Abstract
Background: Little is known about clinical symptom burden, dementia, and social isolation in the last year of life among older adults.
Objective: To describe and contrast the type and severity of symptom burden for older decedents with and without dementia, and whether specific symptoms and presence of dementia are associated with limitations in social participation in the last year of life.
Design: Cross-sectional logistic regression analysis of a population-based study.
Setting/Subjects: A total of 1270 community-dwelling adults of age ≥65 years in the United States participated in the 2011 National Health and Aging Trends Study and died by 2015.
Measurements: Dementia status, 13 clinical symptoms, and limitations in 6 social activities were drawn from the interview preceding death. Severity of sensory, physical, and psychiatric symptom burden was examined in tertiles.
Results: Decedents with dementia (37.3%) had higher prevalence of all symptoms (p's < 0.05), except insomnia and breathing problems. Dementia was associated with greater likelihood of high versus low burden of sensory (odds ratio [OR] 4.52 [95% confidence interval {CI} 3.08–6.63]), physical (OR 3.49 [95% CI 2.48–4.91]), and psychiatric (OR 2.80 [95% CI 1.98–3.95]) symptoms. Dementia and physical symptoms (problems with speaking, leg strength/movement, and balance) were independently associated with limitations in at least three social activities (p's < 0.05 for adjusted ORs).
Conclusion: Symptom burden is higher in patients with dementia. Dementia and physical symptoms are associated with social activity limitations. Older patients with dementia or physical symptoms may benefit from earlier emphasis on palliative care and quality of life.
Keywords: dementia, end of life, hospice, palliative care, symptom burden
Introduction
Maintaining quality of life while minimizing symptom burden becomes primary goals of care toward the end of life. Both palliative care and hospice services are designed to address these goals, although hospice is used by only 48% of older decedents, and median duration of services is estimated to be 24 days.1 Late referrals to hospice delay the benefits of improved symptom management and quality of life to the very end of life.2–4 As a result, older adults often have unrecognized but potentially manageable needs before hospice enrollment.5 At the same time, access to palliative care before hospice is variable and often limited, especially in the outpatient setting.6 Certain sensory, physical, and psychiatric symptoms common in the last year of life may thus receive less attention and ineffective management.7
Specific symptoms and symptom burden may impact social functioning, an aspect of quality of life that may be underemphasized toward the end of life. Dementia may further impact the presence, reporting, and treatment of distressing symptoms and alter social engagement and quality of life for individuals and caregivers.8 Given the prevalence of dementia in older adults, understanding symptoms and quality of life in persons with dementia toward the end of life is important.
Prior research has demonstrated that activity-restricting symptoms are common in the last year of life, increasing notably around four to six months before and steadily increasing until death.8–10 Multimorbidity is associated with increased symptom burden, and symptoms such as pain are common and increasingly prevalent as death nears.9,10 Symptom burden is similar in terminal conditions such as heart failure and cancer.11 Disabilities in basic and instrumental activities of daily living and mobility have also been observed to increase in the last year of life.10
Similar to other terminal diagnoses, patients with dementia experience a high degree of symptom burden at the end of life.12–14 However, symptoms such as pain may be undertreated in patients with dementia versus patients without dementia.15,16 They may also have greater multimorbidity than patients without dementia.17 Dementia, multimorbidity, and symptom burden may each contribute to disability and affect aspects of quality of life. Medicare beneficiaries with dementia receive the greatest number of days under hospice care (median of 54 days).1 Although this length of service exceeds other conditions, it remains less than the last six months of life benefit offered through Medicare, and individuals with dementia are more likely to be disenrolled from hospice.1,18,19 Persons with dementia are also more likely to experience hospitalizations, emergency department visits, and transitions in care in the last years of life, events that may also affect quality of life.20,21 Despite potential benefit, little is known about the use and effectiveness of palliative care in dementia, and numerous barriers affect its use.6,22,23
In this study, we build on prior work by examining specific symptoms, symptom burden, and social participation in the last year of life, with a focus on whether persons with dementia experience these differently.8,10 We used a nationally representative cohort of older Americans who participated in the 2011 National Health and Aging Trends Study (NHATS) and subsequently died over four years of follow-up. Our objectives were to describe and contrast symptoms and symptom burden for older decedents with and without dementia, and further whether specific symptoms and presence of dementia were associated with limitations in social participation in the last year of life.
Methods
Study design and population
NHATS is an ongoing longitudinal study of a population-based nationally representative cohort of Medicare beneficiaries of age 65 years and older in the continental United States. NHATS enrolled 8245 older adults at baseline in 2011, with an overall 71% response rate.24 Participants or proxy respondents have subsequently completed annual interviews. In this study, we identified older adults who died by 2015 and did not reside in a nursing home at the time of the interview preceding death. Dementia status, symptoms, and social participation were assessed from information reported in the last interview before death.
Dementia status
Because dementia is underdiagnosed and clinical evaluations are not feasible in large population-based studies, NHATS investigators have developed an algorithm to identify probable dementia among participants.25,26 The algorithm has been validated against the Aging, Demographics, and Memory Study, which includes more extensive neuropsychological testing and adjudication of clinical dementia based on testing and medical records.25,27 We applied the algorithm, which uses three sources of information in NHATS, to classify probable dementia versus no dementia preceding death.25,26
First, any participant for whom the participant or proxy respondent reported a clinician diagnosis of dementia or Alzheimer's disease was classified as having probable dementia. Proxy respondents also completed the AD-8 dementia screening interview, and participants who met AD-8 criteria for dementia were also classified as having probable dementia.28 Finally, most participants also completed cognitive tests of memory, orientation, and executive function. Cognitive impairment in each domain tested was defined as a score ≥1.5 standard deviations (SDs) below the mean for self-respondents, a method used to define cognitive impairment in prior epidemiologic studies.25,29,30 Participants with impairment in at least two cognitive domains were classified as having probable dementia. Participants who did not meet criteria for probable dementia based on self or proxy report of diagnosis, proxy AD-8 interview, or cognitive testing were classified as no dementia.25
Clinical symptoms and symptom burden
NHATS assesses self- or proxy-reported presence and severity of a range of sensory, physical, and psychiatric symptoms in the month before the interview. We examined all sensory and physical symptoms and impairments included in NHATS, as they have potential to limit activities.31 We further included depression, anxiety, and sleep disturbance given these symptoms are common in dementia and at the end of life.32 Table 1 provides an overview of individual symptoms included, how they were assessed in NHATS, and how individual symptoms and symptom burden were measured in this study. Higher sensory, physical, and psychiatric symptom burden scores indicate greater symptom burden.
Table 1.
Measures of Clinical Symptoms and Symptom Burden
| Symptom | NHATS interview content | Symptom measurement and burden score |
|---|---|---|
| Sensory symptoms | ||
| Hearing impairment | Uses hearing aid or deaf | Presence of hearing or vision impairment measured yes/no |
| Hears well enough to use telephonea | Sensory symptom burden score range 0–8 | |
| Hears conversation with TV ona | Deaf or blind = 4 points | |
| Can have conversation in quiet rooma | Each difficulty = 1 point | |
| Vision impairment | Uses corrective lenses or blind | |
| Sees well enough to recognize person across streeta | ||
| Can watch TV across rooma | ||
| Reads newspaper printa | ||
| Physical symptoms | ||
| Problem chewing or swallowing | Assessed as yes/no | Presence of each symptom measured yes/no |
| Problem speaking or being understood | Physical symptom burden score range 0–14 | |
| Pain | Assessed as yes/no and if present, whether it ever limited activities | Symptom present = 1 point |
| Breathing problems | Symptom limits activities = 1 additional point | |
| Limited strength or movement in upper extremities | ||
| Limited strength or movement in lower extremities | ||
| Low energy or fatigue | ||
| Problems with balance or coordination | ||
| Psychiatric symptoms | ||
| Depression | How often (not at all, several days, more than half the days, nearly every day) did you (1) experience little interest or pleasure in doing things or (2) feel down, depressed, or hopeless? | Participants positive for depression or anxiety if score >2 on respective scales (Patient Health Questionnaire-2 and Generalized Anxiety Disorder-2)51,52 |
| Anxiety | How often (not at all, several days, more than half the days, nearly every day) did you (1) feel nervous, anxious, or on edge or (2) were unable to stop or control worrying? | Participants positive for insomnia if either symptom occurred most or every night |
| Insomnia | How often (never, rarely, some nights, most nights, every night) did you (1) take more than 30 minutes to fall asleep or (2) have trouble falling back asleep if waking up too early? | Presence of depression, anxiety, and insomnia measured yes/no |
| Psychiatric symptom burden score range 0–20 | ||
| For each depression, anxiety, or insomnia symptom, every increase in symptom frequency receives additional point | ||
While using hearing aid or corrective lenses if applicable.
NHATS, National Health and Aging Trends Study.
Social participation limitations
In NHATS, respondents report whether health or functioning kept the participant from six activities in the last month: visiting in person with family/friends not living with them either at the participant's home or family/friend's home; attending religious services; participating in clubs, classes, or other organized activities; going out for enjoyment; doing volunteer work; and engaging in a self-defined favorite activity.
Additional characteristics
Characteristics that may affect symptoms and social participation such as age, gender, education (in nine categories), race/ethnicity (white, non-Hispanic; black, non-Hispanic; Hispanic; other), living alone, and self-reported medical conditions (myocardial infarction, heart disease/failure, hypertension, arthritis, diabetes, lung disease, stroke, and cancer) were considered. We also examined whether the NHATS interview was completed by proxy or participant and time between last interview and death.
Statistical analysis
NHATS has constructed survey weights to reflect the complex sampling strategy and nonresponse bias. We applied survey weights to all analyses, with weights reflecting the survey round before death. Variance estimation used the modified balanced repeated replication method. We first examined descriptive statistics on baseline characteristics, clinical symptoms, and limitations in social participation with the full sample and then compared decedents with no dementia versus probable dementia using chi-square and t test statistics.
We then conducted two sets of multivariable logistic regression models. First, we separately examined whether dementia was associated with sensory, physical, and psychiatric symptom burden level adjusted for participant characteristics, including medical conditions. Because symptom burden is not a standardized construct, we examined symptom burden scores in tertiles of low, medium, and high symptom burden for ease of interpretation (low burden served as reference group). Inclusion of covariates in each symptom burden model was based on identification of the best subset of covariates.
Next, we focused on the association of limitations in social participation for each activity with dementia, individual symptoms, and participant characteristics. Participant characteristics considered in these models (age, gender, arthritis, lung disease, stroke, and cancer) were drawn from final symptom burden models and statistically significant association with any social participation limitation. These characteristics were also conceptually viewed as potentially driving or confounding the relationship between symptoms and social participation limitation. Final inclusion of covariates in these models was based on forward and backward selection. Differences in statistically significant covariates identified in forward versus backward selection (based on p < 0.05) were resolved by selecting the model with the lowest Akaike's information criterion.
As a key variable of interest, dementia status was included in all models. All analyses were conducted using Stata v.14. The study was exempt from Institutional Review Board oversight.
Results
Among 7609 community-dwelling older adults enrolled in NHATS in 2011, there were 1270 deaths over four years. Characteristics of the decedents in the interview before death are displayed in Table 2. In the year before death, 37.3% of decedents had probable dementia. The mean age was 82.3 years (SD 8.1), with a mean age of 80.6 years for persons with no dementia versus 85.0 years for persons with probable dementia. Decedents with probable dementia were older, more likely to be female, non-white race, have less than a high school education, and less likely to live alone. Comparison of medical conditions demonstrated differences by dementia status in myocardial infarction, diabetes, lung disease, and cancer, each more common in decedents with no dementia. Stroke and having a proxy respondent were more common in probable dementia.
Table 2.
Characteristics of Deceased National Health and Aging Trends Study Participants by Dementia Status
| Characteristic, raw n (weighted %) | No dementia | Probable dementia | Overall | p |
|---|---|---|---|---|
| Participants | 728 (62.7) | 542 (37.3) | 1270 (100.0) | — |
| Age, years | ||||
| 65–69 | 31 (6.6) | 10 (4.3) | 41 (5.7) | <0.001 |
| 70–74 | 102 (20.9) | 18 (5.4) | 120 (15.1) | |
| 75–79 | 116 (18.4) | 54 (13.9) | 170 (16.7) | |
| 80–84 | 165 (19.8) | 112 (23.0) | 277 (21.0) | |
| 85–89 | 167 (20.7) | 132 (23.5) | 299 (21.8) | |
| 90+ | 147 (13.6) | 216 (29.9) | 363 (19.7) | |
| Female gender | 386 (52.5) | 326 (59.4) | 712 (55.1) | 0.02 |
| Less than high school education | 223 (61.1) | 236 (71.8) | 459 (65.1) | <0.001 |
| Race/ethnicity | ||||
| White, non-Hispanic | 533 (84.1) | 337 (74) | 870 (80) | 0.003 |
| Black, non-Hispanic | 146 (8.3) | 141 (10.6) | 287 (9.2) | |
| Lives alone | 302 (40.9) | 165 (33.4) | 467 (38.1) | <0.001 |
| Medical conditions | ||||
| Myocardial infarction | 90 (11.7) | 57 (9.7) | 147 (11) | 0.04 |
| Heart disease/failure | 263 (36.0) | 191 (35.2) | 454 (35.7) | 0.16 |
| Hypertension | 543 (73.7) | 385 (70.8) | 928 (72.6) | 0.17 |
| Arthritis | 477 (66.0) | 380 (68.1) | 857 (66.8) | 0.44 |
| Diabetes | 231 (32.4) | 138 (26.8) | 369 (30.3) | 0.05 |
| Lung disease | 200 (30.3) | 120 (21.8) | 320 (27.1) | 0.006 |
| Stroke | 57 (6.6) | 86 (12.7) | 143 (8.9) | <0.001 |
| Cancer | 152 (22.7) | 83 (17.0) | 235 (20.7) | 0.05 |
| Proxy respondent | 28 (3.9) | 344 (62.3) | 372 (25.7) | <0.001 |
| Time since last interview, in months, mean (SD)a | 7.3 (3.6) | 6.0 (4.0) | 6.8 (3.8) | <0.001 |
Missing for 67 participants.
SD, standard deviation.
Time between the last NHATS interview and death ranged from 0 to 17 months (mean 6.8 months). The time interval was 1.3 months greater for participants with no dementia versus probable dementia (p < 0.001).
Clinical symptoms and symptom burden
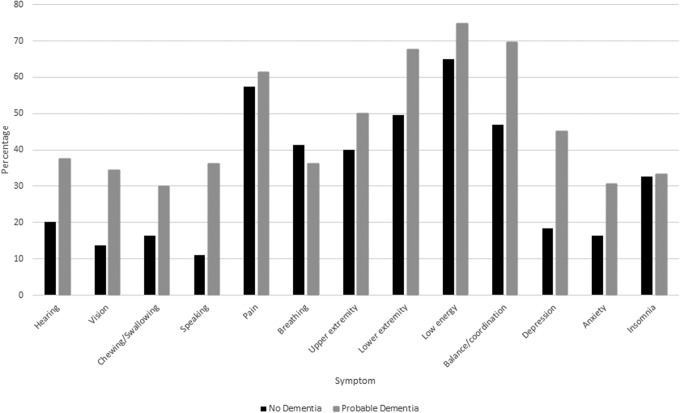
The most commonly reported symptoms were low energy (68.6%), pain (58.9%), limited lower extremity strength or movement (56.3%), and poor balance or coordination (55.5%). All clinical symptoms examined were significantly more common in individuals with probable dementia compared with no dementia (Fig. 1), except for trouble breathing and insomnia. When the association between symptom burden and dementia status was examined after adjusting for sociodemographic and medical factors (Table 3), the presence of probable dementia was associated with significantly greater likelihood of high versus low burden of sensory, physical, and psychiatric symptoms. A similar pattern was seen for medium versus low burden of symptoms, with the exception of no difference in medium versus low burden of psychiatric symptoms between persons with probable dementia versus no dementia.
FIG. 1.
Symptoms in the year before death by dementia status. Upper extremity, limited upper extremity strength/movement; lower extremity, limited lower extremity strength/movement. No statistically significant difference for trouble breathing (p = 0.31) or insomnia (p = 0.36). Pain p = 0.01, for upper extremity strength/movement and low energy (<0.01) and for all other symptoms p < 0.001.
Table 3.
Multivariable-Adjusted Odds Ratios for Level of Symptom Burden by Dementia Status in the Year before Death
| Symptom level (Ref = low) | Crude OR (95% CI) in dementia vs. no dementia | pa | Adjusted OR (95% CI) in dementia vs. no dementiab | pa |
|---|---|---|---|---|
| Sensory symptoms | ||||
| Medium | 1.93 (1.36–2.74) | <0.001 | 1.77 (1.23–2.54) | 0.002 |
| High | 5.12 (3.45–7.58) | <0.001 | 4.52 (3.08–6.63) | <0.001 |
| Physical symptoms | ||||
| Medium | 1.65 (1.18–2.32) | 0.004 | 1.84 (1.30–2.61) | 0.001 |
| High | 2.93 (2.14–4.00) | <0.001 | 3.49 (2.48–4.91) | <0.001 |
| Psychiatric symptoms | ||||
| Medium | 1.05 (0.76–1.47) | 0.76 | 1.06 (0.76–1.48) | 0.71 |
| High | 2.54 (1.88–3.43) | <0.001 | 2.80 (1.98–3.95) | <0.001 |
p-Value calculated by Wald test.
Sensory symptom burden adjusted for gender and age.
Physical symptom burden adjusted for gender, arthritis, diabetes, lung disease, stroke, cancer, and living arrangements. Psychiatric symptom burden adjusted for gender, age, education, arthritis, and lung disease.
CI, confidence interval; OR, odds ratio.
Limitations in social participation
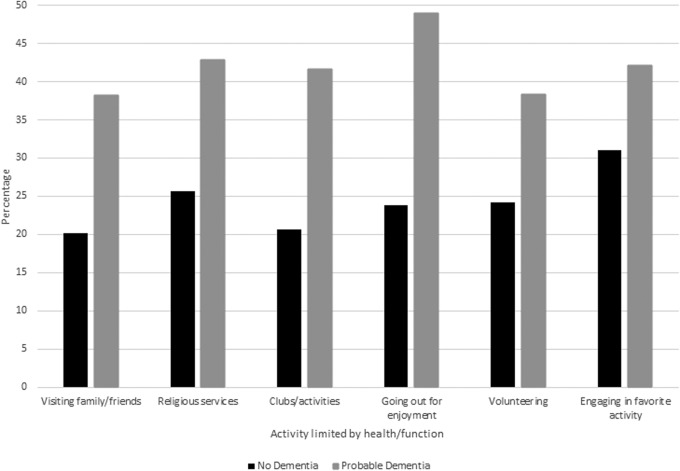
For each social activity examined, participation was limited in 25%–33% of decedents. As shown in Figure 2, limitations in participation for each activity were greater in individuals with probable dementia. For the entire population as well as in persons with probable dementia, limitation was most commonly reported in going out for enjoyment. After multivariable adjustment for individual clinical symptoms, sociodemographic, and medical factors, probable dementia remained independently associated with limitations in three activities: participating in clubs, classes, or organized activities; going out for enjoyment; and volunteering (Table 4). Problems speaking or communicating, poor balance and coordination, and limited lower extremity strength or movement were also associated with limitations in three or more social activities.
FIG. 2.
Participation limitation in the year before death by dementia status. p-Value <0.001 for all activities, except for engaging in favorite activity (p = 0.004).
Table 4.
Multivariable-Adjusted Odds Ratios with 95% Confidence Intervals for Limitation in Social Participation by Symptoms and Dementia Status
| Symptom or condition | Visiting family or friends | Religious services | Clubs or activities | Going out for enjoyment | Volunteering | Favorite activity |
|---|---|---|---|---|---|---|
| Hearing | 1.70* (1.19–2.44) | — | — | — | — | — |
| Vision | 1.36 (0.88–2.11) | 1.20 (0.88–1.62) | — | 1.31 (0.91–1.88) | — | — |
| Chewing | — | — | — | — | — | — |
| Speaking | 1.69** (1.12–2.54) | — | 2.01*** (1.44–2.81) | 2.25*** (1.43–3.55) | 2.11*** (1.42–3.16) | 1.73* (1.23–2.44) |
| Pain | — | — | — | — | — | — |
| Breathing | — | 1.35 (0.92–1.98) | — | 1.46 (0.99–2.15) | — | — |
| Upper extremity | — | — | — | 1.89*** (1.35–2.65) | — | — |
| Lower extremity | 1.51 (1.00–2.28) | 1.33 (0.91–1.95) | 1.81* (1.23–2.68) | 1.69** (1.05–2.72) | 1.59*** (1.11–2.29) | 1.96*** (1.34–2.86) |
| Low energy | 1.66** (1.07–2.57) | — | — | — | 1.51 (0.97–2.35) | 1.82* (1.19–2.79) |
| Balance/coordination | 2.18*** (1.40–3.38) | 1.79* (1.23–2.61) | 1.22 (0.82–1.81) | 1.61** (1.02–2.54) | 1.34 (0.87–2.07) | — |
| Depression | 1.42 (0.95–2.10) | 1.64** (1.12–2.40) | 1.81*** (1.33–2.45) | — | — | — |
| Anxiety | — | — | — | 1.40 (0.99–1.98) | — | 1.28 (0.93–1.75) |
| Insomnia | — | — | — | — | — | — |
| Dementia | 1.36 (0.91–2.02) | 1.44 (0.97–2.13) | 1.84*** (1.34–2.53) | 2.38*** (1.58–3.57) | 1.55** (1.08–2.24) | 1.20 (0.84–1.71) |
Predictors for each social participation limitation model based on forward and backward selection of significant symptoms, sociodemographic, and medical factors. Visiting family or friends also included lung disease. Attending religious services also included gender and age. Attending clubs, classes, or activities also included gender and lung disease. Going out for enjoyment also included arthritis and lung disease. Engaging in a favorite activity also included lung disease.
p < 0.01, **p < 0.05, ***p ≤ 0.001.
Discussion
In a nationally representative cohort of older adults, sensory, psychiatric, and especially physical symptoms were common in the year before death. The most prevalent symptoms were pain, lower extremity weakness, low energy, and imbalance, each of which was experienced by more than half of decedents. Probable dementia was also common, affecting ∼40% of older decedents, and was associated with greater prevalence of most symptoms, high symptom burden, and limitations in social activities. Dementia was independently associated with greater odds of participation limitations in clubs/activities, volunteering, and going out for enjoyment. Of note, physical symptoms that were independently associated with participation limitations in the remaining social activities (visiting family/friends, attending religious services, and engaging in a favorite activity) are commonly seen in advanced dementia (problems speaking, poor balance/coordination, and limited lower extremity strength or movement).33
Prior studies have investigated specific symptoms, impact on activities, and symptoms in dementia. Pain has been shown to lead to activity restriction in prior studies though it was not associated with social participation limitations in our study.34,35 This finding may be due to coping mechanisms, chronicity of pain, the notion that pain is a natural part of aging, or adequate treatment of pain. Consistent with prior research, we found greater burden of sensory impairments in decedents with dementia. Cognitive and sensory impairments may be caused by common neuropathologic processes and lead to greater difficulty performing activities, increasing dependency, and social isolation.36 Vision loss, although burdensome, was not associated with decreased social participation in our study, consistent with previous research.37
Neuropsychiatric symptoms including depression, anxiety, and insomnia are commonly seen in dementia.38 Although anxiety and insomnia were not associated with limited social participation, depression was associated with limitations in attending religious services and clubs/activities. Depression is associated with dissatisfaction with life in cognitively normal and impaired older adults; furthermore, self-reported mood is one of the strongest determinants of quality of life in dementia.39–41 Thus, addressing depression in older adults with and without dementia may improve both subjective quality of life and social participation.
Our findings bring attention not only to the importance of specific symptoms and symptom burden but also to social participation. Problems speaking emerged as the symptom most consistently associated with limitations in social participation, with significantly greater limitations in all activities except for attending religious services. This finding may be due to the central role of communication in social interactions, stigma, or problems speaking as a marker of advanced debilitating illness. Lower extremity weakness and poor balance or coordination were also associated with multiple participation limitations. These symptoms may similarly be markers of advanced illness. However, these symptoms may also be associated with stigma that affects social participation and underscore the role of mobility in participation. Participation in dementia may be limited by physical and psychiatric symptoms increasingly common as dementia progresses. Our study suggests, however, that cognitive impairment itself is also associated with limitations in social participation. This relationship may be both direct and indirect. For example, executive dysfunction and difficulty carrying out complex tasks may limit the ability to participate in clubs or activities, whereas stigma may limit going out for enjoyment.
In the context of our study, a goal-oriented approach to patient care may be an optimal paradigm, especially in the last years of life. A goal-oriented approach would focus medical care on the symptoms and activities that are prioritized as most important to the individual with the goal of improving quality of life.42 Symptoms that are limiting desired social participation may be best addressed through interdisciplinary care as traditional medical interventions are likely to have limited efficacy in addressing symptoms such as lower extremity weakness and poor balance. Physical therapists can help identify and coach patients or caregivers on assistive devices that might improve mobility. Physical activity programs are also feasible and improve function (transfers, walking, and balance) in many older adults, including those with mild to moderate dementia, and could also potentially improve participation.43 Occupational or recreational therapists can help identify creative solutions to maintain participation in valued activities. For example, technology can facilitate “visits” with family or friends and attendance at religious services.
Hospice and palliative care services already use an interdisciplinary goal-oriented approach to care that can also focus on social activities that are meaningful to patients. Thus, earlier enrollment in hospice or greater access to palliative care for patients who do not meet eligibility criteria for hospice may benefit older adults, including those with dementia, in the last years of life. Given higher symptom burden in decedents with dementia, earlier palliative care should be considered in a disease where life expectancy can be challenging to predict.
Our study has several limitations. The exact duration of symptoms was not examined nor was the prevalence of symptoms in prior years; limitations in social participation may have also been present in prior years. Future research might examine trajectories of symptoms and participation to better understand when and how they change as well as their relationship over time. In our study, older adults with probable dementia were also more likely to have a proxy respondent. Concordance between patient and proxy reporting regarding symptom burden and desired participation in various activities is unclear. Prior research has demonstrated that patients typically rate their quality of life better than proxy respondents.41,44–46 Therefore, symptom burden and reduced quality of life may be overestimated in those with proxy respondents.44,47 Fear of falling was not examined but could lead to self-imposed activity restriction and loss of physical independence, reducing social participation.48 There may also be other relevant symptoms not assessed in NHATS that may affect social participation.
Furthermore, we do not have data on cause of death; symptom burden and trajectory may differ depending on the illness. Classification of dementia in an epidemiologic study is also a limitation though probable dementia versus no dementia based on the NHATS algorithm has been found to correspond well to clinical diagnostic assessment.25 Nonetheless, some participants may be misclassified as probable dementia due to other cognitive impairment, low education, or other factors that affect performance on cognitive tests. Finally, it is possible that social participation and activities are not viewed as important in late stage dementia. Patient and caregiver goals of care and values should be elicited in individual cases to confirm that social participation is not important to each patient and family rather than making an assumption, however. Limitation in older adults' social participation may also lead to limitations in caregiver participation.
Greater social engagement is associated with better health outcomes and represents a component of quality of life that may be overlooked toward the end of life.49 Palliative care should emphasize both symptom management and maintenance of activities important to individuals and potentially caregivers.50 For persons who value social participation, interdisciplinary palliative care can address symptoms that may be limiting participation. Older adults with dementia are particularly at risk for high symptom burden and limited social participation. This growing population may benefit from targeted interventions and emphasis on improving quality of life even before their last months of life.
Author Disclosure Statement
No competing financial interests exist.
The views expressed in this article are those of the authors and do not reflect the official policy of the Department of Army/Navy/Air Force, Department of Defense, or the U.S. Government.
References
- 1. NHPCO Facts and Figures: Hospice Care in America. Alexandria, VA: National Hospice and Palliative Care Organization, rev. ed., April 2018 [Google Scholar]
- 2. Diamond EL, Russell D, Kryza-Lacombe M, et al. : Rates and risks for late referral to hospice in patients with primary malignant brain tumors. Neuro Oncol 2016;18:78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cheng S-Y, Dy S, Hu W-Y, et al. : Factors affecting the improvement of quality of dying of terminally ill patients with cancer through palliative care: A ten-year experience. J Palliative Med 2012;15:854–862 [DOI] [PubMed] [Google Scholar]
- 4. Rickerson E, Harrold J, Kapo J, et al. : Timing of hospice referral and families' perceptions of services: Are earlier hospice referrals better? J Am Geriatr Soc 2005;53:819–823 [DOI] [PubMed] [Google Scholar]
- 5. Bakitas M, Lyons KD, Hegel MT, et al. : Effects of a palliative care intervention on clinical outcomes in patients with advanced cancer. JAMA 2009;302:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Meier DE: Increased access to palliative care and hospice services: Opportunities to improve value in health care. Milbank Q 2011;89:343–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Eckerblad J, Theander K, Ekdahl AW, et al. : Symptom trajectory and symptom burden in older people with multimorbidity, secondary outcome from the RCT AGe-FIT study. J Adv Nurs 2016;72:2773–2783 [DOI] [PubMed] [Google Scholar]
- 8. Gill TM, Han L, Leo-Summers L, et al. : Distressing symptoms, disability, and hospice Services at the end of life: Prospective cohort study. J Am Geriatr Soc 2017;66:41–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Smith AK, Cenzer IS, Knight SJ, et al. : The epidemiology of pain during the last 2 years of life. Ann Intern Med 2010;153:563–569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chaudhry SI, Murphy TE, Gahbauer E, et al. : Restricting symptoms in the last year of life. JAMA Intern Med 2013;173:1534–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bekelman DB, Rumsfeld JS, Havranek EP, et al. : Symptom burden, depression, and spiritual well-being: A comparison of heart failure and advanced cancer patients. J Gen Intern Med 2009;24:592–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. McCarthy M, Addington-Hall J, Altmann D. The experience of dying with dementia: A retrospective study. Int J Geriatr Psychiatry 1997;12:404–409 [PubMed] [Google Scholar]
- 13. Pinzon LCE, Claus M, Perrar KM, et al. : Dying with dementia. Dtsch Arztebl Int 2013;110:195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hendriks SA, Smalbrugge M, Hertogh CMPM, et al. : Dying with dementia: Symptoms, treatment, and quality of life in the last week of life. J Pain Sympt Manag 2014;47:710–720 [DOI] [PubMed] [Google Scholar]
- 15. Shen C, Zhao X, Dwibedi N, et al. : Opioid use and the presence of Alzheimer' s disease and related dementias among elderly Medicare beneficiaries diagnosed with chronic pain conditions. Alzheimer's Dement Transl Res Clin Interv 2018;4:661–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Husebo BS, Strand LI, Moe-Nilssen R, et al. : Who suffers most? Dementia and pain in nursing home patients: A cross-sectional study. J Am Med Dir Assoc 2008;9:427–433 [DOI] [PubMed] [Google Scholar]
- 17. Clague F, Mercer SW, Mclean G, et al. : Comorbidity and polypharmacy in people with dementia: Insights from a large, population-based cross-sectional analysis of primary care data. Age Ageing 2017;46:33–39 [DOI] [PubMed] [Google Scholar]
- 18. Aldridge MD, Bradley EH: Epidemiology and patterns of care at the end of life: Rising complexity, shifts in care patterns and sites of death. Health Aff (Millwood) 2017;36:1175–1183 [DOI] [PubMed] [Google Scholar]
- 19. Teno JM, Plotzke M, Gozalo P, et al. : A national study of live discharges from hospice. J Palliat Med 2014;17:1121–1127 [DOI] [PubMed] [Google Scholar]
- 20. Feng Z, Coots LA, Kaganova Y, et al. : Hospital and ED use among Medicare beneficiaries with dementia varies by setting and proximity to death. Health Aff (Millwood) 2014;33:683–690 [DOI] [PubMed] [Google Scholar]
- 21. Callahan CM, Arling G, Tu W, et al. : Transitions in care for older adults with and without dementia. J Am Geriatr Soc 2012;60:813–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Torke AM, Holtz LR, Hui S, et al. : Palliative care for patients with dementia: A national survey. J Am Geriatr Soc 2010;58:2114–2121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murphy E, Froggatt K, Connolly S, O'Shea E, et al. : Palliative care interventions in advanced dementia. Cochrane Database Syst Rev 2016;12:CD011513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Montaquila J, Freedman V, Edwards B, et al. : National Health and Aging Trends Study round 1 sample design and selection. Technical Paper No. 1, 2012;1–8 [Google Scholar]
- 25. Kasper JD, Freedman VA, Spillman BC. Classification of persons by dementia status in the National Health and Aging Trends Study. Technical Paper No. 5, 2013;1–14 [Google Scholar]
- 26. Amjad H, Roth DL, Sheehan OC, et al. : Underdiagnosis of dementia: An observational study of patterns in diagnosis and awareness in US older adults. J Gen Intern Med 2018;33:1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langa KM, Plassman BL, Wallace RB, et al. : The aging, demographics, and memory study: Study design and methods. Neuroepidemiology 2005;25:181–191 [DOI] [PubMed] [Google Scholar]
- 28. Galvin JE, Roe CM, Powlishta KK, et al. : The AD8: A brief informant interview to detect dementia. Neurology 2005;65:559–564 [DOI] [PubMed] [Google Scholar]
- 29. Crimmins EM, Kim JK, Langa KM, et al. : Assessment of cognition using surveys and neuropsychological assessment: The health and retirement study and the aging, demographics, and memory study. J Gerontol Ser B 2011;66B(Suppl. 1):i162–i171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Langa KM, Kabeto M WD: Report on race and cognitive impairment using the HRS. 2010 Alzheimer's disease facts and figures. Alzheimer's Dement 2010;6:158–194 [DOI] [PubMed] [Google Scholar]
- 31. Freedman VA, Stafford F, Schwarz N, et al. : Disability, participation, and subjective wellbeing among older couples. Soc Sci Med 2012;74:588–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryan T, Ingleton C, Gardiner C, et al. : Symptom burden, palliative care need and predictors of physical and psychological discomfort in two UK hospitals. BMC Palliat Care 2013;12:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mitchell SL: Advanced dementia. N Engl J Med 2015;372:2533–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Williamson GM, Schulz R: Activity restriction mediates the association between pain and depressed affect: A study of younger and older adult cancer patients. Psychol Aging 1995;10:369–378 [DOI] [PubMed] [Google Scholar]
- 35. Cubukcu D, Sarsan A, Alkan H: Relationships between pain, function and radiographic findings in osteoarthritis of the knee: A cross-sectional study. Arthritis 2012;2012:1–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin FB, Metter J, O'Brien R, et al. : Hearing loss and incident dementia. Johns Hopkins Med 2011;68:214–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alma MA, van der Mei SF, Melis-Dankers BJM, et al. : Participation of the elderly after vision loss. Disabil Rehabil 2011;33:63–72 [DOI] [PubMed] [Google Scholar]
- 38. Lyketsos CG, Lopez O, Jones B, et al. : Prevalence of neuropsychiatric symptoms results from the cardiovascular health study. JAMA 2002;288:1475–1483 [DOI] [PubMed] [Google Scholar]
- 39. Helvik A-S, Engedal K, Krokstad S, et al. : A comparison of life satisfaction in elderly medical inpatients and the elderly in a population-based study: Nord-Trondelag Health Study 3. Scand J Public Health 2011;39:337–344 [DOI] [PubMed] [Google Scholar]
- 40. Woods RT, Nelis SM, Martyr A, et al. : What contributes to a good quality of life in early dementia? Awareness and the QoL-AD: A cross-sectional study. Health Qual Life Outcomes 2014;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gómez-Gallego M, Gómez-Amor J, Gómez-García J: Determinants of quality of life in Alzheimer's disease: Perspective of patients, informal caregivers, and professional caregivers. Int Psychogeriatr 2012;24:1805–1815 [DOI] [PubMed] [Google Scholar]
- 42. Reuben DB, Tinetti ME: Goal-oriented patient care—An alternative health outcomes paradigm. N Engl J Med 2012;366:777–779 [DOI] [PubMed] [Google Scholar]
- 43. Hauer K, Schwenk M, Zieschang T, et al. : Physical training improves motor performance in people with dementia: A randomized controlled trial. J Am Geriatr Soc 2012;60:8–15 [DOI] [PubMed] [Google Scholar]
- 44. Schulz R, Cook TB, Beach SR, et al. : Magnitude and causes of bias among family caregivers rating Alzheimer disease patients. Am J Geriatr Psychiatry 2013;21:14–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Black B, Johnston D, Morrison A, et al. : Quality of life of community-residing persons with dementia based on self-rated and caregiver-rated measures. Qual Life Res 2012;21:1379–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Bravo G, Sene M, Arcand M: Surrogate inaccuracy in predicting older adults' desire for life-sustaining interventions in the event of decisional incapacity: Is it due in part to erroneous quality-of-life assessments? Int Psychogeriatr 2017;29:1061–1068 [DOI] [PubMed] [Google Scholar]
- 47. Silveira MJ, Given CW, Given B, et al. : Patient-caregiver concordance in symptom assessment and improvement in outcomes for patients undergoing cancer chemotherapy. Chronic Illn 2010;6:46–56 [DOI] [PubMed] [Google Scholar]
- 48. Hsu Y, Alfermann D, Lu FJH, et al. : Pathways from fear of falling to quality of life: The mediating effect of the self-concept of health and physical independence. Aging Ment Health 2013;17:816–822 [DOI] [PubMed] [Google Scholar]
- 49. Bath PA, Deeg D: Social engagement and health outcomes among older people: Introduction to a special section. Eur J Ageing 2005;2:24–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Beernaert K, Deliens L, De Vleminck A, et al. : Is there a need for early palliative care in patients with life-limiting illnesses? Interview study with patients about experienced care needs from diagnosis onward. Am J Hosp Palliat Med 2016;33:489–497 [DOI] [PubMed] [Google Scholar]
- 51. Löwe B, Kroenke K, Gräfe K: Detecting and monitoring depression with a two-item questionnaire (PHQ-2). J Psychosom Res 2005;58:163–171 [DOI] [PubMed] [Google Scholar]
- 52. Kroenke K, Spitzer RL, Williams JB, et al. : Anxiety disorders in primary care: Prevalence, impairment, comorbidity, and detection. Ann Intern Med 2007;146:317–325 [DOI] [PubMed] [Google Scholar]