Abstract
Setting:
A response to an outbreak of multidrug-resistant tuberculosis (MDR-TB) on Daru Island, South Fly District (SFD), Western Province, Papua New Guinea (PNG) was implemented by a national emergency response taskforce.
Objective:
To describe programmatic interventions for TB in SFD and evaluate characteristics of TB case notifications, drug resistance and treatment outcomes.
Design:
This was a retrospective cohort study based on routine programmatic data for all patients enrolled on TB treatment at Daru General Hospital from 2014 to 2017.
Results:
The response involved high-level political commitment, joint planning, resource mobilisation, community engagement and strengthening TB case detection and treatment. Of 1548 people enrolled on TB treatment, 1208 (78%) had drug-susceptible TB (DS-TB) and 333 (21.5%) had MDR-TB. There was an increase in MDR-TB as a proportion of all TB. Treatment success rates increased over the study period from 55% to 86% for DS-TB, and from 70% to 81% for MDR-TB from 2014 to 2015. The 2014 case notification rate for TB in SFD was 1031/100 000, decreasing to 736/100 000 in 2017.
Conclusion:
The outbreak was stabilised through the response from the national and provincial governments and international partners. Additional interventions are needed to decrease the TB burden in Daru.
Keywords: MDR-TB, outbreak, Daru, Papua New Guinea
Abstract
Contexte :
Reconnaissant une flambée de TB multirésistante (MDR-TB) en Papouasie Nouvelle Guinée (PNG), un groupe de travail d'urgence nationale a mis en œuvre une riposte sur l'ile de Daru, district de South Fly (SFD), province de l'Ouest.
Objectif :
Décrire les interventions des programmes relatives à la TB dans le SFD et évaluer les caractéristiques des notifications de cas de TB, la pharmacorésistance et les résultats du traitement.
Schema :
Une étude rétrospective de cohorte à partir de données de routine des programmes pour tous les patients enrôlés en traitement de TB à l'hôpital général de Daru de 2014 à 2017.
Resultats :
La riposte a impliqué un engagement politique de haut niveau, une planification conjointe, une mobilisation des ressources, un engagement des communautés et un renforcement de la détection des cas de TB et de leur traitement. Sur 1548 personnes enrôlées en traitement de TB, 1208 (78%) ont été des TB pharmacosensibles (DS) et 333 (21,5%) ont été des MDR-TB. Il y a eu une augmentation de la proportion des MDR-TB. Les taux de succès du traitement ont augmenté au long de la période d'étude de 55% à 86% pour la DS-TB et de 70% à 81% pour la MDR-TB en 2014–2015. Le taux de notification des cas de TB dans le SFD en 2014 a été de 1031 par 100 000, diminuant à 736 par 100 000 en 2017.
Conclusion :
La stabilisation de la flambée a été obtenue grâce à la riposte des gouvernements national et provinciaux, et des partenaires internationaux. Des interventions supplémentaires pourraient être requises pour réduire le poids de la TB à Daru.
Abstract
Marco de Referencia:
Al reconocer un brote epidémico de tuberculosis multirresistente (MDR-TB) en Papúa Nueva Guinea, el grupo especial de respuesta nacional al brote instauró una respuesta en la Isla Daru, del distrito South Fly, de la Provincia Occidental.
Objetivo:
Describir las intervenciones programáticas en materia de TB realizadas en el distrito South Fly y evaluar las características de las notificaciones de casos de tuberculosis, la farmacorresistencia y los desenlaces terapéuticos.
Método:
Se llevó a cabo un estudio retrospectivo de cohortes a partir de los datos programáticos corrientes de todos los pacientes inscritos para tratamiento en el Hospital General de la Isla Daru del 2014 al 2017.
Resultados:
La respuesta exigió un compromiso político de alto nivel, planeación conjunta, movilización de recursos, participación comunitaria y fortalecimiento de la detección y el tratamiento de los casos de TB. De las 1548 personas inscritas para recibir tratamiento antituberculoso, 1208 presentaban TB farmacosensible (78%) y 333 MDR-TB (21,5%). Se observó un aumento de la proporción de MDR-TB con respecto a todos los casos de TB. Las tasas de éxito terapéutico aumentaron durante el período del estudio de 55% a 86% para la tuberculosis farmacosensible y de 70% a 81% para la MDR-TB, a partir del 2014 y el 2015. La tasa de notificación de casos de TB en distrito South Fly en el 2014 fue 1031 por 100 000 habitantes y se redujo a 736 por 100 000 en el 2017.
Conclusión:
Se logró la estabilización del brote epidémico gracias a la respuesta del gobierno nacional y provincial y de los asociados internacionales. Es posible que se precisen nuevas intervenciones a fin de disminuir la carga de morbilidad por TB en Daru.
Multidrug-resistant tuberculosis (MDR-TB, defined as TB resistant to at least isoniazid and rifampicin) is a continuing global public health crisis and a major threat to achieving the World Health Organization's (WHO) target to end the TB epidemic by 2035. Globally, 458 000 new cases of MDR-TB were reported in 2017, with evidence of increasing incidence each year.1 MDR-TB emerges and spreads in populations served by weak health systems, and transmission continues due to gaps in detection, treatment and prevention. The burden of MDR-TB at the regional, country and sub-national levels is often unknown due to the lack of adequate diagnostics and surveillance, which requires molecular testing or specialised laboratories. As approximately 10% of MDR-TB globally is classified as extensively drug-resistant TB (XDR-TB, i.e., TB resistant to the fluoroquinolones and second-line injectable drugs) and 30% as either fluoroquinolone-resistant or injectable-resistant (pre-XDR-TB), further resistance would be a matter of concern.2
Papua New Guinea (PNG) is one of only 14 countries classified by the WHO as having the triple high burden of TB, MDR-TB and TB-human immunodeficiency virus (HIV) co-infection.1 The first report of MDR-TB in PNG was based on an audit of TB case notifications from clinics in the Australian Torres Strait Islands, bordering the South Fly District (SFD) of Western Province, which showed a dramatic increase in cases among PNG nationals between 1998 and 2002.3 Subsequent reports from the Torres Strait Islands clinics demonstrated alarming rates of MDR-TB, with evidence of person-to-person spread or primary transmission.4 In late 2011, Queensland Health (Brisbane, QLD, Australia) commenced the transfer of care of 92 PNG nationals with MDR-TB to the provincial hospital, Daru General Hospital (DGH) in SFD. This was in conjunction with specific financial support from the Australian Government to improve the management of TB in Western Province through the provision of clinical staff, a community engagement partner and the establishment of key infrastructure and service agreements (communications centre, TB ward, drug supply, laboratory support and an outreach boat).5 Case notifications of MDR-TB in Daru were increasing each year: from 59 cases in 2012, 61 in 2013, to 84 in 2014.6 In 2013, SFD reported TB case notifications were almost twice as high as the national rate. The treatment success rates for drug-susceptible TB (DS-TB) and MDR-TB in 2013 were respectively 65% and 50%.7
Recognising the alarming rates of MDR-TB indicating an outbreak, the emergence of XDR-TB and the need for a concerted programmatic response, the PNG National Department of Health (NDoH) convened an Emergency Response Taskforce for MDR-TB in Western Province and other ‘hot spot’ provinces, such as the Gulf District and the National Capital District, in August 2014.8 The response in SFD involved a multi-stakeholder partnership under the leadership of the provincial government and the NDoH.
The aim of the present study was to describe programmatic interventions for TB in SFD during the emergency response, from January 2014 to December 2017, and to evaluate the burden, trends and characteristics of TB case notifications, drug resistance and treatment outcomes during this period.
METHODS
Study design and study population
This is a retrospective cohort study of routine programmatic data from 1 January 2014 to 31 December 2017. The study participants were all registered for TB treatment at the Daru TB Basic Management Unit, which provides diagnosis and treatment for people from SFD.
Setting
Western Province is the largest but most sparsely populated province in PNG.9 The remoteness and limited roads make access to and delivery of health and essential services challenging. Health indicators, where available, are some of the worst in PNG.10 The Fly River divides the province into three districts, North, Middle and South Fly. Daru Island (population 15 142) is the main residential and service town in SFD (population 59 152) and is notably crowded for an island of approximately 15 km2 (average household size 7.5).11 DGH, the provincial referral hospital, is the only facility to provide TB diagnosis and treatment in SFD and is managed under the NDoH. It is a 100-bed provincial referral hospital providing inpatient and outpatient services, including specialist care. It has a large TB department with a 40-bed inpatient unit and ambulatory TB services. The Daru TB Basic Management Unit has management, supervision and monitoring responsibility for SFD. The provincial government leads the TB programme in Western Province with support from the Australian Government through international partners and the National TB Programme (NTP).
Diagnostic methods and treatment model
Two sputum samples were collected from presumptive TB patients where possible, and microscopy was performed at the DGH laboratory using Ziehl–Nielsen staining. If either specimen was smear-positive, one sample would undergo testing with Xpert® MTB/RIF (Cepheid, Sunnyvale, CA, USA) at DGH. From 2016, all presumptive TB patients were tested using Xpert and smear as the initial test. All samples with detected rifampicin resistance were transported by air to the supranational reference laboratory in Brisbane, QLD, Australia, for culture and/or drug susceptibility testing (DST), and from January 2017, the national TB reference laboratory in Port Moresby, PNG, for culture. The methods used for DST are described elsewhere.6 TB patients were managed according to the national protocols and WHO guidelines, which were revised for MDR-TB in 2016.12–14
Data collection, variables and analysis
Programme activities and interventions implemented over the study period were identified from implementation plans and project reports. Data on treatment cohorts were extracted from the programme's electronic medical records system (Bahmni v.0.87; Thoughtworks, Chicago, IL, USA) for MDR-TB and from the TB treatment registers for DS-TB. The data were extracted using automated scripts and imported into Stata v.15 (StataCorp, College Station, TX, USA) for validation, coding and analysis. All treatment episodes for TB were included, unless they were subsequently found not to have TB, in which case they were excluded. The data variables collected included age, sex, place of residence at time of registration, date of TB treatment initiation, TB site, registration category, diagnostic test results, outcomes, culture monitoring and DST results.
The current WHO definitions (2017 PNG guidelines) were used for DS-TB and MDR-TB enrolment and outcomes,15 with one modification: patients who had a permanent regimen change of at least two anti-tuberculosis drugs due to adverse drug reactions were not strictly classified as treatment failures if the patient was responding to treatment with no evidence of clinical or microbiological failure. In a retrospective review, patients who met this criterion had a change of the injectable to bedaquiline (which became part of the PNG protocol in 2016) or a drug not considered to be core in the regimen (e.g., para-amino salicylic or pyrazinamide). The denominator for case notification rates was taken from the last available PNG national census (2011).10 For 2014, case-level data on DS-TB patients were not available, and aggregate numbers from routine programme reports to the NTP were used instead. Numbers and proportions were calculated for cases registered and treatment outcomes. For geospatial analysis of disease burden, residence was linked to local-level government areas, and 2015 case notification rates for DS-TB and MDR-TB were used. The data were imported into MapInfo Pro v.16 (Pitney Bowes; Stamford, CT, USA) to generate maps.
Ethics
Ethics approval was provided by the PNG Medical Research Advisory Council, Port Moresby, PNG, and the Alfred Hospital Ethics Committee, Melbourne, VIC, Australia.
RESULTS
Joint strategic and implementation planning was conducted by the provincial TB programme and partners under the stewardship of the provincial and national governments in July 2015. The strategic interventions of the implementation plan, which was aimed to reduce TB transmission, were 1) to establish a model of patient-centred TB care for diagnosis, treatment and prevention of DS-TB and MDR-TB in Daru; 2) to strengthen health systems building blocks, adapted from the WHO framework,16 to enable a functional model of TB care; 3) to improve service utilisation and TB prevention through community engagement; 4) to use programme data for effective action; and, 5) to establish decentralised TB care at two sites in SFD in 2017. Table 1 shows the programmatic interventions implemented since the emergency response in 2014, grouped according to a framework used for response planning and monitoring.
TABLE 1.
Programmatic interventions undertaken for the TB emergency response in Daru, Western Province, Papua New Guinea, 2014–2017
| Strategic interventions | Programme status pre-response (2011–2014) | Emergency response (2014–2017) |
|---|---|---|
| Establish a model of patient-centred TB care for diagnosis, treatment and prevention of DS-TB and MDR-TB at Daru | ||
| Technical support | Supervisory visits from NTP | 2014: Standardised operating procedures for the clinical and programmatic management of TB introduced. Remote support from international TB and health systems experts |
| WHO monitoring missions | 2015: Daru-based international TB experts for clinical and programmatic mentoring and training | |
| Infection control plan and committee established WHO MDR-TB officer at national level. WHO regional Green Light Committee missions in 2015 and 2016 | ||
| Diagnosis, treatment, care and prevention | Model of care in line with DOTS strategy until 2011 when MDR-TB care initiated. Microscopy as frontline test | 2014: Strengthened standardised MDR-TB regimen with linezolid and clofazimine |
| Community-treatment support for DS-TB | 2015: Xpert® MTB/RIF adopted as front-line test for presumptive TB. DS-TB case management team established. Introduction of compassionate use bedaquiline. Standardised patient education and counselling | |
| No contact tracing or preventive therapy | 2016: Child MDR-TB protocols (gastric aspirate, dosing, nutrition) developed. Community treatment sites and supporters for MDR-TB with patient support package (nutrition and transport). Contact tracing for MDR-TB commenced. Bedaquiline donation programme accessed | |
| 2017: Introduction of shorter treatment regimens for MDR-TB. Scale up of contact tracing with expansion to DS-TB index case. Scale up of preventive therapy for DS-TB for children aged <5 years | ||
| Strengthen health systems building blocks to enable a functional model of TB care | ||
| Governance and co-ordination | Limited coordination due to complex governance structures: NTP for policy, guidelines and supervision. PHO for community services and DGH for facility care. Cross-border clinical collaborative group for TB | 2014: Formation of Provincial TB Core Group for programme management and implementation team meeting. NDoH Emergency Response Taskforce formed for stewardship, government/donor coordination, advocacy and resource mobilisation. Provincial TB physician leads the programme |
| 2015: Joint statement from NDoH and WHO on scale of Daru DR-TB outbreak. Provincial TB strategic plan and SFD implementation plan developed | ||
| 2016–2017: Annual implementation planning and review of progress | ||
| Financing | Minimal provincial financing for TB. Australian Government support from 2012 | 2014: Increase in Australian Government funding to engaged technical partner and additional human resources for TB programme |
| 2015: District health services funding for vehicles. Provincial funding for outreach and logistics | ||
| 2016: PNG Government emergency funding. Further Australian Government funding for community treatment support and research capacity building programme | ||
| 2017: Australian Government and World Bank/NDOH Emergency TB Project announced | ||
| Human resources | Daru TB Unit staff of 7 (including 2 medical officers) supported by a network of volunteer treatment supporters | 2015: Mobilisation of additional 11 TB health workers—including 2 medical officers, health extension officers, nurses, counsellor, laboratory officer, supply chain officer, data officer |
| Specialist TB medical officer commenced in 2011 | 2016: Treatment supporters were re-engaged with remuneration. Further 6 TB health workers recruited for contact tracing and decentralisation. International nursing educators engaged. DGH recruits medical and nursing staff (departmental structures re-established) | |
| 2017: Peer counselling team established | ||
| Information systems, supply chain and laboratory systems | Paper-based registers and reporting | 2015: Remote connectivity for Xpert. Laboratory protocols, indicators and training established |
| Routine stock outs of consumables and medical supplies | 2016: Electronic medical record system introduced for TB patient care. Electronic stock management tools National reference laboratory commenced culture | |
| Delays in lab turnaround time and results to patients | 2017: Electronic medical records system for TB care rolled out at Daru General Hospital | |
| Improve service utilisation and TB prevention through community engagement | ||
| Community engagement | Community advocacy and social mobilisation conducted by faith-based organisation | 2015: Formation of community stakeholder alliance in Daru, including community leaders and private sector |
| 2016: TB patient representatives elected. | ||
| 2017: TB survivors trained as peer counsellors | ||
| Programme data is utilised for effective action | ||
| Data utilisation and operational research | Routine NTP reporting | 2014: Routine reporting, data cleaning and case-level data collection for MDR-TB |
| 2015: Programme process indicators for continuous quality improvement established | ||
| 2016: Research prioritisation. Structured Operational Research Training Initiative (SORT-IT) conducted in Daru | ||
| 2017: Sociobehavioural research conducted and protocol for MDR-TB preventive therapy pilot developed | ||
| Decentralised TB care is established in SFD | ||
| Decentralised care and treatment | TB patients routinely accessing care in Australian Torres Strait until 2012. Medical sea ambulance for patient transport | 2014: District TB Officer commenced decentralised DS-TB care with outreach and follow-up. Retrieval of MDR-TB patients lost to care |
| 2016: Outreach boats (outboard) for patient transport and follow-up in South Fly. Decentralised MDR-TB care commenced in one site | ||
| 2017: Health facility assessments for decentralisation | ||
TB = tuberculosis; DS-TB = drug-susceptible TB; MDR-TB = multidrug-resistant TB; NTP = National TB Programme; WHO = World Health Organization; NDoH = National Department of Health; SFD = South Fly District; PNG = Papua New Guinea; PHO = Provincial Health Office; DGH = Daru General Hospital.
The case notification rate for all TB in SFD in 2014 was 1031 per 100 000 population and 736/100 000 in 2017. Figure 1 displays the annual number of enrolments for TB treatment for the 2196 people enrolled on TB treatment over the study period. Table 2 shows the demographic and clinical characteristics of the enrolled individuals. Among the 1541 patients from annual cohorts (2015–2017) with complete case-level data available, 1088 (70.6%) were from Daru and 26 (1.7%) were HIV-positive. The majority (68.7%) were aged 0–34 years; 18.9% (291/1540) were aged 10–19 years (adolescents, as defined by the WHO). MDR-TB accounted for 413 (18.8%) of all TB cases. Among all MDR-TB patients, 224 (54.2%) were registered as new, indicating that they had not been previously treated for TB. Among the 2017 MDR-TB patients, 81 (78.6%) were registered as new. Thirty-three (8%) of the MDR-TB patients included in the study had pre-XDR- or XDR-TB. The proportion of patients with bacteriological confirmation (smear, Xpert or culture positivity) was 85.4% (286/335) for drug-resistant TB and 50.5% (609/1206) for DS-TB from 2015 to 2017.
FIGURE 1.
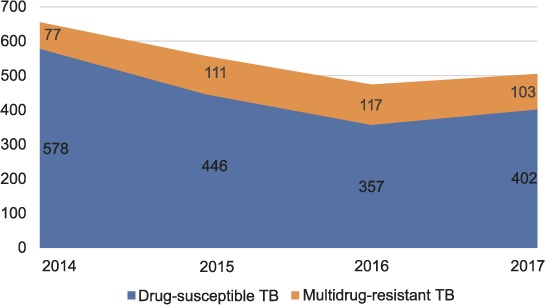
TB enrolments at Daru Basic Management Unit, Daru, Papua New Guinea, 2014–2017. TB = tuberculosis.
TABLE 2.
Baseline demographic and clinical characteristics of patients treated for TB at Daru Basic Management Unit, Daru, Papua New Guinea, 2014–2017
| Total n = 2196 n (%) | 2014* n = 655 n (%) | 2015 n = 561 n (%) | 2016 n = 475 n (%) | 2017 n = 505 n (%) | |
|---|---|---|---|---|---|
| Age group, years | |||||
| 0–4 | 117 (7.6) | 34 (6.1) | 39 (8.2) | 44 (8.7) | |
| 5–14 | 192 (12.5) | 80 (14.3) | 54 (11.4) | 58 (11.5) | |
| 15–34 | 749 (48.6) | 273 (48.7) | 228 (48.0) | 248 (49.1) | |
| 35–54 | 380 (24.7) | 142 (25.3) | 120 (25.3) | 118 (23.4) | |
| ⩾55 | 97 (6.3) | 27 (4.8) | 34 (7.2) | 36 (7.1) | |
| Unrecorded | 6 (0.4) | 5 (0.9) | 0 | 1 (0.2) | |
| Sex | |||||
| Male | 793 (51.5) | 277 (49.4) | 251 (52.8) | 265 (52.5) | |
| Female | 747 (48.5) | 283 (50.4) | 224 (47.2) | 240 (47.5) | |
| Unrecorded | 1 (0.1) | 1 (0.2) | 0 | 0 | |
| Residence | |||||
| In Daru | 1088 (70.6) | 397 (70.8) | 334 (70.3) | 357 (70.7) | |
| Outside Daru | 446 (28.9) | 162 (28.9) | 140 (29.5) | 144 (28.5) | |
| Not recorded | 7 (0.5) | 2 (0.4) | 1 (0.2) | 4 (0.8) | |
| HIV status | |||||
| Negative | 1057 (69.3) | 333 (59.9) | 310 (66.1) | 414 (82.6) | |
| Positive | 26 (1.7) | 8 (1.4) | 5 (1.1) | 13 (2.6) | |
| Not recorded | 443 (29.0) | 215 (38.7) | 154 (32.8) | 74 (14.8) | |
| Resistance | |||||
| DS-TB | 1783 (81.2) | 578 (88.2) | 446 (79.5) | 357 (75.2) | 402 (79.6) |
| RR-TB | 109 (5.0) | 28 (4.3) | 19 (3.4) | 20 (4.2) | 42 (8.3) |
| MDR-TB | 271 (12.3) | 47 (7.2) | 87 (15.5) | 88 (18.5) | 49 (9.7) |
| Pre-XDR-TB | 13 (0.6) | 0 | 1 (0.2) | 4 (0.8) | 8 (1.6) |
| XDR-TB | 20 (0.9) | 2 (0.3) | 8 (1.4) | 6 (1.3) | 4 (0.8) |
| Disease site | |||||
| Pulmonary | 994 (64.5) | 341 (60.8) | 302 (63.6) | 351 (69.5) | |
| Extrapulmonary | 542 (35.2) | 220 (39.2) | 173 (36.4) | 149 (29.5) | |
| Unrecorded | 5 (0.3) | 0 | 0 | 5 (1.0) | |
| Category | |||||
| New | 1262 (81.9) | 412 (73.4) | 392 (82.5) | 458 (90.7) | |
| Retreatment | 239 (15.5) | 137 (24.4) | 67 (14.1) | 35 (6.9) | |
| Other | 33 (2.1) | 6 (1.1) | 16 (3.4) | 11 (2.2) | |
| Unrecorded | 7 (0.5) | 6 (1.1) | 0 | 1 (0.2) | |
| Diagnosis | |||||
| Bacteriological | 895 (58.1) | 233 (41.5) | 317 (66.7) | 345 (68.3) | |
| Clinical | 643 (41.7) | 328 (58.5) | 158 (33.3) | 157 (31.1) | |
| Not recorded | 3 (0.2) | 0 | 0 | 3 (0.6) | |
* Case-level data were not available for 2014 therefore aggregate reports were used.
TB = tuberculosis; HIV = human immunodeficiency virus; DS-TB = drug-susceptible TB; RR-TB = rifampicin-resistant TB; MDR-TB = multidrug-resistant TB; XDR-TB = extensively drug-resistant TB.
The geospatial analyses of 2015 case notification rates by local-level government are shown on the map in Figure 2. Figure 3 shows the treatment outcomes in DS-TB patients enrolled on treatment from 1 January 2014 to 31 July 2017. The treatment success rate increased from 54% in 2014 to 86% in 2017. Figure 4 gives the MDR-TB treatment outcomes for the 2014 and 2015 annual cohorts. The treatment success rate increased from 70% to 81%. The 2016 semester 1 cohort (n = 67) had similar outcomes, with 82% having a successful outcome, 13% having died and 4% who remained in care at the time of analysis. There were no treatment failures or individuals lost to follow-up (LTFU).
FIGURE 2.

Geo-spatial distribution of TB patients enrolled on treatment at Daru Basic Management Unit, Daru, Papua New Guinea, 2015. LLG = local-level government; DR-TB = drug-resistant tuberculosis; CNR = case notification rate; DS-TB = drug-susceptible TB.
FIGURE 3.

Treatment outcomes for drug-susceptible TB patients enrolled on treatment at Daru Basic Management Unit, Daru, Papua New Guinea, 1 January 2014–31 July 2017. LTFU = lost to follow-up; TB = tuberculosis.
FIGURE 4.

Treatment outcomes for multidrug-resistant TB for patients enrolled on treatment at Daru Basic Management Unit, Daru, Papua New Guinea, 1 January 2014–31 December 2015. LTFU = lost to follow-up; TB = tuberculosis.
DISCUSSION
There is a very high burden of TB in SFD, Western Province. The case notification rates for MDR-TB on Daru Island are some of the highest reported at a subnational level, particularly in a setting with low HIV prevalence.17 The emergency response resulted in the establishment of a comprehensive programmatic response to TB and improved treatment outcomes for DS-TB and MDR-TB. Case notification trends indicate an overall stabilisation of the TB outbreak, however, with a decrease in DS-TB and increase in MDR-TB.
We report alarming TB case notification rates in Daru of 2021/100 000 for DS-TB and 594/100 000 for MDR-TB in 2015. The available epidemiological data on TB in PNG indicates that there is a TB epidemic nationally with a heterogeneous distribution of drug resistance.18 A drug resistance survey with a sample of 1182 patients from four provinces reported an MDR-TB rate in 2.7% of new and in 19.1% of previously treated patients.6 Of the total proportion of MDR-TB patients included in the study, 37% were from the National Capital District and 34% from Daru. Our findings of a geographical clustering of TB in Daru (71%), the high proportion of ‘new’ MDR-TB cases (82%), along with the high proportion of TB in children (20.1%) and adolescents (18.9%) is consistent with an epidemic driven by person-to-person transmission. A recent molecular epidemiology study has confirmed this to be an outbreak strain of MDR-TB with a long evolutionary history of over 30 years and a unique resistance profile.19 The study also highlighted the more recent emergence of XDR-TB over 10 years.19 A major factor likely to be contributing to TB transmission in Daru is overcrowding in homes and in the settlements on the island. This is compounded by the influx of people from surrounding villages in SFD who access services, including those who travel for compensation payments to mining-affected communities and to visit relatives.10 The contribution of other potential pathogens or host factors (such as diabetes, malnutrition), or transmission through contact outside the household, needs further investigation. It is notable that this outbreak does not appear to be driven by a co-existing HIV epidemic.
Programme reports demonstrate an emergency response focussed on the establishment of a comprehensive programmatic response to TB that has considered the models of care required, as well as the underlying needs to strengthen health systems that underpin the delivery of TB services. Subsequent to this response, increases in the proportion of patients successfully treated for DS-TB and MDR-TB (from 55% to 86% for DS-TB cohorts and from 70% to 82% for MDR-TB cohorts) and, notably, a very low LTFU rate were observed during the study period. Retention in care was achieved through a patient-centred care approach implemented by dedicated case managers (patient monitoring, care coordination), an education and counselling model that trained TB survivors or peers and treatment supporters (Table 1). Ambulatory care was provided at community treatment sites, along with nutritional transportation support as enablers. Community engagement was conducted through community chiefs, patient representatives and health promotion in congregate settings (market, churches and schools). The majority of patients resided on Daru Island or would stay with relatives during the initial period of treatment. The high death rates are of concern and need further investigation, although they may not be comparable to other cohorts where individuals LTFU may have died.
While there were significant challenges at all levels in implementation, the successful elements of the response were the confluence of high-level political commitment; the mobilisation of additional funding and resources to implement the response plan; and collaboration between technical partners and community engagement partners with government stakeholders under the leadership of provincial and hospital authorities. The NDoH, the WHO and partners in PNG called for additional funding in a ‘joint statement’ on the Daru outbreak.20,21 There were delays in resource mobilisation and funding for the joint plan developed after the emergency was declared in 2014 (Table 1), prompting advocacy.20 The response in the study period focused on detection and treatment of TB, as well as the reinforcement of facility and community-based systems with the introduction of diagnostic algorithms and treatment strategies that brought the programme into line with the evolving current global guidelines for MDR-TB. This included initiating patients on MDR-TB treatment using five effective drugs (including linezolid and clofazimine) and introducing bedaquiline into the programme in late 2015.
Case notification trends indicate a stabilisation of the TB outbreak with an observed increase in MDR-TB as a proportion of all TB cases during the study period. Responses to outbreaks of MDR-TB in Micronesia, in Tugela Ferry in South Africa and in industrialised countries have demonstrated that MDR-TB, and even XDR-TB outbreaks, can be rapidly controlled if there is a concerted effort on the part of governments who have high levels of political commitment coupled with support of multiple national and international agencies.22–24 These responses highlight the possibility of further reducing the TB burden in Daru by using comprehensive approaches that include patient-centred care and community-based case finding and preventive treatment, including for MDR-TB.25 In each of these outbreaks, responses need to be implemented through fragile health systems that are the antecedent cause. As such, common challenges exist at each level of the response and were also noted in Daru—political commitment from governments, resource mobilisation, the implementation of a comprehensive technical strategy, including research, building local capacity and engaging the affected community.
The strengths of our study included the fact that high-quality data were available from the programme's electronic medical records system, and from regular reports to describe and characterise the response and the TB outcomes. All enrolled patients on TB treatment at DGH over a 4-year period were recorded, with few missing data and enhanced collection of clinical and demographic characteristics. Furthermore, the programme documentation of interventions was comprehensive.
A key limitation was that we could not discern the effect of individual interventions on programme outcomes or determine what had the greatest effect or impact. The trends in case notification are influenced by the case detection strategy used. This was consistent since 2016, when Xpert was introduced as the front-line test, which may have led to the proportional increase in MDR-TB. Due to the lack of active case finding for TB outside Daru and limited access to TB services, there may have been an over-reporting bias of residents from Daru.
The MDR-TB outbreak was stabilised through the provision of effective interventions that improved patient care and treatment as a result of concerted efforts from the NDoH, the provincial government and international partners over several years. The developed model of care serves as a template for other provinces in PNG, and has already informed the 2017 national MDR-TB guidelines. While there has been significant progress, the TB burden in Daru and SFD remains extremely high; more ambitious approaches will be required to have a dramatic and swift impact. The situation remains fragile and vulnerable to interruptions in resources or programme delivery. The rates of unfavourable outcomes (death, treatment failure) need to be reduced, and further research to determine who is at risk would be useful. Population screening for active TB commenced on Daru Island in 2018 and the next steps planned by the programme are scaling up a key intervention of household contact tracing and preventive treatment, including high-risk MDR-TB contacts, instituting a capacity building framework to enable transition to the provincial programme and planning for delivery of MDR-TB services outside Daru.
Additional interventions are needed to reverse the TB outbreak in Daru, decentralise care and build capacity of the health system to sustainably manage the response. With the goal to end the global TB epidemic by 2035, there is an opportunity to promote the rapid uptake of novel tools and intervention strategies to expedite MDR-TB outbreak responses such as new molecular TB diagnostics, shorter, all-oral treatments, scaling up treatment for tuberculous infection and empowering community-driven service models. Research and innovation need to be at the forefront of the TB response and delivered equitably to vulnerable communities and health systems where the needs are greatest.
Acknowledgments
This research was conducted as part of the first Operational Research Course for Tuberculosis in Papua New Guinea (PNG). The specific training programme that resulted in this publication was developed and implemented by the Burnet Institute (Melbourne, VIC, Australia) in collaboration with the PNG Institute of Medical Research (Goroka) and University of PNG (Port Moresby), and supported by the PNG National Department of Health Emergency Response Taskforce for MDR and XDR-TB, the National TB Programme and Western Provincial Health Office, Daru, PNG. The model is based on the Structured Operational Research and Training Initiative (SORT IT), a global partnership led by the Special Programme for Research and Training in Tropical Diseases at the World Health Organization (WHO/TDR).
The authors acknowledge the support of the following individuals and institutions that contributed to this research and/or programme from which the operational research was conducted: the Fly River Provincial Government (Daru, PNG), the National Department of Health (Port Moresby, PNG), the Australian High Commission (Port Moresby, PNG), the Health and HIV Implementation Services Provider (Port Moresby, PNG), World Vision International (Port Moresby, PNG), the Burnet Institute (Melbourne, Australia), the World Health Organization (Port Moresby, PNG), A Nakamole, S Madjus, K Tal, R Raphael, P Quinlan, L Gonzales, P Wallis, K Chani, T Adepoyibi, J English, C Coulter, E Lavu, K Johnson, L John, S Bieb and P Kase. The training programme was delivered as part of the Tropical Disease Research Regional Collaboration Initiative, which is supported by the Australian Government and implemented by Menzies School of Health Research (Darwin, NT, Australia) and the Burnet Institute.
The views expressed in this publication are the authors' alone and are not necessarily the views of the Australian or PNG Governments. The funder had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Footnotes
Conflicts of interest: none declared.
References
- 1.World Health Organization Global tuberculosis report, 2018. Geneva, Switzerland: WHO; 2018. WHO/CDS/TB/2018.20. http://www.who.int/tb/publications/global_report/ Accessed July 2019. [Google Scholar]
- 2.Dheda K, Gumbo T, Maartens G et al. The epidemiology, pathogenesis, transmission, diagnosis, and management of multidrug-resistant, extensively drug-resistant, and incurable tuberculosis. Lancet Respir Med. 2017;5(4):291–360. doi: 10.1016/S2213-2600(17)30079-6. [DOI] [PubMed] [Google Scholar]
- 3.Simpson G, Clark P, Knight T. Changing patterns of tuberculosis in Far North Queensland. Med J Aust. 2006;184(5):252. doi: 10.5694/j.1326-5377.2006.tb00219.x. [DOI] [PubMed] [Google Scholar]
- 4.Gilpin CM, Simpson G, Vincent S et al. Evidence of primary transmission of multidrug-resistant tuberculosis in the Western Province of Papua New Guinea. Med J Aust. 2008;188(3):148–152. doi: 10.5694/j.1326-5377.2008.tb01557.x. [DOI] [PubMed] [Google Scholar]
- 5.Australian Government Tackling tuberculosis in Western Province, Papua New Guinea. Canberra, ACT, Australia: AusAid; 2012. https://dfat.gov.au/about-us/publications/Documents/western-province-tb-strategy-summary.pdf Accessed July 2019. [Google Scholar]
- 6.Aia P, Kal M, Lavu E et al. The burden of drug-resistant tuberculosis in Papua New Guinea: results of a large population-based survey. PLoS One. 2016;11(3) doi: 10.1371/journal.pone.0149806. e0149806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Government of Papua New Guinea Monitoring and evaluation, annual report 2013. Port Morseby, Papua New Guinea: Tuberculosis Control Program; 2014. [Google Scholar]
- 8.Pascoe K, Dakulala P, Bieb S. Outbreak of multidrug-resistant tuberculosis on Daru Island: an update. Lancet Respir Med. 2016;4(8):e40. doi: 10.1016/S2213-2600(16)30094-7. [DOI] [PubMed] [Google Scholar]
- 9.National Statistical Office Demographic and Health Survey. 2006 National Report. Port Moresby, Papua New Guinea: NSO; 2009. https://www.nso.gov.pg/index.php/projects/demographic-health-survey/48-dhs Accessed July 2019. [Google Scholar]
- 10.Busilacchi S, Butler J, Van Putten I, Maru Y, Posu J. Asymmetrical development across transboundary regions: the case of the Torres Strait Treaty Region (Australia and Papua New Guinea) Sustainability. 2018;10(11) 4200. https://www.mdpi.com/2071-1050/10/11/4200/htm Accessed July 2019. [Google Scholar]
- 11.National Statistics Office 2011 Housing and Population Census of Papua New Guinea—final figures. Port Moresby, Papua New Guinea: NSO; 2014. https://www.nso.gov.pg/index.php/projects/censuses Accessed July 2019. [Google Scholar]
- 12.National Department of Health Papua New Guinea country guidelines for the programmatic management of drug-resistant tuberculosis. Port Moresby, Papua New Guinea: National TB programme unit, Disease Control Branch, National Department of Health; 2011. [Google Scholar]
- 13.Falzon D, Jaramillo E, Schünemann HJ et al. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J. 2011;38(3):516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 14.World Health Organization WHO guidelines for the treatment of drug-resistant tuberculosis. 2016 update. Geneva, Switzerland: WHO; 2016. WHO/HTM/TB/2016.04. [Google Scholar]
- 15.World Health Organization Definitions and reporting framework for tuberculosis—2013 revision. Geneva, Switzerland: WHO; 2013. WHO/HTM/TB/2013.2. http://www.who.int/tb/publications/definitions/en/ Accessed July 2019. [Google Scholar]
- 16.World Health Organization: Everybody's business: strengthening health systems to improve health outcomes. WHO's Framework for Action. Geneva, Switzerland: WHO; 2007. [Google Scholar]
- 17.Furin J, Cox H. Outbreak of multidrug-resistant tuberculosis on Daru Island. Lancet Respir Med. 2016;4(5):347–349. doi: 10.1016/S2213-2600(16)00101-6. [DOI] [PubMed] [Google Scholar]
- 18.Aia P, Wangchuk L, Morishita F et al. Epidemiology of tuberculosis in Papua New Guinea : analysis of case notification and treatment-outcome data, 2008–2016. Western Pac Surveill Response J. 2018;9(2):9–19. doi: 10.5365/wpsar.2018.9.1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bainomugisa A, Lavu E, Hiashiri S et al. Multi-clonal evolution of multi-drug-resistant/extensively drug-resistant Mycobacterium tuberculosis in a high-prevalence setting of Papua New Guinea for over three decades. Microb Genom. 2018;4(2) doi: 10.1099/mgen.0.000147. e000147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papua New Guinea Department of Health, World Health Organization Drug-resistant TB: an extraordinary situation requires extraordinary measures. Port Morseby, Papua New Guinea: DOH; 2015. https://www.burnet.edu.au/system/asset/file/2085/Joint_Statement_TB-Mtg_25Nov2015_1_pdf Accessed July 2019. [Google Scholar]
- 21.World Health Organization, Western Pacific Regional Office Update on the situation of drug-resistant tuberculosis in Papua New Guinea, with special emphasis on Daru Island. Port Moresby, Papua New Guinea: WHO papua New Guinea Representative Office; 2016. http://www.wpro.who.int/papuanewguinea/areas/tb_leprosy/daru_update/en/ Accessed July 2019. [Google Scholar]
- 22.Centers for Disease Control and Prevention Two simultaneous outbreaks of multidrug-resistant tuberculosis—Federated States of Micronesia, 2007–2009. MMWR Morb Mortal Wkly Rep. 2009;58(10):253–256. [PubMed] [Google Scholar]
- 23.Gandhi NR, Weissman D, Moodley P et al. Nosocomial transmission of extensively drug-resistant tuberculosis in a rural hospital in South Africa. J Infect Dis. 2013;207(1):9–17. doi: 10.1093/infdis/jis631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frieden TR, Fujiwara PI, Washko RM, Hamburg MA. Tuberculosis in New York City—turning the tide. N Engl J Med. 1995;333(4):229–233. doi: 10.1056/NEJM199507273330406. [DOI] [PubMed] [Google Scholar]
- 25.Bamrah S, Brostrom R, Dorina F et al. Treatment for LTBI in contacts of MDR-TB patients, Federated States of Micronesia, 2009–2012. Int J Tuberc Lung Dis. 2014;18(8):912–918. doi: 10.5588/ijtld.13.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]


