ABSTRACT
S100A4 is particularly associated with the progression and metastasis of numerous human malignancies. This study was designed to examine the clinicopathologic significance of S100A4 in gastrointestinal stromal tumor (GISTs). The level of OPNS100A4 expression in a large cohort of resectable GISTs was evaluated with immunohistochemistry. Its correlation with the clinicopathologic parameters of patients with resectable GISTs was analyzed. A survival analysis was performed to evaluate the prognostic significance of S100A4 expression using Kaplan-Meier method. Results: In 108 patients with resectable GISTs, the most high-risk GISTs had a strong level of S100A4 expression. Strong S100A4 expression was significantly associated with tumor size, mitosis, and recurrence, but not gender and age. Patients with weak S100A4 expression had a relatively longer disease-free survival compared to patients with strong S100A4 expression.Therefore, S100A4 expression is a putative marker for tumor progression and an adverse prognosis in GISTs.
KEYWORDS: S100A4, gastrointestinal stromal tumors, immunohistochemistry, prognosis
1. Introduction
Gastrointestinal stromal tumors (GISTs) are the most common mesenchymal tumors located in the alimentary tract and its usual manifestation is gastrointestinal bleeding [1]. The majority of studies have reported an increase in incidence since 2000; nevertheless, this may be a consequence of improvements in diagnostic criteria rather than a true increase in incidence [2]. To date, complete resection without lymph node clearance is the standard curative treatment for primary localized GISTs [3]. However, the optimal surgical procedure for duodenal GISTs is not well defined due to their complex anatomy around the pancreaticoduodenal region [4,5]. The limited resection (LR) is reported to be a technically feasible and oncologically sound procedure for duodenal GISTs, while the pancreaticoduodenectomy (PD) is also warranted in some cases due to the anatomical considerations of the proximity of critical structures, including the papilla, pancreas and biliary and pancreatic ducts [6]. However, the survival impact of surgical procedure on duodenal GISTs still remains controversial [7,8].
Geneticists estimate that about 10% of all individuals suffer from deleterious gene mutations, and current research shows that the development of GISTs is associated with multiple gene mutations, such as c-kit and PDGFRα (platelet derived growth factor receptor alpha) mutations [9]. Recent studies have shown that adjuvant therapy with imatinib, a small molecule tyrosine kinase inhibitor, can prolong both survival and time to metastasis following surgery [10]. However, most micro-GISTs (less than 1 cm in diameter) have little malignancy potential despite the presence of KIT or PDGFRA mutations [11].Together, this demonstrates the need for additional prognostic molecular biomarkers to better characterize tumor prognosis and guide treatment strategy.
S100A4, an important member of S100 family proteins, functions to increase the tumor progression and metastasis, and the molecular mechanisms of S100A4 involving in the progression and metastasis are diverse in various malignant tumors [12,13]. Several studies have also documented that increased S100A4 expression contributes to the aggressive behaviors of gastric cancer cells, which is also useful as a biomarker for poor prognosis of gastric cancer [14,15]. However, the role of S100A4 in GISTs is not investigated up to date.
In this study, we examined the expression level of S100A4 in resectable GIST specimens and evaluated the relationship between S100A4 expression and the clinical parameters and prognosis of GIST patients.
2. Materials and methods
2.1. Patients and specimens
Between January 2008 and December 2012, 138 patients who received complete surgical treatment for primary GISTs in the Qilu Hospital of Shandong University and the affiliated hospital of Qingdao University were enrolled in this study. Informed consent was obtained from all patients. The present study was conducted in accordance with the ethical standards of the Helsinki Declaration in 1975, after approval of the Institutional Review Board of Qilu Hospital of Shandong University (QL-2007–083) and the affiliated hospital of Qingdao University (1999-ES-028). The diagnosis of GISTs was pathologically and clinically proven. To eliminate possible interference factors, we excluded all cases that met any one of these criteria: resections with positive margins, adjuvant imatinib treatment, a family history of GISTs, and a history of other malignancies. Demographic data and pathologic stage were collected. GISTs were categorized into different grades according to the National Institutes of Health (NIH) Consensus Criteria for GIST risk stratification: very-low-risk, low-risk, intermediate-risk and high-risk [16]. Patients were regularly followed at our outpatient department with abdominal computed tomography (CT) every 3 months or 6 months for the first 3 years after surgery depending on high-risk and non-high-risk grade, respectively. The follow-up thereafter for all patients was every 6 months.
2.2. Immunohistochemistry
Immunohistochemical staining for protein S100A4 was performed using a standard avidin-biotin complex (ABC) method. In brief, all sections were deparaffinized by using a series of xylene baths and then hydrated using a graded alcohol series. They were then placed in citric acid buffer (10 mmol/L) and heated in a microwave oven (700 W) for 12 min to retrieve the antigenicity. The sections were then immersed in methanol, containing 0.3% hydrogen peroxide, for 20 min to block endogenous peroxidase activity. The sections were then washed three times in phosphate-buffered saline (PBS) and incubated in 2.5% normal goat serum for 20 min to reduce nonspecific antibody binding. After washing with PBS, the sections were incubated with primary antibodies for 30 min at room temperature. Rabbit polyclonal antibodies against protein S100A4 (Ab-8, Neomarker, 1:100) was used. The tonsil was used the internal positive controls. The reaction products were visualized with diaminobenzidine as a chromogen, and counterstained with commercial hematoxylin.
2.3. Scoring criteria
Two specialized pathologists evaluated and graded the degree of immunohistochemical staining independently. Consensus was reached through rescoring when there were grading discrepancies. PositiveS100A4 staining was defined as brown-yellow cytoplasmic staining. Semiquantitative evaluation was performed to establish the grade of immunohistochemical staining. For each section, five adjacent fields at a magnification of × 400 were observed using light microscopy (Figure 1). The staining intensity was scored as negative (0), weak (1), moderate (2) and strong (3). The percentage of positive staining cells was scored as ≤ 5% (0), 6%-25% (1), 26%-50% (2), 51%-75% (3) and > 75% (4). The terminal score of each field was determined by adding together the staining intensity and the percentage of positive staining cells. A terminal score of 3 or less was considered weak expression. An immunohistochemical staining score greater than 3 was considered strong expression.
Figure 1.
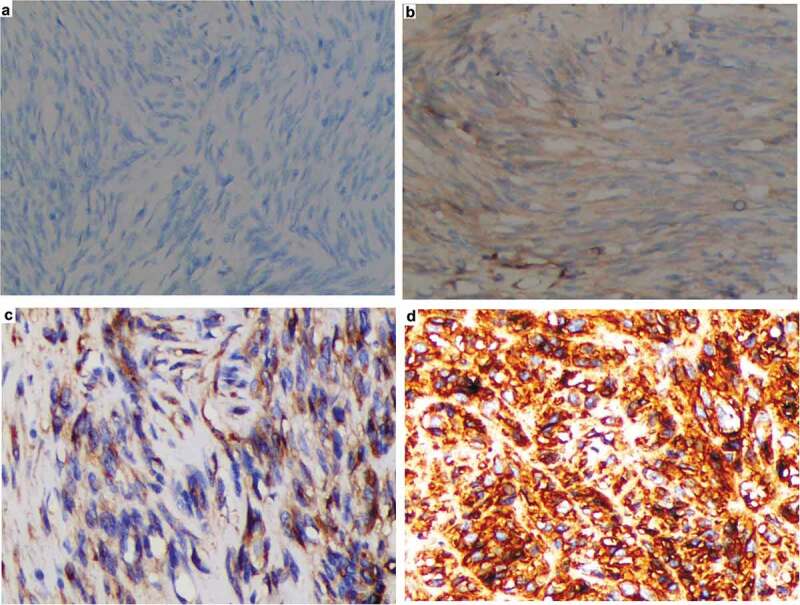
Representative immunohistochemical staining of S100A4 in resectable GISTs. Positive staining for S100A4 was defined as brown-yellow cytoplasmic staining. The staining intensity was scored as negative (A), weak (B), moderate (C) and strong (D); Original magnification (× 200).
2.4. Statistical analysis
All statistical analyses were performed using the SPSS 22.0 package. Descriptive data are expressed as median ± SEM. Categorical variables were compared between groups using the χ2 test, while continuous variables were compared with an independent sample t test. A survival analysis was computed with the KaplanMeier method, and disease-free survival (DFS) and overall survival(OS) was compared using the log-rank test. The Cox proportional hazard model was applied to the multivariate analysis. A probability value of less than 0.05 (P < 0.05) was considered statistically significant.
3. Results
3.1. Demographic and clinicopathologic characteristics of patients with resectable GISTs
We collected 138 patients who underwent complete surgical resections without adjuvant imatinib. The cohort included 61 women and 77 men with a mean age of 63.6 ± 11.4 years (median: 63 years, range: 35 years to 81 years). Maximum tumor diameter varied from 0.6 cm to 16.8 cm (median = 3.8 cm), and mitotic counts varied from 1/50 HPFs to 25/HPF (median = 5/50 HPFs). According to the NIH Consensus Criteria, GIST patients were categorized into very-low-risk (n = 20), low-risk (n = 47), intermediate-risk (n = 18) and high-risk (n = 53) groups.
3.2. Clinicopathologic significance of S100A4 expression in patients with resectable GISTs
We evaluated the relative levels of S100A4 expression in GIST specimens using immunohistochemistry (Figure 1). Patients with strong S100A4 expression had significantly larger tumor sizes and increased mitoses (P = 0.012 and P < 0.001, respectively). However, there was no significant difference between risk status and S100A4 expression (χ2 = 6.18, P = 0.073). There were also no significant differences in S100A4 expression between different gender and age groups (χ2 = 0.018, P = 0.333 and χ2 = 0.746, P = 0.258, respectively). Notably, strong S100A4 expression was clearly related to an increased recurrence rate of resectable GISTs (χ2 = 6.84, P = 0.0053) (Table 1). The results showed that there was a predominance of strong S100A4 expression in patients with high-risk GISTs, despite no significant difference between risk groups as defined by the NIH Consensus Criteria. We further compared the S100A4 expression between high-risk and non-high-risk (including the very-low-risk, low-risk and intermediate-risk) GISTs, and the results were significantly different (χ2 = 6.92, P = 0.0035).
Table 1.
Demographics and clinicopathologic characteristics in patients with resectable GIST based on S100A4 expression
| S100A4 expression |
||||
|---|---|---|---|---|
| Characteristic | Number | Weak ≤ 3 | Strong > 3 | p-value |
| Age (yr) | 0.333 | |||
| < 63 | 59 | 36 | 23 | |
| ≥ 63 | 79 | 49 | 30 | |
| Gender | 0.258 | |||
| Male | 77 | 46 | 31 | |
| Female | 61 | 39 | 22 | |
| Tumor size (cm) (mean ± SD) | 4.86 ± 3.58 | 4.07 ± 2.80 | 5.88 ± 4.21 | 0.012 |
| Mitosis (HPF)(mean ± SD) | 5.62 ± 4.25 | 4.07 ± 1.70 | 7.64 ± 5.57 | <0.001 |
| Risk group | 0.073 | |||
| Very Low | 20 | 11 | 6 | |
| Low | 53 | 29 | 14 | |
| Intermediate | 18 | 8 | 6 | |
| High | 47 | 13 | 21 | |
| Recurrence | 0.0053 | |||
| Yes | 30 | 2 | 18 | |
| No | 99 | 52 | 29 | |
3.3. Survival analysis of S100A4 expression in patients with resectable GISTs
Of 138 GIST patients, 9 were lost to follow-up, and 129 were followed at the time of this study (range: 9–67 months). The disease free survival (DFS) rate of all the patients was poor for high risk GIST (p < 0.001), tumor size ≥10 cm (p = 0.003), mitoses ≥10/50 HPFs (p = 0.002) and strong S100A4 expression (p < 0.001) (Figure 2). The overall survival rate (OS) was also poor for tumor size ≥10 cm (p = 0.046) (Figure 2).
Figure 2.
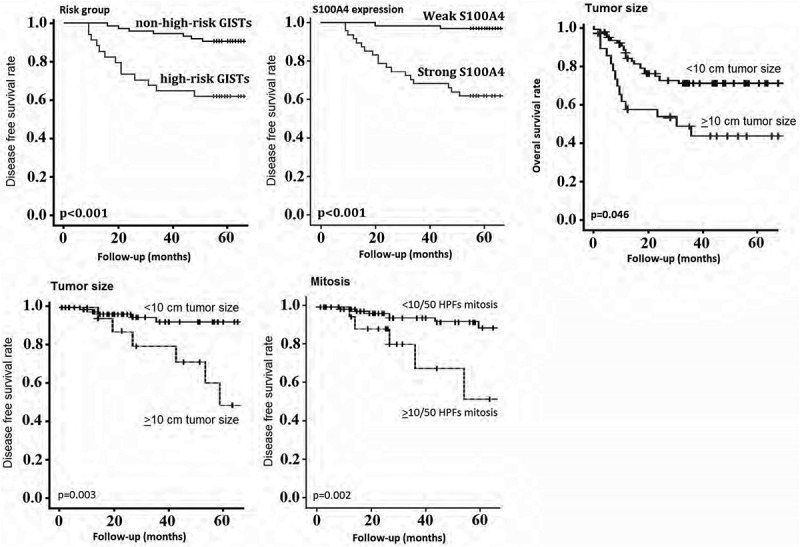
Survival analysis using the Kaplan-Meier method. Disease free survival (DFS) rate of all patients was poor in GIST with high risk GIST, tumor size ≥10 cm, mitoses ≥10/50 HPFs and strong S100A4 positivity;Overall survival (OS) rate was also poor in tumor size ≥10 cm.
On the multivariate analysis, strong S100A4 expression (P = 0.017), tumor size ≥10 cm (p = 0.003) and mitotic figures ≥10/50 HPFs (p = 0.018) were independent prognostic factors for poor DFS, whear tumor size ≥10 cm (p = 0.036) was independent prognostic factors for poor OS.
4. Discussion
GISTs are the main cohort of neoplasms that originate from the mesenchymal tissues of the digestive tract. The clinical manifestations of GISTs are nonspecific and cover a broad spectrum of clinical presentations. In the past, GISTs were frequently misdiagnosed as leiomyomas, leiomyosarcomas, leiomyoblastomas, schwannomas, and so on [17]. Until the discovery and affirmation of the c-KIT and PDGFRα genetic mutations in 1983, GISTs were not an independent entity and family [18]. Over several years, advancements in genetic and immu?nohistochemical features have led to advancements in the diagnosis and therapy of GISTs. Most GISTs (approximately 95%) are positive for CD117, which is the main diagnostic bio?marker [17]. DOG-1 positive staining is another significant immunohistochemical biomarker that can help diagnose GISTs in cases with negative CD117 [19]. Imatinib administration has significantly improved the prognosis of patients with advanced or unresectable GISTs [20,21].It is valuable to explore correlations between potential biomarkers and GIST diagnosis and prognosis.
Here, we conducted the largest study thus far to assess the role of S100A4 in GISTs by analyzing the S100A4 expression levels of 138 resected GIST specimens, which were categorized into different risk statuses according to the NIH Consensus Criteria. Our immunohistochemical findings showed that high-risk GISTs had a relatively stronger S100A4 expression compared to non-high-risk GISTs. We did not find any significant differences in S100A4 expression between different age and gender groups. These results suggest that S100A4 has a tumor-promoting role in the progression of GISTs. To investigate the potential of S100A4 as a prognostic marker, we conducted a regular follow-up for these patients with resectable GISTs. We found that S100A4 expression was positively correlated with recurrence rate. Our survival analysis further suggested that patients with weak S100A4 expression had a relatively longer DFS compared to control group with strong S100A4 expression. The findings of our multivariate analysis showed that strong S100A4 expression, tumor size ≥10 cm and mitotic figures ≥10/50 HPFs were independent prognostic factors for poor DFS, whear tumor size ≥10 cm was independent prognostic factors for poor OS.
It is well established that S100A4 plays a very important role in malignant transformation and contributes to the progression of most human malignancies. S100A4 was also reported to be a prognostic biomarker for gastric cancer [14–16]. Our study’s results established a role of S100A4 in GISTs, which was consistent with the earlier reports mentioned above.
In the clinic, monitoring postoperative GIST patients for recurrence has solely relied on imaging, which is not convenient or economic. No special tumor markers can serve in this duty in the way that CEA and AFP are valuable in the postoperative monitoring of colorectal cancer and hepatic cancer, respectively. Additionally, imatinib is often preoperatively administered to make certain unresectable GISTs better suitable for R0 resection. How can one confirm the best time of resection and manage the duration of imatinib? This is always difficult using imaging alone. By confirming the role of S100A4 in GIST progression and adverse prognosis, S100A4 as a kind of protein might be a potential candidate for monitoring tumor progression and recurrence.
In conclusion, the present study identified that strong S100A4 expression was consistent with GIST progression and that S100A4 was an independent predictor of an adverse prognosis of patients with resectable GISTs. In the era of imatinib, whether S100A4 is a valuable biomarker for the progression and prognosis of GISTs has not been investigated. We did not enroll patients who had received imatinib administration. Further investigation on relationship between S100A4 expression and imatinib treatment would contribute to a much better recognition of the diagnostic and prognostic values of S100A4.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Corless CL, Barnett CM, Heinrich MC.. Gastrointestinal stromal tumours: origin and molecular oncology. Nat Rev Cancer. 2011;11:1–5. [DOI] [PubMed] [Google Scholar]
- [2].Ma GL, Murphy JD, Martinez ME, et al. Epidemiology of gastrointestinal stromal tumors in the era of histology codes: results of a population-based study. Cancer Epidemiol Biomarkers Prev. 2015;24:298–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Singer S, Rubin BP, Lux ML, et al. Prognostic value of KIT mutation type, mitotic activity, and histologic subtype in gastrointestinal stromal tumors. J Clin Oncol. 2002;20:3898–3905. [DOI] [PubMed] [Google Scholar]
- [4].Tien YW, Lee CY, Huang CC, et al. Surgery for gastrointestinal stromal tumors of the duodenum. Ann Surg Oncol. 2010;17:109–114. [DOI] [PubMed] [Google Scholar]
- [5].Goh BK, Chow PK, Ong HS, et al. Gastrointestinal stromal tumor involving the second and third portion of the duodenum: treatment by partial duodenectomy and Roux-en-Y duodenojejunostomy. J Surg Oncol. 2005;91:273–275. [DOI] [PubMed] [Google Scholar]
- [6].Lee SY, Goh BK, Sadot E, et al. Surgical strategy and outcomes in duodenal gastrointestinal stromal tumor. Ann Surg Oncol. 2017;24:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Johnston FM, Kneuertz PJ, Cameron JL, et al. Presentation and management of gastrointestinal stromal tumors of the duodenum: a multi-institutional analysis. Ann Surg Oncol. 2012;19:3351–3360. [DOI] [PubMed] [Google Scholar]
- [8].Zhang Q, Shou CH, Yu JR, et al. Prognostic characteristics of duodenal gastrointestinal stromal tumours. Br J Surg. 2015;102:959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hirota S. Gastrointestinal stromal tumors: their origin and cause. Int J Clin Oncol. 2001;6:1–5. [DOI] [PubMed] [Google Scholar]
- [10].Corless CL, Ballman KV, Antonescu CR, et al. Pathologic and molecular features correlate with long-term outcome after adjuvant therapy of resected primary GI stromal tumor: the ACOSOG Z9001 trial. J Clin Oncol. 2014;32:1563–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Demetri GD, Benjamin RS, Blanke CD, et al. NCCN Task Force report: management of patients with gastrointestinal stromal tumor (GIST)–update of the NCCN clinical practice guidelines. J Natl Compr Canc Netw. 2007;5:S1-29;quiz S30. [PubMed] [Google Scholar]
- [12].Mishra SK, Siddique HR, Saleem M. S100A4 calcium-binding protein is key player in tumor progression and metastasis: preclinical and clinical evidence. Cancer Metastasis Rev. 2012;31:163–172. [DOI] [PubMed] [Google Scholar]
- [13].Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Guo J, Bian Y, Wang Y, et al. S100A4 influences cancer stem cell-like properties of MGC803 gastric cancercells by regulating GDF15 expression. Int J Oncol. 2016;49:559–568. [DOI] [PubMed] [Google Scholar]
- [15].Ling Z, Li R. Clinicopathological and prognostic value of S100A4 expression in gastric cancer: a meta-analysis. Int J Biol Markers. 2014;29:e99–e111. [DOI] [PubMed] [Google Scholar]
- [16].Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Hum Pathol. 2002;33:459–465. [DOI] [PubMed] [Google Scholar]
- [17].Miettinen M, Lasota J. Gastrointestinal stromal tumors (GISTs): definition, occurrence, pathology, differential diagnosis and molecular genetics. Pol J Pathol. 2003;54:3–24. [PubMed] [Google Scholar]
- [18].Kitamura Y. Gastrointestinal stromal tumors: past, present, and future. J Gastroenterol. 2008;43:499–508. [DOI] [PubMed] [Google Scholar]
- [19].Miettinen M, Wang ZF and Lasota J.. DOG1 an?tibody in the differential diagnosis of gastrointestinal stromaltumors: a study of 1840 cases. Am J Surg Pathol. 2009;33:1401–1408. [DOI] [PubMed] [Google Scholar]
- [20].Manley PW, Cowan-Jacob SW, Buchdunger E, et al. Imatinib: a selective tyrosine kinase inhibitor. Eur J Cancer. 2002;38(Suppl 5):S19–27. [DOI] [PubMed] [Google Scholar]
- [21].Wu PC, Langerman A, Ryan CW, et al. Surgical treatment of gastro?intestinal stromal tumors in the imatinib (STI-571) era. Surgery. 2003;134:656–665. [DOI] [PubMed] [Google Scholar]


