Abstract
Immunotherapy has shown promising results in cancer treatment. Research shows that most patients might be resistant to these therapies. So, new immune therapies are needed. OX40 (CD134) and OX40 ligand (OX40L), costimulatory molecules, express on different types of immune cells. The interaction between OX40 and OX40L (OX40/OX40L) induces the expansion and proliferation of T cells and decreases the immunosuppression of regulatory T (Treg) cells to enhance the immune response to the specific antigen. For the important role OX40 takes in the process of immunity, many clinical trials are focusing on OX40 to find out whether it may have active effects in clinical cancer treatment. The results of clinical trials are still not enough. So, we reviewed the OX40 and its ligand (OX40L) function in cancer, clinical trials with OX40/OX40L and the correlation between OX40/OX40L and other immune checkpoints to add more ideas to tumor feasible treatment.
Keywords: cancer, immune checkpoints, OX40/OX40L, immunotherapy
Immunotherapy has shown promising results in cancer treatment,1 cancer immune checkpoint blockades also have got good results.2–5 It was demonstrated that combining cancer vaccines or checkpoint inhibitors with different immunotherapeutic agents could augment the anti-tumor effects and get better results in cancer patients.6,7
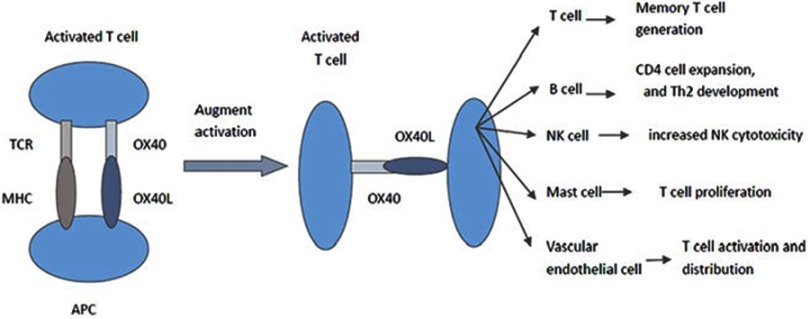
Tumor necrosis factor receptor superfamily member 4 (OX40) (CD134) and OX40 ligand (OX40L) (CD134L) (CD252) are on chromosome 1. The OX40 and OX40L could be expressed by endothelial cells, mast cells, activated natural killer (NK) cells, dendritic cells (DCs), B cells, microglial cells, activated T cells and Foxp3+ regulatory T cells.8–10 OX40L could initiate OX40 signals in activated T cells. OX40L on T cells could provide signals via the interactions between T cells and upregulate the anti-apoptotic protein on T cells to enhance T cell survival, cytokine production and induce the CD4 memory T cell expansion.11–16 The co-stimulation in B cells through the OX40/OX40L pathway contributed to CD4 cell generation, survival and T helper 2 (Th2) development.17 OX40/OX40L could promote NK cell activation, cytokine production and cytotoxicity and enhance targeted cells lysis.18,19 Mast cell via the OX40/OX40L pathway could induce T cell proliferation.20,21 OX40 on Treg cells played an important role in Treg cell development and homeostasis.22,23 We made a figure to clarify the function of OX40-OX40L pathway (Figure 1).
Figure 1.
OX40–OX40L interaction model.
Abbreviations: Th2, T helper 2; NK, natural killer; TCR, T cell receptor; MHC, major histocompatibility complex; APC, antigen presenting cell.
OX40/OX40L and diseases
Many diseases were associated with OX40/OX40L, so many researchers focused on it to find new way of treatment. The activation of OX40 promoted the generation and expansion of activated T cells and memory T cells, thus aggravating autoimmune diseases like Graves’ disease, autoimmune arthritis and uveitis.24–27 OX40 was critically important in sustaining the anti-viral immune response during the viral infection.19,28–30 OX40–OX40L signaling increased the adaptive immune response to an allograft by promoting effector and memory T cell survival. And blockade of OX40–OX40L interaction could decrease the T cells infiltration in the targeted organs to prevent allograft rejection.31–34 OX40L could promote the inflammatory cells infiltration into lesional tissues, leading to the pathological fibrosis in skin and internal organs. And blocking OX40–OX40L regressed the fibrosis.35,36 OX40–OX40L interaction on immune cells might contribute to idiopathic inflammatory myopathies through different pathways in the inflamed muscle.37 OX40/OX40L pathway was involved in the pathological process of Crohn’s disease (CD). And blockade anti-OX40 might be beneficial for the treatment by controlling the T cell-mediated inflammatory in vivo.38,39 Data implicated that OX40/OX40L participated in pathophysiology of acute myeloid leukemiaand also enhanced NK cell cytotoxicity.18
OX40/OX40L and cancer
OX40 was expressed on the tumor-infiltrating lymphocytes (TIL) in head and neck squamous cell carcinoma, ovarian cancer, gastric cancer, cutaneous squamous cell carcinoma, breast cancer and colorectal cancer.40–45 Agonistic anti-OX40 antibodies had anti-tumor effects.46–52 OX40 triggering regressed Treg cells, allowing DCs to reach the draining lymph nodes and prime the specific CD8 lymphocytes response to the tumor.48,53 Many research focused on the anti-tumor immunotherapy, based on activating costimulatory molecules OX40 and OX40L. Here, we showed some of them (Table 1).
Table 1.
OX40/OX40L and cancer
| Disease | Finding | References |
|---|---|---|
| Cancer | Anti-OX40L delayed the tumor progression and even eradicated tumors. | 54 |
| Breast cancer | Activation of OX40 receptor+ CD4+ T cells could stimulate the anti-tumor immune response in mammary cancer. | 55 |
| Colon cancer | High levels of OX40 positive lymphocytes were correlated with better survival in colon cancers. | 56 |
| Cancer | OX40L fusion protein could inhibit the tumor by direct intra-tumor injection. | 9 |
| Cancer | OX40L-transduced tumor cells could elicit tumor-specific Th1 immune responses, generate anti-tumor immunity and inhibit the tumor growth in vivo. | 57 |
| Cancer | OX40 agnostic therapy contributed to anti-tumor CD8 effector T (Teff) cells priming and enhanced CD8 T cell response to the antigen tumor derived. | 58–60 |
| Cancer | Intra peritoneal injection of OX40L-immunoglobulin fusion protein could inhibit tumor growth. | 61 |
| Cancer | OX40L on DCs could induce anti-tumor immunity via binding OX40 on CD4+ T cells and NK T cells. | 62 |
| Advanced cancer | Agonistic anti-OX40 increased circulating T cells, B cells and intratumoral Tregs, enhancing tumor-specific immune responses. | 49 |
| Cancer | Agonist anti-OX40 therapy combined with cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) blockade augmented antigen-specific CD8 T cells and limited the Th2 cells polarization, eliciting potent anti-tumor immunity. | 63,64 |
| Cancer | OX40 agonistic and IDO (indoleamine-(2,3)-dioxygenase) inhibitor produced a synergistic effect on the tumor immune response. | 65 |
| Glioma | Agonist anti-OX40 immunotherapy was active against intracranial glioma. | 66 |
| Metastatic ovarian cancer | Combining anti-OX40 and anti-CD73 immunostimulants increased cytotoxic T cell infiltration and decreased tumor promoting immune cells. | 67 |
Abbreviations: NK, natural killer; DCs, dendritic cells; Th2, T helper 2.
Clinical trials of OX40/OX40L
Based on the role of OX40 and OX40L in the immune system, more and more research focused on its therapeutic effects. Many companies detected the immune checkpoints OX40 and OX40L, searching for the new approaches to treat tumors and autoimmune diseases, many of which are now making great advance in clinical development (Table 2). The results of clinical trials showed the OX40, as a potent immune-stimulating target, played an important role in anti-tumor therapy. The agonist anti-OX40 increased CD4 FoxP3− and CD8 T cells proliferation and the response to the tumor-specific antigen, enhancing both humoral and cellular immunity in cancer treatment.49
Table 2.
Clinical trials with anti-OX40
| Year | Drug | Phase | Company | Type | Objective | Clinical trial.gov identifier |
|---|---|---|---|---|---|---|
| 2012 | OX40 mAb | I | Providence Health & Services | Anti-OX40 | OX40 in patients with advanced cancer | NCT01644968 |
| 2014 | Anti-OX40 | II | Ludwig Institute for Cancer Research | Anti-OX40 | Combining a mouse monoclonal anti-OX40 and Ipilimumab in metastatic melanoma patients | NCT01689870 |
| 2014 | MEDI6469 | I | Providence Health & Services | Anti-OX40 | MEDI6469 applied pre-surgical resection patients with oral, head and neck squamous-cell carcinoma | NCT02274155 |
| 2014 | Pf-04518600 | I | Pfizer | OX40 Agonist | Pf-04518600 and Pf-05082566 in selected partially advanced or metastatic cancers | NCT02315066 |
| 2015 | MOXR0916 | I | Genentech, Inc. | Anti-OX40 | MOXR0916 and atezolizumab (anti-PD-L1) in locally advanced or metastatic tumors | NCT02410512 |
| 2015 | MEDI6469 | I | Providence Health & Services | Anti-OX40 | MEDI6469 in patients with metastatic colorectal cancer | NCT02559024 |
| 2017 | PF-04518600 | II | University of Southern California | Anti-OX40 | PF-04518600 in combination with axitinib versus axitinib in metastatic renal cell carcinoma and exposed to immune checkpoint inhibitor | NCT03092856 |
| 2017 | MEDI0562 | I | Providence Health & Services | Anti-OX40 | MEDI0562 administered pre-surgical resection in melanoma or squamous cell carcinoma | NCT03336606 |
| 2018 | BMS 986178 | I | Ronald Levy | Anti-OX40 | Intratumoral injection of SD-101 and BMS-986178 combined with local radiation in patients with low-grade B cell lymphomas | NCT03410901 |
Correlation of OX40/OX40L and other immune checkpoints
The results of studies suggested that some diseases were not sensitive to antibody therapy alone. So, it was necessary to study on the relationship between checkpoints to work out more effective treatment. CTLA-4, a molecule on T cells, inhibited the proliferation of T cells and cytokine production, thus limiting the lymphocyte immune reaction.68–72 Anti-CTLA-4 blockade induced the depletion of Treg cells within tumor and activation of Teff cells.71,73–76 Combining agonist anti-OX40 and antagonist anti-CTLA-4 further enhanced CD4 and CD8 T cells responses to antigen, indicating they had synergistic effects in improving tumor regression.77–79 And the cytokine of Th1 and Th2 CD4 T cells increased significantly.64 Whether the combination therapy altered the suppressive function of Treg cells remained deeper exploration.63,64 The combination was still more than the sum of its part.80
Programmed death-1 (PD-1) is a molecule that suppresses the immune reaction, inducing T cell exhaustion and apoptosis. Programmed death-ligand 1 (PD-L1), expressed on tumor cells or other tumor-related immune cells, could suppress anti-tumor immune response.81–84 The function of PD-1 and PD-L1 was affected by the complex immunoregulation. PD-1 blockade had already been used in cancer treatment and got a satisfying result.82,84 It was reported that PD-1 inhibitor added at the initiation of the cancer treatment could reduce the effects of OX40 agonist antibody, for it might cause the antigen-specific CD8+ T cell diminishment.85 And timing of PD-1 blockade using might determine whether it was effective immunotherapy when combined with OX40 therapy.81 In most cases, OX40 agonist and PD-1 blockade had a synergistic effect in disease treatment. OX40, combined with CD27 mediated co-stimulation, could synergize with PD-L1 inhibitor by activating CD8+ T cells.86 Combining OX40 stimulation and PD-L1 blockade could synergistically augment hepatitis B virus (HBV)-specific CD4 T cell responses by promoting Th cells to secrete IFN-γ and IL-21 in patients with HBV infection.87 In some poorly immunogenic tumors, combining PD-1 blockade and OX40 stimulation had an anti-tumor effect by inducing cytotoxic T lymphocyte, increasing the Teff cells and decreasing the immunosuppressive cells, while individual did not.41
4-1BB (CD137), member of the TNFR family enhanced T cell proliferation, effector function and cytokines production, and induced maturation of DC, thus increasing the immune reaction.88–93 Agonistic anti- 4-1 BB increased the TIL within tumor and upregulated the expression of 4-1 BB on the immune cells, augmenting anti-tumor reaction.90,94,95 The costimulatory pathway of OX40–OX40L and 4-1 BB-4-1 BBL functioned independently to enhance immune cells response.88 The combination of OX40 agonist and 4-1BB agonist induced profound expansion of CD8 T cell.96,97 But the response of CD4 T cell to the dual costimulation seemed to be additive instead of synergistic.98 On the whole, the combination therapy could synergistically inhibit cancer by producing more enhanced signals.98,99
Acknowledgment
This study was supported in part by grants from National Natural Science Foundation of China (81802255), Shanghai Pujiang Program (17PJD036), Shanghai Municipal Commission of Health and Family Planning Program (20174Y0131), National Key Research & Development Project (2016YFC0902300), Major Disease Clinical Skills Enhancement Program of three year action plan for promoting clinical skills and clinical innovation in municipal hospitals, Shanghai Shen Kang Hospital Development Center Clinical Research Plan of SHDC (16CR1001A),“Dream Tutor" Outstanding Young Talents Program (fkyq1901), Key Disciplines of Shanghai Pulmonary Hospital (2017ZZ02012), and Shanghai Science and Technology Commission (16JC1405900).
Summary
Immune checkpoints play vital roles in cancer treatment. It was proved that the agonist anti-OX40/OX40L could enhance anti-tumor response by promoting the function of immune cells. More and more researchers focused on OX40/OX40L in cancer immunotherapy. But until now, the effects of OX40/OX40L treatment are still limited. Researchers are devoted to combine OX40/OX40L with other immune checkpoints in cancer treatment, which had also made some achievements, but the mechanisms of the synergy between OX40/OX40L and other immune checkpoints still need to be further studied.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;342:1432–1433. doi: 10.1126/science.342.6165.1432 [DOI] [PubMed] [Google Scholar]
- 2.He Y, Rivard CJ, Rozeboom L, et al. Lymphocyte-activation gene-3, an important immune checkpoint in cancer. Cancer Sci. 2016;107:1193–1197. doi: 10.1111/cas.12986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Y, Rozeboom L, Rivard CJ, et al. PD-1, PD-L1 protein expression in non-small cell lung cancer and their relationship with tumor-infiltrating lymphocytes. Med Sci Monit. 2017;23:1208–1216. doi: 10.12659/msm.899909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.He Y, Rozeboom L, Rivard CJ, et al. MHC class II expression in lung cancer. Lung Cancer. 2017;112:75–80. doi: 10.1016/j.lungcan.2017.07.030 [DOI] [PubMed] [Google Scholar]
- 5.He Y, Yu H, Rozeboom L, et al. LAG-3 protein expression in non-small cell lung cancer and its relationship with PD-1/PD-L1 and tumor-infiltrating lymphocytes. J Thorac Oncol. 2017;12:814–823. doi: 10.1016/j.jtho.2017.01.019 [DOI] [PubMed] [Google Scholar]
- 6.Bilusic M, Madan RA, Gulley JL. Immunotherapy of prostate cancer: Facts and hopes. Clin Cancer Res. 2017;23:6764–6770. doi: 10.1158/1078-0432.ccr-17-0019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dushyanthen S, Teo ZL, Caramia F, et al. Agonist immunotherapy restores T cell function following MEK inhibition improving efficacy in breast cancer. Nat Commun. 2017;8:606. doi: 10.1038/s41467-017-00728-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carboni S, Aboul-Enein F, Waltzinger C, Killeen N, Lassmann H, Peña-Rossi C. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145:1–11. [DOI] [PubMed] [Google Scholar]
- 9.Ali SA, Ahmad M, Lynam J, et al. Anti-tumour therapeutic efficacy of OX40L in murine tumour model. Vaccine. 2004;22:3585–3594. doi: 10.1016/j.vaccine.2004.03.041 [DOI] [PubMed] [Google Scholar]
- 10.Kim BS, Kim JY, Kim EJ, et al. Role of thalidomide on the expression of OX40, 4-1BB, and GITR in T cell subsets. Transplant Proc. 2016;48:1270–1274. doi: 10.1016/j.transproceed.2015.12.088 [DOI] [PubMed] [Google Scholar]
- 11.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176:5975–5987. doi: 10.4049/jimmunol.176.10.5975 [DOI] [PubMed] [Google Scholar]
- 12.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15:445–455. [DOI] [PubMed] [Google Scholar]
- 13.Jones RG, Parsons M, Bonnard M, et al. Protein kinase B regulates T lymphocyte survival, nuclear factor kappaB activation, and Bcl-X(L) levels in vivo. J Exp Med. 2000;191:1721–1734. doi: 10.1084/jem.191.10.1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kinnear G, Wood KJ, Marshall D, Jones ND. Anti-OX40 prevents effector T-cell accumulation and CD8+ T-cell mediated skin allograft rejection. Transplantation. 2010;90:1265–1271. doi: 10.1097/TP.0b013e3181fe5396 [DOI] [PubMed] [Google Scholar]
- 15.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188:297–304. doi: 10.1084/jem.188.2.297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vu MD, Clarkson MR, Yagita H, Turka LA, Sayegh MH, Li XC. Critical, but conditional, role of OX40 in memory T cell-mediated rejection. J Immunol. 2006;176:1394–1401. doi: 10.4049/jimmunol.176.3.1394 [DOI] [PubMed] [Google Scholar]
- 17.Linton P-J, Bautista B, Biederman E, et al. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nuebling T, Schumacher CE, Hofmann M, et al. The immune checkpoint modulator OX40 and its ligand OX40L in NK-cell immunosurveillance and acute myeloid leukemia. Cancer Immunol Res. 2018;6:209–221. doi: 10.1158/2326-6066.CIR-17-0212 [DOI] [PubMed] [Google Scholar]
- 19.Pollmann J, Götz -J-J, Rupp D, et al. Hepatitis C virus-induced natural killer cell proliferation involves monocyte-derived cells and the OX40/OX40L axis. J Hepatol. 2018;68:421–430. doi: 10.1016/j.jhep.2017.10.021 [DOI] [PubMed] [Google Scholar]
- 20.Turaj AH, Cox KL, Penfold CA, et al. Augmentation of CD134 (OX40)-dependent NK anti-tumour activity is dependent on antibody cross-linking. Sci Rep. 2018;8:2278. doi: 10.1038/s41598-018-20656-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li F, Wang Y, Lin L, et al. Mast cell-derived exosomes promote Th2 cell differentiation via OX40L-OX40 ligation. J Immunol Res. 2016;2016:1–10. doi: 10.1155/2016/3623898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Takeda I, Ine S, Killeen N, et al. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580 [DOI] [PubMed] [Google Scholar]
- 23.Piconese S, Pittoni P, Burocchi A, et al. A non-redundant role for OX40 in the competitive fitness of Treg in response to IL-2. Eur J Immunol. 2010;40:2902–2913. doi: 10.1002/eji.201040505 [DOI] [PubMed] [Google Scholar]
- 24.Wang Q, Shi B-M, Xie F, et al. Enhancement of CD4(+) T cell response and survival via coexpressed OX40/OX40L in Graves’ disease. Mol Cell Endocrinol. 2016;430:115–124. doi: 10.1016/j.mce.2016.04.008 [DOI] [PubMed] [Google Scholar]
- 25.Jiang J, Liu C, Liu M, et al. OX40 signaling is involved in the autoactivation of CD4(+)CD28(-) T cells and contributes to the pathogenesis of autoimmune arthritis. Arthritis Res Ther. 2017;19:67. doi: 10.1186/s13075-017-1261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu X, Rosenbaum JT, Adamus G, et al. Activation of OX40 prolongs and exacerbates autoimmune experimental uveitis. Invest Ophthalmol Vis Sci. 2011;52:8520–8526. doi: 10.1167/iovs.11-7664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshioka T, Nakajima A, Akiba H, et al. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30:2815–2823. doi: [DOI] [PubMed] [Google Scholar]
- 28.Boettler T, Moeckel F, Cheng Y, et al. OX40 facilitates control of a persistent virus infection. PLoS Pathog. 2012;8:e1002913. doi: 10.1371/journal.ppat.1002913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahiliani V, Hutchinson TE. OX40 cooperates with ICOS to amplify follicular th cell development and germinal center reactions during infection. J Immunol. 2017;198:218–228. doi: 10.4049/jimmunol.1601356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boettler T, Choi YS, Salek-Ardakani S, et al. Exogenous OX40 stimulation during lymphocytic choriomeningitis virus infection impairs follicular Th cell differentiation and diverts CD4 T cells into the effector lineage by upregulating Blimp-1. J Immunol. 2013;191:5026–5035. doi: 10.4049/jimmunol.1300013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Curry AJ, Chikwe J, Smith XG, et al. OX40 (CD134) blockade inhibits the co-stimulatory cascade and promotes heart allograft survival. Transplantation. 2004;78:807–814. doi: 10.1097/01.tp.0000131670.99000.54 [DOI] [PubMed] [Google Scholar]
- 32.Tripathi T, Yin W, Xue Y, et al. Central roles of OX40L-OX40 interaction in the induction and progression of human T cell-driven acute graft-versus-host disease. ImmunoHorizons. 2019;3:110–120. doi: 10.4049/immunohorizons.1900001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li T, Ma R, Zhu J, Wang F, Huang L, Leng X. Blockade of the OX40/OX40L pathway and induction of PD-L1 synergistically protects mouse islet allografts from rejection. Chin Med J. 2014;127:2686–2692. [PubMed] [Google Scholar]
- 34.Kinnear G, Wood KJ, Fallah-Arani F, Jones ND. A diametric role for OX40 in the response of effector/memory CD4+ T cells and regulatory T cells to alloantigen. J Immunol. 2013;191:1465–1475. doi: 10.4049/jimmunol.1300553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Elhai M, Avouac J, Hoffmann-Vold AM, et al. OX40L blockade protects against inflammation-driven fibrosis. Proc Natl Acad Sci U S A. 2016;113:E3901–E3910. doi: 10.1073/pnas.1523512113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boleto G, Allanore Y, Avouac J. Targeting costimulatory pathways in systemic sclerosis. Front Immunol. 2018;9:2998. doi: 10.3389/fimmu.2018.02998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Papadopoulos C, Terzis G, Papadimas GK, Manta P. OX40-OX40L expression in idiopathic inflammatory myopathies. Anal Quant Cytol Histol. 2013;35:17–26. [PubMed] [Google Scholar]
- 38.Totsuka T, Kanai T, Uraushihara K, et al. Therapeutic effect of anti-OX40L and anti-TNF-alpha MAbs in a murine model of chronic colitis. Am J Physiol Gastrointest Liver Physiol. 2003;284:G595–G603. doi: 10.1152/ajpgi.00450.2002 [DOI] [PubMed] [Google Scholar]
- 39.Griseri T, Asquith M, Thompson C, Powrie F. OX40 is required for regulatory T cell-mediated control of colitis. J Exp Med. 2010;207:699–709. doi: 10.1084/jem.20091618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bell RB, Leidner RS, Crittenden MR, et al. OX40 signaling in head and neck squamous cell carcinoma: overcoming immunosuppression in the tumor microenvironment. Oral Oncol. 2016;52:1–10. doi: 10.1016/j.oraloncology.2015.11.009 [DOI] [PubMed] [Google Scholar]
- 41.Guo Z, Wang X, Cheng D, et al. PD-1 blockade and OX40 triggering synergistically protects against tumor growth in a murine model of ovarian cancer. PLoS One. 2014;9:e89350. doi: 10.1371/journal.pone.0089350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Martins MR, Santos RLD, Jatahy KDN, et al. Could OX40 agonist antibody promote activation of the anti-tumor immune response in gastric cancer? J Surg Oncol. 2018;117:840–844. doi: 10.1002/jso.25001 [DOI] [PubMed] [Google Scholar]
- 43.Lai C, August S, Albibas A, et al. OX40+ regulatory T cells in cutaneous squamous cell carcinoma suppress effector T-cell responses and associate with metastatic potential. Clin Cancer Res. 2016;22:4236–4248. doi: 10.1158/1078-0432.ccr-15-2614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamidinia M, Ghafourian Boroujerdnia M, Talaiezadeh A, Solgi G, Taghdiri M, Khodadadi A. Concomitant increase of OX40 and FOXP3 transcripts in peripheral blood of patients with breast cancer. Iran J Immunol. 2013;10:22–30. doi:IJIv10i1A3 [PubMed] [Google Scholar]
- 45.Weixler B, Cremonesi E, Sorge R, et al. OX40 expression enhances the prognostic significance of CD8 positive lymphocyte infiltration in colorectal cancer. Oncotarget. 2015;6:37588–37599. doi: 10.18632/oncotarget.5940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang P, Tu GH, Wei J, et al. Ligand-Blocking and Membrane-Proximal Domain Targeting Anti-OX40 Antibodies Mediate Potent T Cell-Stimulatory and Anti-Tumor Activity. Cell Rep. 2019;27:3117–3123.e5. doi: 10.1016/j.celrep.2019.05.027 [DOI] [PubMed] [Google Scholar]
- 47.Aspeslagh S, Postel-Vinay S, Rusakiewicz S, Soria J-C, Zitvogel L, Marabelle A. Rationale for anti-OX40 cancer immunotherapy. Eur J Cancer. 2016;52:50–66. doi: 10.1016/j.ejca.2015.08.021 [DOI] [PubMed] [Google Scholar]
- 48.Buchan SL, Rogel A, Al-Shamkhani A. The immunobiology of CD27 and OX40 and their potential as targets for cancer immunotherapy. Blood. 2018;131:39–48. doi: 10.1182/blood-2017-07-741025 [DOI] [PubMed] [Google Scholar]
- 49.Curti BD, Kovacsovics-Bankowski M, Morris N, et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 2013;73:7189–7198. doi: 10.1158/0008-5472.CAN-12-4174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Foote JB, Kok M, Leatherman JM, et al. A STING agonist given with OX40 receptor and PD-L1 modulators primes immunity and reduces tumor growth in tolerized mice. Blood. 2017;5:468–479. doi: 10.1182/blood-2017-07-741025; 10.1158/2326-6066.cir-16-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moran AE, Kovacsovics-Bankowski M, Weinberg AD. The TNFRs OX40, 4-1BB, and CD40 as targets for cancer immunotherapy. Curr Opin Immunol. 2013;25:230–237. doi: 10.1016/j.coi.2013.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanmamed MF, Pastor F, Rodriguez A, et al. Agonists of co-stimulation in cancer immunotherapy directed against CD137, OX40, GITR, CD27, CD28, and ICOS. Semin Oncol. 2015;42:640–655. doi: 10.1053/j.seminoncol.2015.05.014 [DOI] [PubMed] [Google Scholar]
- 53.Weinberg AD, Rivera MM, Prell R, et al. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164:2160–2169. doi: 10.4049/jimmunol.164.4.2160 [DOI] [PubMed] [Google Scholar]
- 54.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60:5514–5521. [PubMed] [Google Scholar]
- 55.Morris A, Vetto JT, Ramstad T, et al. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67:71–80. [DOI] [PubMed] [Google Scholar]
- 56.Petty JK, He K, Corless CL, Vetto JT, Weinberg AD. Survival in human colorectal cancer correlates with expression of the T-cell costimulatory molecule OX-40 (CD134). Am J Surg. 2002;183:512–518. doi: 10.1016/s0002-9610(02)00831-0 [DOI] [PubMed] [Google Scholar]
- 57.Andarini S, Kikuchi T, Nukiwa M, et al. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64:3281–3287. [DOI] [PubMed] [Google Scholar]
- 58.Song A, Tang X, Harms KM, Croft M. OX40 and Bcl-xL promote the persistence of CD8 T cells to recall tumor-associated antigen. J Immunol. 2005;175:3534–3541. doi: 10.4049/jimmunol.175.6.3534 [DOI] [PubMed] [Google Scholar]
- 59.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68:5206–5215. doi: 10.1158/0008-5472.CAN-07-6484 [DOI] [PubMed] [Google Scholar]
- 60.Pham Minh N, Murata S, Kitamura N, et al. In vivo antitumor function of tumor antigen-specific CTLs generated in the presence of OX40 co-stimulation in vitro. Int J Cancer. 2018;142:2335–2343. doi: 10.1002/ijc.31244 [DOI] [PubMed] [Google Scholar]
- 61.Assudani DP, Ahmad M, Li G, Rees RC, Ali SA. Immunotherapeutic potential of DISC-HSV and OX40L in cancer. Cancer Immunol Immunother. 2006;55:104–111. doi: 10.1007/s00262-005-0004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zaini J, Andarini S, Tahara M, et al. OX40 ligand expressed by DCs costimulates NKT and CD4+ Th cell antitumor immunity in mice. J Clin Invest. 2007;117:3330–3338. doi: 10.1172/JCI32693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Cancer Immunol Res. 2014;2:142–153. doi: 10.1158/2326-6066.CIR-13-0031-T [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Linch SN, Kasiewicz MJ, McNamara MJ, Hilgart-Martiszus IF, Farhad M, Redmond WL. Combination OX40 agonism/CTLA-4 blockade with HER2 vaccination reverses T-cell anergy and promotes survival in tumor-bearing mice. Proc Natl Acad Sci U S A. 2016;113:E319–E327. doi: 10.1073/pnas.1510518113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Berrong Z, Mkrtichyan M, Ahmad S, et al. Antigen-specific antitumor responses induced by OX40 agonist are enhanced by the IDO inhibitor indoximod. Cancer Immunol Res. 2018;6:201–208. doi: 10.1158/2326-6066.CIR-17-0223 [DOI] [PubMed] [Google Scholar]
- 66.Jahan N, Talat H, Curry WT. Agonist OX40 immunotherapy improves survival in glioma-bearing mice and is complementary with vaccination with irradiated GM-CSF-expressing tumor cells. Neuro-oncology. 2018;20:44–54. doi: 10.1093/neuonc/nox125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Virani NA, Thavathiru E, McKernan P, Moore K, Benbrook DM, Harrison RG. Anti-CD73 and anti-OX40 immunotherapy coupled with a novel biocompatible enzyme prodrug system for the treatment of recurrent, metastatic ovarian cancer. Cancer Lett. 2018;425:174–182. doi: 10.1016/j.canlet.2018.03.027 [DOI] [PubMed] [Google Scholar]
- 68.Chikuma S. CTLA-4, an essential immune-checkpoint for T-cell activation. Curr Top Microbiol Immunol. 2017;410:99–126. doi: 10.1007/82_2017_61 [DOI] [PubMed] [Google Scholar]
- 69.Chen X, Shao Q, Hao S, et al. CTLA-4 positive breast cancer cells suppress dendritic cells maturation and function. Oncotarget. 2017;8:13703–13715. doi: 10.18632/oncotarget.14626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Shi L, Meng T, Zhao Z, et al. CRISPR knock out CTLA-4 enhances the anti-tumor activity of cytotoxic T lymphocytes. Gene. 2017;636:36–41. doi: 10.1016/j.gene.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 71.van Hooren L, Sandin LC, Moskalev I, et al. Local checkpoint inhibition of CTLA-4 as a monotherapy or in combination with anti-PD1 prevents the growth of murine bladder cancer. Eur J Immunol. 2017;47:385–393. doi: 10.1002/eji.201646583 [DOI] [PubMed] [Google Scholar]
- 72.Zhao Y, Yang W, Huang Y, Cui R, Li X, Li B. Evolving roles for targeting CTLA-4 in cancer immunotherapy. Cell Physiol Biochem. 2018;47:721–734. doi: 10.1159/000490025 [DOI] [PubMed] [Google Scholar]
- 73.Selby MJ, Engelhardt JJ, Quigley M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1:32–42. doi: 10.1158/2326-6066.cir-13-0013 [DOI] [PubMed] [Google Scholar]
- 74.Subrahmanyam PB, Dong Z, Gusenleitner D, et al. Distinct predictive biomarker candidates for response to anti-CTLA-4 and anti-PD-1 immunotherapy in melanoma patients. J Immunother Cancer. 2018;6:18. doi: 10.1186/s40425-018-0328-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wang B, Qin L, Ren M, Sun H. Effects of combination of anti-CTLA-4 and anti-PD-1 on gastric cancer cells proliferation, apoptosis and metastasis. Cell Physiol Biochem. 2018;49:260–270. doi: 10.1159/000492876 [DOI] [PubMed] [Google Scholar]
- 76.Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and anti-PD-1 checkpoint blockade. Cell. 2017;170:1120–1133.e17. doi: 10.1016/j.cell.2017.07.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goncalves-Lopes RM, Lima NF, Carvalho KI, Scopel KKG, Kallás EG, Ferreira MU. Surface expression of inhibitory (CTLA-4) and stimulatory (OX40) receptors by CD4(+) regulatory T cell subsets circulating in human malaria. Microbes Infect. 2016;18:639–648. doi: 10.1016/j.micinf.2016.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Montler R, Bell RB, Thalhofer C, et al. OX40, PD-1 and CTLA-4 are selectively expressed on tumor-infiltrating T cells in head and neck cancer. Clin Transl Immunol. 2016;5:e70. doi: 10.1038/cti.2016.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Redmond WL, Linch SN, Kasiewicz MJ. Combined targeting of costimulatory (OX40) and coinhibitory (CTLA-4) pathways elicits potent effector T cells capable of driving robust antitumor immunity. Proc Natl Acad Sci U S A. 2014;2:142–153. doi: 10.1073/pnas.1510518113; 10.1158/2326-6066.cir-13-0031-t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Linch SN, Redmond WL. Combined OX40 ligation plus CTLA-4 blockade: more than the sum of its parts. Oncoimmunology. 2014;3:e28245. doi: 10.4161/onci.28245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Messenheimer DJ, Jensen SM, Afentoulis ME, et al. Timing of PD-1 blockade is critical to effective combination immunotherapy with anti-OX40. Clin Cancer Res. 2017;23:6165–6177. doi: 10.1158/1078-0432.CCR-16-2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chamoto K, Al-Habsi M, Honjo T. Role of PD-1 in immunity and diseases. Curr Top Microbiol Immunol. 2017;410:75–97. doi: 10.1007/82_2017_67 [DOI] [PubMed] [Google Scholar]
- 83.Kuol N, Stojanovska L, Nurgali K, Apostolopoulos V. PD-1/PD-L1 in disease. Immunotherapy. 2018;10:149–160. doi: 10.2217/imt-2017-0120 [DOI] [PubMed] [Google Scholar]
- 84.Salmaninejad A, Khoramshahi V, Azani A, et al. PD-1 and cancer: molecular mechanisms and polymorphisms. Immunogenetics. 2018;70:73–86. doi: 10.1007/s00251-017-1015-5 [DOI] [PubMed] [Google Scholar]
- 85.Shrimali RK, Ahmad S, Verma V, et al. Concurrent PD-1 blockade negates the effects of OX40 agonist antibody in combination immunotherapy through inducing T-cell apoptosis. Cancer Immunol Res. 2017;5:755–766. doi: 10.1158/2326-6066.CIR-17-0292 [DOI] [PubMed] [Google Scholar]
- 86.Buchan S, Manzo T, Flutter B, et al. OX40- and CD27-mediated costimulation synergizes with anti-PD-L1 blockade by forcing exhausted CD8+ T cells to exit quiescence. J Immunol. 2015;194:125–133. doi: 10.4049/jimmunol.1401644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Jacobi FJ, Wild K, Smits M, et al. OX40 stimulation and PD-L1 blockade synergistically augment HBV-specific CD4 T cells in patients with HBeAg-negative infection. J Hepatol. 2019;70:1103–1113. doi: 10.1016/j.jhep.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 88.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173:5944–5951. doi: 10.4049/jimmunol.173.10.5944 [DOI] [PubMed] [Google Scholar]
- 89.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J Immunol. 2001;167:1313–1324. doi: 10.4049/jimmunol.167.3.1313 [DOI] [PubMed] [Google Scholar]
- 90.Chester C, Ambulkar S, Kohrt HE. 4-1BB agonism: adding the accelerator to cancer immunotherapy. Cancer Immunol Immunother. 2016;65:1243–1248. doi: 10.1007/s00262-016-1829-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chester C, Sanmamed MF, Wang J, Melero I. Immunotherapy targeting 4-1BB: mechanistic rationale, clinical results, and future strategies. J Biol Chem. 2018;131:49–57. doi: 10.1074/jbc.M117.814905; 10.1182/blood-2017-06-741041. [DOI] [PubMed] [Google Scholar]
- 92.Lu Y, Li C, Du S, et al. 4-1BB signaling promotes alveolar macrophages-mediated pro-fibrotic responses and crystalline silica-induced pulmonary fibrosis in mice. Front Immunol. 2018;9:1848. doi: 10.3389/fimmu.2018.01848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oh HS, Choi BK, Kim YH, et al. 4-1BB signaling enhances primary and secondary population expansion of CD8+ T cells by maximizing autocrine IL-2/IL-2 receptor signaling. PLoS One. 2015;10:e0126765. doi: 10.1371/journal.pone.0126765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bartkowiak T, Curran MA. 4-1BB agonists: multi-potent potentiators of tumor immunity. Front Oncol. 2015;5:117. doi: 10.3389/fonc.2015.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Sakellariou-Thompson D, Forget M-A, Creasy C, et al. 4-1BB agonist focuses CD8(+) tumor-infiltrating T-cell growth into a distinct repertoire capable of tumor recognition in pancreatic cancer. Clin Cancer Res. 2017;23:7263–7275. doi: 10.1158/1078-0432.ccr-17-0831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Munks MW, Mourich DV, Mittler RS, Weinberg AD, Hill AB. 4-1BB and OX40 stimulation enhance CD8 and CD4 T-cell responses to a DNA prime, poxvirus boost vaccine. Immunology. 2004;112:559–566. doi: 10.1111/j.1365-2567.2004.01917.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hendriks J, Xiao Y, Rossen JWA, et al. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175:1665–1676. doi: 10.4049/jimmunol.175.3.1665 [DOI] [PubMed] [Google Scholar]
- 98.Lee SJ, Myers L, Muralimohan G, et al. 4-1BB and OX40 dual costimulation synergistically stimulate primary specific CD8 T cells for robust effector function. J Immunol. 2004;173:3002–3012. doi: 10.4049/jimmunol.173.5.3002 [DOI] [PubMed] [Google Scholar]
- 99.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177:4464–4472. doi: 10.4049/jimmunol.177.7.4464 [DOI] [PubMed] [Google Scholar]