Abstract
Objective:
It is unknown whether the intravascular ultrasound (IVUS) guidance for percutaneous coronary intervention (PCI) should be routinely used in small-vessel coronary lesions in patients affected by Type 2 diabetes mellitus (T2DM). This study aimed to assess the clinical significance of the IVUS-guided PCI treatment for small-vessel coronary lesions in T2DM.
Methods:
This was a prospective interventional trial. A total of 228 patients affected by T2DM with stable angina and a positive stress test in the presence of coronary arteriography (CAG) involving small vessels [online measurement reference vessel diameter ≤3.0 mm by means of quantitative coronary angiography (QCA)] were recruited and divided into two groups: an IVUS-guided group (n=120) and a CAG-guided group (n=108). Follow-up PCIs were performed via CAG or IVUS criteria, respectively. Between-group comparisons were made for the number of stents implanted, length, diameter, and high-pressure balloons used post-dilatation. Major adverse cardiac events (MACEs) defined as cardiac death, nonfatal myocardial infarction, and target lesion revascularization (TLR) were the primary endpoint. The value of late lumen loss and proportion of in-stent restenosis (ISR) were the secondary endpoint, all of which were also evaluated during the follow-up period.
Results:
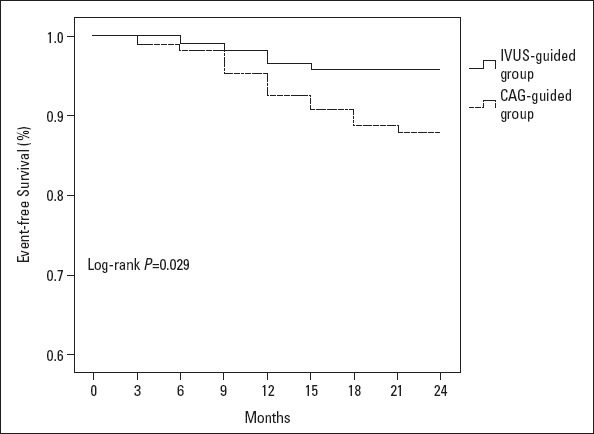
There was an increased lesion length observed using the IVUS measurement when compared with QCA measurements in the IVUS-guided group (p≤0.001). The number of implanted stents, diameter, length, percentage of high-pressure balloons used during post-dilatation, value of late lumen loss, and proportion of ISR decreased in the IVUS-guided group when compared with the CAG-guided group (p=0.002, p=0.001, p=0.003, p=0.004, p=0.007, p=0.001, respectively). After a 2-year follow-up, the Kaplan–Meier curves indicated that the incidence of MACEs was significantly lower in the IVUS-guided group (log-rank p=0.029), mainly because of the TLR reduction (log-rank p=0.037).
Conclusion:
The IVUS-guided PCI treatment improved the event-free survival in small-vessel coronary lesions in patients affected by T2DM.
Keywords: intravascular ultrasound, Type 2 diabetes mellitus, coronary artery disease, small-vessel disease
Introduction
The prevalence of unhealthy lifestyles in modern times has increased the incidence of coronary heart disease (CHD) and diabetes, with many considering the latter to be a CHD equivalent (1). Studies suggest that diabetic coronary heart disease accounts for more than 50% of diabetes complications and, due to the myriad of metabolic complications, patients often develop CHD earlier and faster with an increase in lesion distribution and deteriorating prognosis. Specific coronary lesions associated with diabetes are mainly confined to coronary small-vessels and may be found in patients of all ages (2). Treatment strategies for coronary small vessels (reference vessel diameter less than 3.0 mm) have been an area of contention among practitioners of interventional therapy for CHD. The development of intravascular ultrasound (IVUS) has yielded a more objective and comprehensive basis for clinical decisions regarding the treatment of coronary artery disease. It is unknown whether the IVUS guidance for percutaneous coronary intervention (PCI) should be routinely endorsed in small-vessel coronary lesions in patients affected by Type 2 diabetes mellitus (T2DM), as no pertinent controlled trials are yet available. We tested the hypothesis that IVUS-guided stenosis procedures would result in a significant improvement of the event-free survival when compared to the traditional coronary arteriography (CAG)-guided surgery in small-vessel coronary lesions in patients suffering from T2DM.
Methods
Study population
This investigation was a prospective interventional trial. The study population was selected from patients admitted to the Department of Cardiology of our hospital. A total of 698 consecutive patients with stable angina and a positive stress test were admitted to our hospital and underwent coronary angiography successfully from January to November 2015. Of these patients, 247 patients with T2DM were identified as coronary lesions involving a small vessel [online measurement reference vessel diameter between 2.2 and 3.0 mm by means of quantitative coronary angiography (QCA)]. Nineteen patients were excluded due to the impossibility for the catheter to reach or pass the lesion, especially in advanced tortuous and partially thin vessels. In total, 228 patients who underwent the PCIs treatment were included in the present investigation. Exclusion criteria included a history of myocardial infarction, any acute coronary syndrome, a history of target vessel PCI or coronary artery bypass graft (CABG), severe hepatic and renal dysfunction, hemodialysis, exceeding Class III of the New York Heart Association’s heart failure classification, contraindication to anticoagulation or excessive risk for bleeding, and a life expectancy of less than 2 years. The institutional review board in our hospital approved this study. To ensure the accuracy of the results, it is necessary for those patients in our hospital that CAGs were applied for evaluation at all endpoints or the end of follow-up. Patients enrolled in the study provided consent for participation.
Coronary angiography analysis
Coronary angiography was performed using a standard percutaneous approach through the radial artery, unless this artery was unavailable, following the intracoronary administration of 100 to 200 µg of nitroglycerin (NTG) to prevent coronary spasms and achieve maximal epicardial vasodilatation. The stenosis position and degree of coronary lesion after different projection positions were determined from the angiograms. A QCA analysis was performed using a standard Cardiovascular Angiography Analysis System (CAAS-5; Pie Medical, Maastricht, the Netherlands). Reference vessel diameter (RVD) (<3.0 mm) located in the target lesion was defined as coronary small vessel. The RVD, minimum lumen diameter (MLD), and lesion length (LL) were measured and recorded. The diameter percentage of stenosis was calculated as the ratio of the MLD and the RVD.
Grouping and PCI treatment
Grouping was performed using sealed envelopes that contained the randomization sequence after 228 patients underwent coronary angiography. A total of 108 patients were included in the CAG-guided group, which executed the PCI-guided treatment according to conventional stent implantation standards in the CAG-guided group (3). A total of 120 patients were included in the IVUS-guided group in which the PCI treatment was guided via the following IVUS criterion.
Following the intracoronary administration of 100-200 µg of NTG, IVUS was performed using a 40 MHz IVUS coronary imaging catheter (Ultracross, Boston Scientific Corporation, Natick, MA, USA), which was advanced distally in the target vessel as far as possible and followed by an automatic pullback (0.5 mm/s) according to the criteria of the American College of Cardiology Clinical Expert Consensus Document on IVUS (4). Measurements in the proximal and distal reference segment vessels were selected within 5 mm of each other and were free of intervening side branches. The average RVD, MLD, LL, minimum lumen area (MLA), and the percentage of plaque burden (PB) of the target coronary small vessel were recorded. All of implanted stents were from the same company (Everolimus-Eluting Coronary Stent System, Boston Scientific, USA), and everolimus-eluting stents were implanted in the target lesion according to the results from these evaluations using an anatomic criteria of an MLA ≤2.0 mm2 and a PB ≥70% for defining functionally significant lesions in small coronary arteries (5).
Coronary angiography and IVUS analyses were performed by two independent observers who were blinded to the clinical characteristics of the patients, and a third physician was used in the case of differing results. Following the PCIs, all patients were treated with clopidogrel 75 mg/day for at least 12 months and aspirin 100 mg/day. Other medications such as statins, angiotensin-converting enzyme inhibitors, beta-blockers, and calcium antagonists were the same as the treatment before PCI.
Assessment of short-term effect
The effects of drug-eluting stent (DES) implantation were recorded and evaluated according to the following success criteria: residual stenosis of less than 20% in the presence of thrombolysis in myocardial infarction flow grade 3 after the PCI procedure. Also, there was no coronary perforation, serious aortic dissection, or acute target blood vessel occlusion in either group. The stent diameter can be matched to the diameter of proximal vessels. The ratio of stent diameter to vessels diameter is 1.0:1.1. Coronary arteriography data, IVUS parameters, the number of stents, stent diameter, length, high-pressure balloons used during post-dilatation, and values of post-PCI MLD were recorded between the two groups.
Follow-up and coronary angiography
Follow-up information was obtained using telephone calls and outpatient visits every 3 months. Despite regular visits, patients were advised to seek treatment in case of an emergency. The total follow-up period lasted 2 years, and all patients completed the follow-up. The primary endpoint was when any major adverse cardiac events (MACEs), defined as cardiac death, nonfatal myocardial infarction, and target lesion revascularization (TLR), including PCI or CABG, occurred. Any death was considered to be cardiac unless it could be proven to be noncardiac. MI was diagnosed according to the definition of the European Society of Cardiology/American College of Cardiology consensus document (6). Target vessel revascularization was defined as any clinically driven revascularization of the target vessel, including PCI or CABG. The secondary endpoint was the incidence of in-stent restenosis and the value of late lumen loss, all of which were also evaluated during the follow-up coronary angiography when any major adverse cardiac events or at the end of follow-up in our hospital. The proportion of the in-stent restenosis (ISR) at the follow-up CAG were recorded and analyzed. Restenosis needed treatment is defined as in-stent stenosis following implantation and a distal, proximal 5 mm edge narrowing of the stent over 50%. Unstable angina was the reason of TLR, which was related to the CAG-evaluated lesion and were indicated to be the same as the lesion previously evaluated with follow-up coronary angiography. It was also recorded during the follow-up period. Revascularization was also performed if necessary.
Statistical analysis
All statistical analyses were performed using the SPSS, version 19.0 (SPSS Inc., Chicago, Illinois, USA). Data are presented as means±standard deviations for continuous variables and a frequency (percentage) for discrete variables. An analysis of discrete variables was performed using the chi-squared test or Fisher’s exact test. The Kolmogorov–Smirnov tests were used to examine normality. Statistical results showed that the data in this group were in a normal distribution. Thus, comparisons of continuous variables were performed using the independent samples t-test. The intraclass correlation coefficient was used to measure and evaluate interobserver and interobserver reliability. Cox univariate and multiple regression analysis were performed to predict the MACE incidence. The Kaplan–Meier curves were constructed for MACE. All tests were two tailed with significance set at p<0.05.
Results
Patient characteristics
Between January and November 2015, a total of 228 patients (131 males and 97 females) with T2DM involving small-vessel coronary lesions were included in this study. Among these, 120 patients were from the IVUS-guided group, 108 were from the CAG-guided group. The baseline and clinical characteristics of the study population are summarized in Table 1. Basic characteristics such as age, sex, hypertension, current smoking status, body mass index (BMI), left ventricular ejection fraction (LVEF), hyperlipidemia, and patient’s therapy before CAG (e.g., antiplatelet, statin, calcium antagonists, angiotensin-converting enzyme/angiotensin receptor blocker or beta-blocker use) did not differ significantly between groups (p>0.05 for all).
Table 1.
Basic and clinical characteristics of patients
| IVUS guided | CAG guided | t/x2 | P-value | |
|---|---|---|---|---|
| Age | 57.6±4.5 | 58.1±5.0 | 0.71 | 0.48 |
| Gender (male) | 64 (53.3) | 67 (62.0) | 1.76 | 0.18 |
| Hypertension | 80 (66.7) | 69 (63.9) | 0.19 | 0.66 |
| Current smokers | 52 (43.3) | 50 (46.3) | 0.20 | 0.65 |
| BMI (kg/m2) | 24.1±2.2 | 23.6±1.7 | 1.32 | 0.19 |
| LVEF (%) | ||||
| Medication | 48.2±4.3 | 48.8±4.5 | 1.17 | 0.24 |
| Statins | 105 (87.5) | 99 (84.6) | 0. 41 | 0.52 |
| Aspirin | 109 (90.8) | 99 (91.7) | 0.18 | 0.64 |
| Clopidogrel | 99 (82.5) | 95 (88) | 1.34 | 0.25 |
| ACEI/ARB | 79 (65.8) | 78 (72.2) | 1.08 | 0.30 |
| Calcium antagonists | 51 (42.5) | 38 (35.2) | 1.27 | 0.26 |
| Beta-blocker | 64 (53.3) | 66 (61.1) | 1.40 | 0.24 |
| Oral antidiabetic | 80 (66.7) | 78 (72.2) | 0.82 | 0.36 |
| Insulin | 32 (26.7) | 25 (23.1) | 0.37 | 0.54 |
| Dietary alone | 8 (6.7) | 5 (4.6) | 0.44 | 0.51 |
Data shown are n (%) or mean±standard deviation. ACEI - angiotensin-converting enzyme; ARB - angiotensin receptor blockers; BMI - body mass index; CAG - coronary angiography; IVUS - intravascular ultrasound; LVEF - left ventricular ejection fraction
IVUS and angiographic parameters
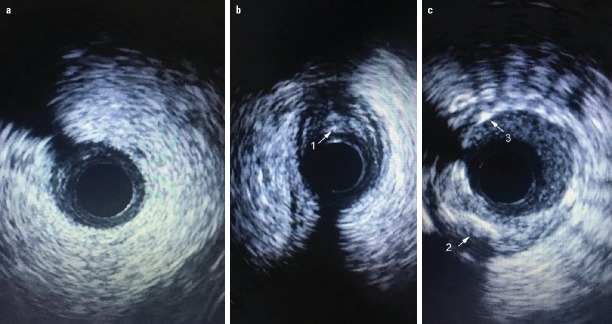
The QCA and IVUS characteristics of the small-vessel coronary lesions between groups are shown in Table 2. Location information (single, double, or triple-vessel lesion), target lesion characteristics (left-anterior descending, left-circumflex, left main coronary artery, calcified, bifurcation and chronic total occlusion lesion), and QCA data did not differ significantly between groups (p>0.05). However, LL was longer under the IVUS measurement when compared to the QCA measurement in the IVUS-guided group (p<0.001). The MLA and the percentage of PB are also shown in Table 2. Pathological features of T2DM involving small-vessel coronary lesions were fibrous plaques and deep calcified plaques. Photographs of these characteristics are shown in Figure 1.
Table 2.
QCA and IVUS parameters
| IVUS guided | CAG guided | t/x2 | P-value | |
|---|---|---|---|---|
| Target lesion | ||||
| Single-vessel lesion | 52 (43.3) | 53 (49.1) | 0.99 | 0.32 |
| Double-vessel lesions | 49 (40.8) | 42 (38.9) | 0.51 | 0.48 |
| Triple-vessel lesions | 19 (15.8) | 13 (12.0) | 0.18 | 0.27 |
| LAD | 57 (38.3) | 41 (31.1) | 1.60 | 0.21 |
| LCX | 30 (20.1) | 28 (21.2) | 0.05 | 0.82 |
| RCA | 37 (24.8) | 40 (30.3) | 1.05 | 0.31 |
| LM | 25 (16.8) | 23 (17.4) | 0.02 | 0.89 |
| Calcified lesions | 83 (69.2) | 73 (67.6) | 1.28 | 0.26 |
| Bifurcation lesions | 45 (37.5) | 39 (36.1) | 0.02 | 0.89 |
| Chronic total | 23 (19.2) | 16 (14.8) | 0.31 | 0.58 |
| occlusion lesions | ||||
| QCA data | ||||
| RVD (mm) | 2.68±0.39 | 2.65±0.35 | 0.63 | 0.45 |
| MLD (mm) | 1.05±0.21 | 1.03±0.29 | 0.71 | 0.08 |
| LL (mm) | 18.57±3.54 | 18.48±3.07 | 0.80 | 0.42 |
| IVUS data | ||||
| RVD (mm) | 2.70±0.32 | 1.37 | 0.17 | |
| MLD (mm) | 1.03±0.24 | 1.73 | 0.09 | |
| LL (mm) | 19.34±3.87* | 4.53 | <0.001 | |
| MLA (mm2) | 1.56±0.49 | |||
| PB (%) | 75.47±5.57 |
Data shown are n (%) or mean±standard deviation. Lesion length in the IVUS-guided group: IVUS data versus QCA data,
P<0.001. CAG - coronary angiography; IVUS - intravascular ultrasound; LAD - left-anterior descending; LCX - left circumflex; LM - left main coronary artery; LL - lesion length; MLA - minimum lumen area; MLD - minimum lumen diameter; PB - plaque burden; QCA - quantitative coronary angiography; RCA - right coronary artery; RVD - reference vessel diameter
Figure 2.
Number at Risk
I IVUS-guided group 120 120 119 118 116 116 116 116 116
CAG-guided group 108107 106 103 101 99 97 97 97
Kaplan–Meier major adverse cardiac event-free curve. Event-free survival in the IVUS-guided group was superior to that in the CAG-guided group (P=0.029). MACE, major adverse cardiac event (cardiac death, nonfatal myocardial infarction, target lesion revascularization)
IVUS - intravascular ultrasound
Characteristics of the implanted stents and follow-up
A total of 120 patients were included in the IVUS-guided group with 149 stents placed, whereas 108 patients were included in the CAG-guided group, which consisted of only 130 stents. A longer stent could be chosen from the beginning when the locations of the two significant lesions were adjacent. Between-group comparisons of the characteristics of the implanted stents are shown in Table 3. Although no statistically significant differences were observed in the coronary angiography before stenting, more implanted stents with a greater diameter and length in the IVUS-guided group were observed when compared with the CAG-guided group (p=0.001, p=0.003, p=0.002). A total of 107 implanted stents (71.8%) in the IVUS-guided group were post-dilatation using high-pressure balloons, while 73 implanted stents (56.2%) were used in the CAG-guided group. The percentage of high-pressure balloons used increased in the IVUS-guided group (p=0.004). Follow-up CAG were performed when any major adverse cardiac events (for the primary endpoint) occurred or at the end of follow-up. Comparison of post-PCI parameters and follow-up CAG parameters and the proportion of ISR were presented in Table 4.
Table 3.
Characteristics of the implanted stents
| IVUS guided | CAG guided | t/x2 | P-value | |
|---|---|---|---|---|
| Stents diameter (mm) | 2.74±0.29 | 2.67±0.23 | 6.87 | 0.001 |
| Stents length (mm) | 19.51±3.00 | 18.63±3.56 | 2.99 | 0.003 |
| Stents number | 1.48±0.72 | 1.36±0.68 | 3.19 | 0.002 |
| Post-dilatation | 107 (71.8) | 73 (56.2) | 8.29 | 0.004 |
Data shown are n (%) or mean±standard deviation. CAG - coronary angiography; IVUS - intravascular ultrasound
Table 4.
Compared the results previous and follow-up CAG parameters
| IVUS guided | CAG guided | t-value | P-value | |
|---|---|---|---|---|
| Post-PCI parameters | ||||
| Post-PCI MLD (mm) | 2.75±0.25 | 2.69±0.21 | 3.09 | 0.002 |
| Follow-up CAG | ||||
| Follow-up MLD (mm) | 2.38±0.26 | 2.23±0.36 | 4.70 | 0.001 |
| Late lumen loss (mm) | 0.37±0.27 | 0.47±0.33 | 2.1 | 0.007 |
| ISR (%) | 15.75±9.34 | 21.83±10.99 | 3.99 | 0.001 |
Data shown are mean±standard deviation.CAG - coronary angiography; IVUS - intravascular ultrasound; ISR - in-stent restenosis; MLD - minimum lumen diameter; PCI - percutaneous coronary intervention
Five implanted stents (3.4%) in the IVUS-guided group experienced ISR, while 12 implanted stents (9.2%) in the CAG-guided group experienced it. The value of late lumen loss and the percentage of ISR (for the secondary endpoint) decreased in the IVUS-guided group (p=0.007, p=0.001). After the Cox univariate regression analysis (Table 5) and the multiple analysis (Table 6) were performed, we could determine the LL (HR/95% CI, p-value, 1.413/1.102–1.812, 0.006), late lumen loss (HR/95% CI, p-value, 1.919/0.015–1.244, 0.039), or ISR (HR/95% CI, p-value, 1.365/1.123–1.659, 0.002) were the independent factors for MACE. The IVUS-guided PCI treatment improved event-free survival (HR/95% CI, p-value, 1.020/0.253–4.104, 0.047), all of which led to a lower incidence of MACE. The case summary of MACE is presented in Table 7. There were 5 patients (4.2% of the group) with MACE in the IVUS-guided group, 1 patient with acute myocardial infarction (AMI), and 4 patients with target lesion revascularization. There were 13 patients (12% of the group) with MACE in the CAG-guided group, 1 patient with AMI, 1 patient with cardiac death, and 11 patients with target lesion revascularization. The Kaplan–Meier curves indicated that the incidence of major adverse clinical events decreased significantly in the IVUS-guided group (log-rank, p=0.029) (Fig. 2), mainly because of reduction of target lesion revascularization (log-rank p=0.037) (Fig. 3).
Table 5.
Cox univariate regression analysis for MACE
| Variable | β | SE | P-value | HR (95% CI) |
|---|---|---|---|---|
| IVUS-guided | -1.090 | 0.526 | 0.038* | 0.036 (0.120, 0.943) |
| LVEF (%) | -0.527 | 0.096 | <0.001* | 0.590 (0.489, 0.713) |
| Lesion length | 0.481 | 0.067 | <0.001* | 1.617 (1.419, 1.843) |
| Post-PCI MLD | -2.975 | 1.226 | 0.015* | 0.051 (0.005, 0.565) |
| Follow-up MLD | -4.651 | 0.646 | <0.001* | 0.010 (0.003, 0.034) |
| Late lumen loss | 29.524 | 5.015 | <0.001* | 6.637 (1.232, 1.575) |
| ISR | 0.239 | 0.038 | <0.001* | 1.270 (1.178, 1.369) |
Data shown are mean±standard deviation.
P<0.05 CI - confidence interval; HR - hazard ratio; IVUS - intravascular ultrasound; ISR - in-stent restenosis; LVEF - left ventricular ejection fraction, MLD - minimum lumen diameter
Table 6.
Cox univariate and multiple regression analysis for MACE
| Variable | β | SE | P-value | HR (95% CI) |
|---|---|---|---|---|
| IVUS-guided | 0.200 | 0.710 | 0.047* | 1.020 (0.253, 4.104) |
| Lesion length | 0.346 | 0.127 | 0.006* | 1.413 (1.102, 1.812) |
| Late lumen loss | 0.652 | 2.474 | 0.039* | 1.919 (0.015, 1.244) |
| ISR | 0.311 | 0.100 | 0.002* | 1.365 (1.123, 1.659) |
Data shown are mean±standard deviation.
P<0.05 CI - confidence interval; HR - hazard ratio; IVUS - intravascular ultrasound; ISR - in-stent restenosis
Table 7.
Case summary of MACE
| Group | Age (year)/gender | Target vessel | Follow-up MLD (mm) | Late lumen loss (mm) | ISR (%) | MACE (month) | Reason for revascularization |
|---|---|---|---|---|---|---|---|
| IVUS-guided group | |||||||
| 1 | 54/F | LAD | 1.38 | 1.42 | 50.71 | 6.0 | ECG(+) |
| 2 | 62/F | LCX | 0.99 | 1.51 | 60.40 | 15.0 | Serum marker(+) |
| 3 | 56/M | LAD | 1.10 | 1.21 | 52.38 | 12.0 | ECG(+) |
| 4 | 63/M | LM | 1.45 | 1.54 | 51.51 | 9.0 | ECG(+) |
| 5 | 59/M | RCA | 1.13 | 1.42 | 55.69 | 12.0 | ECG(+) |
| CAG-guided group | |||||||
| 1 | 58/M | LCX | 0.95 | 1.25 | 56.82 | 3.0 | ECG(+) |
| 2 | 68/F | RCA | 1.20 | 1.50 | 56.00 | 6.0 | ECG(+) |
| 3 | 52/M | LM | 1.15 | 1.60 | 58.19 | 9.0 | ECG(+) |
| 4 | 58/M | LAD | 1.02 | 1.78 | 63.58 | 12.0 | Serum marker(+) |
| 5 | 50/F | LAD | 1.15 | 1.40 | 58.19 | 9.0 | ECG(+) |
| 6 | 64/M | LCX | 1.11 | 1.19 | 51.74 | 12.0 | ECG(+) |
| 7 | 63/F | LAD | 1.20 | 1.30 | 52.00 | 15.0 | ECG(+) |
| 8 | 67/M | RCA | ----- | ------ | ------ | 21.0 | Cardiac death(+) |
| 9 | 60/M | LCX | 1.32 | 1.34 | 50.38 | 18.0 | ECG(+) |
| 10 | 63/F | LAD | 1.03 | 1.22 | 54.22 | 15.0 | ECG(+) |
| 11 | 65/M | RCA | 1.33 | 1.53 | 53.50 | 9.0 | ECG(+) |
| 12 | 59/F | LAD | 0.96 | 1.42 | 59.66 | 18.0 | ECG(+) |
| 13 | 60/M | LAD | 1.08 | 1.24 | 53.45 | 12.0 | ECG(+) |
ACEI - angiotensin-converting enzyme; ARB - angiotensin receptor blockers; BMI - body mass index; CAG - coronary angiography; IVUS - intravascular ultrasound; LVEF - left ventricular ejection fraction; F – female; M – male; MLA - minimum lumen area; MACE - major adverse cardiac events
Figure 3.
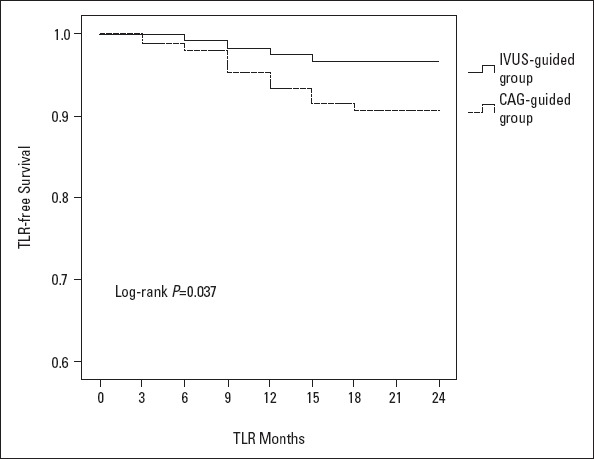
Kaplan–Meier target lesion revascularization event-free curve. Event-free survival in the IVUS-guided group was superior to that in the CAG-guided group (P=0.037)
TLR - target lesion revascularization; IVUS - intravascular ultrasound
Discussion
To the best of our knowledge, the principal novel finding of this investigation was that IVUS-guided PCIs should be routinely endorsed in T2DM with small-vessel coronary lesions. Calcified, diffuse, or multivessel lesions were evident in the coronary vessels of diabetic patients, with small-vessel lesions being particularly evident. Studies have shown that 30%–50% of interventional therapies involve small-vessel lesions (7), which suggests that this investigation defines the direction in our understanding of complications associated with these types of interventions. IVUS-based cross-sectional imaging provides additional information, such as vessel, lumen, and plaque area, and thus aids the efficacy of this percutaneous coronary intervention, making it more effective and safe (8). The results of the present investigation suggest that IVUS guidance ensures appropriate treatment of small-vessel coronary lesions, including the atherosclerotic plaque evaluation, which will likely lead to positive clinical outcomes, especially given the declined incidence of MACE and ISR compared with CAG-guided PCIs.
Status of coronary angiography
Metabolic disorders and disturbances associated with T2DM lead to structural and functional changes in body tissues. Cardiovascular risk factors such as central obesity, hypertension, and lipid metabolism disorders promote the formation of coronary atherosclerotic lesions. Angiography focuses our attention on the narrowing of the coronary lumen as a means to detect atherosclerotic disease and predict coronary events. Glagov et al. (9) showed that coronary remodeling enables patients to develop large atherosclerotic plaques without a reduction in lumen size. Accordingly, angiography would not detect atherosclerosis or accurately assess lesions in these instances, and therefore, the angiogram will seem relatively normal until the disease has progressed significantly. Such diagnostics may overlook some patients who should receive PCI treatment, which could lead to an increased rate of MACE. When coronary angiography underestimates the diameter of the target vessels, the stent may not be expanded sufficiently, or there may be an incomplete stent apposition (ISA), whereas an overestimation of the target vessel diameter can aggravate vascular damage and cause ISR. The detection rate of coronary angiography was low in mildly calcified lesions.
The advantages of using intravascular ultrasound
Before the development and implementation of IVUS guidance, the true diameter of coronary vessels could not be judged accurately, and thus a general assessment of vascular morphology and pathological conditions was lacking. IVUS can qualitatively analyze coronary atherosclerotic plaque, accurately measure the degree of stenosis, and identify the areas of plaque with an elevated risk of rupture. The study (10) showed a 71% ISA whereas a perfected apposition shown in coronary angiography revealed 64% ISA, even in the case of high-pressure expansion. The residual clearance between the mesh stent and intima, the interlayer of the plaque rupture resulting from the expansion of the balloon, can be filled with a contrast agent, resulting in a false impression of completion when angiography is used alone. However, IVUS can monitor the ISA and the effects of the expanded balloon to achieve the best effect of stent release, which has been shown to significantly reduce the incidence of MACE following PCI (11) and provide the basis for appropriate stent re-expansion (12, 13). Most recently, observations from a multicenter study of more than 8,500 patients (14) showed that IVUS-guided PCI in 3,349 patients changed the operative decision in more than 75% of cases, resulting in utilization of longer, more appropriately sized stents. Failure to cover the full length of the diseased coronary arterial segment with a DES was associated with an increased rate of subacute stent thrombosis and late restenosis (15). The American College of Cardiology/American Heart Association Guideline gives a Class IIb recommendation for the IVUS utilization in the left main coronary artery stenting and for the assessment of nonleft main intermediate coronary stenosis (3). The effect of IVUS guidance for PCI in small-vessel coronary lesion with T2DM remained unclear.
PCIs for small-vessel coronary lesion
PCI treatment for small-vessels coronary lesions has always been controversial. This is mainly based on three key points that also represent the typical challenges of PCI in small vessels: (a) it is often considered that small vessels are not worthwhile a stent because of no clinical concern for the patients; (b) high restenosis rate; and (c) difficult deliverability of stents in small vessels. However, studies have shown that 30%–50% of interventional therapies involve small-vessel lesions (7). Thus, more small coronary vessels with symptomatic CAD shall be stented safely and effectively than is currently thought and more clinical benefits shall be given to those patients with really small vessels as primary target for their disease. In small vessels, IVUS has also clearly demonstrated that coronary angiography often mistakenly underestimates the real RVDs, especially in patients with diffuse disease such as diabetics. Briguori et al. (16) showed that the difference between intravascular ultrasound and angiographic artery size (△IVUS-Angio) was ≥0.30 mm. Investigation showed that the LL was longer under the IVUS measurement when compared to QCA measurement (19.34±3.87 vs. 18.57±3.54, p<0.001).
In the present investigation, the small-vessel coronary lesions were calcified and contained eccentric plaque, which is characteristic in patients with diabetes (Fig. 1). Even mildly stenotic segments containing plaque often show features that predict clinical instability (17). This clinical instability may lead to increased cardiovascular events and, in many cases, current interventional therapies adequate to treat small-vessel lesions coronary, especially given the complicated pathological changes associated with T2DM CHD (18, 19). With the widespread use of DES, incidence rate of ISR of DES reduced dramatically compared to that of BMS (20, 21). Although the use of drug-eluting stents has led to a declined ISR, there has been up to a 24%–40% restenosis rate following PCI in small-vessel coronary lesions associated with T2DM (22). This prognostic challenge requires physicians to more accurately analyze and diagnose small-vessel lesions to improve the therapeutic effect of available interventional approaches. The present investigation showed that the addition of IVUS guidance was associated with larger post-PCI MLD and more post-dilatation, which translated into clinically and statistically significant lower rates of target lesion revascularization or ISR after DES implantation.
Figure 1.
Number at risk
IVUS-guided group 120120 119 118 116 115 115 115 115
CAG-guided group 108 107 106 103 100 98 96 95 95
Characteristics of coronary small-vessels, fibrous or calcified plaques and stent in IVUS. Image (a) normal coronary small vessel; Image (b) (1) fibrous plaques; Image (c) (2) deep calcified plaque and (3) stent implant
An analysis of the National Cardiovascular Data Registry data showed that IVUS was used in approximately 20% of total PCI procedures (23). The cost of the equipment and lack of reimbursement are considerations for underutilization (24). Although the economic benefits of IVUS decrease after the first year, the benefits persist during a longer follow-up (11). Alberti et al. (25) suggested that IVUS guidance was not only cost-effective, but may be cost-saving among patients who are at an increased risk of restenosis. This investigation demonstrated that patients in the IVUS-guided group had an increased number of implanted stents, a greater diameter, and length of stents and that the percentage of high-pressure balloons used post-dilatation decreased the incidence of stent restenosis when compared with the CAG-guided group. The Kaplan–Meier curves indicated that the IVUS-guided PCI was associated with a reduction in the risk of MACEs. One mechanism by which IVUS is beneficial is the reduction in ischemia or clinically driven TLR at a mean follow-up of 24 months. The present investigation suggested that the IVUS-guided treatment was a cost-effective method for small-vessel coronary lesions with T2DM.
Combination of CAG and IVUS
The IVUS technology is capable of discerning plaque architecture and composition, and when combined with the angiographic “roadmap”, it may facilitate a tailored stent procedure that treats the disease, rather than just the stenosis. Although IVUS offers a more objective means of assessment than coronary angiography, IVUS can only measure a certain segment of the coronary lesion instead of the whole coronary artery, which means the IVUS image has a precise “point effect”. It is difficult for IVUS imaging to provide the same cross-section image at different temporal points; however, coronary angiography can show the entire lesion of the coronary artery with a rough “comprehensive effect”. So, it is not realistic for IVUS to completely replace coronary angiography. However, these technologies should complement one another as the IVUS measurement based on coronary angiography offers a profound diagnostic insight for patients suffering from diabetes-induced coronary small lesions. A critical finding of this investigation is that the combined use of CAG and IVUS-guided procedures resulted in improved cardiac-event-free survivability, which supports its use as a powerful diagnostic tool.
Study limitations
This study has some limitations. First, it is a single-center study, and the range of this investigation only included patients with diabetes-induced small-vessel coronary lesions. A multicenter study of larger patient populations is warranted to confirm these data, and the included studies cannot generalize the IVUS usage for all lesions. Second, although the QCA-Cardiovascular Measurement System is extensively validated, its potential to detect diffuse disease, often present in small coronary arteries, is limited. Third, how to respond to the IVUS images was left to each operator’s discretion, with no specific guidelines for optimal stenting. Fourth, the sample size was relatively small, which limited the statistical power, and the strength of the conclusions in small-vessels coronary lesions remains challenging in the DES era because of failure to cross the tight lesion (with the undeployed stent) due to vessel tortuosity. This limitation needs to be overcome in the future.
Conclusion
In summary, the results suggest that an IVUS-guided intervention therapy improves the event-free survival in small-vessel coronary lesions in T2DM. The IVUS imaging may be an improved diagnostic tool, thus yielding improved clinical outcomes.
Acknowledgments
Grants, contracts, and financial support: Vital Science and Technology Development Planning Department of Shandong Province, China (Grant no. 2015GSF118045).
Footnotes
Conflict of interest: None declared.
Peer-review: Externally peer-reviewed.
Authorship contributions: Concept – L.L., L.Q.C.; Design – L.L., L.W., L.Q.C.; Supervision – L.W., C.J.Z.; Fundings – C.J.Z., Y.M., J.H.W.; Materials – J.H.W.; Data collection &/or processing – C.J.Z., Y.M.; Analysis &/or interpretation – L.L., L.Q.C.; Literature search – C.J.Z., Y.M.; Writing – L.L., L.W., L.Q.C.; Critical review – L.L., L.Q.C.
References
- 1.Haffner SM, Lehto S, Rönnemaa T, Pyörälä K, Laakso M. Mortality from coronary heart disease in subjects with type 2 diabetes and in nondiabetic subjects with and without prior myocardial infarction. N Engl J Med. 1998;339:229–34. doi: 10.1056/NEJM199807233390404. [DOI] [PubMed] [Google Scholar]
- 2.Banning AP, Westaby S, Morice MC, Kappetein AP, Mohr FW, Berti S, et al. Diabetic and nondiabetic patients with left main and/or 3-vessel coronary artery disease:comparison of outcomes with cardiac surgery and paclitaxel-eluting stents. J Am Coll Cardiol. 2010;55:1067–75. doi: 10.1016/j.jacc.2009.09.057. [DOI] [PubMed] [Google Scholar]
- 3.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. American College of Cardiology Foundation;American Heart Association Task Force on Practice Guidelines;Society for Cardiovascular Angiography and Interventions. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J Am Coll Cardiol. 2001;37:1478–92. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- 5.Lee CH, Tai BC, Soon CY, Low AF, Poh KK, Yeo TC, et al. New set of intravascular ultrasound-derived anatomic criteria for defining functionally significant stenoses in small coronary arteries (results from Intravascular Ultrasound Diagnostic Evaluation of Atherosclerosis in Singapore [IDEAS] study) Am J Cardiol. 2010;105:1378–84. doi: 10.1016/j.amjcard.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. J Am Coll Cardiol. 2000;36:959–69. doi: 10.1016/s0735-1097(00)00804-4. [DOI] [PubMed] [Google Scholar]
- 7.Kastrati A, Schömig A, Dirschinger J, Mehilli J, Dotzer F, von Welser N, et al. A randomized trial comparing stenting with balloon angioplasty in small vessels in patients with symptomatic coronary artery disease. ISAR-SMART Study Investigators. Intracoronary Stenting or Angioplasty for Restenosis Reduction in Small Arteries. Circulation. 2000;102:2593–8. doi: 10.1161/01.cir.102.21.2593. [DOI] [PubMed] [Google Scholar]
- 8.Jensen LO, Thayssen P, Mintz GS, Egede R, Maeng M, Junker A, et al. Comparison of intravascular ultrasound and angiographic assessment of coronary reference segment size in patients with type 2 diabetes mellitus. Am J Cardiol. 2008;101:590–5. doi: 10.1016/j.amjcard.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Glagov S, Weisneberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. N Engl J Med. 1987;316:1371–5. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 10.Brodie BR, Cooper C, Jones M, Fitzgerald P, Cummins F Post dilatation Clinical Compartative Study (POSTIT) Investigators. Is adjunctive balloon postdilatation necessary after coronary stent deployment?Final results from the POSTIT trial. Catheter Cardiovasc Interv. 2003;59:184–92. doi: 10.1002/ccd.10474. [DOI] [PubMed] [Google Scholar]
- 11.Ahn JM, Kang SJ, Yoon SH, Park HW, Kang SM, Lee JY, et al. Meta-analysis of outcomes after intravascular ultrasound-guided versus angiography-guided drugeluting stent implantation in 26, 503 patients enrolled in three randomized trials and 14 observational studies. Am J Cardiol. 2014;113:1338–47. doi: 10.1016/j.amjcard.2013.12.043. [DOI] [PubMed] [Google Scholar]
- 12.Kume T, Waseda K, Koo BK, Yock PG, Botelho R, Verheye S, et al. Intravascular ultrasound analysis of small vessel lesions treated with the Sparrow coronary stent system:results of the CARE II trial. Catheter Cardiovasc Interv. 2014;83:19–24. doi: 10.1002/ccd.24867. [DOI] [PubMed] [Google Scholar]
- 13.Takagi K, Shannon J, Basavarajaiah S, Latib A, Al-Lamee R, Hasegawa T, et al. Discrepancies in vessel sizing between angiography and intravascular ultrasound varies according to the vessel evaluated. Int J Cardiol. 2013;168:3791–6. doi: 10.1016/j.ijcard.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Witzenbichler B, Maehara A, Weisz G, Neumann FJ, Rinaldi MJ, Metzger DC, et al. Relationship between intravascular ultrasound guidance and clinical outcomes after drug-eluting stents:the assessment of dual antiplatelet therapy with drug-eluting stents (ADAPT-DES) study. Circulation. 2014;129:463–70. doi: 10.1161/CIRCULATIONAHA.113.003942. [DOI] [PubMed] [Google Scholar]
- 15.Costa MA, Angiolillo DJ, Tannenbaum M, Driesman M, Chu A, Patterson J, et al. STLLR Investigators. Impact of stent deployment procedural factors onlong-term effectiveness and safety of sirolimus-eluting stents (final results of the multicenter prospective STLLR trial) Am J Cardiol. 2008;101:1704–11. doi: 10.1016/j.amjcard.2008.02.053. [DOI] [PubMed] [Google Scholar]
- 16.Briguori C, Tobis J, Nishida T, Vaghetti M, Albiero R, Di Mario C, et al. Discrepancy between angiography and intravascular ultrasound when analysing small coronary arteries. Eur Heart J. 2002;23:247–54. doi: 10.1053/euhj.2001.2730. [DOI] [PubMed] [Google Scholar]
- 17.Goldstein JA, Maini B, Dixon SR, Brilakis ES, Grines CL, Rizik DG, et al. Detection of lipid-core plaques by intracoronary near-infrared spectroscopy identifies high risk of periprocedural myocardial infarction. Circ Cardiovasc Interv. 2011;4:429–37. doi: 10.1161/CIRCINTERVENTIONS.111.963264. [DOI] [PubMed] [Google Scholar]
- 18.Sugihara M, Miura S, Nishikawa H, Ike A, Mori K, Iwata A, et al. Characteristics of patients and types of lesions in patients with drug-eluting or bare-metal stent implantation in small coronary arteries from the FU-Registry. J Cardiol. 2013;61:117–21. doi: 10.1016/j.jjcc.2012.09.008. [DOI] [PubMed] [Google Scholar]
- 19.Quadri G, D'ascenzo F, Bollati M, Moretti C, Omedé P, Sciuto F, et al. Diffuse coronary disease:short-and long-term outcome after percutaneous coronary intervention. Acta Cardiol. 2013;68:151–60. doi: 10.1080/ac.68.2.2967272. [DOI] [PubMed] [Google Scholar]
- 20.Palmerini T, Benedetto U, Biondi-Zoccai G, Della Riva D, Bacchi-Reggiani L, Smits PC, et al. Long-Term Safety of Drug-Eluting and Bare-Metal Stents:Evidence From a Comprehensive Network Meta-Analysis. J Am Coll Cardiol. 2015;65:2496–507. doi: 10.1016/j.jacc.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 21.Bavry AA, Bhatt DL. Appropriate use of drugeluting stents:balancing the reduction in restenosis with the concern of late thrombosis. Lancet. 2008;371:2134–43. doi: 10.1016/S0140-6736(08)60922-8. [DOI] [PubMed] [Google Scholar]
- 22.Schofer J, Schlüter M, Gershlick AH, Wijns W, Garcia E, Schampaert E, et al. E-SIRIUS Investigators. Sirolmus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries:double-blind, randomised controlled trial (E-SIRIUS) Lancer. 2003;362:1093–9. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- 23.Dattilo PB, Prasad A, Honeycutt E, Wang TY, Messenger JC. Contemporary patterns of fractional flow reserve and intravascular ultrasound use among patients undergoing percutaneous coronary intervention in the United States:insights from the National Cardiovascular Data Registry. J Am Coll Cardiol. 2012;60:2337–9. doi: 10.1016/j.jacc.2012.08.990. [DOI] [PubMed] [Google Scholar]
- 24.Elgendy IY, Choi C, Bavry AA. The Impact of Fractional Flow Reserve on Revascularization. Cardiol Ther. 2015;4:191–6. doi: 10.1007/s40119-015-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Alberti A, Giudice P, Gelera A, Stefanini L, Priest V, Simmonds M, et al. Understanding the economic impact of intravascular ultrasound (IVUS) Eur J Health Econ. 2016;17:185–93. doi: 10.1007/s10198-015-0670-4. [DOI] [PubMed] [Google Scholar]