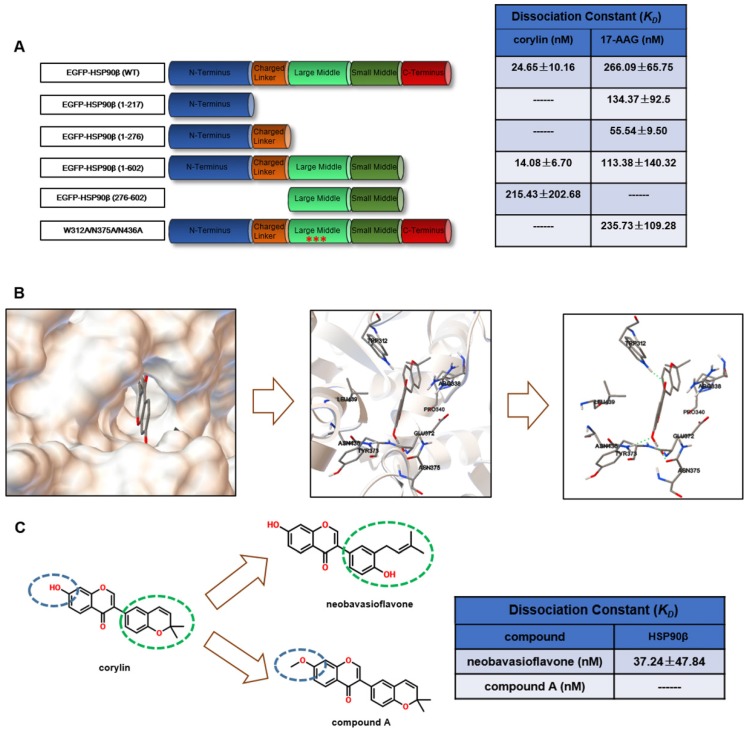
Figure 4.
Corylin Binds specifically to HSP90β. (A) Various EGFP-HSP90β truncation mutant plasmids were constructed and the recombination mutant proteins of EGFP-HSP90β were purified. The interaction between different truncated or a triple mutant (W312A, N375A, N436A) form of HSP90β and 17-AAG or corylin was detected by microscale thermophoresis (MST). (B) Molecular docking model of corylin binding to HSP90β. Molecular modeling suggested that corylin binds to a pocket within MD composed of residues 312 to 439. Hydrogen bonds are formed between corylin and W312, N375, N436 of EGFP-HSP90β. (C) Schematic diagram of key structural difference among corylin, compound A and neobavaisoflavone. The interaction between recombination proteins EGFP-HSP90β and corylin, compound A or neobavaisoflavone was detected by microscale thermophoresis (MST).