Abstract
Background
Brain arteriovenous malformations (AVMs) are the single most common cause of intracerebral haemorrhage in young adults. Brain AVMs also cause seizure(s) and focal neurological deficits (in the absence of haemorrhage, migraine or an epileptic seizure); approximately one‐fifth are incidental discoveries. Various interventions are used in an attempt to eradicate brain AVMs: neurosurgical excision, stereotactic radiosurgery, endovascular embolization, and staged combinations of these interventions. This is an update of a Cochrane Review first published in 2006, and last updated in 2009.
Objectives
To determine the effectiveness and safety of the different interventions, alone or in combination, for treating brain AVMs in adults compared against either each other, or conservative management, in randomized controlled trials (RCTs).
Search methods
The Cochrane Stroke Group Information Specialist searched the Cochrane Stroke Group Trials Register (last searched 7 January 2019), the Cochrane Central Register of Controlled Trials (CENTRAL; 2019, Issue 1) in the Cochrane Library, MEDLINE Ovid (1980 to 14 January 2019), and Embase OVID (1980 to 14 January 2019). We searched international registers of clinical trials, the contents pages of relevant journals, and bibliographies of relevant articles (November 2009). We also contacted manufacturers of interventional treatments for brain AVMs (March 2005).
Selection criteria
We sought RCTs of any intervention for brain AVMs (used alone or in combination), compared against each other or against conservative management, with relevant clinical outcome measures.
Data collection and analysis
One author screened the results of the updated searches for potentially eligible RCTs for this updated review. Both authors independently read the potentially eligible RCTs in full and confirmed their inclusion according to the inclusion criteria. We resolved disagreement by discussion. We assessed the risk of bias in included studies and applied GRADE.
Main results
We included one trial with 226 participants: A Randomized trial of Unruptured Brain Arteriovenous Malformations (ARUBA), comparing intervention versus conservative management for unruptured brain AVMs (that had never bled). The quality of evidence was moderate because we found just one trial that was at low risk of bias other than a high risk of performance bias due to participants and treating physicians not being blinded to allocated treatment. Data on functional outcome and death at a follow‐up of 12 months were provided for 218 (96%) of the participants in ARUBA. In this randomized controlled trial (RCT), intervention compared to conservative management increased death or dependency (modified Rankin Scale score ≥ 2, risk ratio (RR) 2.53, 95% confidence interval (CI) 1.28 to 4.98; 1 trial, 226 participants; moderate‐quality evidence) and the proportion of participants with symptomatic intracranial haemorrhage (RR 6.75, 95% CI 2.07 to 21.96; 1 trial, 226 participants; moderate‐quality evidence), but there was no difference in the frequency of epileptic seizures (RR 1.14, 95% CI 0.63 to 2.06; 1 trial, 226 participants; moderate‐quality evidence). Three RCTs are ongoing.
Authors' conclusions
We found moderate‐quality evidence from one RCT including adults with unruptured brain AVMs that conservative management was superior to intervention with respect to functional outcome and symptomatic intracranial haemorrhage over one year after randomization. More RCTs will help to confirm or refute these findings.
Plain language summary
Interventions for treating abnormal tangles of blood vessels in the brain in adults
Question
Do treatments for adults with abnormal tangles of blood vessels in the brain prevent death, disability and stroke due to bleeding compared to usual medical care?
Background
Abnormal tangles of blood vessels in the brain, known as brain arteriovenous malformations (AVMs) are the single most common cause of stroke due to bleeding in the brain (known as intracerebral haemorrhage, or ICH) in young adults. Brain AVMs can also leave young people disabled for life and cause epilepsy. How they should be treated, if at all, is highly controversial. The main options are: 1) medical treatment of epileptic seizures and headaches (sometimes known as 'conservative management'); or 2) one or more of the following 'interventional' treatments: neurosurgery, endovascular embolization (glue, coils, or particles are lodged within the AVM via a catheter inserted temporarily in the groin), or radiosurgery (a non‐invasive treatment involving focused beams of radiation).
Search date
14 January 2019
Study characteristics
We found one published randomized controlled trial, including 226 adults.
Key results
We found moderate‐quality evidence of harm (stroke due to bleeding in the brain, and death or dependency) over one year of follow‐up from interventional treatments compared to conservative management for adults who had a brain AVM that had never bled. The long‐term risks are unknown.
Quality of the evidence
Overall, the quality of the evidence was moderate because there was just one trial and it did not use blinding. More information will become available from the three trials that are ongoing.
Summary of findings
Summary of findings for the main comparison. Interventions compared to conservative management for brain arteriovenous malformations in adults.
| Interventions compared to conservative management for brain arteriovenous malformations in adults | ||||||
|
Patient or population: adults with a brain arteriovenous malformation
Setting: secondary care
Intervention: interventions (neurosurgery, embolization, or stereotactic radiosurgery, alone or in combination) Comparison: conservative management | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Risk with conservative management | Risk with intervention | |||||
| Death or dependence | Study population | RR 2.53 (1.28 to 4.98) | 213 (1 RCT) | ⊕⊕⊕⊝ Moderate | High risk of performance bias due to participants and treating physicians not being blinded | |
| 95 per 1000 | 241 per 1000 (122 to 474) | |||||
| Symptomatic intracerebral haemorrhage | Study population | RR 6.75 (2.07 to 21.96) | 218 (1 RCT) | ⊕⊕⊕⊕ Moderate | High risk of performance bias due to participants and treating physicians not being blinded | |
| 28 per 1000 | 189 per 1000 (58 to 616) | |||||
| Epileptic seizure | Study population | RR 1.14 (0.63 to 2.06) | 217 (1 RCT) | ⊕⊕⊝⊝ Moderate | High risk of performance bias due to participants and treating physicians not being blinded | |
| 159 per 1000 | 181 per 1000 (100 to 327) | |||||
| Symptomatic radiation necrosis ‒ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| Quality of life ‒ not reported | ‐ | ‐ | ‐ | ‐ | ‐ | |
| *The risk in the intervention group (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio | ||||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect Moderate certainty: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low certainty: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low certainty: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
Background
Description of the condition
Brain arteriovenous malformations (AVMs) are distinguished from other types of intracranial vascular malformation by having a tangled anastomosis of arteries and veins (without intervening capillaries) in the brain parenchyma. Arteriovenous shunting occurs in a central nidus (the point towards which one or more feeding arteries converge and from which one or more veins drain) (Doppman 1971). Brain AVMs are sometimes accompanied by arterial aneurysms within the nidus, on vessels feeding it, or on vessels remote from it. The other types of intracranial vascular malformation (such as cavernous and venous malformations) are classified separately from brain AVMs, not only on the basis of morphological differences, but also because of broad differences in prognosis and response to the different interventions available. A description suitable for non‐medical readers can be found in the information leaflet Vascular malformations of the brain published by the Brain and Spine foundation (freely downloadable in PDF format from www.brainandspine.org.uk).
The cause of brain AVMs is unknown, but it is assumed to be multifactorial with contributions from both genetic polymorphisms and environmental exposures (Lasjaunias 1997). Brain AVMs have long been assumed to be congenital; there is no strong evidence for this although it is possible. Brain AVMs do affect neonates and can arise early in childhood, but most come to light in young adults, and an unknown number remain asymptomatic. We have not addressed the management of brain AVMs in children in this review because their morphology, clinical features, and management tend to differ from AVMs in adults (Fullerton 2005; Lasjaunias 1995).
Technological advances in both non‐invasive imaging of the brain and catheter angiography, and their widening availability, have increased the rate of detection of brain AVMs (Brown 1996). Computed tomography angiography and magnetic resonance angiography appear to have good sensitivity and specificity following ICH for the detection of intracranial vascular malformations (Josephson 2014). A contemporary estimate of the prevalence of brain AVMs is approximately 18 per 100,000 adults (Al‐Shahi 2002), and their incidence in unselected populations is approximately one per 100,000 adults per year (Al‐Shahi 2003; Stapf 2003). It is known that brain AVMs account for 1% to 2% of all strokes, 4% of strokes in young adults, and 9% of subarachnoid haemorrhages. Although brain AVMs are responsible for 4% of all intracerebral haemorrhages, they cause as many as one‐third in young adults (Al‐Shahi 2001). Brain AVMs also cause focal and secondary generalized seizures; and they also seem to cause transient, persistent, or progressive focal neurological deficits (in the absence of haemorrhage, migraine or an epileptic seizure). However, there is uncertainty about their role in causing other symptoms such as cognitive impairment and headache. The overall annual haemorrhage rate of AVMs is 2.3%, which is higher for ruptured (4.8%) than unruptured (1.3%) AVMs (Kim 2014). The long‐term crude annual case fatality is 1% to 1.5% (Stapf 2006a).
Description of the intervention
Small, simple, superficial AVMs with cortical venous drainage in 'non‐eloquent' areas of the brain are amenable to complete microsurgical excision. But neurosurgery carries the risks of a craniotomy and general anaesthetic in addition to the operative hazards of excising a brain AVM. The Spetzler‐Martin grading system is used by many to estimate the risk of surgical intervention by grading AVMs on a five‐point scale, determined by their maximum nidus diameter (< 3 cm = 1 point, 3 cm to 6 cm = 2 points, > 6 cm = 3 points), pattern of venous drainage (superficial only = 0 points, any deep = 1 point), and 'eloquence' of adjacent brain (eloquent = 1 point, non‐eloquent = 0 points) (Spetzler 1986). The use of a single dose of either linear accelerator or gamma knife stereotactic radiotherapy ('radiosurgery') is limited to brain AVMs with a compact nidus of 3 cm diameter or less (with or without prior endovascular embolization), and approximately three years after treatment it achieves radiographic evidence of nidus obliteration in 50% to 80%. Stereotactic radiosurgery leaves people exposed to the risk of haemorrhage before occlusion, which may be incomplete, and radionecrosis of adjacent brain. Endovascular embolization can occlude brain AVMs completely (depending on their vascular anatomy, or 'angioarchitecture'), and is also used for nidus volume reduction prior to radiosurgery or neurosurgery. The benefits of embolization may be offset by the risk of vessel or aneurysm rupture, and the reflux of embolic agents into vessels supplying eloquent areas of the brain.
How the intervention might work
The main target of the intervention (neurosurgery, radiosurgery, endovascular treatment) is to occlude the blood flow in the AVM to prevent rebleeding. However, interventions are associated with significant intervention‐related mortality and morbidity. Neurosurgery is a major undertaking, but the removal of the AVM is considered to be durable. Radiosurgery is a less invasive intervention than neurosurgery, but the effects are slow and it usually takes at least two years for an AVM treated by radiosurgery to be obliterated. Endovascular embolization is less invasive than neurosurgery. It aims to block the artery and to reduce blood flow into the AVM. The major concerns about endovascular embolization are incomplete obliteration of the AVM and vessel rupture.
Why it is important to do this review
ICH may occur in the untreated clinical course ('conservative management') of brain AVMs. Complete obliteration of the brain AVM nidus probably causes a reduction in case fatality and in the subsequent occurrence or recurrence of ICH. These benefits may, of course, be offset by the risks of the interventional treatment itself. Although brain AVM management narrative reviews, guidelines and scientific statements do exist (AVM Study Group 1999; Cenzato 2017; Derdeyn 2017; Ogilvy 2001), an update of this systematic review of randomized controlled trials (RCTs) of interventional treatments for brain AVMs in adults is needed following the publication of a recent RCT (Mohr 2013).
Objectives
To determine the effectiveness and safety of the different interventions, alone or in combination, for treating brain AVMs in adults compared against either each other, or conservative management, in randomized controlled trials (RCTs).
Methods
Criteria for considering studies for this review
Types of studies
Randomized trials, in which one intervention (or combination of interventions) was compared concurrently either against conservative management, or against another intervention (or combination of interventions), whether published in any year or unpublished. Pseudo‐randomized trials were not eligible.
Types of participants
People of either sex, aged 16 years or over with a radiologically definite brain AVM that has not previously been treated, with any type of clinical presentation (haemorrhage, epilepsy, incidental discovery, focal neurological deficit). We excluded any studies involving people with other types of intracranial vascular malformation, such as cavernous malformations (Rigamonti 1987), and venous malformations (Rigamonti 1988), in which arteriovenous shunting does not occur, and we have also excluded studies of AVMs solely involving the dura mater rather than the brain parenchyma (Kobayashi 2014).
Types of interventions
Trials comparing any of the interventions or combination of interventions below. Where possible, we intended to collect information about the concurrent use of medical therapies (e.g. antiepileptic drugs), and the actual or planned use of other interventions after the scheduled treatment period, as these may influence outcome during follow‐up.
Neurosurgical excision
Stereotactic radiosurgery
Endovascular embolization
Aneurysm treatment
Conservative management with medical therapy (e.g. antiepileptic drugs)
Types of outcome measures
We intended to identify the number of people originally randomly allocated to each treatment group with the intention of treating them, and the number who have the following outcomes at set time points or at the end of follow‐up, or both.
Primary outcomes
Death or dependence from any cause, measured on a standard rating scale such as the modified Rankin Scale, at one year after randomization (and preferably later).
Secondary outcomes
Symptomatic intracranial haemorrhage (confirmed by computed tomography (CT), magnetic resonance imaging (MRI), blood in the cerebrospinal fluid, or by autopsy after clinical deterioration), measured as the time to haemorrhage or as its occurrence at one year after randomization (and preferably later).
Epilepsy: time to first epileptic seizure (for people without seizures before randomization); time to 12‐month remission of epilepsy after randomization (for the subgroup of people presenting with epilepsy).
Symptomatic radiation necrosis, detected on MRI.
Quality of life.
Search methods for identification of studies
See the methods for the Cochrane Stroke Group Specialised register. We searched for trials in all languages and arranged translation of relevant articles if necessary.
Electronic searches
The Cochrane Stroke Group Information Specialist searched the Cochrane Stroke Group Trials Register (last searched 7 January 2019), the Cochrane Central Register of Controlled Trials (CENTRAL; Issue 1, 2019) in the Cochrane Library; Appendix 1), MEDLINE Ovid (1980 to 14 January 2019; Appendix 2), and Embase OVID (1980 to 14 January 2019; Appendix 3).
On 15 January 2019 one review author searched the following international registers of clinical trials.
US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov/ct2/home) (Appendix 4);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP; apps.who.int/trialsearch) (Appendix 5).
Searching other resources
We checked bibliographies of relevant articles in an effort to identify further published, ongoing, and unpublished RCTs.
Data collection and analysis
Selection of studies
One review author (SMZ) screened the abstracts of the updated search results for potentially eligible RCTs for this updated review, and obtained the full published articles or trial registry entries for studies likely to be relevant RCTs. Both review authors (SMZ, RASS) independently read the potentially eligible RCTs in full and confirmed their inclusion according to the inclusion criteria. We resolved disagreements by discussion.
Data extraction and management
Two review authors (SMZ, RASS) used a standard data extraction form to independently extract data on risk of bias, other RCT characteristics, participants (including age at presentation (i.e. the clinical event that led to the diagnosis of a brain AVM), the mode of presentation, demographics), methods, imaging (including angioarchitectural features (maximum nidus diameter, presence and location of aneurysms, and deep/superficial venous drainage)), interventions, results, and outcomes during follow‐up. If required data were not available in a publication, we contacted the principal investigator of the trial for further information.
Assessment of risk of bias in included studies
Two review authors (SMZ, RASS) independently assessed the risk of bias in the included RCTs according to the criteria of the Cochrane 'Risk of bias' tool (Higgins 2011). We resolved any disagreements by discussion and we agreed on the overall quality of the evidence for each outcome, using the GRADE approach (Higgins 2011).
Measures of treatment effect
Because outcome events from brain AVMs are relatively infrequent, and because it is likely that the length of follow‐up in any RCT will be variable, we planned to analyze outcomes at one — and preferably five — years following randomization. Where possible, we intended to calculate risk ratios (RRs) or odds ratios (ORs) (according to the frequencies of outcomes) and absolute risk reductions (using the Peto odds ratio to calculate absolute risks across a variety of control group risks) for each dichotomous outcome, calculate hazard ratios for each time‐to‐event outcome, and use a random‐effects model. If we had identified more than one comparable RCT, we would have calculated a weighted estimate of the odds ratio across studies using the Peto method.
Unit of analysis issues
We planned to refer to guidance in the Cochrane Handbook for Systematic Reviews of Interventions for advice on any analytical issues (Higgins 2011).
We intended to perform an intention‐to‐treat analysis using data on the number of people with each outcome event in each allocated treatment group, regardless of adherence and irrespective of whether or not the patient was subsequently deemed ineligible or otherwise excluded from treatment or follow‐up.
Dealing with missing data
We contacted study authors for unpublished data if required data were missing, and used all the data that were available to us.
Assessment of heterogeneity
We planned to investigate inconsistency between RCTs using the I² statistic. We planned to consider heterogeneity to be significant if I² was greater than 50%, in which case we would explore individual trial characteristics to identify potential sources of heterogeneity.
Assessment of reporting biases
We planned to assess the likelihood of reporting biases through the use of a funnel plot if there were sufficient data (defined as at least 10 trials), and if asymmetry were present, we would have attempted to explore causes of it.
Data synthesis
See Measures of treatment effect.
GRADE and 'Summary of findings' table
We created a 'Summary of findings' table using the following outcomes: death or dependence, symptomatic intracranial haemorrhage, epileptic seizure, symptomatic radiation necrosis, and quality of life. We used the five GRADE considerations (study limitations; consistency of effect; imprecision; indirectness; and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes (Atkins 2004). We used the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011); and we used GRADEpro GDT software. We justified all decisions to downgrade the quality of studies.
Subgroup analysis and investigation of heterogeneity
We planned to analyze the following subgroups (all but two of which are dichotomous) if there were sufficient RCTs to justify this approach.
Clinical presentation: intracranial haemorrhage versus other modes of presentation (epileptic seizure(s), incidental discovery, focal neurological deficit (unrelated to haemorrhage or epileptic seizure), other modes of presentation (e.g. headache, pulsatile tinnitus).
Attributes of angioarchitecture (defined according to the Joint Writing Group).
Presence or absence of co‐existing aneurysm(s).
Existence of any deep venous drainage (versus exclusively superficial venous drainage).
Maximum nidus diameter (less than or equal to 3 cm versus more than 3 cm).
Spetzler‐Martin grading system (grades 1 to 5) for studies of surgical excision (Spetzler 1986).
Sensitivity analysis
We had planned to do sensitivity analyses including only participants:
treated by stereotactic radiosurgery and conservative management;
treated by neurosurgical excision and conservative management;
treated by endovascular embolization and conservative management;
with a Spetzler Martin Grade I‐II AVM.
Results
Description of studies
Results of the search
Our search identified 14 potentially eligible RCTs; see Figure 1 for the flowchart describing the searches done for this update. We excluded 10 studies because they did not met all of our inclusion criteria (ChiCTR1800017616; Frenzel 2008; Lin 2017; MTI Onyx; n‐BCA Trial 2002; NCT00783523; NCT02552459; NCT03076099; NCT03306836; Ornstein 1991). Three RCTs were still ongoing (NCT00857662; NCT03691870; NCT02098252); we will assess these for inclusion with the next update. This left one RCT that satisfied all of the inclusion criteria for this review (Mohr 2013).
1.
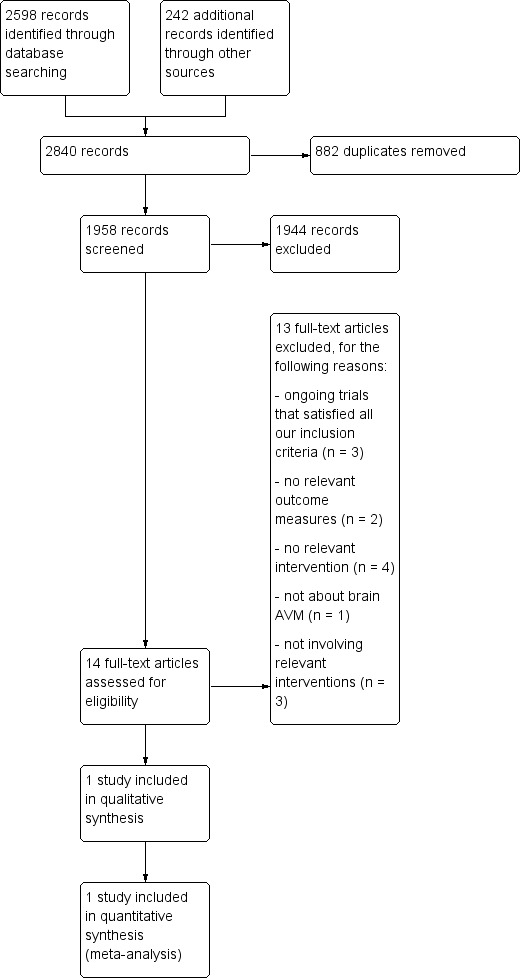
Flow diagram
Included studies
One RCT fulfilled the inclusion criteria for this review (Mohr 2013). A Randomized trial of Unruptured Brain Arteriovenous malformations (ARUBA) (www.arubastudy.org) was an international, multicentre, randomized, controlled, open, prospective clinical trial comparing interventional treatment (endovascular, surgical, and/or radiation therapy) versus conservative management for unruptured brain AVMs in adults.
The intended sample size was a 1:1 random assignment of 800 patients aged 18 years and over, diagnosed with an unruptured brain AVM considered treatable by the local investigators. The endpoint was a composite event of death from any cause or stroke (haemorrhage or infarction confirmed by imaging). Secondary outcomes included risk of death or clinical impairment (modified Rankin Score of 2 or greater) with clinical outcome status measured by the modified Rankin Scale, National Institutes of Health Stroke Scale (NIHSS), and EuroQol. Patients were randomly assigned to best possible interventional treatment (endovascular, surgical, and/or radiation therapy) versus conservative management alone and it was planned that they should be followed for a minimum of five years after randomization. ARUBA's primary aim was to determine whether conservative management was superior (or, alternatively, not inferior) to interventional treatment for preventing the composite outcome of death from any cause or stroke (symptomatic haemorrhage or infarction confirmed by imaging). ARUBA's secondary aim was to determine whether conservative management of unruptured brain AVMs decreased the risk of death or clinical impairment (modified Rankin Score of 2 or greater) at five years after randomization compared to invasive treatment. Randomisation of patients started on 4 April 2007, and stopped on 15 April 2013. The trial was stopped when a data and safety monitoring board appointed by the National Institute of Neurological Disorders and Stroke (part of the US National Institutes of Health) recommended halting randomization because of superiority of conservative management. At that point, outcome data were available for 223 (99%) of 226 randomized participants with a mean follow‐up of 33.3 months. One hundred and fourteen participants were assigned to interventional therapy and 109 participants to conservative management. The halting was based on the results of the second planned interim analysis that showed efficacy of conservative management for the prevention of death or stroke with an observed log‐rank Z statistic of 4.10, exceeding the pre‐specified stopping boundary value of 2.87. We applied to use the ARUBA archived clinical research dataset for this systematic review, because the outcomes in the trial were not reported at the time point we had pre‐specified for analysis in the protocol of this review (www.ninds.nih.gov/Current‐Research/Research‐Funded‐NINDS/Clinical‐Research/Archived‐Clinical‐Research‐Datasets). Summary data from ARUBA for this review's primary and secondary outcomes at the timepoint we had pre‐specified for analysis were provided by Dr Jessica Overbey (Senior Biostatistician at Mount Sinai Medical Center) on 4 March 2019, although complete outcome data were only available for 218 of the trial's participants.
Ongoing studies
We identified three ongoing RCTs that satisfied all of the inclusion criteria for this review. One RCT examines whether AVMs treated by endovascular embolization with Onyx is equivalent to treatment with TRUFILL n‐butyl cyanoacrylate n‐BCA (NCT00857662). Another RCT examines whether conservative management or intervention will reduce the risk of death or debilitating stroke and test if endovascular treatment can improve the safety and efficacy of surgery or radiosurgery (NCT02098252. The third RCT examines transvenous embolization versus transarterial embolization (NCT03691870).
Excluded studies
We excluded 10 studies that did not meet the inclusion criteria of this review ('Characteristics of excluded studies' table).
We excluded two RCTs because the outcome measures failed to meet our inclusion criteria. We have excluded the RCT which intended to test equivalence between the embolic agents n‐Butyl cyanoacrylate (n‐BCA) liquid and polyvinyl alcohol (PVA) particles for the pre‐operative embolization of brain AVMs (n‐BCA Trial 2002). The study was conducted to obtain US Food and Drug Administration (FDA) approval for the use of n‐BCA. Following correspondence with the n‐BCA Trial 2002 principal investigator (Thomas A Tomsick, Department of Radiology, University Hospital, Ohio, USA), the random allocation sequence was contained within consecutively‐numbered randomization envelopes (although we do not know how the sequence was generated); unblinding may have affected participants (after their embolization, which was not performed under general anaesthesia) and the central radiologist determining the degree of vascular occlusion achieved was unblinded because n‐BCA is radiopaque. The trial was funded by Cordis Neurovascular, manufacturers of n‐BCA. None of the primary or secondary outcome measures in the n‐BCA Trial 2002 met our inclusion criteria: the primary outcome was the degree of vascular occlusion achieved (judged by the per cent nidus reduction and number of feeding vessels treated on catheter angiography), and secondary outcomes were the duration of subsequent surgical resection and the number of transfusions required during surgery. Although important clinical outcomes were reported (such as deaths, intracranial haemorrhages, Glasgow Outcome Scale, etc.), absolute numbers were not reported and it was not entirely clear that these outcomes were assessed at a standard time interval after treatment, making a meaningful comparison between treatment arms of the RCT impossible.
Following personal communication with Dr Gary Duckwiler (Department of Radiology, UCLA Medical Center, Los Angeles, USA), we found one unpublished 'non‐inferiority' RCT comparing the liquid embolic agent Onyx with n‐BCA for the pre‐operative embolization of brain AVMs (MTI Onyx). This study was sponsored by Microtherapeutics Inc (MTI), the manufacturers of Onyx, and the preliminary results are available on the US Food and Drug Administration website. We excluded this study too, because its outcome measures failed to meet our inclusion criteria.
We excluded four RCTs because there was no interventional treatment for brain AVMs performed according to our inclusion criteria: one RCT examined the safety and efficacy of fMRI‐guided microsurgery of AVMs in 184 participants receiving surgery for AVMs (Lin 2017); one RCT examined the use of minocycline and doxycycline as medical therapy for AVMs and giant aneurysms (Frenzel 2008); one ongoing RCT examined the effect of intraoperative standard dose heparin sodium versus low dose heparin sodium (NCT03306836); and one ongoing RCT examined the effect of doxycycline therapy to decrease matrix metalloproteinase (MMP) expression in vascular malformation tissue (NCT00783523).
We excluded three RCTs because they did not test interventions meeting our inclusion criteria: one RCT studied three different blood‐pressure‐lowering treatments to induce deliberate hypotension during surgical resection of brain AVMs (Ornstein 1991); one RCT examined the effect of dexmedetomidine on post‐operative blood pressure in participants undergoing brain arteriovenous malformation embolization (NCT03076099); and one RCT examined the effect of combined medication of sufentanil and dexmedetomidine in patient‐controlled analgesia after neurosurgery (NCT02552459).
We excluded one RCT because it did not treat adults with a brain AVM (ChiCTR1800017616).
Risk of bias in included studies
Allocation
The risk of bias in random sequence generation and allocation concealment in the ARUBA trial was low (Mohr 2013). Randomisation was done centrally through a web‐based system that confirmed eligibility before issuing a treatment assignment (Figure 2; Figure 3).
2.
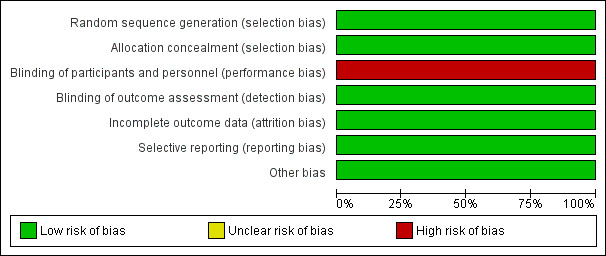
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Blinding
The risk of bias from blinding of participants and personnel in the ARUBA trial was high (Mohr 2013). The study did not blind the intervention and comparator. Assignments were not masked to participants, clinicians, or investigators. Risk of bias from blinding of outcome assessment was high. Detection and reporting of outcome events was non‐blinded, but their adjudication was blinded.
Incomplete outcome data
The risk of bias from incomplete outcome data in the ARUBA trial was low (Mohr 2013). Seven participants (five in the interventional therapy group and two in the control group) discontinued their participation in the trial (3%) during follow‐up.
Selective reporting
Bias from selective outcome reporting in the ARUBA trial was low (Mohr 2013). The study protocol was available.
Other potential sources of bias
None.
Effects of interventions
See: Table 1
The primary and secondary outcomes of this Cochrane Review were available for 218 of the 226 ARUBA trial participants with outcome data available one year after randomization. See Table 1 for the main comparison.
Primary outcome: death or dependence
At one year, 26/108 participants randomized to intervention and 10/105 participants randomized to conservative management were dead or dependent (RR 2.53, 95% CI 1.28 to 4.98; 1 trial, 213 participants; moderate‐quality evidence: Analysis 1.1).
1.1. Analysis.
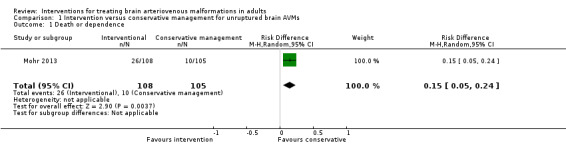
Comparison 1 Intervention versus conservative management for unruptured brain AVMs, Outcome 1 Death or dependence.
Secondary outcome: symptomatic intracranial haemorrhage
Twenty‐one of 111 (18.9%) participants allocated to intervention and 3/107 (2.8%) participants allocated to conservative management experienced a symptomatic intracranial haemorrhage (RR 6.75, 95% CI 2.07 to 21.96; 1 trial, 218 participants; moderate‐quality evidence; Analysis 1.2).
1.2. Analysis.
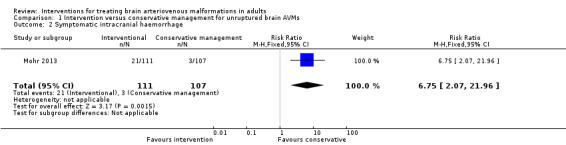
Comparison 1 Intervention versus conservative management for unruptured brain AVMs, Outcome 2 Symptomatic intracranial haemorrhage.
Secondary outcome: epilepsy
At one year, 20/110 participants randomized to intervention and 17/107 participants randomized to conservative management developed at least one seizure (RR 1.14, 95% CI 0.63 to 2.06; 1 trial, 217 participants; moderate‐quality evidence; Analysis 1.3).
1.3. Analysis.
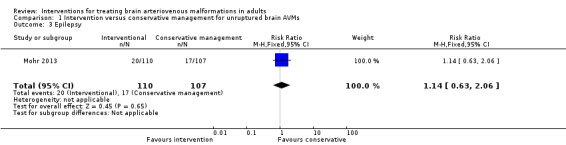
Comparison 1 Intervention versus conservative management for unruptured brain AVMs, Outcome 3 Epilepsy.
We did not conduct sensitivity analysis due to lack of data.
Discussion
Summary of main results
The present review found one trial comparing intervention (or combination of interventions) versus conservative management for adults with a radiologically definite brain AVM that has not previously bled or been treated. We found that intervention for unruptured brain AVMs caused a statistically significant increase in the proportion of participants who were dead or dependent at one‐year follow‐up and a statistically significant increase in the risk of symptomatic intracranial haemorrhage, but no significant differences in seizures.
Overall completeness and applicability of evidence
We included the only published RCT that met our inclusion criteria. Data for the secondary outcome 'epilepsy: time to first epileptic seizure (for people without seizures prior to randomization) and time to 12‐month remission of epilepsy after randomization (for the subgroup of people presenting with epilepsy)' were not available. We therefore used any seizure at one year after randomization. Data for the secondary outcomes 'quality of life' and 'symptomatic radiation necrosis detected on MR imaging' were also not available. Three ongoing RCTs are awaiting completion and publication of their results (Characteristics of ongoing studies). Guidelines have endorsed both intervention and conservative management for unruptured brain AVMs before the results of the ARUBA trial (Ogilvy 2001; Starke 2009). Thereafter, the reception of ARUBA's results has varied, although a scientific statement recently concluded: "The discussion of treatment options with patients should include consideration of these risks weighed carefully against the relative risks of different intervention strategies and life expectancy" (Cenzato 2017; Derdeyn 2017). Brain AVMs still pose a regular management problem because there is still uncertainty about the risks of treatment compared with the long‐term clinical course of people with an untreated brain AVM, and the benefits/risks of one type of intervention compared with others. This uncertainty is reflected by the variation in current treatment practices within and between different countries.
Quality of the evidence
The quality of the one included RCT was moderate, with only a high risk of performance bias due to participants and treating physicians not being blinded.
Potential biases in the review process
We tried to avoid publication bias by using a very comprehensive search strategy and including published and unpublished studies.
Agreements and disagreements with other studies or reviews
There is evidence from observational cohorts that interventional treatment is detrimental for unruptured brain AVMs compared with their untreated clinical course (Al‐Shahi 2014; Mohr 2004; Stapf 2006b; Wedderburn 2008). The findings from these observational cohorts are consistent with the data in this review. The similarities support the generalisability of the results.
NCT02098252, an RCT comparing any form(s) of intervention (with endovascular procedures, neurosurgery, or radiotherapy, alone or in combination) versus conservative management, is now underway. Whether or not conservative management proves no worse, or possibly better than interventional therapy, long‐term follow‐up of the participants in this RCT will be needed since the results of the ARUBA trial.
Authors' conclusions
Implications for practice.
There was evidence from one randomized controlled trial that conservative management was superior over one year compared to one intervention (or combination of interventions) for adults with a radiologically definite brain arteriovenous malformation that had not previously bled or been treated.
Implications for research.
The ongoing TOBAS trial compares intervention (endovascular embolization, neurosurgery, and radiotherapy, alone or in combination) versus conservative management alone for brain arteriovenous malformations (AVMs). Randomized controlled trials (RCTs) are the ideal method of evaluation of interventions because of the uncertainty about whether to intervene at all (e.g. in older people and in subgroups with a more benign prognosis, such as unruptured brain AVMs). RCTs might also settle the uncertainty about which of the interventions to use when treatment seems appropriate (e.g. previously ruptured, small, uncomplicated, superficial AVMs in brain areas that are not eloquent, and which might be equally suited to surgical resection or endovascular embolization). In view of the heterogeneity of brain AVMs, efforts should be made to identify subgroups who may benefit most from conservative management, intervention, or certain types of intervention. In view of this heterogeneity, the rarity of brain AVMs, and relative infrequency of outcome events, future RCTs should be large, inevitably requiring multicentre collaboration (Al‐Shahi 2005). Follow‐up in these RCTs should be long enough to ascertain a sufficient number of early and delayed outcomes to determine effectiveness or equivalence of interventions. To be meaningful and encompass the potential adverse effects of interventions, these outcomes should include case fatality (both all‐cause and brain AVM‐related), death or dependency, first‐ever and recurrent intracranial haemorrhage, first‐ever and recurrent epileptic seizure(s), measures of disability/dependence, quality of life, and an assessment of obliteration/recurrence of the brain AVM. Additionally, treatments must be individually validated as beneficial on their own merits in new RCTs (Magro 2017). There are also grounds for other RCTs comparing different interventions against each other for ruptured brain AVMs.
What's new
| Date | Event | Description |
|---|---|---|
| 22 March 2019 | New search has been performed | Literature searches updated. Inclusion of 1 published RCT (ARUBA) with 226 participants; ongoing trials added. |
| 22 March 2019 | New citation required and conclusions have changed | The conclusion is changed based upon the results of 1 RCT (ARUBA). New first author. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 1, 2006
| Date | Event | Description |
|---|---|---|
| 3 December 2009 | New citation required but conclusions have not changed | New first author. |
| 3 December 2009 | New search has been performed | Updated with the addition of details of one new ongoing RCT (ARUBA). No change to conclusions. |
| 26 August 2008 | Amended | Converted to new review format. |
Acknowledgements
We are grateful to members of the Cochrane Stroke Group editorial team and external peer reviewers for making constructive comments on this review.
Appendices
Appendix 1. CENTRAL search strategy
Cochrane Library Issue 1, 2019
#1 [mh ^"Intracranial Arteriovenous Malformations"]
#2 (AVM or AVMs or bAVM or bAVMs):ti,ab
#3 cerebrovascular malformation*:ti,ab
#4 [mh ^"arteriovenous malformations"] or [mh ^"arteriovenous fistula"]
#5 [mh "cerebral arteries"] or [mh ^"cerebral veins"] or [mh "cerebral ventricles"] or [mh "cerebral arterial diseases"] or [mh ^"intracranial arterial diseases"]
#6 #4 and #5
#7 ((cranial or cerebral or cerebell* or brain* or dural or supratentorial or intracerebral or intracranial) near/5 (arteriovenous or vascular) near/5 (malformation* or fistula*)):ti,ab
#8 ((cranial or cerebral or cerebell* or brain* or dural or supratentorial or intracerebral or intracranial) near/5 arteriovenous near/5 (aneurysm* or shunt* or anomal* or anastomos*)):ti,ab
#9 ((cranial or cerebral or cerebell* or brain* or dural or supratentorial or intracerebral or intracranial) near/5 angioma*):ti,ab
#10 #1 or #2 or #3 or #6 or #7 or #8 or #9
Appendix 2. MEDLINE OVID search strategy
MEDLINE OVID (1980 to 14 January 2019)
1. Intracranial Arteriovenous Malformations/
2. (AVM or AVMs or bAVM or bAVMs).tw.
3. cerebrovascular malformation$.tw.
4. arteriovenous malformations/ or arteriovenous fistula/
5. exp cerebral arteries/ or cerebral veins/ or exp cerebral ventricles/ or exp cerebral arterial diseases/ or intracranial arterial diseases/
6. 4 and 5
7. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 (arteriovenous or vascular) adj5 (malformation$ or fistula$)).tw.
8. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 arteriovenous adj5 (aneurysm$ or shunt$ or anomal$ or anastomos$)).tw.
9. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 angioma$).tw.
10. 1 or 2 or 3 or 6 or 7 or 8 or 9
11. exp animals/ not humans.sh.
12. 10 not 11
13. Randomized Controlled Trials as Topic/
14. random allocation/
15. Controlled Clinical Trials as Topic/
16. control groups/
17. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
18. double‐blind method/
19. single‐blind method/
20. Placebos/
21. placebo effect/
22. Drug Evaluation/
23. randomized controlled trial.pt.
24. controlled clinical trial.pt.
25. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
26. (random$ or RCT or RCTs).tw.
27. (controlled adj5 (trial$ or stud$)).tw.
28. (clinical$ adj5 trial$).tw.
29. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
30. (surgical adj5 (group$ or subject$ or patient$)).tw.
31. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
32. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
33. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
34. (placebo$ or sham).tw.
35. trial.ti.
36. (assign$ or allocat$).tw.
37. controls.tw.
38. or/13‐37
39. 12 and 38
Appendix 3. Embase OVID search strategy
EMBASE OVID (1980 to 14 January 2019)
1. brain arteriovenous malformation/ or cerebrovascular malformation/
2. (AVM or AVMs or bAVM or bAVMs).tw.
3. cerebrovascular malformation$.tw.
4. arteriovenous malformation/ or arteriovenous fistula/
5. exp brain artery/ or brain vein/ or exp brain ventricle/ or cerebral artery disease/
6. 4 and 5
7. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 (arteriovenous or vascular) adj5 (malformation$ or fistula$)).tw.
8. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 arteriovenous adj5 (aneurysm$ or shunt$ or anomal$ or anastomos$)).tw.
9. ((cranial or cerebral or cerebell$ or brain$ or dural or supratentorial or intracerebral or intracranial) adj5 angioma$).tw.
10. 1 or 2 or 3 or 6 or 7 or 8 or 9
11. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
12. 10 not 11
13. Randomized Controlled Trial/
14. Randomization/
15. Controlled Study/
16. control group/
17. clinical trial/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/ or controlled clinical trial/
18. Double Blind Procedure/
19. Single Blind Procedure/ or triple blind procedure/
20. placebo/
21. (random$ or RCT or RCTs).tw.
22. (controlled adj5 (trial$ or stud$)).tw.
23. (clinical$ adj5 trial$).tw.
24. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
25. (surgical adj5 (group$ or subject$ or patient$)).tw.
26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
27. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
28. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
29. (placebo$ or sham).tw.
30. trial.ti.
31. (assign$ or allocat$).tw.
32. controls.tw.
33. or/13‐32
34. 12 and 33
Appendix 4. ClinicalTrials.gov search strategy
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov); Interventional Studies | Arteriovenous Malformations
Appendix 5. World Health Organization International Clinical Trials Registry Platform search strategy
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch) Basic search: arteriovenous malformations Phases are: ALL
Data and analyses
Comparison 1. Intervention versus conservative management for unruptured brain AVMs.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death or dependence | 1 | 213 | Risk Difference (M‐H, Random, 95% CI) | 0.15 [0.05, 0.24] |
| 2 Symptomatic intracranial haemorrhage | 1 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.75 [2.07, 21.96] |
| 3 Epilepsy | 1 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.63, 2.06] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Mohr 2013.
| Methods | International, multicentre, prospective, randomized, controlled, open, adjudicator‐blinded, clinical trial | |
| Participants | 226 patients aged > 18 years diagnosed with an unruptured brain AVM considered treatable by the local investigators were randomized, and 223 were analysed | |
| Interventions | Medical management with interventional therapy (neurosurgery, embolization, or stereotactic radiotherapy, alone or in combination) versus medical management alone | |
| Outcomes | The primary outcome is time to the composite outcome of death from any cause or symptomatic stroke (stroke is defined as a clinically symptomatic event (any new focal neurological deficit, seizure, or new‐onset headache) that is associated with imaging findings of haemorrhage or infarction). The secondary outcome is clinical impairment at 5 years with an mRS score of 2 or higher | |
| Notes | The trial was funded by the US National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done centrally through a web‐based system that confirmed eligibility before issuing a treatment assignment. Participants were assigned in a 1‐to‐1 ratio (random permuted block design using blocks of size 2, 4, or 6, randomly selected with equal probability, stratified by clinical site) |
| Allocation concealment (selection bias) | Low risk | Randomisation was done centrally through a web‐based system that confirmed eligibility before issuing a treatment assignment. Participants were assigned in a 1‐to‐1 ratio (random permuted block design using blocks of size 2, 4, or 6, randomly selected with equal probability, stratified by clinical site) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Assignments are not masked to participants, clinicians, or investigators |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Detection and reporting of outcome events was non‐blinded, but their adjudication was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3% loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Both primary and secondary outcomes were reported |
| Other bias | Low risk | |
AVM: arteriovenous malformations mRS: modified Rankin Scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ChiCTR1800017616 | This was an RCT comparing the effect and outcomes of different embolic agents in the treatment of AVMs for participants with an AVM in head and neck region. It was not a study in adults with a brain AVM |
| Frenzel 2008 | This was a randomized study on the use of minocycline and doxycycline as medical therapy for AVMs and giant aneurysms. The intervention failed to meet our inclusion criteria |
| Lin 2017 | This was a randomized study on the safety and efficacy of fMRI‐guided microsurgery of AVMs in 184 participants receiving surgery for AVMs. It was not a study of an interventional treatment for brain AVMs |
| MTI Onyx | This was a prospective 'non‐inferiority trial', but the methods of randomization were not described, 8 participants were excluded after randomization, and the 'intention to treat' group was not 'as randomized'. It involved participants undergoing pre‐operative endovascular embolization of a brain AVM. The interventions studied were liquid n‐butyl cyanoacrylate (n‐BCA) compared with the liquid embolic agent Onyx. The primary and secondary outcomes in this study did not meet the inclusion criteria for this review |
| n‐BCA Trial 2002 | This was a prospective, multi‐centre, single‐blind, randomized trial. It involved participants undergoing pre‐operative endovascular embolization of a brain AVM. The interventions studied were liquid n‐butyl cyanoacrylate (n‐BCA)/tantalum powder/ethiodized oil mixture, compared with polyvinyl alcohol (PVA) particles ± coils. The primary outcome measures (% reduction of maximum AVM nidus dimensions in 3 planes on post‐embolization catheter angiography, and mean number of feeding vessels embolized) and secondary outcome measures (surgical resection time, transfusion/fluid requirements during surgery) did not meet the selection criteria for this review. Clinical outcome data (Glasgow Outcome Score and NIH Stroke Score) were provided, but at unspecified points following treatment, making meaningful analysis impossible. The reported analysis was not truly intention‐to‐treat |
| NCT00783523 | This is a randomized study on the effect of doxycycline therapy to decrease matrix metalloproteinase (MMP) expression in the vascular malformation tissue. It is not a study of an interventional treatment for brain AVMs |
| NCT02552459 | This is a randomized study of the effect of combined medication of sufentanil and dexmedetomidine in patient‐controlled analgesia after neurosurgery. It is not a study comparing 2 different interventional treatments for brain AVMs |
| NCT03076099 | This is a randomized study of the effect of dexmedetomidine on post‐operative blood pressure in participants undergoing brain AVM embolization. It is not a study comparing 2 different interventional treatments for brain AVMs |
| NCT03306836 | This is a randomized study of the effect of different anticoagulation regimens on activated coagulation time safety coverage rate during surgery. It is not a study comparing 2 different interventional treatments for brain AVMs |
| Ornstein 1991 | This was a randomized study of the safety and efficacy of 3 hypotensive agents in 30 participants undergoing resection of AVMs with deliberate hypotension. It was not a study of an interventional treatment for brain AVMs |
AVM: arteriovenous malformation fMRI: functional magnetic resonance imaging NIH: National Institutes of Health RCT: randomized controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT00857662.
| Trial name or title | US multicenter, randomized controlled study comparing the performance of Onyx(EVOH) and TRUFILL® (n‐BCA) in presurgical embolization of brain arteriovenous malformations (BAVMs) |
| Methods | Multicentre, prospective, randomized, controlled, open label, clinical trial |
| Participants | Patients of any age, diagnosed with a brain AVM (with a Spetzler‐Martin grade of I, II, III, or IV) and the patient is a candidate for surgical resection of the AVM post embolization |
| Interventions | Onyx (investigational device) versus TRUFILL (control device) in the presurgical embolization of brain AVMs |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2001 |
| Contact information | Gary Duckwiler MD |
| Notes | Sponsor: Medtronic Neurovascular Clinical Affairs |
NCT02098252.
| Trial name or title | Treatment Of Brain AVMS (TOBAS) Study: a randomized controlled trial and registry |
| Methods | International, multicentre, prospective, randomized, controlled, open label, clinical trial |
| Participants | Patients aged ≥18 years diagnosed with a brain AVM |
| Interventions | Management may include interventional therapy (with endovascular procedures, neurosurgery, or radiotherapy, alone or in combination) or conservative management |
| Outcomes | Primary outcome measures
Secondary outcome measures
|
| Starting date | May 2014 |
| Contact information | Jean Raymond MD |
| Notes |
NCT03691870.
| Trial name or title | Transvenous Approach for the Treatment of Cerebral Arteriovenous Malformations (TATAM) |
| Methods | Prospective, randomized, open label, clinical trial |
| Participants | Any patient harbouring a brain AVM (ruptured or unruptured) in whom transvenous embolization (TVE) is considered |
| Interventions | Standard transarterial embolization (TAE) versus transvenous embolization (TVE) |
| Outcomes | Primary outcome measures
Secondary outcome measures
(There are 10 more secondary outcomes in addition to those mentioned here) |
| Starting date | August 2018 |
| Contact information | Jean Raymond MD |
| Notes |
AVM: arteriovenous malformation mRS: modified Rankin Scale
Differences between protocol and review
Because we only identified one trial, we could not conduct sensitivity analyses.
Contributions of authors
RA‐SS and Charles Warlow conceived, designed and wrote the first version of this review, which has been updated by JR and RA‐SS, and thereafter by SMZ and RA‐SS.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Remmert Adriaan Laan Foundation, Netherlands.
Foundation for a research fellowship in Edinburgh.
Declarations of interest
We have no personal, political, academic, or financial conflicts of interest with this work. Susanna M Zuurbier: none known. Rustam Al‐Shahi Salman: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Mohr 2013 {published data only}
- Mohr JP, Parides MK, Stapf C, Moquete E, Moy CS, Overbey JR, international ARUBA investigators. Medical management with or without interventional therapy for unruptured brain arteriovenous malformations (ARUBA): a multicentre, non‐blinded, randomised trial. Lancet 2013;383(9917):614‐21. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
ChiCTR1800017616 {unpublished data only}
- ChiCTR1800017616. A randomized controlled trial for comparing the effect and outcomes with different embolic agent: absolute ethanol, onyx and n‐BCA in the treatment of AVM. chictr.org.cn/hvshowproject.aspx?id=13489 (first reported 6 August 2018).
Frenzel 2008 {published data only}
- Frenzel T, Lee CZ, Kim H, Quinnine NJ, Hashimoto T, Lawton MT, et al. Feasibility of minocycline and doxycycline use as potential vasculostatic therapy for brain vascular malformations: pilot study of adverse events and tolerance. Cerebrovascular Diseases (Basel, Switzerland) 2008;25(1‐2):157–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lin 2017 {published data only}
- Lin F, Jiao Y, Wu J, Zhao B, Tong X, Jin Z, et al. Effect of functional MRI‐guided navigation on surgical outcomes: a prospective controlled trial in patients with arteriovenous malformations. Journal of Neurosurgery 2017;126(6):1863‐72. [DOI] [PubMed] [Google Scholar]
MTI Onyx {unpublished data only}
- Microtherapeutics Inc (MTI). FDA website: www.fda.gov/ohrms/dockets/ac/03/minutes/3975m1_Sum%20Min.pdf 2005.
- Loh Y, Duckwiler GR, Onyx Trial Investigators. A prospective, multicenter, randomized trial of the Onyxliquid embolic system and N‐butyl cyanoacrylate embolization of cerebral arteriovenous malformations. Journal of Neurosurgery 2010;113(4):733‐41. [DOI] [PubMed] [Google Scholar]
n‐BCA Trial 2002 {published data only}
- n‐BCA Trial Investigators. N‐butyl cyanoacrylate embolization of cerebral arteriovenous malformations: results of a prospective, randomized, multi‐center trial. American Journal of Neuroradiology 2002;23(5):748‐55. [PMC free article] [PubMed] [Google Scholar]
NCT00783523 {unpublished data only}
- NCT00783523. Influence of matrix metalloproteinase on brain arteriovenous malformation hemorrhage. clinicaltrials.gov/ct2/show/NCT00783523 (first reported 31 October 2008).
NCT02552459 {unpublished data only}
- NCT02552459. Effect of dexmedetomidine combined with sufentanil for postoperative intravenous analgesia in neurosurgery: a randomized controlled study. clinicaltrials.gov/ct2/show/NCT02552459 (first reported 17 September 2015).
NCT03076099 {unpublished data only}
- NCT03076099. Effects of dexmedetomidine on post‐operative blood pressure in patients undergoing brain arteriovenous malformation embolization. clinicaltrials.gov/ct2/show/NCT03076099 (first reported 9 March 2017).
NCT03306836 {unpublished data only}
- NCT03306836. Multi‐center, single blind, prospective randomized controlled trial of exploration of anticoagulation program in cerebral aneurysm and arteriovenous malformations with hybrid operation. clinicaltrials.gov/ct2/show/NCT03306836 (first reported 11 October 2017).
Ornstein 1991 {published data only}
- Ornstein E, Young WL, Ostapkovich N, Matteo RS, Diaz J. Deliberate hypotension in patients with intracranial arteriovenous malformations: esmolol compared with isoflurane and sodium nitroprusside. Anesthesia and Analgesia 1991;72(5):639‐44. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT00857662 {unpublished data only}
- NCT00857662. US multicenter, randomized controlled study comparing the performance of onyx (EVOH) and TRUFILL® (n‐BCA) in presurgical embolization of brain arteriovenous malformations (BAVMs). clinicaltrials.gov/ct2/show/NCT00857662 (first reported 9 March 2009).
NCT02098252 {unpublished data only}
- NCT02098252. Treatment of Brain AVMs (TOBAS) Study (TOBAS). clinicaltrials.gov/ct2/show/NCT02098252 (first reported 27 March 2014).
NCT03691870 {unpublished data only}
- NCT03691870. Transvenous Approach for the Treatment of cerebral Arteriovenous Malformations (TATAM): a randomized controlled trial and registry. clinicaltrials.gov/ct2/show/NCT03691870 (first reported 2 October 2018).
Additional references
Al‐Shahi 2001
- Al‐Shahi R, Warlow CP. A systematic review of the frequency and prognosis of arteriovenous malformations of the brain in adults. Brain 2001;124(10):1900‐26. [DOI] [PubMed] [Google Scholar]
Al‐Shahi 2002
- Al‐Shahi R, Fang JS, Lewis SC, Warlow CP. Prevalence of adults with brain arteriovenous malformations: a community based study in Scotland using capture‐recapture analysis. Journal of Neurology, Neurosurgery, and Psychiatry 2002;73(5):547‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Shahi 2003
- Al‐Shahi R, Bhattacharya JJ, Currie DG, Papanastassiou V, Ritchie V, Roberts RC, et al. Prospective, population‐based detection of intracranial vascular malformations in adults: the Scottish Intracranial Vascular Malformation Study (SIVMS). Stroke 2003;34(5):1163‐9. [DOI] [PubMed] [Google Scholar]
Al‐Shahi 2005
- Al‐Shahi R, Warlow CP. Arteriovenous malformations of the brain: ready to randomise?. Journal of Neurology, Neurosurgery and Psychiatry 2005;76:1327‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Al‐Shahi 2014
- Al‐Shahi Salman R, White PM, Counsell CE, du Plessis J, Beijnum J, Josephson CB, et al. Scottish audit of intracranial vascular malformations collaborators. Outcome after conservative management or intervention for unruptured brain arteriovenous malformations. JAMA 2014;311(16):1661–9. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
AVM Study Group 1999
- The Arteriovenous Malformation Study Group. Arteriovenous malformations of the brain in adults. New England Journal of Medicine 1999;340(23):1812‐8. [DOI] [PubMed] [Google Scholar]
Brown 1996
- Brown RD Jr, Wiebers DO, Torner JC, O'Fallon WM. Incidence and prevalence of intracranial vascular malformations in Olmsted County, Minnesota, 1965 to 1992. Neurology 1996;46(4):949‐52. [DOI] [PubMed] [Google Scholar]
Cenzato 2017
- Cenzato M, Boccardi E, Beghi E, Vajkoczy P, Szikora I, Motti E, et al. European consensus conference on unruptured brain AVMs treatment (Supported by EANS, ESMINT, EGKS, and SINCH). Acta Neurochirurgica 2017;159(6):1059‐64. [DOI] [PubMed] [Google Scholar]
Derdeyn 2017
- Derdeyn CP, Zipfel GJ, Albuquerque FC, Cooke DL, Feldmann E, Sheehan JP, on behalf of the American Heart Association Stroke Council. Management of brain arteriovenous malformations: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2017;48(8):e200‐e224. [DOI] [PubMed] [Google Scholar]
Doppman 1971
- Doppman JL. The nidus concept of spinal cord arteriovenous malformations. A surgical recommendation based upon angiographic observations. British Journal of Radiology 1971;44(526):758‐63. [DOI] [PubMed] [Google Scholar]
Fullerton 2005
- Fullerton HJ, Achrol AS, Johnston SC, McCulloch CE, Higashida RT, Lawton MT, for the UCSF BAVM Study Project. Long‐term hemorrhage risk in children versus adults with brain arteriovenous malformations. Stroke 2005;36(10):2099–104. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Joint Writing Group
- Joint Writing Group. Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke 2001;32(6):1430‐42. [DOI] [PubMed] [Google Scholar]
Josephson 2014
- Josephson CB, White PM, Krishan A, Al‐Shahi Salman R. Computed tomography angiography or magnetic resonance angiography for detection of intracranial vascular malformations in patients with intracerebral haemorrhage. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD009372.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2014
- Kim H, Al‐Shahi Salman R, McCulloch CE, Stapf C, Young WL, MARS Coinvestigators. Untreated brain arteriovenous malformation: patient‐level meta‐analysis of hemorrhage predictors. Neurology 2014;83(7):590‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 2014
- Kobayashi A, Al‐Shahi Salman R. Prognosis and treatment of intracranial dural arteriovenous fistulae: a systematic review and meta‐analysis. International Journal of Stroke 2014;9(6):670‐7. [DOI] [PubMed] [Google Scholar]
Lasjaunias 1995
- Lasjaunias P, Hui F, Zerah M, Garcia‐Monaco R, Malherbe V, Rodesch G, et al. Cerebral arteriovenous malformations in children. Management of 179 consecutive cases and review of the literature. Child's Nervous System : ChNS : Official Journal of the International Society for Pediatric Neurosurgery 1995;11(2):66‐79. [DOI] [PubMed] [Google Scholar]
Lasjaunias 1997
- Lasjaunias P. A revised concept of the congenital nature of cerebral arteriovenous malformations. Interventional Neuroradiology 1997;3(4):275‐81. [DOI] [PubMed] [Google Scholar]
Magro 2017
- Magro E, Gentric JC, Darsaut TE, Ziegler D, Bojanowski MW, Raymond J. Responses to ARUBA: a systematic review and critical analysis for the design of future arteriovenous malformation trials. Journal of Neurosurgery 2017;2(126):486‐94. [DOI] [PubMed] [Google Scholar]
Mohr 2004
- Mohr JP, Stapf C, Sciacca RR, Khaw AV, Mast H, Connolly ES, et al. Natural history versus treatment outcome in patients with unruptured brain arteriovenous malformation (AVM). Stroke 2004;35:328. [Google Scholar]
Ogilvy 2001
- Ogilvy CS, Stieg PE, Awad I, Brown RD Jr, Kondziolka D, Rosenwasser R, et al. Recommendations for the management of intracranial arteriovenous malformations: a statement for healthcare professionals from a special writing group of the Stroke Council, American Stroke Association. Stroke 2001;32(6):1458‐71. [DOI] [PubMed] [Google Scholar]
Rigamonti 1987
- Rigamonti D, Drayer BP, Johnson PC, Hadley MN, Zabramski J, Spetzler RF. The MRI appearance of cavernous malformations (angiomas). Journal of Neurosurgery 1987;67(4):518‐24. [DOI] [PubMed] [Google Scholar]
Rigamonti 1988
- Rigamonti D, Spetzler RF, Drayer BP, Bojanowski WM, Hodak J, Rigamonti KH, et al. Appearance of venous malformations on magnetic resonance imaging. Journal of Neurosurgery 1988;69(4):535‐9. [DOI] [PubMed] [Google Scholar]
Spetzler 1986
- Spetzler RF, Martin NA. A proposed grading system for arteriovenous malformations. Journal of Neurosurgery 1986;65(4):476‐83. [DOI] [PubMed] [Google Scholar]
Stapf 2003
- Stapf C, Mast H, Sciacca RR, Berenstein A, Nelson PK, Gobin YP, et al. The New York Islands AVM Study: design, study progress, and initial results. Stroke 2003;34(5):e29‐e33. [DOI] [PubMed] [Google Scholar]
Stapf 2006a
- Stapf C, Mast H, Sciacca RR, Choi JH, Khaw AV, Connolly ES, et al. Predictors of hemorrhage in patients with untreated brain arteriovenous malformation. Neurology 2006;66(9):1350‐55. [DOI] [PubMed] [Google Scholar]
Stapf 2006b
- Stapf C, Mohr JP, Choi JH, Hartmann A, Mast H. Invasive treatment of unruptured brain arteriovenous malformations is experimental therapy. Current Opinion in Neurology 2006;19(1):63‐8. [DOI] [PubMed] [Google Scholar]
Starke 2009
- Starke RM, Komotar RJ, Hwang BY, Fischer LE, Garrett MC, Otten ML, et al. Treatment guidelines for cerebral arteriovenous malformation microsurgery. British Journal of Neurosurgery 2009;23(4):376‐86. [DOI] [PubMed] [Google Scholar]
Wedderburn 2008
- Wedderburn CJ, Beijnum J, Bhattacharya JJ, Counsell CE, Papanastassiou V, Ritchie V, et al. Outcome after interventional or conservative management of unruptured brain arteriovenous malformations: a prospective, population‐based cohort study. Lancet Neurology 2008;7(3):223‐30. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Al‐Shahi 2006
- Al‐Shahi R, Warlow C. Interventions for treating brain arteriovenous malformations in adults. Stroke 2006;37:1141‐2. [DOI] [PubMed] [Google Scholar]
Al‐Shahi Salman 2006
- Al‐Shahi Salman R, Warlow CP. Interventions for treating brain arteriovenous malformations in adults. Cochrane Database of Systematic Reviews 2006, Issue 1. [DOI: 10.1002/14651858.CD003436.pub2] [DOI] [PubMed] [Google Scholar]
Ross 2010
- Ross J, Al‐Shahi Salman R. Interventions for treating brain arteriovenous malformations in adults. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD003436.pub3] [DOI] [PubMed] [Google Scholar]


