Key Points
Question
Given the increased frequency of cavum septum pellucidum and cavum vergae in fighters who experience repetitive head trauma, is the presence of these imaging findings associated with lower cognitive and mood testing scores and reduced volumes of various brain structures?
Findings
This population-based cohort study demonstrated that fighters with cavum vergae or increased cavum septum pellucidum and cavum vergae length had decreased volumes in various brain structures and lower cognitive scores vs fighters without these imaging findings.
Meaning
The presence of cavum septum pellucidum and cavum vergae was associated with lower regional brain volumes and cognitive performance in a cohort exposed to repetitive head trauma.
Abstract
Importance
Many studies have investigated the imaging findings showing sequelae of repetitive head trauma, with mixed results.
Objective
To determine whether fighters (boxers and mixed martial arts fighters) with cavum septum pellucidum (CSP) and cavum vergae (CV) have reduced volumes in various brain structures or worse clinical outcomes on cognitive and mood testing.
Design, Setting, and Participants
This cohort study assessed participants from the Professional Fighters Brain Health Study. Data were collected from April 14, 2011, to January 17, 2018, and were analyzed from September 1, 2018, to May 23, 2019. This study involved a referred sample of 476 active and retired professional fighters. Eligible participants were at least 18 years of age and had at least a fourth-grade reading level. Healthy age-matched controls with no history of trauma were also enrolled.
Exposures
Presence of CSP, CV, and their total (additive) length (CSPV length).
Main Outcomes and Measures
Information regarding depression, impulsivity, and sleepiness among study participants was obtained using the Patient Health Questionnaire depression scale, Barrett Impulsiveness Scale, and the Epworth Sleepiness Scale. Cognition was assessed using raw scores from CNS Vital Signs. Volumes of various brain structures were measured via magnetic resonance imaging.
Results
A total of 476 fighters (440 men, 36 women; mean [SD] age, 30.0 [8.2] years [range, 18-72 years]) and 63 control participants (57 men, 6 women; mean [SD] age, 30.8 [9.6] years [range, 18-58 years]) were enrolled in the study. Compared with fighters without CV, fighters with CV had significantly lower mean psychomotor speed (estimated difference, –11.3; 95% CI, –17.4 to –5.2; P = .004) and lower mean volumes in the supratentorium (estimated difference, –31 191 mm3; 95% CI, –61 903 to –479 mm3; P = .05) and other structures. Longer CSPV length was associated with lower processing speed (slope, –0.39; 95% CI, –0.49 to –0.28; P < .001), psychomotor speed (slope, –0.43; 95% CI, –0.53 to –0.32; P < .001), and lower brain volumes in the supratentorium (slope, –1072 mm3 for every 1-mm increase in CSPV length; 95% CI, –1655 to –489 mm3; P < .001) and other structures.
Conclusions and Relevance
This study suggests that the presence of CSP and CV is associated with lower regional brain volumes and cognitive performance in a cohort exposed to repetitive head trauma.
This cohort study examines the association of brain structure volume with cognitive and mood test scores among boxers and mixed martial arts fighters with cavum septum pellucidum and cavum vergae.
Introduction
Repetitive head trauma, particularly in the context of contact sports such as US football, is associated with various neurodegenerative disorders.1,2,3,4,5,6 Athletes in these sports sustain repetitive head trauma over the span of their careers, with resulting cognitive and structural changes that are increasingly being studied with imaging and cognitive testing.7,8,9 A wide range of structural brain changes on magnetic resonance imaging findings have been noted in contact sports athletes with histories of repetitive head trauma. These structural brain changes include increased frequency of cerebral microhemorrhages (CMHs),10,11 nonspecific white matter changes (WMCs),12 and the presence of cavum septum pellucidum (CSP) and cavum vergae (CV).13,14,15,16 Other investigations, however, have found no substantial structural abnormalities on imaging results in this population.17,18 These variable findings are confounded by small sample sizes and limited data regarding cognitive changes and clinical outcomes. In addition, few studies have provided longitudinal data on changes in brain structure and function as repetitive head trauma accumulates over time.
The Professional Fighters Brain Health Study (PFBHS) is a longitudinal cohort study of boxers and mixed martial arts fighters that is positioned to elucidate the importance of various structural brain changes caused by cumulative sports-related head trauma and to determine the potential association between structural findings and functional alterations. Changes in various cognitive domains have been reported in professional boxers.8,19 However, few longitudinal studies have explored the association between cognitive and brain structural changes. Previous analyses of data from the PFBHS have demonstrated an association between head trauma and imaging findings of smaller deep gray structures, specifically the caudate and amygdala, as well as decreased function, specifically involving processing speed.9 Increased motor impulsivity has also been reported along with decreased volumes of deep gray structures such as the caudate and thalamus in a subset of this cohort.20
Another recent study examined the frequency of CSP, CV, CMHs, and WMCs among professional fighters vs controls and observed a significantly increased frequency of CSP and CV in fighters21; these findings are similar to those reported in studies of US football players with chronic traumatic encephalopathy.14,17 However, as CSP and CV are also often described as normal variants in imaging studies, the clinical significance of this increased incidence of CSP and CV among fighters is poorly understood. In the present study, we sought to examine associations between CSP and CV, various intracranial volume measurements, and cognitive and mood outcomes.
Methods
Professional Fighters Brain Health Study
The PFBHS is an ongoing observational cohort study of active and retired professional fighters and healthy age-matched controls. Data for this study were collected from April 14, 2011, to January 17, 2018. This study was approved by the Cleveland Clinic Institutional Review Board, and written informed consent was obtained from all study participants. See eAppendix 1 in the Supplement for further details regarding inclusion criteria for fighters and controls.
Cognitive and Mood Assessments
Information regarding depression, impulsivity, and sleepiness among study participants was obtained using the Patient Health Questionnaire depression scale, Barrett Impulsiveness Scale, and the Epworth Sleepiness Scale. Cognition was assessed using raw scores from CNS Vital Signs,22 a computerized test providing robust and reliable measurements of cognition in the clinical realms of verbal memory, processing speed, psychomotor speed, and reaction time. See eAppendix 2 in the Supplement for further details regarding these cognitive and mood assessments.
Imaging
As part of the PFBHS, all fighters and control participants underwent brain imaging on a Verio 3-T magnetic resonance imaging scanner (Siemens). Volumetric segmentation was performed on the MPRAGE image data set using FreeSurfer, version 6.0 (http://surfer.nmr.mgh.harvard.edu/).23,24,25,26,27,28,29,30,31,32,33,34 For the current analysis, 4 of us (neuroradiology experience: J.W., 1 year; J.K.L., 3 years; S.E.J., 12 years; P.R., 26 years) measured the combined length of the CSP and CV (hereafter CSPV length) and the maximum transverse width. The mean measurements from all readers were used for the analysis (eFigure 1 in the Supplement). The readers were blinded to whether the images belonged to a fighter or a control participant. Longitudinal follow-up evaluation was performed by the same 4 neuroradiologists, who were not blinded to the time order of the scans. eFigure 2 in the Supplement shows an example of a CSP and CV. eAppendix 3 in the Supplement provides further details regarding the scan parameters and previous and current analysis techniques.
Statistical Analysis
Statistical analysis was performed from September 1, 2018, to May 23, 2019. For each of the 13 cognitive and mood measures, a linear regression model was used to test the hypothesis that the scores would be different for all study participants with and without CSP. In each model, the score was the outcome variable, the CSP status was the variable of interest, and age, educational level, race, and ethnicity were included as covariates. The resulting 13 P values were adjusted for multiple comparisons using the Holm step-down procedure, applying a significance of .05. This entire process was repeated for the 3 remaining imaging characteristics (CV, WMCs, and CMHs). A similar approach was used to assess the association between imaging findings and the 7 brain volumes of interest (supratentorium, thalamus, corpus callosum, caudate, putamen, hippocampus, and amygdala). A subgroup analysis was repeated separately for active fighters only and retired fighters only.
The intraclass correlation coefficient (ICC) was used to characterize interreader reliability with respect to CSPV length and maximum transverse width of the CSP. Intraclass correlation coefficient estimates and their 95% CIs were calculated based on a single-rater, absolute-agreement, 2-way random effects model. The sample for these estimates consisted of measurements provided by 4 readers from 275 participants with CSP from a prior analysis. In multiple post hoc analyses, associations between CSPV length, fighting status (fighter vs control), and fighter exposure score9 were individually compared with various brain volumes and cognitive scores using a series of linear regression models.
The hypothesis that the association between brain volume and cognitive score would be different for fighters with CV than for fighters without CV was assessed by adding an interaction term (brain volume × CV status) to the model with psychomotor speed and processing speed, which were assessed separately. Follow-up data were also analyzed when available, and the Spearman correlation coefficient was calculated to quantify the association between changes in CSPV length and changes in cognitive measures and brain volumes. See eAppendix 4 in the Supplement for further details regarding these post hoc analyses.
Results
The study population consisted of 476 fighters, with a mean (SD) age of 30.0 (8.2) years (range, 18-72 years); 440 of the fighters were men (92.4%) (eTable 1 in the Supplement). The mean (SD) number of years of education among fighters was 13 (3) (range, 2-25 years). The control population consisted of 63 participants, with a mean (SD) age of 30.8 (9.6) years (range, 18-58 years; P = .41); 57 of the controls (90.5%) were men (P = .46). The mean (SD) number of years of education among control participants was 14 (3) (range, 9-20; P = .15). The cognitive and mood scores among fighters with and without various imaging findings are shown in eTables 2A and 2B in the Supplement.
Agreement among the 4 readers was good with respect to CSPV length (ICC, 0.87; 95% CI, 0.84-0.89) and maximum transverse width of the CSP (ICC, 0.70; 95% CI, 0.64-0.75). Fighters had a significantly increased frequency of CSP (odds ratio, 4.64; 95% CI, 2.36-9.83; P < .001) and CV (odds ratio, 24.8; 95% CI, 3.26-31.94; P = .001), lower volumes in the thalamus (mean difference, –650 mm3; P = .02) and corpus callosum (mean difference, –402 mm3; P < .001), and lower processing speed (mean difference, –7.54; P < .001) and psychomotor speed (mean difference, –20.2; P < .001) vs controls (Table 1). Fighters had a higher volume in the putamen (mean difference, 919 mm3; P < .001) vs controls.
Table 1. Comparison of Imaging and Cognitive Variables in Fighters and Controls.
| Variable | Fighters | Controls | Estimate (95% CI or SE)a | P Valueb | ||
|---|---|---|---|---|---|---|
| Total Participants, No. | Observed Value | Total Participants, No. | Observed Value | |||
| Presence of CSP, No. (%) | 476 | 247 (51.9) | 63 | 11 (17.5) | 4.64 (2.36-9.83) | <.001 |
| Presence of CV, No. (%) | 476 | 67 (14.1) | 63 | 0 | 24.82 (3.26-31.94) | .001 |
| CSPV length, mean (SD), mm | 251 | 16 (14) | 11 | 9 (5) | 3.83 (4.15) | >.99 |
| Transverse width of CSP, mean (SD), mm | 251 | 4 (1) | 11 | 3 (1) | 0.25 (0.38) | >.99 |
| Volume, mean (SD), mm3 | ||||||
| Supratentorial | 441 | 1 066 292 (104 903) | 62 | 1 054 332 (106 459) | 17 828 (13 782) | .79 |
| Thalamic | 441 | 13 739 (1693) | 62 | 14 451 (1465) | −650 (221) | .02 |
| Corpus callosum | 441 | 3037 (540) | 62 | 3490 (553) | −402 (76) | <.001 |
| Caudate | 441 | 7659 (1011) | 62 | 7347 (963) | 349 (142) | .09 |
| Putamen | 441 | 11 918 (1415) | 62 | 11 059 (1427) | 919 (182) | <.001 |
| Hippocampal | 441 | 7943 (805) | 62 | 8162 (732) | −163 (111) | .78 |
| Amygdala | 441 | 3440 (441) | 62 | 3531 (369) | −24 (61) | >.99 |
| Speed, mean (SD) | ||||||
| Processing | 474 | 52 (12) | 63 | 61 (16) | −7.5 (1.6) | <.001 |
| Psychomotor | 474 | 171 (25) | 63 | 191 (60) | −20.2 (4.3) | <.001 |
Abbreviations: CSP, cavum septum pellucidum; CSPV, cavum septum pellucidum and cavum vergae; CV, cavum vergae.
Estimate is the odds ratio (fighter/control) for CSP and CV, and the mean difference (fighter − control) otherwise. All estimates are from models that include age, educational level, race, and ethnicity as covariates.
Adjusted for multiple comparisons using the Holm step-down method.
Fighters with WMCs had higher cognitive complexity scores on the Barrett Impulsiveness Scale vs fighters without WMCs (estimate, 0.9; 95% CI, 0.30-1.44; P = .04). No significant associations were found between presence or absence of CSP or CMHs and the various cognitive measures (Table 2). Fighters with CV had a significantly lower mean psychomotor speed (estimated difference, –11.3; 95% CI, –17.4 to –5.2; P = .004; Table 2) and lower mean brain volumes in the supratentorium (estimated difference, –31 191 mm3; 95% CI, –61 903 to –479 mm3; P = .05) and other structures vs fighters without CV (Table 3).
Table 2. Estimated Mean Differences for Cognitive Examination Scores Among Fighters With and Without Various Imaging Findings.
| Cognitive or Behavioral Measure | Adjusted Mean Difference in Cognitive Examination Score | |||||||
|---|---|---|---|---|---|---|---|---|
| CSP Present vs Absenta,b | P Valuec | CV Present vs Absenta,b | P Valuec | WMCs Present vs Absenta,b | P Valuec | CMH Present vs Absenta,b | P Valuec | |
| PHQ9 | 0.0 | >.99 | 1.2 | .52 | 1.0 | .38 | −0.3 | >.99 |
| ESS | 0.4 | >.99 | 0.7 | >.99 | 0.0 | >.99 | −2.4 | .19 |
| Attention | −0.3 | >.99 | 0.1 | >.99 | 0.3 | >.99 | −1.1 | >.99 |
| Motor | −0.4 | >.99 | −0.4 | >.99 | 0.6 | >.99 | −0.9 | >.99 |
| Self-control | 0.2 | >.99 | 0.3 | >.99 | 1.3 | .053 | 0.4 | >.99 |
| Cognitive complexity | 0.0 | >.99 | 0.2 | >.99 | 0.9 | .04 | −0.7 | >.99 |
| Perseverance | −0.3 | >.99 | −0.1 | >.99 | 0.0 | >.99 | −0.5 | >.99 |
| Cognitive instability | −0.3 | >.99 | 0.0 | >.99 | 0.1 | >.99 | −0.3 | >.99 |
| Total impulsivity | −1.0 | >.99 | 0.1 | >.99 | 3.2 | .24 | −3.2 | >.99 |
| Verbal memory | 0.3 | >.99 | 0.7 | >.99 | −0.7 | >.99 | 0.0 | >.99 |
| Processing speed | −0.3 | >.99 | −3.6 | .34 | −0.3 | >.99 | −2.0 | >.99 |
| Psychomotor speed | −3.7 | >.99 | −11.3 | .004 | 1.8 | >.99 | −4.8 | >.99 |
| Reaction time | 7.5 | >.99 | 38.4 | .23 | 15.9 | >.99 | 32.6 | >.99 |
Abbreviations: CMH, cerebral microhemorrhage; CSP, cavum septum pellucidum; CV, cavum vergae; ESS, Epworth Sleepiness Scale; PHQ9, Patient Health Questionnaire-9; WMCs, white matter changes.
Positive values indicate higher scores when the imaging finding is present; negative values indicate higher scores when the imaging finding is absent.
Estimates adjusted for age, educational level, race, and ethnicity.
Adjusted for multiple comparisons using the Holm step-down method.
Table 3. Estimated Mean Differences for Various Brain Volumes Among Fighters With and Without CSP and CV.
| Location | Adjusted Mean Difference in Brain Volume, mm3 | |||
|---|---|---|---|---|
| CSP Present vs Absent (95% CI)a,b | P Valuec | CV Present vs Absent (95% CI)a,b | P Valuec | |
| Supratentorium | 21 666 (552 to 42 779) | .31 | −31 191 (−61 903 to −479) | .05 |
| Thalamus | −84 (−420 to 252) | >.99 | −706 (−1188 to −223) | .03 |
| Corpus callosum | 17 (−97 to 131) | >.99 | −234 (−398 to −70) | .03 |
| Caudate | −150 (−369 to 69) | >.99 | −508 (−823 to −193) | .01 |
| Putamen | −49 (−322 to 223) | >.99 | −573 (−965 to −181) | .03 |
| Hippocampus | 27 (−142 to 196) | >.99 | −286 (−530 to −42) | .05 |
| Amygdala | 30 (−64 to 123) | >.99 | −165 (−300 to −29) | .05 |
Abbreviations: CSP, cavum septum pellucidum; CV, cavum vergae.
Positive values indicate higher volumes when the imaging finding is present; negative values indicate higher volumes when the imaging finding is absent.
Estimates adjusted for age, educational level, race, and ethnicity.
Adjusted for multiple comparisons using the Holm step-down method.
Longer CSPV was associated with lower processing speed (slope, –0.39; 95% CI, –0.49 to –0.28; P < .001) and psychomotor speed (slope, –0.43; 95% CI, –0.53 to –0.32; P < .001) scores (eFigure 3 in the Supplement). In addition, longer CSPV was associated with lower brain volumes in the supratentorium (slope, –1072 mm3 for every 1-mm increase in CSPV length; 95% CI, –1655 to –489 mm3; P < .001) and other structures (Table 4).
Table 4. Estimated Change in Mean Brain Volumes With Each 1-mm Increase in CSPV Length.
| Location | Estimated Change in Mean Brain Volume per 1-mm Increase in CSPV Length (95% CI), mm3,a,b | P Valuec |
|---|---|---|
| Supratentorium | −1072 (−1655 to −489) | <.001 |
| Thalamus | −22 (−35 to −9) | .005 |
| Corpus callosum | −11 (−16 to −5) | <.001 |
| Caudate | −14 (−24 to −5) | .01 |
| Putamen | −18 (−30 to −6) | .01 |
| Hippocampus | −9 (−16 to −1) | .04 |
| Amygdala | −6 (−10 to −2) | .01 |
Abbreviation: CSPV, cavum septum pellucidum and cavum vergae.
Positive values indicate higher volumes when CSPV length increases; negative values indicate lower volumes when CSPV length increases.
Estimates adjusted for age, educational level, race, ethnicity, and total intracranial volume.
Adjusted for multiple comparisons using the Holm step-down method.
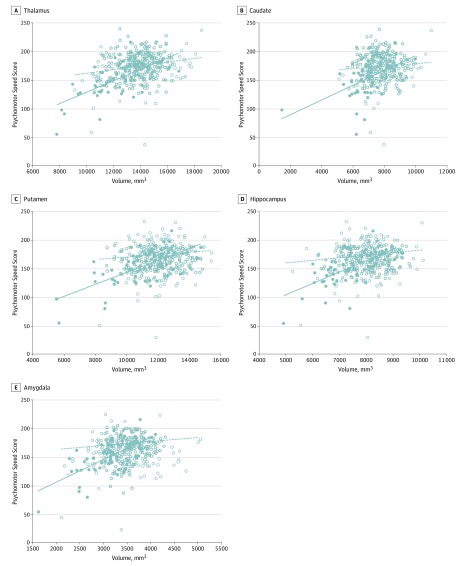
For the thalamus, caudate, putamen, hippocampus, and amygdala, a significant interaction was observed between presence or absence of CV and brain volume with respect to psychomotor speed. In these locations, decreasing volume was associated with decreasing psychomotor speed in fighters with or without CV, but the association was strongest in fighters with CV. No similar significant interaction was seen between corpus callosum volume and psychomotor speed or between the various deep brain structures and processing speed (Figure; eTable 3 in the Supplement). No significant associations were seen between fighter exposure score and the various imaging findings, brain volumes, or cognitive scores (eTable 4 in the Supplement).
Figure. Interaction Between the Associations of Cavum Vergae (CV) With Brain Volume and With Psychomotor Speed Score.
Solid dots represent participants with CV; hollow dots represent participants without CV. Solid lines represent the best fit lines for participants with CV; dotted lines represent the best fit lines for participants without CV. Decreasing brain volume is associated with decreasing psychomotor speed scores in participants both with and without CV, but the slope is more severe for participants with CV. In models that include age and educational level as covariates, the P values for the interaction terms are .03 for the thalamus, .04 for the caudate, .009 for the putamen, .02 for the hippocampus, and .007 for the amygdala (after adjustments are made for multiple testing via the Holm step-down procedure).
A total of 125 fighters with CSP were followed up longitudinally. Median follow-up duration was 2.8 years (range, 0.8-5.7 years). The length of the CSPV increased a mean of 1.0 mm (range, –3.2 to 21.5 mm). Maximum transverse width of the CSP increased a mean of 0.2 mm (range, –0.45 to 2.3 mm) (an example is shown in eFigure 4 in the Supplement). No significant associations were seen between changes in CSP size and changes in brain volumes or cognitive scores, although this study was ultimately not designed to assess these associations (eTables 5 and 6 in the Supplement).
A subanalysis of active and retired fighters (437 active fighters vs 39 retired fighters; eTable 7 in the Supplement) demonstrated many of the same associations as were seen in the main analysis with regard to the control group, although corpus callosum volumes were more influenced in active fighters (eTable 8 in the Supplement) and thalamic volumes were more influenced in retired fighters (eTable 11 in the Supplement). For active fighters, the presence of CV was not associated with decreased intracranial volumes (eTable 9 in the Supplement), but associations between increased CSPV length and brain volumes (eTable 10 in the Supplement) and processing and psychomotor speeds (eFigure 5 in the Supplement) were seen. For retired fighters, associations seen in the main analysis with regard to CV, CSPV length, and CSPV length vs processing and psychomotor speed were no longer observed (eTables 12 and 13, eFigure 6 in the Supplement).
Discussion
In this study, we found that, compared with control participants, fighters had an increased frequency of CSP and CV, impaired cognitive testing results, and decreased volumes in certain brain structures. Within the fighter group, those with CV and increased CSPV lengths demonstrated impaired cognitive testing results and volume loss in various central structures, although fighters with CSP did not. Such a link between cognitive testing scores and intracranial volumes as measured with conventional magnetic resonance imaging sequences has not previously been reported.
The exact mechanism underlying the development of CSP and CV is unknown. One theory is that direct traumatic injury causes tears in the septal leaflets, described in a postmortem examination of a boxer as multiple perforations of the septal leaflets with a lattice-like appearance.35 Subsequently, transient increased intracranial pressure, perhaps from direct trauma or Valsalva maneuvers when the fighter bears down before or during contact, may accentuate these tears and/or cause increased cerebrospinal fluid to accumulate. The development of devices designed to monitor intracranial pressure in real time could help elucidate this theory.36 Another potential mechanism for the development of CSP and CV is the accumulation of various proteins in the subarachnoid space owing to a breakdown of the blood-brain barrier, resulting in impaired cerebrospinal fluid resorption.37 Finally, ex vacuo expansion of the potential space in the septum pellucidum from central volume loss could also exacerbate CSP enlargement in cases of repetitive traumatic injury.14,38 Further evaluation of other potential risk factors, such as environmental and genetic factors, is needed. In addition, it is unclear why certain fighters develop CV and increased CSPV length and others do not. In this study, the fighter exposure score was not associated with the presence of CV, increased CSPV length, intracranial volumes, or cognitive measures. However, our analysis may have been limited because of the high number of hypotheses being tested, leading to increased adjusted P values. Again, further study is needed to explore these potential associations.
Specific brain structures are also affected by repetitive head trauma among fighters. Central structures such as the corpus callosum, thalamus, caudate, putamen, and hippocampus have extensive connections with cortical structures and with each other. Rotational movement of the head due to punches can result in shear strain and diffuse axonal injury in white matter tracts because of the relative fixation of the deep portion of the brain to the superficial aspect. These resultant injuries usually occur at junctions of gray matter and white matter and deep white matter structures.39 Most of these injuries are nonhemorrhagic, which is reflected in the low number of CMHs evident among susceptibility-weighted imaging findings in our cohort.21 However, injury to these white matter tracts can cause Wallerian degeneration and volume loss in various central structures.40,41,42,43 In addition, torsional forces can produce fluid waves in the lateral ventricles that may directly injure adjacent structures that are relatively immobile.44 These 2 hypotheses may explain the volume loss that was seen in fighters with CV and increased CSPV length in our study. This analysis also demonstrated that fighters with WMCs had higher cognitive complexity scores than did fighters without WMCs, and fighters had higher putamen volumes than did control participants. Further study is needed to determine whether these findings are of any clinical significance.
The decreased volume of multiple brain structures in fighters with increased CSPV length may explain the decreases in psychomotor speed and processing speed seen in this analysis. Psychomotor speed, which is a measure of the time needed to complete fine motor tasks requiring precise movement, coordination, and dexterity, is often considered one of the most commonly affected areas of cognition after repetitive head trauma.45 Our study demonstrated that decreased volumes in certain brain structures were associated with decreased psychomotor speed; this association was even stronger in fighters with CV. This study also assessed the association of head trauma with processing speed, which is a measure of the time needed to complete mental tasks that involve comprehension of an instruction and the subsequent reaction; this measure includes components of visual, auditory, and motor functions. The tasks comprised in processing speed involve multiple brain networks. Thus, reduced processing speed may reflect a global disruption such as widespread disturbance of white matter tracts,46,47 as might be seen with repetitive head trauma in fighters.43,48 For instance, decreased processing speed has been associated with a history of multiple concussions.49 However, these associations may be nonlinear, and certain thresholds of volume loss may be necessary to affect cognitive measures; this would account for our finding that presence of CV and increased CSPV length were associated with decreased psychomotor and processing speeds, whereas presence of CSP was not.
In this analysis, mood and behavioral measures were not associated with structural changes. Perhaps cognitive domains are more likely to be affected by cumulative head trauma, or perhaps these domains are more easily measured in the setting of cumulative trauma. In addition, a limited set of mood measures was used in this study; these factors can be difficult to assess accurately as they are self-reported and can vary over time.
Our follow-up analysis also did not demonstrate an association between increased CSPV length and decreased intracranial volumes or cognitive measures; the reasons for this result are unclear. Perhaps the length of follow-up was not sufficient or the degree of change in CSPV length was too small to discern associated changes in regional volumes or cognitive scores. In addition, several fighters demonstrated decreases in CSPV length during follow-up; again, the reasons for this finding are unclear. Longer-term follow-up with this cohort may help to elucidate these results.
Finally, in the separate subanalysis we performed to assess differences between retired fighters and active fighters, some of the associations we observed in our primary analysis were nullified. However, separating the fighters in this way underpowered both groups because of the small number of retired fighters in the study. In addition, the retired fighter group was significantly different from the active fighter group in terms of age and fighting exposure.
Strengths and Limitations
This study had several important strengths. A large cohort of well-characterized fighters was included in this analysis. In addition, all fighters underwent imaging on the same magnetic resonance imaging machine, with images reviewed by several expert neuroradiologists with many years of experience. Furthermore, there will be continued follow-up of this cohort to document further changes in imaging findings or alterations in cognitive and mood assessments.
This study also had several limitations. Because this was primarily a cross-sectional study, causality could not be ascertained. Moreover, it would be premature to suggest that the presence or increased size of CV is an indicator of a specific pathologic process such as chronic traumatic encephalopathy; there is no way to know whether any individuals in the cohort harbored pathologic characteristics of chronic traumatic encephalopathy. Fighters were also self-selected, which may have introduced bias. Finally, the computerized neuropsychiatric tests used in this study are limited in scope and may be influenced by the effort of the participant.
Conclusions
Repetitive traumatic injury in fighters with the imaging findings of CV and increased CSPV length is associated with decreased volumes of various brain structures and impaired cognitive measures. When CSP and CV are identified on routine clinical imaging in fighters, they can be viewed as a risk factor for clinical traumatic brain injury and may warrant further clinical analysis. Continued follow-up in this cohort will be important to evaluate longer-term changes and to determine whether the pathologic process of chronic traumatic encephalopathy will become evident.
eAppendix 1. Professional Fighters Brain Health Study
eAppendix 2. Cognitive and Mood Assessments
eAppendix 3. Imaging
eAppendix 4. Statistics
eFigure 1. Measurement of the Cavum Septum Pellucidum (CSP) in the Maximum Transverse Dimension and Measurement of the CSP and Cavum Vergae (CV) in the Maximum Longitudinal Dimension
eFigure 2. Example of a Cavum Septum Pellucidum and Cavum Vergae in a Fighter and Example of a Cavum Septum Pellucidum in a Fighter
eFigure 3. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score Among 251 Fighters With CSPV Length Available
eFigure 4. Example of a Patient With Increased Cavum Septum Pellucidum/Cavum Vergae Over Time
eFigure 5. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score With Only Active Fighters
eFigure 6. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score With Only Retired Fighters
eTable 1. Characteristics of Study Participants
eTable 2A. Cognitive Scores Among Fighters With and Without CSP and CV
eTable2B. Cognitive Scores Among Fighters With and Without WMC and CMH
eTable 3. Change in Psychomotor Speed With Increase in Various Brain Volumes Without and With CV
eTable 4. Association Between Fighter Exposure Score and Various Imaging Characteristics, Brain Volumes, and Cognitive Scores
eTable 5. Correlation Between Changes in CSPV Length and Changes in Various Cognitive/Behavioral Measures
eTable 6. Correlation Between Changes in CSPV Length and Changes in Various Brain Volumes
eTable 7. Characteristics of Active Versus Retired Fighters
eTable 8. Comparison of Imaging and Cognitive Variables in Active Fighters and Controls
eTable 9. Estimated Mean Differences for Various Brain Volumes Among Active Fighters With and Without CSP and CV
eTable 10. Estimated Change in Mean Brain Volumes With Each 1-mm Enlargement in CSPV Length in Active Fighters
eTable 11. Comparison of Imaging and Cognitive Variables in Retired Fighters and Controls
eTable 12. Estimated Mean Differences for Various Brain Volumes Among Retired Fighters With and Without CSP and CV
eTable 13. Estimated Change in Mean Brain Volumes With Each 1-mm Enlargement in CSPV Length in Retired Fighters
References
- 1.Bieniek KF, Ross OA, Cormier KA, et al. . Chronic traumatic encephalopathy pathology in a neurodegenerative disorders brain bank. Acta Neuropathol. 2015;130(6):877-889. doi: 10.1007/s00401-015-1502-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ling H, Morris HR, Neal JW, et al. . Mixed pathologies including chronic traumatic encephalopathy account for dementia in retired association football (soccer) players. Acta Neuropathol. 2017;133(3):337-352. doi: 10.1007/s00401-017-1680-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McKee AC, Cairns NJ, Dickson DW, et al. ; TBI/CTE group . The first NINDS/NIBIB consensus meeting to define neuropathological criteria for the diagnosis of chronic traumatic encephalopathy. Acta Neuropathol. 2016;131(1):75-86. doi: 10.1007/s00401-015-1515-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKee AC, Stern RA, Nowinski CJ, et al. . The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136(pt 1):43-64. doi: 10.1093/brain/aws307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mez J, Daneshvar DH, Kiernan PT, et al. . Clinicopathological evaluation of chronic traumatic encephalopathy in players of American football. JAMA. 2017;318(4):360-370. doi: 10.1001/jama.2017.8334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tagge CA, Fisher AM, Minaeva OV, et al. . Concussion, microvascular injury, and early tauopathy in young athletes after impact head injury and an impact concussion mouse model. Brain. 2018;141(2):422-458. doi: 10.1093/brain/awx350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCrory P, Zazryn T, Cameron P. The evidence for chronic traumatic encephalopathy in boxing. Sports Med. 2007;37(6):467-476. doi: 10.2165/00007256-200737060-00001 [DOI] [PubMed] [Google Scholar]
- 8.Heilbronner RL, Bush SS, Ravdin LD, et al. . Neuropsychological consequences of boxing and recommendations to improve safety: a National Academy of Neuropsychology education paper. Arch Clin Neuropsychol. 2009;24(1):11-19. doi: 10.1093/arclin/acp005 [DOI] [PubMed] [Google Scholar]
- 9.Bernick C, Banks SJ, Shin W, et al. . Repeated head trauma is associated with smaller thalamic volumes and slower processing speed: the Professional Fighters’ Brain Health Study. Br J Sports Med. 2015;49(15):1007-1011. doi: 10.1136/bjsports-2014-093877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hähnel S, Stippich C, Weber I, et al. . Prevalence of cerebral microhemorrhages in amateur boxers as detected by 3T MR imaging. AJNR Am J Neuroradiol. 2008;29(2):388-391. doi: 10.3174/ajnr.A0799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasiloglu ZI, Albayram S, Selcuk H, et al. . Cerebral microhemorrhages detected by susceptibility-weighted imaging in amateur boxers. AJNR Am J Neuroradiol. 2011;32(1):99-102. doi: 10.3174/ajnr.A2250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levin HS, Lippold SC, Goldman A, et al. . Neurobehavioral functioning and magnetic resonance imaging findings in young boxers. J Neurosurg. 1987;67(5):657-667. doi: 10.3171/jns.1987.67.5.0657 [DOI] [PubMed] [Google Scholar]
- 13.Bodensteiner JB, Schaefer GB. Dementia pugilistica and cavum septi pellucidi: born to box? Sports Med. 1997;24(6):361-365. doi: 10.2165/00007256-199724060-00002 [DOI] [PubMed] [Google Scholar]
- 14.Gardner RC, Hess CP, Brus-Ramer M, et al. . Cavum septum pellucidum in retired American pro-football players. J Neurotrauma. 2016;33(1):157-161. doi: 10.1089/neu.2014.3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aviv RI, Tomlinson G, Kendall B, Thakkar C, Valentine A. Cavum septi pellucidi in boxers. Can Assoc Radiol J. 2010;61(1):29-32. doi: 10.1016/j.carj.2009.09.002 [DOI] [PubMed] [Google Scholar]
- 16.Koerte IK, Hufschmidt J, Muehlmann M, et al. . Cavum septi pellucidi in symptomatic former professional football players. J Neurotrauma. 2016;33(4):346-353. doi: 10.1089/neu.2015.3880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koerte IK, Ertl-Wagner B, Reiser M, Zafonte R, Shenton ME. White matter integrity in the brains of professional soccer players without a symptomatic concussion. JAMA. 2012;308(18):1859-1861. doi: 10.1001/jama.2012.13735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Heier LA, Zimmerman RD, Jordan B, Uluğ AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27(9):2000-2004. [PMC free article] [PubMed] [Google Scholar]
- 19.Mendez MF. The neuropsychiatric aspects of boxing. Int J Psychiatry Med. 1995;25(3):249-262. doi: 10.2190/CUMK-THT1-X98M-WB4C [DOI] [PubMed] [Google Scholar]
- 20.Banks SJ, Mayer B, Obuchowski N, et al. . Impulsiveness in professional fighters. J Neuropsychiatry Clin Neurosci. 2014;26(1):44-50. doi: 10.1176/appi.neuropsych.12070185 [DOI] [PubMed] [Google Scholar]
- 21.Lee JK, Wu J, Banks S, et al. . Prevalence of traumatic findings on routine MRI in a large cohort of professional fighters. AJNR Am J Neuroradiol. 2017;38(7):1303-1310. doi: 10.3174/ajnr.A5175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gualtieri CT, Johnson LG. Reliability and validity of a computerized neurocognitive test battery, CNS Vital Signs. Arch Clin Neuropsychol. 2006;21(7):623-643. doi: 10.1016/j.acn.2006.05.007 [DOI] [PubMed] [Google Scholar]
- 23.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis, I: segmentation and surface reconstruction. Neuroimage. 1999;9(2):179-194. doi: 10.1006/nimg.1998.0395 [DOI] [PubMed] [Google Scholar]
- 24.Dale AM, Sereno MI. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. J Cogn Neurosci. 1993;5(2):162-176. doi: 10.1162/jocn.1993.5.2.162 [DOI] [PubMed] [Google Scholar]
- 25.Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proc Natl Acad Sci U S A. 2000;97(20):11050-11055. doi: 10.1073/pnas.200033797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fischl B, Liu A, Dale AM. Automated manifold surgery: constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Trans Med Imaging. 2001;20(1):70-80. doi: 10.1109/42.906426 [DOI] [PubMed] [Google Scholar]
- 27.Fischl B, Salat DH, Busa E, et al. . Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341-355. doi: 10.1016/S0896-6273(02)00569-X [DOI] [PubMed] [Google Scholar]
- 28.Fischl B, van der Kouwe A, Destrieux C, et al. . Automatically parcellating the human cerebral cortex. Cereb Cortex. 2004;14(1):11-22. doi: 10.1093/cercor/bhg087 [DOI] [PubMed] [Google Scholar]
- 29.Fischl B, Sereno MI, Dale AM. Cortical surface–based analysis, II: inflation, flattening, and a surface-based coordinate system. Neuroimage. 1999;9(2):195-207. doi: 10.1006/nimg.1998.0396 [DOI] [PubMed] [Google Scholar]
- 30.Han X, Jovicich J, Salat D, et al. . Reliability of MRI-derived measurements of human cerebral cortical thickness: the effects of field strength, scanner upgrade and manufacturer. Neuroimage. 2006;32(1):180-194. doi: 10.1016/j.neuroimage.2006.02.051 [DOI] [PubMed] [Google Scholar]
- 31.Jovicich J, Czanner S, Greve D, et al. . Reliability in multi-site structural MRI studies: effects of gradient non-linearity correction on phantom and human data. Neuroimage. 2006;30(2):436-443. doi: 10.1016/j.neuroimage.2005.09.046 [DOI] [PubMed] [Google Scholar]
- 32.Ségonne F, Dale AM, Busa E, et al. . A hybrid approach to the skull stripping problem in MRI. Neuroimage. 2004;22(3):1060-1075. doi: 10.1016/j.neuroimage.2004.03.032 [DOI] [PubMed] [Google Scholar]
- 33.Reuter M, Fischl B. Avoiding asymmetry-induced bias in longitudinal image processing. Neuroimage. 2011;57(1):19-21. doi: 10.1016/j.neuroimage.2011.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reuter M, Schmansky NJ, Rosas HD, Fischl B. Within-subject template estimation for unbiased longitudinal image analysis. Neuroimage. 2012;61(4):1402-1418. doi: 10.1016/j.neuroimage.2012.02.084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mawdsley C, Ferguson FR. Neurological disease in boxers. Lancet. 1963;2(7312):795-801. doi: 10.1016/S0140-6736(63)90498-7 [DOI] [PubMed] [Google Scholar]
- 36.Ketchem T, Twedt M, Lim D, Bashford G, Hawks JA. Proof-of-concept prototype for noninvasive intracranial pressure monitoring using ocular hemodynamics under applied force. J Med Device. 2015;9(2):024502. doi: 10.1115/1.4029810 [DOI] [Google Scholar]
- 37.Sweeney MD, Sagare AP, Zlokovic BV. Blood-brain barrier breakdown in Alzheimer disease and other neurodegenerative disorders. Nat Rev Neurol. 2018;14(3):133-150. doi: 10.1038/nrneurol.2017.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shin W, Mahmoud SY, Sakaie K, et al. . Diffusion measures indicate fight exposure-related damage to cerebral white matter in boxers and mixed martial arts fighters. AJNR Am J Neuroradiol. 2014;35(2):285-290. doi: 10.3174/ajnr.A3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hammoud DA, Wasserman BA. Diffuse axonal injuries: pathophysiology and imaging. Neuroimaging Clin N Am. 2002;12(2):205-216. doi: 10.1016/S1052-5149(02)00011-4 [DOI] [PubMed] [Google Scholar]
- 40.Blennow K, Hardy J, Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886-899. doi: 10.1016/j.neuron.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 41.Chappell MH, Brown JA, Dalrymple-Alford JC, Uluğ AM, Watts R. Multivariate analysis of diffusion tensor imaging data improves the detection of microstructural damage in young professional boxers. Magn Reson Imaging. 2008;26(10):1398-1405. doi: 10.1016/j.mri.2008.04.004 [DOI] [PubMed] [Google Scholar]
- 42.Chappell MH, Uluğ AM, Zhang L, et al. . Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging. 2006;24(3):537-542. doi: 10.1002/jmri.20656 [DOI] [PubMed] [Google Scholar]
- 43.Banks S, Shin W, Obuchowski N, Lowe M, Modic M, Bernick C Longitudinal changes in brain health in professional fighters. Presented at: Tenth World Congress on Brain Injury 2014; San Francisco, California. [Google Scholar]
- 44.Corsellis JA, Bruton CJ, Freeman-Browne D. The aftermath of boxing. Psychol Med. 1973;3(3):270-303. doi: 10.1017/S0033291700049588 [DOI] [PubMed] [Google Scholar]
- 45.Collie A, Darby D, Maruff P. Computerised cognitive assessment of athletes with sports related head injury. Br J Sports Med. 2001;35(5):297-302. doi: 10.1136/bjsm.35.5.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Penke L, Muñoz Maniega S, Murray C, et al. . A general factor of brain white matter integrity predicts information processing speed in healthy older people. J Neurosci. 2010;30(22):7569-7574. doi: 10.1523/JNEUROSCI.1553-10.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niogi SN, Mukherjee P, Ghajar J, et al. . Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29(5):967-973. doi: 10.3174/ajnr.A0970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bernick C, Banks S, Shin W, et al. . Structural and functional brain changes in boxers and mixed martial arts fighters are correlated with fight exposure (S54.006). Neurology. 2013;80(7 suppl):S54.006.23479546 [Google Scholar]
- 49.Collins MW, Grindel SH, Lovell MR, et al. . Relationship between concussion and neuropsychological performance in college football players. JAMA. 1999;282(10):964-970. doi: 10.1001/jama.282.10.964 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Professional Fighters Brain Health Study
eAppendix 2. Cognitive and Mood Assessments
eAppendix 3. Imaging
eAppendix 4. Statistics
eFigure 1. Measurement of the Cavum Septum Pellucidum (CSP) in the Maximum Transverse Dimension and Measurement of the CSP and Cavum Vergae (CV) in the Maximum Longitudinal Dimension
eFigure 2. Example of a Cavum Septum Pellucidum and Cavum Vergae in a Fighter and Example of a Cavum Septum Pellucidum in a Fighter
eFigure 3. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score Among 251 Fighters With CSPV Length Available
eFigure 4. Example of a Patient With Increased Cavum Septum Pellucidum/Cavum Vergae Over Time
eFigure 5. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score With Only Active Fighters
eFigure 6. Scatterplot of Cavum Septum Pellucidum and Cavum Vergae (CSPV) Length and Processing Speed (PSS) Score and Psychomotor Speed (PsychoS) Score With Only Retired Fighters
eTable 1. Characteristics of Study Participants
eTable 2A. Cognitive Scores Among Fighters With and Without CSP and CV
eTable2B. Cognitive Scores Among Fighters With and Without WMC and CMH
eTable 3. Change in Psychomotor Speed With Increase in Various Brain Volumes Without and With CV
eTable 4. Association Between Fighter Exposure Score and Various Imaging Characteristics, Brain Volumes, and Cognitive Scores
eTable 5. Correlation Between Changes in CSPV Length and Changes in Various Cognitive/Behavioral Measures
eTable 6. Correlation Between Changes in CSPV Length and Changes in Various Brain Volumes
eTable 7. Characteristics of Active Versus Retired Fighters
eTable 8. Comparison of Imaging and Cognitive Variables in Active Fighters and Controls
eTable 9. Estimated Mean Differences for Various Brain Volumes Among Active Fighters With and Without CSP and CV
eTable 10. Estimated Change in Mean Brain Volumes With Each 1-mm Enlargement in CSPV Length in Active Fighters
eTable 11. Comparison of Imaging and Cognitive Variables in Retired Fighters and Controls
eTable 12. Estimated Mean Differences for Various Brain Volumes Among Retired Fighters With and Without CSP and CV
eTable 13. Estimated Change in Mean Brain Volumes With Each 1-mm Enlargement in CSPV Length in Retired Fighters