ABSTRACT
Background: It is well known from cross-sectional studies that individual coping strategies significantly influence the pathogenesis of posttraumatic stress disorder (PTSD). Equally, undisputed is the role of biological processes, e.g. of the so-called ‘stress hormone’ cortisol for the trajectory of PTSD. Ecological momentary assessment (EMA), the repeated collection of self-reported momentary states via smartphones, is ideal for shedding light upon symptom fluctuations and coping strategies. EMA may also constitute a promising approach to provide closer associations to biomarkers than retrospective self-report. The mobile application ‘CoachPTBS’, created to facilitate transition into health-care systems, bridges waiting periods for trauma-specific psychotherapy. CoachPTBS offers tools akin to EMA that could elucidate coping with stress symptoms. Moreover, the app’s self-management tools may improve coping strategies. However, these processes have never been examined in a combined, longitudinal fashion.
Objective: The aim of the current study is to assess symptom fluctuations, coping strategies and long-term endocrine correlates of PTSD by a longitudinal, multimodal approach, combining traditional, online and EMA self-report with hair cortisol data and CoachPTBS as a possible novel mHealth tool.
Method: 120 participants waiting for PTSD psychotherapy will be randomly grouped. After in-situ assessment and hair sample collection, 40 will receive CoachPTBS, using it daily throughout 4 weeks. A parallel group of 40 will participate in EMA, completing daily questionnaires on symptoms and coping. In between, online surveys will be conducted. After 6 weeks, a final interview and another hair sample collection will follow. Comparisons between these groups and waitlist-control, also consisting of 40 PTSD participants, and 40 non-traumatized participants assessed via EMA regarding aversive emotions and coping are planned.
Discussion: Novel insights into the interplay of biological and coping strategies in PTSD are expected due to the innovative multimodal study design. Results will further explore benefits of eHealth tools on coping with PTSD.
KEYWORDS: Posttraumatic stress disorder, eHealth, ecological momentary assessment, mHealth, coping, hair cortisol
HIGHLIGHTS
• Study Protocol presents a multimodal approach, combining traditional measures, those of Ecological Momentary Assessment (EMA) including CoachPTBS, and hair cortisol concentrations (HCC) as a biomarker of traumatic stress.• Effects on Posttraumatic stress disorder (PTSD) symptomology by participants’ differences in intra-individual coping strategies to be explored.• Effects of coping behaviour taught through mHealth tools, such as CoachPTBS, to be explored.• Contrasting retrospective recall and instant assessment through EMA in regarding PTSD symptoms and coping behaviour.
Abstract
Antecedentes: Es bien sabido por los estudios transversales que las estrategias de afrontamiento individuales influyen significativamente en la patogénesis del estrés postraumático (TEPT). Igualmente es indiscutible el rol de los procesos biológicos, por ej. De la llamada ‘hormona del estrés’, el cortisol para la trayectoria del TEPT. La evaluación ecológica del momento (EMA, por sus siglas en ingles), la que consiste en la colección repetida de estados momentáneos auto-reportados a través de teléfonos inteligentes, es ideal para arrojar luz sobre las fluctuaciones de los síntomas y estrategias de afrontamiento. La EMA puede constituir tambien un enfoque prometedor para proporcionar asociaciones más cercanas a los biomarcadores que el auto-reporte retrospectivo. La aplicación móvil ‘CoachPTBS’, creada para facilitar la transición en los sistemas de atención médica, une los periodos de espera para la psicoterapia específica para el trauma. CoachPTBS ofrece herramientas similares a EMA que podrían dilucidar el manejo de los síntomas de estrés. Además, las herramientas de autogestión de la aplicación pueden mejorar las estrategias de afrontamiento. Sin embargo, estos procesos nunca han sido examinados de manera longitudinal combinada.
Objetivo: El objetivo de este estudio es evaluar las fluctuaciones de los síntomas, las estrategias de afrontamiento y los correlatos endocrinos a largo plazo del TEPT mediante un enfoque longitudinal y multimodal que combina el auto-reporte tradicional, en línea y EMA con información de cortisol capilar y CoachPTBS como una posible herramienta novedosa de salud móvil.
Métodos: Se agruparán 120 participantes que esperan psicoterapia para TEPT. Después de una evaluación in situ y toma de muestra de cabello, 40 recibirán CoachPTBS, usándola diariamente durante cuatro semanas. Un grupo paralelo de 40 participará en EMA, completando cuestionarios diariamente acerca de los síntomas y afrontamiento. En el proceso, se realizaran encuestas en línea. Después de seis semanas, se realizará una entrevista final y otra recolección de muestras de cabello. Se planea una comparación entre estos grupos y la lista de espera, tambien consistente en 40 participantes con TEPT, y 40 participantes no traumatizados evaluados a través de EMA en relación a emociones adversas y afrontamiento.
Discusión: Se esperan novedosas ideas en la interacción entre lo biológico y las estrategias de afrontamiento en TEPT debido al diseño innovador del estudio multimodal. Los resultados exploraran con mayor profundidad los beneficios de las herramientas de Salud ‘electrónica’ para hacer frente al TEPT.
PALABRAS CLAVES: Trastorno de Estrés postraumático, Salud electrónica, Evaluación Ecológica del momento, Salud móvil, afrontamiento, Cortisol en cabello
Abstract
背景:众所周知,横断面研究支持个体应对策略会显著影响创伤后应激障碍(PTSD)的发病机制。生物过程(例如被称作‘应激激素’的皮质醇影响PTSD的轨迹)的作用也已达成共识。生态瞬时评估(EMA),通过智能手机反复自我报告瞬时状态,是解决症状波动和应对策略的理想选择。与回顾性自我报告相比,EMA比自评报告与生物标志物联系更紧密,具有一定前景。移动应用程序‘CoachPTBS’旨在促进向医疗保健系统的过渡,填补创伤特定心理治疗的等待期。 CoachPTBS提供类似于EMA的工具,可以阐明应对压力症状的方法。此外,应用程序的自我管理工具可以改善应对策略。然而,这些过程从未以组合的、纵向方式进行检验。
目的:本研究的目的是通过纵向、多模式方法评估创伤后应激障碍的症状波动,应对策略和长期内分泌相关因素,将传统、在线和EMA自我报告结合头发皮质醇数据和CoachPTBS作为一种可能的新型mHealth工具的数据。
方法:等待PTSD心理治疗的120名参与者将被随机分组。在原位(in-situ)评估和头发样本采集后,40人将接受CoachPTBS,每天使用持续四周。另一个40人的平行小组将参加EMA,们日完成关于症状和应对的问卷。在此期间,还进行了在线调查。六周后,将进行最后一次采访和第二次头发样本采集。这些组与等待名单控制组(包括40名创伤后应激障碍参与者)以及40名通过EMA评估厌恶情绪和应对的非创伤暴露参与者进行比较。
讨论:本研究使用创新的多模式研究设计,提出对PTSD中生物和应对策略的相互作用的新见解。结果将进一步探讨eHealth在应对 PTSD方面的贡献。
关键词: 创伤后应激障碍, eHealth, 生态瞬时评估, 移动健康, 应对, 头发皮质醇
1. Traumatic events, PTSD and coping
Whereas acute stress reactions are fairly common after experiencing a traumatic event, characterized by exposure to actual or threatened death, serious injury or sexual violence (American Psychiatric Association, 2013), only a minority of exposed individuals will suffer long-term consequences such as posttraumatic stress disorder (PTSD) or other trauma-associated symptoms. Numbers on exposure to at least one traumatic event vary and can reach a prevalence of over 70% worldwide (Benjet et al., 2016). Influenced by varying pre-, peri-, and posttraumatic factors, about 10% of those develop a clinical PTSD (Breslau, 2009), with symptoms such as intrusive memories, hyperarousal, avoidance behaviour and changes in cognition and mood (American Psychiatric Association, 2013). According to meta-analytic data, about 44% of the individuals suffering from PTSD experience spontaneous remission (Morina, Wicherts, Lobbrecht, & Priebe, 2014). Of those seeking treatment, however, up to 54% do not respond to or drop out of care (Bradley, Greene, Russ, Dutra, & Westen, 2005; Najavits, 2015). This heterogeneity shows the relevance of individual factors for development and maintenance of PTSD. Therefore, research on factors leading to less severe pathogenesis as well as enhancing remission, such as individual coping, is crucial for progress in PTSD therapy.
One major progress in psychological research is the possibility to examine underlying biological mechanisms of psychopathology, enabling researchers to further explore disease mechanisms, but also future treatment options. In PTSD, a current focus is on the so-called ‘stress hormone’ cortisol and other parameters associated with the hypothalamic-pituitary-adrenal (HPA) axis central for the endocrine stress response (Olff, Langeland, & Gersons, 2005; Olff & van Zuiden, 2017; Steudte-Schmiedgen, Kirschbaum, Alexander, & Stalder, 2016; van Zuiden et al., 2019). While the assessment of cortisol levels is possible in urine, saliva, or blood, these measures reflect a rather short time span of HPA axis activity with higher variability due to situational influences. Over the past decade, analysis of hair cortisol concentrations (HCC) has thus been successfully implemented as a valid and reliable marker of cumulative, long-term cortisol secretion (Stalder & Kirschbaum, 2012). Although due to the method’s novelty, no normative values are available so far, its aptness for longitudinal research questions has greatly increased the feasibility of examining psychopathology (Stalder & Kirschbaum, 2012). One main insight from HCC in the context of PTSD and trauma research is the assumption of a characteristic time course of cortisol secretion in response to trauma, namely elevated levels immediately after traumatization and a down-regulation with increasing time since the traumatic event (i.e. hypocortisolism; Steudte-Schmiedgen et al., 2016). Notably, those processes do not seem to be constrained to psychopathologies after traumatic events, but were also shown, albeit less pronounced, in individuals who had experienced traumatic events without having developed PTSD (e.g. Steudte et al., 2013). Associations of lower HCC have further been found with more severe intrusion (e.g. Steudte et al., 2013) and avoidance symptoms (e.g. Steudte-Schmiedgen et al., 2015; Wang et al., 2015).
However, as existing studies so far mostly focused on pathogenetic facets in PTSD, associations of endocrine markers with more salutogenic perspectives on factors contributing to less severe symptomatology or quicker and more stable remission remained largely neglected.
‘Although it has proven difficult to document unequivocally, coping research argues that how people deal with stress can reduce or amplify the effects of adverse life events and conditions … ’ (Skinner, Edge, Altman, & Sherwood, 2003). Coping with the traumatic experience itself, but also with trauma sequelae like PTSD symptoms plays an important role in several influential PTSD models (Foa, Hembree, & Rothbaum, 2007), most clearly in the ‘Cognitive Model of PTSD’ (Ehlers & Clark, 2000). According to this framework, strategies like, e.g. avoidance, self-medication, and safety behaviour in reaction to symptom experiencing have an adverse influence on the course of illness (Brewin & Holmes, 2003; Ehlers & Clark, 2000). Moreover, the most common and arguably most adverse way of coping with PTSD symptoms, avoidance, is reflected in the diagnostic criteria for PTSD (American Psychiatric Association, 2013), and is also the focus of therapeutic interventions, e.g. in Prolonged Exposure (Foa et al., 2007), making it both a central PTSD symptom and a factor contributing to the maintenance of symptomatology (Foa & Kozak, 1986).
While there are important data on coping with PTSD symptomatology (Clohessy & Ehlers, 1999; Dempsey, Stacy, & Moely, 2000; Hassija, Garvert, & Cloitre, 2015; Olff et al., 2005; Street, Gibson, & Holohan, 2005; Sullivan, Weiss, Price, Pugh, & Hansen, 2018), their interpretation is limited by the purely retrospective character of assessing coping strategies. Capturing detailed intra-individual fluctuations free from the impact of biases caused by retrospective recall (Schwarz, 2012;) is often impossible, limiting the ecological validity of those results. Well-known effects are, e.g. generalization bias, context, primacy or recency effects or the influence of later events on recall (Smyth & Stone, 2003). Additionally, symptoms of and coping with PTSD are considered to fluctuate over time. All this underlines the necessity to turn to novel methods in order to enrich traditional, retrospective self-report.
2. Ecological momentary assessment (EMA) and the network approach
EMA is a research tool that collects data in an ambulatory fashion in participants’ natural environments, usually via smartphones. In recent years, especially with the advance of mobile technology that allowed for the extended collection of EMA data, many interesting studies have been published proving the feasibility of the method in clinical research and giving insights into the dynamics of psychological conditions following traumatization (Gaher et al., 2014; Pfaltz, Michael, Grossman, Margraf, & Wilhelm, 2010; Possemato et al., 2012; Walz, Nauta, & Aan Het Rot, 2014). However, research has yet to make the most of the novel technologies by, on the one hand, combining innovative self-report like EMA with newly-quantifiable endocrine correlates of PTSD and, on the other, to draw on the full potential of EMA by applying innovative statistical approaches.
Prominently, network analyses have gained much popularity in psychological science by adding new approaches to classical disease models. Here, the understanding of psychopathology differs in some aspects from the traditional views (for two reviews, see Fried et al., 2017; McNally, 2016), mainly put forward by Borsboom and colleagues (e.g. Borsboom & Cramer, 2013). They postulate that psychopathological episodes do not necessarily covariate due to an underlying latent variable, but rather ‘emerge[s] from the dynamic, causal interactions among symptoms themselves’ (McNally, 2016, p. 1). Thus, an episode of a disorder is explained by activation of a number of highly interconnected symptoms. Targeting central symptoms or their links to other symptoms (e.g. by therapeutic intervention) may cause deactivation and thus lead to recovery (McNally, 2016). Comorbidities are explained by the model through the introduction of the so-called bridge symptoms (Fried et al., 2017). For instance, Robinaugh and colleagues identified a feeling that life is empty or meaningless as well as loneliness to be bridge symptoms that influence both depression and persistent complex bereavement disorder (Robinaugh, LeBlanc, Vuletich, & McNally, 2014). Although the use of network analyses in psychology is still in its early stages and needs further development (Epskamp, Borsboom, & Fried, 2017), interesting studies already lead the direction.
Examples of network models in PTSD research include cross-sectional studies following mass violence identifying ‘anger’ and ‘intrusion’ as central symptoms (Sullivan, Smith, Lewis, & Jones, 2018), or on victims of a workplace terrorist attack with ‘numbness’ as core symptom (Birkeland & Heir, 2017). Results from a clinical sample of refugees revealed ‘emotional cue reactivity’ (Spiller et al., 2017), while U.S. veteran data identified ‘negative emotions’ to be central (Armour, Fried, Deserno, Tsai, & Pietrzak, 2017). Those results impressively show the heterogeneity of PTSD, with different core aspects emerging for different populations with different traumatic experiences.
However, currently, only few network analyses for longitudinal PTSD data are available (Bryant et al., 2017; Greene, Gelkopf, Epskamp, & Fried, 2018). One model identified a central role of the ‘startle response’ as a predictor for PTSD symptoms in the following time point (Greene et al., 2018). The other found ‘intrusive memories’ to be central during acute traumatization (Bryant et al., 2017).
To the best of our knowledge, data on the role of coping strategies within a network model of PTSD including self-report and endocrine markers are lacking.
3. From eAssessment to eHealth: CoachPTBS
The mobile application CoachPTBS is an e-mental health tool for German-speaking countries that was created based on the success of the U.S. version ‘PTSD Coach’ (Hoffman et al., 2011). CoachPTBS was developed for German military service personnel and their relatives, and has been a joint project by the Universität der Bundeswehr in Munich, the Technische Universität Dresden, and the German Armed Forces Center for Military Mental Health in Berlin. CoachPTBS was released in June 2016 by the head of the German Ministry of Defense. Among information and psychoeducation, the app lists addresses for support and a few short checklists. for screening and subsequently suggesting assistance if necessary. Importantly, like PTSD Coach, the German app also features tools for self-help, mostly alluding to third-wave and mindfulness techniques (Kahl, Winter, & Schweiger, 2012). The majority of them represents re-worked and re-implemented versions of the U.S.-American counterpart and encompass exercises like audio-guided breathing, and motivational text to increase activation (e.g. suggesting activities). The user’s three highest-rated tools are also assembled in an emergency kit (Kuhn et al., 2018)
The second interesting aspect of the app for purposes of this study is the ‘logbook’ specifically implemented for the German CoachPTBS. This category represents an EMA tool in its early stages. Its main feature is a ‘momentary assessment’ which lets the user choose between six emotional states and comment on it. This feature, too, conveys the idea of the CoachPTBS: The app itself should not be considered a stand-alone intervention but rather a companion tool, by collecting data useful for therapy during the user’s everyday life.
CoachPTBS’ archetype, the PTSD Coach (Hoffman et al., 2011), has already been subject to a feasibility study (Possemato et al., 2016). It was found that clinician-supported use of the app resulted in more specialty PTSD care use after intervention (i.e. the app seemed to have motivated or taught people how to acquire further help) and also possibly resulted in greater reductions in PTSD symptoms than in a similar group using PTSD Coach only by themselves, so even self-guided use of the app lead to benefits. These encouraging findings led to the first RCT on the PTSD Coach (Kuhn et al., 2017). Participants of this study, after using PTSD Coach for 3 months, had significantly greater improvements in PTSD, as well as depression symptoms and psychosocial functioning, compared to waitlist participants; however, at posttreatment, there were no significant mean differences in outcomes between conditions. The effects were still considered a success since mobile applications – after initial development and given proper maintenance (cf. Schellong, Lorenz, & Weidner, 2019) – can reach a much broader clientele (Kuhn et al., 2017).
These strategies may, at least in part, address long waiting periods for psychotherapy. On average, people in Germany wait for 19.9 weeks on guideline-oriented psychotherapy (BPtK, 2018). E-Mental Health tools can bridge this gap, e.g. by focusing patients’ attentions to their coping strategies and assessing changes that can be subsequently addressed during therapy.
4. The proposed study
4.1. Aim
The primary goal is to examine CoachPTBS regarding its suitability and effects on individual well-being, symptomatology and coping, the secondary aims are to examine the interplay between PTSD, coping, and HCC. The current study thus combines different facets of longitudinal assessment, i.e. EMA and data collected alongside CoachPTBS, and HCC as a marker for stress-associated long-term endocrine changes in order to increase knowledge about everyday aspects of PTSD and strategies to cope with it. EMA data collection will be implemented in order to further explore the associations between patients’ inter- and intra-individual variability of symptom severity, psychoendocrine aberrance and coping strategies. The application CoachPTBS will be employed as a means to possibly improve upon patients’ coping techniques while waiting on a trauma-specific psychotherapy and additionally assist in acquisition of momentary data by means of its logbook tools. In order to explore those aims, four groups will be recruited (see Figure 1 and Table 1): PTSD participants who will use the application CoachPTBS (group A), PTSD participants who will take part in an EMA phase (group B), PTSD participants without any smartphone-based application (group C) and healthy controls (group D).
Figure 1.
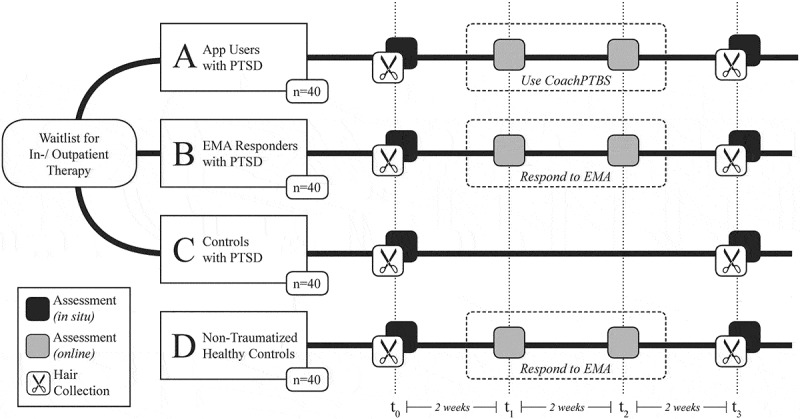
Experimental branches, conditions and time line. PTSD = posttraumatic stress disorder. EMA = ecological momentary assessment.
Table 1.
Schedule of assessments.
| Retrospective assessments (in situ and online) |
Ambulatory phase |
||
|---|---|---|---|
|
A | App Users with PTSD |
|
| B | EMA Responders with PTSD |
|
|
| C | Controls with PTSD | - | |
| D | Non-Traumatized Healthy Control |
|
|
Sociodemographic and hair-related data: Self-developed questionnaires; Trauma and PTSD severity: Posttraumatic Stress Disorder Checklist for DSM-5 (PCL-5) (Blevins, Weathers, Davis, Witte, & Domino, 2015), Posttraumatic Cognitions Inventory (PTCI) (Foa, Ehlers, Clark, Tolin, & Orsillo, 1999), Questionnaire on Dissociative Symptoms (FDS-20) (Spitzer, Mestel, Klingelhofer, Gansicke, & Freyberger, 2004) and Childhood Trauma Questionnaire (CTQ); Health and well-being: Short Form-8 Health Survey (SF-8) (Beierlein, Morfeld, Bergelt, Bullinger, & Brähler, 2012), Patient Health Questionnaire (PHQ-D) (Gräfe, Zipfel, Herzog, & Löwe, 2004); Coping strategies and efficacy: BriefCOPE (Carver, 1997), Difficulties in Emotion Regulation Scale (DERS) (Gratz & Roemer, 2004); Manual App Usage Diary: Self-developed tool; Experiencing of PTSD symptoms: Primary Care PTSD Screen for DSM-V (PC-PTSD-V) (Prins et al., 2016) Applied coping strategies and efficacy: BriefCOPE (Carver, 1997); Aversive emotions: Positive and Negative Affect Schedule (PANAS) (Watson, Clark, & Tellegen, 1988); Study feedback: Self-developed questionnaires (attrition, compliance, usability of the applications, use of therapeutic or self-help offers)
In a first step, insights on unique features of PTSD regarding self-report (symptomatology, emotion regulation, quantity and quality of coping strategies) and HCC are to be expected when compared to the non-traumatized controls (A, B and C vs. D). As a second step, the implementation of EMA leads to expect novel insights into the inter- and intra-individual variability of symptom severity and coping strategies within the PTSD participants (group B) as well as into differences regarding the efficacy and heterogeneity of coping strategies in comparison to the healthy controls (B vs. D). Additionally, the study makes it feasible to analyse possible methodological issues of EMA like reactivity or attrition effects by a comparison of parallel groups of PTSD participants with CoachPTBS, with and without EMA (A vs. B vs. C). Last, but not least, for the purposes of research into CoachPTBS as a primary goal, the study design can yield the beneficial effects of the application regarding symptomatology, quality of life (QoL) and coping strategies. This is acquired by gathering participants’ feedback after the CoachPTBS phase (group A), as well as by contrasting group A with the waiting control (A vs. C), and the EMA group (A vs. B). Notwithstanding its overall usefulness, only limited PTSD symptom change occurred through PTSD Coach (Kuhn et al., 2017). The present study thusly additionally explores QoL as a more fine-grained measurement. Additionally, the app could enhance coping strategies in a qualitative (i.e. increased diversity in coping strategies) as well as in a quantitative fashion (i.e. increased frequency of use). Since the CoachPTBS works exclusively offline, we can only simulate EMA-acquisition for group A by using digital and manual diary entries to correlate changes with usage. In addition, due to the CoachPTBS’ functionality to count and single out the three top-rated tools used we will infer how the app is individually utilized. Moreover, by employing HCC analyses the relationship between patients’ perceived benefit of CoachPTBS after 4 weeks and symptom severity at baseline, acquired from biological markers, will become comparable. Changes in stress levels achieved by improving coping skills and possibly reducing symptoms and/or increasing QoL might thus be backed up by biological markers. However, due to the expected limited effect sizes of the CoachPTBS on PTSD, the latter remains an exploratory analysis.
4.2. Recruitment and inclusion criteria
PTSD participants (n = 120) on the waiting list for an in- or outpatient treatment (trauma-specific outpatient clinic, University Hospital in Dresden) will be recruited. Eligible persons will be informed during their treatment-related admission interview by a psychotherapist or via flyers and posters (within the in-/outpatient clinic) about the possibility to participate in the study to bridge waiting time until the start of psychotherapy. Participants are eligible if they fulfil the A criterion (exposure to a traumatic stressor) according to DSM-5 (American Psychiatric Association, 2013). The PCL-5 is obligatory for every participant as well (see methods), so inclusion criteria for PTSD (criteria B through E) can be further determined. Exclusion criteria will be insufficient proficiency in German and, because of HCC analyses, hair length shorter than 1 cm, signs of hair loss or baldness, current use of glucocorticoid-containing medication, pregnancy or breast-feeding in women, current shift work or jet lag or smoking more than 15 cigarettes per day. Furthermore, participants should not have any lifetime history of schizophrenia or addictive disorders (self-report). Additionally, age- and gender-matched non-traumatized healthy participants (n = 40) will be recruited via flyers and posters.
These sample sizes of 40 participants per group were considered apt for several reasons. First, because of their intense longitudinal design with many data points generated by each individual, EMA studies bear high statistical power also if the sample sizes are small to medium (Bolger & Laurenceau, 2013). Furthermore, n = 40 should be sufficient to depict significant differences between PTSD participants and non-traumatized controls regarding HCC at an α-level of .05 and a statistical power of .95, as existing data yield effect sizes of d = .87 (Steudte et al., 2013). Additionally, for EMA studies, dropout rates are rather low (e.g. Moskowitz & Young, 2006); with PTSD-focused studies reporting dropout rates between 0% and 8.3% (e.g. Dewey et al., 2015; Naragon-Gainey, Simpson, Moore, Varra, & Kaysen, 2012). Finally, many of the study’s central research questions are based on an exploratory approach expected to yield interesting results also in medium-sized samples. In case of dropout, recruitment will continue to reach the planned total sample size.
4.3. Procedure
The study will be conducted in accordance with the latest version of the declaration of Helsinki and has been approved by the local Ethics Committee of the Medical Faculty of the Technische Universität Dresden (EK335082017, EK282062019). After receiving information on the study and giving their informed consent, PTSD participants will be randomly assigned to one out of three PTSD study groups, with the fourth group consisting of age- and gender-matched non-traumatized participants (see Figure 1). After an in-situ assessment (t0), where self-report data and hair samples will be obtained, participants will follow different further procedures during the ambulatory phase of 4 weeks according to their respective group (see below). All participants, however, will participate in two online assessments regarding symptom severity and coping after 2 weeks (t1) and after 4 weeks (t2), respectively. The final assessment (t3) is similar for all groups: After 6 weeks, an interview and another collection of hair samples will be carried out and participants will be reimbursed depending on their compliance.
The ambulatory phase will proceed as follows: Assisted by the study personnel, group A will receive CoachPTBS for their mobile phone. Throughout 4 weeks of using the application on a daily basis and marking their progress in a diary, they will teach themselves about PTSD and coping strategies. Group B will receive the EMA application movisensXS (movisens GmbH, Karlsruhe, Germany) for their mobile phones, which will be used to complete one questionnaire regarding PTSD symptoms and individual coping strategies each evening on workdays, and additional four assessments on two random days each week. Findings will be compared with the waitlist-control group C, who will neither receive CoachPTBS nor EMA during the ambulatory phase, and also with group D, consisting of healthy non-traumatized controls. Group D will also take part in an EMA assessment regarding coping strategies when confronted with aversive emotions using movisensXS. This is to compare the quality or quantity of coping strategies employed by a healthy versus clinical group.
The study procedure is illustrated in Figure 1.
4.4. Clinical and HCC assessments
For the assessment of HCC, hair samples with a diameter of ~3 mm will be taken (at t0 and t3) as close as possible to the scalp from a posterior vertex position (Stalder & Kirschbaum, 2012), the area with the proposedly most uniform hair growth rate (Pragst & Balikova, 2006). HCC will be analysed in the 1 cm segment most proximal to the scalp. Based on an average hair growth rate of 1 cm per month (Wennig, 2000), this segment is considered to reflect cortisol secretion of the previous month prior to hair sampling. The laboratory analyses will be conducted following the published liquid chromatography-tandem mass spectrometry (LC-MS/MS) protocol (Gao et al., 2013). This method has been shown to achieve excellent sensitivity, specificity and reliability (intra- and inter-assay CVs between 3.7% and 8.8%) (Gao et al., 2013).
Psychological variables like PTSD symptomatology, coping styles and efficacy and subjective well-being will be assessed via three different modes: traditional paper-pencil-based self-report during the in-situ assessments (t0, t3; all groups), retrospective online-based assessments (t1, t2; all groups) and EMA-based self-report during the ambulatory phase (groups B and D). Additionally, for group A, every participant will manually mark their app usage (time spent total and per each of the five main chapters) in a diary. The app itself also collects the number of uses per tool and the three most liked tools. The following table illustrates the data collection plan.
4.5. Data analyses
Statistical analyses will be conducted partly in an inferential statistical and partly in an exploratory way using SPSS 25.0 for Windows (IBM Corporation, NY, USA). Differences between the study groups regarding baseline symptomatology, coping efficacy and HCC will be examined via t-tests or ANOVAs, respectively.
Analyses regarding the EMA part of the study will be mostly conducted using Multilevel Modelling (Bolger & Laurenceau, 2013) because of the hierarchical structure of the data (level 1 = measurement time, level 2 = measurement day, level 3 = participant) and the aptness for dealing with varying time intervals between assessments and missing data. For depicting systematic (i.e. changing with time) or unsystematic (i.e. symptom variability) inter- and intraindividual variations in symptom severity and coping strategies, intraclass correlation coefficients (ICCs) will be used, while associations of symptomatology, coping efficacy and HCC will be analysed using multilevel correlation and regression analyses.
In an exploratory fashion, data acquired through EMA tools (see Table 1) will be used to create a network model as proposed by Borsboom and Cramer (2013). At its core, analyses will be bivariate partial correlations, suited for dealing with missing values, between symptoms, but also coping strategies, assessed in groups B and D.
4.6. Discussion
To our knowledge, this is the first study to longitudinally assess the interplay between symptomatology, coping strategies, endocrine markers and a self-help tool with the aim of gaining valuable insights in pathogenetic, but also coping strategies regarding PTSD.
Findings may lead to a more exact description regarding the interplay of coping mechanisms and symptomology, whether or not, or under which circumstances (e.g. magnitude of symptomatology or endocrine aberrances), coping skills can be successfully improved through the usage of eHealth and in which parameters this will be reflected (HCC, QoL assessment, symptom changes). Furthermore, it might spark further studies addressing the effect of traumatization versus PTSD, or the exact mechanisms of the proposed bi-directionality between coping and PTSD in a multi-method fashion.
In conclusion, the big strength of the presented study is the multimodal approach, combining traditional, online and EMA self-report measures with HCC as a biomarker of traumatic stress and the mobile application CoachPTBS as a possible novel mHealth tool.
Acknowledgments
We acknowledge support by the Open Access Publication Funds of the SLUB/TU Dresden. Lena Schindler was supported by the German Academic Scholarship Foundation.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- American Psychiatric Association (2013). Diagnostic and statistical manual of mental disorders: DSM-5 (5th ed.). Washington, DC: American Psychiatric Publishing. [Google Scholar]
- Armour C., Fried E. I., Deserno M. K., Tsai J., & Pietrzak R. H. (2017). A network analysis of DSM-5 posttraumatic stress disorder symptoms and correlates in US military veterans. Journal of Anxiety Disorders, 45, 49–9. [DOI] [PubMed] [Google Scholar]
- Beierlein V., Morfeld M., Bergelt C., Bullinger M., & Brähler E. (2012). Messung der gesundheitsbezogenen Lebensqualität mit dem SF-8. Diagnostica, 58(3), 145–153. doi: 10.1026/0012-1924/a000068 [DOI] [Google Scholar]
- Benjet C., Bromet E., Karam E., Kessler R., McLaughlin K., Ruscio A., … Hill E. (2016). The epidemiology of traumatic event exposure worldwide: Results from the World Mental Health Survey Consortium. Psychological Medicine, 46(2), 327–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkeland M. S., & Heir T. (2017). Making connections: Exploring the centrality of posttraumatic stress symptoms and covariates after a terrorist attack. European Journal of Psychotraumatology, 8(sup3), 1333387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blevins C. A., Weathers F. W., Davis M. T., Witte T. K., & Domino J. L. (2015). The posttraumatic stress disorder checklist for DSM-5 (PCL-5): Development and initial psychometric evaluation. Journal of Traumatic Stress, 28(6), 489–498. [DOI] [PubMed] [Google Scholar]
- Bolger N., & Laurenceau J. (2013). Intensive longitudinal methods. New York, NY: Guilford. [Google Scholar]
- Borsboom D., & Cramer A. O. J. (2013). Network analysis: An integrative approach to the structure of psychopathology. Annual Review of Clinical Psychology, 9, 91–121. [DOI] [PubMed] [Google Scholar]
- Bradley R., Greene J., Russ E., Dutra L., & Westen D. (2005). A multidimensional meta-analysis of psychotherapy for PTSD. The American Journal of Psychiatry, 162(2), 214–227. [DOI] [PubMed] [Google Scholar]
- Breslau N. (2009). The epidemiology of trauma, PTSD, and other posttrauma disorders. Trauma, Violence, & Abuse, 10(3), 198–210. [DOI] [PubMed] [Google Scholar]
- Brewin C. R., & Holmes E. A. (2003). Psychological theories of posttraumatic stress disorder. Clinical Psychology Review, 23(3), 339–376. [DOI] [PubMed] [Google Scholar]
- Bryant R. A., Creamer M., O’Donnell M., Forbes D., McFarlane A. C., Silove D., & Hadzi-Pavlovic D. (2017). Acute and chronic posttraumatic stress symptoms in the emergence of posttraumatic stress disorder: A network analysis. Jama Psychiatry, 74(2), 135–142. [DOI] [PubMed] [Google Scholar]
- Bundespsychotherapeutenkammer (BPtK) (2018). BPtK-Studie: Ein Jahr nach der Reform der Psychotherapie-Richtlinie. Wartezeiten 2018. Retrieved from http://www.bptk.de/publikationen/bptk-studie.html
- Carver C. S. (1997). You want to measure coping but your protocol’s too long: Consider the brief COPE. International Journal of Behavioral Medicine, 4(1), 92–100. [DOI] [PubMed] [Google Scholar]
- Clohessy S., & Ehlers A. (1999). PTSD symptoms, response to intrusive memories and coping in ambulance service workers. British Journal of Clinical Psychology, 38(3), 251–265. [DOI] [PubMed] [Google Scholar]
- Dempsey M., Stacy O., & Moely B. (2000). “Approach” and “avoidance” coping and PTSD symptoms in innercity youth. Current Psychology, 19(1), 28–45. [Google Scholar]
- Dewey D., McDonald M. K., Brown W. J., Boyd S. J., Bunnell B. E., & Schuldberg D. (2015). The impact of ecological momentary assessment on posttraumatic stress symptom trajectory. Psychiatry Research, 230(2), 300–303. [DOI] [PubMed] [Google Scholar]
- Ehlers A., & Clark D. M. (2000). A cognitive model of posttraumatic stress disorder. Behaviour Research and Therapy, 38(4), 319–345. [DOI] [PubMed] [Google Scholar]
- Epskamp S., Borsboom D., & Fried E. I. (2017). Estimating psychological networks and their accuracy: A tutorial paper. Behavior Research Methods, 50, 195–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E., Ehlers A., Clark D. M., Tolin D. F., & Orsillo S. M. (1999). The Posttraumatic Cognitions Inventory (PTCI): Development and validation. Psychological Assessment, 11(3), 303–314. [Google Scholar]
- Foa E., Hembree E., & Rothbaum B. O. (2007). Prolonged exposure therapy for PTSD: Emotional processing of traumatic experiences therapist guide. Treatments that work. New York, NY: Oxford University Press. [Google Scholar]
- Foa E. B., & Kozak M. J. (1986). Emotional processing of fear: Exposure to corrective information. Psychological Bulletin, 99(1), 20–35. [PubMed] [Google Scholar]
- Fried E. I., van Borkulo C. D., Cramer A. O., Boschloo L., Schoevers R. A., & Borsboom D. (2017). Mental disorders as networks of problems: A review of recent insights. Social Psychiatry and Psychiatric Epidemiology, 52(1), 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaher R. M., Simons J. S., Hahn A. M., Hofman N. L., Hansen J., & Buchkoski J. (2014). An experience sampling study of PTSD and alcohol-related problems. Psychology of Addictive Behaviors, 28(4), 1013–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Stalder T., Foley P., Rauh M., Deng H., & Kirschbaum C. (2013). Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. Journal of Chromatography B, 928, 1–8. [DOI] [PubMed] [Google Scholar]
- Gräfe K., Zipfel S., Herzog W., & Löwe B. (2004). Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”. Diagnostica, 50(4), 171–181. [Google Scholar]
- Gratz K. L., & Roemer L. (2004). Multidimensional assessment of emotion regulation and dysregulation: Development, factor structure, and initial validation of the difficulties in emotion regulation scale. Journal of Psychopathology and Behavioral Assessment, 26(1), 41–54. [Google Scholar]
- Greene T., Gelkopf M., Epskamp S., & Fried E. (2018). Dynamic networks of PTSD symptoms during conflict. Psychological Medicine, 48, 2409–2417. [DOI] [PubMed] [Google Scholar]
- Hassija C. M., Garvert D. W., & Cloitre M. (2015). Brief report: symptoms of PTSD, coping strategies, and social adjustment among survivors of early life interpersonal trauma. Journal of Aggression, Maltreatment & Trauma, 24(5), 520–531. [Google Scholar]
- Hoffman J. E., Wald L. J., Kuhn E., Greene C., Ruzek J. I., & Weingardt K. (2011). PTSD coach (Mobile Application). US Department of Veterans Affairs. Retrieved August 27, 2019, from https://itunes.apple.com/de/app/ptsd-coach/id430646302?mt=8 [Google Scholar]
- Kahl K. G., Winter L., & Schweiger U. (2012). The third wave of cognitive behavioural therapies: What is new and what is effective? Current Opinion in Psychiatry, 25(6), 522–528. [DOI] [PubMed] [Google Scholar]
- Kuhn E., Kanuri N., Hoffman J. E., Garvert D. W., Ruzek J. I., & Taylor C. B. (2017). A randomized controlled trial of a smartphone app for posttraumatic stress disorder symptoms. Journal of Consulting and Clinical Psychology, 85(3), 267–273. [DOI] [PubMed] [Google Scholar]
- Kuhn E., van der Meer C., Owen J., Hoffman J. E., Cash R., Carrese P., … Iversen T. (2018). PTSD coach around the world. mHealth, 4, 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally R. J. (2016). Can network analysis transform psychopathology? Behaviour Research and Therapy, 86, 95–104. [DOI] [PubMed] [Google Scholar]
- Morina N., Wicherts J. M., Lobbrecht J., & Priebe S. (2014). Remission from post-traumatic stress disorder in adults: A systematic review and meta-analysis of long term outcome studies. Clinical Psychology Review, 34(3), 249–255. [DOI] [PubMed] [Google Scholar]
- Moskowitz D. S., & Young S. N. (2006). Ecological momentary assessment: What it is and why it is a method of the future in clinical psychopharmacology. Journal of Psychiatry and Neuroscience, 31(1), 13. [PMC free article] [PubMed] [Google Scholar]
- Najavits L. M. (2015). The problem of dropout from “gold standard” PTSD therapies. F1000prime Reports, 7, 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naragon-Gainey K., Simpson T. L., Moore S. A., Varra A. A., & Kaysen D. L. (2012). The correspondence of daily and retrospective PTSD reports among female victims of sexual assault. Psychological Assessment, 24(4), 1041–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olff M., Langeland W., & Gersons B. P. R. (2005). The psychobiology of PTSD: Coping with trauma. Psychoneuroendocrinology, 30(10), 974–982. [DOI] [PubMed] [Google Scholar]
- Olff M., & van Zuiden M. (2017). Neuroendocrine and neuroimmune markers in PTSD: Pre-, peri-and post-trauma glucocorticoid and inflammatory dysregulation. Current Opinion in Psychology, 14, 132–137. [DOI] [PubMed] [Google Scholar]
- Pfaltz M. C., Michael T., Grossman P., Margraf J., & Wilhelm F. H. (2010). Instability of physical anxiety symptoms in daily life of patients with panic disorder and patients with posttraumatic stress disorder. Journal of Anxiety Disorders, 24(7), 792–798. [DOI] [PubMed] [Google Scholar]
- Possemato K., Kaier E., Wade M., Lantinga L. J., Maisto S. A., & Ouimette P. (2012). Assessing daily fluctuations in posttraumatic stress disorder symptoms and substance use with interactive voice response technology: Protocol compliance and reactions. Psychological Services, 9(2), 185–196. [DOI] [PubMed] [Google Scholar]
- Possemato K., Kuhn E., Johnson E., Hoffman J. E., Owen J. E., Kanuri N., & Brooks E. (2016). Using PTSD coach in primary care with and without clinician support: A pilot randomized controlled trial. General Hospital Psychiatry, 38, 94–98. doi: 10.1016/j.genhosppsych.2015.09.005 [DOI] [PubMed] [Google Scholar]
- Pragst F., & Balikova M. A. (2006). State of the art in hair analysis for detection of drug and alcohol abuse. Clinica Chimica Acta, 370(1–2), 17–49. [DOI] [PubMed] [Google Scholar]
- Prins A., Bovin M. J., Smolenski D. J., Marx B. P., Kimerling R., Jenkins-Guarnieri M. A., … Tiet Q. Q. (2016). The primary care PTSD screen for DSM-5 (PC-PTSD-5): Development and evaluation within a veteran primary care sample. Journal of General Internal Medicine, 31(10), 1206–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinaugh D. J., LeBlanc N. J., Vuletich H. A., & McNally R. J. (2014). Network analysis of persistent complex bereavement disorder in conjugally bereaved adults. Journal of Abnormal Psychology, 123(3), 510–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellong J., Lorenz P., & Weidner K. (2019). Proposing a standardized, step-by-step model for creating post-traumatic stress disorder (PTSD) related mobile mental health apps in a framework based on technical and medical norms. European Journal of Psychotraumatology, 10(1), 1611090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N. (2012). Why researchers should think “real-time”: A cognitive rationale In Mehl M. R. & Conner T. S. (Eds.), Handbook of research methods for studying daily life (pp. 22–42). New York, NY: Guilford Press. [Google Scholar]
- Skinner E. A., Edge K., Altman J., & Sherwood H. (2003). Searching for the structure of coping: A review and critique of category systems for classifying ways of coping. Psychological Bulletin, 129(2), 216–269. [DOI] [PubMed] [Google Scholar]
- Smyth J. M., & Stone A. A. (2003). Ecological momentary assessment research in behavioral medicine. Journal of Happiness Studies, 4(1), 35–52. [Google Scholar]
- Spiller T. R., Schick M., Schnyder U., Bryant R. A., Nickerson A., & Morina N. (2017). Symptoms of posttraumatic stress disorder in a clinical sample of refugees: A network analysis. European Journal of Psychotraumatology, 8(sup3), 1318032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitzer C., Mestel R., Klingelhofer J., Gansicke M., & Freyberger H. J. (2004). Screening und Veranderungsmessung dissoziativer Psychopathologie: Psychometrische Charakteristika der Kurzform des Fragebogens zu dissoziativen Symptomen (FDS-20). Psychotherapie, Psychosomatik, medizinische Psychologie, 54(3–4), 165–172. [DOI] [PubMed] [Google Scholar]
- Stalder T., & Kirschbaum C. (2012). Analysis of cortisol in hair–state of the art and future directions. Brain, Behavior, and Immunity, 26(7), 1019–1029. [DOI] [PubMed] [Google Scholar]
- Steudte S., Kirschbaum C., Gao W., Alexander N., Schonfeld S., Hoyer J., & Stalder T. (2013). Hair cortisol as a biomarker of traumatization in healthy individuals and posttraumatic stress disorder patients. Biological Psychiatry, 74(9), 639–646. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S., Kirschbaum C., Alexander N., & Stalder T. (2016). An integrative model linking traumatization, cortisol dysregulation and posttraumatic stress disorder: Insight from recent hair cortisol findings. Neuroscience & Biobehavioral Reviews, 69, 124–135. [DOI] [PubMed] [Google Scholar]
- Steudte-Schmiedgen S., Kirschbaum C., Gao W., Alexander N., Schönfeld S., Hoyer J., & Stalder T. (2015). Reply to: Linking hair cortisol levels to phenotypic heterogeneity of posttraumatic stress symptoms in highly traumatized Chinese women. Biological Psychiatry, 77(4), e23–e24. [DOI] [PubMed] [Google Scholar]
- Street A. E., Gibson L. E., & Holohan D. R. (2005). Impact of childhood traumatic events, trauma-related guilt, and avoidant coping strategies on PTSD symptoms in female survivors of domestic violence. Journal of Traumatic Stress, 18(3), 245–252. [DOI] [PubMed] [Google Scholar]
- Sullivan C. P., Smith A. J., Lewis M., & Jones R. T. (2018). Network analysis of PTSD symptoms following mass violence. Psychological Trauma: Theory, Research, Practice, and Policy, 10(1), 58–66. [DOI] [PubMed] [Google Scholar]
- Sullivan T. P., Weiss N. H., Price C., Pugh N., & Hansen N. B. (2018). Strategies for coping with individual PTSD symptoms: Experiences of African American victims of intimate partner violence. Psychological Trauma: Theory, Research, Practice and Policy, 10(3), 336–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zuiden M., Savas M., Koch S. B. J., Nawijn L., Staufenbiel S. M., Frijling J. L., … Olff M. (2019). Associations among hair cortisol concentrations, posttraumatic stress disorder status, and amygdala reactivity to negative affective stimuli in female police officers. Journal of Traumatic Stress, 32(2), 238–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz L. C., Nauta M. H., & Aan Het Rot M. (2014). Experience sampling and ecological momentary assessment for studying the daily lives of patients with anxiety disorders: A systematic review. Journal of Anxiety Disorders, 28(8), 925–937. [DOI] [PubMed] [Google Scholar]
- Wang L., Cao C., Wang W., Xu H., Zhang J., Deng H., & Zhang X. (2015). Linking hair cortisol levels to phenotypic heterogeneity of posttraumatic stress symptoms in highly traumatized chinese women. Biological Psychiatry, 77(4), e21e–22. [DOI] [PubMed] [Google Scholar]
- Watson D., Clark L. A., & Tellegen A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–1070. [DOI] [PubMed] [Google Scholar]
- Wennig R. (2000). Potential problems with the interpretation of hair analysis results. Forensic Science International, 107(1–3), 5–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Bundespsychotherapeutenkammer (BPtK) (2018). BPtK-Studie: Ein Jahr nach der Reform der Psychotherapie-Richtlinie. Wartezeiten 2018. Retrieved from http://www.bptk.de/publikationen/bptk-studie.html


