Key Points
Question
What is the prognostic value of tumor-infiltrating lymphocytes (TILs) in head and neck squamous cell carcinoma?
Findings
In this cohort study that included 464 patients, higher levels of TILs were associated with improved survival in multivariable models.
Meaning
Scoring of TILs could be included into clinicopathologic prognostic models for patients with head and neck squamous carcinoma.
This cohort study examines the prognostic value of specific classes of tumor-infiltrating lymphocytes in head and neck squamous cell carcinoma pretreatment biopsies.
Abstract
Importance
Biomarkers that reflect prognosis and cellular immunity in patients with head and neck squamous cell carcinoma (HNSCC) are a prerequisite for improving individualized treatment that limits the intensity and morbidity of conventional treatment and may be useful in the introduction of new immunotherapy regimens.
Objective
To determine if specific classes of tumor-infiltrating lymphocytes (TILs) in pretreatment biopsy specimens have prognostic value for outcomes in a large training and validation cohort of patients with HNSCC.
Design, Setting, and Participants
In this prospective, epidemiologic study with a median follow-up of 47.5 months, in 464 previously untreated patients with available tissue for construction of tissue microarray, HNSCC disease sites included oral cavity (228), oropharynx (147), larynx (74), and hypopharynx (15). The training cohort consisted of 241 patients and the validation cohort consisted of 223 patients. Overall tumor stage was I (55), II (69), III (71), or IV (269). Patients were enrolled between November 2008 to October 2014. Data were analyzed between October 2018 to April 2019.
Main Outcomes and Measures
Semiquantitative levels of CD4, CD8, and FoxP3 lymphocytes were assessed by immunohistologic analysis and correlations with clinical prognostic factors, initial treatment modality, and overall survival (OS) and disease-specific (DSS) survival were determined. A principal component analysis was performed to generate a combined TIL-weighted sum score (TILws).
Results
Of the 464 participants, 135 (29%) were women; mean (SD) age was 61.1 (11.8) years. Higher CD8 counts were associated with improved OS in both training and validation sets (HR, 0.94; 95% CI, 0.90-0.98; and HR, 0.97; 95% CI, 0.95-0.99, respectively). Higher TILws levels were associated with improved OS and DSS in both the training set (HR, 0.91; 95% CI, 0.86-0.96; and HR, 0.93; 95% CI, 0.87-0.99, respectively) and validation set (HR, 0.96; 95% CI, 0.93-0.99; and HR, 0.94; 95% CI, 0.89-0.99, respectively). A multivariable Cox model controlling for batch, age, clinical stage, disease site, comorbidities, HPV status, and smoking, showed that higher TILws levels were associated with improved OS and DSS (HR, 0.94; 95% CI, 0.92-0.97; and HR, 0.94; 95% CI, 0.90-0.98, respectively). When grouped by treatment (surgery vs chemoradiation) and tested for interaction, treatment was found to be an effect modifier for CD4 levels and OS. Low CD4 levels were showed greater association with decreased survival in the chemoradiation cohort than the surgery cohort.
Conclusions and Relevance
The findings from this large cohort study suggest that levels of TILs are an independent prognostic factor in patients with HNSCC. Subsets of TILs and combined TIL scores may be clinically useful predictive and prognostic factors.
Introduction
The presence of tumor-infiltrating lymphocytes (TIL) is increasingly recognized as an important biomarker in head and neck squamous cell carcinoma (HNSCC). Consistent observations using a variety of techniques show that higher levels of T cells in the tumor microenvironment (TME) are associated with improved outcomes.1,2,3,4,5 Since the discovery of subpopulations of TILs in the 1980s, understanding of the location, functional activity, and cell types has expanded.6 Although TILs appear to have some prognostic value in HNSCC, their usefulness as a biomarker to determine treatment type, or impact on outcome in heterogeneous head and neck cancer subsites (oral cavity, oropharynx, larynx) has yet to be elucidated. Thus, a more accurate analysis of the potential benefit of assessing microenvironment T-cell infiltrates requires a homogenous sample of large numbers of patients with tumors of similar site with detailed information regarding other prognostic clinical characteristics, treatment regimens, and specific outcomes. Furthermore, a better understanding of TILs as a biomarker is a necessary prerequisite for improving individualized treatment that limits the intensity and morbidity of conventional treatment, and may be useful in advancing the introduction of new immunotherapy regimens.
In this study, we first confirmed our previous work, by examining TILs in a training and validation cohort of patients with HNSCC. Second, we combined our training and validation cohorts to evaluate the largest population of previously untreated patients with HNSCC, to our knowledge. Finally, we stratified participants by treatment type and subsite to examine biologically different tumors (oral cavity, oropharynx, larynx) and determine the association, if any, of treatment modality and survival.
Methods
Patient Population
After written informed consent was obtained, we prospectively enrolled 1042 patients from November 2008 to October 2014 into our Head and Neck Specialized Program of Research Excellence (SPORE) epidemiologic database, which was approved by the University of Michigan Medical School institutional review board. For this study, only previously untreated patients with biopsy-proven squamous carcinoma arising from the 4 major sites of oral cavity, oropharynx, larynx, or hypopharynx and with adequate tissue for creation of tissue microarrays (TMA) were included. A total of 464 patients met the inclusion criteria. Demographics were obtained through baseline questionnaires, and updates to patient status (tumor events) were determined by prospective data collection at routine follow-up visits supplemented by review of outside medical records and follow-up visits in the electronic medical record along with annual surveys. Deaths were confirmed through the Social Security Death Index. There were a total of 127 tumor recurrences, 43 second primary tumors (12 head and neck), and 150 deaths (91 disease-specific). The median follow-up for the study population was 47.5 months.
For the purposes of this study, we divided the population into training and validation cohorts based on the enrollment into SPORE. This was done because each cohort represented a different set of tissue arrays and underwent staining at different times. The first cohort was determined to be the training set and included patients treated from November 10, 2008, to October 12, 2012, and included 241 participants. We previously reported on a subset of this training group with shorter follow-up and showed that TILs have an important prognostic value in HNSCC.1 Methodology for assessing TIL counts at that time did not include a normalization for the amount of tumor represented in each core in the initial report. We believed it necessary to repeat these calculations to normalize TIL counts and repeat the overall survival (OS) analysis as a training cohort prior to expanding the participant group to include the second cohort that was used as validation of the findings. The validation set included patients treated from October 12, 2012 to October 6, 2014, and included 223 participants. For the current study we improved standardization of quantification by normalizing all TIL counts to cells/mm2 rather than cells/microcore as described herein. The baseline characteristics of the patient population are shown in Table 1 stratified by the training and validation sets. Patients in the training cohort were more likely to be younger (59.3 vs 63.0 years) and more likely to use alcohol. All patients were discussed at our multidisciplinary tumor board to standardize treatment recommendations.
Table 1. Demographics and Clinical Characteristics by Training and Validation Cohorts.
| Variable | Combined, No. (%) | Cohort | |
|---|---|---|---|
| Training | Validation | ||
| Patients, No. | 464 | 241 | 223 |
| Age, mean (SD), y | 61.1 (11.8) | 59.3 (12.0) | 63.0 (11.3) |
| Sex | |||
| Male | 329 (71) | 176 (73) | 153 (69) |
| Female | 135 (29) | 65 (27) | 70 (31) |
| Stage | |||
| 1 | 55 (12) | 26 (11) | 29 (13) |
| 2 | 69 (15) | 29 (12) | 40 (18) |
| 3 | 71 (15) | 39 (16) | 32 (14) |
| 4 | 269 (58) | 147 (61) | 122 (55) |
| T stage | |||
| 1 | 97 (21) | 53 (22) | 44 (20) |
| 2 | 139 (30) | 69 (28) | 70 (31) |
| 3 | 69 (15) | 39 (16) | 30 (13) |
| 4 | 159 (34) | 80 (33) | 79 (35) |
| N stage | |||
| 0 | 213 (46) | 100 (41) | 113 (51) |
| 1 | 59 (13) | 31 (13) | 28 (13) |
| 2 | 177 (38) | 101 (42) | 76 (34) |
| 3 | 15 (3) | 9 (4) | 6 (3) |
| M stage | |||
| 0 | 464 (100) | 241 (100) | 223 (100) |
| Disease site | |||
| Larynx | 74 (16) | 45 (19) | 29 (13) |
| Oral cavity | 228 (49) | 114 (47) | 114 (51) |
| Oropharynx | 147 (32) | 77 (32) | 70 (31) |
| Hypopharynx | 15 (3) | 5 (2) | 10 (5) |
| Initial treatment | |||
| Surgery | 268 (58) | 138 (57) | 130 (58) |
| Chemoradiation | 134 (29) | 77 (32) | 57 (26) |
| Radiation | 23 (5) | 12 (5) | 11 (5) |
| Palliation | 39 (7) | 11 (5) | 19 (9) |
| Chemotherapy | 9 (2) | 3 (1) | 6 (3) |
| ACE Comorbidities score | |||
| None | 114 (25) | 70 (29) | 44 (20) |
| Mild | 224 (48) | 112 (46) | 112 (50) |
| Moderate | 83 (18) | 39 (16) | 44 (20) |
| Severe | 41 (9) | 19 (8) | 22 (10) |
| Missing | 2 | 1 | 1 |
| HPV (p16) status | |||
| Negative | 301 (67) | 159 (69) | 142 (64) |
| Positive | 151 (33) | 72 (31) | 79 (36) |
| Unknown | 12 | 10 | 2 |
| HPV (p16) status among OP only | |||
| Negative | 29 (20) | 17 (23) | 12 (17) |
| Positive | 115 (80) | 58 (77) | 57 (83) |
| Unknown | 3 | 2 | 1 |
| Drinker (n = 443) | |||
| Never | 54 (12) | 21 (9) | 33 (16) |
| Current | 279 (63) | 161 (67) | 118 (58) |
| Former (quit >12 mo) | 110 (25) | 59 (24) | 51 (25) |
| Missing | 21 | 0 | 21 |
| Smoker (cigarettes) (n = 445) | |||
| Never | 102 (23) | 52 (22) | 50 (25) |
| Current | 496 (44) | 112 (46) | 84 (41) |
| Former (quit >12 mo) | 147 (33) | 77 (32) | 70 (34) |
| Missing | 19 | 0 | 19 |
Abbreviations: ACE, Adult Comorbidity Evaluation; HPV, human papillomavirus.
Immunohistologic Analyses
Pretreatment biopsies were obtained and representative hematoxylin-eosin slides screened and reviewed by an expert (J.B.M.) to identify areas of tumor with more than 70% cellularity for TMA construction. We extracted 0.7-mm tumor cores in triplicate from identified areas in the biopsy blocks for each patient.7 Blocks with insufficient histology or size were excluded from the study. Antibody staining was performed by our previously described methods.1 Briefly, TMA sections were incubated overnight at 65 °C. They were then deparaffinized, rehydrated, and antigen retrieval was carried out by a heat-induced epitope retrieval method. The slides were incubated in a preheated pressure cooker with citrate buffer pH6 or Tris-EDTA buffer pH9 and blocked with horse serum (30 minutes at 25 °C). Immunohistochemical staining was completed on separate slides of the TMA on a DAKO autostainer using liquid streptavidin biotin horseradish peroxidase and DBA (DAKO labeled avidin-biotin-peroxidase kits) as chromogens. Deparaffinized sections were stained with 4 monoclonal antibodies at the following titrations: CD4-1:250 (Abcam Ab846); CD8-1:40 (Nova Castra VP-C320); and FoxP3 -1:200 (Abcam Ab20034); and CD104 -1:50 (eBioscience 438-9b). Appropriate negative (without primary antibodies) and positive (tonsillar tissue and various carcinomas) controls were stained concurrently on the same slides.
Only the most common subsets of T lymphocytes that previously were thought to be prognostic were considered in the analysis and included CD4 (helper T lymphocytes), CD8 (cytotoxic/suppressor T lymphocytes), FoxP3 (regulatory T lymphocytes that are a subset of CD4 cells). Correlations with outcome for these subsets have varied with some studies suggesting that cytotoxic/suppressor T cells (CD8) are important, whereas others suggest that helper T lymphocytes (CD4) are associated with favorable prognosis and both favorable and unfavorable correlations with prognosis have been reported for FoxP3 cell counts. In this study we investigated methods to account for variations in each of these cell populations by principal components analysis.
TIL Quantification
The TMA slides were imaged and interrogated using Aperio ImageScope software (version 12, Aperio). The TIL counts were performed by manually counting cells at 200 × magnification by a technician blinded to patient clinical outcome. The CD104 staining (β4 integrin) for each core was examined first to locate and estimate the extent of the carcinoma in the tissue cores. To normalize the lymphocyte density counts among tissue cores with differing amounts of tumor, cores were characterized as having 25%, 50%, 75%, or 100% tumor tissue. Cores with less than 25% tumor tissue were excluded. This was performed by visual estimation by a naïve reader and the percentage of tumor was recorded for each core. This allowed for evaluation of tissue cores that had partial tissue loss, folding of cores, or cores with no tumor present. Semiquantitative TIL counts for each core could then be calculated by dividing the intraparenchymal T-cell counts by the percentage tumor tissue.1,8,9 Triplicate cores for each patient were averaged. Counts were normalized to 100% of the core before averaging by dividing the counts by the estimated percentage of tumor tissue in each core. To further standardize final counts to the areal size of the core, the averaged TIL count was divided by the area of the 0.7-mm core (πr2) so that the cell counts would be represented as number of cells per mm2. This provided data that could potentially be duplicated by other investigators counting TILs per high-power microscopic field in whole tissue sections.
Human Papillomavirus Testing
Human Papilloma virus (HPV)-positive tumors were identified by staining for p16INK4a and was performed using a kit (CINtec p16INK4a Histology Kit; MTM Laboratories) by our previously described method.10,11,12 Antibody binding was scored by an experienced head and neck pathologist (J.M.), using a continuous scale (10%, 30%, 90%, etc) for the proportion of tumor cells demonstrating nuclear and cytoplasmic p16 staining. The proportions were then divided into a quartile scale of 1 to 4: 1 was less than 5%; 2, 5% to 20%; 3, 21% to 50%; and 4, 51% to 100% tumor staining. Intensity was also scored on a scale of 1 to 4: 1 equal to no staining; 2, low intensity; 3, moderate; and 4, high intensity. Staining results for p16 were considered positive when the proportion score was equal to 4 and the intensity score was 3 or 4. The HPV (p16) testing was performed in 452 patients and yielded positive results in 80% of oropharynx patients.
Statistical Analysis
The TIL counts per mm2 for CD4, CD8, and FoxP3 cells were analyzed for associations with clinical variables and correlations among one another using Kruskal-Wallis tests and Spearman correlation coefficients, respectively. Positive correlations between TILs were observed for all pairs. A principal component analysis was performed in the training set to reduce the dimension of the TIL data. The first principal component explained 59% of the variability in the TIL data. The eigenvector for this principal component was used to create scaled weights that sum to 1 for use in a summary score on the same scale as the individual TIL cells per mm2. The TIL weighted sum score (TILws) was calculated with the formula:
| TILws = 0.35 × CD4 + 0.35 × CD8 + 0.3 × FoxP3 |
The same weights were applied in the validation set. Time-to-event outcomes were defined from date of initial diagnosis to date of last follow-up, or death from any cause (OS), or death from cancer (disease-specific survival [DSS]). The Kaplan-Meier method and log-rank tests were used to evaluate univariable outcomes based on high or low levels of each biomarker both dichotomized at the median or split into tertiles. Single-variable and multivariable Cox proportional hazard models were used to test associations between TILs and time-to-event outcomes treating each biomarker as a continuous variable. Hazard ratios are presented for increases of 10 cells per mm2. Multivariable models included covariate adjustments for age, stage, disease site, p16 status, Adult Comorbidity Evaluation (ACE) comorbidity score, batch effect, and smoking history. The interrelationship of initial primary treatment modality and outcome in these models was explored using multivariable Cox proportional hazard models including an interaction term for each biomarker by primary treatment group. All statistical analyses were conducted in SAS statistical software (version 9.3; SAS Institute, Inc) and graphed in R statistical software (version 3.0.3, R Foundation).
Results
Comparison of Training and Validation Cohorts
We first sought to confirm the prognostic value of TILs between the training and validation cohorts. The mean levels of TIL counts in the combined cohort and each set are presented in Table 2. The CD8 counts and combined TILws were higher in the validation set. There were no significant differences between CD4 or FoxP3 cell counts. There were also no differences in other clinical characteristics or prognostic factors other than age and alcohol use (Table 1).
Table 2. Tumor-Infiltrating Lymphocytes by Training and Validation Cohorts.
| Variable | Patients, No. | Cohort, Lymphocyte Count, Mean (SD) [range] | ||
|---|---|---|---|---|
| Entire | Training | Validation | ||
| CD4 | 457 | 85.5 (128.5) [0-948] | 83.1 (94.6) [0-468] | 88.0 (156.6) [0-948] |
| CD8 | 457 | 123.4 (225.2) [0-3045] | 80.8 (104.5) [0-629] | 168.1 (298.1) [0-3045] |
| FoxP3 | 460 | 84.8 (113.2) [0-919] | 93.9 (114.2) [0-919] | 74.8 (111.4) [0-754] |
| TILws | 451 | 97.3 (127.5) [0-1489] | 83.9 (76.9) [0-453] | 111.3 (163.7) [0-1489] |
Abbreviation: TILws, tumor-infiltrating lymphocytes weighted sum score.
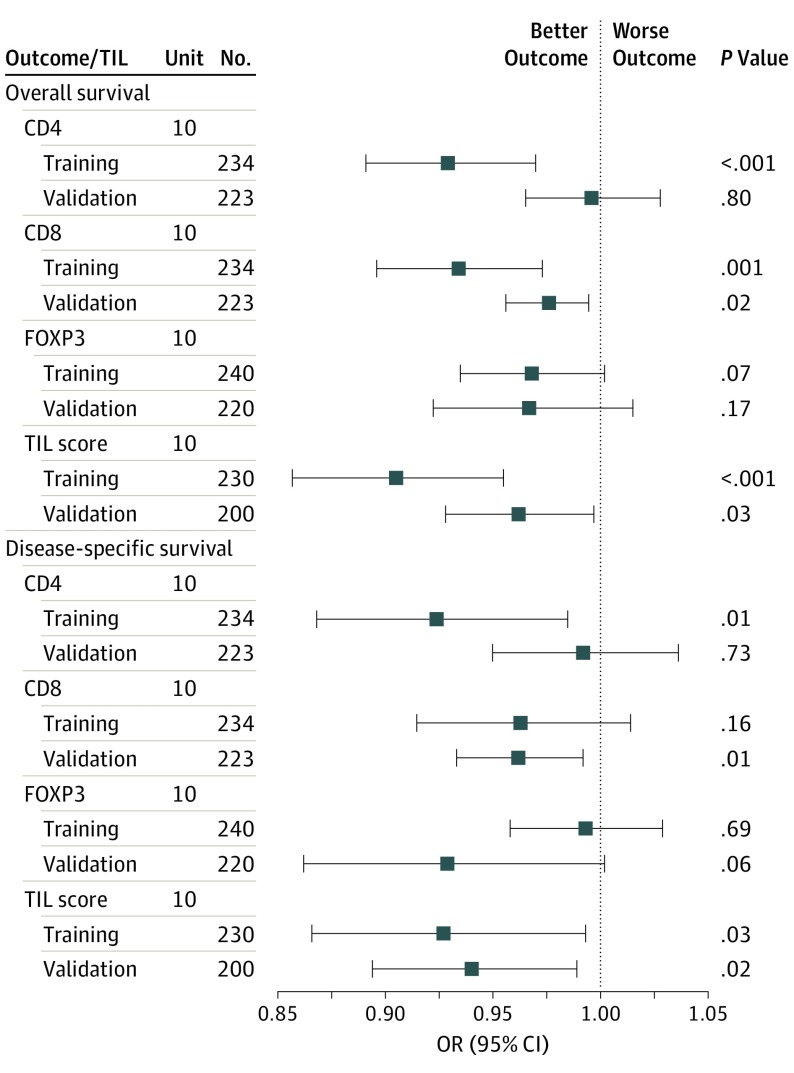
Both univariable and multivariable Cox modeling were performed to evaluate the prognostic value of TILs in the training and validation cohorts. In univariable analysis, CD8 and TILws were each associated with reduced risk of death or death from cancer in both sets of patients. The results of the multivariable models that included covariate adjustments for age, stage, disease site, HPV status, batch effect, and smoking history are presented in Figure 1. Higher CD8 counts were associated with improved OS in both the training set (HR, 0.94; 95% CI, 0.90-0.98) and validation set (HR, 0.97; 95% CI, 0.95-0.99). Higher TILws counts were associated with improved OS and DSS in both the training set (HR, 0.91; 95% CI, 0.86-0.96; and HR, 0.93; 95% CI, 0.87-0.99; respectively) and validation set (HR, 0.96; 95% CI, 0.93-0.99; and HR, 0.94; 95% CI, 0.89-0.99; respectively).
Figure 1. Forest Plot of Overall Survival and Disease-Specific Survival Within Subclasses of Tumor-Infiltrating Lymphocytes (TILs) Stratified by the Training and Validation Cohorts.
Analysis of TILs by Treatment Type
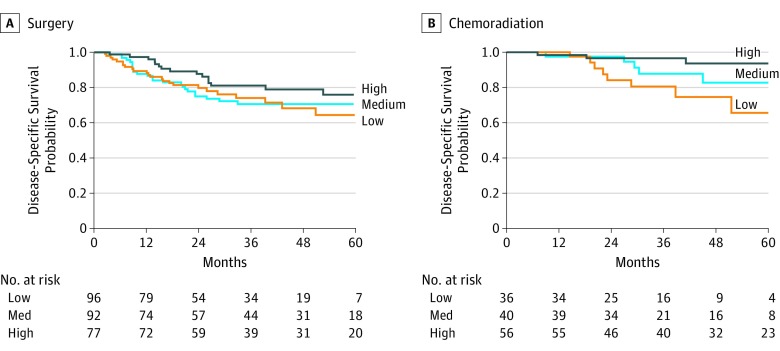
To explore the effect of TILs by primary treatment type (surgery vs chemoradiation) and outcomes, we combined the training and validation cohorts and eliminated patients who underwent palliative chemotherapy (9 patients) or nontreatment (30 patients). Figure 2 shows the Kaplan-Meier DSS curves for patients treated with primary surgery (Figure 2A) or primary chemoradiation (Figure 2B) stratified by high or low CD4 counts using tertiles. Multivariable modeling controlling for batch, age, clinical stage, disease site, comorbidities, HPV status, and smoking showed that only CD4 counts showed a significant interaction with primary treatment type. By Cox regression analysis, CD4 counts did not show a significant difference in outcome risks after surgery, whereas patients with higher CD4 counts had significantly decreased risk of death after primary chemoradiation (HR, 0.88; 95% CI, 0.80-0.96). The findings were similar when TILws counts were analyzed (HR, 0.85; 95% CI, 0.77-0.93).
Figure 2. Survival by Treatment Method.
Kaplan-Meier survival curves showing the disease-specific survival for patients whose primary treatment was surgery (A) and chemoradiation (B). CD4 is stratified at tertiles. There was improved survival in patients treated with chemoradiation with high pretreatment CD4 counts.
Multivariable Modeling of TILs on Survival
Multivariable Cox models were used to understand the association of TILws and clinical variables with survival. The results of Cox models for OS and DSS survival are shown in Table 3. The TILws was highly prognostic of DSS (HR, 0.94), along with advanced stage, severe comorbidities, laryngeal disease site, and p16 status. This confirms the independence of TILws score from known clinical prognostic variables. We also found TILws was associated with an improved OS in multivariable analysis. In addition, patients with lower stage, lower comorbidities, HPV-positive status, and never smoking had improved OS.
Table 3. Multivariable Cox Model for Overall Survival (OS) and Disease-Specific Survival (DSS).
| Parameter | HR (95% CI) | |
|---|---|---|
| OS | DSS | |
| TILws | ||
| 10 U | 0.94 (0.92-0.97) | 0.94 (0.90-0.98) |
| Age, y | ||
| 1 | 1.02 (1.00-1.03) | 0.98 (0.95-1.00) |
| Stage | ||
| 1 | 0.29 (0.13-0.64) | 0.14 (0.04-0.45) |
| 2 | 0.62 (0.35-1.10) | 0.22 (0.08-0.58) |
| 3 | 0.56 (0.31-1.00) | 0.63 (0.29-1.34) |
| 4 | 1 [Reference] | 1 [Reference] |
| Comorbidities | ||
| None | 0.35 (0.16-0.74) | 0.41 (0.16-1.05) |
| Mild | 0.56 (0.31-1.00) | 0.42 (0.19-0.94) |
| Moderate | 0.73 (0.37-1.42) | 0.40 (0.15-1.08) |
| Severe | 1 [Reference] | 1 [Reference] |
| Disease site | ||
| Hypopharynx | 0.68 (0.19-2.38) | 0.66 (0.14-3.20) |
| Larynx | 0.57 (0.27-1.23) | 0.33 (0.12-0.92) |
| Oral cavity | 1.34 (0.65-2.76) | 1.04 (0.43-2.55) |
| Oropharynx | NA | NA |
| HPV status | ||
| Negative | 2.00 (1.14-3.49) | 6.02 (2.53-14.30) |
| Positive | 1 [Reference] | 1 [Reference] |
| Smoking status | ||
| Current | 1.96 (1.05-3.66) | 1.03 (0.50-2.11) |
| Former | 2.45 (1.31-4.60) | 1.84 (0.89-3.79) |
| Never | 1 [Reference] | 1 [Reference] |
Abbreviations: HPV, human papillomavirus; NA, not applicable.
Analysis of TILs by Disease Site
Because of the biological differences in head and neck cancers arising at different disease sites in HNSCC, we evaluated the correlation between TILs and clinical variables in the most common disease sites (oropharynx, oral cavity, larynx) and determined the prognostic value of TILs. There were significant differences in TILws between subsites (oropharynx > oral cavity/larynx), suggesting that these differences could influence the prognostic value of TILs, necessitating further analysis.
TILs in Oropharynx
For patients with oropharynx cancer (n = 147), CD8 counts and TILws were associated with higher overall stage, lower T class, and HPV-positive status. In multivariable analysis controlling for known prognostic factors including HPV status, higher levels of CD4, CD8, FoxP3, and TILws were associated with an improved OS. Higher levels of FoxP3 and TILws were also associated with an improved DSS.
TILs in Oral Cavity
For patients with oral cavity cancer (n = 228), there were sex differences seen in cell counts of CD4, CD8, FoxP3, and TILws, with higher levels in women. Higher CD4 counts were associated with advanced stage, and higher CD8 levels were associated with increasing age. In multivariable analysis controlling for known prognostic factors, only higher levels of CD8 were associated with an improved OS (HR, 0.98; 95% CI, 0.96-1.00) with a trend for improved DSS (HR, 0.97; 95% CI, 0.95-1.00).
TILs in Larynx
For patients with cancer of the larynx (n = 74), there were significant differences in TIL levels between comorbidities, with higher cell counts of CD4, CD8, and TILws associated with lower ACE comorbidity scores. Higher CD4 counts were also associated with lower T class. In multivariable analysis controlling for known prognostic factors, higher levels of CD8 (HR, 0.88; 95% CI, 0.79-0.98) and TILws (HR, 0.86; 95% CI, 0.74-1.00) were associated with improved OS.
Discussion
To our knowledge, this is currently the largest study in the literature examining the prognostic value of TILs in patients with head and neck cancer. The most significant finding was that we were able to confirm our previous findings and validate that cell counts continue to be prognostic when comparing our training and validation cohorts. We also defined a novel semiquantitative cell count parameter (TILws) that combined levels of individual T-cell subsets and was highly and independently significant. This could allow better standardization of TIL counts per mm2 of tumor parenchyma, which would be an important step in bringing TIL evaluation into the mainstream of reportable findings by pathologists. Further, our work confirms and extends other reports of successful quantitation of TILs in tissue that show correlations with outcomes despite potential issues with sampling variations in using biopsy tissue and creating TMAs.13,14,15,16
In our multivariable model, we showed that in a large cohort of 464 patients, a combined TILws can predict prognosis independent of clinical variables. This provides further evidence that suggests that TIL assessments should be integrated into clinicopathologic prognostic models for patients with HNSCC. This has been suggested in breast cancer, as a pooled analysis by Loi and colleagues examining 2148 patients with early stage triple negative disease showed TILs added prognostic value to known clinical variables.17 In addition, the International Immuno-Oncology Biomarker Working Group on Breast Cancer has suggested a methodology for pathologists to count TILs in the postneoadjuvant residual disease setting.18 This type of standardization will be a necessary step to broaden the use of TILs in HNSCC. A recent meta-analysis that included 19 studies confirmed the favorable prognostic role for T-cell infiltration, but also emphasized the lack of large studies of homogenous patient cohorts that controlled for tumor site, stage, and treatment.19
In the subset analysis of TILs by disease site, important differences between patients with oropharyngeal, oral cavity, and laryngeal primary tumors were found. In the oropharyngeal cohort, not only were the number of TILs increased, but all subpopulations of TILs were prognostic, confirming the results of our previous findings and others in the literature.2,4,9,20 The immunogenicity of HPV-related tumors in the lymphoid-rich oropharynx may explain improved survival reported in these patients, but it is unclear why there are certain patients with immune-depleted tumors who do not share this prognostic benefit. In addition, the improved survival seen with increased levels of FoxP3, a marker of T regulatory cells, strengthens the argument that overall immunogenicity of HPV-related tumors drives the survival advantage. The critical mechanisms that regulate the recruitment and retention of functional T cells in the TME remain unclear but are actively under investigation. The oral cavity and larynx cancers typically have a less robust immune reactive mucosal environment compared with the oropharynx, and only CD8 infiltrate levels predicted OS in all 3 groups. The characterization of CD8 as a prognostic cytotoxic lymphocyte has been confirmed in other cancer types and has been a consistent finding.21,22
The stratification of DSS by CD4 counts in both the surgically treated and chemoradiation-treated patient populations that was initially noted in our training set was confirmed in our combined cohort and may have important ramifications. It is notable that CD4 levels appear to be more predictive of primary treatment results than purely prognostic. Currently, there are no treatment paradigm selection differences based on tissue biomarkers. Our data suggest that CD4-rich tumors may be better candidates for treatment with chemoradiation compared with CD4-depleted tumors that had similar outcomes after primary surgery as did patients with CD4-rich tumors. The precise role of CD4 cells in the TME is unknown but includes a variety of subsets responsible for cytokine production and T-cell regulation. We have previously shown that pretreatment CD4 levels in the serum of patients with advanced laryngeal cancer show an improved response to induction chemotherapy.23 In addition, we have shown that surgically treated patients with recurrent laryngeal cancer also have an improved survival with higher levels of CD4, CD8, and CD103 TILs.24 Thus, there may be differences in treatment stratification by types of immune cells in different tumors, and further study is required to determine how to expand our understanding of how these differences relate to treatment options for patients with HNSCC.
The expanding role of immunotherapy in patients with recurrent or metastatic HNSCC provides a promising novel treatment pathway for patients with potentially curable HNSCC.25,26,27 The characterization of immune status in the TME is a critical prerequisite for understanding which patients may benefit from immune modulation and will be very important to the introduction of immunotherapy. How best to measure immunity in the TME that is cost-effective, reliable, and timely is currently unclear. Simple histologic methods offer advantages because they are readily available and rapid. It is remarkable that correlations of TILs and survival have been consistently demonstrated using a variety of scoring methods and tumor samples. Correlations of tumor genetics and the immune inflammatory response have been described recently that could help characterize distinct populations of patients who may be selectable for more personalized treatment.28 Patients with immune cell-depleted tumors have different responses to cytoreductive therapy,29,30 and thus could present varying treatment options for patients whose tumors may appear the same clinically and radiographically. Assessing changes in immune cells in the TME after chemotherapy and/or immunotherapy may also play an important role in evaluating responses or drug choices/delivery.
Limitations
A limitation of this study is its need for external validation because this was a single-institution study with prior extensive experience in TIL counts. There are also sample- and treatment-related biases because modality selection was based on tumor site and extent independent of biomarker considerations. Further, there may be additional subsite differences when even larger cohorts of patients are examined because the current subsite analysis, although the largest in the literature, could have been limited by patient numbers.
Conclusions
The semiquantitative immunohistologic assessment of TILs in biopsy specimens appears to be an important independent prognostic factor for patients with HNSCC. Despite the fact that there are confounding issues and interactions of tumor site and HPV status with treatment, analysis of subsets of TILs could be useful in selecting a primary treatment modality. The findings suggest that some form of combined TIL scoring should be considered for incorporation into clinicopathologic prognostic models for patients with HNSCC.
References
- 1.Nguyen N, Bellile E, Thomas D, et al. ; Head and Neck SPORE Program Investigators . Tumor-infiltrating lymphocytes and survival in patients with head and neck squamous cell carcinoma. Head Neck. 2016;38(7):1074-1084. doi: 10.1002/hed.24406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balermpas P, Rödel F, Rödel C, et al. CD8+ tumour-infiltrating lymphocytes in relation to HPV status and clinical outcome in patients with head and neck cancer after postoperative chemoradiotherapy: A multicentre study of the German cancer consortium radiation oncology group (DKTK-ROG). Int J Cancer. 2016;138(1):171-181. doi: 10.1002/ijc.29683 [DOI] [PubMed] [Google Scholar]
- 3.Badoual C, Hans S, Rodriguez J, et al. Prognostic value of tumor-infiltrating CD4+ T-cell subpopulations in head and neck cancers. Clin Cancer Res. 2006;12(2):465-472. doi: 10.1158/1078-0432.CCR-05-1886 [DOI] [PubMed] [Google Scholar]
- 4.Balermpas P, Michel Y, Wagenblast J, et al. Tumour-infiltrating lymphocytes predict response to definitive chemoradiotherapy in head and neck cancer. Br J Cancer. 2014;110(2):501-509. doi: 10.1038/bjc.2013.640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lei Y, Xie Y, Tan YS, et al. Telltale tumor-infiltrating lymphocytes (TIL) in oral, head & neck cancer. Oral Oncol. 2016;61:159-165. doi: 10.1016/j.oraloncology.2016.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolf GT, Hudson JL, Peterson KA, Miller HL, McClatchey KD. Lymphocyte subpopulations infiltrating squamous carcinomas of the head and neck: correlations with extent of tumor and prognosis. Otolaryngol Head Neck Surg. 1986;95(2):142-152. doi: 10.1177/019459988609500203 [DOI] [PubMed] [Google Scholar]
- 7.Nocito A, Kononen J, Kallioniemi OP, Sauter G. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer. 2001;94(1):1-5. doi: 10.1002/ijc.1385 [DOI] [PubMed] [Google Scholar]
- 8.Wolf GT, Chepeha DB, Bellile E, Nguyen A, Thomas D, McHugh J; University of Michigan Head and Neck SPORE Program . Tumor-infiltrating lymphocytes (TIL) and prognosis in oral cavity squamous carcinoma: a preliminary study. Oral Oncol. 2015;51(1):90-95. doi: 10.1016/j.oraloncology.2014.09.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wansom D, Light E, Thomas D, et al. ; UM Head Neck SPORE Program . Infiltrating lymphocytes and human papillomavirus-16--associated oropharyngeal cancer. Laryngoscope. 2012;122(1):121-127. doi: 10.1002/lary.22133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walline HM, Komarck C, McHugh JB, et al. High-risk human papillomavirus detection in oropharyngeal, nasopharyngeal, and oral cavity cancers: comparison of multiple methods. JAMA Otolaryngol Head Neck Surg. 2013;139(12):1320-1327. doi: 10.1001/jamaoto.2013.5460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV Titer, Bcl-xL and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26(19):3128-3137. doi: 10.1200/JCO.2007.12.7662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vainshtein J, McHugh JB, Spector ME, et al. Human papillomavirus-related oropharyngeal cancer: HPV and p16 status in the recurrent versus parent tumor. Head Neck. 2015;37(1):8-11. doi: 10.1002/hed.23548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Distel LV, Fickenscher R, Dietel K, et al. Tumour infiltrating lymphocytes in squamous cell carcinoma of the oro- and hypopharynx: prognostic impact may depend on type of treatment and stage of disease. Oral Oncol. 2009;45(10):e167-e174. doi: 10.1016/j.oraloncology.2009.05.640 [DOI] [PubMed] [Google Scholar]
- 14.Pretscher D, Distel LV, Grabenbauer GG, Wittlinger M, Buettner M, Niedobitek G. Distribution of immune cells in head and neck cancer: CD8+ T-cells and CD20+ B-cells in metastatic lymph nodes are associated with favourable outcome in patients with oro- and hypopharyngeal carcinoma. BMC Cancer. 2009;9:292. doi: 10.1186/1471-2407-9-292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ward MJ, Thirdborough SM, Mellows T, et al. Tumour-infiltrating lymphocytes predict for outcome in HPV-positive oropharyngeal cancer. Br J Cancer. 2014;110(2):489-500. doi: 10.1038/bjc.2013.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim HR, Ha SJ, Hong MH, et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep. 2016;6:36956. doi: 10.1038/srep36956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loi S, Drubay D, Adams S, et al. Tumor-Infiltrating Lymphocytes and Prognosis: A Pooled Individual Patient Analysis of Early-Stage Triple-Negative Breast Cancers. J Clin Oncol. 2019;37(7):559-569. doi: 10.1200/JCO.18.01010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dieci MV, Radosevic-Robin N, Fineberg S, et al. ; International Immuno-Oncology Biomarker Working Group on Breast Cancer . Update on tumor-infiltrating lymphocytes (TILs) in breast cancer, including recommendations to assess TILs in residual disease after neoadjuvant therapy and in carcinoma in situ: A report of the International Immuno-Oncology Biomarker Working Group on Breast Cancer. Semin Cancer Biol. 2018;52(Pt 2):16-25. doi: 10.1016/j.semcancer.2017.10.003 [DOI] [PubMed] [Google Scholar]
- 19.de Ruiter EJ, Ooft ML, Devriese LA, Willems SM. The prognostic role of tumor infiltrating T-lymphocytes in squamous cell carcinoma of the head and neck: A systematic review and meta-analysis. Oncoimmunology. 2017;6(11):e1356148. doi: 10.1080/2162402X.2017.1356148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wansom D, Light E, Worden F, et al. Correlation of cellular immunity with human papillomavirus 16 status and outcome in patients with advanced oropharyngeal cancer. Arch Otolaryngol Head Neck Surg. 2010;136(12):1267-1273. doi: 10.1001/archoto.2010.211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahmoud SM, Paish EC, Powe DG, et al. Tumor-infiltrating CD8+ lymphocytes predict clinical outcome in breast cancer. J Clin Oncol. 2011;29(15):1949-1955. doi: 10.1200/JCO.2010.30.5037 [DOI] [PubMed] [Google Scholar]
- 22.Anitei MG, Zeitoun G, Mlecnik B, et al. Prognostic and predictive values of the immunoscore in patients with rectal cancer. Clin Cancer Res. 2014;20(7):1891-1899. doi: 10.1158/1078-0432.CCR-13-2830 [DOI] [PubMed] [Google Scholar]
- 23.Dewyer NA, Wolf GT, Light E, et al. Circulating CD4-positive lymphocyte levels as predictor of response to induction chemotherapy in patients with advanced laryngeal cancer. Head Neck. 2014;36(1):9-14. doi: 10.1002/hed.23263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mann JE, Smith JD, Birkeland AC, et al. Analysis of tumor-infiltrating CD103 resident memory T-cell content in recurrent laryngeal squamous cell carcinoma. Cancer Immunol Immunother. 2019;68(2):213-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab vs investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 2018;81:45-51. doi: 10.1016/j.oraloncology.2018.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bauml J, Seiwert TY, Pfister DG, et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J Clin Oncol. 2017;35(14):1542-1549. doi: 10.1200/JCO.2016.70.1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Saada-Bouzid E, Peyrade F, Guigay J. Immunotherapy in recurrent and or metastatic squamous cell carcinoma of the head and neck. Curr Opin Oncol. 2019;31(3):146-151. doi: 10.1097/CCO.0000000000000522 [DOI] [PubMed] [Google Scholar]
- 28.Bai S, Zhang P, Zhang JC, et al. A gene signature associated with prognosis and immune processes in head and neck squamous cell carcinoma. Head Neck. 2019;41(8):2581-2590. doi: 10.1002/hed.25731 [DOI] [PubMed] [Google Scholar]
- 29.Denkert C, von Minckwitz G, Brase JC, et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J Clin Oncol. 2015;33(9):983-991. doi: 10.1200/JCO.2014.58.1967 [DOI] [PubMed] [Google Scholar]
- 30.Bradford CR, Kumar B, Bellile E, et al. Biomarkers in advanced larynx cancer. Laryngoscope. 2014;124(1):179-187. doi: 10.1002/lary.24245 [DOI] [PMC free article] [PubMed] [Google Scholar]