Key Points
Question
Does the addition of metformin hydrochloride to standard epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors for EGFR-mutated lung adenocarcinoma improve progression-free survival compared with EGFR–tyrosine kinase inhibitors therapy alone?
Findings
In this phase 2 randomized clinical trial that included 139 patients, the addition of metformin to standard EGFR–tyrosine kinase inhibitors significantly improved both progression-free survival and overall survival.
Meaning
In this phase 2 trial of patients with EGFR-mutated lung adenocarcinoma, the addition of metformin increased survival, warranting the design of a larger phase 3 study.
This randomized clinical trial assesses the progression-free survival in patients with advanced lung adenocarcinoma who received treatment with epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKIs) plus metformin compared with those who received EGFR-TKIs alone.
Abstract
Importance
Metformin hydrochloride is emerging as a repurposed anticancer drug. Preclinical and retrospective studies have shown that it improves outcomes across a wide variety of neoplasms, including lung cancer. Particularly, evidence is accumulating regarding the synergistic association between metformin and epidermal growth factor receptor (EGFR)–tyrosine kinase inhibitors (TKIs).
Objective
To assess the progression-free survival (PFS) in patients with advanced lung adenocarcinoma who received treatment with EGFR-TKIs plus metformin compared with those who received EGFR-TKIs alone.
Design, Setting, and Participants
Open-label, randomized, phase 2 trial conducted at the Instituto Nacional de Cancerología (INCan), Mexico City, Mexico. Eligible patients were 18 years or older, had histologically confirmed stage IIIB-IV lung adenocarcinoma with an activating EGFR mutation.
Interventions
Patients were randomly allocated to receive EGFR-TKIs (erlotinib hydrochloride, afatinib dimaleate, or gefitinib at standard dosage) plus metformin hydrochloride (500 mg twice a day) or EGFR-TKIs alone. Treatment was continued until occurrence of intolerable toxic effects or withdrawal of consent.
Main Outcomes and Measures
The primary outcome was PFS in the intent-to-treat population. Secondary outcomes included objective response rate, disease control rate, overall survival (OS), and safety.
Results
Between March 31, 2016, and December 31, 2017, a total of 139 patients (mean [SD] age, 59.4 [12.0] years; 65.5% female) were randomly assigned to receive EGFR-TKIs (n = 70) or EGFR-TKIs plus metformin (n = 69). The median PFS was significantly longer in the EGFR-TKIs plus metformin group (13.1; 95% CI, 9.8-16.3 months) compared with the EGFR-TKIs group (9.9; 95% CI, 7.5-12.2 months) (hazard ratio, 0.60; 95% CI, 0.40-0.94; P = .03). The median OS was also significantly longer for patients receiving the combination therapy (31.7; 95% CI, 20.5-42.8 vs 17.5; 95% CI, 11.4-23.7 months; P = .02).
Conclusions and Relevance
To our knowledge, this is the first study to prospectively show that the addition of metformin to standard EGFR-TKIs therapy in patients with advanced lung adenocarcinoma significantly improves PFS. These results justify the design of a phase 3, placebo-controlled study.
Trial Registration
ClinicalTrials.gov identifier: NCT03071705
Introduction
Lung cancer remains a major public health problem, with more than 1.6 million deaths every year; it is the leading cause of cancer-related deaths worldwide.1,2 In addition to the alarming incidence rate, 75% of patients with lung cancer are diagnosed in the late-stage setting, when therapeutic options are often inefficient at achieving long-term disease control.3 Nonetheless, the discovery of targetable molecular alterations has outlined a subset of patients with lung cancer who are sensitive to targeted therapy.4 Particularly, the epidermal growth factor receptor gene (EGFR [OMIM 131550]), a common genetic alteration in lung cancer,4 renders potential sensitivity to specific tyrosine kinase inhibitors (TKIs).5 The use of epidermal growth factor receptor (EGFR)-TKIs has dramatically improved patient outcomes; however, patients ultimately progress due to acquired resistance.6 Current research is focusing on therapeutic combinations that might increase long-term efficacy of EGFR-TKIs, including the addition of antiangiogenics, immune checkpoint inhibitors, and poly–adenosine diphosphate ribose polymerase (PARP) inhibitors, among others. Nevertheless, some of these combinations come with a high risk of toxic effects and in some cases have failed to show a survival advantage.7,8,9
Previous reports have identified that metformin hydrochloride, an oral biguanide used for treating type 2 diabetes, might be beneficial when repurposing its use as an antineoplastic agent.10,11,12,13,14 Metformin affects the mitochondrial respiration rate, as well as adenosine triphosphate (ATP) production. In addition, it activates adenosine monophosphate (AMP)–activated protein kinase (AMPK) by liver kinase 1 (LKB1), which decreases cellular proliferation (eFigure 1 in Supplement 1). A recent phase 2 trial15 showed a significant benefit in progression-free survival (PFS) for patients treated with carboplatin, paclitaxel, bevacizumab, and metformin compared with historical controls treated without metformin, without added toxic effects. Evidence has also been accumulating in terms of the synergistic association between metformin and EGFR-TKIs both in vitro and in retrospective studies.16,17,18 However, the efficacy of metformin use on the outcomes of patients with lung adenocarcinoma has not been previously validated to our knowledge in prospective trials among specific oncogene-addicted subsets. We present the results from a randomized, phase 2 trial that evaluated the effect of adding metformin to a standard treatment with EGFR-TKIs in patients with EGFR-mutated lung adenocarcinoma.
Methods
Study Design and Participants
This prospective, open-label, randomized, phase 2 trial recruited patients at the Instituto Nacional de Cancerología (INCan), Mexico City, Mexico, between March 31, 2016, and December 31, 2017. Eligible patients were 18 years or older and had histologically confirmed stage IIIB/IV lung adenocarcinoma with an activating EGFR mutation. All patients were naive to EGFR-TKIs treatment and had an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or less, a life expectancy of at least 12 weeks, and measurable lesions based on the Response Evaluation Criteria in Solid Tumors (RECIST) guideline (version 1.1). Main exclusion criteria included the presence of Thr790Met mutation, history of diabetes, and the use of any hypoglycemic drug. This study was performed in accord with the Declaration of Helsinki19 and the principles of good clinical practice. All patients provided written informed consent to participate, and the study was approved by the scientific and bioethical committees of the Instituto Nacional de Cancerología (INCan) (CEI/1019/16). The trial protocol is available in Supplement 2. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guidelines.
Sample Size
The sample size was calculated for a 2-sample comparison PFS function with the log-rank test using the method by Freedman20 to observe an effect size corresponding to a 0.47 hazard ratio (HR)16 between the therapeutic arms to prove the next hypothesis for H0: S1(t) = S2(t), where H0 indicates the null hypothesis; S1(t), the survival function for the experimental arm; and S2(t), the survival function for the control arm. In addition, study power was set at 0.80, and the 2-sided type I error (α) was set at 0.20. Therefore, the estimated sample size was 124. Finally, 14 patients (10%) were added to the whole sample size to account for losses, with an expected sample of 138.
Randomization and Masking
Eligible patients were randomly assigned in a 1:1 ratio using a random-numbers table to receive treatment with EGFR-TKIs or EGFR-TKIs plus metformin. The method was simple randomization; allocation was not masked, so both the patient and health care provider were aware of the assignment.
Outcomes
The primary outcome was PFS in the intent-to-treat population. Secondary outcomes included objective response rate (ORR), disease control rate (DCR), overall survival (OS), and safety. For PFS, time to event was defined and calculated from the date of randomization until radiographic disease progression or treatment discontinuation due to either unacceptable toxic effects or death by any cause. For OS, time to event was defined as time from randomization until death by any cause or loss to follow-up. Observations for patients who did not experience an event were censored at patient-specific last follow-up. The radiographic response was assessed by 2 independent masked radiooncologists (M.Y.R. and a nonauthor) according to the RECIST guideline (version 1.1) by comparing the pretreatment and posttreatment images. Any in-field tumor progression or the appearance of new malignant lesions denoted progressive disease. The ORR was defined as the sum of complete and partial response, whereas DCR was defined as the sum of ORR and stable disease. In addition, an exploratory analysis was performed to evaluate the usefulness of LKB1 as a prognostic biomarker in patients with non–small cell lung cancer (NSCLC) treated with EGFR-TKIs and metformin.
Procedures
Patients in the EGFR-TKIs group received treatment with erlotinib hydrochloride, afatinib dimaleate, or gefitinib at standard dosage according to physician preference. Patients in the experimental arm received treatment with EGFR-TKIs plus metformin hydrochloride (500 mg twice a day). Metformin was started concomitantly with EGFR-TKIs therapy. Treatment was continued until intolerable adverse events or withdrawal of consent. Toxic effects were assessed according to the Common Terminology Criteria for Adverse Events (version 4.0) at each visit. Patients were clinically assessed every 4 weeks. A dosage reduction for metformin hydrochloride (500 mg daily) was permitted in the case of intolerable grade 3 to 4 drug-related adverse events. Patients who progressed to EGFR-TKIs were switched to a different systemic therapy; however, metformin treatment was continued until occurrence of intolerable toxic effects. A 28-day treatment interruption to recover from toxic effects was allowed. Patients in both groups were assessed for progression every 8 weeks (±1 week) with computed tomography or magnetic resonance imaging. After disease progression, patients continued to receive metformin, in addition to further therapy recommended by their treating physician. Additional information regarding laboratory methods can be found in eMethods 1 in Supplement 1.
Statistical Analysis
Continuous variables were summarized as arithmetic means with standard deviations or medians and ranges according to the data distribution assessed with the Kolmogorov-Smirnov test. For descriptive purposes, categorical variables were summarized as frequencies and percentages. Cross-sectional inferential comparisons were made using the Mann-Whitney test. Paired comparisons were performed using the Wilcoxon rank sum test. The χ2 test or the Fisher exact test was used for assessing statistical significance of categorical variables. Both PFS and OS were analyzed with the Kaplan-Meier method. Comparisons among the subgroups were analyzed using the log-rank test. Statistically significant (P < .05) and clinically relevant variables were included for adjustment in the multivariable Cox proportional hazards regression model, and HRs were calculated with their corresponding 95% CIs as a measure of association. Statistical significance was set at P < .05 using a 2-tailed test. SPSS software (version 20; SPSS Inc) was used for statistical analysis, and Stata (release 13; StataCorp LP) was used for plotting. This study was registered at ClinicalTrials.gov (NCT03071705) (an error occurred when uploading the information to ClinicalTrials.gov, which incorrectly lists OS as the primary end point). Further information regarding the statistical analysis is available in eMethods 2 in Supplement 1.
Results
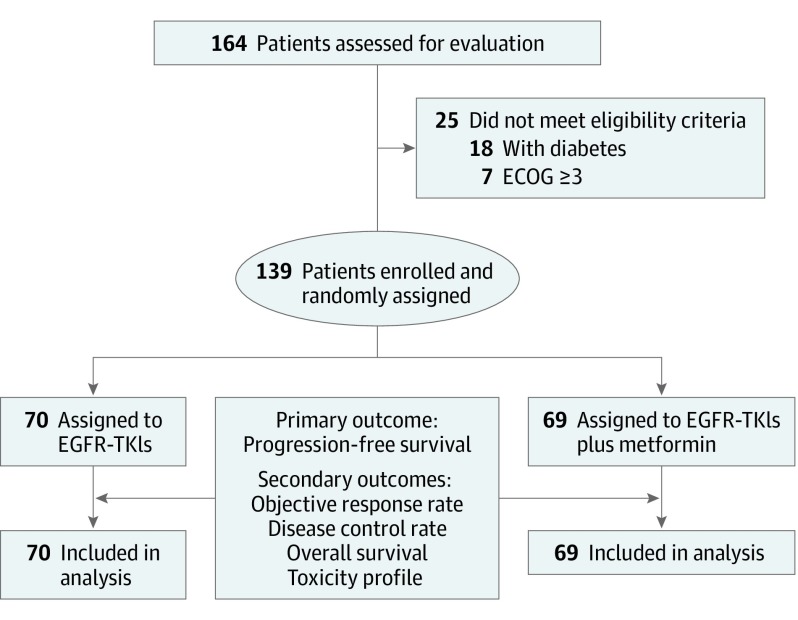
A total of 164 patients were assessed for eligibility. Among them, 139 patients (mean [SD] age, 59.4 [12.0] years; 65.5% female) met inclusion criteria and were enrolled. Participants were randomly assigned to receive EGFR-TKIs (n = 70) or EGFR-TKIs plus metformin (n = 69) (Figure 1). Baseline characteristics were equally distributed between the treatment groups. There were no patients with recurrent disease who met eligibility criteria during the recruitment period; therefore, the study population includes only patients with recently diagnosed lung adenocarcinoma (Table 1). The median follow-up for the entire population was 16.9 (95% CI, 7.0-24.9) months. The median PFS was 10.9 (95% CI, 9.4-12.5) months. In the univariate analysis, the addition of metformin to EGFR-TKIs treatment was associated with a better PFS (13.1; 95% CI, 9.8-16.3 vs 9.9; 95% CI, 7.5-12.2 months; P = .01). Patients allocated to receive EGFR-TKIs plus metformin had 40% less risk of progression to EGFR-TKIs (HR, 0.60; 95% CI, 0.40-0.94; P = .03) (Table 2 and Figure 2A and B). In both the univariate and multivariable analyses, the presence of liver metastases was the only clinical factor associated with a worse PFS (HR, 2.71; 95% CI, 1.20-6.30; P = .02) (Table 2).
Figure 1. Trial CONSORT Diagram.
CONSORT indicates Consolidated Standards of Reporting Trials; ECOG, Eastern Cooperative Oncology Group; and EGFR-TKIs, epidermal growth factor receptor–tyrosine kinase inhibitors.
Table 1. Baseline Characteristics of the Study Population.
| Characteristic | No. (%) | P Value | |
|---|---|---|---|
| EGFR-TKIs (n = 70) | EGFR-TKIs Plus Metformin (n = 69) | ||
| Sex | |||
| Male | 20 (28.6) | 28 (40.6) | .14 |
| Female | 50 (71.4) | 41 (59.4) | |
| Age, y | |||
| Mean (SD) | 60.4 (13.3) | 58.4 (1.6) | .33 |
| <60 | 37 (52.9) | 39 (56.5) | .66 |
| ≥60 | 33 (47.1) | 30 (43.5) | |
| Tobacco exposure | |||
| Absent | 52 (74.3) | 49 (71.0) | .67 |
| Present | 18 (25.7) | 20 (29.0) | |
| Wood smoke exposure | |||
| Absent | 39 (55.7) | 48 (69.6) | .09 |
| Present | 31 (44.3) | 21 (30.4) | |
| Predominant histological pattern | |||
| Lepidic | 9 (12.9) | 3 (4.3) | .26 |
| Acinar | 15 (21.4) | 16 (23.2) | |
| Papillary | 7 (10.0) | 6 (8.7) | |
| Micropapillary | 0 | 3 (4.3) | |
| Solid | 16 (22.9) | 14 (20.3) | |
| Unspecified | 23 (32.9) | 27 (39.1) | |
| Metastases | |||
| Bone | |||
| Absent | 43 (61.4) | 39 (56.5) | .56 |
| Present | 27 (38.6) | 30 (43.5) | |
| Pleura | |||
| Absent | 48 (68.6) | 44 (63.8) | .55 |
| Present | 22 (31.4) | 25 (36.2) | |
| Contralateral lung | |||
| Absent | 50 (71.4) | 42 (60.9) | .19 |
| Present | 20 (28.6) | 27 (39.1) | |
| Adrenal glands | |||
| Absent | 64 (91.4) | 66 (95.7) | .49a |
| Present | 6 (8.6) | 3 (4.3) | |
| Central nervous system | |||
| Absent | 42 (60.0) | 44 (63.8) | .65 |
| Present | 28 (40.0) | 25 (36.2) | |
| Liver | |||
| Absent | 66 (94.3) | 64 (92.8) | .75a |
| Present | 4 (5.7) | 5 (7.2) | |
| EGFR mutational profile | |||
| Exon 18, G719X | 0 | 5 (7.2) | .07 |
| Exon 19, deletion | 47 (67.1) | 42 (60.9) | |
| Exon 21, L858R | 23 (32.9) | 22 (31.9) | |
| EGFR-TKIs treatment | |||
| Gefitinib | 28 (40.0) | 22 (31.9) | .60 |
| Afatinib dimaleate | 30 (42.9) | 33 (47.8) | |
| Erlotinib hydrochloride | 12 (17.1) | 14 (20.3) | |
| Tumor Response | |||
| Radiooncologist 1 | |||
| Complete response | 3 (4.3) | 8 (11.6) | .08 |
| Partial response | 35 (50.0) | 41 (59.4) | |
| Stable response | 27 (38.6) | 19 (27.5) | |
| Disease progression | 5 (7.1) | 1 (1.4) | |
| Radiooncologist 2 | |||
| Complete response | 3 (4.3) | 10 (14.5) | .02 |
| Partial response | 35 (50.0) | 41 (59.4) | |
| Stable response | 26 (37.1) | 17 (24.6) | |
| Disease progression | 6 (8.6) | 1 (1.4) | |
| Objective Response Rate | |||
| Radiooncologist 1 | |||
| Responders | 38 (54.3) | 49 (71.0) | .04 |
| Nonresponders | 32 (45.7) | 20 (29.0) | |
| Objective response rate (95% CI) | 0.48 (0.24-0.97) | .04 | |
| Radiooncologist 2 | |||
| Responders | 38 (54.3) | 51 (73.9) | .02 |
| Nonresponders | 32 (45.7) | 18 (26.1) | |
| Objective response rate (95% CI) | 0.42 (0.20-0.85) | .02 | |
| Disease Control Rate | |||
| Radiooncologist 1 | |||
| Responders | 65 (92.9) | 68 (98.6) | .10 |
| Nonresponders | 5 (7.1) | 1 (1.4) | |
| Disease control rate (95% CI) | 0.19 (0.02-1.68) | .14 | |
| Radiooncologist 2 | |||
| Responders | 64 (91.4) | 68 (98.6) | .06 |
| Nonresponders | 6 (8.6) | 1 (1.4) | |
| Disease control rate (95% CI) | 0.16 (0.01-1.34) | .09 | |
Abbreviation: EGFR-TKIs, epidermal growth factor receptor–tyrosine kinase inhibitors.
Fisher exact test. Categorical variables with more than 5 observations per group were compared with the χ2 test, comparisons between means were performed with the t test, and comparisons between medians were performed with the Mann-Whitney test.
Table 2. Univariate and Multivariable Analyses of the Clinicopathological Factors Associated With Progression-Free Survival.
| Characteristic | No. of Patients (n = 139) | No. of Events (n = 83) | Progression-Free Survival, Median (95% CI), mo | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 48 | 27 | 10.3 (8.2-12.5) | .98 | NA | NA |
| Female | 91 | 56 | 10.9 (8.9-12.9) | |||
| Age, y | ||||||
| <60 | 76 | 47 | 10.1 (8.9-12.1) | .42 | NA | NA |
| ≥60 | 63 | 36 | 11.2 (9.4-13.0) | |||
| Tobacco exposure | ||||||
| Absent | 101 | 61 | 10.9 (9.5-12.3) | .44 | NA | NA |
| Present | 38 | 22 | 11.5 (8.8-14.0) | |||
| Wood smoke exposure | ||||||
| Absent | 87 | 50 | 11.8 (7.8-15.8) | .52 | NA | NA |
| Present | 52 | 33 | 10.9 (9.7-12.1) | |||
| Predominant histological pattern | ||||||
| Well/moderately differentiated | 56 | 37 | 11.2 (9.2-11.6) | .17 | 0.82 (0.50-1.30) | .42 |
| Poorly/nondifferentiated | 83 | 46 | 10.4 (9.2-11.6) | |||
| Metastases | ||||||
| Bone | ||||||
| Absent | 82 | 49 | 12.5 (9.0-15.9) | .53 | NA | NA |
| Present | 57 | 34 | 10.1 (8.9-11.3) | |||
| Pleura | ||||||
| Absent | 92 | 50 | 11.2 (8.5-13.9) | .55 | NA | NA |
| Present | 47 | 33 | 10.1 (8.6-11.6) | |||
| Contralateral lung | ||||||
| Absent | 92 | 53 | 10.9 (9.2-12.8) | .76 | NA | NA |
| Present | 47 | 30 | 10.3 (7.9-12.7) | |||
| Adrenal glands | ||||||
| Absent | 130 | 78 | 10.9 (9.0-12.8) | .92 | NA | NA |
| Present | 9 | 5 | 11.2 (9.7-12.7) | |||
| Central nervous system | ||||||
| Absent | 86 | 46 | 12.7 (10.5-14.9) | .08 | 1.54 (0.90-2.40) | .06 |
| Present | 53 | 37 | 9.9 (8.8-11.2) | |||
| Liver | ||||||
| Absent | 130 | 76 | 11.2 (9.0-13.4) | .03 | 2.71 (1.20-6.30) | .02 |
| Present | 9 | 7 | 6.8 (4.1-9.6) | |||
| EGFR mutational profile | ||||||
| Exon 18, G719X | 5 | 2 | 9.9 (NR) | .48 | NA | NA |
| Exon 19, deletion | 89 | 51 | 11.2 (9.5-12.9) | |||
| Exon 21, L858R | 45 | 30 | 10.3 (7.6-12.9) | |||
| EGFR-TKIs treatment | ||||||
| Gefitinib | 50 | 27 | 10.1 (8.2-11.9) | .96 | NA | NA |
| Afatinib dimaleate | 63 | 34 | 11.5 (8.4-14.6) | |||
| Erlotinib hydrochloride | 26 | 22 | 10.4 (8.4-12.4) | |||
| Objective response rate | ||||||
| Responders | 87 | 53 | 13.1 (10.8-15.4) | .003 | NA | NA |
| Nonresponders | 52 | 30 | 9.0 (5.7-12.3) | |||
| Disease control rate | ||||||
| Responders | 133 | 78 | 11.2 (9.0-13.4) | <.001 | 4.81 (0.80-12.90) | .002 |
| Nonresponders | 6 | 5 | 3.8 (1.8-5.8) | |||
| Treatment arm | ||||||
| EGFR-TKIs | 70 | 49 | 9.9 (7.5-12.2) | .01 | 0.60 (0.40-0.94) | .03 |
| EGFR-TKIs plus metformin | 69 | 34 | 13.1 (9.8-16.3) | |||
| LKB1 expression | ||||||
| Negative | 8 | 8 | 10.3 (5.8-14.8) | .09 | NA | NA |
| Positive | 16 | 10 | 13.1 (11.9-14.3) | |||
| Second-line therapy | ||||||
| Absent | 78 | 37 | 12.7 (10.4-14.3) | .09 | NA | NA |
| Present | 61 | 46 | 9.8 (8.7-11.5) | |||
| Third-line therapy | ||||||
| Present | 122 | 67 | 11.2 (9.5-13.3) | .20 | NA | NA |
| Absent | 17 | 16 | 9.9 (5.2-13.1) | |||
Abbreviations: EGFR-TKIs, epidermal growth factor receptor–tyrosine kinase inhibitors; HR, hazard ratio; LKB1, liver kinase 1; NA, not applicable; NR, not reached.
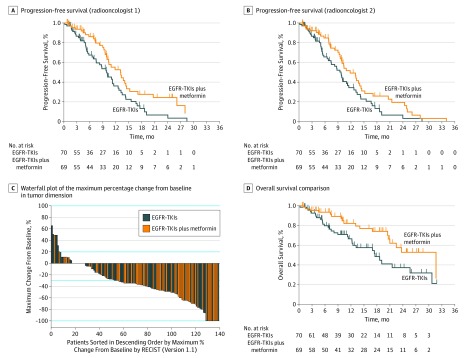
Figure 2. Kaplan-Meier Curves of Study Outcomes for Comparison Between Therapy With EGFR-TKIs vs EGFR-TKIs Plus Metformin.
A, Median progression-free survival is 9.9 (95% CI, 7.5-12.2) months for EGFR-TKIs vs 13.1 (95% CI, 9.8-16.3) months for EGFR-TKIs plus metformin (HR, 0.60; 95% CI, 0.40-0.94; P = .03). B, Median progression-free survival is 9.7 (95% CI, 5.1-14.3) months for EGFR-TKIs vs 11.8 (7.3-20.6) months for EGFR-TKIs plus metformin (HR, 0.64; 95% CI, 0.41-0.99; P = .049). C, Median objective response rate is 54.3% (38 of 70) for EGFR-TKIs vs 71.0% (49 of 69) for EGFR-TKIs plus metformin (P = .04). D, Median overall survival is 17.5 (95% CI, 11.4-23.7) months for EGFR-TKIs vs 31.7 (95% CI, 20.5-42.8) months for EGFR-TKIs plus metformin (HR, 0.50; 95% CI, 0.28-0.90; P = .02). EGFR-TKIs indicates epidermal growth factor receptor–tyrosine kinase inhibitors; HR, hazard ratio; and RECIST, Response Evaluation Criteria in Solid Tumors guideline (version 1.1).
Patients allocated to receive EGFR-TKIs plus metformin were more likely to respond to EGFR-TKIs therapy (71.0% [49 of 69] vs 54.3% [38 of 70]; P = .04). Grade 3 or 4 adverse events in both groups included diarrhea, rash, nausea, and mucositis; however, the frequency was similar across both arms. Indeed, the addition of metformin to EGFR-TKIs was associated with a lower risk of not responding to EGFR-TKIs (ORR, 0.48; 95% CI, 0.24-0.97; P = .04) (Table 1 and Figure 2C). An additional independent validation assessment of the radiographic outcomes was performed by a second masked radiooncologist, with a concordance agreement percentage of 91.7% and a κ value of 0.784 (eFigure 2 and eTables 1, 2, and 3 and in Supplement 1).
The median OS was 21.5 (95% CI, 7.9-25.1) months. Patients in the experimental arm of the trial had a statistically significant longer median OS of 31.7 (95% CI, 20.5-42.8) months compared with those in the control arm, who had a median OS of 17.5 (95% CI, 11.4-23.7) months (P = .02). In the univariate analysis, the addition of metformin to EGFR-TKIs was the only statistically significant factor associated with a better OS (Figure 2D). Disease progression to EGFR-TKIs was associated with a worse OS (5.5; 95% CI, 0.0-19.3 vs 22.5; 95% CI, 17.6-23.4 months; P = .02). In the multivariable analysis, the addition of metformin to EGFR-TKIs was the only factor independently associated with a better OS, decreasing the hazard of death by 48% (HR, 0.52; 95% CI, 0.30-0.90; P = .04) (Table 3). After progression, patients in our study were mostly treated with standard platinum-based chemotherapy. Patients in the experimental group who did not receive second-line therapy had lower hazards of death (HR, 0.32; 95% CI, 0.14-0.70; P = .005).
Table 3. Univariate and Multivariable Analyses of the Clinicopathological Factors Associated With Overall Survival.
| Characteristic | No. of Patients (n = 139) | No. of Events (n = 50) | Overall Survival, Median (95% CI), mo | P Value | HR (95% CI) | P Value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Male | 48 | 15 | 25.7 (18.4-32.9) | .44 | NA | NA |
| Female | 91 | 35 | 20.6 (14.8-26.4) | |||
| Age, y | ||||||
| <60 | 76 | 24 | 30.7 (20.8-40.7) | .10 | 1.76 (0.90-3.30) | .06 |
| ≥60 | 63 | 26 | 19.1 (10.4-21.8) | |||
| Tobacco exposure | ||||||
| Absent | 101 | 39 | 20.9 (17.8-24.2) | .21 | 0.74 (0.40-1.50) | .40 |
| Present | 38 | 11 | 23.7 (NR) | |||
| Wood smoke exposure | ||||||
| Absent | 87 | 30 | 23.7 (17.3-30.0) | .75 | NA | NA |
| Present | 52 | 20 | 20.5 (18.1-22.8) | |||
| Predominant histological pattern | ||||||
| Well/moderately differentiated | 56 | 20 | 25.7 (NR) | .17 | 0.75 (0.40-1.40) | .36 |
| Poorly/nondifferentiated | 83 | 30 | 20.6 (17.6-23.6) | |||
| Metastases | ||||||
| Bone | ||||||
| Absent | 82 | 32 | 21.5 (17.4-25.7) | .58 | NA | NA |
| Present | 57 | 18 | 25.7 (13.4-37.9) | |||
| Pleura | ||||||
| Absent | 92 | 33 | 22.5 (18.7-26.2) | .97 | NA | NA |
| Present | 47 | 17 | NR (NR) | |||
| Contralateral lung | ||||||
| Absent | 92 | 35 | 20.9 (17.7-24.3) | .59 | NA | NA |
| Present | 47 | 15 | 31.7 (13.1-50.3) | |||
| Adrenal glands | ||||||
| Absent | 130 | 47 | 22.5 (17.9-22.0) | .69 | NA | NA |
| Present | 9 | 3 | NR (NR) | |||
| Central nervous system | ||||||
| Absent | 86 | 27 | 25.7 (16.6-34.7) | .35 | NA | NA |
| Present | 53 | 23 | 20.5 (16.5-24.3) | |||
| Liver | ||||||
| Absent | 130 | 45 | 22.5 (16.0-28.9) | .33 | NA | NA |
| Present | 9 | 5 | 20.9 (0.0-48.5) | |||
| EGFR mutational profile | ||||||
| Exon 18, G719X | 5 | 0 | NA | .23 | NA | NA |
| Exon 19, deletion | 89 | 33 | 20.9 (11.2-30.7) | |||
| Exon 21, L858R | 45 | 17 | 20.6 (10.3-NR) | |||
| EGFR-TKIs treatment | ||||||
| Gefitinib | 50 | 21 | 17.5 (10.4-24.6) | .27 | NA | NA |
| Afatinib dimaleate | 63 | 19 | 25.7 (17.9-33.3) | |||
| Erlotinib hydrochloride | 26 | 10 | 23.7 (18.3-28.9) | |||
| Objective response rate | ||||||
| Responders | 87 | 24 | 31.7 (17.0-46.3) | .001 | NA | NA |
| Nonresponders | 52 | 26 | 16.9 (9.6-24.3) | |||
| Disease control rate | ||||||
| Responders | 133 | 45 | 22.5 (17.6-23.4) | .02 | 2.48 (0.90-7.10) | .09 |
| Nonresponders | 6 | 5 | 5.5 (0.0-19.3) | |||
| Treatment arm | ||||||
| EGFR-TKIs | 70 | 32 | 17.5 (11.4-23.7) | .02 | 0.52 (0.30-0.90) | .04 |
| EGFR-TKIs plus metformin | 69 | 18 | 31.7 (20.5-42.8) | |||
| LKB1 expression | ||||||
| Negative | 8 | 5 | 25.7 (13.2-38.1) | .94 | NA | NA |
| Positive | 16 | 7 | 20.5 (NR) | |||
| Second-line therapy | ||||||
| Absent | 78 | 32 | 14.0 (11.2-22.5) | .005 | NA | NA |
| Present | 61 | 18 | 25. 7 (20.6-NR) | |||
| Third-line therapy | ||||||
| Present | 122 | 44 | 20.6 (14.6-NR) | .08 | NA | NA |
| Absent | 17 | 6 | 30.7 (18.6-NR) | |||
Abbreviations: EGFR-TKIs, epidermal growth factor receptor–tyrosine kinase inhibitors; HR, hazard ratio; LKB1, liver kinase 1; NA, not applicable; NR, not reached.
The frequency and severity of adverse events were evaluated among all participants. Overall, adverse events were similar in patients treated with EGFR-TKIs plus metformin and patients treated with EGFR-TKIs. Details regarding adverse events are listed in eTable 4 in Supplement 1. eTable 5 in Supplement 1 summarizes metformin dosage reductions, discontinuations, and continuations after progression. A total of 61 patients received second-line therapy (31 in the EGFR-TKIs group and 30 in the EGFR-TKIs plus metformin group). Only 17 patients received a third line of treatment (eTable 6 in Supplement 1). An additional stratified analysis was performed to assess the effect of second-line therapy on OS (eFigure 3, eTable 7, and eTable 8 in Supplement 1).
As a post hoc analysis, LKB1 expression on tissue biopsy specimens, as well as serological levels of glucose, glycated hemoglobin, interleukin 6 (IL-6), and insulinlike growth factor (IGF), was assessed in a subset of patients (n = 24) enrolled in the trial. The overall frequency of LKB1 loss of expression was 33.3%. No characteristic was associated with PFS in the stratified univariate analysis (eTable 9 in Supplement 1). When assessing OS stratified on LKB1 expression, LKB1-positive patients seemed to have a better OS when metformin was added to EGFR-TKIs therapy (not reached vs 6.7 months; P = .002). (eFigure 4 and eTable 10 in Supplement 1). The findings regarding glycated hemoglobin, IL-6, and IGF levels are shown in eFigure 5 in Supplement 1.
Discussion
To the best of our knowledge, this is the first report that prospectively evaluates the use of metformin combined with EGFR-TKIs in patients with advanced NSCLC harboring EGFR mutations. Our results show that patients who receive treatment with EGFR-TKIs combined with metformin have a significantly improved PFS and ORR and a significantly longer OS compared with those who received treatment with EGFR-TKIs alone. Multivariable analysis showed that treatment with metformin is independently associated with longer PFS and OS.
Since 2005, evidence regarding the antineoplastic effectiveness of metformin has been accumulating,21,22 with the results showing that metformin use reduced the risk of pancreatic, prostate, and lung cancer,22 even when adjusting for age, smoking status, and glycated hemoglobin levels. Recently, the beneficial effectiveness of metformin in survival outcomes for patients with lung cancer has been documented in several retrospective trials, including a study16 of metformin use in patients receiving EGFR-TKIs treatment. The survival outcomes we report in our study are in concordance with the results reported in that study, with a similar ORR (71.0% vs 70.5%) and OS (31.7 vs 32.0 months) in patients treated with EGFR-TKIs plus metformin. This evidence suggests that the use of metformin as concomitant treatment of lung adenocarcinoma can be a valuable addition to improve clinical outcomes.14 Findings from in vitro studies10,23 have suggested a synergistic association between metformin and EGFR-TKIs in EGFR-mutated lung cancer cell lines, and the combination of metformin plus erlotinib or gefitinib has been shown to resensitize cell lines with EGFR-TKIs resistance. Although the exact mechanism of action remains unknown, it has been reported that the most potent antineoplastic effectiveness of metformin is by activating the LKB1-AMPK signaling pathway, which leads to an increased ratio of AMP to ATP and to AMPK activation, thus suppressing the mammalian target of rapamycin (mTOR) signaling pathway and leading to inhibition of tumor cell proliferation.24,25
LKB1 (OMIM 602216) mutations result in the loss of AMPK functions and have been associated with several types of cancer, including lung cancer.26,27 Deletions of LKB1 are the third most common type of mutation-associated NSCLC expression, ranging from 10% to 50%,28 and LKB1 loss is associated with a more aggressive phenotype in NSCLC.29,30
Strengths and Limitations
There are several strengths of this study, including the selection of a homogeneous population of patients with EGFR mutations. In addition, the study design reaffirms previous reports regarding the benefit of the addition of metformin to the EGFR-TKIs, suggesting the potential use of this drug in combination with osimertinib mesylate for first-line treatment, as supported by a recent preclinical study.31
However, the findings in this study should also be interpreted in light of its limitations, including the lack of randomization stratification according to smoking status, the EGFR mutation profile, or the EGFR-TKIs. Also, the fact that OS data might appear to subperform compared with recently published trials of first-line EGFR-TKIs (eg, the FLAURA study)32 could be explained by the high incidence of central nervous system metastases at the beginning of our trial or the tumor mutation burden in the patients studied. In the FLAURA study, 18-month survival was 71% (95% CI, 65%-76%) in the standard EGFR-TKIs group, which included patients treated with first-generation and second-generation EGFR-TKIs. However, 43% of these patients crossed over to the osimertinib arm of the trial as second-line treatment after their first progression, which might have influenced OS data. After progression, patients in our study were mostly treated with standard platinum-based chemotherapy (eTable 6 in Supplement 1). Patients in the experimental group who did not receive second-line therapy had lower hazards of death (HR, 0.32; 95% CI, 0.14-0.70; P = .005). Another important limitation is the fact that our trial was not designed as a double-blind study; therefore, several biases might be present. The presence of immortal bias must be acknowledged because patients had received previous non-TKIs treatments before the study. Therefore, despite the use of adjustment methods for survival analyses using the Cox proportional hazards regression model, future considerations of this drawback must be taken into account when designing additional phase 3 trials. It is of utmost importance that future phase 3 trials be designed as double-blind studies; they should include a stratified randomization with treatment-naive nondiabetic patients and with a mixed design that allows the simultaneous assessment of potential biomarkers to avoid these relevant limitations.
Conclusions
The addition of metformin to a standard EGFR-TKIs treatment in patients with EGFR-mutated lung adenocarcinoma significantly prolongs PFS and OS, without significantly increasing adverse events. The results from this phase 2 study warrant the design of a larger, phase 3, placebo-controlled study to draw more robust conclusions.
eFigure 1. Molecular effects of metformin on lung cancer
eFigure 2. Progression-free survival (radio-oncologist 2)
eFigure 3. (A) Kaplan-Meier curves for overall survival among patients who received second-line treatment and (B) patients who did not receive a second-line of treatment
eFigure 4. Kaplan-Meier curves for (A) Progression-free survival comparison between patients with negative LKB1 expression according to the therapeutic arm. (B) Overall survival comparison between patients with negative LKB1 expression according to the received treatment. (C) Progression-free survival comparison between patients with positive LKB1 expression according to the received treatment. (D) Overall survival comparison between patients with positive LKB1 expression according to therapeutic arm
eFigure 5. Box-plots of the serum levels of Glucose (A), HbA1c (B), Interleukin-6 (C), and Insulin growth factor receptor (D) at baseline, the endo of 1st, and 2nd EGFR-TKIs cycle
eMethods 1. Supplementary laboratory methods
eMethods 2. Supplementary log file for statistical analysis
eTable 1. Concordance agreement assessment among radio-oncologists (n = 2) for the radiographic evaluation of the best response rate through RECIST v1.1
eTable 2. Univariate and multivariable analysis of the clinic-pathological factors associated with progression-free survival according to radio-oncologist 2
eTable 3. Crude and adjusted Hazard Ratios for PFS by Radio-oncologists 1 and 2
eTable 4. Adverse events
eTable 5. Details of metformin treatment
eTable 6. Further lines of treatment
eTable 7. Overall survival among patients who did not receive a second-line of therapy after intervention
eTable 8. Overall survival among patients who received a second-line of therapy after intervention
eTable 9. Univariate and LKB1-stratified analysis of the clinic-pathological factors associated with progression-free survival
eTable 10. Univariate and LKB1-stratified analysis of the clinic-pathological factors associated with overall survival
Trial Protocol
Data Sharing Statement
References
- 1.Torre LA, Siegel RL, Jemal A. Lung cancer statistics In: Advances in Experimental Medicine and Biology. Basel, Switzerland: Springer; 2016:-. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 3.Walters S, Maringe C, Coleman MP, et al. ; ICBP Module 1 Working Group . Lung cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK: a population-based study, 2004-2007. Thorax. 2013;68(6):551-564. doi: 10.1136/thoraxjnl-2012-202297 [DOI] [PubMed] [Google Scholar]
- 4.Mok TSK. Personalized medicine in lung cancer: what we need to know. Nat Rev Clin Oncol. 2011;8(11):661-668. doi: 10.1038/nrclinonc.2011.126 [DOI] [PubMed] [Google Scholar]
- 5.Arrieta O, Cardona AF, Martín C, et al. Updated frequency of EGFR and KRAS mutations in non–small-cell lung cancer in Latin America: the Latin-American Consortium for the Investigation of Lung Cancer (CLICaP). J Thorac Oncol. 2015;10(5):838-843. doi: 10.1097/JTO.0000000000000481 [DOI] [PubMed] [Google Scholar]
- 6.Hirsch FR, Scagliotti GV, Mulshine JL, et al. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389(10066):299-311. doi: 10.1016/S0140-6736(16)30958-8 [DOI] [PubMed] [Google Scholar]
- 7.Tran G, Zafar SY. Financial toxicity and implications for cancer care in the era of molecular and immune therapies. Ann Transl Med. 2018;6(9):166. doi: 10.21037/atm.2018.03.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Campelo R, Arrieta O, Massuti B, Rodriguez-Abreu D. Combination of gefitinib and olaparib versus gefitinib alone in EGFR mutant non–small-cell lung cancer (NSCLC): a randomized phase 2 study (GOAL, Spanish Lung Cancer Group). J Clin Oncol. 2018;suppl:36. [DOI] [PubMed] [Google Scholar]
- 9.Hosomi Y, Seto T, Nishio M, et al. Erlotinib plus bevacizumab (EB) versus erlotinib alone (E) as first-line treatment for advanced non-squamous non–small-cell lung cancer (NSCLC) with activating EGFR mutation (mt): JO25567 exploratory subgroup analysis. Ann Oncol. 2015;26:ix127 https://academic.oup.com/annonc/article/26/suppl_9/ix127/2801051. Published December 19, 2015. Accessed September 2018. [Google Scholar]
- 10.Yousef M, Tsiani E. Metformin in lung cancer: review of in vitro and in vivo animal studies. Cancers (Basel). 2017;9(5):45. doi: 10.3390/cancers9050045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Provinciali N, Lazzeroni M, Cazzaniga M, Gorlero F, Dunn BK, DeCensi A. Metformin: risk-benefit profile with a focus on cancer. Expert Opin Drug Saf. 2015;14(10):1573-1585. doi: 10.1517/14740338.2015.1084289 [DOI] [PubMed] [Google Scholar]
- 12.Guo Q, Liu Z, Jiang L, et al. Metformin inhibits growth of human non–small cell lung cancer cells via liver kinase B-1–independent activation of adenosine monophosphate–activated protein kinase. Mol Med Rep. 2016;13(3):2590-2596. doi: 10.3892/mmr.2016.4830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arrieta O, Varela-Santoyo E, Soto-Perez-de-Celis E, et al. Metformin use and its effect on survival in diabetic patients with advanced non–small cell lung cancer. BMC Cancer. 2016;16:633. doi: 10.1186/s12885-016-2658-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao X, Wen ZS, Wang XD, Li Y, Liu KY, Wang X. The clinical effect of metformin on the survival of lung cancer patients with diabetes: a comprehensive systematic review and meta-analysis of retrospective studies. J Cancer. 2017;8(13):2532-2541. doi: 10.7150/jca.19750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marrone KA, Zhou X, Forde PM, et al. A randomized phase II study of metformin plus paclitaxel/carboplatin/bevacizumab in patients with chemotherapy-naïve advanced or metastatic nonsquamous non–small cell lung cancer. Oncologist. 2018;23(7):859-865. doi: 10.1634/theoncologist.2017-0465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen H, Yao W, Chu Q, et al. Synergistic effects of metformin in combination with EGFR-TKI in the treatment of patients with advanced non–small cell lung cancer and type 2 diabetes. Cancer Lett. 2015;369(1):97-102. doi: 10.1016/j.canlet.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 17.Morgillo F, Sasso FC, Della Corte CM, et al. Synergistic effects of metformin treatment in combination with gefitinib, a selective EGFR tyrosine kinase inhibitor, in LKB1 wild-type NSCLC cell lines. Clin Cancer Res. 2013;19(13):3508-3519. doi: 10.1158/1078-0432.CCR-12-2777 [DOI] [PubMed] [Google Scholar]
- 18.Barrios-Bernal P, Hernandez-Pedro NY, Soca-Chafre G, Orozco-Morales M, Arrieta O. Abstract 3554: the combined effect of afatinib and metformin on glycolytic regulation in EGFR-mutant non–small cell lung cancer. Cancer Res. 2017;77:3554. doi: 10.1158/1538-7445.AM2017-3554 [DOI] [Google Scholar]
- 19.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 20.Freedman LS. Tables of the number of patients required in clinical trials using the logrank test. Stat Med. 1982;1(2):121-129. [DOI] [PubMed] [Google Scholar]
- 21.Evans JMM, Donnelly LA, Emslie-Smith AM, Alessi DR, Morris AD. Metformin and reduced risk of cancer in diabetic patients. BMJ. 2005;330(7503):1304-1305. doi: 10.1136/bmj.38415.708634.F7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mazzone PJ, Rai H, Beukemann M, Xu M, Jain A, Sasidhar M. The effect of metformin and thiazolidinedione use on lung cancer in diabetics. BMC Cancer. 2012;12:410. doi: 10.1186/1471-2407-12-410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li L, Han R, Xiao H, et al. Metformin sensitizes EGFR-TKI–resistant human lung cancer cells in vitro and in vivo through inhibition of IL-6 signaling and EMT reversal. Clin Cancer Res. 2014;20(10):2714-2726. doi: 10.1158/1078-0432.CCR-13-2613 [DOI] [PubMed] [Google Scholar]
- 24.Pernicova I, Korbonits M. Metformin: mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10(3):143-156. doi: 10.1038/nrendo.2013.256 [DOI] [PubMed] [Google Scholar]
- 25.Storozhuk Y, Hopmans SN, Sanli T, et al. Metformin inhibits growth and enhances radiation response of non–small cell lung cancer (NSCLC) through ATM and AMPK. Br J Cancer. 2013;108(10):2021-2032. doi: 10.1038/bjc.2013.187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Momcilovic M, Shackelford DB. Targeting LKB1 in cancer: exposing and exploiting vulnerabilities. Br J Cancer. 2015;113(4):574-584. doi: 10.1038/bjc.2015.261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gill RK, Yang SH, Meerzaman D, et al. Frequent homozygous deletion of the LKB1/STK11 gene in non–small cell lung cancer. Oncogene. 2011;30(35):3784-3791. doi: 10.1038/onc.2011.98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanchez-Cespedes M. The role of LKB1 in lung cancer. Fam Cancer. 2011;10(3):447-453. doi: 10.1007/s10689-011-9443-0 [DOI] [PubMed] [Google Scholar]
- 29.Cao C, Gao R, Zhang M, et al. Role of LKB1-CRTC1 on glycosylated COX-2 and response to COX-2 inhibition in lung cancer. J Natl Cancer Inst. 2014;107(1):358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calles A, Sholl LM, Rodig SJ, et al. Immunohistochemical loss of LKB1 is a biomarker for more aggressive biology in KRAS-mutant lung adenocarcinoma. Clin Cancer Res. 2015;21(12):2851-2860. doi: 10.1158/1078-0432.CCR-14-3112 [DOI] [PubMed] [Google Scholar]
- 31.Martin MJ, Eberlein C, Taylor M, Ashton S, Robinson D, Cross D. Inhibition of oxidative phosphorylation suppresses the development of osimertinib resistance in a preclinical model of EGFR-driven lung adenocarcinoma. Oncotarget. 2016;7(52):86313-86325. doi: 10.18632/oncotarget.13388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soria JC, Ohe Y, Vansteenkiste J, et al. FLAURA Investigators. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. N Engl J Med. 2018;378(2):113-125. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Molecular effects of metformin on lung cancer
eFigure 2. Progression-free survival (radio-oncologist 2)
eFigure 3. (A) Kaplan-Meier curves for overall survival among patients who received second-line treatment and (B) patients who did not receive a second-line of treatment
eFigure 4. Kaplan-Meier curves for (A) Progression-free survival comparison between patients with negative LKB1 expression according to the therapeutic arm. (B) Overall survival comparison between patients with negative LKB1 expression according to the received treatment. (C) Progression-free survival comparison between patients with positive LKB1 expression according to the received treatment. (D) Overall survival comparison between patients with positive LKB1 expression according to therapeutic arm
eFigure 5. Box-plots of the serum levels of Glucose (A), HbA1c (B), Interleukin-6 (C), and Insulin growth factor receptor (D) at baseline, the endo of 1st, and 2nd EGFR-TKIs cycle
eMethods 1. Supplementary laboratory methods
eMethods 2. Supplementary log file for statistical analysis
eTable 1. Concordance agreement assessment among radio-oncologists (n = 2) for the radiographic evaluation of the best response rate through RECIST v1.1
eTable 2. Univariate and multivariable analysis of the clinic-pathological factors associated with progression-free survival according to radio-oncologist 2
eTable 3. Crude and adjusted Hazard Ratios for PFS by Radio-oncologists 1 and 2
eTable 4. Adverse events
eTable 5. Details of metformin treatment
eTable 6. Further lines of treatment
eTable 7. Overall survival among patients who did not receive a second-line of therapy after intervention
eTable 8. Overall survival among patients who received a second-line of therapy after intervention
eTable 9. Univariate and LKB1-stratified analysis of the clinic-pathological factors associated with progression-free survival
eTable 10. Univariate and LKB1-stratified analysis of the clinic-pathological factors associated with overall survival
Trial Protocol
Data Sharing Statement