Key Points
Question
Does adding doxorubicin to sorafenib therapy improve overall survival in patients with advanced hepatocellular cancer?
Findings
This phase 3 clinical trial randomized 356 eligible patients to treatment with doxorubicin plus sorafenib vs sorafenib alone. Results demonstrated no difference in median overall survival (9.3 months for the doxorubicin plus sorafenib arm and 9.4 months for the sorafenib arm).
Meaning
The addition of doxorubicin to sorafenib therapy did not improve overall survival and resulted in higher toxicity; the combination of doxorubicin and sorafenib should not be used for the treatment of advanced hepatocellular cancer.
Abstract
Importance
Previous communication has reported significant improvement in overall survival (OS) when using doxorubicin plus sorafenib in the treatment of advanced hepatocellular cancer (HCC).
Objective
To determine if doxorubicin added to sorafenib therapy improves OS, with stratification for locally advanced and metastatic disease.
Design, Setting, and Participants
This unblinded randomized phase 3 clinical trial was led by Alliance in collaboration with Eastern Cooperative Oncology Group–American College of Radiology Imaging Network, Canadian Cancer Trials Group, and Southwest Oncology Group. It was launched in February 2010 and completed in May 2015; data were also analyzed during this time frame. Patients with histologically proven advanced HCC, no prior systemic therapy, Child-Pugh grade A score, Eastern Cooperative Oncology Group performance status of 0 to 2 (later amended to 0-1), and adequate hematologic, hepatic, renal, and cardiac function were eligible. The OS primary end point had a final analysis planned with 364 events observed among 480 total patients with 90% power to detect a 37% increase in median OS.
Interventions or Exposures
Patients received either 60 mg/m2 of doxorubicin every 21 days plus 400 mg of sorafenib orally twice daily or the sorafenib alone, adjusted to half doses for patients with bilirubin levels of 1.3 to 3.0 mg/dL.
Main Outcomes and Measures
The primary end point was OS, and progression-free survival (PFS) was a secondary end point.
Results
Of 356 patients included in the study, the mean (SD) age was 62 (10.1) years, and 306 (86.0%) were men. Although it was planned to include 480 patients, the study was halted after accrual of 356 patients (180 patients treated with doxorubicin plus sorafenib and 176 with sorafenib alone) with a futility boundary crossed at a planned interim analysis. Median OS was 9.3 months (95% CI, 7.3-10.8 months) in the doxorubicin plus sorafenib arm and 9.4 months (95% CI, 7.3-12.9 months) in the sorafenib alone arm (hazard ratio, 1.05; 95% CI, 0.83-1.31). The median PFS was 4.0 months (95% CI, 3.4-4.9 months) in the doxorubicin plus sorafenib arm and 3.7 months (95% CI, 2.9-4.5 months) in the sorafenib alone arm (hazard ratio, 0.93; 95% CI, 0.75-1.16). Grade 3 or 4 neutropenia and thrombocytopenia adverse events occurred in 61 (36.8%) and 29 (17.5%) patients, respectively, being treated with doxorubicin plus sorafenib vs 1 (0.6%) and 4 (2.4%) patients treated with sorafenib.
Conclusions and Relevance
This multigroup study of the addition of doxorubicin to sorafenib therapy did not show improvement of OS or PFS in patients with HCC.
Trial Registration
ClinicalTrials.gov identifier: NCT01015833
This phase 3 randomized clinical trial follows treatment of advanced hepatocellular cancer with doxorubicin plus sorafenib to determine if it improves overall survival in patients vs treatment with sorafenib alone.
Introduction
The tyrosine kinase inhibitor sorafenib was shown in 2 randomized phase 3 clinical trials to lead to superior overall survival (OS) compared with placebo in the treatment of patients with advanced hepatocellular carcinoma (HCC).1,2 Doxorubicin has also been considered an effective systemic therapy for HCC.3 A phase 1 clinical trial proved the feasibility and tolerability of sorafenib in combination with doxorubicin.4 A randomized, double-blind, phase 2 study of doxorubicin plus sorafenib and doxorubicin plus placebo followed and demonstrated a significant improvement in OS with doxorubicin plus sorafenib.5 Cancer and Leukemia Group B (CALGB), now part of the Alliance for Clinical Trials in Oncology, designed CALGB 80802 to determine if doxorubicin plus sorafenib improved survival compared with the single agent sorafenib in the treatment of advanced HCC.
Methods
This Alliance-led National Clinical Trials Network multigroup trial was approved by the institutional review boards at all sites and/or the National Cancer Institute (NCI) central institutional review board and was registered at ClinicalTrials.gov (NCT01015833). The study was conducted in accordance with the US Department of Health and Human Services guidelines. Written informed consent was obtained from all patients. The Alliance Data and Safety Monitoring Board (DSMB) reviewed this trial semiannually for toxic effects and scheduled interim efficacy analyses. The trial protocol is available in Supplement 1.
Patients’ Eligibility
Patients with measurable, histologically proven, locally advanced (with disease not amenable to curative interventions) or metastatic HCC who had received no prior systemic therapy for HCC were eligible. Patients with known central nervous system tumors, including brain metastases, were ineligible. In view of the differing causes and backgrounds of HCC, ethnicity was reported by participants. Patients were required to have an Eastern Cooperative Oncology Group performance status of 0 to 2 (later amended to 0-1), Child-Pugh grade A score status, an absolute neutrophil count of 1500/mm3 or greater, a platelet count of 75 × 109/L or greater, a hemoglobin level of 8.5 g/dL or greater, and a prothrombin time–international normalized ratio of 1.7 or less. Patients who were therapeutically anticoagulated were allowed to participate provided that no prior evidence of underlying abnormality in prothrombin time–international normalized ratio existed. Adequate hepatic (bilirubin, ≤3 mg/dL; alanine transaminase and aspartate aminotransferase, ≤5 × upper limit of normal) and renal (serum creatinine, ≤1.5 × upper limit of normal or creatinine clearance, ≥60 cc/min) function were required.
Any hypertension must have been well controlled (<140/90 mm Hg). Patients with known history of congestive heart failure greater than New York Heart Association class II, cardiac arrhythmias requiring antiarrhythmic therapy other than beta blockers or digoxin, or myocardial infarction within 6 months prior to study entry were not eligible. Patients were required to have an absolute left ventricular ejection fraction (LVEF) of 45% or greater or the normal lower limit of the specific institution at which they were seen.
Patients could have had prior liver-directed treatment provided there was a measurable target lesion that had not been subjected to local therapy and/or progressed since last treatment. Such therapy must have been completed 4 or more weeks prior to study entry. Patients having undergone liver transplantation were not eligible.
Current antiviral therapy was allowed except for interferon. Patients with known HIV were not eligible.
Treatment and Dose Modifications
All patients received sorafenib and were randomly assigned on a 1:1 basis to receive doxorubicin or not using a permuted block allocation procedure.6 Randomization was stratified by extent of disease (locally advanced vs metastatic). Hepatitis status (no hepatitis, hepatitis B, hepatitis C, or hepatitis B and C) was considered as a covariate. Patients received either 60 mg/m2 of doxorubicin intravenously every 21 days (1 cycle) for a maximum total dose of 360 mg/m2, plus 400 mg of sorafenib orally twice daily or 400 mg of sorafenib orally twice daily alone. Three dose reductions were allowed for doxorubicin (45, 30, and 22.5 mg/m2) and 2 for sorafenib (400 mg daily and 400 mg every other day) for drug-related toxic effects based on the NCI Common Toxicity Criteria, version 4.0.
Patients with bilirubin levels between 1.3 and 3.0 mg/dL received either 30 mg/m2 of doxorubicin intravenously every 21 days for a maximum of 360 mg/m2 plus 400 mg of sorafenib orally once daily or 400 mg of sorafenib orally once daily.7 Only 1 dose reduction was allowed for doxorubicin (22.5 mg/m2) and sorafenib (400 mg every other day) for drug-related toxic effects.
After the maximal doxorubicin dose, patients continued treatment with single-agent sorafenib. In approved circumstances when a patient was benefitting from therapy and continued to have normal ejection fraction, treatment with doxorubicin was allowed up to a maximum total dose of 450 mg/m2, following which sorafenib could be continued as a single agent. Cardiac function was followed with multigated acquisition scans obtained at baseline before start of doxorubicin therapy and every 3 cycles, then every cycle after the cumulative dose reached 360 mg/m2. Patients were removed from protocol therapy in cases of clinical heart failure (eg, left ventricular systolic dysfunction ≥ grade 3) or LVEF value decline by a relative 20% from baseline (eg, a decline in LVEF from 55% to 44%).
Specific sorafenib dose modifications were implemented for hypertension (eTable 1 in Supplement 2). Specific sorafenib dose modifications were used for hand-foot skin reaction, palmar-plantar erythrodysesthesia (eTable 2 in Supplement 2),8 and for hepatic toxicity (eTable 3 in Supplement 2).
If dose reductions beyond the lowest dose level were required, or either agent was held for more than 3 weeks, all protocol therapy was to be discontinued.
Disease Assessments and Follow-up
Patients were evaluated at the start of every cycle. Imaging was performed every 2 cycles. Posttreatment survival and progression follow-up was conducted every 3 months for 1 year, then every 6 months until 3 years after registration. Survival follow-up was conducted through available means, including but not limited to clinic visits, phone calls, and death reports.
Statistical Methods
Efficacy analysis was based on an intent-to-treat principle with all eligible patients belonging to the treatment arm in which they were randomized. The primary outcome measure was OS, defined as the time from the date of randomization to the date of death due to any cause. Patients who were alive at the primary analysis time were censored at the time they were last known to be alive. Assuming a median OS of 10.7 months in the sorafenib alone group, 480 patients enrolled over 2 years and followed up for 15 months were required to achieve 90% power to detect a 37% increase in median OS in the sorafenib plus doxorubicin arm (ie, 10.7-14.7 months; hazard ratio [HR], 0.73), using stratified log-rank test at a 1-sided significance level of α = .05. A total of 364 deaths were expected at the time of the final analysis. Formal interim analyses for OS began when 15% of expected events were observed and subsequently occurred every 6 months. Futility interim analyses based on the OS end point were conducted using a confidence interval approach. The Lan-DeMets boundaries9 and O’Brien-Fleming10 analogue were used to test the superiority and futility hypotheses at each interim (1-sided α = .05). A preplanned early-stopping analysis based on progression-free survival (PFS) was conducted when 130 events were observed, which provided 90% power to detect a 50% increase in median PFS (ie, 4.0-6.0 months in the sorafenib alone and sorafenib plus doxorubicin arms, respectively [HR, 0.66]), at a 1-sided significance level α = .15. If PFS was not statistically superior on the sorafenib plus doxorubicin arm vs the sorafenib alone arm at α = .15, the trial would have closed to further accrual. Cardiac toxicity was monitored by a formal statistical plan among patients randomized to treatment with sorafenib plus doxorubicin beginning when 33 patients were enrolled on the combination treatment arm and subsequently every 6 months coinciding with Alliance DSMB meetings. Cardiac toxicity was defined as the development of a grade 3 or higher decrease in ejection fraction per Common Terminology Criteria for Adverse Events, version 4.0.
Secondary end points were PFS, time to progression (TTP), and response by Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1.11 The PFS was defined as time from randomization to disease progression or death due to any cause. The TTP was measured from randomization to documented disease progression, and patients who died without progression were censored at time of death. For both PFS and TTP, patients lost to follow-up were censored at the date of their most recent disease assessment (or contact).
The Kaplan-Meier method was used to estimate the distributions of time-to-event end points.12 Stratified log-rank test was used to compare time-to-event end points between treatment groups.13,14,15 A stratified Cox proportional hazards regression model was used to estimate HRs and 95% CIs.16 For all analyses regarding time-to-event end point (eg, log-rank test and Cox model), extent of disease (locally advanced vs metastatic) was included as the stratification factor for controlling confounding effect. Hepatitis status was considered as a covariate. A P < .05 level was considered statistically significant. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Center. Data quality was ensured by review of data by the Alliance Statistics and Data Center and by the study chairperson (G.K.A.) following Alliance policies. All analyses were based on the study database frozen on December 19, 2017. Analyses were performed by using SAS, version 9.4 (SAS Institute Inc.).
Correlative Studies
A series of correlative studies were planned and are being completed. These include the evaluation of tumor necrotic areas using a new volumetric method of assessing nonviable tumor as a correlate for response,17 the effect of sorafenib on hepatitis C viral titers, quasispecies in patients with virologic failure, and correlation with radiologic evaluation. Results from these studies will be reported separately.
Results
Study Population
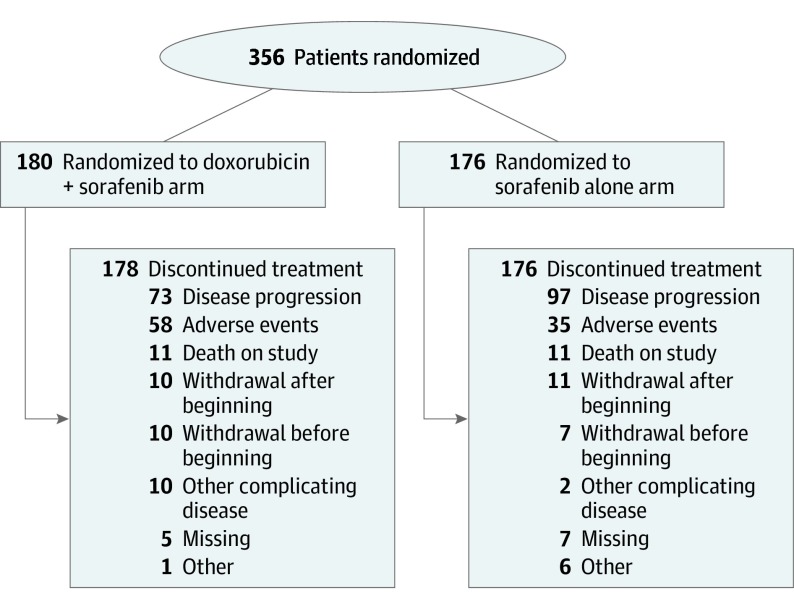
Patient enrollment began February 15, 2010, and was halted on May 21, 2015, per recommendation of the DSMB after the fifth interim analysis demonstrated a low probability that OS of the combination group would surpass that of the sorafenib alone group. A total of 356 patients were enrolled from Alliance, Eastern Cooperative Oncology Group (now part of the Eastern Cooperative Oncology Group–American College of Radiology Imaging Network), National Cancer Institute of Canada (now Canadian Cancer Trials Group), and Southwest Oncology Group sites. All results are based on an intent-to-treat population of 356 patients (Figure 1). A total of 180 patients were randomized to the doxorubicin plus sorafenib arm and 176 to the sorafenib alone arm. All patients were evaluated for the toxicity analysis. Baseline demographic and disease characteristics of randomized patients are summarized in Table 1 and were similar between the 2 study arms.
Figure 1. Cohort Flowchart.
Table 1. Patient Demographics and Disease Characteristics by Treatment Arm.
| Characteristic | No. (%) | |
|---|---|---|
| Doxorubicin Plus Sorafenib (n = 180) | Sorafenib (n = 176) | |
| Age, median (range), y | 62.0 (21.0-80.0) | 61.5 (30.0-85.0) |
| Gender | ||
| Male | 153 (85.0) | 153 (86.9) |
| Female | 27 (15.0) | 23 (13.1) |
| Race | ||
| Unknown | 3 (1.7) | 6 (3.4) |
| White | 121 (67.2) | 118 (67.0) |
| Black or African American | 24 (13.3) | 26 (14.8) |
| Asian | 27 (15.0) | 23 (13.1) |
| American Indian or Alaska Native | 2 (1.1) | 1 (0.6) |
| Not reported | 3 (1.7) | 2 (1.1) |
| ECOG performance status | ||
| 0 | 65 (36.1) | 70 (39.8) |
| 1 | 111 (61.7) | 100 (56.8) |
| 2 | 4 (2.2) | 6 (3.4) |
| Extent of disease | ||
| Locally advanced | 75 (41.7) | 75 (42.6) |
| Metastatic | 105 (58.3) | 101 (57.4) |
| Hepatitis status | ||
| Missing | 81 (45.0) | 86 (48.9) |
| None | 36 (20.0) | 37 (21.0) |
| Hepatitis B | 16 (8.9) | 17 (9.7) |
| Hepatitis C | 38 (21.1) | 32 (18.2) |
| Hepatitis B and C | 9 (5.0) | 4 (2.3) |
| Histologic grade | ||
| Missing | 3 | 5 |
| Grade cannot be assessed | 33 (18.6) | 39 (22.8) |
| Well differentiated | 41 (23.2) | 39 (22.8) |
| Moderately differentiated | 68 (38.4) | 58 (33.9) |
| Poorly differentiated | 35 (19.8) | 31 (18.1) |
| Undifferentiated | 0 | 4 (2.3) |
| Baseline AFP, median (range), ng/mL | 137.0 (0.0-514 014.0) | 134.4 (0.0-494 082.0) |
| Prior therapy | ||
| Surgery | 47 (26.6) | 51 (29.5) |
| Locoregional therapy | 33 (18.6) | 33 (19.1) |
| Adjuvant | 4 (2.3) | 1 (0.6) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; AFP, alpha-fetoprotein.
Dose and Duration of Therapy
In the doxorubicin plus sorafenib arm, the median total dose of doxorubicin administered was 237.5 mg (range, 0-1036 mg) given over a median of 3 cycles (range, 1-22 cycles), and the median daily dose of sorafenib was 433 mg (range, 19-895 mg). In the sorafenib arm, the mean daily dose was 495 mg (range, 38-994 mg). The median duration of treatment was 8.9 weeks (range, 0.6-71.3 weeks) in the doxorubicin plus sorafenib arm and 11.7 weeks (range, 0.6-223.4 weeks) in the sorafenib arm.
Overall Survival
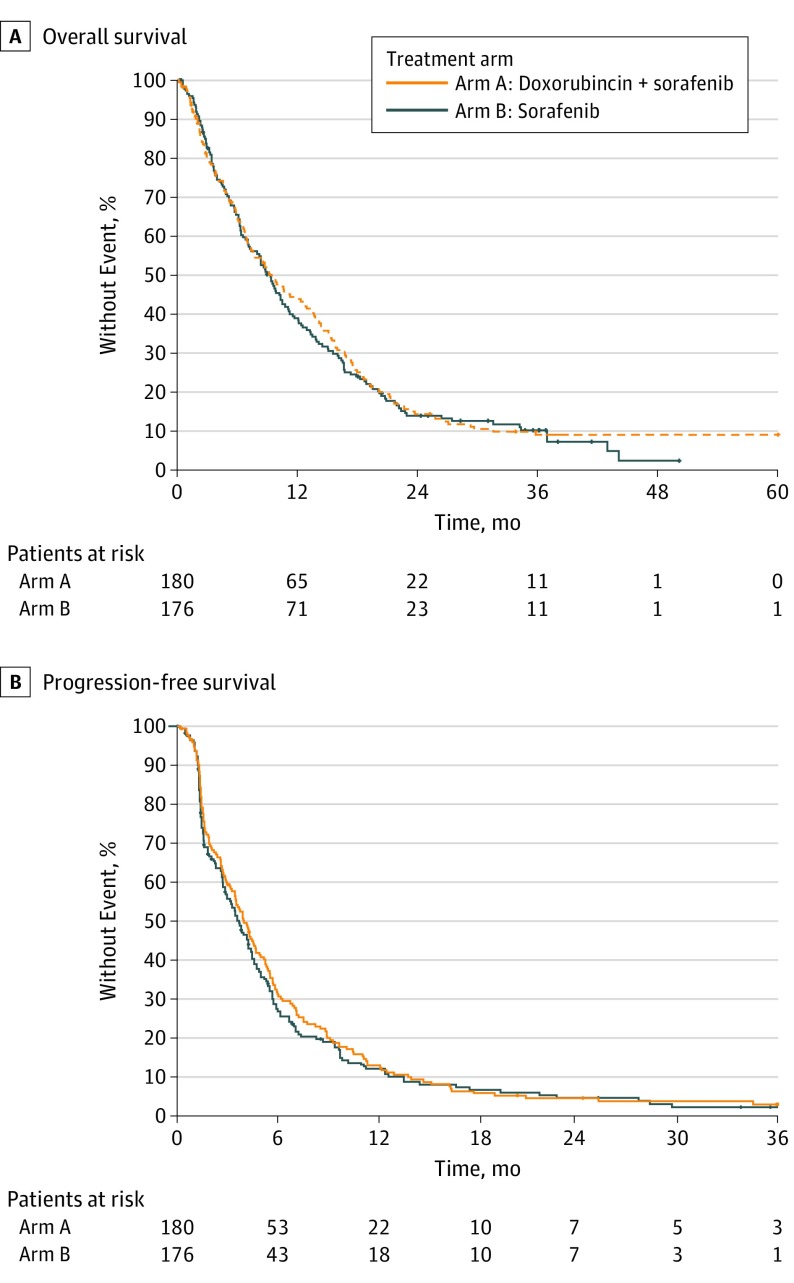
A total of 302 deaths (154 in the doxorubicin plus sorafenib arm and 148 in the sorafenib alone arm) were observed after a median follow-up of 36.1 months. The median OS was 9.3 months (95% CI, 7.3-10.8 months) in patients treated with doxorubicin plus sorafenib compared with 9.4 months (95% CI, 7.3-12.9 months) among those who received sorafenib alone. The stratified HRs were 1.05 (95% CI, 0.83-1.31; P = .68) and 1.03 (95% CI, 0.82-1.29; P = .83) without and with adjustment by hepatitis status, respectively (Figure 2A).
Figure 2. Overall and Progression-Free Survival.
A, The median overall survival was 9.3 months in patients treated with doxorubicin plus sorafenib compared with 9.4 months among those treated with sorafenib alone. B, The median progression-free survival was 4.0 months among patients treated with doxorubicin plus sorafenib compared with 3.7 months among those treated with sorafenib alone.
Progression-Free Survival
The preplanned PFS interim analysis was completed per protocol in July 2013 after the first 170 patients were enrolled and 130 PFS events were observed. At that time, median PFS for patients randomized to the doxorubicin plus sorafenib arm was 4.5 months (95% CI, 2.6-6.0 months) and median PFS for patients randomized to the sorafenib alone arm was 3.5 months (95% CI, 2.6-5.0 months). The 1-sided log-rank P value was .12, which is significant at α = .15 level. This satisfied the preplanned threshold for activity of the doxorubicin plus sorafenib arm, and the study continued accrual. With updated database information available as of December 2017, the median PFS was 4.0 months (95% CI, 3.4-4.9 months) among patients treated with doxorubicin plus sorafenib compared with 3.7 months (95% CI, 2.9-4.5 months) among those who received sorafenib alone. The HR estimated by Cox model was 0.93 (95% CI, 0.75-1.16) with stratified log-rank test (P = .54) (Figure 2B).
Time to Progression
The Kaplan-Meier estimate of the median TTP was 4.7 months (95% CI, 4.1-5.6 months) in patients treated with doxorubicin plus sorafenib compared with 4.2 months (95% CI, 3.4-5.4 months) for those who received sorafenib alone. The HR estimated by Cox model was 0.92 (95% CI, 0.71-1.18) with log-rank test (P = .49).
Response Rate
There was 1 complete response in the doxorubicin plus sorafenib arm. Partial response was noted in 14 (9.3%) patients in the doxorubicin plus sorafenib arm, and 8 (5.4%) in the sorafenib alone arm (χ24, 3.21; P = .52). Table 2 shows the detailed RECIST response data. There was no significant difference in tumor response between the doxorubicin plus sorafenib and sorafenib alone arms.
Table 2. Observed Treatment Responses (RECIST 1.1) by Regimen.
| Characteristic | No. (%) | |
|---|---|---|
| Doxorubicin Plus Sorafenib (n = 180) | Sorafenib (n = 176) | |
| Missing, No. | 30 | 28 |
| Complete response | 1 (0.7) | 0 |
| Partial response | 14 (9.3) | 8 (5.4) |
| Stable disease | 85 (56.7) | 89 (60.1) |
| Progressive disease | 44 (29.3) | 47 (31.8) |
| Not evaluable | 6 (4.0) | 4 (2.7) |
Abbreviation: RECIST, Response Evaluation Criteria in Solid Tumors, version 1.1.
Toxic Events
Grade 3 or 4 neutropenia and thrombocytopenia occurred in 61 (36.8%) and 29 (17.5%) patients, respectively, being treated with doxorubicin plus sorafenib vs 1 (0.6%) and 4 (2.4%) patients, respectively, taking sorafenib; nonhematologic adverse events were comparable (138 [83.1%] and 118 [76.9%] patients, respectively). Observed nonhematologic grade 3 and 4 toxicities that occurred with an incidence of more than 4% in at least one arm included fatigue (21 [12.6%] patients in the doxorubicin plus sorafenib arm, and 17 [10.1%] in the sorafenib only arm), hypertension (8 [4.8%] and 23 [13.6%] patients), hand-foot skin reaction (22 [13.3%] and 24 [14.2%] patients), nausea (11 [6.6%] and 12 [7.1%] patients), mucositis (15 [9.0%] and 4 [2.4%] patients), abdominal pain (8 [4.8%] and 14 [8.3%] patients), and diarrhea (12 [7.2%] and 12 [7.1%] patients) (eTable 4 in Supplement 2).
Grade 3 and 4 cardiac toxic events were limited to patients in the doxorubicin plus sorafenib arm. Left ventricular systolic dysfunction and decreased ejection fraction rates were noted in 5 (3.0%) and 8 (4.8%) patients taking doxorubicin plus sorafenib, respectively.
Of 46 deaths (22 in the doxorubicin plus sorafenib arm and 24 in the sorafenib arm), 7 in the doxorubicin plus sorafenib arm, and 3 in the sorafenib arm, were possibly related to treatment.
Discussion
This phase 3 randomized clinical trial evaluating the addition of doxorubicin to sorafenib in the treatment of advanced HCC is a landmark study. It represents, to our knowledge, the first US national effort of a phase 3 clinical trial for advanced HCC that was led by the Alliance National Clinical Trials Network group collaborating successfully with Eastern Cooperative Oncology Group–American College of Radiology Imaging Network, Canadian Cancer Trials Group, and Southwest Oncology Group. This is also to our knowledge the first published product of collaborative work of the NCI Hepatobiliary Task Force that was established around the same time with oversight from the NCI Gastrointestinal Cancer Steering Committee.
This study demonstrates that the addition of doxorubicin to sorafenib treatment does not improve OS and also strongly suggests that doxorubicin does not have a role as a systemic therapy for patients with advanced HCC.18 This also substantiates a previous finding that the addition of doxorubicin to therapy does not improve outcomes when combined with locoregional intrahepatic embolization.19
The experimental arm was designed following the results from a randomized phase 2 trial3 that combined doxorubicin and sorafenib based on laboratory evidence of the deactivation of the multidrug resistance pathway by the Ras/Raf/MEK/ERK pathway20 and bFGF-mediated activation of Raf-1 promoting the formation of antiapoptotic Raf-1 and ASK1 complex, induced by anthracyclines.21,22 The OS for those on sorafenib monotherapy was in the expected range (9.4 months), but the patients receiving the doxorubicin and sorafenib combination had a survival of just 9.3 months; that contrasts with the OS of 13.7 months in the cohort of patients treated with doxorubicin and sorafenib in the preceding randomized phase 2 trial.5
Although this study, like almost every other contemporary study in HCC, was restricted to patients who could have no worse than Child-Pugh grade A cirrhosis, HCC is a very heterogeneous disease, and similar patient populations can have very different outcomes (eg, the SHARP North American/European and the Asia-Pacific sorafenib phase III trials).1,2
After 130 (of the planned 480) patients experienced disease progression, a statistically significant difference in PFS favoring the doxorubicin plus sorafenib arm was mandatory for the study to continue accrual. The study met that metric, yet with continued accrual, OS did not prove to be any better when accrual was complete.
The doxorubicin plus sorafenib combination can be added to a long list of phase 2 treatments in any cancer that were not sustained in phase 3 trials. Beyond the possible confounders listed above, it appears that the toxicity of doxorubicin was a major factor despite the observed adverse events of both doxorubicin and sorafenib as previously noted,3 with an observed and expected increase in the rates of grade 3 or greater adverse events for the doxorubicin plus sorafenib arm compared with the sorafenib alone arm. The increased rate of doxorubicin-associated cardiac toxic events in this study was not unexpected, and it is unclear whether it was attributable to a sorafenib-induced increase in doxorubicin area under the curve or to other factors.23
Limitations
Regardless of the outcome, this study has certain limitations. Raf inhibition of sorafenib may play a limited role compared with other attributes of the drug that would not necessarily support the combination. It is also a reminder of the criticality of phase 3 trials in the setting of promising phase 2 data.5 Despite the detailed descriptors of the study population with Child-Pugh scores, further details delineating portal vein thrombosis among others would have been helpful. The relatively low median daily dose of sorafenib and starting dose based on bilirubin level may also explain the poor outcome of the study.
Conclusions
Despite the negative outcomes of this study, the data obtained from patients enrolled in the sorafenib control arm are of key importance. They will help provide insights into a series of correlative research questions that are being studied that will provide data pertinent to patients with HCC. Ongoing planned analyses include a prospective evaluation of radiographic tumor necrosis for association with treatment response building on prior studies,17,24 studies of hepatitis C viral load and quasispecies changes on therapy, and baseline platelets level and correlation with biology and outcome,18 as described in the methods section.
The study is timely considering the continued rising incidence of HCC in the United States25 and the critical need for better treatments for patients with advanced HCC.
Trial Protocol
eTable 1. Sorafenib dose modifications for hypertension
eTable 2. Sorafenib dose modifications for hand-foot skin reaction HFSR, palmar-plantar erythrodysesthesia
eTable 3. Dose modifications for hepatic toxicity
eTable 4. Observed adverse events regardless of attribution by grade and treatment
arm
Data Sharing Statement
References
- 1.Llovet JM, Ricci S, Mazzaferro V, et al. ; SHARP Investigators Study Group . Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390. doi: 10.1056/NEJMoa0708857 [DOI] [PubMed] [Google Scholar]
- 2.Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25-34. doi: 10.1016/S1470-2045(08)70285-7 [DOI] [PubMed] [Google Scholar]
- 3.Yeo W, Mok TS, Zee B, et al. A randomized phase III study of doxorubicin versus cisplatin/interferon alpha-2b/doxorubicin/fluorouracil (PIAF) combination chemotherapy for unresectable hepatocellular carcinoma. J Natl Cancer Inst. 2005;97(20):1532-1538. doi: 10.1093/jnci/dji315 [DOI] [PubMed] [Google Scholar]
- 4.Richly H, Henning BF, Kupsch P, et al. Results of a phase I trial of sorafenib (BAY 43-9006) in combination with doxorubicin in patients with refractory solid tumors. Ann Oncol. 2006;17(5):866-873. doi: 10.1093/annonc/mdl017 [DOI] [PubMed] [Google Scholar]
- 5.Abou-Alfa GK, Johnson P, Knox JJ, et al. Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA. 2010;304(19):2154-2160. doi: 10.1001/jama.2010.1672 [DOI] [PubMed] [Google Scholar]
- 6.Zelen M. The randomization and stratification of patients to clinical trials. J Chronic Dis. 1974;27(7-8):365-375. doi: 10.1016/0021-9681(74)90015-0 [DOI] [PubMed] [Google Scholar]
- 7.Miller AA, Murry DJ, Owzar K, et al. Phase I and pharmacokinetic study of sorafenib in patients with hepatic or renal dysfunction: CALGB 60301. J Clin Oncol. 2009;27(11):1800-1805. doi: 10.1200/JCO.2008.20.0931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lacouture ME, Wu S, Robert C, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13(9):1001-1011. doi: 10.1634/theoncologist.2008-0131 [DOI] [PubMed] [Google Scholar]
- 9.Lan KKG, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70(3):659-663. doi: 10.2307/2336502 [DOI] [Google Scholar]
- 10.O’Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35(3):549-556. doi: 10.2307/2530245 [DOI] [PubMed] [Google Scholar]
- 11.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228-247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457-481. doi: 10.1080/01621459.1958.10501452 [DOI] [Google Scholar]
- 13.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50(3):163-170. [PubMed] [Google Scholar]
- 14.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc [Ser A]. 1972;135(2):185-207. doi: 10.2307/2344317 [DOI] [Google Scholar]
- 15.Harrington D. Linear rank tests in survival analysis In: Armitage P, Colton T, eds. Encyclopedia of Biostatistics. Cambridge, England: Wiley Interscience; 2005. [Google Scholar]
- 16.Cox DR. Regression models and life-tables. J R Stat Soc B. 1972;34(2):187-220. [Google Scholar]
- 17.Abou-Alfa GK, Schwartz L, Ricci S, et al. Phase II study of sorafenib in patients with advanced hepatocellular carcinoma. J Clin Oncol. 2006;24(26):4293-4300. doi: 10.1200/JCO.2005.01.3441 [DOI] [PubMed] [Google Scholar]
- 18.Olweny CL, Toya T, Katongole-Mbidde E, Mugerwa J, Kyalwazi SK, Cohen H. Treatment of hepatocellular carcinoma with adriamycin: preliminary communication. Cancer. 1975;36(4):1250-1257. doi: [DOI] [PubMed] [Google Scholar]
- 19.Brown KT, Do RK, Gonen M, et al. Randomized trial of hepatic artery embolization for hepatocellular carcinoma using doxorubicin-eluting microspheres compared with embolization with microspheres alone. J Clin Oncol. 2016;34(17):2046-2053. doi: 10.1200/JCO.2015.64.0821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCubrey JA, Steelman LS, Abrams SL, et al. Roles of the RAF/MEK/ERK and PI3K/PTEN/AKT pathways in malignant transformation and drug resistance. Adv Enzyme Regul. 2006;46:249-279. doi: 10.1016/j.advenzreg.2006.01.004 [DOI] [PubMed] [Google Scholar]
- 21.Alavi AS, Acevedo L, Min W, Cheresh DA. Chemoresistance of endothelial cells induced by basic fibroblast growth factor depends on Raf-1-mediated inhibition of the proapoptotic kinase, ASK1. Cancer Res. 2007;67(6):2766-2772. doi: 10.1158/0008-5472.CAN-06-3648 [DOI] [PubMed] [Google Scholar]
- 22.Song S, Wientjes MG, Gan Y, Au JL. Fibroblast growth factors: an epigenetic mechanism of broad spectrum resistance to anticancer drugs. Proc Natl Acad Sci USA. 2000;97(15):8658-8663. doi: 10.1073/pnas.140210697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schneider C, Wallner M, Kolesnik E, et al. The anti-cancer multikinase inhibitor sorafenib impairs cardiac contractility by reducing phospholamban phosphorylation and sarcoplasmic calcium transients. Sci Rep. 2018;8(1):5295. doi: 10.1038/s41598-018-23630-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abou-Alfa GK, Zhao B, Capanu M, et al. Tumor necrosis as a correlate for response in subgroup of patients with advanced hepatocellular carcinoma (HCC) treated with sorafenib [ESMO abstract 547P]. Ann Oncol. 2008:19(suppl 8):viii178. [Google Scholar]
- 25.Petrick JL, Kelly SP, Altekruse SF, McGlynn KA, Rosenberg PS. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34(15):1787-1794. doi: 10.1200/JCO.2015.64.7412 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eTable 1. Sorafenib dose modifications for hypertension
eTable 2. Sorafenib dose modifications for hand-foot skin reaction HFSR, palmar-plantar erythrodysesthesia
eTable 3. Dose modifications for hepatic toxicity
eTable 4. Observed adverse events regardless of attribution by grade and treatment
arm
Data Sharing Statement