Abstract
Setting:
Mendi Provincial Hospital, Southern Highlands Province, Papua New Guinea (PNG).
Background:
PNG is a high burden country for tuberculosis (TB) and TB-human immunodeficiency virus (HIV). TB is the second most common cause of death in PNG.
Objective:
To identify the number of adult inpatients with TB who died between 1 January 2015 and 30 August 2017; describe these patients' characteristics and identify contributing factors that could be modified.
Design:
This was a retrospective case series review.
Results:
Among 905 inpatients with TB during the study period, there were 90 deaths. The patients who died were older than those who survived (median age 40 years vs. 32 years, P = 0.011). The majority of patients who died lived less than 3 hours from the hospital (71%), were diagnosed after admission (79%) and were clinically diagnosed (77%). HIV status was not known in 50% of the deaths. Of patients with a known status, 27% (12/45) were HIV-positive. The median symptom duration prior to presentation was 28 days, with females presenting later than males (84 vs. 28 days, P = 0.008).
Conclusion:
This study highlights areas where community and hospital-based management of TB could be improved to potentially reduce TB mortality, including earlier detection and treatment, improved bacteriological diagnosis and increased HIV testing.
Keywords: death audit, preventable deaths, risk factors
Abstract
Lieu :
Hôpital provincial de Mendi, Papouasie Nouvelle Guinée (PNG).
Contexte :
La PNG est un pays fortement touché par la tuberculose (TB) et la TB-virus de l'immunodéficience humaine (VIH). La TB est la deuxième cause de décès en termes de fréquence en PNG.
Objectif :
Identifier le nombre de patients adultes hospitalisés pour TB qui sont décédés ente le 1e janvier 2015 et le 30 août 2017, décrire les caractéristiques de ces patients et identifier les facteurs qui ont contribué au décès et qui pourraient être modifiés.
Schema :
Une revue rétrospective d'une série de cas.
Resultats :
Il y a eu 90 décès parmi 905 patients hospitalisés avec TB pendant la durée de l'étude. Les patients qui sont décédés ont été plus âgés que ceux qui ont survécu (âge médian 40 contre 32 ans, P = 0,011). La majorité des patients qui sont décédés habitaient à moins de 3 heures de l'hôpital (71%), ont été diagnostiqués après l'admission (79%) et ont eu un diagnostic clinique (77%). Le statut VIH est resté inconnu pour 50% des patients décédés. Parmi les patients de statut connu, 26,7% (12/45) ont été VIH positifs. La durée médiane des symptômes avant la présentation a été de 28 jours, les femmes se présentant plus tard que les hommes (84 contre 28 jours, P = 0,008).
Conclusion :
Cette étude met en lumière les domaines où la prise en charge communautaire et hospitalière de la TB pourrait être améliorée afin de réduire la mortalité liée à la TB grâce à une détection et à un traitement plus précoces, à une amélioration du diagnostic bactériologique et à une augmentation de la couverture du test VIH.
Abstract
Marco de Referencia:
El Hospital Provincial de Mendi en Papua Nueva Guinea.
Marco de Referencia:
Papua Nueva Guinea es un país con una carga de morbilidad alta por tuberculosis y coinfección por tuberculosis (TB) y el virus de la inmunodeficiencia humana (VIH). La TB es la segunda causa de muerte en el país.
Objetivo:
Determinar el número de pacientes adultos hospitalizados que fallecieron del 1 de enero del 2015 al 30 de agosto del 2017, describir las características de estos pacientes y definir los factores contribuyentes que podrían modificarse.
Metodo:
Fue esta una revisión retrospectiva de series de casos.
Resultados:
Hubo 90 defunciones en los 905 pacientes hospitalizados con TB durante el período del estudio. Los pacientes que fallecieron eran mayores que los sobrevivientes (mediana de la edad 40 años contra 32 años; P = 0,011). La mayoría de los pacientes que murieron, permaneció menos de 3 horas en el hospital (71 %), recibió el diagnóstico después de la hospitalización (79%) y su diagnóstico fue clínico (77%). La situación frente al VIH se desconocía en 50% de los pacientes fallecidos. De los pacientes cuya situación se conocía, el 26,7 % (12 de 45) era positivo frente al VIH. La mediana de la duración de los síntomas antes de acudir al hospital fue 28 días y las mujeres acudían más tarde que los hombres (84 días contra 28 días; P = 0,008).
Conclusion:
El presente estudio destaca las esferas del manejo comunitario y hospitalario de la TB que podrían mejorarse con el fin de reducir la mortalidad por esta enfermedad, entre las cuales se cuenta la detección y el tratamiento más tempranos, un mejor diagnóstico bacteriológico y una mayor detección sistemática de la infección por el VIH.
The World Health Organization (WHO) End TB Strategy aims to reduce tuberculosis (TB) mortality by 95% by 2035 compared to 2015, with the vision of achieving zero deaths from TB by 2050.1 Globally, TB is the ninth most common cause of death and the most common relating to an infectious disease.2 In people with the human immunodeficiency virus (HIV), TB is the most common cause of death.2
In 2016, Papua New Guinea (PNG) had the highest estimated TB mortality rate of countries in the Western Pacific Region, accounting for respectively 44 and 10 deaths per 100 000 population among HIV-negative and HIV-positive TB patients.2 However, deaths from TB are believed to be under-reported in PNG.3 In 2016, only 986 of an estimated 4420 deaths related to TB were reported, with 18.6% of these in HIV-positive individuals.4,5 TB is estimated to be the second most common cause of death in PNG, after lower respiratory tract infections.6
TB-HIV coinfection,7 failure of diagnosis,8 poor living conditions,9 smoking,10 diabetes mellitus,11 multi-drug-resistant TB,12 advanced age12 and residence in low- to middle-income countries13 have been associated with mortality among all people with TB. High mortality rates have been reported in patients hospitalised with TB,14,15 however, there is less understanding regarding risk factors for death in the subset of people with TB who are inpatients, especially in low- and middle-income countries similar to PNG.16 Clinical, programmatic and societal barriers to preventing mortality have not been explored in PNG, with no data available on the factors surrounding deaths in TB patients.
In 2016, Southern Highlands Province (SHP) of PNG reported a case notification rate of 145/100 000, with the Highlands region of PNG estimated to have a TB mortality of about 5%; similar to the national figure.4 The prevalence of TB-HIV and drug-resistant TB in SHP have not been reported; however, 7.9% of TB patients tested for HIV were positive in 2016.4 Mendi Provincial Hospital (MPH) is the main hospital for SHP and treats patients with TB from SHP and some nearby districts from surrounding provinces. Implementation of a mortality audit at MPH since March 2015 identified a number of deaths in people with TB, including many who were diagnosed clinically. To further understand the burden and drivers of TB mortality in inpatients at MPH, this study aimed to identify the number of adult inpatients with TB at MPH who died between January 2015 and August 2017, to describe these patients' demographic and clinical characteristics and to explore potentially modifiable factors that could reduce mortality.
METHODS
Study design
This study was a retrospective, descriptive case series involving a chart review of inpatients at MPH with TB and presumptive TB who died between January 2015 and August 2017.
Setting
SHP is located at the western end of the Highlands region of PNG. With an estimated population of 510 245, it is one of the most populated provinces of PNG.17 The majority of the population lives in rural areas, with a smaller proportion in urban and semi-urban areas. Peripheral districts are connected by roads; however, these are generally poorly maintained, which, in addition to cost of travel, make it difficult for many people to access health services. Mendi is the provincial capital.
The study was undertaken in the Internal Medicine Division of MPH. The hospital has a 320 bed-capacity, of which the Internal Medicine division manages 75 beds. The division has two wards, a general medical ward (35 beds) and a TB-HIV ward (40 beds). The Internal Medicine and TB wards at MPH were staffed by 20 nurses, 3 doctors (one internal medicine physician and two registrars) and a Health Extension Officer. Ward rounds were conducted every alternate day of the week when there was only one doctor available and daily when two or more doctors were available.
TB management at Mendi Provincial Hospital
There are ten Basic Management Units for TB in SHP, of which MPH is the largest. Presumptive TB patients presenting to MPH are admitted to the medical wards for clinical assessment and management by the internal medicine team. Smear microscopy, Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA), chest X-ray, HIV serology and full blood examination are available, although there are intermittent stockouts and difficulties maintaining equipment. For example, during most of 2015, the hospital's laboratory services were unable to undertake sputum smear and Xpert testing due to a faulty biosafety cabinet. As a result, most patients with presumptive TB were diagnosed clinically over this period. Following a clinical diagnosis of TB, patients are empirically commenced on first-line TB treatment.
Study population
Adult patients (aged ⩾15 years) admitted under the internal medicine division at MPH between 1 January 2015 and 31 August 2017 with TB or presumed TB were considered to be the accessible population. The subset of patients who died during this period were eligible for inclusion in the study and were defined as the study population. Patients who left the ward alive included patients who were discharged, absconded or voluntarily left the hospital.
Data sources and data collection
Patients diagnosed with TB or presumptive TB were identified from the existing admissions register for the adult medical wards. Patients who died were then cross-linked to patient records using the medical registration number to allow further data extraction. Data were collected using a structured paper data collection tool, before entry into EpiData v.3.1 (EpiData Association, Odense, Denmark) and validation.
Variables extracted from the admissions register included admission date, discharge date, demographics (e.g. age, sex), diagnosis and outcomes (e.g. death, discharge). This record is updated daily by the medical team at MPH. Variables extracted from the patient chart included duration of symptoms prior to presentation, timing of TB diagnosis, mode of TB diagnosis, timing of initiation of TB treatment, HIV status at admission, HIV testing and result during admission, use of antiretroviral therapy and cotrimoxazole if HIV-positive, haemoglobin, and review by a doctor within 24 h prior to death.
Statistical analysis
Analysis was undertaken using EpiData v.3.1 and Stata v.15 (StataCorp, College Station, TX, USA). Basic demographics were compared between patients with TB who died and patients who survived. The inpatient mortality rate among inpatients with TB was calculated quarterly with the number of TB inpatients who left the hospital (including those who died) used as the denominator. Categorical variables were described by frequency, and numerical variables by median and interquartile range [IQR]. Results were stratified by sex with comparisons undertaken using the χ2 test for categorical variables and the Wilcoxon rank sum test for continuous variables. Significance was set at P = 0.05.
Ethics
Ethical approval to conduct this study was obtained from the Medical Research Advisory Committee of the PNG National Department of Health (Port Moresby, PNG) and the Alfred Hospital Ethics Committee (Melbourne, VIC, Australia).
RESULTS
A total of 3517 inpatients left the internal medicine ward at MPH between 1 January 2015 and 31 August 2017, 467 of whom died. There were 905 inpatients with known or presumptive TB, of whom 815 (90%) left the ward alive and 90 died (10%). The total number of inpatients increased towards the end of 2015, however, the inpatient mortality rate remained relatively constant (Figure).
FIGURE.
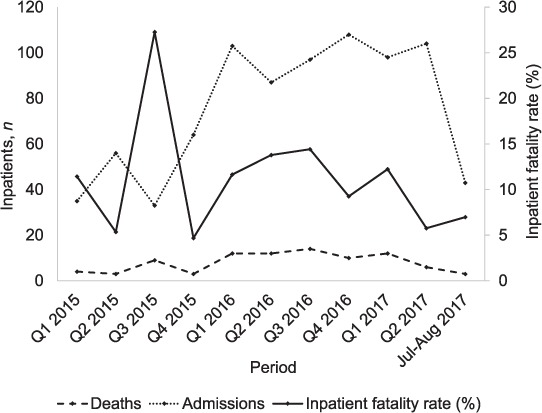
Deaths among adult inpatients with known or presumptive tuberculosis in Mendi Provincial Hospital, Mendi, Papua New Guinea, by quarter, from January 2015 to August 2017.
The median age of the 90 patients who died was 40 years [IQR 28–50]: 63% (57) were male (Table 1). Inpatients who survived were younger (median age 32 years [IQR 25–40], P = 0.011) but a similar proportion were male (57%, 461/815, P = 0.798). The majority of patients who died lived a distance of less than 3 h from the hospital (71%, 65/90), were diagnosed after admission (79%, 71/90), were clinically-diagnosed (77%, 69/90) and had extrapulmonary TB (49% with extrapulmonary TB alone and 12% combined with pulmonary TB). The commonest sites of extrapulmonary TB were the meninges (26.2%, 17/65) and abdomen (23.1%, 15/65). One half of the patients had an unknown HIV status at death and the majority of deaths were considered to be directly related to TB (57%, 51/90). Compared to female patients, males were significantly more likely to be HIV-positive (19% [11/57] vs. 3% [1/33], P = 0.046), to report a shorter duration of symptoms before admission (median 28 vs. 84 days, P = 0.008) and have a lower number of days from symptom onset to death (51 vs. 98 days, P = 0.004; Table 1).
TABLE 1.
Demographic and clinical characteristics of adult inpatients with known or presumptive TB who died, Mendi Provincial Hospital, Mendi, Papua New Guinea, January 2015 to August 2017
| Characteristics | Overall n (%) | Female n (%) | Male n (%) | P value* |
|---|---|---|---|---|
| Total | 90 | 33 | 57 | |
| Demographics | ||||
| Age, years, median [IQR] | 40 [28–50] | 37 [28–40] | 40 [30–60] | 0.186 |
| Time to residence from Mendi Provincial Hospital, h | ||||
| 0–<3 | 65 (71) | 20 (61) | 44 (77) | 0.131 |
| 3–24 hours | 22 (24) | 10 (30) | 12 (21) | |
| Not recorded | 4 (4) | 3 (9) | 1 (2) | |
| Clinical characteristics | ||||
| Timing of TB diagnosis | ||||
| Before admission | 16 (18) | 6 (18) | 10 (18) | 0.990 |
| On or after admission | 71 (79) | 26 (79) | 45 (79) | |
| Not recorded | 3 (3) | 1 (3) | 2 (4) | |
| Mode of TB diagnosis | ||||
| Bacteriologically-confirmed | 8 (9) | 0 | 8 (14) | 0.078 |
| Clinically-diagnosed | 69 (77) | 28 (85) | 41 (72) | |
| Not recorded | 13 (14) | 5 (15) | 8 (14) | |
| Type of TB | ||||
| Pulmonary TB only | 34 (38) | 8 (24) | 26 (46) | 0.143 |
| Extrapulmonary TB | 44 (49) | 19 (58) | 25 (42) | |
| Pulmonary TB + Extrapulmonary TB | 11 (12) | 6 (18) | 5 (9) | |
| Not recorded | 1 (1) | 0 | 1 (2) | |
| Duration of symptoms at admission,† days, median [IQR] | 28 [21–99] | 84 [28–112] | 28 (14–84) | 0.008 |
| Days from symptom onset to death,† median [IQR] | 63 [30–118] | 98 [42–129] | 51 [16–91] | 0.004 |
| Days from admission to death,‡ median [IQR] | 9 [3–18] | 12 [4–18] | 5 [2–17] | 0.108 |
| Days of TB treatment prior to death,§ median [IQR] | 12 [4–29] | 12.5 [5–32) | 10 [2–29] | 0.385 |
| HIV status at death | ||||
| Negative | 33 (37) | 16 (49) | 17 (30) | 0.046 |
| Positive | 12 (13) | 1 (3) | 11 (19) | |
| Unknown | 45 (50) | 16 (49) | 29 (51) | |
| Haemoglobin on admission; g/dL (median [IQR])¶ | 11 [10–14] | 10 [9–12] | 13 [11–15] | 0.005 |
| Cause of death | ||||
| Directly related to TB | 51 (57) | 21 (64) | 30 (53) | 0.510 |
| Associated with TB | 19 (21) | 5 (15) | 14 (25) | |
| Unlikely to be related to TB | 20 (22) | 7 (21) | 13 (23) | |
* Categorical variables analysed using the χ2 test for differences across all categories; continuous variables analysed using the Wilcoxon rank sum test.
† Data not available for one patient.
‡ Data not available for 22 patients.
§ Data not available for 28 patients.
¶ 20 missing (4 females, 16 males).
TB = tuberculosis; IQT = interquartile range; HIV = human immunodeficiency virus.
Table 2 shows the potentially modifiable characteristics. There was a median of 60 days [IQR 1–161] from the start of TB treatment to admission in patients diagnosed with TB prior to admission. Patients diagnosed with TB after admission were diagnosed rapidly (median 1 day [IQR 0–4]). Of the 45 patients with a known HIV status 12 (27 %) were HIV-positive. Eleven (12%) patients were known to be HIV-positive prior to admission. Of these 11, CD4+ testing was performed in four (36%) patients during admission and results ranged from 4 to 230 cells/μL. One patient was diagnosed with HIV after admission. The majority of patients (74%, 67/90) were reviewed by a doctor within the 24 h prior to their death. Xpert testing for rifampicin resistance was undertaken for one patient with drug-sensitive TB.
TABLE 2.
Potentially modifiable factors in adult inpatients with known or presumptive TB who died from January 2015 to August 2017, Mendi Provincial Hospital, Mendi, Papua New Guinea
| Clinical characteristics | n (%) |
|---|---|
| Timeliness of TB diagnosis | |
| Days from start of TB treatment to admission if TB was diagnosed before admission (n = 16), median [IQR] | 60 [1–161] |
| Days from admission to start of TB treatment if TB was diagnosed after admission, (n = 50), median [IQR] | 1 [0–4]* |
| Diagnosis and management of HIV | |
| Known HIV-positive pre-admission | 11 (12) |
| CD4 count performed during admission† | 4 (36) |
| On antiretroviral therapy | 2 (18)‡ |
| On cotrimoxazole preventive therapy | 4 (36)§ |
| Tested for HIV during admission (n = 79) | 36 (46) |
| Newly diagnosed with HIV during admission¶ | 1 (3) |
| Reviewed by a doctor <24 h prior to death | |
| No | 11 (12) |
| Yes | 67 (74) |
| Not recorded | 12 (13) |
* Data not available for 21 patients.
† Denominator is the number of patients known to be HIV-positive on admission.
‡ Results not recorded for nine patients.
§ Results not recorded for seven patients.
¶ Denominator is the number of patients tested for HIV during admission; results were not recorded for two patients.
TB = tuberculosis; IQR = interquartile range; HIV = human immunodeficiency virus.
DISCUSSION
This retrospective case series of the deaths of 90 inpatients with TB or presumptive TB at MPH provides one of the first detailed assessments of deaths in patients with TB from a provincial setting in PNG, where the burden of TB has not been previously well-described. The study identified a number of modifiable components of patient care, both prior to and after hospital admission, which have the potential to improve patient outcomes. In particular, there was a long time between symptom onset and treatment, many patients did not have bacteriologically-confirmed TB diagnosis and HIV testing was not performed in one half of the patients.
A recent systematic review identified 11 cohort or case-control studies that had investigated predictors of in-hospital mortality among patients with pulmonary TB. Malignancy was the only factor significantly associated with mortality on meta-analysis, with insufficient evidence to demonstrate an association with sex, diabetes or sputum smear negativity.16 Similarly, the current study did not identify sex to be associated with mortality, despite an apparent increased duration of symptoms prior to admission and TB diagnosis in females. Patients who died were older than those who survived in the current study, and although a pooled estimation of the risk of age on mortality was unable to be assessed in the systematic review, this finding is consistent with studies from Japan and Israel.18,19
The current study did not investigate the prevalence of malignancy in our patient population due to limitations in diagnostic testing at MPH, with no availability to undertake CT scans or detailed biochemical and histologic investigations. However, given that the majority of patients were clinically diagnosed it is possible that a number of these had a malignancy, either as their primary cause of death or as a comorbidity.
The TB management of patients at MPH and in SHP can be informed by these findings. Patients presented relatively late, having been symptomatic for a median of 28 days prior to admission and 84 days if female. They then died relatively quickly after treatment was commenced (median 12 days). Although many patients were diagnosed and treated rapidly after admission, outcomes may be difficult to change if the patient was unwell for a long period prior to presentation. To improve patient outcomes, prior to hospitalisation there is a need for better identification of symptomatic individuals, introduction of early treatment and improved health education. Future qualitative studies into health-seeking behaviour would inform our understanding of sex differences, including symptom reporting and barriers to seeking care.
HIV testing is vital, as patients with HIV are at risk of acquiring TB20 and patients with TB and HIV have an increased risk of mortality.7,21 In patients with an unknown HIV status on admission, about 50% were not tested during admission; well under the target of 100% set by the National TB Programme.3 Stockouts of HIV point-of-care diagnostic tests likely contributed to this, as did the rapid death of many patients (24% died within 48 h). Despite these constraints, the standardised HIV testing of inpatients with TB also needs to be improved and TB-HIV collaborative activities could be strengthened.
As HIV has been associated with increased rates of sputum smear-negative pulmonary TB,22,23 the presence of a high proportion of patients dying without bacteriologically-confirmed TB at MPH also needs investigation. While low diagnostic rates may partly relate to equipment malfunction and diagnostic stockouts in the earlier part of the study period, it is possible that a proportion of TB smear-negative results was due to patients being co-infected with HIV, or that TB was over-diagnosed. Improved microscopy services, increased use of Xpert testing, the introduction of the urine lateral flow lipoarabinomannan test or X-ray screening with computer-aided detection could improve diagnostic accuracy. In addition, the high prevalence of drug-resistant TB in some parts of PNG24 highlights the need for bacteriological confirmation with tools such as Xpert for exclusion of drug resistance. Post-mortem examinations may provide additional information about the true number of patients with TB dying and the causes of death in this population.
The presence of a doctor within 24 h before death was used as a crude measure of the medical care. Although a doctor was recorded as reviewing the patient within 24 h in 74% of cases, over 10% of cases did not have a review in this period suggesting further improvement is possible.
The primary limitation of this study was the lack of a detailed comparison group, preventing many baseline characteristics and differences in management from being compared to inpatients with TB who did not die. Additional limitations are that the study relied on data that were retrospectively collected and there were considerable missing data. The assessment of symptom duration relied upon patient history, which risks recall bias. As patients were only included if they were a registered inpatient, results cannot be generalised to the outpatient setting and the proportion who died can only be used as a measure of hospital outcomes. Furthermore, the study did not capture deaths after discharge or readmissions in patients surviving to discharge. Some potentially modifiable factors were not explored in this study, including diagnosis and management of opportunistic infections associated with advanced HIV. Future case control or prospective cohort studies would provide further clarity on the risk factors associated with mortality in inpatients with TB.
A strength of this study was that all patient records were available for the patients who died during the study period. In addition, the cohort size and detail of the data collected provides important information on this population.
CONCLUSION
This study of 90 inpatients at MPH who died with known or presumed TB highlights the challenges of managing TB patients in a semi-urban, provincial setting in PNG, and of successfully achieving the END TB Strategy aim of zero deaths by 2050 in PNG. The study highlights areas where TB management could be improved both within and outside of the hospital setting. Despite most deaths occurring in patients who lived close to the hospital, there was a significant delay between symptom onset and presentation, demonstrating a need for improved community education and early identification of symptomatic individuals. In addition, many patients died without HIV testing and the majority of patients were clinically diagnosed, meaning that true TB cases may be over- or under-ascertained, or that there may be other drivers such as HIV causing these deaths. Reliable and systematic testing for HIV and TB, including increased use of Xpert as part of an evidence-based package of interventions, and the adoption of new innovations would assist in addressing these concerns.
Acknowledgments
This research was conducted as part of the first Operational Research Course for Tuberculosis in Papua New Guinea (PNG). The specific training programme that resulted in this publication was developed and implemented by the Burnet Institute (Melbourne, VIC, Australia) in collaboration with the PNG Institute of Medical Research (Goroka) and University of PNG (Port Moresby), and supported by the PNG National Department of Health Emergency Response Taskforce for MDR and XDR-TB, the National TB Programme and Western Provincial Health Office, Daru, PNG. The model is based on the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR).
The authors acknowledge the support of the Southern Highlands Provincial Health Authority for allowing the study to be conducted and assisting with patient records and information access. The authors also thank all the study participants and their families.
The training programme was delivered as part of the Tropical Disease Research Regional Collaboration Initiative, which is supported by the Australian Government and implemented by Menzies School of Health Research (Darwin, NT, Australia) and the Burnet Institute.
The views expressed in this publication are the authors' alone and are not necessarily the views of the Australian or PNG Governments. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization End TB Strategy. Geneva, Switzerland: WHO; 2014. [Google Scholar]
- 2.World Health Organization Global tuberculosis report, 2017. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 3.Papua New Guinea Government National Tuberculosis National Strategic Plan (NSP) for Papua New Guinea 2015–2020. Port Moresby, PNG: PNG Government; 2014. [Google Scholar]
- 4.Aia P, Wanchuk L, Morishita F, Kisomb J, Yasi R, Kal M. Epidemiology of tuberculosis in Papua New Guinea; analysis of case notification and treatment outcome data, 2008–2016. Western Pac Surveill Response J. 2018;9(2):9–19. doi: 10.5365/wpsar.2018.9.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization Papua New Guinea tuberculosis profile 2016. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 6.World Health Organization Papua New Guinea: WHO statistical profile. Geneva, Switzerland: WHO; 2015. [Google Scholar]
- 7.Domingos M P, Caiaffa W T, Colosimo E A. Mortality, TB-HIV coinfection, and treatment dropout: predictors of tuberculosis prognosis in Recife, Pernambuco State, Brazil. Cad Saude Publica. 2008;24(4):887–896. doi: 10.1590/s0102-311x2008000400020. [DOI] [PubMed] [Google Scholar]
- 8.Enarson D A, Gryzbowski S, Dorken E. Failure of diagnosis as a factor in tuberculosis mortality. Can Med Assoc J. 1978;118(12):1520–1522. [PMC free article] [PubMed] [Google Scholar]
- 9.Zürcher K, Ballif M, Zwahlen M, Reider H L, Egger M, Fenner L. Tuberculosis mortality and poor living conditions in Bern, Switzerland, 1856–1950. PLoS One. 2016;11(2) doi: 10.1371/journal.pone.0149195. e0149195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gajalakshmi V, Peto R, Kanaka T S, Jha P. Smoking and mortality from tuberculosis and other diseases in India: retrospective study of 43 000 adult male deaths and 35 000 controls. Lancet. 2003;362(9383):507–515. doi: 10.1016/S0140-6736(03)14109-8. [DOI] [PubMed] [Google Scholar]
- 11.Baker A B, Harries D, Jeon C Y et al. The impact of diabetes on tuberculosis treatment outcomes: a systemic review. BMC Med. 2011;9:81. doi: 10.1186/1741-7015-9-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lefebvre N, Falzon D. Risk factors for deaths among tuberculosis cases: analysis of European surveillance data. Eur Respir J. 2008;31(6):1256–1260. doi: 10.1183/09031936.00131107. [DOI] [PubMed] [Google Scholar]
- 13.World Health Organization Tuberculosis. Geneva, Switzerland: WHO; 2018. http://www.who.int/news-room/fact-sheets/detail/tuberculosis Accessed July 2019. [Google Scholar]
- 14.Kirenga B J, Levin J, Ayakaka I et al. Treatment outcomes of new tuberculosis patients hospitalized in Kampala, Uganda: a prospective cohort study. PLoS One. 2014;9(3) doi: 10.1371/journal.pone.0090614. e90614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Silva D R, Menegotto D M, Schulz L F, Gazzana M B, Dalcin Pde T. Factors associated with mortality in hospitalized patients with newly diagnosed tuberculosis. Lung. 2010;188(1):33–41. doi: 10.1007/s00408-009-9224-9. [DOI] [PubMed] [Google Scholar]
- 16.de Almeida C P B, Ziegelmann P K, Couban R, Wang L, Busse J W, Silva D R. Predictors of in-hospital mortality among pulmonary TB patients: a systemic review and meta-analysis. Sci Rep. 2018;8 doi: 10.1038/s41598-018-25409-5. 7230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Statistics Office 2011 National Population and Housing Census of Papua New Guinea—Final Figures. Port Moresby, Papua New Guinea: National Statistics Office; 2014. [Google Scholar]
- 18.Lubart E, Lidgi M, Leibovitz A, Rabinovitz C, Segal R. Mortality of patients hospitalized for active tuberculosis in Israel. Isr Med Assoc J. 2007;9(12):870–873. [PubMed] [Google Scholar]
- 19.Horita N, Miyazawa N, Yoshiyama T et al. Development and validation of a tuberculosis prognostic score for smear-positive in-patients in Japan. Int J Tuberc Lung Dis. 2013;17(1):54–60. doi: 10.5588/ijtld.12.0476. [DOI] [PubMed] [Google Scholar]
- 20.Guelar A, Gatell J M, Verdejo J et al. A prospective study of the risk of tuberculosis among HIV-infected patients. AIDS. 1993;7(10):1345. doi: 10.1097/00002030-199310000-00007. [DOI] [PubMed] [Google Scholar]
- 21.Corbett E L, Watt C J, Walker N et al. The growing burden of tuberculosis: global trends and interactions with the HIV epidemic. Arch Intern Med. 2003;163(9):1009. doi: 10.1001/archinte.163.9.1009. [DOI] [PubMed] [Google Scholar]
- 22.Elliott A M, Namaambo K, Allen B W et al. Negative sputum smear results in HIV-positive patients with pulmonary tuberculosis in Lusaka, Zambia. Tuberc Lung Dis. 1993;74:191–194. doi: 10.1016/0962-8479(93)90010-U. [DOI] [PubMed] [Google Scholar]
- 23.Johnson J L, Vjecha M J, Okwera A et al. Impact of human immunodeficiency virus type-1 infection on the initial bacteriologic and radiographic manifestations of pulmonary tuberculosis in Uganda. Int J Tuberc Lung Dis. 1998;2:397–404. [PubMed] [Google Scholar]
- 24.Aia P, Kal M, Lavu E et al. The burden of drug-resistant tuberculosis in Papua New Guinea: Results of a large population-based survey. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0149806. e0149806. [DOI] [PMC free article] [PubMed] [Google Scholar]


