Abstract
Background: Physician Orders for Life-Sustaining Treatment (POLST) is an advance care planning tool that is designed to document end-of-life (EoL) care wishes of those living with limited life expectancies. Although positive impacts of POLST program has been studied, variations in state-specific POLST programs across the nation remain unknown.
Objective: Identify state variations in POLST forms and determine if variations are associated with program maturity status.
Design: Environmental scan.
Measurements: Using the national POLST website, state-specific POLST program characteristics were examined. With available sample POLST forms, EoL care options were abstracted.
Results: Of all 51 states (50 United States states and Washington, D.C examined), the majority (n = 48, 98%) were actively participating in POLST; 3 states (5.9%) had Mature status, 19 states and District of Columbia (39.2%) were Endorsed, 24 states were in the developing phase (47.1%), and 4 states (7.8%) were nonconforming. Forty-five states (88.2%) had forms available for review. Antibiotic and intravenous fluid options were identified in 32 (71.1%), and 33 (73.3%) POLST forms, respectively. Hospital transfer and use of oxygen were mentioned in all forms. Use of respiratory devices (i.e., continuous positive airway pressure and bi-level positive airway pressure) were mentioned on 27 (60%) forms, whereas ventilator or intubation use were mentioned in 36 POLST forms (80%). No associations were found between POLST maturity status and provision of treatment options.
Conclusions: Variations in integration of infection and symptom management options were identified. Further research is needed to determine if there are regional factors associated with provision of treatment options on POLST forms and if there are differences in actual rates of infection or symptoms reported.
Keywords: advance directives, environmental scan, physician orders for life-sustaining treatment, POLST, state variations
Introduction
Advances in medical technologies, combined with an aging population, have resulted in an increased number of individuals living with complex health issues. Previous researchers found that the number of elderly Americans suffering from chronic illnesses and comorbidities has increased drastically.1–3 Many of these people are at the end of life (EoL) and at risk for infection, which is often terminal but results in burdensome hospitalizations.4,5 Despite wishes to remain at home and avoid aggressive treatments, many individuals die in acute care settings, including emergency rooms or intensive care units.6–8 Delivering care that reflects individual's values is a priority of care at EoL.
Discussions eliciting patients' preferences for interventions at EoL are difficult. As many as 70% of individuals at the EoL lack the capacity to communicate their preferences due to the progressive and advanced nature of their illnesses (e.g., dementia or stroke).9 Advance care planning, a process of documenting individual's preferences for medical care, is one way to understand individual's preferences in the face of incapacity. In United States, living wills are the most widely used advance care planning tool.10 Since the passing of the Patient Self-Determination Act in 1990, which promoted the use of advance directives, public awareness on advance directives has increased; however, this has not translated into an increased proportion of individuals who actually complete advance directives.11 Although it has been nearly six decades since advance directives were first introduced, the proportion of individuals completing advance directives remains low (i.e., <30% completion rate), and EoL care remains suboptimal.12–14
Recognizing shortcomings of conventional advance directives, and to fulfill the need for an alternative tool that can help honor a patient's EoL care wishes, a group of medical ethicists from Oregon formulated a new advance care planning tool called the Medical Treatment Coversheet in 1991.15 After the instrument was validated and pilot tested, it was renamed “Physician Orders for Life-Sustaining Treatment (POLST)” and released for use in Oregon in 1995.
Although POLST and advance directives share similar aims to document individual's EoL care wishes, they differ in important ways. Advance directives are designed for any adult 18+ years of age; POLST targets people who are suffering from advanced, progressive illnesses and/or frailty, and living with limited life expectancy.16,17 By targeting intended users with advanced illness and who are close to death, POLST offers the opportunity for dying patients to articulate their care preferences with the knowledge of their on-going medical conditions. In addition, medical interventions documented on POLST forms become a set of portable medical orders upon completion, which increase the likelihood that the patient's preferred treatment options will be honored across care settings.18 The POLST paradigm was designed not only to preserve the autonomy of terminally ill individuals, but also to facilitate much needed EoL conversations between a dying patient and treating medical providers.
While the effectiveness of the POLST program and the positive impact it has had on EoL care is well documented in previous studies, implementation of POLST has been driven by states, resulting in state-level variation in content, timing, and rates of adoption. The consequences of these variations have not been addressed adequately.19–21 Currently, the National POLST Paradigm Task Force (NPPTF) supports implementation and operation of state POLST programs, requiring only that the general tenets of POLST Form Usage Policy be followed (i.e., POLST must be a voluntary tool and be used within the intended population). This has resulted in wide variation in POLST design and content across the country, including EoL treatment options that are discussed and care preferences documented.22–24 A close examination of this variation is an important step in identifying best practices in POLST programs.
The NPPTF monitors and designates the “maturity” of state-specific POLST programs using four categories, each representing different stages of program development and implementation (see http://polst.org/programs-in-your-state/).25
Developing status indicates that the state coalitions have contacted NPPTF to develop a state-specific POLST program and are currently working toward the goal of implementing statewide POLST program. States with developing status can be at any stage of program development, with activities ranging from designing the POLST forms to on-going regional pilot studies with POLST program.
Endorsed status is for the states where POLST programs have been implemented, and have met key criteria (i.e., presence of a single POLST form per state). Different issues relevant to the state-level POLST program (i.e., legal, regulatory, education, and quality improvement) must also be addressed.
Mature status is the highest level of POLST recognition. It is reserved for states where the POLST programs have been endorsed as a part of the standard of care. Mature status is obtained after NPPTF confirms that the POLST program is being used in more than 50% of all medical facilities (i.e., hospitals, nursing homes, and hospices).
Lastly, for POLST programs that are already developed, but failed to comply with requirements in either structural component of POLST forms, or how the programs are being implemented within a state (e.g., voluntary), nonconforming status is assigned. This status indicates that the state's POLST program is not on a pathway to be endorsed by NPPTF.
The purposes of this research study were to: (1) identify state variations in how EoL treatment options were captured on POLST forms through an environmental scan, and (2) determine if variation in EoL treatment options on the POLST forms was associated with the maturity status of the program. Environmental scanning is a research method in which publicly available information is gathered systematically and is used to evaluate both internal and external environments of organizations, organizational practices, and health programs. It produces important insights on current trends and occurrences based on existing resources and can assist with the development of evidence-based policies in future practices.
Methods
An environmental scan was conducted using the national POLST website (www.polst.org), states' Department of Health websites and by searching the internet to identify the most up-to-date information on POLST programs in all 50 United States states and the District of Columbia (hereby referred to as states). Data collection occurred between August 2017 and February 2018. When available, sample POLST forms were obtained using state POLST websites and/or by internet search.
A standardized data collection tool was developed (available upon request) after reviewing the national POLST website, published research describing the POLST program, and consultations with experts.26 The following data were obtained: (1) name of each state POLST program; (2) POLST program maturity status; (3) year POLST program began; (4) year POLST program was endorsed or distinguished as mature (when applicable), and (5) availability of a sample POLST form (Y/N). When the POLST program had a nonconforming maturity status, we further identified the reason (i.e., specific tenet it violated).
When a POLST form was available, we examined how EoL treatment management options were captured, including antibiotics use, intravenous (IV) fluids, hospital transfer, medication administration by any route, oxygen use, and utilization of less-invasive respiratory devices (i.e., bi-level positive airway pressure [BiPAP] or continuous positive airway pressure [CPAP]) and invasive respiratory devices (i.e., ventilation/intubation). Because treatment options can be found in multiple different places in a POLST form (i.e., under Comfort Measures, Limited Treatment and Full Treatment and/or under a separate assessment section), for each treatment option we assessed (1) the frequency the treatment option listed, and (2) the location(s) where the treatment options were found on the form.
A double data collection process was performed; for every five states in which data were collected by the first data collector (A.T.), a second data collector (M.A.) randomly selected one state and independently extracted data. During the data collection period, all authors met weekly to discuss findings, review data collection progress, and to clarify any discrepancies. Inter-rater agreement was calculated using the kappa statistic. Distributions and descriptive statistics were computed, and chi square tests were used to test for associations between POLST maturity status and treatment options.
Results
Data were collected from all 51 state POLST programs (i.e., 50 states and Washington, D.C.). The inter-rater agreement was excellent (Kappa = 0.77).27
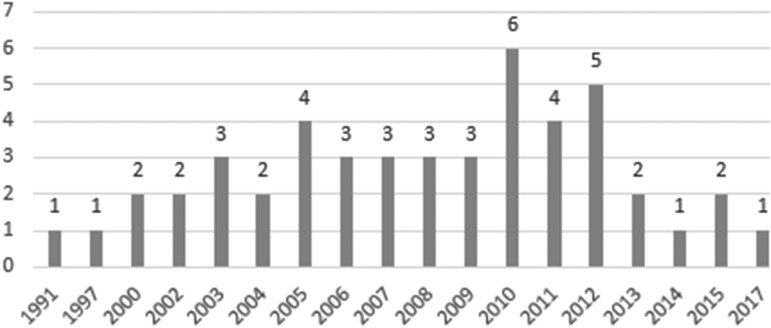
Table 1 presents the characteristics of the programs. The distribution of state POLST program start years is presented in Figure 1. The first program began in 1991 (Oregon) and the most recent began in 2017 (Arkansas). Excluding three states that did not specify the start year (i.e., Maryland, South Dakota, and Wyoming), half (n = 24) of all state programs began between the years of 1991 and 2008, and the rest in the years of 2009–2017.
Table 1.
State Physician Orders for Life-Sustaining Treatment Characteristics
| State | POLST maturity status | Program name | POLST started (year) | POLST endorsed (year) | POLST matured (year) | Reason for nonconforming status |
|---|---|---|---|---|---|---|
| Alabamaa | Developing | TOPP | 2004 | N/A | N/A | |
| Alaskaa | Developing | MOLST | 2015 | N/A | N/A | |
| Arizona | Developing | AzMOST | 2012 | N/A | N/A | |
| Arkansas | Developing | POLST | 2017 | N/A | N/A | |
| California | Mature | POLST | 2007 | 2009 | 2016 | |
| Colorado | Endorsed | MOST | 2005 | 2011 | N/A | |
| Connecticut | Developing | MOLST | 2012 | N/A | N/A | |
| Delaware | Developing | DMOST | 2010 | N/A | N/A | |
| Florida | Developing | POLST | 2003 | N/A | N/A | |
| Georgia | Endorsed | POLST | 2012 | 2013 | N/A | |
| Hawaii | Endorsed | POLST | 2009 | 2009 | N/A | |
| Idaho | Endorsed | POST | 2007 | 2011 | N/A | |
| Illinois | Developing | POLST | 2010 | N/A | N/A | |
| Indiana | Endorsed | POST | 2013 | 2017 | N/A | |
| Iowa | Endorsed | IPOST | 2006 | 2015 | N/A | |
| Kansas | Endorsed | TPOPP | 2008 | 2016 | N/A | |
| Kentucky | Developing | MOST | 2010 | N/A | N/A | |
| Louisiana | Endorsed | LaPOST | 2011 | 2012 | N/A | |
| Maine | Endorsed | POLST | 2008 | 2015 | N/A | |
| Maryland | Nonconforming | MOLST | N/S | N/A | N/A | POLST form not voluntary |
| Massachusetts | Nonconforming | MOLST | 2010 | N/A | N/A | Lacking limited intervention section |
| Michigan | Developing | POST | 2011 | N/A | N/A | |
| Minnesota | Developing | POLST | 2009 | N/A | N/A | |
| Mississippi | Developing | POST | 2014 | N/A | N/A | |
| Missouri | Endorsed | TPOPP | 2008 | 2016 | N/A | |
| Montana | Endorsed | POLST | 2010 | 2011 | N/A | |
| Nebraskaa | Nonconforming | POLST | 2005 | N/A | N/A | Lacking core elements of POLST form |
| Nevada | Developing | POLST | 2009 | N/A | N/A | |
| New Hampshire | Endorsed | POLST | 2003 | 2017 | N/A | |
| New Jersey | Developing | POLST | 2011 | N/A | N/A | |
| New Mexico | Developing | MOST | 2012 | N/A | N/A | |
| New York | Endorsed | MOLST | 2003 | 2006 | N/A | |
| North Carolina | Endorsed | MOST | 2004 | 2008 | N/A | |
| North Dakota | Developing | POLST | 2010 | N/A | N/A | |
| Ohio | Developing | MOLST | 2006 | N/A | N/A | |
| Oklahoma | Developing | OkPOLST | 2007 | N/A | N/A | |
| Oregon | Mature | POLST | 1991 | 2004 | 2013 | |
| Pennsylvania | Endorsed | PAPOLST | 2000 | 2011 | N/A | |
| Rhode Island | Developing | MOLST | 2011 | N/A | N/A | |
| South Carolina | Developing | POST | 2012 | N/A | N/A | |
| South Dakotaa | Developing | N/S | N/S | N/A | N/A | |
| Tennessee | Endorsed | POST | 2005 | 2009 | N/A | |
| Texas | Developing | MOST | 2013 | N/A | N/A | |
| Utah | Endorsed | POLST | 2002 | 2011 | N/A | |
| Vermont | Nonconforming | COLST | 2005 | N/A | N/A | Lacking core elements of POLST form |
| Virginia | Endorsed | POST | 2006 | 2016 | N/A | |
| Washington | Endorsed | POLST | 2000 | 2005 | N/A | |
| West Virginia | Mature | POST | 2002 | 2005 | 2013 | |
| Wisconsina | Endorsed | POLST | 1997 | 2008 | N/A | |
| Wyoming | Developing | WyoPOLST | N/S | N/A | N/A | |
| Washington D.C.a | Developing | MOST | 2015 | N/A | N/A |
POLST form not available for review.
N/A, not applicable; N/S, not specified; AzMOST, Arizona Medical Orders for Scope of Treatment; COLST, Clinician Orders for Life-Sustaining Treatment; DMOST, Delaware Medical Orders for Scope of Treatment; IPOST, Iowa Physician Orders for Scope of Treatment; LaPOST, Louisiana Physician Orders for Scope of Treatment; MOLST, Medical Orders for Life-Sustaining Treatment; MOST, Medical Orders for Scope of Treatment; OkPOLST, Oklahoma Physician Orders for Life-Sustaining Treatment; POLST, Physician Orders for Life-Sustaining Treatment; POST, Physician Orders for Scope of Treatment; TOPP, Transportable Orders for Patient Preferences; TPOPP, Transportable Physician Orders for Patient Preferences; WyoPOLST, Wyoming Providers Orders for Life-Sustaining Treatment.
FIG. 1.
Year Physician Orders for Life-Sustaining Treatment program started (N = 48).
Three states (i.e., California, Oregon, and West Virginia, 5.88%) had mature status, 20 states (39.22%) were endorsed, 24 states (47.06%) were developing, and 4 states (7.84%) were nonconforming. Reasons for nonconforming included: missing core elements (Massachusetts, Vermont), omitting limited intervention section on the form (Nebraska) and mandating completion to certain patient population (Maryland).
The state program's endorsement date was identifiable for 23 states. Between years of 2004 and 2017, 20 states obtained and maintained their endorsed status, while 3 states went on to obtain a higher (i.e., mature) status. The average time it took for a state program from the start year to the receipt of endorsed status was 6 years (standard deviation [SD] = 4.09, median = 5). New Hampshire's POLST program took the longest time to transition from start to endorsed status (14 years), whereas Hawaii's POLST program took less than a year.
Of the three states that went on to obtain mature status, two states (Oregon and West Virginia) obtained mature status in 2013. States took an average of 14 years to transition from starting the POLST program to obtaining mature status and 8 years (SD = 1) to transition from endorsed to mature status (max = 9, min = 7 years). Oregon maintained endorsed status for 13 years before it obtained mature status, and West Virginia maintained endorsed status for 3 years. California obtained mature status in 2016, after having endorsed status for 9 years.
There were several different names used. The majority (n = 18, 35.29%) used the name POLST, followed by Physician Orders for Scope of Treatments (n = 8, 15.69%). Seven states (13.73%) used Medical Orders for Life-Sustaining Treatment and six states (11.76%) used Medical Orders for Scope of Treatment (MOST) with the “M” standing for Medical. Two programs (3.92%) were called Transportable Physician Orders for Patient Preferences. Nine states (17.65%) used state-specific names (e.g., AzMOST for Arizona, DMOST for Delaware, OkPOLST for Oklahoma, WyoPOLST for Wyoming). The program name for one state (i.e., South Dakota, 1.96%) was not specified.
Forty-five states had forms available for review. The six states that did not have sample POLST forms were Alabama, Alaska, Nebraska, South Dakota, Wisconsin, and Washington D.C.). All mature programs (n = 3) had a sample form available; there were 19 from endorsed programs, 20 from developing, and 3 forms from nonconforming programs.
Frequencies and specific locations for the EoL treatment options are presented in Table 2. Patient preferences for antibiotic therapies were assessed on 32 forms (71.11%), including 2/3 programs from mature status (66.67%), 14/19 (73.68%) endorsed and developing, and 2/3 (66.67%) nonconforming status. Most forms assessed antibiotic preferences only once (n = 28, 62.22%); and, it was most frequently listed under the full treatment section (n = 15, 33.33%), followed by the comfort measures section (n = 13, 28.89%). Four forms (8.89%) contained antibiotics use under two different sections; these sections were comfort and limited section (n = 3) or limited and separate section (n = 1).
Table 2.
Variations in End-of-Life Treatment Options Presented on Physician Orders for Life-Sustaining Treatment Forms (n = 45)
| Antibiotics | IV fluids | Transfer to hospital | Medication by any route | Oxygen | BiPAP/CPAP | Intubation/ventilation | |
|---|---|---|---|---|---|---|---|
| POLST maturity status | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) |
| POLST maturity status | |||||||
| Mature | 2 (66.67) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Endorsed | 14 (73.68) | 13 (68.42) | 19 (100) | 19 (100) | 19 (100) | 18 (94.74) | 18 (94.74) |
| Developing | 14 (70.00) | 16 (80.00) | 20 (100) | 20 (100) | 20 (100) | 18 (90) | 17 (85) |
| Nonconforming | 2 (66.67) | 1 (33.33) | 3 (100) | 3 (100) | 3 (100) | 3 (100) | 3 (100) |
| Frequency mentioned and locations | |||||||
| Mentioned once | |||||||
| Comfort Measures | 13 (28.89) | 0 | 0 | 45 (100) | 45 (100) | 0 | 0 |
| Limited treatment | 0 | 28 (62.22) | 0 | 0 | 0 | 1 (2.22) | 0 |
| Full treatment | 15 (33.33) | 0 | 0 | 0 | 0 | 10 (22.22) | 36 (80) |
| Separate section | 0 | 0 | 4 (8.89) | 0 | 0 | 0 | 5 (11.11) |
| Mentioned twice | |||||||
| Comfort + limited treatment | 3 (6.67) | 0 | 7 (15.56) | 0 | 0 | 0 | 0 |
| Limited + full treatment | 0 | 5 (11.11) | 0 | 0 | 0 | 26 (57.78) | 0 |
| Limited + separate section | 1 (2.22) | 0 | 0 | 0 | 0 | 0 | 0 |
| Full + separate section | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Mentioned three time | |||||||
| Comfort + limited + full treatment | 0 | 0 | 34 (75.56) | 0 | 0 | 0 | 0 |
| Limited + full + separate section | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Total mentioned | 32 (71.11) | 33 (73.33) | 45 (100) | 45 (100) | 45 (100) | 42 (93.33) | 41 (91.11) |
| Not mentioned at all | 13 (28.89) | 12 (26.67) | 0 | 0 | 0 | 3 (6.67) | 4 (8.89) |
| Total | 45 (100) | 45 (100) | 45 (100) | 45 (100) | 45 (100) | 45 (100) | 45 (100) |
BiPAP, bi-level positive airway pressure; CPAP, continuous positive airway pressure; IV, intravenous.
Preferences for IV fluids use at EoL were assessed on 33 forms (73.33%), which included all forms from mature programs (n = 3, 100%), more than half of forms from endorsed (n = 13, 68.42%) and developing status (n = 16, 80%), and 1 from nonconforming program. Similar to antibiotics use, preferences for IV fluids were mostly mentioned once per form (n = 28, 62.22%); however, it was listed under the limited treatment section. Five forms (11.11%) assessed IV fluid use option twice per form, all under a limited and full treatment section.
Patient preferences for the hospital transfer at EoL were assessed in all forms (n = 45, 100%). Most state forms (n = 34) captured the transfer option three times under all medical intervention sections (i.e., comfort, limited, and full treatment). When this option was mentioned twice (n = 5, 11.11%), they were listed under a comfort and limited treatment section. When mentioned only once on the form (n = 4, 8.89%), all were located under a separate section.
All forms assessed patient preferences for medication administration by any route, as well as the options to receive oxygen for respiratory symptom relief, all captured under the comfort measures section. All forms from mature and nonconforming status contained the option to use respiratory devices (i.e., BiPAP/CPAP and intubation/ventilation). Program maturity status was not related to the assessment of BiPAP/CPAP preferences, as the option was available most of the time. Forms that contained preferences for intubation and ventilation use (n = 41, 91.11%) mentioned this option only once and this was mostly under full treatment sections (80%), or under a separate section (11.11%).
Because there was no variation in three treatment options (i.e., transfer to hospital, medication by any route, oxygen) associations between maturity status and treatment options could only be compared with antibiotic use, IV fluid use, BiPAP/CPAP, intubation/ventilation use. We did not find any significant associations between treatments mentioned and POLST maturity status (data not shown).
Discussion
This is the first comprehensive examination of how POLST forms vary across the nation. Variations in types, interventions, locations, or frequencies of options captured on forms may be explained by the lack of consensus on specific EoL treatment care options that should be addressed. Maturity status of the program was not related to the variation in the forms.
Previous researchers largely focused on the use in clinical care settings (i.e., nursing homes), or lessons learned from implementing a program in a single state.28–30 Recently, Hickman and Critser reported their findings on the national and state level variations in POLST programs.31 However, their study aimed to identify whether the state forms were adherent to the national standards by identifying inclusion of specific sections (e.g., medical order) and exclusion of language that is prohibited by NPPTF. These investigators only examined the sample POLST forms from endorsed or mature programs, excluding information from developing or nonconforming POLST programs from their final analysis.31
A large number of unnecessary and burdensome hospital transfers occur near EoL.32 These transitions become a source of disconcordant care that increases both psychological and physical burdens for a dying patient. They are also closely related to the overutilization of aggressive treatments that may contradict a dying patient's EoL care wishes.33,34 Many elderly individuals with advanced illness transferred to hospitals die within weeks of hospitalization.35–37 While conventional advance directives do not assess individuals' preference for hospitalization near death, all POLST forms we examined contained a hospital transfer option. Most forms contained hospital transfer under all three sections (i.e., comfort, limited, and full treatment sections).
Decision making surrounding antibiotic use at EoL is a difficult part. Due to ethical concerns, examining outcomes (e.g., quality of life) among dying patients with or without antibiotic use is not feasible through randomized control trials. As a result, the evidence is based on retrospective cohort designs, with no comparison groups.38–41 Lack of guidelines, and the absence of high-level scientific evidence on antibiotic use at EoL adds challenges to determining best practices in the infection management among elderly and frail individuals.42,43 In addition, Oregon included an antibiotics option when POLST was introduced but, nearly a decade after the program's initiation, removed it after research evidence found little difference in actual use of antibiotics regardless of written preferences.18
An aim of POLST is to facilitate advance care planning that can enhance quality of life for those who are dying. By using a standardized national tool, one should be able to receive care that is documented and desired, regardless of physical location (e.g., care institution located in a different state). Even if the care transfer was made near the time of death, across states, POLST documentation should always be easily identifiable and patient wishes respected. The variations we observed make interstate transfer of POLST orders unlikely.
Limitations
This environmental scan was limited to EoL treatment options that were relevant to infection and/or symptom management. Discussing treatment options outside infection/symptom management (such as tube feedings) were out of scope of this study. While we attempted to be comprehensive and current, not all forms were available. Our findings represent a cross-sectional view and does not imply causality. The forms used during data collection may have been revised, and/or maturity status changed after data collection was conducted; however, Oregon was the only state where such changes are reported on an ongoing basis.
Oregon has recently separated from NPPTF, due to differences in views for receipt of industry funding.44 Although the national POLST website indicated that Oregon's POLST program was mature, this information has subsequently been removed.44 Nevertheless, we classified Oregon's POLST program status as mature program throughout our data collection and analysis.
Directions for the future research
We highlighted a gap in knowledge in current status of POLST program implementation. Future research is recommended to identify how variations in EoL care options, particularly antibiotics preferences and other infection-related care options, addressed on advance care planning tools impact appropriate use of medication at EoL. Determining if EoL care wishes on POLST forms were honored for individuals who relocate to a different state, close to the time of death, is also needed.
This study yielded information that can inform policy makers, researchers, and clinicians. Close monitoring of POLST program for improvements and dissemination of new research findings on areas that can be improved will facilitate further success of the program. In addition, it will provide a platform for increased public awareness on the importance of formulating patient-centered EoL plans for terminally ill patients.
Acknowledgments
This research was funded by the Study of Infection Management and Palliative care at the End of Life (SIMP-EL, R01 NR013687), the Comparative and Cost-effectiveness Research Training for Nurse Scientists (CER2, T32 NR014205), and the Center for Improving Palliative Care for Vulnerable Adults with MCC (CIPC, P20 NR018072). Ms. A.T. is also funded by Jonas Center for Nursing and Veterans Health care.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Tinetti ME, Fried TR, Boyd CM: Designing health care for the most common chronic condition—multimorbidity. JAMA 2012;307:2493–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson G: Chronic Care: Making the Case for Ongoing Care. Robert Wood Johnson Foundation, Princeton, NJ, 2010 [Google Scholar]
- 3. The Coalition to Transform Advanced Care Policy Agenda: Options to Transform Advanced Care. 2015. https://thectac.org/wp-content/uploads/2016/03/C_TAC_Policy-Agenda-Summary.pdf (last accessed September21, 2018)
- 4. Institute of Medicine: Dying in America: Improving quality and honoring individual preferences near the end of life. Mil Med 2015;180:365–367 [DOI] [PubMed] [Google Scholar]
- 5. Cardona-Morrell M, Kim JCH, Brabrand M, et al. : What is inappropriate hospital use for elderly people near the end of life? A systematic review. Eur J Intern Med 2017;42:39–50 [DOI] [PubMed] [Google Scholar]
- 6. Howell DA, Wang HI, Roman E, et al. : Preferred and actual place of death in haematological malignancy. BMJ Support Palliat Care 2017;7:150–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rainsford S, MacLeod RD, Glasgow NJ: Place of death in rural palliative care: A systematic review. Palliat Med 2016;30:745–763 [DOI] [PubMed] [Google Scholar]
- 8. Tang ST: When death is imminent: Where terminally ill patients with cancer prefer to die and why. Cancer Nurs 2003;26:245–251 [DOI] [PubMed] [Google Scholar]
- 9. Silveira MJ, Kim SYH, Langa KM: Advance directives and outcomes of surrogate decision making before death. N Engl J Med 2010;362:1211–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Green MJ, Levi BH: Development of an interactive computer program for advance care planning. Health Expect 2009;12:60–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Robinson MK, DeHaven MJ, Koch KA: Effects of the patient self-determination act on patient knowledge and behavior. J Fam Pract 1993;37:363–368 [PubMed] [Google Scholar]
- 12. Fagerlin A, Schneider CE: Enough. The failure of the living will. Hastings Cent Rep 2004;34:30–42 [PubMed] [Google Scholar]
- 13. Habal MV, Micevski V, Greenwood S, et al. : How aware of advanced care directives are heart failure patients, and are they using them? Can J Cardiol 2011;27:376–381 [DOI] [PubMed] [Google Scholar]
- 14. Hickey DP: The disutility of advance directives: We know the problems, but are there solutions? J Health Law 2003;36:455–473 [PubMed] [Google Scholar]
- 15. Dunn PM, Schmidt TA, Carley MM, et al. : A method to communicate patient preferences about medically indicated life-sustaining treatment in the out-of-hospital setting. J Am Geriatr Soc 1996;44:785–791 [DOI] [PubMed] [Google Scholar]
- 16. Perkins HS: Controlling death: The false promise of advance directives. Ann Intern Med 2007;147:51–57 [DOI] [PubMed] [Google Scholar]
- 17. Vearrier L: Failure of the current advance care planning paradigm: Advocating for a communications-based approach. HEC Forum 2016;28:339–354 [DOI] [PubMed] [Google Scholar]
- 18. Hickman SE, Nelson CA, Moss AH, et al. : The consistency between treatments provided to nursing facility residents and orders on the physician orders for life-sustaining treatment form. J Am Geriatr Soc 2011;59:2091–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wenger NS, Citko J, O'Malley K, et al. : Implementation of physician orders for life sustaining treatment in nursing homes in California: Evaluation of a novel statewide dissemination mechanism. J Gen Intern Med 2013;28:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sebastian P, Freitas B, Fischberg D: Provider orders for life-sustaining treatment implementation and training in nursing facilities in Hawai'i. Hawai'i J Med Public Health 2015;74(9 Suppl 2):8–11 [PMC free article] [PubMed] [Google Scholar]
- 21. Hickman SE, Sudore RL, Sachs GA, et al. : Use of the physician orders for scope of treatment program in Indiana nursing homes. J Am Geriatr Soc 2018;66:1096–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. U.S. Department of Health and Human Services: Electronic End-of-Life and Physician Orders for Life-Sustaining Treatment (POLST) Documentation Access through Health Information Exchange. Office of the National Coordinator for Health Information Technology, Washington, DC, 2017 [Google Scholar]
- 23. Bierman M, Hadley J, Pendleton K, Garrison A: National POLST Paradigm. In Program Development Guide. 2015. http://polst.org/wp-content/uploads/2015/07/POLST-Program-Development-Guide.pdf (last accessed August1, 2018)
- 24. Moss AH, Zive DM, Falkenstine EC, Dunithan C: The quality of POLST completion to guide treatment: A 2-state study. J Am Med Dir Assoc 2017;18:810.e5–810.e9 [DOI] [PubMed] [Google Scholar]
- 25. National POLST Paradigm: POLST Program Status. 2018. http://polst.org/wp-content/uploads/2018/08/2018.04.11-POLST-Program-Status.pdf (Last accessed May21, 2018)
- 26. Hickman SE, Keevern E, Hammes BJ: Use of the physician orders for life-sustaining treatment program in the clinical setting: A systematic review of the literature. J Am Geriatr Soc 2015;63:341–350 [DOI] [PubMed] [Google Scholar]
- 27. Fleiss JL: Measuring nominal scale agreement among many raters. Psychol Bull 1971;76:378–382 [Google Scholar]
- 28. Bomba PA, Orem K: Lessons learned from New York's community approach to advance care planning and MOLAT. Ann palliat Med 2015;4:10–21 [DOI] [PubMed] [Google Scholar]
- 29. Fritz ZBM, Barclay SI: Patients' resuscitation preferences in context: Lessons from POLST. Resuscitation 2014;85:444–445 [DOI] [PubMed] [Google Scholar]
- 30. Schmidt TA, Zive D, Fromme EK, et al. : Physician orders for life-sustaining treatment (POLST): Lessons learned from analysis of the Oregon POLST Registry. Resuscitation 2014;85:480–485 [DOI] [PubMed] [Google Scholar]
- 31. Hickman SE, Critser R: National standards and state variation in physician orders for life-sustaining treatment forms. J Palliat Med 2018;21:978–986 [DOI] [PubMed] [Google Scholar]
- 32. Cardona-Morrell M, Kim JCH, Turner RM, et al. : Non-beneficial treatments in hospital at the end of life: A systematic review on extent of the problem. Int J Qual Health Care 2016;28:456–469 [DOI] [PubMed] [Google Scholar]
- 33. Marik PE: The cost of inappropriate care at the end of life: Implications for an aging population. Am J Hosp Palliat Med 2014;32:703–708 [DOI] [PubMed] [Google Scholar]
- 34. Gozalo P, Teno JM, Mitchell SL, et al. : End-of-life transitions among nursing home residents with cognitive issues. N Engl J Med 2011;365:1212–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Menec VH, Nowicki S, Blandford A, Veselyuk D: Hospitalizations at the end of life among long-term care residents. J Gerontol A Biol Sci Med Sci 2009;64:395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Houttekier D, Vandervoort A, Van den Block L, et al. : Hospitalizations of nursing home residents with dementia in the last month of life: Results from a nationwide survey. Palliat Med 2014;28:1110–1117 [DOI] [PubMed] [Google Scholar]
- 37. Teno JM, Gozalo PL, Bynum JPW, et al. : Change in end-of-life care for medicare beneficiaries: Site of death, place of care, and health care transitions in 2000, 2005, and 2009. JAMA 2013;309:470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Helde-Frankling M, Bergqvist J, Bergman P, Bjorkhem-Bergman L: Antibiotic treatment in end-of-life cancer patients-a retrospective observational study at a palliative care center in Sweden. Cancer (Basel) 2016;8:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Servid SA, Noble BN, Fromme EK, Furuno JP: Clinical intentions of antibiotics prescribed upon discharge to hospice care. J Am Geriatr Soc 2018;66:565–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Lam PT, Chan KS, Tse CY, Leung MW: Retrospective analysis of antibiotic use and survival in advanced cancer patients with infections. J Pain Symptom Manage 2005;30:536–543 [DOI] [PubMed] [Google Scholar]
- 41. Thompson AJ, Silveira MJ, Vitale CA, Malani PN: Antimicrobial use at the end of life among hospitalized patients with advanced cancer. Am J Hosp Palliat Care 2012;29:599–603 [DOI] [PubMed] [Google Scholar]
- 42. Juthani-Mehta M, Malani PN, Mitchell SL: Antimicrobials at the end of life: An opportunity to improve palliative care and infection management. JAMA 2015;314:2017–2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Leibovici L, Paul M, Ezra O: Ethical dilemmas in antibiotic treatment. J Antimicrob Chemother 2012;67:12–16 [DOI] [PubMed] [Google Scholar]
- 44. Oregon POLST: Statement on Oregon POLST Separation from National POLST. 2018. https://static1.squarespace.com/static/52dc687be4b032209172e33e/t/5b05ff462b6a28e3e73cabac/1527119687088/2018.05.23+Statement+on+Oregon+POLST+separation+from+National+POLST.pdf (Last accessed April6, 2018)