Abstract
Setting:
Xpert® MTB/RIF was introduced in Papua New Guinea in 2012 for the diagnosis of tuberculosis (TB) and of rifampicin-resistant TB (RR-TB), a marker of multi-drug-resistant TB (MDR-TB).
Objective:
To assess the concordance of Xpert with phenotypic drug susceptibility testing (DST) performed at the supranational reference laboratory and to describe the patterns of drug-resistant TB observed.
Design:
This was a retrospective descriptive study of laboratory data collected from April 2012 to December 2017.
Results:
In 69 months, 1408 specimens with Xpert results were sent for mycobacterial culture and DST; Mycobacterium tuberculosis was cultured from 63% (884/1408) and DST was completed in 99.4%. The concordance between Xpert and culture for M. tuberculosis detection was 98.6%. Of 760 RR-TB cases, 98.7% were detected using Xpert; 98.5% of 620 MDR-TB cases were identified using phenotypic DST. Phenotypic resistance to second-line drugs was detected in 59.4% (522/879) of specimens tested, including 29 with fluoroquinolone resistance; the majority were from the National Capital District and Daru Island.
Conclusion:
The high concordance between phenotypic DST and Xpert in identifying RR-TB cases supports the scale-up of initial Xpert testing in settings with high rates of drug resistance. However, rapid DST in addition to the detection of RR-TB is required.
Keywords: multidrug-resistant tuberculosis, Xpert® MTB/RIF, drug susceptibility testing, Papua New Guinea
Abstract
Contexte :
L'Xpert® MTB/RIF a été introduit en Papouasie Nouvelle Guinée en 2012 pour le diagnostic de la tuberculose (TB) et de la TB résistante à la rifampicine (RR-TB), un marqueur de TB multirésistante (MDR-TB).
Objectif :
Evaluer la concordance de l'Xpert avec le test de pharmacosensibilité phénotypique (DST) réalisé au laboratoire de référence supranational et décrire les profils de TB pharmacorésistante observés.
Schema :
Une étude rétrospective descriptive de données de laboratoire recueillies entre avril 2012 et décembre 2017.
Resultats :
En 69 mois, 1408 échantillons comportant des résultats d'Xpert ont été envoyés au laboratoire pour une culture de mycobactéries et un DST. Mycobacterium tuberculosis a été cultivé sur 63% (884/1408) et le DST a été réalisé sur 99,4% de ces derniers. La concordance entre l'Xpert et la culture pour la détection de M. tuberculosis a été de 98,6%. L'Xpert a détecté 98,7% de 760 cas de RR-TB et 98,5% de 620 cas de MDR-TB identifiés par DST phénotypique. La résistance phénotypique aux médicaments de deuxième ligne a été détectée dans 59,4% (522/879) des échantillons testés, dont 29 avaient une résistance aux fluoroquinolones, la majorité émanant du district de la capitale nationale et de l'île de Daru, Province de l'Ouest.
Conclusion :
La concordance entre DST phénotypique et Xpert pour la RR-TB est en faveur de l'accélération du test initial par Xpert dans les contextes de taux élevés de pharmacorésistance. Il y a cependant un besoin de test rapide de pharmacorésistance pour la détection de la résistance aux médicaments autres que la rifampicine.
Abstract
Marco de Referencia:
La prueba Xpert® MTB/RIF se introdujo en Papúa Nueva Guinea en el 2012 para el diagnóstico de la tuberculosis (TB) y la TB resistente a rifampicina (RR-TB), que es un marcador de la TBmultirresistente (MDR-TB).
Objetivo:
Evaluar la concordancia de la prueba Xpert con las pruebas fenotípicas de sensibilidad realizadas en el Laboratorio Supranacional de Referencia y describir los tipos de TB farmacorresistente observados.
Método:
Fue este un estudio descriptivo retrospectivo de los datos de laboratorio recogidos de abril del 2012 a diciembre del 2017.
Resultados:
En 69 meses, se enviaron 1408 muestras con resultados de la prueba Xpert para cultivo micobacteriano y pruebas fenotípicas de resistencia. Se cultivó el Mycobacterium tuberculosis del 63% de las muestras (884/1408) y en 99,4% de estas se completaron las pruebas fenotípicas de resistencia. La concordancia entre la prueba Xpert y el cultivo de micobacterias para la detección de M. tuberculosis fue de 98,6%. Con la prueba Xpert se detectó el 98,7% de 760 casos de RR-TBy el 98,5% de 620 casos de MDR-TB detectados con las pruebas fenotípicas. En el 59,4% (522/879) de las muestras examinadas se detectó resistencia fenotípica a los fármacos de segunda línea, incluidos 29 casos con resistencia a fluoroquinolona y la mayor parte provenía del Distrito Capital Nacional y la Isla Daru de la Provincia Occidental.
Conclusión:
La alta concordancia entre las pruebas fenotípicas de sensibilidad a los medicamentos y la prueba Xpert con respecto a rifampicina respalda la ampliación de escala de la utilización de la prueba Xpert hacia entornos con tasas altas de farmacorresistencia. Sin embargo, se precisan pruebas rápidas de detección de la resistencia a otros fármacos diferentes de la rifampicina.
Tuberculosis (TB) is the leading cause of global mortality due to an infectious agent, and the World Health Organization (WHO) estimates that 10 million people worldwide developed TB in 2017.1 The emergence and spread of drug-resistant TB (DR-TB) is a public health crisis. An estimated 558 000 people developed rifampicin-resistant TB (RR-TB) in 2017, 82% of whom had multidrug-resistant TB (MDR-TB), defined as additional resistance to at least isoniazid (INH). Less than 30% of the estimated RR-TB cases were detected and notified to the WHO.1 This proportion was even lower (19%) in Papua New Guinea (PNG), where many patients do not have access to reliable TB diagnostics or drug susceptibility testing (DST). PNG is among the 14 WHO high-burden countries for TB, MDR-TB and TB plus human immunodeficiency virus (HIV) co-infection. PNG has an estimated TB incidence of 432 (uncertainty interval 352–521) per 100 000 population and 23 (uncertainty interval 15–24)/100 000 for RR/MDR-TB; 7% of tested TB patients were HIV-positive in 2017.1
Information about the MDR-TB burden in PNG has been limited due to a lack of laboratory facilities to perform DST. MDR-TB was first reported in patients from the Western Province, who were diagnosed through cross-border travel to Australia in 1984, but not reported until 2000.2,3 A subnational drug resistance survey of people presenting to 14 diagnostic centres in PNG in 2012–2014 found the prevalence of MDR-TB to be respectively 2.7% and 19.1% in new and retreatment cases.4 Wide variations in MDR-TB prevalence was noted between the different centres, with none detected in some centres and 32% of cases identified at Daru General Hospital, South Fly District, Western Province, the site of an ongoing MDR-TB outbreak.5,6
In the past, smear microscopy was the cornerstone of TB diagnosis in PNG. However, low rates of testing and of bacteriological confirmation (25.9% of all treated pulmonary TB cases in 2016), as well as inadequate quality assurance in remote settings posed major challenges.7 In 2012, the Xpert® MTB/RIF assay (Cepheid, Sunnyvale, CA, USA) was introduced into PNG and has since been installed in 17 of the country's 22 provinces. Culture and DST is available through specimen referral to the Queensland Mycobacterial Reference Laboratory (QMRL; Brisbane, QLD, Australia). The Xpert assay is more sensitive and specific than smear microscopy in detecting Mycobacterium tuberculosis (MTB); it also detects rifampicin (RIF) resistance as a surrogate marker for MDR-TB with a rapid turn-around time.8,9 Xpert therefore has the potential to improve case detection and reduce time to effective treatment initiation in countries such as PNG, where access to diagnostic services is limited.10
The aim of the present study was to assess the concordance of Xpert with phenotypic DST performed at the QMRL and to describe the patterns of DR-TB observed in PNG.
METHODS
Study design, setting and population
We conducted a retrospective analysis of the diagnostic samples initially tested using Xpert in PNG, followed by submission to the QMRL for culture and DST from 1 April 2012 to 31 December 2017. Specimens were first sent to the Central Public Health Laboratory (CPHL; Port Moresby, PNG), which acts as the PNG national reference laboratory for TB, HIV, malaria and vaccine-preventable diseases. It also provides supervision and external quality assurance programmes to other provincial and peripheral laboratories, and participates in international quality assurance programmes. The QMRL serves as the WHO supranational reference laboratory for PNG.
From 2012 until 2017, Xpert instruments were installed in 30 sites in PNG, with the cloud-based Gx-Alert data monitoring system (SystemOne, Boston, MA, USA) available in 25 sites.11 All but two of the instruments were purchased by non-governmental agencies, the majority by The Global Fund (Geneva, Switzerland). Xpert instruments were first installed in the CPHL, Daru Island and Goroka (Eastern Highlands). In 2013, five more sites were included, followed by nine more in 2014. By the end of 2017, all but five (22.7%) of PNG's 22 provinces had at least one Xpert instrument. The National TB Programme (NTP) installed 26 of the 30 Xpert instruments; 4 were installed by non-government health services.
Only the initial sample collected at the time of presentation for diagnosis is included in this analysis. Presumptive TB patients are routinely requested to provide two sputum samples (one spot and one morning) for smear microscopy, but this is rarely adhered to in settings with access to Xpert. In accordance with the relevant NTP diagnostic algorithm in place at the time, a single specimen from all eligible patients was tested in sites with Xpert. From 2012 to 2016, only presumptive MDR-TB patients, including TB treatment failures, contacts of MDR-TB patients and people living with HIV were tested using Xpert. From 2017, the protocol recommended Xpert as a frontline test for all presumptive TB cases, in parallel with smear microscopy. In provinces with high MDR-TB burdens, such as the National Capital District (NCD) and the Western and Gulf Provinces, Xpert was performed on all sputum smear-positive samples, as well as sputum from smear-negative patients with presumptive DR-TB over the study period. Extrapulmonary samples (e.g., from the lymph nodes or cerebrospinal fluid) were tested using Xpert upon clinical request. The NTP protocol requires all specimens that are RIF-resistant on Xpert to be sent to the CPHL and then to the QMRL for mycobacterial culture and DST. In addition, culture and DST were sometimes requested in Xpert-negative cases who were presumed to have MDR-TB.
Laboratory procedures
Routine sputum smear microscopy was performed in peripheral health facilities using the standard Ziehl-Neelsen technique. The Xpert assay was performed using the G4 cartridge; results were recorded as MTB detected/not detected and RIF resistance detected/not detected/indeterminate. In the QMRL, samples were culture tested on solid and liquid media using Löwenstein-Jensen slopes with pyruvate and the BACTEC™MGIT™960 (Becton Dickinson Diagnostics, Sparks, MD, USA). Phenotypic DST was performed using RIF (1.0 μg/ml), INH (0.1 μg/ml, low-level; 0.4 μg/ml, high-level), streptomycin (1.0 μg/ml), ethambutol (5.0 μg/ml) and pyrazinamide (PZA; 100 μg/ml) using an automated MGIT960 system. For isolates identified as MDR-TB, second-line DST was performed using amikacin (1.0 μg/ml), capreomycin (2.5 μg/ml), kanamycin (2.5 μg/ml), ethionamide (ETH) (5.0 μg/ml) and ofloxacin (2.0 μg/ml).
Data sources, variables and analysis
Data were extracted from the electronic TB laboratory database at the CPHL. Variables extracted included age, sex, health facility and province of diagnosis, results of acid-fast bacilli (AFB) microscopy and Xpert recorded from the peripheral diagnostic facility, and of the culture and DST data from the QMRL. The data were carefully reviewed and all analyses were performed using Stata v.12 (StataCorp, College Station, TX, USA).
Ethical approval
Ethical approval to conduct this study was obtained from the PNG Medical Research Advisory Committee, Port Moresby, PNG (MRAC no 17.42).
RESULTS
A total of 1408 specimens with Xpert results were sent to the QMRL for culture and DST. Of these, RR-TB was detected using Xpert in 1070 (76%) (Figure). MTB was cultured from 884 (63%) samples, 879 (99.4%) of which were subjected to DST. Eight (0.6%) culture samples grew non-tuberculous mycobacteria (NTM). An additional 233 samples were RR-TB-positive on Xpert, but were not sent to the CPHL (and therefore not to QMRL), including 105 (45%) from NCD and 42 (18%) from Daru island. This analysis therefore includes 82% of all 1303 RR-TB cases detected in PNG during the study period.
FIGURE.
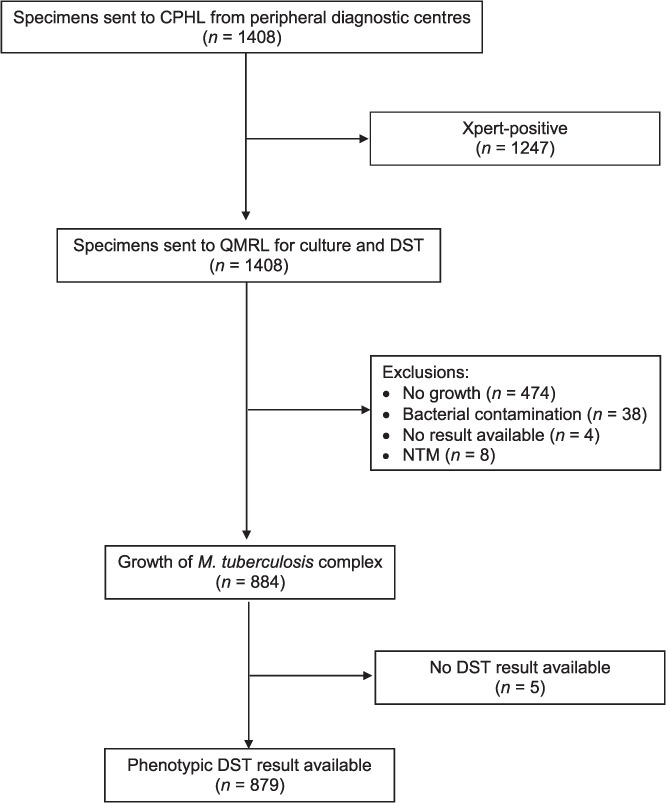
Specimen flow from peripheral laboratories to the CPHL (Port Moresby, Papua New Guinea) and to the supranational QMRL (Brisbane, QLD, Australia). CPHL = Central Public Health Laboratory; QMRL = Queensland Mycobacterial Reference Laboratory; DST = drug susceptibility testing; NTM = non-tuberculous mycobacteria.
Table 1 shows the patient characteristics and laboratory results by culture result. The majority (n = 1200, 85.2%) of the samples came from diagnostic facilities located in the southern region, i.e., NCD, Western Province, (458/461, 99.3% from Daru General Hospital) and the Gulf Province. Nearly half (45.8%) of the patients were aged 15–34 years; 117 (10%) of all samples were from children (aged <15 years), and the vast majority (89.3%) of the samples were sputum.
TABLE 1.
Characteristics of population and of sample laboratory results with proportion of Mycobacterium culture results by characteristic
| Characteristic | Total (n = 1408) n (%) | MTB culture-positive (n = 884, 59.9) n (%) | MTB culture no growth (n = 474, 33.7) n (%) | Other* = n (50, 3.6) n (%) |
|---|---|---|---|---|
| Age group, years | ||||
| 0–4 | 40 (2.8) | 11 (27.5) | 27 (67.5) | 2 (5.0) |
| 5–14 | 77 (5.5) | 43 (55.8) | 30 (39.0) | 4 (5.2) |
| 15–24 | 304 (21.6) | 213 (70.1) | 81 (26.6) | 10 (3.3) |
| 25–34 | 341 (24.2) | 217 (63.6) | 112 (32.8) | 12 (3.5) |
| 35–44 | 198 (14.1) | 121 (61.1) | 70 (35.4) | 7 (3.5) |
| 45–54 | 133 (9.4) | 80 (60.2) | 50 (37.6) | 3 (2.3) |
| 55–64 | 66 (4.7) | 46 (69.7) | 18 (27.3) | 2 (3.0) |
| ⩾65 | 16 (1.1) | 10 (62.5) | 6 (37.5) | 0 (0.0) |
| Missing | 233 (16.5) | 143 (61.4) | 80 (34.3) | 10 (4.3) |
| Sex | ||||
| Male | 566 (40.2) | 349 (61.7) | 198 (35.0) | 19 (3.4) |
| Female | 610 (43.3) | 393 (64.4) | 196 (35.0) | 21 (3.4) |
| Missing | 232 (16.5) | 142 (61.2) | 80 (34.5) | 10 (4.3) |
| Province | ||||
| NCD/Central | 590 (41.9) | 406 (68.8) | 163 (27.6) | 21 (3.6) |
| Western | 461 (32.7) | 292 (63.3) | 155 (33.6) | 14 (3.0) |
| Gulf | 149 (10.6) | 71 (47.7) | 71 (47.7) | 7 (4.7) |
| Other provinces† | 208 (14.8) | 115 (55.3) | 85 (40.9) | 8 (3.8) |
| Site | ||||
| Pulmonary (sputum) | 1258 (89.3) | 790 (62.8) | 421 (33.5) | 47 (3.7) |
| Extrapulmonary | ||||
| Lymph node aspirates | 94 (6.7) | 54 (57.4) | 39 (41.5) | 1 (1.1) |
| Gastric aspirates | 25 (1.8) | 5 (20.0) | 20 (80.0) | 0 (0.0) |
| CSF | 8 (0.6) | 3 (37.5) | 5 (62.5) | 0 (0.0) |
| Pleural fluid | 8 (0.6) | 2 (25.0) | 6 (75.0) | 0 (0.0) |
| Ascitic fluid | 5 (0.4) | 0 (0.0) | 5 (100.0) | 0 (0.0) |
| Other aspirates | 9 (0.6) | 5 (55.6) | 3 (33.3) | 1 (11.1) |
| AFB smear result | ||||
| AFB-negative | 238 (16.9) | 88 (37.0) | 142 (59.7) | 8 (3.4) |
| AFB-positive | 384 (27.3) | 315 (82.0) | 54 (14.1) | 15 (3.9) |
| Missing | 784 (55.7) | 481 (61.4) | 278 (35.5) | 25 (3.2) |
| Xpert MTB | ||||
| Negative | 161 (11.4) | 12 (7.5) | 138 (85.7) | 11 (6.8) |
| Positive | 1247 (88.6) | 872 (69.9) | 336 (26.9) | 39 (3.1) |
| Xpert RIF resistance | ||||
| Not detected | 147 (10.4) | 89 (60.5) | 53 (36.1) | 5 (3.4) |
| Detected | 1070 (76.0) | 773 (72.2) | 263 (24.6) | 34 (3.2) |
| Indeterminate | 30 (2.1) | 10 (33.3) | 20 (66.7) | 0 (0.0) |
* Non-tuberculous mycobacteria, contaminants, no test.
† Madang, Milne Bay, Morobe, Oro, East Sepik, West Sepik, Enga, Eastern Highlands, Hela, Jiwaka, Western Highlands, Southern Highlands, Autonomous Region of Bougainville, East New Britain, West New Britain and Sandaun.
MTB = Mycobacterium tuberculosis; NCD = National Capital District; CSF = cerebospinal fluid; AFB = acid-fast bacilli; RIF = rifampicin.
A smear microscopy result was recorded for only 624 (44.3%) specimens. Of the 403 specimens with recorded AFB smear and culture results, 315 (78.2%) AFB smear-positive specimens were also culture positive. MTB was cultured in 884 (62.8%) specimens, including 12/161 (7.5%) specimens from patients with a clinical presumption of MDR-TB who were Xpert-negative; 3/12 (25.0%) had MDR-TB confirmed using DST. Among 1247 Xpert-positive specimens for MTB, 335 (26.9%) were culture-negative, 32 (2.6%) had bacterial contamination, 4 (0.3%) had missing results and 3 (0.2%) had NTM. Among 884 specimens culture-positive for MTB, 872 (98.6%) were Xpert-positive.
Table 2 shows the concordance between genotypic (Xpert) and phenotypic DST results. Of the 879 cases with a phenotypic DST result, 760 (86.5%) were identified as RIF-resistant and 620 (70.5%) were MDR-TB. RIF resistance was detected using Xpert in 750/760 (98.7%) cases confirmed by DST, and in 611/620 (98.5%) cases with confirmed MDR-TB. RIF resistance was not detected on Xpert in 10 (1.3%) of the 760 cases confirmed using DST; 5 were ‘MTB detected, RIF not detected’; 2 were ‘MTB detected, RIF indeterminate’ and 3 were ‘MTB-negative’. Of 162 samples reported as ‘MTB-negative’ on Xpert, 3 (1.9%) were found to be MDR-TB cases on culture and DST.
TABLE 2.
Concordance between genotypic (Xpert® MTB/RIF) and phenotypic DST results for RIF and multidrug resistance
| Xpert | Phenotypic DST n (%) | |||
|---|---|---|---|---|
| RIF-resistant | RIF-susceptible | MDR-TB | Non-MDR-TB* | |
| MTB | ||||
| Not detected | 3 (0.4) | 9 (7.6) | 3 (0.5) | 9 (3.5) |
| Detected | 757 (99.6) | 110 (92.4) | 617 (99.5) | 250 (96.5) |
| RIF resistance | ||||
| Not detected | 5 (0.6) | 83 (69.7) | 5 (0.8) | 83 (32.0) |
| Detected | 750 (98.7) | 19 (16.0) | 611 (98.5) | 158 (61.0) |
| Indeterminate | 2 (0.3) | 8 (6.7) | 1 (0.2) | 9 (3.5) |
| Total | 760 | 119 | 620 | 259 |
* Included 119 RIF-susceptible on DST and 140 RIF-monoresistant (INH-susceptible).
DST = drug susceptibility testing; RIF = rifampicin; MTB = Mycobacterium tuberculosis; MDR-TB = multidrug-resistant TB.
Table 3 provides an overview of the phenotypic DST results by province. Among those with DST results, 140/879 (15.9%) were RIF-resistant but INH-susceptible; 94 were RIF-monoresistant and 46 were resistant to other drugs. Of the 140 strains that were RIF-resistant but not MDR-TB, 98 (70.0%) were from NCD. The proportions of all samples with detected drug resistance that were RIF-resistant but not MDR-TB (i.e., INH-susceptible) varied by province: they were highest in samples from Gulf Province, followed by NCD, other provinces and Western Province (respectively 14/31, 42.4%; 98/358, 27.4%; 23/110, 20.7%; and 5/268, 1.9%. Of the 620 MDR-TB cases, 484 (78.1%) had resistance to at least one second-line drug (most commonly ETH). Shared resistance to RIF, high-dose INH, ETH and PZA was seen in 232/879 (26.3%) cases, and was especially common in isolates from Daru Island, comprising 160/461 (55.0%) of all the Daru DST results. There were 13 cases of extensively drug-resistant (XDR) TB (defined as MDR-TB plus resistance to any fluoroquinolone and a second-line injectable) and 21 with pre-XDR-TB, i.e. resistance to a fluoroquinolone (n = 16) or a second-line injectable (n = 5). Of the 34 cases of XDR-TB or pre-XDR-TB, 29 (85.3%) were from recognised ‘MDR/XDR hotspots’ (Port Moresby and Daru island).
TABLE 3.
DST results by PNG province
| NCD/Central n (%) | Western Province n (%) | Gulf Province n (%) | Others* n (%) | Total n (%) | |
|---|---|---|---|---|---|
| Samples sent | n = 590 | n = 461 | n = 149 | n = 208 | n = 1408 |
| DST completed | 403 (68.3) | 291 (63.1) | 70 (47.0) | 115 (55.3) | 879 (62.4) |
| Drug resistance detected† | 358 (88.8) | 268 (92.1) | 33 (47.1) | 111 (96.5) | 770 (87.6) |
| Specific drug resistance: n resistant/n with reported result for the specific drug (%) | |||||
| First-line drugs | |||||
| RIF | 358/403 (88.8) | 261/291 (89.7) | 31/70 (44.3) | 110/115 (95.7) | 760/879 (86.5) |
| INH 0.1 μg/ml | 259/402 (64.4) | 263/291 (90.4) | 19/70 (27.1) | 89/115 (77.4) | 630/878 (71.8) |
| INH 0.4 μg/ml | 169/315 (53.7) | 206/257 (80.2) | 13/28 (46.4) | 56/104 (53.8) | 444/704 (63.1) |
| Ethambutol | 57/401 (14.2) | 72/290 (24.8) | 4/70 (5.7) | 19/113 (16.8) | 152/874 (17.4) |
| Pyrazinamide | 68/402 (16.9) | 161/290 (55.5) | 6/69 (8.7) | 19/115 (16.5) | 254/876 (29.0) |
| Streptomycin | 173/402 (43.0) | 256/290 (88.3) | 15/70 (21.4) | 58/114 (50.9) | 502/876 (57.3) |
| Second-line drugs | |||||
| Ethionamide | 184/262 (70.2) | 242/259 (93.4) | 18/31 (58.1) | 60/116 (51.7) | 504/668 (75.4) |
| OFX | 8/352 (2.3) | 18/262 (6.9) | 0/31 (0.0) | 3/112 (2.7) | 29/757 (3.8) |
| Amikacin | 2/353 (0.6) | 0/262 (0.0) | 0/31 (0.0) | 0/112 (0.0) | 2/758 (0.3) |
| Capreomycin | 7/352 (2.0) | 10/262 (3.8) | 0/31 (0.0) | 2/112 (1.8) | 19/757 (2.5) |
| Kanamycin | 2/353 (0.6) | 1/262 (0.4) | 0/31 (0.0) | 1/112 (0.9) | 4/758 (0.5) |
| Drug resistance category: n resistant/DST completed (n = 879), n (%) | |||||
| Monoresistance | |||||
| RIF | 69 (17.1) | 4 (1.4) | 6 (8.6) | 15 (13.0) | 94 (10.7) |
| INH | 0 | 1 (0.3) | 0 | 1 (0.9) | 2 (0.2) |
| Poly resistance | |||||
| H + other than R | 1 (0.2) | 5 (1.7) | 2 (2.9) | 0 | 8 (0.9) |
| R + other than H | 29 (7.2) | 1 (0.3) | 8 (11.4) | 8 (7.0) | 46 (5.2) |
| MDR-TB | 259 (64.3) | 257 (88.3) | 17 (24.3) | 87 (75.7) | 620 (70.5) |
| Pre-XDR-TB or XDR-TB: n resistant/all MDR-TB (n = 620), n (%) | |||||
| HROfx, not SLIDs | 6 (2.3) | 6 (2.3) | 0 (0.0) | 4 (4.6) | 16 (2.6) |
| HR+SLIDs, not OFX | 4 (1.5) | 0 (0.0) | 0 (0.0) | 1 (1.1) | 5 (0.8) |
| All pre-XDR-TB | 10 (3.9) | 6 (2.3) | 0 (0.0) | 5 (5.7) | 21 (3.4) |
| XDR-TB | 3 (1.2) | 10 (3.9) | 0 (0.0) | 0 (0.0) | 13 (2.1) |
* Madang, Milne Bay, Morobe, Oro, East Sepik, West Sepik, Enga, Eastern Highlands, Hela, Jiwaka, Western Highlands, Southern Highlands, Autonomous Region of Bougainville, East New Britain, West New Britain and Sandaun.
† Among 109 with no drug resistance detected on DST, 99 were fully drug-susceptible and 10 were mono-resistant to streptomycin (i.e., fully susceptible to all current first-line drugs).
DST = drug susceptibility testing; PNG = Papua New Guinea; NCD = National Capital District; RIF, R = rifampicin; INH, H = isoniazid; OFX = ofloxacin; MDR-TB = multidrug-resistant TB; SLID = second-line injectable drug; XDR-TB = extensively drug-resistant TB.
DISCUSSION
This descriptive analysis of routine laboratory data following the introduction of Xpert testing in PNG provides important epidemiological data on TB drug resistance with implications for programmatic implementation. The high degree of concordance between Xpert and phenotypic DST for RIF resistance, while not unexpected given the high pre-test probability of resistance due to sample selection bias, supports the use of Xpert for the early detection of RR-TB patients in PNG, a country endemic for MDR-TB but currently lacking in-country facilities for phenotypic DST. The study also presents the first data on resistance to a broad range of TB drugs from multiple settings in PNG .
The study had a number of significant limitations. There was inherent sample selection bias in that the findings simply reflect the numbers and patterns of DR-TB among samples that were sent for culture and phenotypic DST to the QMRL. DST was mainly performed on samples found to be RR-TB on Xpert or selected by clinicians using variable criteria. Samples were also mainly from better-resourced centres that were able to send their samples to the CPHL. These centres may have a higher DR-TB prevalence than other settings in the country and may also represent areas with better access to second-line drugs in the past. Due to the specimen selection bias, it is probable that the study does not accurately reflect DR-TB prevalence in PNG, nor does it represent the full spectrum of resistance to drugs other than RIF, including INH. Nonetheless, our findings highlight the presence of DR-TB strains in PNG and the risk of ongoing transmission of these strains beyond the currently recognised hot spots, emphasising the critical need for more collaborative efforts to combat DR-TB in PNG.
The overall yield from mycobacterial culture was only 62%, although MTB was detected on Xpert in the majority of the samples before transport. As expected, the culture yield was significantly higher from specimens that were MTB-positive on Xpert than from those that were MTB-negative. The low culture yield reflects particular challenges with adequate storage and the time to transfer samples, with culture yield lowest in samples from Gulf Province, where laboratory services and transport logistics are the most challenging. The failure to send 18% of samples with ‘RR detected’ to the CPHL may be associated with a lack of International Air Transportation Association permits in some provinces, a lack of communication around sample collection and the absence of an effective monitoring system. Factors that decrease adherence to laboratory guidelines and increase transport time in this challenging environment require further research. The findings from this study support the need to further develop pragmatic guidelines for optimal Xpert implementation and ongoing quality assurance. The culture yield in this study was lower than that in reports from similar remote settings, and strategies to increase yield, such as site decontamination, could be explored; however, bacterial contamination rates were remarkably low.12–15 There is a need to perform careful cost-benefit analyses for new diagnostics in PNG and adequate in-country support is vital to ensure the reliability and sustainability of Xpert.16,17
Despite these limitations, the study provides a unique overview of DR-TB patterns observed in PNG. Data on first- and second-line drug resistance are required to inform the development of effective regimens for the programmatic management of RR/MDR-TB in different areas of PNG. For example, the combination of RIF resistance and INH susceptibility was most prevalent in DR-TB samples from Gulf Province and NCD. The reasons for this are not known. Contributing factors may have been the use of RIF to treat large numbers of leprosy cases from NCD and Gulf Province over a period of many decades, as well as historic drug stock-outs or inferior quality drugs that compromised first-line treatment, but this is speculative. MDR-TB with additional second-line drug resistance was observed in all provinces. Resistance to high-dose INH was very common in samples from Daru, compared to studies performed elsewhere.18 The co-resistance of ETH, streptomycin, PZA and ethambutol was also common in Daru. The unique DST pattern of this outbreak strain has been previously reported,6 and, given the treatment challenges of this unique strain, it is a matter of concern that it was also identified in NCD and other provinces. The national guidelines were revised in 2017 to exclude ETH from the standardised MDR-TB regimens in PNG.
CONCLUSION
The use of Xpert has increased the detection of RR-TB and MDR-TB in PNG. The high frequency of resistance to multiple drugs emphasises the need to accelerate the in-country availability of DST. Ongoing surveillance of DST patterns is essential to improve treatment guidelines, especially in the context of recent major changes to the WHO MDR-TB treatment guidelines.19
Acknowledgments
The authors thank the management of the Queensland Mycobacterium Reference Laboratory (Brisbane, QLD, Australia); the Central Public Health Laboratory and the provincial laboratories in PNG; and especially M Kaupa, J Dume and M Keket for their support .
This research was supported and facilitated by the first Operational Research Course for Tuberculosis in PNG, a specific training programme that was developed and implemented by the Burnet Institute (Melbourne, VIC, Australia) in collaboration with the PNG Institute of Medical Research (Goroka) and University of PNG (Port Moresby), and supported by the PNG National Department of Health Emergency Response Taskforce for MDR and XDR-TB, the National TB Programme and Western Provincial Health Office, Daru, PNG.
The training programme was delivered as part of the Tropical Disease Research Regional Collaboration Initiative, which is supported by the Australian Government and implemented by Menzies School of Health Research (Darwin, NT, Australia) and the Burnet Institute.
The views expressed in this publication are the authors' alone and are not necessarily the views of the Australian or PNG Governments. The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization Global tuberculosis report, 2018. Geneva, Swizterland: WHO; 2018. WHO/CDS/TB/2018.20. [Google Scholar]
- 2.Gilpin CM, Simpson G, Vincent S et al. Evidence of primary transmission of multidrug-resistant tuberculosis in the Western Province of Papua New Guinea. Med J Aust. 2008;188:148–152. doi: 10.5694/j.1326-5377.2008.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 3.Simpson G, Coulter C, Weston J et al. Resistance patterns of multidrug-resistant tuberculosis in Western Province, Papua New Guinea. Int J Tuberc Lung Dis. 2011;15:551–552. doi: 10.5588/ijtld.10.0347. [DOI] [PubMed] [Google Scholar]
- 4.Aia P, Kal M, Lavu E et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLoS One. 2016;11 doi: 10.1371/journal.pone.0149806. e0149806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kase P, Dakulala P, Bieb S. Outbreak of multidrug-resistant tuberculosis on Daru Island: an update. Lancet Respir Med. 2016;4:e40. doi: 10.1016/S2213-2600(16)30094-7. [DOI] [PubMed] [Google Scholar]
- 6.Bainomugisa A, Lavu E, Hiashiri S et al. Multi-clonal evolution of multi-drug-resistant/extensively drug-resistant Mycobacterium tuberculosis in a high-prevalence setting of Papua New Guinea for over three decades. Microb Genom. 2018;4:1–11. doi: 10.1099/mgen.0.000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aia P, Wangchuk L, Morishita F et al. Epidemiology of tuberculosis in Papua New Guinea: analysis of case notification and treatment-outcome data, 2008–2016. Western Pac Surveill Response J. 2018;9:1–11. doi: 10.5365/wpsar.2018.9.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boehme CC, Nicol MP, Nabeta P et al. Feasibility, diagnostic accuracy, and effectiveness of decentralised use of the Xpert MTB/RIF test for diagnosis of tuberculosis and multidrug resistance: a multicentre implementation study. Lancet. 2011;377:1495–1505. doi: 10.1016/S0140-6736(11)60438-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kohli M, Schiller I, Dendukuri N et al. Xpert® MTB/RIF assay for extrapulmonary tuberculosis and rifampicin resistance. Cochrane Database Syst Rev. 2018;8 doi: 10.1002/14651858.CD012768.pub2. CD012768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auld A, Fielding K, Gupta-Wright A, Lawn S. Xpert MTB/RIF—why the lack of morbidity and mortality impact in intervention trials? Trans R Soc Trop Med Hyg. 2016;110:432–444. doi: 10.1093/trstmh/trw056. [DOI] [PubMed] [Google Scholar]
- 11.Banamu J, Lavu E, Johnson K et al. Impact of GxAlert on management of rifampicin-resistant tuberculosis patients, Port Moresby, Papua New Guinea. Public Health Action. 2019:000–000. doi: 10.5588/pha.18.0067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ralph AP, Waramori G, Pontororing GJ et al. L-arginine and vitamin D adjunctive therapies in pulmonary tuberculosis: a randomised, double-blind, placebo-controlled trial. PLoS One. 2013;8 doi: 10.1371/journal.pone.0070032. e70032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lumb R, Ardian M, Waramori G et al. An alternative method for sputum storage and transport for Mycobacterium tuberculosis drug resistance surveys. Int J Tuberc Lung Dis. 2006;10:172–177. [PMC free article] [PubMed] [Google Scholar]
- 14.Rashid Ali MR, Parameswaran U, William T et al. A prospective study of mycobacterial viability in refrigerated, unpreserved sputum batched for up to 8 weeks. Int J Tuberc Lung Dis. 2015;19:620–621. doi: 10.5588/ijtld.14.0938. [DOI] [PubMed] [Google Scholar]
- 15.Maharjan B, Shrestha B, Weirich A, Stewart A, Kelly-Cirino CD. A novel sputum transport solution eliminates cold chain and supports routine tuberculosis testing in Nepal. J Epidemiol Glob Health. 2016;6:257–265. doi: 10.1016/j.jegh.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khaparde S, Raizada N, Nair SA et al. Scaling-up the Xpert MTB/RIF assay for the detection of tuberculosis and rifampicin resistance in India: an economic analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0184270. e0184270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albert H, Nathavitharana RR, Isaacs C, Pai M, Denkinger CM, Boehme CC. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur Respir J. 2016;48:516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization Multidrug-resistant TB (MDR-TB). 2017 update. Geneva, Switzerland: WHO; 2017. [Google Scholar]
- 19.World Health Organization WHO treatment guidelines for multidrug- and rifampicin-resistant tuberculosis. 2018 update. Geneva, Switzerland: WHO; 2018. [Google Scholar]


