Abstract
18F-fluorodihydrotestosterone (18F-FDHT) PET/CT potentially provides a noninvasive method for assessment of androgen receptor expression in patients with metastatic castration-resistant prostate cancer (mCRPC). The objective of this study was to assess simplified methods for quantifying 18F-FDHT uptake in mCRPC patients and to assess effects of tumor perfusion on these 18F-FDHT uptake metrics. Methods: Seventeen mCRPC patients were included in this prospective observational multicenter study. Test and retest 30-min dynamic 18F-FDHT PET/CT scans with venous blood sampling were performed in 14 patients. In addition, arterial blood sampling and dynamic 15O-H2O scans were obtained in a subset of 6 patients. Several simplified methods were assessed: Patlak plots; SUV normalized to body weight (SUVBW), lean body mass (SUVLBM), whole blood (SUVWB), parent plasma activity concentration (SUVPP), area under the parent plasma curve (SUVAUC,PP), and area under the whole-blood input curve (SUVAUC,WB); and SUVBW corrected for sex hormone–binding globulin levels (SUVSHBG). Results were correlated with parameters derived from full pharmacokinetic 18F-FDHT and 15O-H2O. Finally, the repeatability of individual quantitative uptake metrics was assessed. Results: Eighty-seven 18F-FDHT–avid lesions were evaluated. 18F-FDHT uptake was best described by an irreversible 2-tissue-compartment model. Replacing the continuous metabolite-corrected arterial plasma input function with an image-derived input function in combination with venous sample data provided similar Ki results (R2 = 0.98). Patlak Ki and SUVAUC,PP showed an excellent correlation (R2 > 0.9). SUVBW showed a moderate correlation to Ki (R2 = 0.70, presumably due to fast 18F-FDHT metabolism. When calculating SUVSHBG, correlation to Ki improved (R2 = 0.88). The repeatability of full kinetic modeling parameters was inferior to that of simplified methods (repeatability coefficients > 36% vs. < 28%, respectively). 18F-FDHT uptake showed minimal blood flow dependency. Conclusion: 18F-FDHT kinetics in mCRPC patients are best described by an irreversible 2-tissue-compartment model with blood volume parameter. SUVAUC,PP showed a near-perfect correlation with the irreversible 2-tissue-compartment model analysis and can be used for accurate quantification of 18F-FDHT uptake in whole-body PET/CT scans. In addition, SUVSHBG could potentially be used as an even simpler method to quantify 18F-FDHT uptake when less complex scanning protocols and accuracy are required.
Keywords: FDHT, PET/CT, prostate cancer, quantification
Prostate cancer is the most frequently diagnosed form of cancer in developed parts of the world and the second most common cause of cancer-related mortality in U.S. men, leading to about 29,000 annual deaths (1,2). The androgen receptor (AR) plays a central role in both early and later stages of prostate cancer, including metastatic castration-resistant prostate cancer (mCRPC). For mCRPC, several mechanisms of AR-signaling persistence have been proposed, including persisting androgen production, AR overexpression, AR-splice variation, and AR transcription via alternative signaling pathways (3). Several agents have been developed that specifically target the AR (e.g., enzalutamide and abiraterone). In mCRPC patients, both with and without prior treatment with docetaxel, these drugs have been shown to result in improved survival and quality of life (4,5).
18F-fluorodihydrotestosterone (18F-FDHT) is a positron-emitting tracer that provides a means to image the AR in vivo in mCRPC patients (6,7). Therefore, 18F-FDHT PET/CT could potentially be used as an imaging biomarker to evaluate AR status and pharmacologic targeting on a lesion-by-lesion level. This potential is of particular significance because mechanisms of persistent AR signaling can differ between metastatic lesions (8,9). In mCRPC, direct assessment of AR using 18F-FDHT might aid AR-targeted drug development, and more personalized treatment planning, thereby potentially preventing unnecessary toxicities and costs.
Accurate quantification is required for objective evaluation of 18F-FDHT uptake in mCRPC lesions. The gold standard for quantification of tracer uptake is pharmacokinetic modeling using nonlinear regression (NLR) in combination with a metabolite-corrected arterial plasma input function (10). At present, NLR is incompatible with the whole-body acquisitions typically required in patients with metastatic disease. Development of total-body PET scanners may overcome this problem. Moreover, because of its complexity, this method it is not well suited for daily clinical practice or large multicenter studies, in which simpler methods such as SUVs are preferred. Although pharmacokinetic assessment of 18F-FDHT uptake has been performed by Beattie et al. (6), a population-based input function was used rather than individually measured arterial input functions. This method could have confounded results because of intersubject differences in individual arterial input function.
Full understanding of 18F-FDHT kinetics is essential for developing simplified methods to quantify 18F-FDHT uptake in clinical practice. Therefore, the objectives of this study were, first, to identify the optimal pharmacokinetic model for quantifying 18F-FDHT kinetics in mCRPC patients using individually measured arterial input function; second, to comprehensively investigate whether simplified methods can be used for accurate quantification of 18F-FDHT uptake; third, to measure the repeatability of 18F-FDHT uptake metrics; and fourth, to assess potentially confounding effects of perfusion on these simplified 18F-FDHT uptake metrics.
MATERIALS AND METHODS
Patients
Between July 2014 and October 2017, 17 histologically proven mCRPC patients were included at the VU University Medical Center and at Memorial Sloan Kettering Cancer Center. Fifteen patients were also included in a previous publication assessing the repeatability of whole-body quantitative 18F-FDHT uptake metrics (11). Patient eligibility criteria were castration-resistant prostate cancer (castrate levels of serum testosterone < 1.7 nmol·L−1 [50 ng·dL−1]), no treatment with enzalutamide or other antiandrogens within 4 wk before study entry, no other malignancies, at least 1 lesion within the field of view positioned over the ascending aorta, and progressive disease based on any of the following: a rise in serum PSA through 3 consecutive measurements, RECIST 1.1 imaging evidence of progressive disease, or a bone scan showing at least 2 new metastatic lesions not attributable to the flare phenomenon. Patients without orchiectomy remained on androgen depletion therapy with a gonadotropin-releasing hormone analog or inhibitor during the study. This study was approved by the institutional review boards of both centers, and all participants gave written informed consent before study enrollment. This trial is registered at clinicaltrials.gov (NCT00588185; this number applies only to Memorial Sloan Kettering, the only United States–based site).
PET Imaging
18F-FDHT PET/CT scans were obtained using a GE Healthcare 690 or 710 or a Philips Gemini TF 64 PET/CT scanner. All participants underwent double baseline 18F-FDHT scans on 2 consecutive days. Sex hormone–binding globulin levels were determined on the day of the first scan. First, a low-dose CT scan (120–140 kV) was performed during tidal breathing, directly followed by a 30-min dynamic 18F-FDHT PET scan over the thorax starting simultaneously with intravenous 18F-FDHT administration. The tracer was administered either manually at a rate of 0.5 mL·s−1 during 3–10 s followed by more than 40 mL of saline in 30–60 s, or using an automated injector (Medrad) flushed with 40 mL of saline (5 mL at 0.8 mL·s−1 followed by 35 mL at 2 mL·s−1). After injection, residual activity in the syringe and lines was measured. Dynamic 18F-FDHT data were reconstructed into 19 frames (6 × 5, 3 × 10, 4 × 60, 2 × 150, and 4 × 300 s) and corrected for detector inhomogeneity, dead time, decay, scatter, random coincidences, and photon attenuation, the last of these using the low-dose CT scan. Ordered-subset expectation maximization was used for reconstruction of the images. In addition, during the dynamic 18F-FDHT scan, 3 manual venous samples were drawn from a separate intravenous cannula at 5, 10, and 30 min after injection (12). For all samples, whole-blood and plasma activity concentrations were measured, as well as parent and metabolite fractions of 18F-FDHT. Radiometabolite analysis was performed using high-performance liquid chromatography at the VU University Medical Center. At Memorial Sloan Kettering Cancer Center, a simplified method was used. High-performance liquid chromatography was performed for only the first and last blood samples; for the other samples, an extraction technique was used, and combined with the high-performance liquid chromatography data, the parent fraction was determined. Details on the radiometabolite analysis method used can be found in a previous publication by Beattie et al. (6).
Furthermore, a subset of patients underwent continuous arterial sampling at 5 mL·min−1 during the first 5 min and at 2.5 mL·min−1 thereafter until the end of the dynamic 18F-FDHT scan. Continuous sampling was interrupted at 5, 10, 15, 20, 30, and 45 min after injection to obtain manual arterial samples for determination of whole-blood activity concentrations, plasma activity concentrations, and parent and metabolite fractions. These patients also underwent a dynamic 10-min 15O-H2O scan before the first dynamic 18F-FDHT scan. 15O-H2O (370 MBq) was administered using an automated injector (Medrad) and flushed using 40 mL of saline (5 mL at 0.8 mL·s−1 followed by 35 mL at 2 mL·s−1). After the 15O-H2O PET scan, a low-dose CT scan (120 kV) was acquired for attenuation correction. The dynamic 15O-H2O data were reconstructed into 26 frames (1 × 10, 8 × 5, 4 × 10, 2 × 15, 3 × 20, 2 × 30, and 6 × 60 s) using the same correction and reconstruction methods as for the dynamic 18F-FDHT scans.
Data Analysis
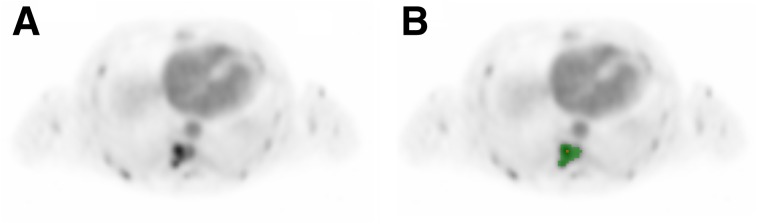
All 18F-FDHT–avid tumors were delineated on an averaged image generated from the last 15 min of the dynamic 18F-FDHT scan, using a 50% isocontour of SUVpeak (sphere of 1.2-cm diameter, positioned to maximize its mean value) corrected for local background to obtain volumes of interest (Fig. 1) (12). Time–activity curves were produced by projecting tumor volumes of interest on the dynamic 18F-FDHT and, when applicable, 15O-H2O scans. In addition, image-derived input functions (IDIFs) were obtained from 18F-FDHT and 15O-H2O scans by placing a 2 × 2 voxel volume of interest centrally on the ascending aorta in 5 consecutive planes using an early frame in which the blood pool was clearly visible. Corresponding time–activity curves were generated by projecting these volumes of interest onto 18F-FDHT and 15O-H2O scans.
FIGURE 1.
(A) Example of averaged image generated from last 15 min of dynamic 18F-FDHT scan. (B) All tumors were delineated using 50% isocontour of SUVpeak (sphere of 1.2-cm diameter, positioned to maximize its mean value) corrected for local background. Yellow voxel indicates hottest voxel in volume of interest.
Both arterially sampled input curves and IDIFs (600–2,000 s) were calibrated using the manual arterial blood samples. Subsequently, these calibrated input curves were corrected for plasma–to–whole-blood ratios and metabolites to generate parent plasma input functions using a multiexponential fit and Hill fit (13), respectively. For IDIFs, this procedure was repeated using the manual venous blood samples (IDIFvenous).
Pharmacokinetic modeling was performed using in-house–developed software in MATLAB (MathWorks Inc.). 15O-H2O scans were analyzed using the standard single-tissue reversible arterial input model with an additional blood volume fraction parameter, and the kinetic rate constant K1 was used as the outcome parameter (14). 18F-FDHT data were analyzed using 1-tissue and both irreversible and reversible 2-tissue-compartment models, all with an additional blood volume fraction parameter consisting of whole-blood activity (10,15). Net influx rate (Ki) and volume of distribution (VT) were calculated from fitted kinetic rate constants: 2-tissue irreversible Ki = K1·k3/(k2 + k3), 2-tissue reversible 2-compartment VT = K1/k2·(1 + k3/k4), and 2-tissue reversible 1-compartment VT = K1/k2. The optimal fit was obtained from the best among 20 constrained fits, each initialized with randomly chosen starting parameters. The constraints of the pharmacokinetic parameters were 0–2 for K1, 0–100 for k2, 0.025–100 for k3, and 0–100 for k3/k4. Furthermore, several simplified uptake metrics were derived from the 18F-FDHT data: Patlak Ki (t* = 5 min after injection); SUV normalized to body weight (SUVBW), lean body mass (SUVLBM), whole-blood activity concentration (SUVWB), parent plasma concentration (SUVPP), area under the whole-blood input curve (SUVAUC,WB), and area under the parent plasma input curve (SUVAUC,PP); and SUVBW corrected for serum SHBG levels (SUVSHBG = SUVBW/serum SHBG). All SUV uptake intervals were set to 20–30 min after injection (6).
Statistical Analysis
Normality of the data was assessed visually using a quantile–quantile plot and histogram analysis. The Akaike criterion was used to select the preferred model for describing kinetics of 18F-FDHT in patients undergoing arterial blood sampling (16). Pharmacokinetic outcome measures calculated using the metabolite-corrected arterial plasma input functions were correlated against pharmacokinetic outcome measures obtained using IDIFs and perfusion metrics. Performance of simplified uptake metrics was assessed in a head-to-head comparison with pharmacokinetic outcome measures from NLR. These analyses were performed using linear regression analysis, intraclass correlation coefficients, and Bland–Altman plots. In addition, when applicable, the repeatability of all outcome measures was assessed using repeatability coefficients (RCs) calculated as 1.96 × SD of the relative differences per lesion. Levene testing was performed to assess differences in RCs between outcome measures. Differences were deemed significant if P was less than 0.05. All statistical analyses were performed using SPSS 22.0.
RESULTS
Pharmacokinetic Modeling
Fourteen patients, with a total of 87 lesions, were enrolled (Table 1). Three patients were excluded because of incomplete or missing blood sample data. Overall, plasma-to-blood ratios remained stable over time; however, 18F-FDHT underwent fast metabolism and about 90% was metabolized at 30 min after injection (Supplemental Fig. 1; supplemental materials are available at http://jnm.snmjournals.org).
TABLE 1.
Patient Characteristics (n = 14)
| Characteristic | Median | n |
| Age (y) | 69 (58–85) | |
| Length (cm) | 180 (170–194) | |
| Weight (kg) | 83 (65–125) | |
| Gleason score | 8 (5–10) | |
| P-specific antigen (ng/mL) | 102.5 (0.5–1,602) | |
| SHBG (nmol/L) | 41 (19–81) | |
| Injected activity (MBq) | ||
| Test | 197 (174–337) | |
| Retest | 196 (186–342) | |
| Residual dose (MBq) | ||
| Test | 31.4 (18.2–55.7) | |
| Retest | 34.5 (6.1–53.5) | |
| Lesions | ||
| Bone | 75 | |
| Lymph node | 12 | |
| Location | ||
| Thoracic vertebrae | 36 | |
| Ribs | 24 | |
| Sternum | 8 | |
| Scapulae | 6 | |
| Humerus | 1 | |
| Mediastinal lymph nodes | 12 | |
| Axillary lymph nodes | 1 | |
| Scanner type | ||
| Philips Gemini TF 64 | 11 | |
| GE Healthcare 690 or 710 | 3 | |
| Sampling | ||
| Arterial | 6 | |
| Venous | 14 |
Data in parentheses are ranges.
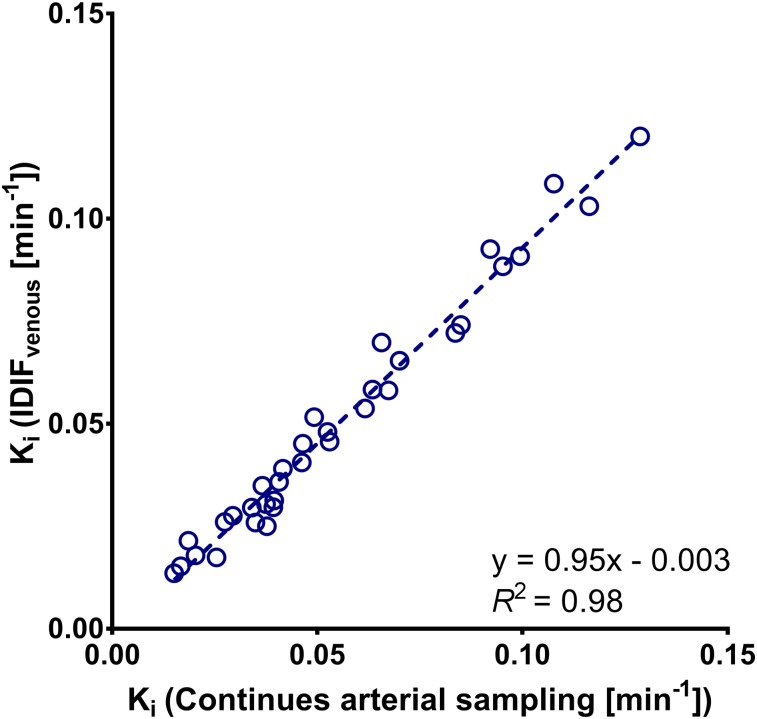
Continuous arterial blood sampling in combination with manual arterial sampling was performed in a subset of 6 patients with 44 18F-FDHT–avid lesions. On the basis of the Akaike criterion, tumor time–activity curves were best described by an irreversible 2-tissue model in 34%, a reversible 2-tissue 2-compartment model in 27% and a reversible 2-tissue 1-compartment model in 39% of the lesions. In 52% of the lesions, the difference in Akaike criterion between the pharmacokinetic models was less than 15 points. All individual Ki values were within reference range, whereas VT values suffered from outliers in 36% of the cases for the reversible 2-tissue 2-compartment model and 7% for the reversible 2-tissue 1-compartment model. Therefore, the irreversible 2-tissue model was used for further evaluation of 18F-FDHT (Fig. 2). Replacing the continuous arterial plasma input function with IDIFvenous provided similar Ki results (R2 = 0.98; intraclass correlation coefficient, 0.99) (Fig. 3). The results of full kinetic modeling and simplified methods are shown in Supplemental Table 1.
FIGURE 2.
Typical example of 18F-FDHT uptake in metastatic prostate cancer lesions fitted using irreversible 2-tissue model with blood volume fraction parameter.
FIGURE 3.
18F-FDHT Ki obtained using IDIF corrected using venous blood samples (IDIFvenous) plotted against those obtained using continuous arterial sampling (n = 34).
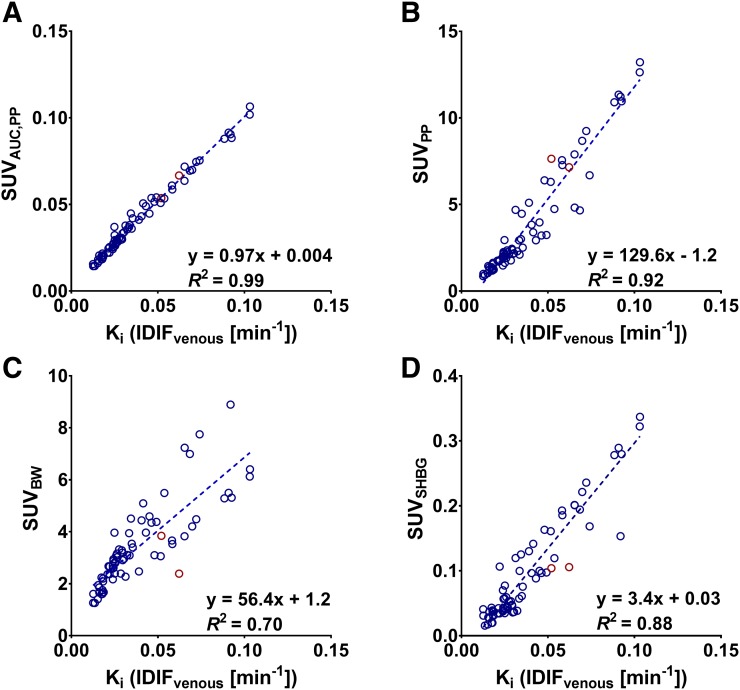
Considering the strong correlation with Ki obtained using continuous arterial sampling, Ki obtained using IDIFvenous was used for validation of simplified methods. Fourteen lesions were excluded because of unrealistically high k2 values and SDs (k2 > 1). An excellent correlation was found between Patlak Ki and NLR-derived Ki (R2 = 0.99; intraclass correlation coefficient, 0.99). This was also the case for SUVAUC,PP, but the performance of more simplified methods was poorer (Fig. 4; Table 2; Supplemental Fig. 2). No significant differences were found in accuracy between SUVBW and SUVLBM. When SUVBW was corrected for serum SHBG levels (SUVSHBG), overall correlation with full kinetic modeling improved (R2 = 0.88). A direct comparison of serum SHBG to the rate of 18F-FDHT metabolism, calculated as the AUC of the parent plasma input function, did not show a strong relationship (R2 = 0.32). All simplified methods reached equilibrium at 30 min after injection, except for SUVpp, which still showed a steep increase.
FIGURE 4.
Scatterplots showing correlation of 18F-FDHT SUVAUC,PP (A), SUVPP (B), SUVBW (C), and SUVSHBG (D) with Ki obtained using IDIF corrected for metabolites using venous blood samples (IDIFvenous) (n = 87). Blue = Philips Gemini TF 64; red = GE Healthcare 690 or 710.
TABLE 2.
Correlation of Simplified Methods with Ki Obtained Using Pharmacokinetic Modeling
| Continuous arterial sampling |
IDIFvenous |
|||||
| Method | R2 | Slope | Intercept | R2 | Slope | Intercept |
| Patlak Ki | 0.99 | 0.90 | 0.00 | 0.99 | 0.93 | 0.00 |
| SUVAUC,PP | 0.99 | 0.94 | 0.00 | 0.99 | 0.97 | 0.00 |
| SUVAUC,WB | 0.83 | 0.41 | 0.01 | 0.83 | 0.45 | 0.00 |
| SUVPP | 0.96 | 116.99 | −0.46 | 0.92 | 129.57 | −1.16 |
| SUVWB | 0.77 | 10.88 | 0.24 | 0.77 | 11.69 | 0.29 |
| SUVBW | 0.76 | 55.91 | 0.81 | 0.70 | 56.40 | 1.23 |
| SUVLBM | 0.73 | 41.64 | 0.75 | 0.70 | 40.97 | 1.06 |
| SUVSHBG | 0.80 | 2.88 | −0.30 | 0.88 | 3.19 | −0.03 |
IDIFvenous = NLR using IDIF corrected using venous blood samples.
Repeatability
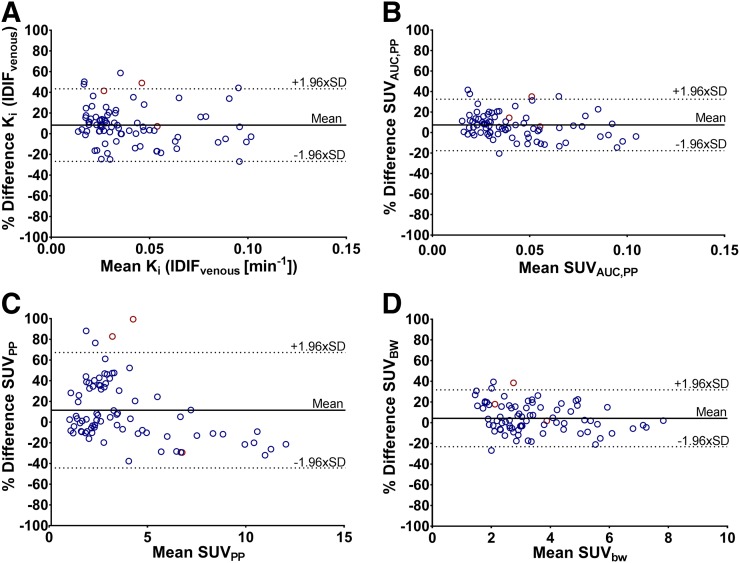
Repeated baseline scans were available in 10 patients with a total of 80 lesions. Median plasma-to-blood and parent plasma fractions at 30 min were not significantly different between test and retest scans (P > 0.7) (Supplemental Fig. 1). The correlation between test and retest scans was strong for all quantitative metrics (R2 = 0.86–0.93; intraclass correlation coefficient, >0.95). The RC of NLR-derived Ki using IDIF corrected with venous sample data was 36% (Fig. 5). Except for SUVpp, quantitatively assessing 18F-FDHT uptake using simplified methods reduced variability (RC, 23%–31%) (Table 3; Fig. 5; Supplemental Table 2; Supplemental Fig. 3). The repeatability of all uptake metrics showed a trend toward dependency on lesion size. In addition, for SUVpp and SUVBW, repeatability appeared to improve for higher SUVs.
FIGURE 5.
Bland–Altman plots showing relative differences in 18F-FDHT uptake between test and retest Ki obtained using IDIF corrected using venous blood samples (A), SUVAUC,PP (B), SUVPP (C), and SUVBW (D) (n = 80). Blue = Philips Gemini TF 64; red = GE 690 or GE710.
TABLE 3.
RCs of Several Quantitative 18F-FDHT Quantitative Uptake Metrics per Lesion
| Absolute difference |
Relative difference |
|||
| Metric | Mean | RC | Mean (%) | RC (%) |
| IDIFvenous Ki | 0.003 | 0.037 | 8.3 | 35.0 |
| Patlak Ki | 0.003 | 0.034 | 8.2 | 31.3 |
| SUVAUC,PP | 0.003 | 0.038 | 7.4 | 25.1 |
| SUVAUC,WB | 0.002 | 0.023 | 3.1 | 23.7 |
| SUVPP | 0.089 | 4.178 | 11.4 | 55.8 |
| SUVWB | 0.062 | 0.660 | 2.4 | 25.1 |
| SUVBW | 0.277 | 2.878 | 4.2 | 27.1 |
| SUVLBM | 0.230 | 2.239 | 4.3 | 27.1 |
IDIFvenous = NLR using IDIF corrected using venous blood samples.
Perfusion
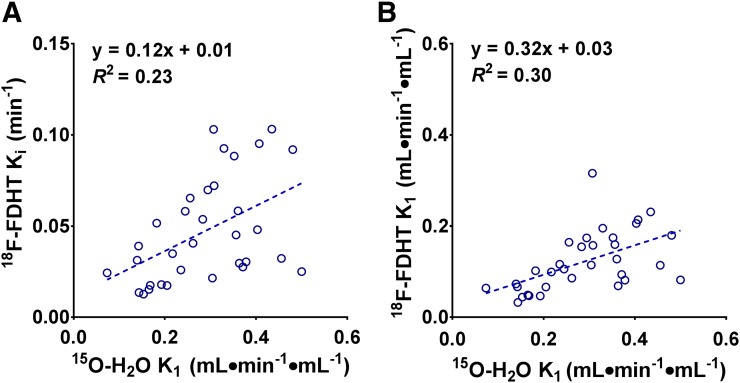
Thirty-five lesions (30 bone and 5 lymph node metastases) were available for assessing the correlation between perfusion and quantitative 18F-FDHT uptake metrics. Ki values obtained using the irreversible 2-tissue-compartment model with IDIFvenous as well as 18F-FDHT plasma extraction showed minimal blood flow dependency (R2 = 0.23 and 0.30, respectively) (Fig. 6). In addition, the effects of perfusion on the discrepancy between SUV- and NLR-based Ki for 18F-FDHT were assessed by plotting the ratio of SUV/Ki against 15O-H2O–derived K1. This plot showed no correlation with blood flow for any of the SUVs (R2 ≤ 0.01) (Supplemental Fig. 4).
FIGURE 6.
NLR-based Ki (A) and K1 (B) using venous blood sampling and SUVSHBG plotted against 15O-H2O based K1.
DISCUSSION
This multicenter study addressed the important clinical question of whether simplified uptake metrics can be used to measure 18F-FDHT uptake in mCRPC both accurately and precisely. An irreversible 2-tissue model with blood volume fraction parameter was preferred for characterizing tumor 18F-FDHT kinetics. This is congruent with previous findings from Beattie et al. (6), for whom both irreversible 1- and 2-tissue models provided the best fits in most cases. The preference for the irreversible 2-tissue model is also logical from a physiologic perspective. In this model, K1 presumably represents influx of 18F-FDHT into the cell. After influx, 18F-FDHT binds to AR (the presumptive second compartment described by k3) and is then transported into the nucleus. It could therefore be argued that k3 is a more appropriate measure for assessment of AR expression, as it describes the binding of 18F-FDHT to the AR rather than uptake in the prostate cancer cell. Finally, k2 represents efflux of unbound 18F-FDHT out of the prostate cancer cell. It has been suggested that 18F-FDHT binding might be reversible at later time points (6,17) and that slow reversibility may potentially develop more than 1 h after injection of 18F-FDHT.
Pharmacokinetic measures obtained using an IDIFvenous correlated well with those obtained using a continuous arterial plasma input function. There was a slight negative bias (5%), which could be due to temporal differences between the 2 input functions. In addition, no significant differences were found between plasma-to-blood ratios and parent fractions of venous and arterial blood samples. This indicates that arterial blood sampling is not required in the case of 18F-FDHT quantification. There was an almost perfect correlation between SUVAUC,PP and Ki derived from pharmacokinetic analysis, eliminating the need for dynamic scanning. Nevertheless, SUVAUC,PP still requires an additional 30-min static PET scan over the chest together with metabolite analysis of several venous blood samples. This enables accurate quantification of lesions outside the thorax, although metabolite analysis may limit its feasibility in multicenter studies and daily clinical practice. Automation of 18F-FDHT metabolite analysis could potentially overcome these limitations.
As an alternative, SUV requires only a single static whole-body scan without any blood sampling. However, in line with Beattie et al. (6), SUV showed only a moderate correlation with pharmacokinetic outcome measures. This poorer correlation was primarily caused by 1 subject with very extensive disease (>40 lesions) and relatively low SHBG levels. In blood, most dihydrotestosterone is bound to proteins (mainly SHBG). Yet, as postulated in the free hormone hypothesis, only free circulating dihydrotestosterone is able to bind to the AR (18,19). In a murine prostate cancer model, Larimer et al. (17) showed that differences in tissue-to-blood ratios between free circulating and SHBG-bound 18F-FDHT were small at 1 h after injection. However, blood-pool activity was significantly higher in SHBG-bound 18F-FDHT at 1 h after injection, indicating a decreased metabolic rate compared with freely circulating FDHT and an increased tumor uptake, as tissue-to-blood ratios are comparable. Normalizing SUV for interpatient differences in serum SHBG levels significantly improved correlation with NLR-derived Ki. Nevertheless, the rate of 18F-FDHT metabolism levels only showed a moderate correlation with serum SHBG (data not shown). Determining serum SHBG just before the 18F-FDHT scan could potentially be used as a surrogate for more cumbersome parent plasma fraction measurements. SHBG measurements are widely available, which would facilitate application in clinical practice or larger trials. However, further research is needed before SHBG can be used as a surrogate for metabolite analysis.
Changes in tumor perfusion due to physiologic variability or treatment could potentially affect tracer uptake when it is perfusion-limited. For Ki, a poor correlation was found with 15O-H2O–derived K1, and therefore Ki does not seem to depend on tumor perfusion. Even though 18F-FDHT is rapidly cleared from blood plasma, k3 values were relatively small compared with k2 values, thereby limiting the effects of perfusion on 18F-FDHT uptake. SUVBW and SUVSHBG showed somewhat stronger and weaker correlations with 15O-H2O K1, respectively. However, discrepancies of SUVBW and SUVSHBG with 18F-FDHT Ki were not due to differences in perfusion.
Before quantitative uptake metrics can be used in a response assessment setting, repeatability should be known. A highly accurate parameter cannot be used for response measurements if precision is poor. RCs found for SUV in the present study were similar to those found in a previous study for whole-body quantitative 18F-FDHT uptake metrics and in line with those of other 18F-labeled tracers (11,20,21). The repeatability of full kinetic modeling parameters obtained using an irreversible 2-tissue-compartment model showed higher variability than those of more simplified methods, with the exception of SUVPP. NLR analysis is known to be more vulnerable to noise, but it can account for changes in pharmacokinetics after therapy. Pharmacokinetic assessment of quantitative tracer uptake should therefore be performed before simplified methods can be used in a response evaluation setting. In the present study, no RCs could be calculated for SUVSHBG as SHBG levels were determined before only the first 18F-FDHT scan. Nevertheless, previous studies found small fluctuations in SHBG levels within 2 consecutive days, and the influence of SHBG on repeatability is therefore expected to be minimal (22).
In the present study, validation of simplified 18F-FDHT uptake metrics was performed in mCRPC patients. This is an essential step in the development of 18F-FDHT as an imaging biomarker for prognosis, response, and AR-targeted drug development by direct evaluation of AR status on a lesion-by-lesion level. SUVAUC,PP and, to a lesser extent, SUVSHBG seemed to be the preferred simplified methods for quantification of 18F-FDHT uptake. SUVSHBG is more attractive in clinical practice and for larger multicenter trials, as it requires only a single whole-body 18F-FDHT scan and SHBG blood sample. However, the exact RCs of this uptake measure still need to be determined. The correlation of SUVAUC,PP with Ki derived from full pharmacokinetic analysis was much stronger than for SUVSHBG, although at the cost of an additional early scan over the chest and metabolite analysis to obtain the parent plasma input function. This method is preferred when high accuracy is required. An additional advantage of including SUVAUC,PP in an investigational setting is that more simplified methods can also be assessed. SUVAUC,PP and SUVSHBG can both be used for whole-body acquisitions, which is essential in mCRPC because most lesions are located outside of the thorax. As a next step in the development of 18F-FDHT PET/CT as an imaging biomarker, the performance of these quantitative uptake metrics need to be assessed in biologic and clinical validation studies.
The small number of patients is an inherent limitation to pharmacokinetic modeling studies. High patient burden and costly procedures limit the number of scans that can be acquired. Nevertheless, we performed a multicenter study and double baseline scanning to maximize the reliability of the pharmacokinetic 18F-FDHT modeling. Unfortunately, most scans were obtained from 1 center; however, multicenter pharmacokinetic studies are unusual, and most pharmacokinetic studies are performed in single-center setting.
CONCLUSION
An irreversible 2-tissue-compartment model with blood volume parameter best described 18F-FDHT kinetics in mCRPC patients. SUVAUC,PP correlated nearly perfectly with Ki obtained using full pharmacokinetic analysis and can be used for accurate quantification of 18F-FDHT uptake in whole-body PET/CT scans. Therefore, SUVAUC,PP is recommended when high accuracy is required. In addition, SUVSHBG also showed a strong correlation with Ki and might be considered when less accuracy is required.
DISCLOSURE
This study was funded by a Movember Foundation Global Action Plan award. The Memorial Sloan Kettering Cancer Center is supported by NIH/NCI Cancer Center Support Grant P30 CA008748. Ian Davis is supported by an Australian NHMRC Practitioner Fellowship (APP1102604). No other potential conflict of interest relevant to this article was reported.
Supplementary Material
KEY POINTS
QUESTION: Can simplified methods be used for accurate quantification of 18F-FDHT uptake in metastatic castration-resistant prostate cancer lesions?
PERTINENT FINDINGS: In this prospective observational multicenter study, we found that 18F-FDHT uptake can be accurately quantified using SUV corrected for area under the venous parent plasma curve (R2 = 0.99). SUV normalized to body weight shows a moderate correlation (R2 = 0.70); however, correction for sex hormone–binding globulin levels improves results (R2 = 0.88).
IMPLICATIONS FOR PATIENT CARE: This study is an essential step in the development of 18F-FDHT PET/CT as an imaging biomarker for prognosis, response, and AR-targeted drug development by direct evaluation of AR status on a lesion-by-lesion level.
REFERENCES
- 1.Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 3.Ferraldeschi R, Welti J, Luo J, Attard G, de Bono JS. Targeting the androgen receptor pathway in castration-resistant prostate cancer: progresses and prospects. Oncogene. 2015;34:1745–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187–1197. [DOI] [PubMed] [Google Scholar]
- 5.Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in men with chemotherapy-naive metastatic castration-resistant prostate cancer: extended analysis of the phase 3 PREVAIL study. Eur Urol. 2017;71:151–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beattie BJ, Smith-Jones PM, Jhanwar YS, et al. Pharmacokinetic assessment of the uptake of 16beta-18F-fluoro-5alpha-dihydrotestosterone (FDHT) in prostate tumors as measured by PET. J Nucl Med. 2010;51:183–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonasera TA, O’Neil JP, Xu M, et al. Preclinical evaluation of fluorine-18-labeled androgen receptor ligands in baboons. J Nucl Med. 1996;37:1009–1015. [PubMed] [Google Scholar]
- 8.Larson SM, Morris M, Gunther I, et al. Tumor localization of 16beta-18F-fluoro-5alpha-dihydrotestosterone versus 18F-FDG in patients with progressive, metastatic prostate cancer. J Nucl Med. 2004;45:366–373. [PubMed] [Google Scholar]
- 9.Vargas HA, Wassberg C, Fox JJ, et al. Bone metastases in castration-resistant prostate cancer: associations between morphologic CT patterns, glycolytic activity, and androgen receptor expression on PET and overall survival. Radiology. 2014;271:220–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gunn RN, Gunn SR, Cunningham VJ. Positron emission tomography compartmental models. J Cereb Blood Flow Metab. 2001;21:635–652. [DOI] [PubMed] [Google Scholar]
- 11.Vargas HA, Kramer GM, Scott AM, et al. Reproducibility and repeatability of semiquantitative 18F-fluorodihydrotestosterone uptake metrics in castration-resistant prostate cancer metastases: a prospective multicenter study. J Nucl Med. 2018;59:1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frings V, Yaqub M, Hoyng LL, et al. Assessment of simplified methods to measure 18F-FLT uptake changes in EGFR-mutated non-small cell lung cancer patients undergoing EGFR tyrosine kinase inhibitor treatment. J Nucl Med. 2014;55:1417–1423. [DOI] [PubMed] [Google Scholar]
- 13.Gunn RN, Sargent PA, Bench CJ, et al. Tracer kinetic modeling of the 5-HT1A receptor ligand [carbonyl-11C]WAY-100635 for PET. Neuroimage. 1998;8:426–440. [DOI] [PubMed] [Google Scholar]
- 14.van der Veldt AA, Hendrikse NH, Harms HJ, et al. Quantitative parametric perfusion images using 15O-labeled water and a clinical PET/CT scanner: test-retest variability in lung cancer. J Nucl Med. 2010;51:1684–1690. [DOI] [PubMed] [Google Scholar]
- 15.Yaqub M, Boellaard R, Kropholler MA, Lammertsma AA. Optimization algorithms and weighting factors for analysis of dynamic PET studies. Phys Med Biol. 2006;51:4217–4232. [DOI] [PubMed] [Google Scholar]
- 16.Akaike H. A new look at the statistical model identification. IEEE Trans Automat Contr. 1974;19:716–723. [Google Scholar]
- 17.Larimer BM, Dubois F, Bloch E, et al. Specific 18F-FDHT accumulation in human prostate cancer xenograft murine models is facilitated by prebinding to sex hormone-binding globulin. J Nucl Med. 2018;59:1538–1543. [DOI] [PubMed] [Google Scholar]
- 18.Swerdloff RS, Dudley RE, Page ST, Wang C, Salameh WA. Dihydrotestosterone: biochemistry, physiology, and clinical implications of elevated blood levels. Endocr Rev. 2017;38:220–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Selby C. Sex hormone binding globulin: origin, function and clinical significance. Ann Clin Biochem. 1990;27:532–541. [DOI] [PubMed] [Google Scholar]
- 20.Kramer GM, Frings V, Hoetjes N, et al. Repeatability of quantitative whole-body 18F-FDG PET/CT uptake measures as function of uptake interval and lesion selection in non-small cell lung cancer patients. J Nucl Med. 2016;57:1343–1349. [DOI] [PubMed] [Google Scholar]
- 21.Oprea-Lager DE, Kramer G, van de Ven PM, et al. Repeatability of quantitative 18F-fluoromethylcholine PET/CT studies in prostate cancer. J Nucl Med. 2016;57:721–727. [DOI] [PubMed] [Google Scholar]
- 22.Brambilla DJ, Matsumoto AM, Araujo AB, McKinlay JB. The effect of diurnal variation on clinical measurement of serum testosterone and other sex hormone levels in men. J Clin Endocrinol Metab. 2009;94:907–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.