain desired and new fiber properties. The FGF5 gene, which is a dominant inhibitor of fiber length and growth, is an attractive target. In sheep, CRISPR/Cas9 has been applied to disrupt the normal function of the FGF5 gene, resulting in 3/18 (16.6%) mutated founders that carried a disruption in FGF5 and showed increased wool length (Hu et al., 2017). Another publication has also reported the generation of FGF5-disrupted sheep; 16/20 (80%) mutated founders carried both monoallelic and biallelic mutations in FGF5 and showed increased wool length and quantity (Li et al., 2017c). Recently, Zhang et al. have also confirmed that the disruption of FGF5 in sheep can lead to an increased wool length and average wool growth rate (Zhang et al., 2019c). The results of these studies confirm the functional role of FGF5 and its desired disrupting effect. In an interesting study related to fiber characteristics, Zhang et al. introduced the targeted disruption of the ASIP gene by using CRISPR/Cas9 (Zhang et al., 2017a). The resultant founders that carried disruption within ASIP have shown various coat color patterns versus the white coat color of wild-type individuals of the same breed (the Chinese merino). This highlights the critical role of the ASIP gene in coat color determination in sheep.
In the MSTN/FGF5 knockout goat model (Wang et al., 2015), further confirmation of the gene-editing event of the resultant founders has been performed. The simultaneous occurrence of FGF5 disruption at both the morphological and genetic levels has been confirmed and an enhancement in fiber length, as well as an increase in the number of secondary hair follicles were obtained (Wang et al., 2016a). CRISPR/Cas9 has also been used to generate ectodysplasin receptor (EDAR) gene knockout goats by SCNT to investigate the disruption effects on the phenotype, hair follicle growth and development (Hao et al., 2018). EDAR-knockout founders showed abnormal primary hair follicles and an absence of hair on the top of their heads; these characteristics are distinctive features of EDAR mutants. These generated founders provide a useful model for the study of the relationship between the EDAR gene and hair follicle growth and development. Investigating the functional roles of genes that are directly associated with hair growth and development is of great importance in wool- and cashmere-producing breeds. Recently, CRISPR-mediated base editing has been applied by our team to introduce nonsense codon introgression into the FGF5 gene to enhance the yield of cashmere hair in goats (Li et al., 2018b). Five newborns out of 22 transferred embryos were generated that carried at least one nonsense mutation or other mutational types that were induced by the base editing system. In addition to our recent report regarding the applications of base editing in sheep (Zhou et al., 2019), these results highlight the potential of base editor application to the genomes of farm animals to obtain desirable economical traits that are harbored by single bases. CRISPR/Cas9 has shown potential for disrupting genes that inhibit fiber desirable phenotypes in sheep and goats, which establishes a new platform to rapidly achieve the aims of animal breeding based on gene editing.
Molecular Manipulation of Milk Components
Manipulation of milk components and the expression of desired transgenes in milk with the aim to enrich its components with valuable proteins are among the main aims in livestock genetic modification programs. CRISPR/Cas9 has been applied in sheep and goats to alter the characteristics of milk by inhibiting the expression of undesired proteins or by applying gene knockin strategies to enrich the milk with new desired expression products. Initially, CRISPR/Cas9 has been used to target BLG in goat primary fibroblasts (19%) (Ni et al., 2014), and later, gene-edited goats with disrupted BLG gene have been generated (Zhou et al., 2017). Four founders out of 26 (15.38%) were BLG edits, which have shown decreased expression of BLG and abolished BLG protein production in milk (Zhou et al., 2017). The generated founders of this study provide a useful model for the study of the relationship between BLG and other milk proteins. Furthermore, this study provides a useful caprine model that can generate BLG-free milk.
Induction of genetic integration into the genomes is of great interest, especially for the introduction of new expressional characteristics in milk. CRISPR/Cas9 has shown the ability to introduce knockin of the marker gene turbo GFP (tGFP) in the Rosa26 locus of sheep genome (Wu et al., 2016). This study also indicates the Rosa26 locus as a potential site for exogenous gene expression in sheep. Another example of CRISPR/Cas9 application for the manipulation of milk components includes the generation of gene-edited sheep with an enriched production of melatonin in milk (Ma et al., 2017). In this approach, Cas9 mRNA, sgRNA, and the linearized vectors carrying arylalkylamine N-acetyltransferase (AANAT) and acetylserotonin methyltransferase (ASMT) for the expression of melatonin were cytoplasmically co-injected into pronuclear embryos. Of 34 transgenic founders, seven carried AANAT, two carried ASMT, and 25 carried both of AANAT and ASMT genes. Founders carrying these genes produced melatonin-enriched milk. These models might be a good source of melatonin, which has various nutritional and medicinal values and uses.
CRISPR/Cas9 has also been used to investigate the functional role of genes such as stearoyl-CoA desaturase 1 (SCD1) in goat mammary epithelial cells (Tian et al., 2018a) and acetyl CoA acyltransferase 2 (ACAA2) in sheep precursor adipocyte cells (Zhang et al., 2019d). These genes are directly or indirectly related to milk traits and affect the fatty acid metabolism. CRISPR/Cas9 has facilitated the generation of gene-modified sheep and goats with specific milk characteristics which might also facilitate the large-scale production of useful proteins and pharmaceuticals in milk.
Promotion of Reproductive Performance
Improving the reproductive performance is an important direction of livestock breeding. Desirable traits related to reproductivity such as litter size have been suggested as goals for introduction to farm animals using gene-editing tools instead of the comparatively tedious conventional breeding strategies. Mutations in the sheep BMPR-IB (FecB) gene has shown to be responsible on an increased ovulation rate and consequently larger litter size (Fabre et al., 2006). Initially, CRISPR/Cas9 has been reported to target sheep BMPR-IB in vitro, resulting in gene-edited embryos that can be characterized by a variety of indels at the BMPR-IB/FecB locus (Zhang et al., 2017b). Sheep BMPR-1B has also been targeted by our team using an ssODN-based approach to introduce defined point mutations. Gene-edited founders have been produced. Seven out of 21 delivered lambs contained editing events, five of which have been determined to carry the intended nucleotide substitution (Zhou et al., 2018). The targeting efficiency was 33.3% (7/21) in the generated founders, and the efficiency of the intended single-nucleotide substitution was 23.8% (5/21).
In goats, another defined point mutation has also been introduced by our team in the growth differentiation factor 9 (GDF9) gene, which exerts a large effect on both ovulation rate and litter size (Niu et al., 2018). In this study, four out of 18 delivered kids carried the intended mutation (22.2% targeting efficiency). In summary, the results of both reports highlight the role of CRISPR/Cas9-induced HDR with an ssODN template for introducing reliable and defined point mutations in livestock. In another experiment, the effects of open pulled straw (OPS) vitrification as a method to preserve microinjected embryos on AANAT-microinjected embryo development and the reproductive capacity of produced AANAT-transgenic offspring have been investigated (Tian et al., 2018b). In this study, a number of both frozen and non-frozen microinjected sheep embryos have been used to compare and generate live transgenic offspring. The results of this study showed no significant differences between the frozen and non-frozen AANAT-microinjected embryos. Furthermore, AANAT-transgenic individuals have shown improved reproductive capacity. CRISPR/Cas9 has also been applied to investigate the biological role of the glucocorticoid receptor (NR3C1) in sheep conceptus elongation using recovered elongating conceptuses (Brooks et al., 2015). However, the study highlighted the essential roles of other factors rather than NR3C1 in conceptus elongation in sheep. CRISPR/Cas9 has been applied to develop desirable traits for the improvement of the reproductive performance of sheep and goats.
Generation of Disease-Resistant Animals
Gene editing has been suggested as a robust tool that can be used to generate disease-resistant animals. Consequently, genetically modified resistant farm animals have been produced. These offer good models to investigate and understand disease pathogenesis and offer a potential source for the spread of disease resistance traits in commercial flocks. The CRISPR/Cas9 system has been applied in sheep and goats to achieve aims related to improving animal health and welfare. Several disease-related genes have been disrupted using CRISPR/Cas9. The PrPc is directly associated with the pathogenesis of the transmissible spongiform encephalopathies, which occur in humans and a number of livestock species including sheep and goats (Yu et al., 2006). PrP-resistant animals can be produced by suppressing the expression of PrP (Golding et al., 2006). CRISPR/Cas9 has been applied to target PrP in goat fibroblasts with the aim to generate PrP-knockout donor cells that can be used in SCNT for the production of PrP-resistant goats (Ni et al., 2014; Hu et al., 2015; Fan et al., 2019). The targeting efficiency of PrP increased by 70% in goat fibroblasts and by 20% (9/45) for the simultaneous targeting of PrP and MSTN. Moreover, of the nine dual gene mutant colonies, five had mutations in all four alleles of both genes. These results suggest the use of the CRISPR/Cas9 system to target genes to confer potential disease resistance in farm animals.
Menchaca et al. indicated the application of the CRISPR/Cas9 system to induce the loss of function of hyaluronidase 2 (HYAL2), which can be used by Jaagsiekte sheep retrovirus as a cell entry receptor, resulting in ovine pulmonary adenocarcinoma syndrome (Menchaca et al., 2018). As indicated by the authors, generation of HYAL2-knockout lambs will validate the possibility to apply the CRISPR/Cas9 system for producing virus-resistant farm animals and will help to investigate the functional roles of HYAL2. It seems that the research of genetic engineering via CRISPR systems for the generation of disease-resistant sheep and goats is ongoing, and maybe varieties of genetically modified resistant sheep and goats will be reported soon, thus enriching the field with novel and valuable models.
Generation of Models for Human Diseases
Sheep and goats have been used as interesting models in biomedical research. Compared to experimental rodents, sheep and goats offer the advantage of being more suitable mimics for human diseases due to their similar size and anatomy. CRISPR/Cas9 has been applied in sheep and goats to provide biomedical research with useful models to investigate human diseases. In mice, mutations within NUP155 were associated with atrial fibrillation and early sudden cardiac death (Zhang et al., 2008b). Large NUP155-knockout animals could be useful models for research in cardiac physiology. Initially, CRISPR/Cas9 has been applied to target the NUP155 gene in goat fibroblasts to generate NUP155-knockout donor cells, which can be used in the SCNT program to produce NUP155-knockout goat model (Hu et al., 2014; Ni et al., 2014; Fan et al., 2019).
In humans, during pregnancy, Zika virus (ZIKV) infection can lead to fetal infection, causing microcephaly and other severe congenital neurological symptoms. Type I interferons (IFNs) are central for host resistance against ZIKV, and interferon α/β receptor IFNAR-deficient mice have been shown to be highly susceptible to ZIKV infection (Yockey et al., 2018). Fan et al. reported the generation of IFNAR-knockout sheep by applying CRISPR/Cas9 in combination with SCNT to provide highly susceptible ZIKV large animal model (Fan et al., 2017).
Fan et al. have also provided an interesting sheep model for the investigation of human cystic fibrosis (CF) by employing both CRISPR/Cas9 and SCNT (Fan et al., 2018). CF is an inherited disorder that affects mostly the lungs and other organs in the body. The authors have introduced targeting of the CF transmembrane conductance regulator (CFTR) gene and CFTR–/– as well as CFTR+/– lambs have been produced with a severe phenotype of CF pathology similar to that of humans.
Additionally, Williams et al. have also reported an interesting sheep model, recapitulating human hypophosphatasia (HPP; a rare metabolic bone disease) by applying CRISPR/Cas9 (Williams et al., 2018). In this study, a single point mutation in the tissue-nonspecific alkaline phosphatase (TNSALP) gene (ALPL, alkaline phosphatase, biomineralization associated) has been introduced. The thus generated gene-edited lambs accurately phenocopied human HPP, providing a useful large animal model for the study of rare human bone diseases. The results of these reports corroborate the great potential of the CRISPR/Cas9 system to generate gene-edited sheep and goats that recapitulate human diseases.
Xenotransplantation and Generation of Hosts for the Growth of Human Organs
One of the most promising strategies to solve the shortage of organ donors worldwide is to generate human organs inside large animals by applying a technique called interspecies blastocyst complementation (De Los Angeles et al., 2018). In this technique, large animals act as hosts and grow human organs. The procedure of this technique is based on a combination of gene-edited embryos of large animals and human pluripotent stem cells (PSCs). In this case, gene-editing tools are used to generate large animal embryos with genetically engineered “organ niches” by disabling a specific gene or a number of genes that are responsible for the formation of a target organ, thus allowing human PSCs to colonize the vacant niche and generate the desired organ “gest-derived organ” (Rashid et al., 2014).
In this context, an interesting study reported the creation of pancreatogenesis-disabled sheep as a step toward achieving interspecies blastocyst complementation–based xenotransplantation between human and large animals (Vilarino et al., 2017; Vilarino et al., 2018). In this experiment, CRISPR/Cas9 has been applied to target pancreatic and duodenal homeobox protein 1 (PDX1), which is a necessary gene for pancreatic development. CRISPR/Cas9 combined with dual sgRNAs and direct oocyte microinjection approach was used to generate PDX1 disruption, and a resulting fetus with PDX1-,- mutation lacked a pancreas. The results of these promising attempts highlight the potential of gene-edited sheep to be applied in interspecies organ generation as candidate hosts for the growth of human organs. Integrative programs that employ CRISPR/Cas9 and PSC complementation offer a potential solution for the shortage of organ donors.
Conclusions and Future Perspectives
In the current era, genome engineering systems have become indispensable tools of basic research, biomedicine, and agriculture. Genome engineering tools, especially the recently emerged CRISPR systems, offer the potential to revolutionize all biological research fields including research on agriculture and farm animals. Applying genome engineering tools to farm animals including sheep and goats is of significant importance. Currently, gene-edited sheep and goats are generated with relative ease using CRISPR systems, providing valuable models for agricultural, veterinary, and biomedical research. The list of sheep and goat genes targeted by CRISPR systems is continuously growing. In the future, a large number of genes will be targeted, and more valuable models will be generated, thus widening our understanding about these genes and their corresponding phenotypes and functions. This basic research is only the beginning; the potent potential of genome engineering will become apparent in the future.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
This study was supported by the National Natural Science Foundation of China (31772571, 31572369, 31872332), and Local Grants (NXTS2018-001, 2017NY-072, and 2018KJXX-009). Xiaolong Wang is a Tang Scholar at Northwest A&F University, China.
Conflict of Interest Statement
Author TS was employed by Recombinetics Inc. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors wish to thank all the colleagues in Prof. Chen Yulin’s laboratory for their valuable comments during the preparation of the manuscript. The authors also wish to thank Mr. Rauan Shotkin for his efforts in editing and producing the figures in this manuscript.
References
- Abbasi H., Hosseini S. M., Hajian M., Nasiri Z., Bahadorani M., Tahmoorespur M., et al. (2015). Lentiviral vector-mediated transduction of goat undifferentiated spermatogonia. Anim. Reprod. Sci. 163, 10–17. 10.1016/j.anireprosci.2015.09.002 [DOI] [PubMed] [Google Scholar]
- Adli M. (2018). The CRISPR tool kit for genome editing and beyond. Nat. Commun. 9, 1911. 10.1038/s41467-018-04252-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akshey Y. S., Malakar D., De A. K. (2008). 23 Production of handmade cloned goat blastocysts using fetal fibroblast cells [Abstract]. Reprod. Fertil. Dev. 20, 91–92. 10.1071/RDv20n1Ab23 [DOI] [Google Scholar]
- Amoah E. A., Gelaye S. (1997). Biotechnological advances in goat reproduction. J. Anim. Sci. 75, 578–585. 10.2527/1997.752578x [DOI] [PubMed] [Google Scholar]
- An L. Y., Yuan Y. G., Yu B. L., Yang T. J., Cheng Y. (2012). Generation of human lactoferrin transgenic cloned goats using donor cells with dual markers and a modified selection procedure. Theriogenology 78, 1303–1311. 10.1016/j.theriogenology.2012.05.027 [DOI] [PubMed] [Google Scholar]
- Andreas S., Schwenk F., Küter-Luks B., Faust N., Kühn R. (2002). Enhanced efficiency through nuclear localization signal fusion on phage ϕC31-integrase: activity comparison with Cre and FLPe recombinase in mammalian cells. Nucleic Acids Res. 30, 2299–2306. 10.1093/nar/30.11.2299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. S., Kennan W. S., Gould M. N., Bremel R. D. (1994). Human growth hormone (hGH) secretion in milk of goats after direct transfer of the hGH gene into the mammary gland by using replication-defective retrovirus vectors. Proc. Natl. Acad. Sci. 91, 6840–6844. 10.1073/pnas.91.15.6840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguisi A., Behboodi E., Melican D. T., Pollock J. S., Destrempes M. M., Cammuso C., et al. (1999). Production of goats by somatic cell nuclear transfer. Nat. Biotechnol. 17, 456–461. 10.1038/8632 [DOI] [PubMed] [Google Scholar]
- Bai D. P., Yang M. M., Qu L., Chen Y. L. (2017). Generation of a transgenic cashmere goat using the piggyBac transposition system. Theriogenology 93, 1–6. 10.1016/j.theriogenology.2017.01.036 [DOI] [PubMed] [Google Scholar]
- Bai D., Yang M., Qu L., Chen Y. (2012). PiggyBac transposon-mediated gene transfer in cashmere goat fetal fibroblast cells. Biosci. Biotechnol. Biochem. 76, 933–937. 10.1271/bbb.110939 [DOI] [PubMed] [Google Scholar]
- Bedell V. M., Wang Y., Campbell J. M., Poshusta T. L., Starker C. G., Krug R. G., II, et al. (2012). In vivo genome editing using a high-efficiency TALEN system. Nature 491, 114–118. 10.1038/nature11537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behboodi E., Ayres S. L., Memili E., O’coin M., Chen L. H., Reggio B. C., et al. (2005). Health and reproductive profiles of malaria antigen-producing transgenic goats derived by somatic cell nuclear transfer. Cloning Stem Cells 7, 107–118. 10.1089/clo.2005.7.107 [DOI] [PubMed] [Google Scholar]
- Bevacqua R. J., Canel N. G., Hiriart M. I., Sipowicz P., Rozenblum G. T., Vitullo A., et al. (2013). Simple gene transfer technique based on I-SceI meganuclease and cytoplasmic injection in IVF bovine embryos. Theriogenology 80, 104–113.e29. 10.1016/j.theriogenology.2013.03.017 [DOI] [PubMed] [Google Scholar]
- Bevacqua R. J., Fernandez-Martin R., Canel N. G., Gibbons A., Texeira D., Lange F., et al. (2017). Assessing Tn5 and Sleeping Beauty for transpositional transgenesis by cytoplasmic injection into bovine and ovine zygotes. PLoS One 12, e0174025. 10.1371/journal.pone.0174025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettcher M., McManus M. T. (2015). Choosing the right tool for the job: RNAi, TALEN, or CRISPR. Mol. Cell 58, 575–585. 10.1016/j.molcel.2015.04.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanove A. J., Voytas D. F. (2011). TAL effectors: customizable proteins for DNA targeting. Science 333, 1843–1846. 10.1126/science.1204094 [DOI] [PubMed] [Google Scholar]
- Boulanger L., Pannetier M., Gall L., Allais-Bonnet A., Elzaiat M., Le Bourhis D., et al. (2014). FOXL2 is a female sex-determining gene in the goat. Curr. Biol. 24, 404–408. 10.1016/j.cub.2013.12.039 [DOI] [PubMed] [Google Scholar]
- Boutros M., Ahringer J. (2008). The art and design of genetic screens: RNA interference. Nat. Rev. Genet. 9, 554–566. 10.1038/nrg2364 [DOI] [PubMed] [Google Scholar]
- Brackett B. G., Baranska W., Sawicki W., Koprowski H. (1971). Uptake of heterologous genome by mammalian spermatozoa and its transfer to ova through fertilization. Proc. Natl. Acad. Sci. 68, 353–357. 10.1073/pnas.68.2.353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford B. J., Cooper C. A., Tizard M. L., Doran T. J., Hinton T. M. (2017). RNA interference-based technology: what role in animal agriculture? Anim. Prod. Sci. 57, 1–15. 10.1071/AN15437 [DOI] [Google Scholar]
- Brinster R. L., Avarbock M. R. (1994). Germline transmission of donor haplotype following spermatogonial transplantation. Proc. Natl. Acad. Sci. 91, 11303–11307. 10.1073/pnas.91.24.11303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Zimmermann J. W. (1994). Spermatogenesis following male germ-cell transplantation. Proc. Natl. Acad. Sci. 91, 11298–11302. 10.1073/pnas.91.24.11298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks K., Burns G., Spencer T. E. (2015). Biological roles of hydroxysteroid (11-beta) dehydrogenase 1 (HSD11B1), HSD11B2, and glucocorticoid receptor (NR3C1) in sheep conceptus elongation. Biol. Reprod. 93, 38. 10.1095/biolreprod.115.130757 [DOI] [PubMed] [Google Scholar]
- Campbell K. H. S., McWhir J., Ritchie W. A., Wilmut I. (1996). Sheep cloned by nuclear transfer from a cultured cell line. Nature 380, 64–66. 10.1038/380064a0 [DOI] [PubMed] [Google Scholar]
- Chen K., Gao C. (2013). TALENs: customizable molecular DNA scissors for genome engineering of plants. J. Genet. Genomics 40, 271–279. 10.1016/j.jgg.2013.03.009 [DOI] [PubMed] [Google Scholar]
- Choulika A., Perrin A., Dujon B., Nicolas J. F. (1995). Induction of homologous recombination in mammalian chromosomes by using the I-SceI system of Saccharomyces cerevisiae. Mol. Cell. Biol. 15, 1968–1973. 10.1128/MCB.15.4.1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. J. (2002). “Generation of transgenic livestock by pronuclear injection,” in Transgenesis Techniques: Principles and Protocols (Totowa, NJ: Springer; ), 273–287. 10.1385/1-59259-178-7:273 [DOI] [PubMed] [Google Scholar]
- Clark A. J., Bessos H., Bishop J. O., Brown P., Harris S., Lathe R., et al. (1989). Expression of human anti-hemophilic factor IX in the milk of transgenic sheep. BioTechnology 7, 487–492. 10.1038/nbt0589-487 [DOI] [Google Scholar]
- Clements J. E., Wall R. J., Narayan O., Hauer D., Schoborg R., Sheffer D., et al. (1994). Development of transgenic sheep that express the visna virus envelope gene. Virology 200, 370–380. 10.1006/viro.1994.1201 [DOI] [PubMed] [Google Scholar]
- Colman A. (1999). Dolly, Polly and other “ollys”: likely impact of cloning technology on biomedical uses of livestock. Genet. Anal. Biomol. Eng. 15, 167–173. 10.1016/S1050-3862(99)00022-4 [DOI] [PubMed] [Google Scholar]
- Cong L., Ran F. A., Cox D., Lin S., Barretto R., Habib N., et al. (2013). Multiplex genome engineering using CRISPR/Cas systems. Science 339, 819–823. 10.1126/science.1231143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornetta K., Tessanne K., Long C., Yao J., Satterfield C., Westhusin M. (2013). Transgenic sheep generated by lentiviral vectors: safety and integration analysis of surrogates and their offspring. Transgenic Res. 22, 737–745. 10.1007/s11248-012-9674-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispo M., Mulet A. P., Tesson L., Barrera N., Cuadro F., Santos-neto P. C., (2015. a). Efficient generation of myostatin knock-out sheep using CRISPR/Cas9 technology and microinjection into zygotes. PLoS One 10, e0136690. 10.1371/journal.pone.0136690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crispo M., Vilarino M., dos Santos-Neto P. C., Nunez-Olivera R., Cuadro F., Barrera N., et al. (2015. b). Embryo development, fetal growth and postnatal phenotype of eGFP lambs generated by lentiviral transgenesis. Transgenic Res. 24, 31–41. 10.1007/s11248-014-9816-x [DOI] [PubMed] [Google Scholar]
- Cui C., Song Y., Liu J., Ge H., Li Q., Huang H., et al. (2015). Gene targeting by TALEN-induced homologous recombination in goats directs production of β-lactoglobulin-free, high-human lactoferrin milk. Sci. Rep. 5, 10482. 10.1038/srep10482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daboussi F., Stoddard T. J., Zhang F., (2015). “Engineering meganuclease for precise plant genome modification,” in Advances in new technology for targeted modification of plant genomes (New York, NY: Springer; ), 21–38. 10.1007/978-1-4939-2556-8_2 [DOI] [Google Scholar]
- Damak S., Jay N. P., Barrell G. K., Bullock D. W. (1996. a). Targeting gene expression to the wool follicle in transgenic sheep. Nat. Biotechnol. 14, 181–184. 10.1038/nbt0296-181 [DOI] [PubMed] [Google Scholar]
- Damak S., Su H., Jay N. P., Bullock D. W. (1996. b). Improved wool production in transgenic sheep expressing insulin-like growth factor 1. Nat. Biotechnol. 14, 185–188. 10.1038/nbt0296-185 [DOI] [PubMed] [Google Scholar]
- De Los Angeles A., Pho N., Redmond D. E. (2018). Generating human organs via interspecies chimera formation: advances and barriers. Yale J. Biol. Med. 91, 333–342. [PMC free article] [PubMed] [Google Scholar]
- Deng S., Li G., Yu K., Tian X., Wang F., Li W., et al. (2017). RNAi combining Sleeping Beauty transposon system inhibits ex vivo expression of foot-and-mouth disease virus VP1 in transgenic sheep cells. Sci. Rep. 7, 10065. 10.1038/s41598-017-09302-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Li G., Zhang J., Zhang X., Cui M., Guo Y., et al. (2013). Transgenic cloned sheep overexpressing ovine toll-like receptor 4. Theriogenology 80, 50–57. 10.1016/j.theriogenology.2013.03.008 [DOI] [PubMed] [Google Scholar]
- Deng S., Wu Q., Yu K., Zhang Y., Yao Y., Li W., et al. (2012. a). Changes in the relative inflammatory responses in sheep cells overexpressing of toll-like receptor 4 when stimulated with LPS. PLoS One 7, e47118. 10.1371/journal.pone.0047118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng S., Yu K., Zhang B., Yao Y., Liu Y., He H., et al. (2012. b). Effects of over-expression of TLR2 in transgenic goats on pathogen clearance and role of up-regulation of lysozyme secretion and infiltration of inflammatory cells. BMC Vet. Res. 8, 196. 10.1186/1746-6148-8-196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denning C., Burl S., Ainslie A., Bracken J., Dinnyes A., Fletcher J., et al. (2001). Deletion of the α (1,3)galactosyl transferase (GGTA1) gene and the prion protein (PrP) gene in sheep. Nat. Biotechnol. 19, 559–562. 10.1038/89313 [DOI] [PubMed] [Google Scholar]
- Dent J., McGovern P. T., Hancock J. L. (1971). Immunological implications of ultrastructural studies of goat × sheep hybrid placentae. Nature 231, 116–117. 10.1038/231116a0 [DOI] [PubMed] [Google Scholar]
- Doudna J. A., Charpentier E. (2014). The new frontier of genome engineering with CRISPR-Cas9. Science 346, 1258096. 10.1126/science.1258096 [DOI] [PubMed] [Google Scholar]
- Du C., Wang Z., Zhang W., Fu S., Liu B., Liu Z., et al. (2018). Construction of lentiviral RNAi vector of PPARγ gene in cashmere goats and comparison with the transcriptome analysis of adipose cells treatments. Small Rumin. Res. 164, 87–93. 10.1016/j.smallrumres.2018.04.008 [DOI] [Google Scholar]
- Duan B., Cheng L., Gao Y., Yin F. X., Su G. H., Shen Q. Y., et al. (2012). Silencing of fat-1 transgene expression in sheep may result from hypermethylation of its driven cytomegalovirus (CMV) promoter. Theriogenology 78, 793–802. 10.1016/j.theriogenology.2012.03.027 [DOI] [PubMed] [Google Scholar]
- Ebert K. M., Selgrath J. P., DiTullio P., Denman J., Smith T. E., Memon M. A., et al. (1991). Transgenic production of a variant of human tissue-type plasminogen activator in goat milk: generation of transgenic goats and analysis of expression. Nat. Biotechnol. 9, 835–838. 10.1038/nbt0991-835 [DOI] [PubMed] [Google Scholar]
- Edmunds T., Van Patten S. M., Pollock J., Hanson E., Bernasconi R., Higgins E., et al. (1998). Transgenically produced human antithrombin: structural and functional comparison to human plasma-derived antithrombin. Blood 91, 4561–4571. [PubMed] [Google Scholar]
- Eid A., Alshareef S., Mahfouz M. M. (2018). CRISPR base editors: genome editing without double-stranded breaks. Biochem. J. 475, 1955–1964. 10.1042/BCJ20170793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabre S., Pierre A., Mulsant P., Bodin L., Di Pasquale E., Persani L., et al. (2006). Regulation of ovulation rate in mammals: contribution of sheep genetic models. Reprod. Biol. Endocrinol. 4, 20–25. 10.1186/1477-7827-4-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan W., Plaut K., Bramley A. J., Barlow J. W., Kerr D. E. (2002). Adenoviral-mediated transfer of a lysostaphin gene into the goat mammary gland. J. Dairy Sci. 85, 1709–1716. 10.3168/jds.S0022-0302(02)74244-6 [DOI] [PubMed] [Google Scholar]
- Fan Z., Perisse I. V., Cotton C. U., Regouski M., Meng Q., Domb C., et al. (2018). A sheep model of cystic fibrosis generated by CRISPR/Cas9 disruption of the CFTR gene. JCI Insight 3, e123529. 10.1172/jci.insight.123529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Z., Regouski M., Yang M., Gash K., Meng Q., Davies C. J., et al. (2017). Generation of interferon α/β receptor knockout sheep using CRISPR/Cas9 and SCNT techniques [Abstract]. In: program and abstracts of the 14th transgenic technology meeting (TT2017). Transgenic Res. 26, 1–45. 10.1007/s11248-017-0033-2 [DOI] [PubMed] [Google Scholar]
- Fan Z., Yang M., Regouski M., Polejaeva I. A., (2019). “Gene knockouts in goats using CRISPR/Cas9 system and somatic cell nuclear transfer,” in Microinjection (New York, NY: Springer; ), 373–390. 10.1007/978-1-4939-8831-0_22 [DOI] [PubMed] [Google Scholar]
- Fehilly C. B., Willadsen S. M., Tucker E. M. (1984. a). Experimental chimaerism in sheep. Reproduction 70, 347–351. 10.1530/jrf.0.0700347 [DOI] [PubMed] [Google Scholar]
- Fehilly C. B., Willadsen S. M., Tucker E. M. (1984. b). Interspecific chimaerism between sheep and goat. Nature 307, 634–636. 10.1038/307634a0 [DOI] [PubMed] [Google Scholar]
- Freitas V. J. F., Melo L. M., Teixeira D. I. A., Bhat M. H., Serova I. A., Andreeva L. E. et al., (2016). “The use of reproductive technologies to produce transgenic goats,” in Insights from Animal Reproduction (London, UK: IntechOpen; ), 67–84. 10.5772/62447 [DOI] [Google Scholar]
- Fu Y. T., Zheng X., He Q., Jia X. Y., Guo Z. X., Yao R. Y., et al. (2015). Silencing FKBP38 gene by siRNA induces activation of mTOR signaling in goat fetal fibroblasts. Genet. Mol. Res. 14, 9675–9682. 10.4238/2015.August.14.30 [DOI] [PubMed] [Google Scholar]
- Gaj T., Gersbach C. A., Barbas C. F. (2013). ZFN, TALEN, and CRISPR/Cas-based methods for genome engineering. Trends Biotechnol. 31, 397–405. 10.1016/j.tibtech.2013.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Guo J., Kato Y., Sirk S. J., Barbas C. F. (2012). Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nat. Methods 9, 805–807. 10.1038/nmeth.2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T., Sirk S. J., Barbas C. F. (2014). Expanding the scope of site-specific recombinases for genetic and metabolic engineering. Biotechnol. Bioeng. 111, 1–15. 10.1002/bit.25096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galetto R., Duchateau P., Pâques F. (2009). Targeted approaches for gene therapy and the emergence of engineered meganucleases. Expert Opin. Biol. Ther. 9, 1289–1303. 10.1517/14712590903213669 [DOI] [PubMed] [Google Scholar]
- Gatica R., Boland M. P., Crosby T. F., Gordon I. (1984). Micromanipulation of sheep morulae to produce monozygotic twins. Theriogenology 21, 555–560. 10.1016/0093-691X(84)90440-0 [DOI] [PubMed] [Google Scholar]
- Ge H., Cui C., Liu J., Luo Y., Quan F., Jin Y., et al. (2016). The growth and reproduction performance of TALEN-mediated β-lactoglobulin-knockout bucks. Transgenic Res. 25, 721–729. 10.1007/s11248-016-9967-z [DOI] [PubMed] [Google Scholar]
- Golding M. C., Long C. R., Carmell M. A., Hannon G. J., Westhusin M. E. (2006). Suppression of prion protein in livestock by RNA interference. Proc. Natl. Acad. Sci. 103, 5285–5290. 10.1073/pnas.0600813103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon J. W., Ruddle F. H. (1981). Integration and stable germ line transmission of genes injected into mouse pronuclei. Science 214, 1244–1246. 10.1126/science.6272397 [DOI] [PubMed] [Google Scholar]
- Gordon J. W., Scangos G. A., Plotkin D. J., Barbosa J. A., Ruddle F. H. (1980). Genetic transformation of mouse embryos by microinjection of purified DNA. Proc. Natl. Acad. Sci. U. S. A. 77, 7380–7384. 10.1073/pnas.77.12.7380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo R., Wan Y., Xu D., Cui L., Deng M., Zhang G., et al. (2016). Generation and evaluation of myostatin knock-out rabbits and goats using CRISPR/Cas9 system. Sci. Rep. 6, 29855. 10.1038/srep29855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R. M., Musunuru K. (2014). Expanding the genetic editing tool kit: ZFNs, TALENs, and CRISPR-Cas9. J. Clin. Invest. 124, 4154–4161. 10.1172/JCI72992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajian M., Hosseini S. M., Forouzanfar M., Abedi P., Ostadhosseini S., Hosseini L., et al. (2011). “Conservation cloning” of vulnerable Esfahan mouflon (Ovis orientalis isphahanica): in vitro and in vivo studies. Eur. J. Wildl. Res. 57, 959–969. 10.1007/s10344-011-0510-5 [DOI] [Google Scholar]
- Hammer R. E., Pursel V. G., Rexroad C. E., Jr., Wall R. J., Bolt D. J., Ebert K. M., et al. (1985). Production of transgenic rabbits, sheep and pigs by microinjection. Nature 315, 680–683. 10.1038/315680a0 [DOI] [PubMed] [Google Scholar]
- Han H., Ma Y., Wang T., Lian L., Tian X., Hu R., et al. (2014). One-step generation of myostatin gene knockout sheep via the CRISPR/Cas9 system. Front. Agric. Sci. Eng. 1, 2–5. 10.15302/J-FASE-2014007 [DOI] [Google Scholar]
- Han Z. S., Li Q. W., Zhang Z. Y., Xiao B., Gao D. W., Wu S. Y., et al. (2007). High-level expression of human lactoferrin in the milk of goats by using replication-defective adenoviral vectors. Protein Expr. Purif. 53, 225–231. 10.1016/j.pep.2006.11.019 [DOI] [PubMed] [Google Scholar]
- Han Z., Wu S., Li Q., Li J., Gao D., Li K., et al. (2009). Efficient human growth hormone gene expression in the milk of non-transgenic goats. Folia Biol. 55, 17–22. [PubMed] [Google Scholar]
- Hao F., Yan W., Li X., Wang H., Wang Y., Hu X., et al. (2018). Generation of cashmere goats carrying an EDAR gene mutant using CRISPR-Cas9-mediated genome editing. Int. J. Biol. Sci. 14, 427–436. 10.7150/ijbs.23890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hati Boruah J. L., Ranjan R., Gogoi H., Pandey S. K., Kumar D., Phukan A. J., et al. (2016). Effect of co-transfection of anti-myostatin shRNA constructs in caprine fetal fibroblast cells. Anim. Biotechnol. 27, 44–51. 10.1080/10495398.2015.1074915 [DOI] [PubMed] [Google Scholar]
- He X., Li H., Zhou Z., Zhao Z., Li W. (2012). Production of brown/yellow patches in the SLC7A11 transgenic sheep via testicular injection of transgene. J. Genet. Genomics 39, 281–285. 10.1016/j.jgg.2012.04.005 [DOI] [PubMed] [Google Scholar]
- He Z., Lu R., Zhang T., Jiang L., Zhou M., Wu D., et al. (2018. a). A novel recombinant human plasminogen activator: efficient expression and hereditary stability in transgenic goats and in vitro thrombolytic bioactivity in the milk of transgenic goats. PLoS One 13, e0201788. 10.1371/journal.pone.0201788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z., Zhang T., Jiang L., Zhou M., Wu D., Mei J., et al. (2018. b). Use of CRISPR/Cas9 technology efficiently targetted goat myostatin through zygotes microinjection resulting in double-muscled phenotype in goats. Biosci. Rep. 38, BSR20180742. 10.1042/BSR20180742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill J. R., Dobrinski I. (2006). Male germ cell transplantation in livestock. Reprod. Fertil. Dev. 18, 13–18. 10.1071/RD05123 [DOI] [PubMed] [Google Scholar]
- Hoess R. H., Abremski K. (1984). Interaction of the bacteriophage P1 recombinase Cre with the recombining site loxP. Proc. Natl. Acad. Sci. 81, 1026–1029. 10.1073/pnas.81.4.1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A., Behboodi E., Blash S., Megee S. O., Dobrinski I. (2003. a). Germ cell transplantation in goats. Mol. Reprod. Dev. 64, 422–428. 10.1002/mrd.10205 [DOI] [PubMed] [Google Scholar]
- Honaramooz A., Behboodi E., Megee S. O., Overton S. A., Galantino-Homer H., Echelard Y., et al. (2003. b). Fertility and germline transmission of donor haplotype following germ cell transplantation in immunocompetent goats. Biol. Reprod. 69, 1260–1264. 10.1095/biolreprod.103.018788 [DOI] [PubMed] [Google Scholar]
- Honaramooz A., Megee S., Zeng W., Destrempes M. M., Overton S. A., Luo J., et al. (2008). Adeno-associated virus (AAV)-mediated transduction of male germ line stem cells results in transgene transmission after germ cell transplantation. FASEB J. 22, 374–382. 10.1096/fj.07-8935com [DOI] [PubMed] [Google Scholar]
- Hosseini S. M., Hajian M., Forouzanfar M., Moulavi F., Abedi P., Asgari V., et al. (2012). Enucleated ovine oocyte supports human somatic cells reprogramming back to the embryonic stage. Cell. Reprogram. 14, 155–163. 10.1089/cell.2011.0061 [DOI] [PubMed] [Google Scholar]
- Hsu P. D., Lander E. S., Zhang F. (2014). Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278. 10.1016/j.cell.2014.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu R., Fan Z. Y., Wang B. Y., Deng S. L., Zhang X. S., Zhang J. L., et al. (2017). Rapid communication: generation of FGF5 knockout sheep via the CRISPR/Cas9 system. J. Anim. Sci. 95, 2019–2024. 10.2527/jas.2017.1503 [DOI] [PubMed] [Google Scholar]
- Hu S., Ni W., Sai W., Zhang H., Cao X., Qiao J., et al. (2011). Sleeping Beauty-mediated knockdown of sheep myostatin by RNA interference. Biotechnol. Lett. 33, 1949–1953. 10.1007/s10529-011-0667-8 [DOI] [PubMed] [Google Scholar]
- Hu S., Ni W., Sai W., Zi H., Qiao J., Wang P., et al. (2013). Knockdown of myostatin expression by RNAi enhances muscle growth in transgenic sheep. PLoS One 8, e58521. 10.1371/journal.pone.0058521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S., Wang Z., Polejaeva I. (2014). 40 knockout of goat nucleoporin 155 (NUP155) gene using CRISPR/Cas9 systems [Abstract]. Reprod. Fertil. Dev. 26, 134. 10.1071/RDv26n1Ab40 [DOI] [Google Scholar]
- Hu S., Yang M., Polejaeva I. (2015). 360 Double knockout of goat myostatin and prion protein gene using clustered regularly interspaced short palindromic repeat (CRISPR)/Cas9 systems [Abstract]. Reprod. Fertil. Dev. 27, 268. 10.1071/RDv27n1Ab360 [DOI] [Google Scholar]
- Hua S., Zhang Y., Song K., Song J., Zhang Z., Zhang L., et al. (2008). Development of bovine-ovine interspecies cloned embryos and mitochondria segregation in blastomeres during preimplantation. Anim. Reprod. Sci. 105, 245–257. 10.1016/j.anireprosci.2007.03.002 [DOI] [PubMed] [Google Scholar]
- Huang S., Zhang K., Huang Y., Chen M., Li H., Lu D., et al. (1998). Secretion of biologically active human factor IX protein in the milk of transgenic goats. Chin. Sci. Bull. 43, 1317–1319. 10.1007/BF02884150 [DOI] [Google Scholar]
- Huang Y. J., Huang Y., Baldassarre H., Wang B., Lazaris A., Leduc M., et al. (2007). Recombinant human butyrylcholinesterase from milk of transgenic animals to protect against organophosphate poisoning. Proc. Natl. Acad. Sci. 104, 13603–13608. 10.1073/pnas.0702756104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudecek M., Izsvák Z., Johnen S., Renner M., Thumann G., Ivics Z. (2017). Going non-viral: the Sleeping Beauty transposon system breaks on through to the clinical side. Crit. Rev. Biochem. Mol. Biol. 52, 355–380. 10.1080/10409238.2017.1304354 [DOI] [PubMed] [Google Scholar]
- Ivics Z., Hackett P. B., Plasterk R. H., Izsvák Z. (1997). Molecular reconstruction of Sleeping Beauty, a Tc1-like transposon from fish, and its transposition in human cells. Cell 91, 501–510. 10.1016/S0092-8674(00)80436-5 [DOI] [PubMed] [Google Scholar]
- Jacobsen J. C., Bawden C. S., Rudiger S. R., McLaughlan C. J., Reid S. J., Waldvogel H. J., et al. (2010). An ovine transgenic Huntington’s disease model. Hum. Mol. Genet. 19, 1873–1882. 10.1093/hmg/ddq063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain H., Singh S., Kadam M., Sarkhel B. C. (2010). Knockdown of the myostatin gene by RNA interference in caprine fibroblast cells. J. Biotechnol. 145, 99–102. 10.1016/j.jbiotec.2009.10.017 [DOI] [PubMed] [Google Scholar]
- Jain S. K., Jain H., Kumar D., Bedekar M. K., Pandey A. K., Sarkhel B. C. (2015). Quantitative evaluation of myostatin gene in stably transfected caprine fibroblast cells by anti-myostatin shRNA. Appl. Biochem. Biotechnol. 177, 486–497. 10.1007/s12010-015-1757-1 [DOI] [PubMed] [Google Scholar]
- Joung J. K., Sander J. D. (2013). TALENs: a widely applicable technology for targeted genome editing. Nat. Rev. Mol. Cell Biol. 14, 49–55. 10.1038/nrm3486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keefer C. L., Baldassarre H., Keyston R., Wang B., Bhatia B., Bilodeau A. S., et al. (2001). Generation of dwarf goat (Capra hircus) clones following nuclear transfer with transfected and nontransfected fetal fibroblasts and in vitro-matured oocytes. Biol. Reprod. 64, 849–856. 10.1095/biolreprod64.3.849 [DOI] [PubMed] [Google Scholar]
- Keefer C. L., Keyston R., Lazaris A., Bhatia B., Begin I., Bilodeau A. S., et al. (2002). Production of cloned goats after nuclear transfer using adult somatic cells. Biol. Reprod. 66, 199–203. 10.1095/biolreprod66.1.199 [DOI] [PubMed] [Google Scholar]
- Khan S., Tali M., Khan A., Bhat S., Ashraf A., Bhat M., et al. (2018). Comparison of efficiency of in vitro cloned sheep embryo production by conventional somatic cell nuclear transfer and handmade cloning technique. Reprod. Domest. Anim. 53, 512–518. 10.1111/rda.13138 [DOI] [PubMed] [Google Scholar]
- Ko J. H., Lee C. S., Kim K. H., Pang M. G., Koo J. S., Fang N., et al. (2000). Production of biologically active human granulocyte colony stimulating factor in the milk of transgenic goat. Transgenic Res. 9, 215–222. 10.1023/A:1008972010351 [DOI] [PubMed] [Google Scholar]
- Komor A. C., Badran A. H., Liu D. R. (2017). CRISPR-based technologies for the manipulation of eukaryotic genomes. Cell 168, 20–36. 10.1016/j.cell.2016.10.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komor A. C., Kim Y. B., Packer M. S., Zuris J. A., Liu D. R. (2016). Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 533, 420–424. 10.1038/nature17946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Singh S. P., Kumari P., Mitra A. (2014). Small interfering RNA (siRNA)-mediated knockdown of myostatin influences the expression of myogenic regulatory factors in caprine foetal myoblasts. Appl. Biochem. Biotechnol. 172, 1714–1724. 10.1007/s12010-013-0582-7 [DOI] [PubMed] [Google Scholar]
- Kumar R., Singh S. P., Mitra A. (2018). Short-hairpin mediated myostatin knockdown resulted in altered expression of myogenic regulatory factors with enhanced myoblast proliferation in fetal myoblast cells of goats. Anim. Biotechnol. 29, 59–67. 10.1080/10495398.2017.1299744 [DOI] [PubMed] [Google Scholar]
- Lagutina I., Lazzari G., Duchi R., Turini P., Tessaro I., Brunetti D., et al. (2007). Comparative aspects of somatic cell nuclear transfer with conventional and zona-free method in cattle, horse, pig and sheep. Theriogenology 67, 90–98. 10.1016/j.theriogenology.2006.09.011 [DOI] [PubMed] [Google Scholar]
- Lan J., Song Y., Hua S., Liu Y., Liu J., Zhang H., et al. (2010). cDNA cloning of goat DNA methyltransferase 1, screening of shRNA vectors and influences to development of nuclear transfer embryos. Agric. Sci. China 9, 1035–1040. 10.1016/S1671-2927(09)60187-5 [DOI] [Google Scholar]
- Largaespada D. A. (2003). Generating and manipulating transgenic animals using transposable elements. Reprod. Biol. Endocrinol. 1, 80. 10.1186/1477-7827-1-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavitrano M., Busnelli M., Cerrito M. G., Giovannoni R., Manzini S., Vargiolu A. (2006). Sperm-mediated gene transfer. Reprod. Fertil. Dev. 18, 19–23. 10.1071/RD05124 [DOI] [PubMed] [Google Scholar]
- Lavitrano M., Camaioni A., Fazio V. M., Dolci S., Farace M. G., Spadafora C. (1989). Sperm cells as vectors for introducing foreign DNA into eggs: genetic transformation of mice. Cell 57, 717–723. 10.1016/0092-8674(89)90787-3 [DOI] [PubMed] [Google Scholar]
- Li C., Zhou S., Li Y., Li G., Ding Y., Li L., et al. (2018. a). Trio-based deep sequencing reveals a low incidence of off-target mutations in the offspring of genetically edited goats. Front. Genet. 9, 499. 10.3389/fgene.2018.00449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G., Zhou S., Li C., Cai B., Yu H., Ma B., et al. (2018. b). Base pair editing of goat embryos: nonsense codon introgression into FGF5 to improve cashmere yield. bioRxiv 348441. 10.1101/348441 [DOI]
- Li H., Wang G., Hao Z., Zhang G., Qing Y., Liu S., et al. (2016). Generation of biallelic knock-out sheep via gene-editing and somatic cell nuclear transfer. Sci. Rep. 6, 33675. 10.1038/srep33675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Ma A. T., Zhang Y. J., Liu Y. Q., Wang S. Y., Shi X. F., et al. (2017. a). Effects of silencing INHα gene by RNAi on the mRNA expressions of StAR, FST, INHβB, and FSHR genes in cultured sheep granulosa cells. Small Rumin. Res. 157, 23–26. 10.1016/j.smallrumres.2017.10.002 [DOI] [Google Scholar]
- Li W., He S., Liu C., Zhang X., Wang L., Lin J., et al. (2017. b). Ectopic expression of FGF5s induces wool growth in Chinese merino sheep. Gene 627, 477–483. 10.1016/j.gene.2017.06.037 [DOI] [PubMed] [Google Scholar]
- Li W., Liu C., Zhang X., Chen L., Peng X., He S., et al. (2017. c). CRISPR/Cas9-mediated loss of FGF5 function increases wool staple length in sheep. FEBS J. 284, 2764–2773. 10.1111/febs.14144 [DOI] [PubMed] [Google Scholar]
- Li W., Wang K., Kang S., Deng S., Han H., Lian L., et al. (2015). Tongue epithelium cells from shRNA mediated transgenic goat show high resistance to foot and mouth disease virus. Sci. Rep. 5, 17897. 10.1038/srep17897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Bai Y., Cheng X., Kalds P. G. T., Sun B., Wu Y., et al. (2018. c). Efficient SSA-mediated precise genome editing using CRISPR/Cas9. FEBS J. 285, 3362–3375. 10.1111/febs.14626 [DOI] [PubMed] [Google Scholar]
- Lin J., Zhang Q., Zhu L. Q., Yu Q. H., Yang Q. (2014). The copy number and integration site analysis of IGF-1 transgenic goat. Int. J. Mol. Med. 34, 900–910. 10.3892/ijmm.2014.1841 [DOI] [PubMed] [Google Scholar]
- Liu C., Li W., Zhang X., Zhang N., He S., Huang J., et al. (2012). The critical role of myostatin in differentiation of sheep myoblasts. Biochem. Biophys. Res. Commun. 422, 381–386. 10.1016/j.bbrc.2012.04.151 [DOI] [PubMed] [Google Scholar]
- Liu C., Li W., Zhang X., Zhang N., He S., Huang J., et al. (2014). Knockdown of endogenous myostatin promotes sheep myoblast proliferation. Vitr. Cell. Dev. Biol. Anim. 50, 94–102. 10.1007/s11626-013-9689-y [DOI] [PubMed] [Google Scholar]
- Liu C., Wang L., Li W., Zhang X., Tian Y., Zhang N., et al. (2013. a). Highly efficient generation of transgenic sheep by lentivirus accompanying the alteration of methylation status. PLoS One 8, e54614. 10.1371/journal.pone.0054614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Luo Y., Ge H., Han C., Zhang H., Wang Y., et al. (2013. b). Anti-bacterial activity of recombinant human β-defensin-3 secreted in the milk of transgenic goats produced by somatic cell nuclear transfer. PLoS One 8, e65379. 10.1371/journal.pone.0065379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Luo Y., Zheng L., Liu Q., Yang Z., Wang Y., et al. (2013. c). Establishment and characterization of fetal fibroblast cell lines for generating human lysozyme transgenic goats by somatic cell nuclear transfer. Transgenic Res. 22, 893–903. 10.1007/s11248-013-9688-5 [DOI] [PubMed] [Google Scholar]
- Liu Z., Han Z., Li Q., Yang H., Lu W., Li W. (2010). Enhanced expression of adenovirus encoding rhEPO assisted by BAPTA. Anim. Biotechnol. 21, 164–169. 10.1080/10495391003617838 [DOI] [PubMed] [Google Scholar]
- Loi P., Clinton M., Barboni B., Fulka J., Cappai P., Feil R., et al. (2002). Nuclei of nonviable ovine somatic cells develop into lambs after nuclear transplantation. Biol. Reprod. 67, 126–132. 10.1095/biolreprod67.1.126 [DOI] [PubMed] [Google Scholar]
- Loi P., Ptak G., Barboni B., Fulka J., Cappai P., Clinton M. (2001). Genetic rescue of an endangered mammal by cross-species nuclear transfer using post-mortem somatic cells. Nat. Biotechnol. 19, 962–964. 10.1038/nbt1001-962 [DOI] [PubMed] [Google Scholar]
- Lu J., Sun D., Xu L., Lu G., Zhao F., Wei C., et al. (2012). Selection of an effective small interference RNA to silence myostatin gene expression in sheep fibroblast cells. Biochem. Genet. 50, 838–847. 10.1007/s10528-012-9524-2 [DOI] [PubMed] [Google Scholar]
- Lu J., Wei C., Zhang X., Xu L., Zhang S., Liu J., et al. (2013). The effect of myostatin silencing by lentiviral-mediated RNA interference on goat fetal fibroblasts. Mol. Biol. Rep. 40, 4101–4108. 10.1007/s11033-013-2494-6 [DOI] [PubMed] [Google Scholar]
- Lu R., Zhang T., Wu D., He Z., Jiang L., Zhou M., et al. (2018). Production of functional human CuZn-SOD and EC-SOD in bitransgenic cloned goat milk. Transgenic Res. 27, 343–354. 10.1007/s11248-018-0080-3 [DOI] [PubMed] [Google Scholar]
- Luo J., Zhang Y., Guo Y., Tang H., Wei H., Liu S., et al. (2017). TRIM28 regulates Igf2-H19 and Dlk1-Gtl2 imprinting by distinct mechanisms during sheep fibroblast proliferation. Gene 637, 152–160. 10.1016/j.gene.2017.09.048 [DOI] [PubMed] [Google Scholar]
- Ma H., Ma Q., Lu Y., Wang J., Hu W., Gong Z., et al. (2014). PhiC31 integrase induces efficient site-specific recombination in the Capra hircus genome. DNA Cell Biol. 33, 484–491. 10.1089/dna.2013.2124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L. B., Yang L., Hua S., Cao J. W., Li J. X., Zhang Y. (2008). Development in vitro and mitochondrial fate of interspecies cloned embryos. Reprod. Domest. Anim. 43, 279–285. 10.1111/j.1439-0531.2007.00891.x [DOI] [PubMed] [Google Scholar]
- Ma T., Tao J., Yang M., He C., Tian X., Zhang X., et al. (2017). An AANAT/ASMT transgenic animal model constructed with CRISPR/Cas9 system serving as the mammary gland bioreactor to produce melatonin-enriched milk in sheep. J. Pineal Res. 63, e12406. 10.1111/jpi.12406 [DOI] [PubMed] [Google Scholar]
- Maga E. A., Sargent R. G., Zeng H., Pati S., Zarling D. A., Oppenheim S. M., et al. (2003). Increased efficiency of transgenic livestock production. Transgenic Res. 12, 485–496. 10.1023/A:1024257906647 [DOI] [PubMed] [Google Scholar]
- Mali P., Yang L., Esvelt K. M., Aach J., Guell M., DiCarlo J. E., et al. (2013). RNA-guided human genome engineering via Cas9. Science 339, 823–826. 10.1126/science.1232033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik H. N., Dubey A. K., Singhal D. K., Saugandhika S., Mohapatra S. K., Malakar D. (2014). Generation of handmade cloned embryos from adipose tissue derived mesenchymal stem cells in goat. Small Rumin. Res. 121, 340–350. 10.1016/j.smallrumres.2014.09.004 [DOI] [Google Scholar]
- McCreath K. J., Howcroft J., Campbell K. H. S., Colman A., Schnieke A. E., Kind A. J. (2000). Production of gene-targeted sheep by nuclear transfer from cultured somatic cells. Nature 405, 1066–1069. 10.1038/35016604 [DOI] [PubMed] [Google Scholar]
- McLaren A. (2000). Cloning: pathways to a pluripotent future. Science 288, 1775–1780. 10.1126/science.288.5472.1775 [DOI] [PubMed] [Google Scholar]
- Mehravar M., Shirazi A., Nazari M., Banan M. (2019). Mosaicism in CRISPR/Cas9-mediated genome editing. Dev. Biol. 445, 156–162. 10.1016/j.ydbio.2018.10.008 [DOI] [PubMed] [Google Scholar]
- Meinecke-Tillmann S., Meinecke B. (1984). Experimental chimaeras—removal of reproductive barrier between sheep and goat. Nature 307, 637–638. 10.1038/307637a0 [DOI] [PubMed] [Google Scholar]
- Meir Y. J. J., Wu S. C. Y. (2011). Transposon-based vector systems for gene therapy clinical trials: challenges and considerations. Chang Gung Med. J. 34, 565–579. [PubMed] [Google Scholar]
- Meissner A., Jaenisch R. (2006). Mammalian nuclear transfer. Dev. Dyn. 235, 2460–2469. 10.1002/dvdy.20915 [DOI] [PubMed] [Google Scholar]
- Menchaca A., Anegon I., Whitelaw C. B. A., Baldassarre H., Crispo M. (2016). New insights and current tools for genetically engineered (GE) sheep and goats. Theriogenology 86, 160–169. 10.1016/j.theriogenology.2016.04.028 [DOI] [PubMed] [Google Scholar]
- Menchaca A., Mulet A. P., dos Santos Neto P. C., Cuadro F., Souza M., Anegon I., et al. (2018). CRISPR in sheep: a southern perspective [Abstract]. In: abstracts from the uc davis transgenic animal research conference xi. Transgenic Res. 27, 467–487. 10.1007/s11248-018-0086-x [DOI] [PubMed] [Google Scholar]
- Modric T., Mergia A. (2009). The use of viral vectors in introducing genes into agricultural animal species. Anim. Biotechnol. 20, 216–30. 10.1080/10495390903196380 [DOI] [PubMed] [Google Scholar]
- Mojica F. J. M., Montoliu L. (2016). On the origin of CRISPR-Cas technology: from prokaryotes to mammals. Trends Microbiol. 24, 811–820. 10.1016/j.tim.2016.06.005 [DOI] [PubMed] [Google Scholar]
- Muñoz-López M., García-Pérez J. L. (2010). DNA transposons: nature and applications in genomics. Curr. Genomics 11, 115–128. 10.2174/138920210790886871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray J. D., Nancarrow C. D., Marshall J. T., Hazelton I. G., Ward K. A. (1989). Production of transgenic merino sheep by microinjection of ovine metallothionein-ovine growth hormone fusion genes. Reprod. Fertil. Dev. 1, 147–155. 10.1071/RD9890147 [DOI] [PubMed] [Google Scholar]
- Nakagawa T., Hoogenraad C. C. (2011). “Lentiviral transgenesis,” in Methods in Molecular Biology (Totowa, NJ: Springer; ), 117–142. 10.1007/978-1-60761-974-1_8 [DOI] [PubMed] [Google Scholar]
- Ni W., Hu S., Qiao J., Wang Y., Shi H., Wang Y., et al. (2012). ΦC31 integrase mediates efficient site-specific integration in sheep fibroblasts. Biosci. Biotechnol. Biochem. 76, 2093–2095. 10.1271/bbb.120439 [DOI] [PubMed] [Google Scholar]
- Ni W., Qiao J., Hu S., Zhao X., Regouski M., Yang M., et al. (2014). Efficient gene knockout in goats using CRISPR/Cas9 system. PLoS One 9, e106718. 10.1371/journal.pone.0106718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemann H., Halter R., Espanion G., Wrenzycki C., Herrmann D., Lemme E., et al. (1996). Expression of human blood clotting factor VIII (FVIII) constructs in the mammary gland of transgenic mice and sheep. J. Anim. Breed. Genet. 113, 437–444. 10.1111/j.1439-0388.1996.tb00634.x [DOI] [Google Scholar]
- Niu Y., Jin M., Li Y., Li P., Zhou J., Wang X., et al. (2017). Biallelic β-carotene oxygenase 2 knockout results in yellow fat in sheep via CRISPR/Cas9. Anim. Genet. 48, 242–244. 10.1111/age.12515 [DOI] [PubMed] [Google Scholar]
- Niu Y., Zhao X., Zhou J., Li Y., Huang Y., Cai B., et al. (2018). Efficient generation of goats with defined point mutation (I397V) in GDF9 through CRISPR/Cas9. Reprod. Fertil. Dev. 30, 307–312. 10.1071/RD17068 [DOI] [PubMed] [Google Scholar]
- Nuo M. T., Yuan J. L., Yang W. L., Gao X. Y., He N., Liang H., et al. (2016). Promoter methylation and histone modifications affect the expression of the exogenous DsRed gene in transgenic goats. Genet. Mol. Res. 15, gmr.15038560. 10.4238/gmr.15038560 [DOI] [PubMed] [Google Scholar]
- Oliver D., Yuan S., McSwiggin H., Yan W. (2015). Pervasive genotypic mosaicism in founder mice derived from genome editing through pronuclear injection. PLoS One 10, e0129457. 10.1371/journal.pone.0129457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olorunniji F. J., Rosser S. J., Stark W. M. (2016). Site-specific recombinases: molecular machines for the genetic revolution. Biochem. J. 473, 673–684. 10.1042/BJ20151112 [DOI] [PubMed] [Google Scholar]
- Park K.-E., Kaucher A. V., Powell A., Waqas M. S., Sandmaier S. E. S., Oatley M. J., et al. (2017). Generation of germline ablated male pigs by CRISPR/Cas9 editing of the NANOS2 gene. Sci. Rep. 7, 40176. 10.1038/srep40176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker M. H., Birck-Wilson E., Allard G., Masiello N., Day M., Murphy K. P., et al. (2004). Purification and characterization of a recombinant version of human α-fetoprotein expressed in the milk of transgenic goats. Protein Expr. Purif. 38, 177–183. 10.1016/j.pep.2004.07.007 [DOI] [PubMed] [Google Scholar]
- Patel A. K., Tripathi A. K., Patel U. A., Shah R. K., Joshi C. G. (2014). Myostatin knockdown and its effect on myogenic gene expression program in stably transfected goat myoblasts. Vitr. Cell. Dev. Biol. Anim. 50, 587–596. 10.1007/s11626-014-9743-4 [DOI] [PubMed] [Google Scholar]
- Patel U. A., Patel A. K., Joshi C. G. (2015). Stable suppression of myostatin gene expression in goat fetal fibroblast cells by lentiviral vector-mediated RNAi. Biotechnol. Prog. 31, 452–459. 10.1002/btpr.2022 [DOI] [PubMed] [Google Scholar]
- Pereira A. F., Feltrin C., Almeida K. C., Carneiro I. S., Avelar S. R. G., Neto A. S. A., et al. (2013). Analysis of factors contributing to the efficiency of the in vitro production of transgenic goat embryos (Capra hircus) by handmade cloning (HMC). Small Rumin. Res. 109, 163–172. 10.1016/j.smallrumres.2012.07.020 [DOI] [Google Scholar]
- Pereyra-Bonnet F., Fernández-Martín R., Olivera R., Jarazo J., Vichera G., Gibbons A., et al. (2008). A unique method to produce transgenic embryos in ovine, porcine, feline, bovine and equine species. Reprod. Fertil. Dev. 20, 741–749. 10.1071/RD07172 [DOI] [PubMed] [Google Scholar]
- Pereyra-Bonnet F., Gibbons A., Cueto M., Sipowicz P., Fernández-Martín R., Salamone D. (2011). Efficiency of sperm-mediated gene transfer in the ovine by laparoscopic insemination, in vitro fertilization and ICSI. J. Reprod. Dev. 57, 188–196. 10.1262/jrd.10-063A [DOI] [PubMed] [Google Scholar]
- Petersen B. (2017). Basics of genome editing technology and its application in livestock species. Reprod. Domest. Anim. 52, 4–13. 10.1111/rda.13012 [DOI] [PubMed] [Google Scholar]
- Peura T. T., Vajta G. (2003). A comparison of established and new approaches in ovine and bovine nuclear transfer. Cloning Stem Cells 5, 257–277. 10.1089/153623003772032772 [DOI] [PubMed] [Google Scholar]
- Pfeifer A., Hofmann A. (2009). “Lentiviral transgenesis,” in Methods in Molecular Biology (Totowa, NJ: Springer; ), 391–405. 10.1007/978-1-59745-471-1_21 [DOI] [PubMed] [Google Scholar]
- Pfister E. L., DiNardo N., Mondo E., Borel F., Conroy F., Fraser C., et al. (2018). Artificial miRNAs reduce human mutant huntingtin throughout the striatum in a transgenic sheep model of Huntington’s Disease. Hum. Gene Ther. 29, 663–673. 10.1089/hum.2017.199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polejaeva I. A., Ranjan R., Davies C. J., Regouski M., Hall J., Olsen A. L., et al. (2016). Increased susceptibility to atrial fibrillation secondary to atrial fibrosis in transgenic goats expressing transforming growth factor-β1. J. Cardiovasc. Electrophysiol. 27, 1220–1229. 10.1111/jce.13049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polzin V. J., Anderson D. L., Anderson G. B., BonDurant R. H., Butler J. E., Pashens R. L., et al. (1987). Production of sheep-goat chimeras by inner cell mass transplantation. J. Anim. Sci. 65, 325–330. 10.2527/jas1987.651325x [DOI] [PubMed] [Google Scholar]
- Pramod R. K., Kumar R., Mitra A. (2016). Transgenic expression of green fluorescent protein in caprine embryos produced through electroporation-aided sperm-mediated gene transfer. Gene 576, 505–511. 10.1016/j.gene.2015.10.066 [DOI] [PubMed] [Google Scholar]
- Pramod R. K., Mitra A. (2018). Intratesticular injection followed by electroporation allows gene transfer in caprine spermatogenic cells. Sci. Rep. 8, 3169. 10.1038/s41598-018-21558-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot C., Carlson D. F., Huddart R., Long C. R., Pryor J. H., King T. J., et al. (2015). Genome edited sheep and cattle. Transgenic Res. 24, 147–153. 10.1007/s11248-014-9832-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y., Chen H., Zhang Y., Zhu C., Gao B., Yin Y., et al. (2013). Cloning of the Xuhuai goat PPARγ gene and the preparation of transgenic sheep. Biochem. Genet. 51, 543–553. 10.1007/s10528-013-9585-x [DOI] [PubMed] [Google Scholar]
- Qin Y., Zhang Y., Yin Y., Xu F., Gao B., Shi Q., et al. (2012). Cloning of Xuhuai goat lipoprotein lipase gene and the preparation of transgenic sheep. Mol. Biol. Rep. 39, 8439–8446. 10.1007/s11033-012-1697-6 [DOI] [PubMed] [Google Scholar]
- Rafferty S. A., Quinn T. A. (2018). A beginner’s guide to understanding and implementing the genetic modification of zebrafish. Prog. Biophys. Mol. Biol. 138, 3–19. 10.1016/j.pbiomolbio.2018.07.005 [DOI] [PubMed] [Google Scholar]
- Raina A., Kumar S., Shrivastava R., Mitra A. (2015). Testis mediated gene transfer: in vitro transfection in goat testis by electroporation. Gene 554, 96–100. 10.1016/j.gene.2014.10.030 [DOI] [PubMed] [Google Scholar]
- Rashid T., Kobayashi T., Nakauchi H. (2014). Revisiting the flight of Icarus: making human organs from PSCs with large animal chimeras. Cell Stem Cell 15, 406–409. 10.1016/j.stem.2014.09.013 [DOI] [PubMed] [Google Scholar]
- Reh W. A., Maga E. A., Collette N. M., Moyer A., Conrad-Brink J. S., Taylor S. J., et al. (2004). Hot topic: using a stearoyl-CoA desaturase transgene to alter milk fatty acid composition. J. Dairy Sci. 87, 3510–3514. 10.3168/jds.S0022-0302(04)73486-4 [DOI] [PubMed] [Google Scholar]
- Ren Y., Wu H., Ma Y., Yuan J., Liang H., Liu D. (2014). Potential of adipose-derived mesenchymal stem cells and skeletal muscle-derived satellite cells for somatic cell nuclear transfer mediated transgenesis in Arbas cashmere goats. PLoS One 9, e93583. 10.1371/journal.pone.0093583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rexroad C. E., Hammer R. E., Bolt D. J., Mayo K. E., Frohman L. A., Palmiter R. D., et al. (1989). Production of transgenic sheep with growth-regulating genes. Mol. Reprod. Dev. 1, 164–169. 10.1002/mrd.1080010304 [DOI] [PubMed] [Google Scholar]
- Rexroad C. E., Jr., Mayo K., Bolt D. J., Elsasser T. H., Miller K. F., Behringer R. R., et al. (1991). Transferrin- and albumin-directed expression of growth-related peptides in transgenic sheep. J. Anim. Sci. 69, 2995–3004. 10.2527/1991.6972995x [DOI] [PubMed] [Google Scholar]
- Ritchie W. A., King T., Neil C., Carlisle A. J., Lillico S., McLachlan G., et al. (2009). Transgenic sheep designed for transplantation studies. Mol. Reprod. Dev. 76, 61–64. 10.1002/mrd.20930 [DOI] [PubMed] [Google Scholar]
- Ritchie W. A., Taylor J. E., Gardner J. O., Wilmut I., Carlisle A., Neil C., et al. (2005). Live lambs born from zona-pellucida denuded embryos. Cloning Stem Cells 7, 178–182. 10.1089/clo.2005.7.178 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa J. R., Silvertown J. D., Foster R. A., Medin J. A., Hahnel A. (2009). Transduction and transplantation of spermatogonia into the testis of ram lambs through the extra-testicular rete. Reprod. Domest. Anim. 44, 612–620. 10.1111/j.1439-0531.2007.01030.x [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa R. J., Dobson H., Hahnel A. (2006). Isolation and transplantation of spermatogonia in sheep. Theriogenology 66, 2091–2103. 10.1016/j.theriogenology.2006.03.039 [DOI] [PubMed] [Google Scholar]
- Rouet P., Smih F., Jasin M. (1994). Introduction of double-strand breaks into the genome of mouse cells by expression of a rare-cutting endonuclease. Mol. Cell. Biol. 14, 8096–8106. 10.1128/MCB.14.12.8096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salabi F., Nazari M., Chen Q., Nimal J., Tong J., Cao W. G. (2014). Myostatin knockout using zinc-finger nucleases promotes proliferation of ovine primary satellite cells in vitro. J. Biotechnol. 192, 268–280. 10.1016/j.jbiotec.2014.10.038 [DOI] [PubMed] [Google Scholar]
- Sánchez O., Barrera M., Farnós O., Parra N. C., Salgado E. R., Saavedra P. A., et al. (2014). Effectiveness of the E2-classical swine fever virus recombinant vaccine produced and formulated within whey from genetically transformed goats. Clin. Vaccine Immunol. 21, 1628–1634. 10.1128/CVI.00416-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez O., Toledo J. R., Rodríguez M. P., Castro F. O. (2004). Adenoviral vector mediates high expression levels of human growth hormone in the milk of mice and goats. J. Biotechnol. 114, 89–97. 10.1016/j.jbiotec.2004.06.009 [DOI] [PubMed] [Google Scholar]
- Sanjana N. E., Cong L., Zhou Y., Cunniff M. M., Feng G., Zhang F. (2012). A transcription activator-like effector toolbox for genome engineering. Nat. Protoc. 7, 171–192. 10.1038/nprot.2011.431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnieke A. E., Kind A. J., Ritchie W. A., Mycock K., Scott A. R., Ritchie M., et al. (1997). Human factor IX transgenic sheep produced by transfer of nuclei from transfected fetal fibroblasts. Science 278, 2130–2133. 10.1126/science.278.5346.2130 [DOI] [PubMed] [Google Scholar]
- Seidel G. E. (1983). Production of genetically identical sets of mammals: cloning? J. Exp. Zool. 228, 347–354. 10.1002/jez.1402280217 [DOI] [PubMed] [Google Scholar]
- Selokar N. L., George A., Saha A. P., Sharma R., Muzaffer M., Shah R. A., et al. (2011). Production of interspecies handmade cloned embryos by nuclear transfer of cattle, goat and rat fibroblasts to buffalo (Bubalus bubalis) oocytes. Anim. Reprod. Sci. 123, 279–282. 10.1016/j.anireprosci.2011.01.008 [DOI] [PubMed] [Google Scholar]
- Sha H. Y., Chen J. Q., Chen J., Zhang P. Y., Wang P., Chen L. P., et al. (2009). Fates of donor and recipient mitochondrial DNA during generation of interspecies SCNT-derived human ES-like cells. Cloning Stem Cells 11, 497–507. 10.1089/clo.2009.0021 [DOI] [PubMed] [Google Scholar]
- Shadanloo F., Najafi M. H., Hosseini S. M., Hajian M., Forouzanfar M., Ghaedi K., et al. (2010). Sperm status and DNA dose play key roles in sperm/ICSI-mediated gene transfer in caprine. Mol. Reprod. Dev. 77, 868–875. 10.1002/mrd.21228 [DOI] [PubMed] [Google Scholar]
- Shelton J. N., Szell A. (1988). Survival of sheep demi-embryos in vivo and in vitro. Theriogenology 30, 855–863. 10.1016/S0093-691X(88)80047-5 [DOI] [PubMed] [Google Scholar]
- Shen B., Zhang J., Wu H., Wang J., Ma K., Li Z., et al. (2013). Generation of gene-modified mice via Cas9/RNA-mediated gene targeting. Cell Res. 23, 720–723. 10.1038/cr.2013.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi B., Ding Q., He X., Zhu H., Niu Y., Cai B., et al. (2017). Tβ4-overexpression based on the piggyBac transposon system in cashmere goats alters hair fiber characteristics. Transgenic Res. 26, 77–85. 10.1007/s11248-016-9988-7 [DOI] [PubMed] [Google Scholar]
- Singh N. K., Singh S., Jain S. K., Sarkhel B. C. (2012). Evaluation of interferon response induced by anti-myostatin shRNA constructs in goat (Capra hircus) fetal fibroblasts by quantitative real time-polymerase chain reaction. Anim. Biotechnol. 23, 174–183. 10.1080/10495398.2012.664598 [DOI] [PubMed] [Google Scholar]
- Smith K. R., editor. (2012). Sperm-mediated gene transfer: concepts and controversies. Sharjah, UAE: Bentham Science Publishers. 10.2174/97816080523701120101 [DOI] [Google Scholar]
- Song Y., Cui C., Zhu H., Li Q., Zhao F., Jin Y. (2015). Expression, purification and characterization of zinc-finger nuclease to knockout the goat beta-lactoglobulin gene. Protein Expr. Purif. 112, 1–7. 10.1016/j.pep.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Sun N., Zhao H. (2013). Transcription activator-like effector nucleases (TALENs): a highly efficient and versatile tool for genome editing. Biotechnol. Bioeng. 110, 1811–1821. 10.1002/bit.24890 [DOI] [PubMed] [Google Scholar]
- Sun R., Li Q., Yan H., Zhao J., Gao X. (2012). Bzw2 promotes proliferation and lactation of mammary epithelial cell in dairy goat. J. Integr. Agric. 11, 1884–1891. 10.1016/S2095-3119(12)60194-4 [DOI] [Google Scholar]
- Tan W., Carlson D. F., Lancto C. A., Garbe J. R., Webster D. A., Hackett P. B., et al. (2013). Efficient nonmeiotic allele introgression in livestock using custom endonucleases. Proc. Natl. Acad. Sci. 110, 16526–16531. 10.1073/pnas.1310478110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Proudfoot C., Lillico S. G., Whitelaw C. B. A. (2016). Gene targeting, genome editing: from Dolly to editors. Transgenic Res. 25, 273–287. 10.1007/s11248-016-9932-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang D., Zhu H., Wu J., Chen H., Zhang Y., Zhao X., et al. (2012). Silencing myostatin gene by RNAi in sheep embryos. J. Biotechnol. 158, 69–74. 10.1016/j.jbiotec.2012.01.008 [DOI] [PubMed] [Google Scholar]
- Tao J., Yang M., Wu H., Ma T., He C., Chai M., et al. (2018). Effects of AANAT overexpression on the inflammatory responses and autophagy activity in the cellular and transgenic animal levels. Autophagy 14, 1850–1869. 10.1080/15548627.2018.1490852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y., Cheng L., Zhang M., Li B., Ding J., Zhang Y., et al. (2008). Ultrastructural changes in goat interspecies and intraspecies reconstructed early embryos. Zygote 16, 93–110. 10.1017/S0967199407004492 [DOI] [PubMed] [Google Scholar]
- Tavares K. C. S., de Oliveira Dias A. C., Lazzarotto C. R., Neto S. G., de Sá Carneiro I., Ongaratto F. L., et al. (2016). Transient expression of functional glucocerebrosidase for treatment of Gaucher’s disease in the goat mammary gland. Mol. Biotechnol. 58, 47–55. 10.1007/s12033-015-9902-1 [DOI] [PubMed] [Google Scholar]
- Tian H., Luo J., Zhang Z., Wu J., Zhang T., Busato S., et al. (2018. a). CRISPR/Cas9-mediated stearoyl-CoA desaturase 1 (SCD1) deficiency affects fatty acid metabolism in goat mammary epithelial cells. J. Agric. Food Chem. 66, 10041–10052. 10.1021/acs.jafc.8b03545 [DOI] [PubMed] [Google Scholar]
- Tian X., Lv D., Ma T., Deng S., Yang M., Song Y., et al. (2018. b). AANAT transgenic sheep generated via OPS vitrified-microinjected pronuclear embryos and reproduction efficiency of the transgenic offspring. PeerJ 6, e5420. 10.7717/peerj.5420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Li W., Wang L., Liu C., Lin J., Zhang X., et al. (2013). Expression of 2A peptide mediated tri-fluorescent protein genes were regulated by epigenetics in transgenic sheep. Biochem. Biophys. Res. Commun. 434, 681–687. 10.1016/j.bbrc.2013.04.009 [DOI] [PubMed] [Google Scholar]
- Toledo J. R., Sánchez O., Montesino R., Farnos O., Rodríguez M. P., Alfonso P., et al. (2008). Highly protective E2–CSFV vaccine candidate produced in the mammary gland of adenoviral transduced goats. J. Biotechnol. 133, 370–376. 10.1016/j.jbiotec.2007.09.014 [DOI] [PubMed] [Google Scholar]
- Toledo J. R., Sánchez O., Seguí R. M., García G., Montañez M., Zamora P. A., et al. (2006). High expression level of recombinant human erythropoietin in the milk of non-transgenic goats. J. Biotechnol. 123, 225–235. 10.1016/j.jbiotec.2005.10.019 [DOI] [PubMed] [Google Scholar]
- Tripathi A. K., Ramani U. V., Patel A. K., Rank D. N., Joshi C. G. (2013). Short hairpin RNA-induced myostatin gene silencing in caprine myoblast cells in vitro. Appl. Biochem. Biotechnol. 169, 688–694. 10.1007/s12010-012-0021-1 [DOI] [PubMed] [Google Scholar]
- Tsunoda Y., Tokunaga T., Sugie T., Katsumata M. (1985). Production of monozygotic twins following the transfer of bisected embryos in the goats. Theriogenology 24, 337–343. 10.1016/0093-691X(85)90225-0 [DOI] [PubMed] [Google Scholar]
- Tucker E. M., Moor R. M., Rowson L. E. A. (1974). Tetraparental sheep chimaeras induced by blastomere transplantation: changes in blood type with age. Immunology 26, 613–621. [PMC free article] [PubMed] [Google Scholar]
- Udy G. B. (1987). Commercial splitting of goat embryos. Theriogenology 28, 837–847. 10.1016/0093-691X(87)90035-5 [DOI] [Google Scholar]
- Urnov F. D., Rebar E. J., Holmes M. C., Zhang H. S., Gregory P. D. (2010). Genome editing with engineered zinc finger nucleases. Nat. Rev. Genet. 11, 636–646. 10.1038/nrg2842 [DOI] [PubMed] [Google Scholar]
- Vajta G. (2007). Handmade cloning: the future way of nuclear transfer? Trends Biotechnol. 25, 250–253. 10.1016/j.tibtech.2007.04.004 [DOI] [PubMed] [Google Scholar]
- Vajta G., Gjerris M. (2006). Science and technology of farm animal cloning: state of the art. Anim. Reprod. Sci. 92, 211–230. 10.1016/j.anireprosci.2005.12.001 [DOI] [PubMed] [Google Scholar]
- Vajta G., Lewis I. M., Hyttel P., Thouas G. A., Trounson A. O. (2001). Somatic cell cloning without micromanipulators. Cloning 3, 89–95. 10.1089/15204550152475590 [DOI] [PubMed] [Google Scholar]
- Vajta G., Lewis I. M., Tecirlioglu R. T., (2006). “Handmade somatic cell cloning in cattle,” in Nuclear Transfer Protocols (Totowa, NJ: Springer; ), 183–195. 10.1007/978-1-59745-154-3_12 [DOI] [PubMed] [Google Scholar]
- Vilarino M., Rashid S. T., Suchy F. P., McNabb B. R., Meulen T., Fine E. J., et al. (2017). CRISPR/Cas9 microinjection in oocytes disables pancreas development in sheep. Sci. Rep. 7, 17472. 10.1038/s41598-017-17805-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilarino M., Suchy F. P., Rashid S. T., Lindsay H., Reyes J., McNabb B. R., et al. (2018). Mosaicism diminishes the value of pre-implantation embryo biopsies for detecting CRISPR/Cas9 induced mutations in sheep. Transgenic Res. 27, 525–537. 10.1007/s11248-018-0094-x [DOI] [PubMed] [Google Scholar]
- Wall R. J. (2002). New gene transfer methods. Theriogenology 57, 189–201. 10.1016/S0093-691X(01)00666-5 [DOI] [PubMed] [Google Scholar]
- Wan Y. J., Zhang Y. L., Zhou Z. R., Jia R. X., Li M., Song H., et al. (2012). Efficiency of donor cell preparation and recipient oocyte source for production of transgenic cloned dairy goats harboring human lactoferrin. Theriogenology 78, 583–592. 10.1016/j.theriogenology.2012.03.004 [DOI] [PubMed] [Google Scholar]
- Wang H., Yang H., Shivalila C. S., Dawlaty M. M., Cheng A. W., Zhang F., et al. (2013). One-step generation of mice carrying mutations in multiple genes by CRISPR/Cas-mediated genome engineering. Cell 153, 910–918. 10.1016/j.cell.2013.04.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Cai B., Zhou S., Zhu H., Qu L., Wang X., et al. (2017). RNA-seq reveals transcriptome changes in goats following myostatin gene knockout. PLoS One 12, e0187966. 10.1371/journal.pone.0187966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Peng T., Zhu H., Lv Z., Liu T., Shuai Z., et al. (2007). In vitro development of reconstructed ibex (Capra ibex) embryos by nuclear transfer using goat (Capra hircus) oocytes. Small Rumin. Res. 73, 135–141. 10.1016/j.smallrumres.2006.12.009 [DOI] [Google Scholar]
- Wang X., Cai B., Zhou J., Zhu H., Niu Y., Ma B., et al. (2016. a). Disruption of FGF5 in cashmere goats using CRISPR/Cas9 results in more secondary hair follicles and longer fibers. PLoS One 11, e0164640. 10.1371/journal.pone.0164640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Dai B., Duan E., Chen D. (2001). Advances in interspecific pregnancy. Chin. Sci. Bull. 46, 1772–1778. 10.1007/BF02900547 [DOI] [Google Scholar]
- Wang X., Liu J., Niu Y., Li Y., Zhou S., Li C., et al. (2018. a). Low incidence of SNVs and indels in trio genomes of Cas9-mediated multiplex edited sheep. BMC Genomics 19, 397. 10.1186/s12864-018-4712-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Niu Y., Zhou J., Yu H., Kou Q., Lei A., et al. (2016. b). Multiplex gene editing via CRISPR/Cas9 exhibits desirable muscle hypertrophy without detectable off-target effects in sheep. Sci. Rep. 6, 32271. 10.1038/srep32271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Niu Y., Zhou J., Zhu H., Ma B., Yu H., et al. (2018. b). CRISPR/Cas9-mediated MSTN disruption and heritable mutagenesis in goats causes increased body mass. Anim. Genet. 49, 43–51. 10.1111/age.12626 [DOI] [PubMed] [Google Scholar]
- Wang X., Yu H., Lei A., Zhou J., Zeng W., Zhu H., et al. (2015). Generation of gene-modified goats targeting MSTN and FGF5 via zygote injection of CRISPR/Cas9 system. Sci. Rep. 5, 13878. 10.1038/srep13878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Zhou X. Y., Xiang P. Y., Wang L. L., Tang H., Xie F., et al. (2014). The meganuclease I-SceI containing nuclear localization signal (NLS-I-SceI) efficiently mediated mammalian germline transgenesis via embryo cytoplasmic microinjection. PLoS One 9, e108347. 10.1371/journal.pone.0108347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinthal D., Tovkach A., Zeevi V., Tzfira T. (2010). Genome editing in plant cells by zinc finger nucleases. Trends Plant Sci. 15, 308–321. 10.1016/j.tplants.2010.03.001 [DOI] [PubMed] [Google Scholar]
- White K. L., Bunch T. D., Mitalipov S., Reed W. A. (1999). Establishment of pregnancy after the transfer of nuclear transfer embryos produced from the fusion of argali (Ovis ammon) nuclei into domestic sheep (Ovis aries) enucleated oocytes. Cloning 1, 47–54. 10.1089/15204559950020085 [DOI] [PubMed] [Google Scholar]
- Whitelaw C., Lillico S., King T. (2008). Production of transgenic farm animals by viral vector-mediated gene transfer. Reprod. Domest. Anim. 43, 355–358. 10.1111/j.1439-0531.2008.01184.x [DOI] [PubMed] [Google Scholar]
- Willadsen S. M. (1979). A method for culture of micromanipulated sheep embryos and its use to produce monozygotic twins. Nature 277, 298–300. 10.1038/277298a0 [DOI] [PubMed] [Google Scholar]
- Willadsen S. M. (1980). The viability of early cleavage stages containing half the normal number of blastomeres in the sheep. J. Reprod. Fertil. 59, 357–362. 10.1530/jrf.0.0590357 [DOI] [PubMed] [Google Scholar]
- Willadsen S. M. (1981). The developmental capacity of blastomeres from 4- and 8-cell sheep embryos. Development 65, 165–172. [PubMed] [Google Scholar]
- Willadsen S. M. (1986). Nuclear transplantation in sheep embryos. Nature 320, 63–65. 10.1038/320063a0 [DOI] [PubMed] [Google Scholar]
- Willadsen S. M. (1989). Cloning of sheep and cow embryos. Genome 31, 956–962. 10.1139/g89-167 [DOI] [PubMed] [Google Scholar]
- Williams D. K., Pinzón C., Huggins S., Pryor J. H., Falck A., Herman F., et al. (2018). Genetic engineering a large animal model of human hypophosphatasia in sheep. Sci. Rep. 8, 16945. 10.1038/s41598-018-35079-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmut I., Schnieke A. E., McWhir J., Kind A. J., Campbell K. H. S. (1997). Viable offspring derived from fetal and adult mammalian cells. Nature 385, 810–813. 10.1038/385810a0 [DOI] [PubMed] [Google Scholar]
- Wilmut I., Schnieke E., McWhir J., Kind A. J., Colman A., Campbell K. H. S., (1999). “Nuclear transfer in the production of transgenic farm animals,” in Transgenic Animals in Agriculture (Wallingford, UK: CAB International; ), 67–78. [Google Scholar]
- Wright G., Carver A., Cottom D., Reeves D., Scott A., Simons P., et al. (1991). High level expression of active human alpha-1-antitrypsin in the milk of transgenic sheep. Nat. Biotechnol. 9, 830–834. 10.1038/nbt0991-830 [DOI] [PubMed] [Google Scholar]
- Wu J., Kandavelou K., Chandrasegaran S. (2007). Custom-designed zinc finger nucleases: what is next? Cell. Mol. Life Sci. 64, 2933–2944. 10.1007/s00018-007-7206-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Du L., Liu R., Wei C., Wang X., Wang Y., et al. (2018). Double-muscled phenotype in mutant sheep directed by the CRISPR-Cas9 system. Cloning Transgenes. 7, 1000161. 10.4172/2168-9849.1000161 [DOI] [Google Scholar]
- Wu M., Wei C., Lian Z., Liu R., Zhu C., Wang H., et al. (2016). Rosa26-targeted sheep gene knock-in via CRISPR-Cas9 system. Sci. Rep. 6, 24360. 10.1038/srep24360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao B., Li Q., Feng B., Han Z., Gao D., Zhao R., et al. (2009). Expression of recombinant human nerve growth factor beta in milk of goats by recombinant replication-defective adenovirus. Appl. Biochem. Biotechnol. 157, 357–366. 10.1007/s12010-008-8346-5 [DOI] [PubMed] [Google Scholar]
- Xiong K., Li S., Zhang H., Cui Y., Yu D., Li Y., et al. (2013). Targeted editing of goat genome with modular-assembly zinc finger nucleases based on activity prediction by computational molecular modeling. Mol. Biol. Rep. 40, 4251–4256. 10.1007/s11033-013-2507-5 [DOI] [PubMed] [Google Scholar]
- Xu Y., Liu S., Yu G., Chen J., Chen J., Xu X., et al. (2008). Excision of selectable genes from transgenic goat cells by a protein transducible TAT-Cre recombinase. Gene 419, 70–74. 10.1016/j.gene.2008.04.020 [DOI] [PubMed] [Google Scholar]
- Yang H., Li Q., Han Z., Hu J., Li W., Liu Z. (2009). Recombinant human antithrombin expressed in the milk of non-transgenic goats exhibits high efficiency on rat DIC model. J. Thromb. Thrombolysis 28, 449–457. 10.1007/s11239-009-0347-x [DOI] [PubMed] [Google Scholar]
- Yang H., Wang H., Shivalila C. S., Cheng A. W., Shi L., Jaenisch R. (2013). One-step generation of mice carrying reporter and conditional alleles by CRISPR/Cas-mediated genome engineering. Cell 154, 1370–1379. 10.1016/j.cell.2013.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yockey L. J., Jurado K. A., Arora N., Millet A., Rakib T., Milano K. M., et al. (2018). Type I interferons instigate fetal demise after Zika virus infection. Sci. Immunol. 3, eaao1680. 10.1126/sciimmunol.aao1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong Z., Jianchen W., Jufen Q., Zhiming H. (1991). Nuclear transplantation in goats [Abstract]. Theriogenology 35, 299. 10.1016/0093-691X(91)90275-I [DOI] [Google Scholar]
- Yong Z., Yuqiang L. (1998). Nuclear-cytoplasmic interaction and development of goat embryos reconstructed by nuclear transplantation: production of goats by serially cloning embryos. Biol. Reprod. 58, 266–269. 10.1095/biolreprod58.1.266 [DOI] [PubMed] [Google Scholar]
- Yu B., Lu R., Yuan Y., Zhang T., Song S., Qi Z., et al. (2016). Efficient TALEN-mediated myostatin gene editing in goats. BMC Dev. Biol. 16, 26. 10.1186/s12861-016-0126-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu G., Chen J., Yu H., Liu S., Chen J., Xu X., et al. (2006). Functional disruption of the prion protein gene in cloned goats. J. Gen. Virol. 87, 1019–1027. 10.1099/vir.0.81384-0 [DOI] [PubMed] [Google Scholar]
- Yu H., Chen J., Liu S., Zhang A., Xu X., Wang X., et al. (2013. a). Large-scale production of functional human lysozyme in transgenic cloned goats. J. Biotechnol. 168, 676–683. 10.1016/j.jbiotec.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Yu H., Guo Z., Guo S., Qi D., Yang X., Yue P., et al. (2011). Interspecies embryo reconstruction in Tibetan antelope Pantholops hodgsonii by handmade cloning. Afr. J. Biotechnol. 10, 2360–2365. [Google Scholar]
- Yu H., Wang X., Zhu L., He Z., Liu G., Xu X., et al. (2013. b). Establishment of a rapid and scalable gene expression system in livestock by site-specific integration. Gene 515, 367–371. 10.1016/j.gene.2012.10.017 [DOI] [PubMed] [Google Scholar]
- Yuan Y., Cheng Y., Wang J., Peng Q. (2016). Targeted mutagenesis of beta-lactoglobulin gene in caprine fetal fibroblasts by context-dependent assembly zinc-finger nucleases. Open Acess Libr. J. 3, e2813. 10.4236/oalib.1102813 [DOI] [Google Scholar]
- Yuan Y. G., An L., Yu B., Song S., Zhou F., Zhang L., et al. (2014). Expression of recombinant human alpha-lactalbumin in the milk of transgenic goats using a hybrid pomoter/enhancer. J. Anal. Methods Chem. 2014, 281031. 10.1155/2014/281031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y. G., Song S. Z., Zhu M. M., He Z. Y., Lu R., Zhang T., et al. (2017). Human lactoferrin efficiently targeted into caprine beta-lactoglobulin locus with transcription activator-like effector nucleases. Asian Australas. J. Anim. Sci. 30, 1175–1182. 10.5713/ajas.16.0697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W., Tang L., Bondareva A., Luo J., Megee S. O., Modelski M., et al. (2012). Non-viral transfection of goat germline stem cells by nucleofection results in production of transgenic sperm after germ cell transplantation. Mol. Reprod. Dev. 79, 255–261. 10.1002/mrd.22014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Wang L., Ren G., Li Z., Ren C., Zhang T., et al. (2014. a). Targeted disruption of the sheep MSTN gene by engineered zinc-finger nucleases. Mol. Biol. Rep. 41, 209–215. 10.1007/s11033-013-2853-3 [DOI] [PubMed] [Google Scholar]
- Zhang G., Deng M., Zhang Y., Fan Y., Wan Y., Nie H., et al. (2016. a). Effect of PGC-1α overexpression or silencing on mitochondrial apoptosis of goat luteinized granulosa cells. J. Bioenerg. Biomembr. 48, 493–507. 10.1007/s10863-016-9684-6 [DOI] [PubMed] [Google Scholar]
- Zhang H. X., Zhang Y., Yin H. (2019. a). Genome editing with mRNA encoding ZFN, TALEN, and Cas9. Mol. Ther. 27, 735–746. 10.1016/j.ymthe.2019.01.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Cui M. L., Nie Y. W., Dai B., Li F. R., Liu D. J., et al. (2018. a). CRISPR/Cas9-mediated specific integration of fat-1 at the goat MSTN locus. FEBS J. 285, 2828–2839. 10.1111/febs.14520 [DOI] [PubMed] [Google Scholar]
- Zhang J., Li L., Cai Y., Xu X., Chen J., Wu Y., et al. (2008. a). Expression of active recombinant human lactoferrin in the milk of transgenic goats. Protein Expr. Purif. 57, 127–135. 10.1016/j.pep.2007.10.015 [DOI] [PubMed] [Google Scholar]
- Zhang J., Liu J., Yang W., Cui M., Dai B., Dong Y., et al. (2019. b). Comparison of gene editing efficiencies of CRISPR/Cas9 and TALEN for generation of MSTN knock-out cashmere goats. Theriogenology 132, 1–11. 10.1016/j.theriogenology.2019.03.029 [DOI] [PubMed] [Google Scholar]
- Zhang P., Liu P., Dou H., Chen L., Chen L., Lin L., et al. (2013). Handmade cloned transgenic sheep rich in omega-3 fatty acids. PLoS One 8, e55941. 10.1371/journal.pone.0055941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q., Chen J. Q., Lin J., Yu Q. H., Yu H. Q., Xu X. J., et al. (2014. b). Production GH transgenic goat improving mammogenesis by somatic cell nuclear transfer. Mol. Biol. Rep. 41, 4759–4768. 10.1007/s11033-014-3347-7 [DOI] [PubMed] [Google Scholar]
- Zhang R., Wu H., Lian Z. (2019. c). Bioinformatics analysis of evolutionary characteristics and biochemical structure of FGF5 Gene in sheep. Gene 702, 123–132. 10.1016/j.gene.2019.03.040 [DOI] [PubMed] [Google Scholar]
- Zhang S., Xiong K., Xie Z., Nan W., Liu H., Chen J. (2012). Stable silencing of β-lactoglobulin (BLG) gene by lentivirus-mediated RNAi in goat fetal fibroblasts. Genet. Mol. Biol. 35, 680–685. 10.1590/S1415-47572012005000042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Chen S., Yoo S., Chakrabarti S., Zhang T., Ke T., et al. (2008. b). Mutation in nuclear pore component NUP155 leads to atrial fibrillation and early sudden cardiac death. Cell 135, 1017–1027. 10.1016/j.cell.2008.10.022 [DOI] [PubMed] [Google Scholar]
- Zhang X., Li W., Liu C., Peng X., Lin J., He S., et al. (2017. a). Alteration of sheep coat color pattern by disruption of ASIP gene via CRISPR Cas9. Sci. Rep. 7, 8149. 10.1038/s41598-017-08636-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Li W., Wu Y., Peng X., Lou B., Wang L., et al. (2017. b). Disruption of the sheep BMPR-IB gene by CRISPR/Cas9 in in vitro-produced embryos. Theriogenology 91, 163–172. 10.1016/j.theriogenology.2016.10.025 [DOI] [PubMed] [Google Scholar]
- Zhang X., Wang L., Wu Y., Li W., An J., Zhang F., et al. (2016. b). Knockout of myostatin by zinc-finger nuclease in sheep fibroblasts and embryos. Asian Australas. J. Anim. Sci. 29, 1500–1507. 10.5713/ajas.16.0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. S., Du Y. C., Sun L. R., Wang X. H., Liu S. B., Xi J. F., et al. (2018. b). A genetic method for sex determination in Ovis spp. by interruption of the zinc finger protein, Y-linked (ZFY) gene on the Y chromosome. Reprod. Fertil. Dev. 30, 1161–1168. 10.1071/RD17339 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Wang X., Ji Y., Cheng S., Wang M., et al. (2019. d). Acetyl-coenzyme A acyltransferase 2 promote the differentiation of sheep precursor adipocytes into adipocytes. J. Cell. Biochem. 120, 8021–8031. 10.1002/jcb.28080 [DOI] [PubMed] [Google Scholar]
- Zhang Y., Wang Y., Yulin B., Tang B., Wang M., Zhang C., et al. (2018. c). CRISPR/Cas9-mediated sheep MSTN gene knockout and promote sSMSCs differentiation. J. Cell. Biochem. 120, 1794–1806. 10.1002/jcb.27474 [DOI] [PubMed] [Google Scholar]
- Zhang Z., Xu F., Zhang Y., Li W., Yin Y., Zhu C., et al. (2014. c). Cloning and expression of MyoG gene from Hu sheep and identification of its myogenic specificity. Mol. Biol. Rep. 41, 1003–1013. 10.1007/s11033-013-2945-0 [DOI] [PubMed] [Google Scholar]
- Zhao X., Ni W., Chen C., Sai W., Qiao J., Sheng J., et al. (2016). Targeted editing of myostatin gene in sheep by transcription activator-like effector nucleases. Asian Australas. J. Anim. Sci. 29, 413–418. 10.5713/ajas.15.0041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Wei H., Wang Y., Wang L., Yu M., Fan J., et al. (2010). Production of transgenic goats by sperm-mediated exogenous DNA transfer method. Asian Australas. J. Anim. Sci. 23, 33–40. 10.5713/ajas.2010.90216 [DOI] [Google Scholar]
- Zhao Z. J., Li R. C., Cao H. H., Zhang Q. J., Jiang M. X., Ouyang Y. C., et al. (2007). Interspecies nuclear transfer of Tibetan antelope using caprine oocyte as recipient. Mol. Reprod. Dev. 74, 412–419. 10.1002/mrd.20608 [DOI] [PubMed] [Google Scholar]
- Zheng H. L., Liu X. M., Yu Q., Xing R. F., Yang Z. Y., Luo J. (2013). Construction of recombinant adenoviruses carrying the optimal shRNA template against goat PTHrP and successful suppression of PTHrP expression in mammary epithelial cells. Genet. Mol. Res. 12, 3363–3374. 10.4238/2013.September.4.2 [DOI] [PubMed] [Google Scholar]
- Zhong B., Zhang Y., Yan Y., Wang Z., Ying S., Huang M., et al. (2014). MicroRNA-mediated myostatin silencing in caprine fetal fibroblasts. PLoS One 9, e107071. 10.1371/journal.pone.0107071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Cai B., He C., Wang Y., Ding Q., Liu J., et al. (2019). Programmable base editing of the sheep genome revealed no genome-wide off-target mutations. Front. Genet. 10, 215. 10.3389/fgene.2019.00215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou S., Yu H., Zhao X., Cai B., Ding Q., Huang Y., et al. (2018). Generation of gene-edited sheep with a defined Booroola fecundity gene (FecBB) mutation in bone morphogenetic protein receptor type 1B (BMPR1B) via clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated (Cas) 9. Reprod. Fertil. Dev. 30, 1616–1621. 10.1071/RD18086 [DOI] [PubMed] [Google Scholar]
- Zhou W., Wan Y., Guo R., Deng M., Deng K., Wang Z., et al. (2017). Generation of beta-lactoglobulin knock-out goats using CRISPR/Cas9. PLoS One 12, e0186056. 10.1371/journal.pone.0186056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Hu L., Liu J., Chen H., Cui C., Song Y., et al. (2016. a). Generation of β-lactoglobulin-modified transgenic goats by homologous recombination. FEBS J. 283, 4600–4613. 10.1111/febs.13950 [DOI] [PubMed] [Google Scholar]
- Zhu H., Liu J., Cui C., Song Y., Ge H., Hu L., et al. (2016. b). Targeting human α-lactalbumin gene insertion into the goat β-lactoglobulin locus by TALEN-mediated homologous recombination. PLoS One 11, e0156636. 10.1371/journal.pone.0156636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X., Chen Y., Wang Y., Luo J., Zhang Q., Zhang X., et al. (2001). Production of cloned goats from enucleated oocytes injected with cumulus cell nuclei or fused with cumulus cells. Cloning 3, 31–37. 10.1089/152045501300189312 [DOI] [PubMed] [Google Scholar]
- Zou X., Wang Y., Cheng Y., Yang Y., Ju H., Tang H., et al. (2002). Generation of cloned goats (Capra hircus) from transfected foetal fibroblast cells, the effect of donor cell cycle. Mol. Reprod. Dev. 61, 164–172. 10.1002/mrd.1143 [DOI] [PubMed] [Google Scholar]